Abstract
We examined 41 human and animal rotavirus strains representative of all known P genotypes for their dependency on cellular N-acetylneuraminic (sialic) acid (SA) residues for infectivity. Our results showed that all rotaviruses studied, whether of animal or human origin, belonging to P genotypes [1], [2], [3], and [7] depended on SA residues on the cell surface for efficient infectivity but that all human and animal rotavirus strains representative of the remaining known P genotypes were SA independent. The SA residue requirement for efficient infectivity did not change for reassortant rotavirus strains with altered VP4-VP7 combinations. The initial interaction of rotavirus strains with SA residues on the cell surface correlated with VP4 genotype specifity, not with species of origin or VP7 G serotype specificity (P = 0.001; r2 = 1.00, Pearson's correlation coefficient). In addition to being a requirement for infectivity, the presence of SA residues on the cell surface is a requirement for efficient growth in cell culture; recognition of the association of specific P genotypes with the binding of rotavirus to SA residues will facilitate our understanding of the molecular basis of the early events of rotavirus-cell interactions in cell culture models and of pathogenicity in vivo.
Group A rotaviruses are important viral agents of acute gastroenteritis in young children and many animal species (12). Two surface proteins present on the outer capsids of rotaviruses, VP4 and VP7, have been shown to independently elicit neutralizing antibodies and induce protective immunity (12). Rotavirus serotype designations are based on neutralization determinants, defined by serology, on both VP4 (P, for protease-sensitive protein) and VP7 (G, for glycoprotein) (12). Alternatively, if immunological reagents are lacking, sequence analysis and/or nucleic acid hybridization data can determine the P or G genotype (with the P genotype designated in brackets) (12). Fourteen G serotypes have been identified and shown to correspond with G genotypes. Out of 20 different P genotypes, only 13 P serotypes have been identified with available antisera or anti-VP4 monoclonal antibodies that recognize specific VP4 neutralization epitopes. Unlike G genotypes, P genotypes do not all correspond to P serotypes (12).
The spike protein VP4 (encoded by gene 4) is the virus attachment protein (7, 24), and rotavirus infectivity is enhanced by proteolytic cleavage of VP4 into VP8* and VP5* (10, 11). Additional biologic functions associated with the spike protein VP4 include the ability to agglutinate erythrocytes (hemagglutination [HA]) and bind to N-acetylneuraminic (sialic) acid (SA) residues, functions mapped to a domain within amino acids (aa) 93 to 208 of the VP4 cleavage product VP8* (9, 13, 19, 21). VP4 is also associated with restricted growth in cell culture (17), protease sensitivity associated with plaque formation (3), and virulence (2, 33, 38).
The initial stages of rotavirus binding to cells are complex, and although the identity of the rotavirus cellular receptor(s) remains controversial (for a review, see reference 5), it was thought that animal rotaviruses required the presence of SA residues on the cell surface for efficient binding and infectivity but that human rotaviruses did not require SA residues for such functions (14, 18, 23-28, 35, 36; J. A. López, J. E. Ludert, F. H. Pujol, and F. Liprandi, submitted for publication). This hypothesis was disproved following the testing of a large number of human and animal rotavirus strains, when it was shown that only a minority of animal rotaviruses require the presence of SA residues on the cell surface for efficient infectivity; thus, most animal and all human rotaviruses are SA independent (4). SA-independent variants of animal rotavirus strains isolated from SA-dependent strains bind to and infect cells efficiently, confirming that binding to SA is not an essential step for rotavirus infection (4, 26-28). The binding of SA-dependent rotavirus strains to cells is mediated initially by VP8* through SA residues and then by VP5*, while the binding of SA-independent rotavirus strains is proposed to be mediated directly by VP5* (39).
Human and animal rotavirus strains representative of all P genotypes, with the exception of P genotypes [13], [15], [18], and [19], have previously been tested for their SA dependency during the initial steps of infection. In view of the fact that only animal rotavirus strains belonging to P genotypes [1], [2], [3], and [7] have been shown to be SA dependent (4, 8, 14, 20, 23-28, 35, 36), we tested a total of 41 additional animal and human rotavirus strains of different VP4-VP7 combinations, comprising all known P genotypes, including those never tested before, to determine if there is a correlation between SA dependency and rotavirus VP4 P genotype. Since binding to SA residues may be determined by different or altered VP4-VP7 interactions (27), we tested five reassortant strains, including the bovine (strain UK)-human reassortant candidate vaccine strains (29, 30), and retested the simian (strain RRV)-human rotavirus reassortant candidate vaccine strains (25, 27, 29, 30) to determine if these reassortants exhibit altered SA dependency. Our data show that the requirement of the presence of SA residues on the cell surface for efficient infectivity of rotavirus strains correlates with the VP4 genotype, not with the species of origin.
The species of origin, G serotypes, P genotypes and serotypes (where available), and sources of rotavirus strains used in this study are summarized in Table 1. All virus strains were propagated in embryonic rhesus monkey kidney cells (MA104) in the presence of trypsin as described previously (4, 6). The SA dependence or independence of the infectivities of all virus strains was measured in confluent MA104 cells grown in 96-well plates essentially as described previously (4). Briefly, MA104 cells were treated for 1 h at 37°C with 100 μl of twofold dilutions of neuraminidase from Arthrobacter ureafaciens or Vibrio cholerae purified by affinity chromatography (Sigma Chemical Co., St. Louis, Mo.) starting at 20 mU/ml or with 100 μl of TNC buffer (10 mM Tris [pH 7.5], 140 mM NaCl, 10 mM CaCl2) as a control. Following treatment with neuraminidase, cells were washed with TNC buffer prior to inoculation with a single dilution (depending on the rotavirus strain) of 200 to 500 focus-forming units (FFU). After the virus was allowed to adsorb for 2 h at 37°C, the inoculum was removed and cells were washed with 199 medium (Irvine Scientific, Santa Ana, Calif.) supplemented with 3 mM l-glutamine and 4.5 g of sodium bicarbonate per liter. Cells were incubated for 16 to 18 h at 37°C with 199 medium, washed with phosphate-buffered saline, fixed with cold methanol, and stained for fluorescent focus assay as described previously (4). Infectivity assays (fluorescent focus assays) were performed at least three times with each virus strain. Viral infectivity was expressed as a percentage reflecting the number of FFU in neuraminidase-treated cells relative to the number observed in control (TNC buffer-treated) cells. If infected cells were fixed and stained immediately after the adsorption period, no fluorescent foci were observed (data not shown). Similar reductions in the levels of infectivity of the SA-dependent SA11 Cl3 rotavirus strain were observed following the treatment of MA104 cells with neuraminidase from A. ureafaciens or V. cholerae (data not shown). Therefore, neuraminidase from A. ureafaciens was selected for the experiments performed in this report. In addition, it has recently been shown that the treatment of MA104 cells and polarized epithelial Caco-2, HT-29, and MDCK-1 cells with neuraminidase from A. ureafaciens removes the SA residues required for the efficient binding and infectivity of SA-dependent rotavirus strains (6).
