Abstract
Polyamine transport activities have been described in diverse multicellular systems, but their bioenergetic mechanisms and molecular identity remain unclear. In the present paper, we describe a high-affinity spermine/spermidine transport activity expressed in Drosophila S2 cells. Ion-replacement experiments indicate that polyamine uptake across the cell membrane is Na+-, K+-, Cl−- and Ca2+-independent, but pH-sensitive. Additional experiments using ionophores suggest that polyamine uptake may be H+-coupled. Pharmacological experiments show that polyamine uptake in S2 cells is selectively blocked by MGBG {methylglyoxal bis(guanylhydrazone) or 1,1′-[(methylethanediylidine)-dinitrilo]diguanidine} and paraquat (N,N-dimethyl-4,4′-bipyridylium), two known inhibitors of polyamine uptake in mammalian cells. In addition, inhibitors known to block the Slc22 (solute carrier 22) family of organic anion/cation transporters inhibit spermine uptake in S2 cells. These data and the genetic tools available in Drosophila will facilitate the molecular identification and further characterization of this activity.
Keywords: choline, Drosophila S2 cell, polyamine transport, solute carrier 22 (Slc22), spermine, spermidine
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; CCCP, carbonyl cyanide m-chlorophenylhydrazone; MDM, minimal Drosophila medium; MGBG, methylglyoxal bis(guanylhydrazone); NMDG, N-methyl-D-glucamine; Slc, solute carrier
INTRODUCTION
Polyamines are a family of low-molecular-mass cations containing two to four amine moieties separated by methylene groups. Naturally occurring polyamines include spermine, spermidine, putrescine and cadaverine, and are found in practically all living organisms [1]. Polyamines bind directly to DNA and RNA, acidic phospholipids and some ion channels [2,3] and thereby modulate their function. Additionally, polyamines may modulate signal transduction pathways via mechanisms that remain unclear [4]. In general, the modulatory effects of polyamines have been associated with increased cell growth and proliferation [2], although recent evidence suggests that they may be involved in programmed cell death as well [5].
The effects of polyamines on cell-cycle regulation have made polyamine metabolism an important target for the chemotherapeutic treatment of cancer. Polyamine synthesis, metabolism, substrate binding and uptake are all potential targets for antineoplasic drugs [6,7]. Historically, antiproliferative drugs have targeted biosynthetic enzymes, but endogenous uptake mechanisms rapidly compensate for decreased synthesis, thus limiting the therapeutic effects of these drugs. Therefore more recent efforts have shifted to blocking uptake through competitive antagonism of polyamine transport activities [6,8–10]. However, since the molecular identities of the polyamine transporter(s) in mammals and other complex eukaryotes are not known, the development of drugs that block these activities has been difficult.
In addition to their role in dividing cells, polyamines are critical for the signalling properties of mature neurons. Spermine in particular is an endogenous antagonist of inwardly rectifying K+ channels, which are essential for maintaining the resting potential across the neuronal plasma membrane [3]. In addition, polyamines regulate the function of glutamate receptors: Ca2+-permeable AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid) receptors are inwardly rectifying owing to an intracellular spermine block [11]. Furthermore, polyamine-induced changes in the function of AMPA receptors have been reported to regulate the integrative properties of tectal neurons [12], underscoring the importance that polyamines have in modulating neuronal signalling. Similarly, NMDA (N-methyl-D-aspartate)-type glutamate receptors can be blocked by extracellular spermine [3], and since tetany can eliminate this blockade, this phenomenon has been suggested to contribute to some models of cellular learning [13]. The potential to reduce Ca2+ flux through glutamate receptors also suggests that polyamines may have a neuroprotective effect.
Despite the potential significance of polyamine uptake activity for both neuronal activity and cell growth, polyamine transport in eukaryotes is poorly understood. In mammals, polyamine uptake across the plasma membrane has been proposed to be carrier-mediated, energy-dependent and saturable [14], and many reports suggest that transport may be Na+-independent [15–18]. However, the bioenergetics of polyamine transport and the ionic gradients to which it may be coupled remain unclear. Moreover, although the existence of polyamine transport activities in both prokaryotic and some eukaryotic cells has been thoroughly established [15–17,19–24], only the prokaryote and unicellular eukaryote transporters have been identified [25–30].
In the present study, we report a specific spermine/spermidine transport activity in Drosophila melanogaster S2 (Schneider line 2) cells, the first such activity described in a model genetic system, and we have characterized its kinetics, ionic requirements and pharmacological profile. This activity shows specific affinity for spermine and spermidine, but not putrescine, is dependent on H+, but not Na+, and shows a pharmacological profile strikingly similar to that of the Slc22 (solute carrier 22) family of solute carriers. These data will facilitate further experiments for the molecular identification and characterization of the spermine/spermidine transporter in Drosophila and perhaps in other species as well.
MATERIALS AND METHODS
Reagents
[14C]Spermine tetrahydrochloride (113 Ci/mol) and [14C]spermdine trihydrochoride (112 Ci/mol) were obtained from Amersham Biosciences. Schneider's Drosophila medium and foetal bovine serum were purchased from Gibco, and penicillin/streptomycin (10000 units/ml) was obtained from Cellgro. Agmatine, Ala-Gln, Ala-Gly, L-arginine, L-asparagine, cadaverine, CCCP (carbonyl cyanide m-chlorophenylhydrazone), L-carnitine, L-carnosine, choline, cimetidine, desipramine, L-glutamine, histamine, L-histidine, lactate, L-lysine, NMDG (N-methyl-D-glucamine), MGBG {methylglyoxal bis-(guanylhydrazone) or 1,1′-[(methylethanediylidine)-dinitrilo]diguanidine}, MPP+ (1-methyl-4-phenylpyridinium iodide), nigericin, DL-ornithine, ouabain, paraquat (N,N-dimethyl-4,4′-bipyridylium), L-proline, potassium cyanide, putrescine, pyruvate, quinidine, serotonin, spermidine, spermine, TEA (tetra-ethylammonium), thiamine, valinomycin and verapamil were all obtained from Sigma–Aldrich. The sodium ionophore SQl-Pr was purchased from Calbiochem. Stock solutions for all substrates were prepared in double-distilled water, except quinidine, which was prepared in 100% methanol; cimetidine, which was prepared in 0.1 M HCl; CCCP, nigericin and valinomycin, which were prepared in absolute ethanol; and SQl-Pr, which was prepared in DMSO.
MDM (minimal Drosophila S2 cell medium)
To ensure reliability in the transport assays, we developed an MDM containing only salts and glucose. MDM essentially replicates the concentrations of inorganic salts in Schneider's modified Drosophila medium. All other components were iso-osmotically replaced with glucose to maintain an osmolarity of ∼300 mosM. MDM contains 36 mM NaCl, 21.5 mM KCl, 9.1 mM KH2PO4, 14 mM Na2HPO4, 15 mM MgSO4, 4 mM CaCl2 and 99.4 mM glucose, pH 6.8.