TABLE 1.
Species of origin, P and G serotypes and genotypes, and sources of rotavirus or reassortant rotavirus strains used in this study
| Origin | Virus strain | Serotype
|
Source | |
|---|---|---|---|---|
| Pa | G | |||
| Monkey | SA11 4F | 6[1] | 3 | H. Pereira, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil |
| Cow | RF | 6[1] | 6 | J. Cohen, Institut National de la Recherche Agronomique Jouy-en-Josas, France |
| Cow | BRV033 | 6[1] | 6 | F. Liprandi, Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela |
| Monkey | SA11 C13 | 5B[2] | 3 | H. Malherbe, Gull Laboratories, Salt Lake City, Utah |
| Human | HCR3a | 5A[3] | 3 | H. F. Clark, Wistar Institute, Philadelphia, Pa. |
| Dog | CU-1 | 5A[3] | 3 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Dog | K9 | 5A[3] | 3 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Cat | Cat97 | 5A[3] | 3 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Monkey | RRV | 5B[3] | 3 | H. B. Greenberg, Stanford University, Palo Alto, Calif. |
| Human | KUN | 1B[4] | 2 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | L26 | 1B[4] | 12 | S. Urasawa, Sapporo Medical University, Sapporo, Japan |
| Pig | 4S | 7[5] | 3 | U. Desselberger, Clinical Microbiology and Public Health Laboratory, Cambridge, United Kingdom |
| Cow | UK | 7[5] | 6 | D. R. Snodgrass, Moredum Reserarch Institute, Edinburgh, United Kingdom |
| Cow | WC3 | 7[5] | 6 | H. F. Clark, Wistar Institute, Philadelphia, Pa. |
| Cow | B641 | 7[5] | 6 | M. E. Hardy, Montana State University, Bozeman, Mont. |
| Cow | 678 | 7[5] | 8 | U. Desselberger, Clinical Microbiology and Public Health Laboratory, Cambridge, United Kingdom |
| Human | M37 | 2A[6] | 1 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | 1076 | 2A[6] | 2 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | McN13 | 2A[6] | 3 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Pig | C95 | 9[7] | 1 | N. Mattion, Centro de Virologia Animal, Buenos Aires, Argentina |
| Pig | CRW-8 | 9[7] | 3 | I. H. Holmes, University of Melbourne, Parkville, Australia |
| Pig | SB-1A | 9[7] | 4 | E. Bohl, Ohio Agricultural Research and Development Center, Wooster, Ohio |
| Pig | OSU | 9[7] | 5 | E. Bohl, Ohio Agricultural Research and Development Center, Wooster, Ohio |
| Pig | EE | 9[7] | 5 | E. Bohl, Ohio Agricultural Research and Development Center, Wooster, Ohio |
| Pig | A131 | 9[7] | 3 | F. Liprandi, Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela |
| Pig | A138 | 9[7] | 3 | F. Liprandi, Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela |
| Pig | A411 | 9[7] | 3 | F. Liprandi, Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela |
| Pig | C134 | 9[7] | 5 | N. Mattion, Centro de Virologia Animal, Buenos Aires, Argentina |
| Pig | TFR-41 | 9[7] | 5 | I. H. Holmes, University of Melbourne, Parkville, Australia |
| Pig | A253 | 9[7] | 11 | F. Liprandi, Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela |
| Human | KU | 1A[8] | 1 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | D | 1A[8] | 1 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | P | 1A[8] | 3 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | MO | 1A[8] | 3 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | Hokosawa | 1A[8] | 4 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | K8 | 3[9] | 1 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | O264 | 3[9] | 3 | S. Urasawa, Sapporo Medical University, Sapporo, Japan |
| Cat | Cat2 | 3[9] | 3 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Human | 69M | 4[10] | 8 | S. Matsuno, National Institute of Health, Tokyo, Japan |
| Cow | B223 | 8[11] | 10 | N. R. Blacklow, University of Massachusetts, Worcester, Mass. |
| Human | I321 | 8[11] | 10 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Horse | FI-14 | 4[12] | 3 | M. E. Conner, Baylor College of Medicine, Houston, Tex. |
| Horse | FR5 | 4[12] | 14 | F. Liprandi, Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela |
| Pig | A46 | 13[13] | 5 | F. Liprandi, Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela |
| Human | Mc35 | 11[14] | 10 | S. Urasawa, Sapporo Medical University, Sapporo, Japan |
| Sheep | Lp14 | [15] | 10 | U. Desselberger, Clinical Microbiology and Public Health Laboratory, Cambridge, United Kingdom |
| Mouse | EDIM | 10[16] | 3 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Mouse | EB | 10[16] | 3 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Chicken | Ch-2 | [17] | 7 | Y. Hoshino and M. Gorziglia, National Institutes of Health, Bethesda, Md. |
| Horse | L338 | 12[18] | 13 | D. R. Snodgrass and P. Iša, Moredum Reserarch Institute, Edinburgh, United Kingdom |
| Pig | 4F | [19] | 3 | U. Desselberger, Clinical Microbiology and Public Health Laboratory, Cambridge, United Kingdom |
| Human × humanb | PA169 × DS-1 | 11[14] | 2 | Y. Hoshino, National Institutes of Health, Bethesda, Md. |
| Monkey × humanc | RRV × D | 5B[3] | 1 | H. B. Greenberg, Stanford University, Palo Alto, Calif. |
| Monkey × humand | RRV × DS-1 | 5B[3] | 2 | H. B. Greenberg, Stanford University, Palo Alto, Calif. |
| Monkey × humane | RRV × ST3 | 5B[3] | 4 | H. B. Greenberg, Stanford University, Palo Alto, Calif. |
| Cow × humanc | UK × D | 7[5] | 1 | H. B. Greenberg, Stanford University, Palo Alto, Calif. |
| Cow × humanf | UK × DS-1 | 7[5] | 2 | H. B. Greenberg, Stanford University, Palo Alto, Calif. |
| Cow × humang | UK × P | 7[5] | 3 | H. B. Greenberg, Stanford University, Palo Alto, Calif. |
| Cow × humane | UK × ST3 | 7[5] | 4 | H. B. Greenberg, Stanford University, Palo Alto, Calif. |
P genotype is designated in brackets, while serotype (where known) precedes P genotype (12).
Reassortant whose gene 8 is derived from the human rotavirus strain DS-1, while all others are derived from the human strain PA169 (15).
Reassortant whose segment 9 is derived from the human strain D, while all others are derived from the simian RRV or bovine UK strain (29).
Reassortant whose segments 8 and 5 are derived from the human strain DS-1, while all others are derived from the simian strain RRV (27, 29).
Reassortant whose segment 9 is derived from the human strain ST3, while all others are derived from the simian RRV or bovine UK strain (30).
Reassortant whose segment 8 are derived from the human strain DS-1, while all others are derived from the bovine strain UK (29).
Reassortant whose segment 8 are derived from the human strain P, while all others are derived from the bovine strain UK (29).
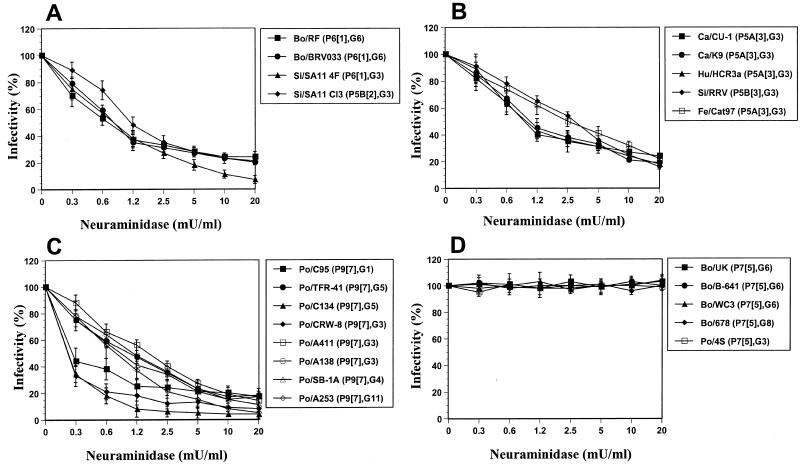
Bovine (RF and BRV033) and simian (SA11 4F) rotavirus strains of the P[1] genotype tested in this study were SA dependent (Fig. 1A), in accordance with the results of previous studies with the P[1] bovine NDCV rotavirus strain (4, 8, 14). As shown previously (4, 14, 35), the P[2] simian SA11 Cl3 rotavirus strain was SA dependent (Fig. 1A). The P[3] simian RRV strain depended on the presence of SA residues on the cell surface for efficient infectivity (Fig. 1B), as shown previously (4, 24-27; López et al., submitted). Testing of additional P[3] canine and feline (CU-1, K9, and Cat97) and human (HCR3a) rotavirus strains (Fig. 1B) showed that the infectivities of these strains were similarly affected by neuraminidase treatment of MA104 cells. The infectivities of P[7] porcine rotavirus strains CRW-8, C95, TFR-41, C134, A138, A411, SB-1A, and A253 (Fig. 1C) and OSU, EE, and A131 (data not shown) were SA dependent (Fig. 1C), as shown previously for P[7] porcine rotavirus strains OSU, CRW-8, and YM and equine rotavirus strain H-1 (4, 20, 23, 28). The SA dependency of the P[7] porcine rotavirus strains was not affected by the association of the VP4 P[7] genotype with five different VP7 G serotype specificities (Table 1) exhibited by the rotavirus strains tested. In addition, rotavirus strains belonging to P genotypes [1], [2], [3], and [7] also have the ability to hemagglutinate (4, 19, 31).
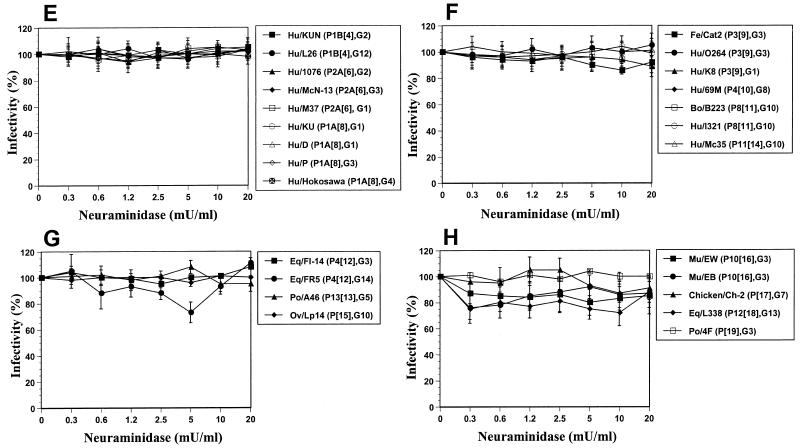
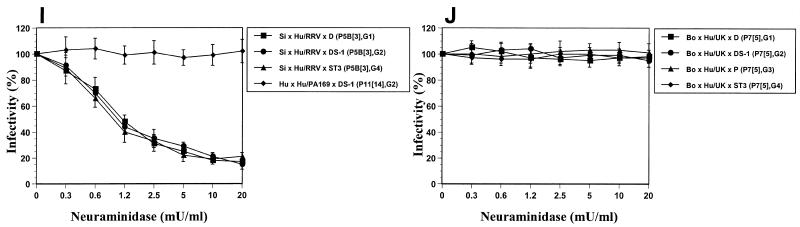
FIG. 1.
Effects of treatment of MA104 cells with neuraminidase (from A. ureafaciens) on the infectivities of P[1] and P[2] bovine and simian rotavirus strains reported previously (A); P[3] human, simian, canine, and feline rotavirus strains (B); P[7] porcine rotavirus strains (C); P[5] bovine and porcine rotavirus strains (D); P[4], P[6], and P[8] human rotavirus strains (E); P[9], P[10], P[11], and P[14] feline, human, and bovine rotavirus strains (F); P[12], P[13], and P[15] equine, porcine, and ovine rotavirus strains (G); P[16], P[17], P[18], and P[19] murine, avian (chicken), equine, and porcine rotavirus strains (H); P11[14] human-human reassortant and P5B[3] RRV-human rotavirus reassortant strains (I); and P7[5] UK-human rotavirus reassortant candidate vaccine strains (J). Viral infectivity was expressed as a percentage reflective of the number of FFU in neuraminidase-treated cells relative to the number observed in control (TNC buffer-treated) cells. Values shown are the arithmetic means of results from at least three replicate experiments. Error bars represent 1 standard error of the mean. The genotype and serotype are given in parentheses for each strain. Abbreviations: Si, simian; Eq, equine; Po, porcine; Ca, canine; Fe, feline; Bo, bovine; Ov, ovine; Mu, murine; Hu, human.