For the ion replacement experiments, NaCl and KCl were iso-osmotically replaced by choline chloride, LiCl, NMDG, sucrose or each other. Cl−-free medium was made with NaNO3, KNO3 and Ca(NO3)2 or sodium gluconate, potassium gluconate and calcium gluconate. Ca2+-free medium was made by replacing CaCl2 with MgCl2. In the Na+/K+-free medium, Mops, pH-adjusted with Ca(OH)2, was used to replace the phosphates. The different pH MDMs were prepared by correspondingly changing the ratio of mono- and di-basic phosphate salts.
Cell cultures
Drosophila S2 cells were cultured at 22 °C (room temperature) in 10 cm cell-culture plates using Schneider's Drosophila medium (Gibco) supplemented with 10% foetal bovine serum (Gibco) and 100 units/ml penicillin/streptomycin (Cellgro). Plates were incubated to confluence before harvesting. The S2 cell medium was aspirated, and the cells were washed gently with 2 ml of normal MDM, pH 6.8, before being resuspended in 10 ml of the appropriate MDM. The final cell densities ranged from 106 to 107 cells/ml. Cells numbers and viability were determined using a haemocytometer and Trypan Blue exclusion. Only cell batches with >95% viability were used for further experiments.
Transport assays
All transport assays were performed using 500 nM [14C]spermine, except the concentration-dependence experiment where 50 nM to 10 μM [14C]spermine or [14C]spermidine were used. When unlabelled substrates were used, they were added immediately before the radiolabelled substrate. All experiments were performed in triplicate.
A 500 μl volume of Drosophila S2 cell suspension was added to 2.0 ml centrifuge tubes. The correct volume of radiolabelled substrate was added directly to the suspension for the desired final concentration. Cells were agitated gently and incubated at 22 °C or on ice (0 °C) for the specified amount of time. Cells were then pelleted by centrifugation at 5000 g for 30 s and washed with 2×1.5 ml of ice-cold MDM. Centrifugation was sufficient to stop the reaction (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/393/bj3930583add.htm). The cell pellets were dissolved in 100 μl of 0.2 M NaOH and 1% (w/v) SDS and transferred to scintillation tubes. Scintillation cocktail (Ecolume, ICN Radiochemicals) was added to the tubes, and counts were obtained using a Packard TriCarb 2300 scintillation counter. The counting efficiency for 14C isotopes was approx. 80%.
For kinetic measurements, we subtracted the values obtained at 0 °C from transport measurements obtained at 22 °C to ensure that all values reflected only uptake rather than non-specific binding. Lineweaver–Burk transformations were used to obtain measurements of Km and Vmax.
Ionophore experiments
[14C]Spermine was added to 500 μl aliquots of S2 cells either immediately after the addition of the drugs (0 min pre-incubation) or after 5 min (5 min pre-incubation), and uptake measurements were taken after an additional 5 min incubation with the radiolabelled substrate in normal MDM. The reactions were stopped as specified above. CCCP, nigericin and valinomycin were tested at concentrations of 500 nM. SQl-Pr [31] and KCN were tested at concentrations of 10 μM and 500 μM respectively. The concentration of vehicle was never larger than 0.1% in any given experiment.
Data analysis
All data collected from the scintillation spectrometry were normalized to cell number and specific activity using Microsoft Excel and are expressed as the means±S.E.M. or means±range. Statistical significance was determined by unpaired, two-tailed Student's t test or a two-way ANOVA with a Tukey's post-hoc test using the Prism 4 statistical package. Linear/non-linear regressions were obtained using SigmaPlot 8.0.
RESULTS
Drosophila S2 cells show spermine and spermidine uptake
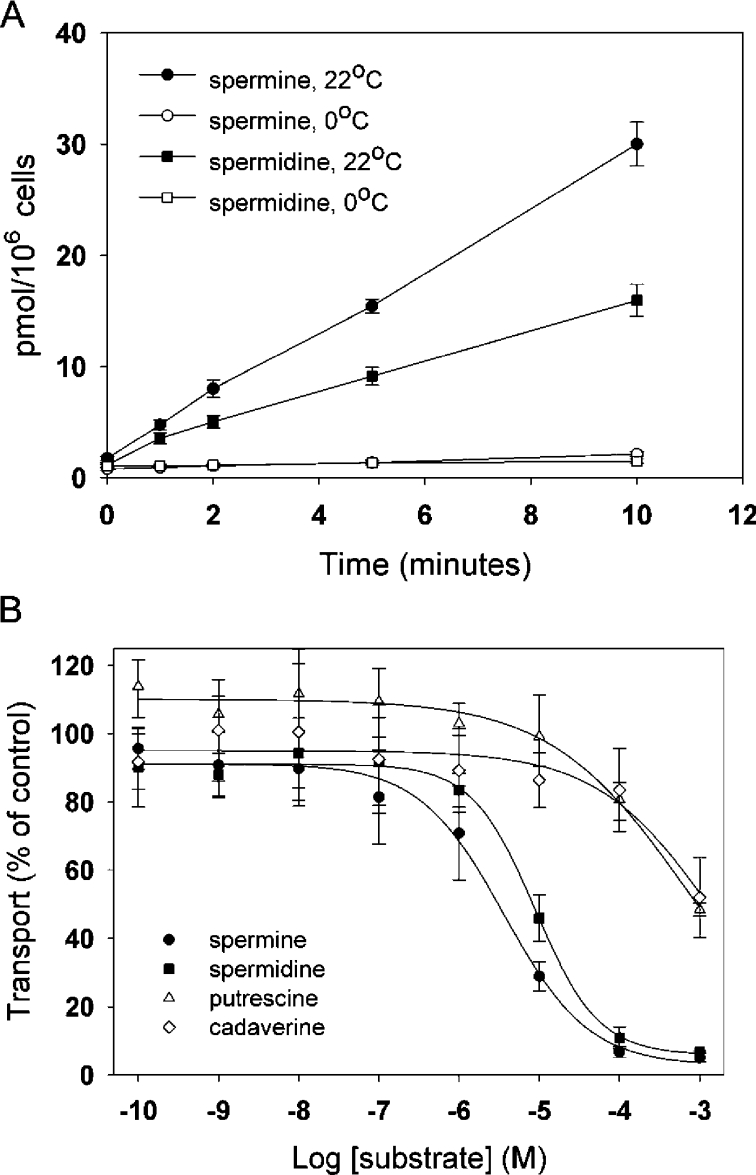
To determine whether S2 cells express a detectable polyamine transport activity, we quantified uptake of radiolabelled substrate into intact cells. Our initial experiments using a filtration-based assay resulted in consistently high background (results not shown). We therefore used a simple and robust centrifugation-based transport assay (see the Materials and methods section). To minimize non-specific inhibition by biological amines, these assays were performed using MDM that contains a more limited set of salts and other osmolytes. Trypan Blue exclusion indicated that at least 95% of the cells were viable in MDM throughout the entire course of each experiment (results not shown). Since most available data suggest that spermine and spermidine share a similar transport mechanism [2], we assayed the uptake of both substrates into S2 cells. We observed transport using either 500 nM [14C]spermine or [14C]spermidine at 22 °C, but not at 0 °C. The effect of temperature indicates that the measurements we obtained are likely to be the result of an energy-dependent transport mechanism rather than non-specific binding of substrate to the plasma membrane (Figure 1A).