Confirming the results of a recent report (8), we found that the infectivity of the P[5] bovine UK rotavirus strain was unaffected by neuraminidase treatment of MA104 cells (Fig. 1D). Testing of additional P[5] bovine (B-641, WC3, and 678) and porcine (4S) strains (Fig. 1D), belonging to three different G serotypes, revealed that the rotaviruses had the same resistance following neuraminidase treatment of MA104 cells. In agreement with the results of previous studies (4, 14, 18), we found that human rotavirus strains KUN (P[4]), MO (P[8]), K8 (P[9]), and 69M (P[10]) and bovine rotavirus strain B223 (P[11]) are SA independent (Fig. 1E and F and data not shown). Additional human and animal P[4] (L26), P[6] (M37, 1076, and McN13), P[8] (KU, D, P, and Hokosawa), P[9] (O264 and Cat2), P[11] (I321), P[12] (FI-14 and FR5), P[13] (A46), P[14] (Mc35), P[15] (Lp14), P[16] (EB and EDIM), P[17] (Ch-2), P[18] (L338), and P[19] (4F) rotavirus strains analyzed in this study did not require the presence of SA residues on the cell surface for efficient infectivity (Fig. 1E to H). Taken together with previous results obtained for human P[4] (DS-1 and S2), P[6] (ST3), P[8] (Wa, YO, Ito, Nemoto, Hochi, VA70, and WI61), and P[14] (PA169 and HAL1166); porcine P[6] (Gottfried); equine P[12] (H-2 and FI-23); lapine P[14] (ALA, C-11, BAP-2, and R-2); murine P[16] (EC and EW); and avian P[17] (Ty-1; turkey) rotavirus strains (4, 18, 20, 28), our results suggest that human and animal rotavirus strains belonging to P genotypes [4] to [6] and [8] to [19] do not require the presence of SA residues on the cell surface for infectivity, regardless of the G serotype specificity of the strain. The P[20] murine strain EHP has also been reported to be SA independent in MA104 cells (24).
SA-independent rotavirus strains belonging to P genotypes [4] to [6], [8] to [14], [16], and [20] do not agglutinate human type O red blood cells, whereas the SA-independent equine rotavirus strain L338 does hemagglutinate (4, 19, 24, 31). The ability of SA-independent P[15], P[17], and P[19] rotavirus strains to hemagglutinate is not known. The infectivities of the SA-independent murine strains EB and EDIM and equine strain L338 (Fig. 1H) were slightly reduced in comparison to those of other SA-independent rotavirus strains, but the degree of infectivity reduction did not depend on the neuraminidase concentration. The same effect was observed for EHP, EW, and EC, the other SA-independent murine strains tested (4, 24). This reduction of infectivity suggests that SA could still be involved in the initial stage of the infectivity process of the SA-independent murine strains EB and EDIM and equine strain L338. The ability of equine rotavirus strain L338, but not murine strain EW, to hemagglutinate (19, 24) suggests that strain L338 may still bind to SA residues but that murine strain EW may not bind to SA residues. However, further analyses are required to determine if the equine P[18] strain L338 and all the murine P[16] and P[20] strains bind to SA residues to initiate infection.
Although our results indicate that the SA dependence and SA independence phenotypes are segregated according to the VP4 P genotype, Méndez et al. (27) reported that SA residue binding may be affected by altered VP4-VP7 interactions. We tested a series of rotavirus strains with different combinations of VP4 and VP7 specificities (Table 1); to further analyze the role of SA residues on the cell surface in initiating rotavirus entry into cells, the single-gene reassortant strain PA169 × DS-1 (P11[14], G2) (15) and the RRV-human and UK-human rotavirus reassortant candidate vaccine strains RRV × D (P5B[3], G1), RRV × DS-1 (P5B[3], G2), RRV × ST3 (P5B[3], G4), UK × D (P7[5], G1), UK × DS-1 (P7[5], G2), UK × P (P7[5], G3), and UK × ST3 (P7[5], G4) (29, 30) were tested for infectivity in neuraminidase-treated MA104 cells. Like the infectivity of the parental RRV strain (Fig. 1B), the infectivities of the reassortant strains RRV × D and RRV × ST3 were still found to depend on the presence of SA residues on the cell surface (Fig. 1I). Contrary to certain previous results (27) but consistent with those of Ludert et al. (25), the RRV × DS-1 reassortant strain was SA dependent, as was the RRV parental strain (Fig. 1I). In addition, reassortant strain PA169 × DS-1 and all four UK-human rotavirus strains were SA independent, as expected (Fig. 1I and J, respectively). Therefore, our results indicate that the dependency on SA residues in rotavirus entry is mediated by VP4 and not by VP7. In fact, the initial interaction of rotavirus strains with SA residues on the cell surface correlated exclusively with the VP4 genotype (P = 0.001; r2 = 1.00, Pearson's correlation coefficient), not with the species of origin, and was not affected by associations with different VP7 G serotype specificities.
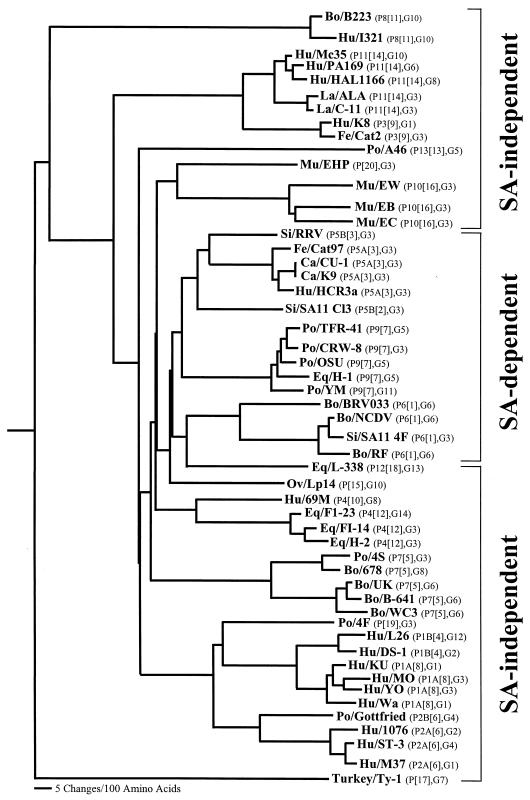
Table 2 summarizes whether all rotavirus and reassortant strains tested to date initially require SA residues on the cell surface for infectivity. To study the relatedness of the P genotype amino acid sequences of SA-dependent and SA-independent animal and human rotavirus strains, the amino acid sequences of the VP8* cleavage products of VP4 were aligned by using the PILEUP program, and parsimonious phylogenetic trees were generated by using the PAUP program (Wisconsin Package, version 9.1; Genetics Computer Group, Madison, Wis.). The validities of the resulting trees were tested by bootstrap analysis, and a consensus tree of 95% majority rule is presented for all VP8* sequences analyzed (Fig. 2). Each of the VP8* sequences of rotavirus strains belonging to each of the 20 P genotypes for which we know whether the presence of SA residues on the cell surface is required for efficient binding and infectivity formed a distinct branch on the phylogenetic tree. The VP8* sequences of SA-dependent rotavirus strains belonging to P genotypes [1], [2], [3], and [7] formed four closely related clusters (one for each P genotype) at the center of the phylogenetic tree. The VP8* sequences of SA-independent rotavirus strains belonging to the other known P genotypes showed a more complex pattern of phylogenetic relatedness, with most rotavirus strains falling into two multipart clusters, one including rotavirus strains from P genotypes [9], [11], [13], [14], [16], and [20] (Fig. 2, top) and the other including rotavirus strains from P genotypes [4], [5], [6], [8], [10], [12], [15], [18], and [19] (bottom). The VP8* sequence of the SA-independent rotavirus strain Ty-1 (P[17]) formed a distinct, independent branch. From the clustering of the VP8* sequence of the single P[18] equine strain L338 (Fig. 2), it would be difficult to predict the SA dependency of strain L338, because both the P[18] strain and all the P[1] strains have a common root in the phylogenetic tree. However, strain L338, which is known to hemagglutinate (19), may still bind SA residues indirectly, in a fashion similar to that of the isolated RRV variants that are no longer SA dependent for infectivity but retain HA activity (26). The VP8* sequence of the SA-independent Lp14 (P[15]) rotavirus strain clustered very closely to that of L338, but whether the P[15] ovine Lp14 rotavirus strain is able to hemagglutinate remains to be determined. Within P genotypes, minimal genetic distances often correspond with a common species of origin, as suggested previously (37), but there was not a tendency toward greater similarity associated with G serotype specificity. Although this could be a sampling artifact, it is suggestive of a relatively well-conserved gene belonging to the same VP4 genotype within a common species of origin. The finding that the amino acid sequences of the VP8* cleavage products of VP4 of all animal and human SA-dependent rotavirus strains formed a major cluster distinct from all other animal and human SA-independent rotavirus strains indicates that the requirement of the SA residue on the cell surface for efficient infectivity is associated with the P type rather than the species of origin, since all P[1], P[2], P[3], and P[7] animal and human rotavirus strains infect cells in an SA-dependent manner.
TABLE 2.
Summary of initial interactions of 76 rotavirus strains, of different P and G types, with SA residues on the surfaces of MA104 cells
| Virus strain(s)a | Serotype
|
SA dependencyc | Reference(s) | |
|---|---|---|---|---|
| Pb | G | |||
| Si/SA11 4Fd | 6[1] | 3 | + | This report |
| Bo/NCDV, Bo/RF,d Bo/BRV033d | 6[1] | 6 | + | This report and references 4, 8, and 14 |
| Si/SA11e | 5B[2] | 3 | + | This report and references 4, 14, and 36 |
| Si/RRV,e Hu/HCR3a,d Ca/CU-1,d Ca/K9,d Fe/Cat97d | 5A[3] | 3 | + | This report and references 4, 24, 26 |
| Hu/DS-1, Hu/S2, Hu/KUN | 1B[4] | 2 | − | This report and references 4, 14, and 18 |
| Hu/L26 | 1B[4] | 12 | − | This report |
| Po/4S | 7[5] | 3 | − | This report |
| Bo/UK, Bo/WC3, Bo/B-641 | 7[5] | 6 | − | This report and reference 8 |
| Bo/678 | 7[5] | 8 | − | This report |
| Hu/M37 | 2A[6] | 1 | − | This report |
| Hu/1076 | 2A[6] | 1 | − | This report |
| Hu/McN13 | 2A[6] | 2 | − | This report |
| Hu/ST3 | 2A[6] | 4 | − | 4 and 25 |
| Po/Gottfried | 2B[6] | 4 | − | 4 |
| Po/C95 | 9[7] | 1 | + | This report |
| Po/CRW-8, Po/A131, Po/A138, Po/A411 | 9[7] | 3 | + | This report and reference 20 |
| Po/SB-1A | 9[7] | 4 | + | This report |
| Po/OSU, Eq/H-1, Po/C134, Po/TFR-41, Po/EE | 9[7] | 5 | + | This report and references 4, 23, and 35 |
| Po/YM, Po/A253 | 9[7] | 11 | + | This report and reference 28 |
| Hu/Wa, Hu/KU, Hu/D | 1A[8] | 1 | − | This report and references 4, 14, and 20 |
| Hu/MO, Hu/YO, Hu/lto, Hu/Nemoto | 1A[8] | 3 | − | This report and references 4 and 14 |
| Hu/VA70, Hu/Hochi, Hu/Hokosawa | 1A[8] | 4 | − | This report and reference 4 |
| Hu/W161 | 1A[8] | 9 | − | 4 |
| Hu/K8 | 3[9] | 1 | − | This report and reference 4 |
| Hu/O264, Fe/Cat2 | 3[9] | 3 | − | This report |
| Hu/69M | 4[10] | 8 | − | This report and reference 4 |
| Bo/B223, Hu/1321 | 8[11] | 10 | − | This report and reference 4 |
| Eq/H-2, Eq/Fl-14 | 4[12] | 3 | − | This report and reference 4 |
| Eq/FI-23, Eq/FR5 | 4[12] | 14 | − | This report and reference 4 |
| Po/A46 | 13[13] | 5 | − | This report |
| La/Ala, La/C-11, La/BAP-2, La/R-2 | 11[14] | 3 | − | 4 |
| Hu/PA169 | 11[14] | 6 | − | 4 |
| Hu/HAL1166 | 11[14] | 8 | − | 4 |
| Hu/Mc35 | 11[14] | 10 | − | This report |
| Ov/Lp14 | [15] | 10 | − | This report |
| Mu/EW, Mu/EC, Mu/EB, Mu/EDIM | 10[16] | 3 | − | This report and references 4 and 24 |
| Turkey/Ty-1, Chicken/Ch-2 | [17] | 7 | − | This report and reference 4 |
| Eq/L338 | 12[18] | 13 | − | This report |
| Po/4F | [19] | 3 | − | This report |
| Mu/EHP | [20] | 3 | − | 24 |
Strain name is preceded by species of origin. Abbreviations: Hu, human; Si, simian; Bo, bovine; Ca, canine; Fe, feline; Eq, equine; Po, porcine; La, lapine; Ov, ovine; Mu, murine.