Figure 1. Drosophila S2 cells show specific spermine and spermidine uptake.
(A) Time course of spermine and spermidine uptake in Drosophila S2 cells. At zero time, 500 nM [14C]spermine (●, ○) or [14C]spermidine (■, □) was added to Drosophila S2 cells as described in the Materials and methods section. A robust time-dependent increase in the amount of label associated with S2 cells was observed at 22 °C, but not at 0 °C, indicating that these measurements reflect temperature-dependent transport rather than non-specific binding. Results are means±S.E.M. for three independent experiments performed in triplicate. (B) Inhibition of [14C]spermine uptake by different polyamines. Varying concentrations of unlabelled spermine (●), spermidine (■), putrescine (△) and cadaverine (◇) were added to Drosophila S2 cells incubated with 500 nM [14C]spermine. Results are means±range for two independent experiments performed in triplicate. See the text for IC50 values for spermine and spermidine.
Transport of both spermine and spermidine appeared to be essentially linear for 1–10 min, suggesting that a convenient intermediate time point might be used for further analyses. To confirm this, we obtained kinetic measurements at 1 and 5 min using 50 nM–10 μM concentrations of [14C]spermidine. The Km (app) and Vmax (app) values for spermidine at 1 and 5 min are nearly identical (see Table 1), thus indicating the validity of using the 5 min time point for additional experiments. We also obtained kinetic measurements for spermine at 5 min (Table 1). These data indicate that spermine and spermidine uptake follows saturable kinetics with a single apparent affinity (results not shown).
Table 1. Endogenous polyamine transport activity of Drosophila S2 cells.
Cell suspensions were assayed for transport activity at both 1 and 5 min. The Km (app) and Vmax (app) values were determined by double-reciprocal plots (Lineweaver–Burk transformation) of transport activity in the presence of increasing concentrations of [14C]spermine or [14C]spermidine. Results are means±S.E.M. for three independent experiments performed in triplicate.
| Assay at 5 min | Assay at 1 min | |||
|---|---|---|---|---|
| Substrate | Km (app) (μM) | Vmax (app) (pmol/min per 106 cells) | Km (app) (μM) | Vmax (app) (pmol/min per 106 cells) |
| Spermine | 3.67±0.54 | 23.47±1.43 | – | – |
| Spermidine | 5.80±1.41 | 21.43±2.52 | 5.91±0.73 | 18.76±1.14 |
Previous reports suggest that eukaryotic cells may express both spermine/spermidine and putrescine transport activities [2,14]. We therefore tested the relative inhibitory potential of spermine, spermidine and putrescine, as well as cadaverine, which shows affinity for putrescine transport activities [23]. As expected, both spermine and spermidine inhibited [14C]spermine transport (Figure 1B), with IC50 values of 3.82±0.93 μM and 8.87±1.44 μM respectively (means±S.E.M.). In contrast, neither putrescence nor cadaverine inhibited transport with high affinity. These data indicate that, unlike some other cultured cell lines [32], the spermine/spermidine transport activity in S2 cells is relatively insensitive to putrescine. We note that this does not preclude the possibility that S2 cells express an independent putrescine transporter.
Ionic requirements of spermine uptake in Drosophila S2 cells
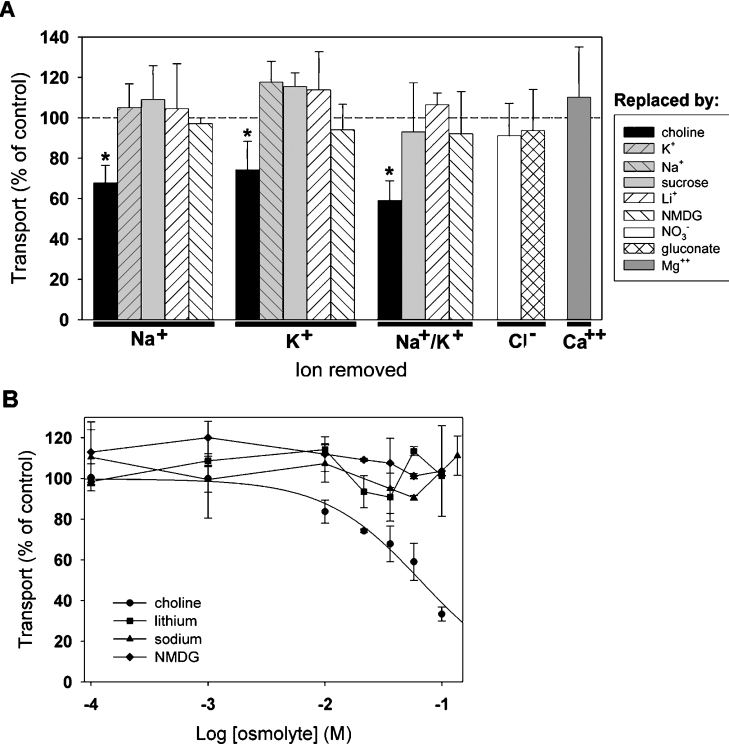
Secondarily active transport mechanisms depend on ion gradients across membranes to accumulate a substrate against a concentration gradient [33]. We find that transport of [14C]spermine in S2 cells is not significantly reduced by replacement of Na+, K+, Cl− or Ca2+ (Figure 2A). Thus, similar to mammalian polyamine transport activities, the transporter responsible for uptake of spermine and spermidine in S2 cells does not require Na+, K+, Cl− or Ca2+ [15–18,23].
Figure 2. Ionic dependence of spermine uptake in Drosophila S2 cells.
(A) Na+, K+, Cl− and Ca2+ were iso-osmotically replaced using either ionic or non-ionic osmolytes. Cells were incubated with 500 nM [14C]spermine for 5 min, and the values obtained for each replacement experiment were normalized to uptake under control conditions using standard MDM (broken line). Transport was not significantly changed by replacement by Na+, K+, Li+, sucrose, NMDG, NO3−, gluconate or Mg2+, but was inhibited by choline (*, P<0.01). (B) Concentration-dependent inhibition of spermine uptake by choline. Increasing concentrations of choline, Na+, Li+ or NMDG were added to cells incubated in standard MDM with 500 nM [14C]spermine. A four-parameter logistic sigmoid curve was fitted to the choline data (R2=0.7879) to yield an IC50 of 66.4±39.8 mM (mean±S.E.M.). Results are means±range for two independent experiments performed in triplicate.