P genotype is designated in brackets, while serotype (where known) precedes P genotype (12).
As measured following treatment of MA104 cells with neuraminidase from A. ureafaciens.
Rotavirus strain tested for the first time in this report.
Rotavirus strain tested previously and in this report.
FIG. 2.
Phylogenetic relationships among the VP8* cleavage products of the VP4 spike proteins of 52 animal and human rotavirus strains representative of the 20 known P genotypes, for which we know if the presence of SA residues is required for efficient adsorption to and infectivity of MA104 cells. The tree shows an analysis based on amino acid sequences deduced from the gene encoding the VP8* cleavage product of VP4 and was constructed by using the PAUP program (Wisconsin Package, version 9.0). A consensus tree of 95% majority rule is presented. The tree is rooted with the VP8* amino acid sequence of the group A avian (turkey) rotavirus strain Ty-1. The vertical distances are arbitrary. VP8* amino acid sequence data were obtained from the following rotavirus strains, with the GenBank accession numbers in parentheses: SA11 4F (X57319), NCDV (VPXRT2), RF (U65924), BRV033 (U62155), SA11 Cl3 (M23188), RRV (M18736), CU-1 (D13401), K9 (D13400), Cat97 (D13402), HCR3a (L19712), DS-1 (P11196), L26 (M58292), UK (P12474), WC3 (AY050271), B-641 (M63267), 678 (D32054), 4S (L10358), Gottfried (M33516), ST3 (L33895), 1076 (P11198), M37 (L20877), OSU (X13190), TFR-41 (L07889), CRW-8 (L07888), H-1 (D16341), YM (P25174), MO (AB008278), YO (AB008279), Wa (L34161), KU (M21014), K8 (D90260), Cat2 (D13403), 69M (M60600), B223 (M92986), I321 (L07657), H-2 (D13397), FI-14 (D13398), FI-23 (D13342), A46 (AY050274), Mc35 (D140032), HAL1166 (L20875), PA169 (L20874), ALA (U62149), C-11 (U62150), Lp14 (L11599), EW (U08429), EB (U08419), EC (U08421), Ty-1 (L41493), L338 (D13399), 4F (L10359), and EHP (U08424). On the figure, the genotype and serotype for each strain are given in parentheses. Abbreviations: Si, simian; La, lapine; Eq, equine; Po, porcine; Ca, canine; Fe, feline; Bo, bovine; Ov, ovine; Mu, murine; Hu, human.
The loss of the SA requirement for the efficient infectivity of the RRV, SA11, and OSU variants segregated with VP4, and sequencing data of the VP4 genes of these variants revealed that mutations were present at aa 150 and 187 (25, 27); 180, 183, and 194 (4); and 100 (López et al., submitted), respectively. Also, a VP4 neutralization escape mutant from RRV, v4B6, identified a mutation at aa 190 that resulted in the loss of the ability to agglutinate erythrocytes (16). The predicted secondary structure of the VP8* cleavage product, which contains the SA-binding domain between aa 93 and 208, reveals the VP8* product as consisting of 11 β-strands separated by loops (9, 19). In this model, the mutations involved in the change from the SA dependence phenotype to the SA independence phenotype by rotavirus strains RRV, SA11, and OSU map to loops 3, 7, and 9 (4, 19, 25; López et al., submitted). Mutations identified in the RRV and SA11 variants are in close proximity to aa 155 and 188 to 190, located in loops 7 and 9, respectively, which have been determined to be essential for the SA-binding function of VP8* (19). Comparison of the VP8* sequences of SA-dependent and SA-independent rotavirus strains at amino acid residues that were identified in SA-independent variants and isolated from SA-dependent rotavirus SA11, RRV, or OSU strains (4, 25, 27; López et al., submitted) failed to identify conserved residues in the sequences of VP8* that might be involved in binding to SA residues (Table 3). However, the amino acid residues at these positions may still be critical for the conformation of VP8* and may alter binding to SA residues, because the mutations responsible for the phenotypic change from SA dependence to SA independence allow the variants to bypass the initial interaction with SA residues and interact with a common cellular molecule for virus entry (4, 19, 28). In addition, VP4 sequences outside the VP8* domain could contribute to SA residue binding. Structural studies of VP4 are under way, and atomic-resolution structures of the VP8* core with and without bound SA, obtained following nuclear magnetic resonance and X-ray crystallographic analyses, should identify the amino acids involved in SA residue binding for the SA dependence phenotype of some rotavirus strains (9).
TABLE 3.
Comparison of the VP8* trypsin cleavage products of VP4 proteinsa
| Amino acid positionb | Amino acid residue in:
|
|
|---|---|---|
| SA-dependent rotavirus strainsc | SA-independent rotavirus strainsd | |
| 100 | N or D | N, D, T, A, I, S, or G |
| 150 | G | G, Q, I, S, D, or N |
| 180 | E, Q, or T | E, D, T, or A |
| 183 | N | D, T, N, Q, H, or R |
| 187 | A, G, D, or K | D, K, R, S, T, A, or N |
| 190e | I or S | T, V, S, P, A, N, F, or L |
| 194 | F or Y | V, L, I, N, or Y |
VP4 proteins were examined at amino acid positions identified as responsible for the loss of the requirement of SA for efficient binding and the infectivity of SA-independent variants isolated from SA-dependent rotavirus strains. Variants v5C4 and nar3 were isolated from RRV (25, 27); 10G6R, 7G6R, and 954/23R were isolated from SA11 (4); and m10 was isolated from OSU (López et al., submitted).
The position of the amino acids is based on the VP4 of SA-dependent rotavirus strains, which contains a total of 776 amino acids, and is adjusted to those of some SA-independent rotavirus strains, which contain 772, 775, or 778 amino acids (12).
Mutation identified in a VP4 neutralization escape mutant of RRV, v4B6, that resulted in the loss of the ability to agglutinate erythrocytes.
This detailed analysis of the requirement of the presence of SA on the cell surface for rotavirus infectivity shows that the majority of animal and human rotavirus strains are SA independent. Out of the 76 human and animal rotavirus strains that have now been tested, only 22 bovine, simian, porcine, and human rotavirus strains of P genotypes [1], [2], [3], and [7] require the presence of SA residues on the cell surface for efficient infectivity. The remaining 54 human and animal rotavirus strains tested, belonging to the other 16 P genotypes, do not require SA residues on the cell surface for efficient infectivity. Since the SA dependency of rotavirus strains correlates with the VP4, it is expected that all rotavirus strains belonging to P genotypes [1], [2], [3], and [7], regardless of their species of origin, will be SA-dependent strains but that those belonging to the other known P genotypes will be SA independent. Dependency on SA binding may be transferred between species, as seen with the human strain HCR3a, belonging to the P[3] genotype and was found to be SA dependent. This strain was thought to have originally been a rotavirus circulating in dogs and cats, and its isolation from humans is thought to be the result of zoonotic transmission (32).
Many of the SA-dependent rotavirus strains (SA11, RRV, NCDV, and OSU) were originally isolated at least 2 decades ago and have been used as prototypes or reference strains in many laboratories because, in general, SA-dependent strains grow in tissue culture at titers 10 to 100 times higher than those of SA-independent strains and are more stable at 4°C (data not shown). Tissue culture adaptation of these P[1], P[2], P[3], and P[7] viruses did not select SA-dependent viruses, because porcine and bovine fecal specimens, presumably containing P[7] and P[1] strains, respectively, are able to form large plaques directly out of stools and they hemagglutinate prior to passage in cell culture. While the requirement of SA residues on the cell surface may be a characteristic necessary for efficient growth in cell culture, interesting questions are why do SA-dependent rotavirus strains grow more efficiently in cell culture than SA-independent rotavirus strains and why does the dichotomy between SA-dependent and SA-independent strains exist? It will also be of interest to determine what property of VP4, in SA-dependent rotavirus strains, is responsible for the efficient growth in cell culture and if this property has relevance to pathogenesis in vivo. Finally, these studies focus interest on testing of whether SA-independent variants isolated from SA-dependent strains are as infectious in animals as the parental SA-dependent strains are. In the cases of mengovirus, transmissible gastroenteritis virus, and mouse cytomegalovirus, the loss of binding activity to SA residues or the occurrence of HA results in a change in pathogenicity (1, 22, 34). Although there is not enough epidemiological information on canine, feline, and simian rotaviruses, the genotype most commonly found circulating in pigs is P[7], while both SA-dependent P[1] and SA-independent P[5] genotypes are equally common in cows (2, 3, 12; López et al., submitted). It is not known whether the prevalence of rotaviruses of genotypes P[7] and P[1] among pig and cow populations, respectively, relates to the fact that they are more commonly associated with disease or that they may spread more rapidly, thereby facilitating their identification at any given time. Future genetic approaches can address possible links between the biological relevance of binding to SA residues, pathogenicity, and transmission as well as continue to probe the molecular mechanisms related to VP4 that affect the early rotavirus-cell interactions in cell culture models.
Acknowledgments
We thank Sharon S. Krater and Noslen Lobo for tissue culture assistance and Sue E. Crawford and Margaret E. Conner for helpful suggestions.
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease grant DK30144, grant DK56338 to the Texas Gulf Coast Digestive Diseases Center, and NHS Executive (Eastern) grant HSR/1199/6.
REFERENCES
- 1.Anderson, K., and C. Bond. 1987. Biological properties of mengovirus: characterization of avirulent, hemagglutination-defective mutants. Arch. Virol. 93:31-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridger, J. C., G. I. Tauscher, and U. Desselberger. 1998. Viral determinants of rotavirus pathogenicity in pigs: evidence that the fourth gene of a porcine rotavirus confers diarrhea in the homologous host. J. Virol. 72:6929-6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke, B., M. A. McCrae, and U. Desselberger. 1994. Sequence analysis of two porcine rotaviruses differing in growth in vitro and in pathogenicity: distinct VP4 sequences and conservation of NS53, VP6, and VP7 genes. J. Gen. Virol. 75:2205-2212. [DOI] [PubMed] [Google Scholar]
- 4.Ciarlet, M., and M. K. Estes. 1999. Human and most animal rotavirus strains do not require the presence of sialic acid on the cell surface for efficient infectivity. J. Gen. Virol. 80:943-948. [DOI] [PubMed] [Google Scholar]
- 5.Ciarlet, M., and M. K. Estes. 2001. Interactions between rotavirus and gastrointestinal cells. Curr. Opin. Microbiol. 4:435-441. [DOI] [PubMed] [Google Scholar]
- 6.Ciarlet, M., S. E. Crawford, and M. K. Estes. 2001. Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains. J. Virol. 75:11834-11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford, S. E., M. Labbé, J. Cohen, M. H. Burroghs, Y.-J. Zhou, and M. K. Estes. 1994. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J. Virol. 68:5945-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delorme, C., H. Brüssow, J. Sidoti, N. Roche, K.-A. Karlsson, J.-R. Neeser, and S. Teneberg. 2001. Glycosphingolipid binding specificities of rotavirus: identification of a sialic acid-binding epitope. J. Virol. 75:2276-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dormitzer, P. R., H. B. Greenberg, and S. C. Harrison. 2001. Proteolysis of monomeric recombinant rotavirus VP4 yields an oligomeric VP5* core. J. Virol. 75:7339-7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espejo, R. T., S. López, and C. Arias. 1981. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J. Virol. 37:156-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estes, M. K., D. Y. Graham, and B. B. Mason. 1981. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J. Virol. 39:879-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747-1785. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 13.Fiore, L., H. B. Greenberg, and E. R. Mackow. 1991. The VP8* fragment of VP4 is the rhesus rotavirus hemagglutinin. Virology 181:553-563. [DOI] [PubMed] [Google Scholar]