We observed a significant decrease in spermine uptake using media in which Na+ or K+ was replaced by choline. However, since choline, like spermine and spermidine, is an organic cation, we speculated that choline might decrease uptake by acting as a low-affinity inhibitor of transport, rather than disrupting the function of a co-transported ion. Therefore we performed additional experiments in which we added excess choline or other ions to normal MDM. Choline inhibited transport with an IC50 of 66.4±39.8 mM (mean±S.E.M.), whereas increasing concentrations of Na+, Li+ or NMDG had no effect (Figure 2B). These data suggest that choline acts as a low-affinity antagonist of spermine uptake in S2 cells. Furthermore, these data may also explain the partial reduction of polyamine transport reported previously in other systems when Na+ and K+ were replaced by this ion [15–18,34].
Dependence of spermine transport on pH
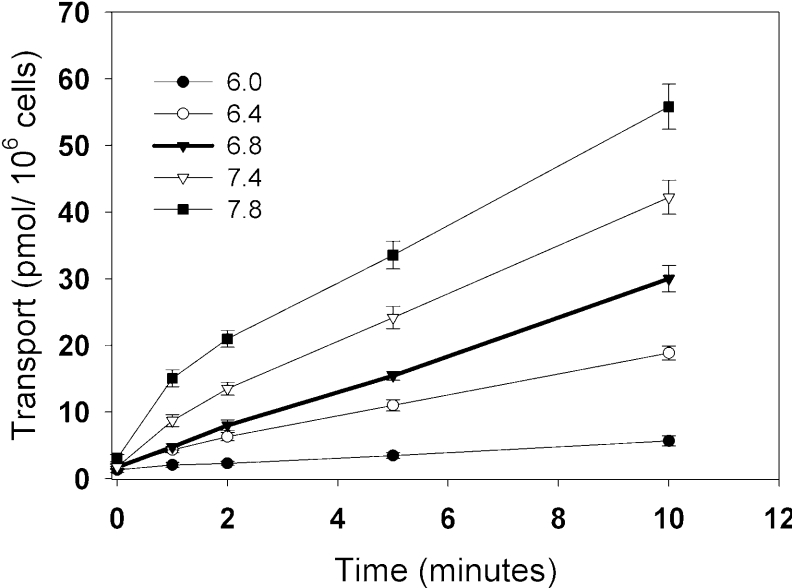
Since transport appeared to be independent of Na+, K+, Cl− and Ca2+, we postulated that a H+ gradient across the plasma membrane might function to drive polyamine uptake. Indeed, we find that [14C]spermine transport is sensitive to pH at all of the time-points tested (Figure 3). More specifically, we observe an inhibition of transport at pHs lower than the control (pH <6.8), and an enhancement of transport at pHs higher than the control (pH >6.8). We note that, at higher pH, transport may not remain linear over the course of 10 min. This may indicate that more than one transport process is affected by the higher pH in the medium.
Figure 3. pH-dependence of spermine uptake in Drosophila S2 cells.
(A) Time course of spermine transport at pH 6.0 (●), pH 6.4 (○), pH 6.8 (▼), pH 7.4 (▽) and pH 7.8 (■). At zero time, 500 nM [14C]spermine was added, and cells were incubated for the times indicated. The pH of standard Schneider's Drosophila medium and MDM is 6.8 and served as a control. Results are means±S.E.M. for three independent experiments performed in triplicate.
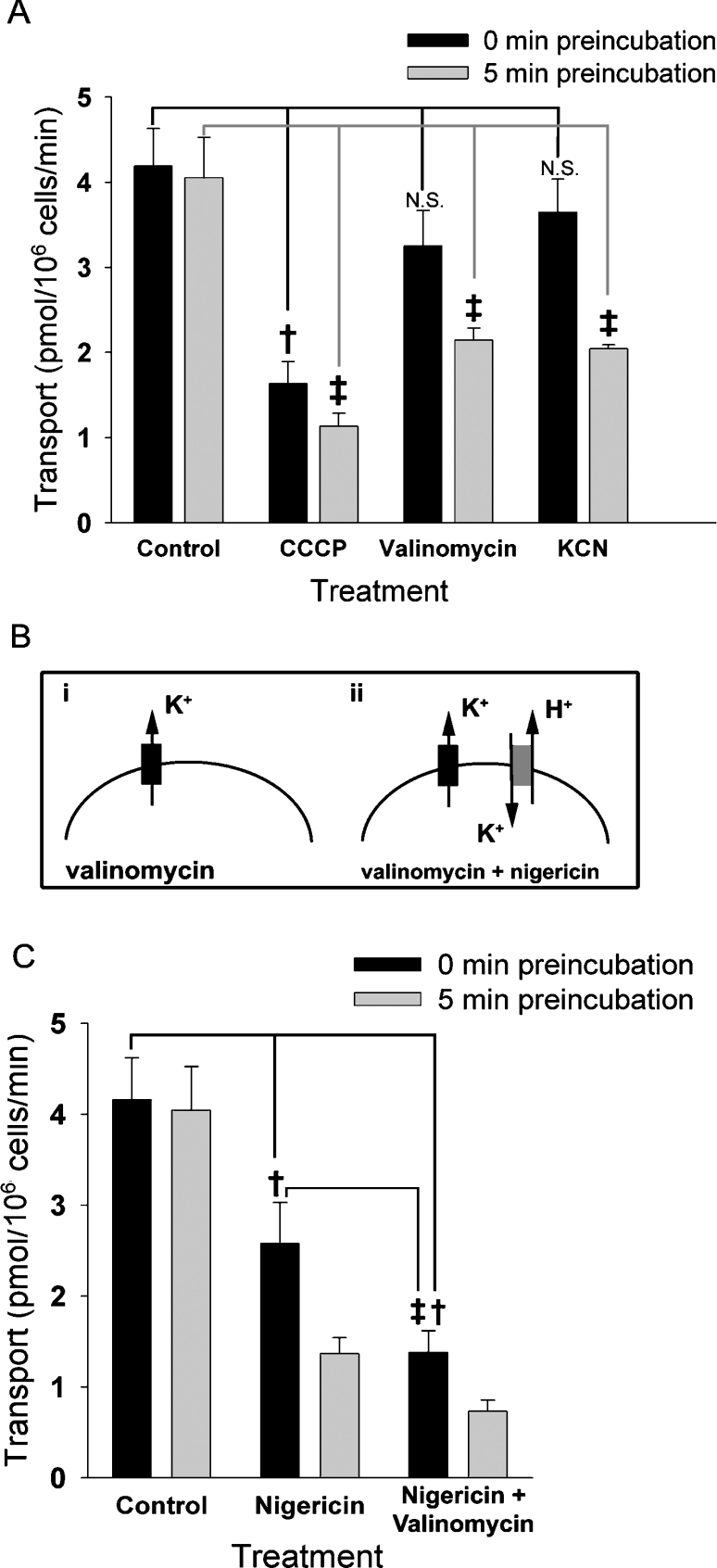
We next performed additional experiments to test the use of specific ionophores to disrupt the pH and other ionic gradients across the plasma membrane. Importantly, these manipulations do not disrupt the bulk pH or ionic concentrations of the medium. To disrupt the pH gradient across the plasma membrane, we first tested the effect of CCCP, an H+-specific ionophore [35]. CCCP reduced spermine transport by 60% when added at the beginning of the transport assay (0 min pre-incubation; Figure 4A). Pre-incubation in CCCP for 5 min further reduces transport only slightly (5 min pre-incubation), indicating that the bulk of the effect of CCCP occurs nearly immediately. Since treatment with CCCP eliminates the H+ gradients without changing the extracellular pH, these data are consistent with the notion that a H+ gradient facilitates spermine transport. Furthermore, since this effect does not require any pre-incubation, it is likely to involve a relatively direct effect, such as disruption of the pH gradient across the plasma membrane, rather than affecting intracellular H+ stores.
Figure 4. Effects of ionophores on spermine transport in Drosophila S2 cells.
(A, C) [14C]Spermine (500 nM) was added to cell suspensions either immediately (black bars) or 5 min (grey bars) after the addition of ionophores. Transport was calculated after 5 min of incubation with the radiolabelled substrate as described in the Materials and methods section. (A) Transport activity after addition of 500 nM valinomycin, 500 nM CCCP or 500 μM KCN. The control group shows transport in the absence of any ionophore. We observed a significant reduction in transport in the absence of pre-incubation using CCCP compared with the control (†, P<0.00001, ANOVA with Tukey's post-hoc test), but not with valinomycin or KCN (not significant, N.S.). Using a 5 min pre-incubation, transport was significantly reduced using all three agents compared with the control (‡, P<0.00001). Post-hoc comparison between values obtained for valinomycin and KCN using 0 min compared with 5 min pre-incubated cells also showed a significant difference (P<0.001, not indicated on the Figure). A smaller reduction was seen for CCCP using 0 min compared with 5 min pre-incubated cells (P<0.05, not indicated on the Figure). (B) Addition of valinomycin (represented by a black box) in K+-free medium will clamp the cells at the equilibrium potential of K+, by allowing K+ ions to flow down their electrochemical gradient (i). If nigericin is added (grey box), H+ is pumped out as the K+ cycles in and out of the cell (ii). (C) Transport activity of [14C]spermine after addition of 500 nM nigericin or 500 nM nigericin+500 nM valinomycin. We detected a significant difference between ionophore treatment and control at 0 min pre-incubation time for both nigericin and nigericin+valinomycin (†, P<0.00001, ANOVA with Tukey's post-hoc test). In addition, we observed significant differences between nigericin compared with nigericin+valinomycin at 0 min pre-incubation time (‡, P<0.00001). Transport using 0 min compared with 5 min pre-incubation also differed for both nigericin and for nigericin+valinomycin (P<0.05, not indicated on the Figure).
CCCP also can affect the H+ gradient across mitochondrial membranes, and thereby inhibit mitochondrial function and ATP production [35]. Therefore, to test the possibility that the effects of CCCP using a 5 min pre-incubation step could result from changes in mitochondrial function, we used valinomycin, a K+ ionophore which depolarizes the mitochondrial membrane [36]. Unlike CCCP, valinomycin yields a minimal reduction of spermine uptake without a pre-incubation step, consistent with our ion-replacement experiments, indicating that K+ is not required for spermine transport across the plasma membrane (see Figure 2A). In contrast, after pre-incubation for 5 min to allow penetration of the ionophore in to the mitochondrial membrane, valinomycin reduces spermine transport by 50% (Figure 4A). These data suggest that changes in mitochondrial function may also inhibit spermine transport.
One important effect of decreasing mitochondrial function is the blockade of ATP production. To specifically test the effect of depleting intracellular ATP, we treated cells with KCN, which inhibits cytochrome c oxidase of the electron-transfer chain [37]. Without a pre-incubation step to allow access to mitochondria, treatment with KCN yields a minimal decrease in spermine uptake. In contrast, when KCN was pre-incubated with the cells for 5 min before the start of the transport assay, we observed a 55% reduction in spermine uptake, indicating that depletion of cellular ATP inhibits spermine transport.
Since polyamine uptake has been reported previously to be dependent on the membrane potential across the plasma membrane [17,18,24,38], we also tested the effects of the Na+-preferential ionophore SQl-Pr [31], which disrupts the Na+ gradient across the plasma membrane, thereby depolarizing the cell. Importantly, since ion-replacement experiments (see Figure 2A) show that Na+ is not required for spermine transport, SQl-Pr functions in these experiments as a method to depolarize the plasma membrane electrochemical potential rather than testing the effect of the Na+ gradient itself. SQl-Pr significantly reduces [14C]spermine uptake without pre-incubation (results not shown), suggesting that, similar to other systems, polyamine uptake in S2 cells is dependent on the electrochemical potential across the plasma membrane.
Taken together, our data using individual ionophores suggest that eliminating the H+ gradient, reducing intracellular ATP and/or depolarizing the membrane potential reduce spermine uptake, but do not clearly distinguish between these factors. Therefore, to more specifically assess the H+ sensitivity of spermine uptake, we used a combination of the ionophores nigericin and valinomycin. Nigericin is an electroneutral K+/H+ exchanger, and, when used alone, equalizes the K+ and H+ concentration gradients across the plasma membrane. This disrupts the H+ gradient, but does not change the membrane potential. In addition, nigericin used in conjunction with the K+ ionophore valinomycin functions to deplete further the H+ gradient. In essence, in the presence of both ionophores and in K+-free medium, K+ is shuttled between the intracellular and extracellular spaces, where it then exchanges for an H+ (Figure 4B). Nigericin significantly reduces transport relative to controls (Figure 4C), and we observed an additional reduction in [14C]spermine transport using the combination of nigericin and valinomycin, which mimics the effects seen with CCCP. Furthermore, similar to CCCP, the effect of nigericin and valinomycin is immediate, consistent with an effect on the H+ gradient across the plasma membrane rather than at an intracellular site.