- 14.Fukudome, K., Y. Osamu, and T. Konno. 1988. Comparison of human, simian, and bovine rotavirus for requirement of sialic acid in hemagglutination and cell adsorption. Virology 172:192-205. [DOI] [PubMed] [Google Scholar]
- 15.Gerna, G., J. Sears, Y. Hoshino, A. D. Steele, O. Nakagomi, A. Sarasini, and J. Flores. 1994. Identification of a new VP4 serotype of human rotaviruses. Virology 200:66-71. [DOI] [PubMed] [Google Scholar]
- 16.Giammarioli, A. M., E. R. Mackow, L. Fiore, H. B. Greenberg, and F. M. Ruggeri. 1996. Production and characterization of murine IgA monoclonal antibodies to the surface antigens of rhesus rotavirus. Virology 225:97-110. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg, H. B., J. Flores, A. Kalica, R. Wyatt, and R. Jones. 1983. Gene coding assignments for growth restriction neutralization and subgroup specificities of the Wa and DS-1 strains of human rotavirus. J. Gen. Virol. 64:313-320. [DOI] [PubMed] [Google Scholar]
- 18.Guo, C., O. Nakagomi, M. Mochizuki, H. Ishida, M. Kiso, Y. Ohta, T. Suzuki, D. Miyamoto, K. Hidari, and Y. Suzuki. 1999. Ganglioside GM(1a) on the cell surface is involved in the infection by human rotavirus KUN and MO strains. J. Biochem. (Tokyo) 126:683-688. [DOI] [PubMed] [Google Scholar]
- 19.Iša, P., S. López, L. Segovia, and C. F. Arias. 1997. Functional and structural analysis of the sialic acid-binding domain of rotaviruses. J. Virol. 71:6749-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jolly, C., B. Beisner, and I. H. Holmes. 2000. Rotavirus infection of MA104 cells is inhibited by Ricinus lectin and separately expressed single binding domains. Virology 275:89-97. [DOI] [PubMed] [Google Scholar]
- 21.Kalica, A., J. Flores, and H. B. Greenberg. 1983. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology 125:194-205. [DOI] [PubMed] [Google Scholar]
- 22.Krempl, C., M. L. Ballesteros, G. Zimmer, L. Enjuanes, H. Klenk, and G. Herrler. 2000. Characterization of the sialic acid binding activity of transmissible gastroenteritis coronavirus by analysis of haemagglutination-deficient mutants. J. Gen. Virol. 81:489-496. [DOI] [PubMed] [Google Scholar]
- 23.Liprandi, F., Z. Moros, M. Gerder, J. E. Ludert, F. H. Pujol, M.-C. Ruiz, F. Michelangeli, A. Charpilienne, and J. Cohen. 1997. Productive penetration of rotavirus in cultured cells induces co-entry of the translation inhibitor α-sarcin. Virology 237:430-438. [DOI] [PubMed] [Google Scholar]
- 24.Ludert, J. E., N. Feng, J. H. Yu, R. L. Broome, Y. Hoshino, and H. B. Greenberg. 1996. Genetic mapping indicates that VP4 is the rotavirus cell attachment protein in vitro and in vivo. J. Virol. 70:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludert, J. E., B. Mason, J. Angel, B. Tang, Y. Hoshino, N. Feng, P. T. Vo, E. R. Mackow, F. M. Ruggeri, and H. B. Greenberg. 1998. Identification of mutations in the rotavirus protein VP4 that alter sialic-acid-dependent infection. J. Gen. Virol. 79:725-729. [DOI] [PubMed] [Google Scholar]
- 26.Méndez, E., C. F. Arias, and S. López. 1993. Binding to sialic acids is not an essential step for the entry of animal rotaviruses to epithelial cells in culture. J. Virol. 67:5253-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Méndez, E., C. F. Arias, and S. López. 1996. Interactions between the two surface proteins of rotavirus may alter the receptor-binding specificity of the virus. J. Virol. 70:1218-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Méndez, E., S. López, M. Cuadras, P. Romero, and C. F. Arias. 1999. Entry of rotaviruses is a multistep process. Virology 263:450-459. [DOI] [PubMed] [Google Scholar]
- 29.Midthun, K., H. B. Greenberg, Y. Hoshino, A. Z. Kapikian, R. G. Wyatt, and R. M. Chanock. 1985. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J. Virol. 53:949-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Midthun, K., Y. Hoshino, A. Z. Kapikian, and R. M. Chanock. 1986. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J. Clin. Microbiol. 24:822-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mochizuki, M., and O. Nakagomi. 1995. Haemagglutination by rotaviruses in relation to VP4 genotypes. Res. Virol. 146:371-374. [DOI] [PubMed] [Google Scholar]
- 32.Nakagomi, T., and O. Nakagomi. 2000. Human rotavirus HCR3 possesses a genomic RNA constellation indistinguishable from that of feline and canine rotaviruses. Arch. Virol. 145:2403-2409. [DOI] [PubMed] [Google Scholar]
- 33.Offit, P. A., G. Blavat, H. B. Greenberg, and H. F. Clark. 1986. Molecular basis of rotavirus virulence: role of gene segment 4. J. Virol. 57:46-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravindranath, R. M. H., and M. C. Graves. 1990. Attenuated murine cytomegalovirus binds to N-acetylglucosamine, and shift to virulence may involve recognition of sialic acids. J. Virol. 64:5430-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rolsma, M. D., H. B. Gelberg, and M. S. Kuhlenschmidt. 1994. Assay for evaluation of rotavirus-cell interactions: identification of an enterocyte ganglioside fraction that mediates group A porcine rotavirus recognition. J. Virol. 68:258-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Superti, F., and G. Donelli. 1991. Gangliosides as binding sites in SA-11 rotavirus infection of LLC-MK2 cells. J. Gen. Virol. 72:2467-2474. [DOI] [PubMed] [Google Scholar]
- 37.Taniguchi, K., T. Urasawa, and S. Urasawa. 1994. Species specificity and interspecies relatedness in VP4 genotypes demonstrated by VP4 sequence analysis of equine, feline, and canine rotavirus strains. Virology 200:390-400. [DOI] [PubMed] [Google Scholar]
- 38.Tauscher, G. I., and U. Desselberger. 1997. Viral determinants of rotavirus pathogenicity in pigs: production of reassortants by asynchronous coinfection. J. Virol. 71:853-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zárate, S., R. Espinosa, P. Romero, E. Méndez, C. F. Arias, and S. López. 2000. The VP5 domain of VP4 can mediate attachment of rotaviruses to cells. J. Virol. 74:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]