Pharmacological profile of the spermine/spermidine transporter in Drosophila
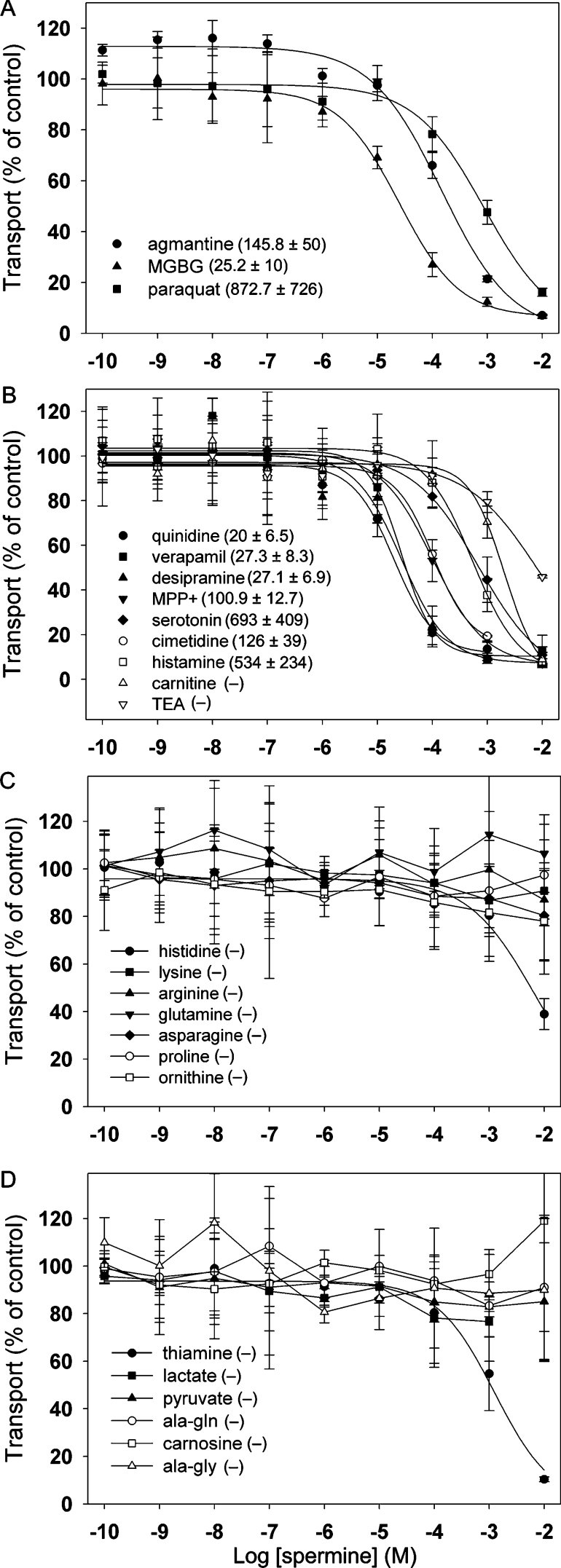
Secondarily active transporters have been catalogued into distinct families using both structural and functional characteristics. Several of these Slc families contain transporters that are known to use H+ in some aspect of transport [33]. Therefore we tested the affinities of several known substrates of H+-dependent transporter families. Similar to mammalian activities, spermine transport in S2 cells is inhibited by MGBG [15,39] and agmatine [34] (Figure 5A). In addition, we find that most, if not all, substrates of the Slc22 cation/anion family of transporters [40] also inhibit spermine transport (Figure 5B). Thiamine (Slc19) also showed a tendency to inhibit spermine uptake at high concentrations. In contrast, the substrates of H+-dependent amino acid permeases (Slc36 and/or Slc38) had minimal effects (Figure 5C). Similarly, we observed low affinity for substrates of Slc15 and Slc16 (Figure 5D). These data suggest that polyamine transport in S2 cells may be mediated by a protein related to the Slc22 family rather than others that employ H+ gradients across the plasma membrane.
Figure 5. Inhibitory profile of spermine transport in Drosophila S2 cells.
Various concentrations of unlabelled inhibitors were added to Drosophila S2 cells incubated for 5 min with 500 nM [14C]spermine. Whenever possible, four-parameter logistic sigmoid curves were fitted to the data, and IC50 values were calculated (shown as means±S.E.M.). IC50 values (in μM) are listed next to each inhibitor, where (−) indicates absence of inhibition or a measurable IC50. Added agents indicated in the Figure include inhibitors of mammalian polyamine transport (A), substrates or inhibitors of the Slc22 family of cation/anion transporters (B), amino acid substrates of H+-dependent permeases (C) and substrates of other H+-dependent transport systems (D). Results are means±range for two independent experiments performed in triplicate.
DISCUSSION
Although polyamine transport has been widely reported, the bioenergetic mechanisms and the transport proteins that are responsible for most of these activities remain unknown [15–17,19–24]. We find that Drosophila S2 cells show high-affinity spermine and spermidine uptake through a temperature-dependent saturable mechanism, and the results of the present study suggest that this activity may be sensitive to the H+ gradients across the plasma membrane. We also report a correlation between inhibition of polyamine transport in S2 cells and inhibition of one family of H+-linked transporters.
The activity that we have identified is similar to polyamine transport in mammalian cells [15–17,22,24] in terms of both its substrate affinity and its inhibition by MGBG, paraquat and agmatine [15,34,39]. A plasma membrane transporter showing affinity for both putrescine and spermidine has been isolated recently from Leishmania [29]. In contrast, the polyamine transport activity in S2 cells has little or no apparent affinity for putrescine, suggesting that the molecule responsible for this activity is likely to be distinct from that expressed in Leishmania and other protists, and perhaps more similar to mammalian transporters.
Consistent with most previous studies [15–18,23], ion-replacement experiments indicated that polyamine transport in S2 cells is Na+-independent and is also independent of K+, Cl− and Ca2+. We detected a moderate reduction in transport when the organic cation choline was used as a replacement for the inorganic cations Na+ or K+; however, we found that choline also reduces transport when added to standard media, and suggest that choline may function as a direct inhibitor of the polyamine uptake activity. Thus choline and possibly other organic cations may not be suitable osmolytes to replace inorganic cations in polyamine uptake experiments.
We did not observe any measurable increase in spermine uptake when iso-osmotically replacing either Na+ or K+ with sucrose. Although the increases in transport activity reported in other studies remains unclear [15–17,21,41], they could conceivably be explained by a change in membrane potential caused by the removal of extracellular ions. Indeed, the sensitivity of polyamine uptake to membrane potential has been reported in mammalian cells depolarized with high extracellular K+ [17,24] or treatment with Na+-preferential ionophores [18,38]. Similarly, we observed a decrease in spermine transport using the Na+-specific ionophore SQl-Pr. It remains unclear whether polyamine uptake is also electrogenic and involves a net transfer of charge across the plasma membrane [42].
Although we found that valinomycin decreases spermine transport in S2 cells, the mechanism is likely to differ from that of mammalian cells treated with high extracellular K+ [18,38]. In the presence of high extracellular K+, valinomycin is likely to depolarize the plasma membrane. In contrast, under the normal haemolymph-like conditions of our experiments [43,44], it is less likely that valinomycin would depolarize the plasma membrane. Moreover, since the effects of valinomycin require a pre-incubation step, it is likely that the observed effect of valinomycin is due to depolarization of mitochondria [36].
Importantly, we find that spermine transport is increased when S2 cells are exposed to high extracellular pH (>6.8) and decreased in the presence of low extracellular pH (<6.8). Although previous studies have suggested that pH may influence polyamine uptake, the authors argued that pH changed either non-specific binding [45] or polyamine protonation [46]. We detected minimal non-specific binding to the plasma membrane at 0 °C, and we observe pH sensitivity at pH 6.0–7.4, a range in which spermine is fully protonated, thus arguing against either of the previously proposed mechanisms. In addition, others have used ionophores to disrupt the H+ gradient [18,38,47], but have argued that changes in transport were due to the disruption of the plasma membrane potential. We have attempted to tease apart the effects of membrane potential and the H+ gradient using several different ionophores, including CCCP, nigericin and valinomycin. Since nigericin, as well as CCCP, significantly reduces spermine uptake, it is likely that polyamine uptake is sensitive to the H+ gradient, independent of the membrane potential.
We emphasize that, despite abolishing the H+ gradient with H+ ionophores, we did not completely abolish spermine transport. Therefore, in S2 cells and perhaps in other systems, spermine transport might not be strictly H+-coupled. Similar to monocarboxylic transporters, it is possible that polyamine uptake is mediated by a non-obligatory H+-driven transporter [48]. Likewise, there might be additional non-H+-dependent uptake mechanisms present in S2 cells. Interestingly, several studies using mammalian cell lines have suggested that polyamines can be taken up by proteoglycans [10,49].
Additional information on the pharmacological sensitivity of the polyamine transporter may facilitate its identification. Since the spermine/spermidine transport activity in S2 cells is H+ dependent, we reasoned that we might help to determine the transporter family to which it belongs by pharmacologically screening agents that are known to inhibit other H+-coupled transporters. We find that spermine transport activity in Drosophila shows affinity for most agents that are found to interact with identified members of the Slc22 family. Conversely, the addition of increasing concentrations of various amino acids, dipeptides or monocarboxylic acids and substrates of other H+-dependent transporters does not inhibit spermine uptake. Therefore we suggest that the Drosophila spermine carrier may be related to the Slc22 family.
We speculate that these data may assist future attempts to clone the polyamine transporters in Drosophila and perhaps other complex eukaryotes. Furthermore, our results showing that the Drosophila spermine transport may require a H+ gradient across the plasma membrane are likely to be relevant to our general understanding of this important class of molecules. Finally, it is likely that the study of polyamine transport, and, more generally, the function of polyamines, will be aided by the use of a genetically tractable model organism such as Drosophila.
Online Data
Acknowledgments
We thank Michael W. Quick and H. Ron Kaback for many helpful suggestions on the manuscript. This work was supported by a grant from the EJLB Foundation (D.E.K.), a Diversity Program in Neuroscience (DPN) predoctoral fellowship from the American Psychological Association (R.R.-C.) and the Shirley & Stefan Hatos Neuroscience Research Foundation (R.R.-C.).
References
- 1.Gerner E. W., Meyskens F. L., Jr Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 2.Wallace H. M., Fraser A. V., Hughes A. A perspective of polyamine metabolism. Biochem. J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams K. Interactions of polyamines with ion channels. Biochem. J. 1997;325:289–297. doi: 10.1042/bj3250289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachrach U., Wang Y. C., Tabib A. Polyamines: new cues in cellular signal transduction. News Physiol. Sci. 2001;16:106–109. doi: 10.1152/physiologyonline.2001.16.3.106. [DOI] [PubMed] [Google Scholar]
- 5.Pignatti C., Tantini B., Stefanelli C., Flamigni F. Signal transduction pathways linking polyamines to apoptosis. Amino Acids. 2004;27:359–365. doi: 10.1007/s00726-004-0115-3. [DOI] [PubMed] [Google Scholar]
- 6.Thomas T., Thomas T. J. Polyamine metabolism and cancer. J. Cell. Mol. Med. 2003;7:113–126. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace H. M., Fraser A. V. Inhibitors of polyamine metabolism: review article. Amino Acids. 2004;26:353–365. doi: 10.1007/s00726-004-0092-6. [DOI] [PubMed] [Google Scholar]
- 8.Wallace H. M., Fraser A. V. Polyamine analogues as anticancer drugs. Biochem. Soc. Trans. 2003;31:393–396. doi: 10.1042/bst0310393. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell J. L., Simkus C. L., Thane T. K., Tokarz P., Bonar M. M., Frydman B., Valasinas A. L., Reddy V. K., Marton L. J. Antizyme induction mediates feedback limitation of the incorporation of specific polyamine analogues in tissue culture. Biochem. J. 2004;384:271–279. doi: 10.1042/BJ20040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belting M., Persson S., Fransson L. A. Proteoglycan involvement in polyamine uptake. Biochem. J. 1999;338:317–323. [PMC free article] [PubMed] [Google Scholar]
- 11.Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell. Signalling. 1997;9:1–13. doi: 10.1016/s0898-6568(96)00089-7. [DOI] [PubMed] [Google Scholar]
- 12.Aizenman C. D., Munoz-Elias G., Cline H. T. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron. 2002;34:623–634. doi: 10.1016/s0896-6273(02)00674-8. [DOI] [PubMed] [Google Scholar]
- 13.Pellegrini-Giampietro D. E. An activity-dependent spermine-mediated mechanism that modulates glutamate transmission. Trends Neurosci. 2003;26:9–11. doi: 10.1016/s0166-2236(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 14.Seiler N., Delcros J. G., Moulinoux J. P. Polyamine transport in mammalian cells: an update. Int. J. Biochem. Cell Biol. 1996;28:843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 15.Dot J., Lluch M., Blanco I., Rodriguez-Alvarez J. Polyamine uptake in cultured cerebellar granule neurons. Neurochem. Int. 2004;44:549–556. doi: 10.1016/j.neuint.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Dot J., Lluch M., Blanco I., Rodriguez-Alvarez J. Polyamine uptake in cultured astrocytes: characterization and modulation by protein kinases. J. Neurochem. 2000;75:1917–1926. doi: 10.1046/j.1471-4159.2000.0751917.x. [DOI] [PubMed] [Google Scholar]
- 17.Paz J. C., Sanchez-Jimenez F., Medina M. A. Characterization of spermine uptake by Ehrlich tumour cells in culture. Amino Acids. 2001;21:271–279. doi: 10.1007/s007260170013. [DOI] [PubMed] [Google Scholar]
- 18.Poulin R., Lessard M., Zhao C. Inorganic cation dependence of putrescine and spermidine transport in human breast cancer cells. J. Biol. Chem. 1995;270:1695–1704. doi: 10.1074/jbc.270.4.1695. [DOI] [PubMed] [Google Scholar]
- 19.Khan N. A., Quemener V., Havouis R., Moulinoux J. P. Mechanism of polyamine spermidine uptake by Xenopus laevis oocytes. Biochem. Int. 1990;21:607–613. [PubMed] [Google Scholar]
- 20.Aziz S. M., Lipke D. W., Olson J. W., Gillespie M. N. Role of ATP and sodium in polyamine transport in bovine pulmonary artery smooth cells. Biochem. Pharmacol. 1994;48:1611–1618. doi: 10.1016/0006-2952(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 21.Fajardo I., Urdiales J. L., Paz J. C., Chavarria T., Sanchez-Jimenez F., Medina M. A. Histamine prevents polyamine accumulation in mouse C57.1 mast cell cultures. Eur. J. Biochem. 2001;268:768–773. doi: 10.1046/j.1432-1327.2001.01930.x. [DOI] [PubMed] [Google Scholar]
- 22.Srinath P., McQuarrie S. A., Suresh M. R. Comparative uptake of polyamines by prostate and non-prostate cancer cell lines. Nucl. Med. Biol. 2002;29:497–503. doi: 10.1016/s0969-8051(02)00287-1. [DOI] [PubMed] [Google Scholar]
- 23.Adeola O., Ram J. I., Maenz D. D., Classen H. L. Transport of putrescine across duodenal, jejunal and ileal brush-border membrane of chicks (Gallus domesticus) Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2003;135C:235–247. doi: 10.1016/s1532-0456(03)00121-2. [DOI] [PubMed] [Google Scholar]
- 24.Masuko T., Kusama-Eguchi K., Sakata K., Kusama T., Chaki S., Okuyama S., Williams K., Kashiwagi K., Igarashi K. Polyamine transport, accumulation, and release in brain. J. Neurochem. 2003;84:610–617. doi: 10.1046/j.1471-4159.2003.01558.x. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwagi K., Hosokawa N., Furuchi T., Kobayashi H., Sasakawa C., Yoshikawa M., Igarashi K. Isolation of polyamine transport-deficient mutants of Escherichia coli and cloning of the genes for polyamine transport proteins. J. Biol. Chem. 1990;265:20893–20897. [PubMed] [Google Scholar]
- 26.Igarashi K., Kashiwagi K. Polyamine transport in bacteria and yeast. Biochem. J. 1999;344:633–642. [PMC free article] [PubMed] [Google Scholar]
- 27.Tomitori H., Kashiwagi K., Sakata K., Kakinuma Y., Igarashi K. Identification of a gene for a polyamine transport protein in yeast. J. Biol. Chem. 1999;274:3265–3267. doi: 10.1074/jbc.274.6.3265. [DOI] [PubMed] [Google Scholar]
- 28.Tomitori H., Kashiwagi K., Asakawa T., Kakinuma Y., Michael A. J., Igarashi K. Multiple polyamine transport systems on the vacuolar membrane in yeast. Biochem. J. 2001;353:681–688. doi: 10.1042/0264-6021:3530681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasne M. P., Ullman B. Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J. Biol. Chem. 2005;280:15188–15194. doi: 10.1074/jbc.M411331200. [DOI] [PubMed] [Google Scholar]
- 30.Uemura T., Tachihara K., Tomitori H., Kashiwagi K., Igarashi K. Characteristics of the polyamine transporter TPO1 and regulation of its activity and cellular localization by phosphorylation. J. Biol. Chem. 2005;280:9646–9652. doi: 10.1074/jbc.M410274200. [DOI] [PubMed] [Google Scholar]
- 31.Jankowski A., Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential: quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J. Biol. Chem. 1999;274:26098–26104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- 32.Minchin R., Raso A., Martin R., Ilett K. Evidence for the existence of distinct transporters for the polyamines putrescine and spermidine in B16 melanoma cells. Eur. J. Biochem. 1991;200:457–462. doi: 10.1111/j.1432-1033.1991.tb16204.x. [DOI] [PubMed] [Google Scholar]
- 33.Hediger M. A., Romero M. F., Peng J. B., Rolfs A., Takanaga H., Bruford E. A. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins: introduction. Pflugers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 34.Satriano J., Isome M., Casero R. A., Jr, Thomson S. C., Blantz R. C. Polyamine transport system mediates agmatine transport in mammalian cells. Am. J. Physiol. Cell Physiol. 2001;281:C329–C334. doi: 10.1152/ajpcell.2001.281.1.C329. [DOI] [PubMed] [Google Scholar]
- 35.Terada H. The interaction of highly active uncouplers with mitochondria. Biochim. Biophys. Acta. 1981;639:225–242. doi: 10.1016/0304-4173(81)90011-2. [DOI] [PubMed] [Google Scholar]
- 36.Pressman B. C. Biological applications of ionophores. Annu. Rev. Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- 37.Egekeze J. O., Oehme F. W. Cyanides and their toxicity: a literature review. Tijdschr. Diergeneeskd. 1980;105(suppl. 2):104–114. [PubMed] [Google Scholar]
- 38.Kakinuma Y., Hoshino K., Igarashi K. Characterization of the inducible polyamine transporter in bovine lymphocytes. Eur. J. Biochem. 1988;176:409–414. doi: 10.1111/j.1432-1033.1988.tb14297.x. [DOI] [PubMed] [Google Scholar]
- 39.Byers T. L., Kameji R., Rannels D. E., Pegg A. E. Multiple pathways for uptake of paraquat, methylglyoxal bis(guanylhydrazone), and polyamines. Am. J. Physiol. 1987;252:C663–C669. doi: 10.1152/ajpcell.1987.252.6.C663. [DOI] [PubMed] [Google Scholar]
- 40.Koepsell H., Endou H. The SLC22 drug transporter family. Pflugers Arch. 2004;447:666–676. doi: 10.1007/s00424-003-1089-9. [DOI] [PubMed] [Google Scholar]
- 41.Molderings G. J., Bonisch H., Gothert M., Bruss M. Agmatine and putrescine uptake in the human glioma cell line SK-MG-1. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363:671–679. doi: 10.1007/s002100100418. [DOI] [PubMed] [Google Scholar]
- 42.Poulin R., Zhao C., Verma S., Charest-Gaudreault R., Audette M. Dependence of mammalian putrescine and spermidine transport on plasma-membrane potential: identification of an amiloride binding site on the putrescine carrier. Biochem. J. 1998;330:1283–1291. doi: 10.1042/bj3301283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jan L. Y., Jan Y. N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J. Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart B. A., Atwood H. L., Renger J. J., Wang J., Wu C. F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi M., Iseki K., Saitoh H., Miyazaki K. Uptake characteristics of polyamines into rat intestinal brush-border membrane. Biochim. Biophys. Acta. 1992;1105:177–183. doi: 10.1016/0005-2736(92)90177-n. [DOI] [PubMed] [Google Scholar]
- 46.Fukumoto G., Byus C. A kinetic characterization of putrescine and spermidine uptake and export in human erythrocytes. Biochim. Biophys. Acta. 1996;1282:48–56. doi: 10.1016/0005-2736(96)00036-3. [DOI] [PubMed] [Google Scholar]
- 47.Sakata K., Kashiwagi K., Igarashi K. Properties of a polyamine transporter regulated by antizyme. Biochem. J. 2000;347:297–303. [PMC free article] [PubMed] [Google Scholar]
- 48.Hertz L., Dienel G. A. Lactate transport and transporters: general principles and functional roles in brain cells. J. Neurosci. Res. 2005;79:11–18. doi: 10.1002/jnr.20294. [DOI] [PubMed] [Google Scholar]
- 49.Belting M., Borsig L., Fuster M. M., Brown J. R., Persson L., Fransson L. A., Esko J. D. Tumor attenuation by combined heparan sulfate and polyamine depletion. Proc. Natl. Acad. Sci. U.S.A. 2002;99:371–376. doi: 10.1073/pnas.012346499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.