Abstract
Breast-feeding-associated protection against calicivirus diarrhoea is associated with the presence of high levels of 2-linked oligosaccharides in mother's milk, and human calicivirus strains including the NV (Norwalk virus) use gut 2-linked fucosylated glycans as receptors, suggesting the presence of decoy receptors in milk. Our aim was to analyse the ability of human milk to inhibit the attachment of rNV VLPs (recombinant NV-like particles) to their carbohydrate ligands and to characterize potential inhibitors found in milk. Milk from women with the secretor phenotype was strongly inhibitory, unlike milk from women that are non-secretors, which is devoid of 2-linked fucosylated structures. At least two fractions in human milk acted as inhibitors for the NV capsid attachment. The first fraction corresponded to BSSL (bile-salt-stimulated lipase) and the second to associated mucins MUC1 and MUC4. These proteins present tandem repeat O-glycosylated sequences that should act as decoy receptors for the NV, depending on the combined mother/child secretor status.
Keywords: bile-salt-stimulated lipase (BSSL), mucin, norovirus, fucosyltransferase, histo-blood-group, gastroenteritis
Abbreviations: BSDL, bile-salt-dependent lipase; BSSL, bile-salt-stimulated lipase; Le, Lewis blood-group-antigen; mAb, monoclonal antibody; NV, Norwalk virus; rNV VLP, recombinant NV virus-like particle; TMB, 5-tetramethylbenzidine
INTRODUCTION
Gastroenteritis constitutes a major cause of morbidity and mortality in developing countries and remains a major health problem in developed countries. Recent epidemiological studies revealed that the majority of outbreaks of non-bacterial gastroenteritis were caused by members of the norovirus genus in the calicivirus family [1,2]. These viruses, which infect people of all ages have also been reported to be involved in a large number of sporadic cases, particularly among children [3,4]. Although it is considered that norovirus gastroenteritis is of moderate severity, recent reports suggest that in people under stressful conditions or in young children it could be more severe than expected [5].
We and others have recently shown that some caliciviruses attach to host epithelial cells through carbohydrates of the histo-blood-group family. Thus NV (Norwalk virus), the prototypical norovirus, binds to 2-fucosylated structures based on the blood-group-antigen H type 1 trisaccharide Fucα2Galβ3GlcNAc [6–8]. Synthesis of this ligand requires an active α1,2-fucosyltransferase, which in most epithelial cells is the FUT2 enzyme. The presence of this enzymatic activity characterizes the so-called secretor phenotype. Inactivating mutations in the FUT2 gene, occurring in approx. 20% of the population with European ancestry, are responsible for the non-secretor phenotype, characterized by an absence of 2-fucosylated structures, and hence of ABO(H) blood-group antigens in secretions such as saliva and on most epithelial cells [9]. Consistent with the attachment of NV to H type 1-containing glycans, volunteers challenged with the virus become infected only if they are secretors [10,11].
Breast-feeding has been known for a long time to protect infants from diarrhoea and a number of potential protective factors in human milk have been characterized. These include maternal antibodies, lactoferrin, BSSL (bile-salt-stimulated lipase), lactadherin, lysozyme, cytokines, oligosaccharides and mucins [12,13]. Unlike cows' milk, human milk contains large amounts of fucosylated oligosaccharides, the amount of 2-linked fucose depending upon the FUT2 status of the mother, with large variations during the course of lactation [14–16]. Human milk additionally contains glycans in the form of glycolipids or glycoproteins that can be fucosylated in a genetically determined manner. We observed previously that the milk from secretor mothers could inhibit the binding of rNV VLPs (recombinant NV virus-like particles) to their carbohydrate ligands [7] and hypothesized that the 2-fucosylated milk component could have a protective effect, acting as a decoy receptor, depending upon the combined FUT2 status of the mother and child [17]. Thus a non-secretor child would be genetically resistant to NV whereas a secretor child that is potentially sensitive would be protected by his mother's milk providing she is of the secretor-type. As a first step towards testing this hypothesis, the present study was undertaken in order to characterize the components of human milk that inhibit NV-binding to its carbohydrate ligand.
MATERIALS AND METHODS
Collection and treatment of milk samples
Human milk was obtained from the milk bank of the Nantes University Hospital in accordance with its ethical guidelines. Milk was collected from 15 anonymous, healthy mothers at 3 weeks lactation, labelled from 1 to 15 and stored at −20 °C prior to analysis. Samples labelled A, from an additional mother, were collected at 3 and 5 weeks post-partum. Inactivation of the milk antibodies and enzymes was performed by boiling 100 μl samples in a water bath in the presence of 5% (v/v) β-mercaptoethanol.
To prepare fractions, frozen human milk was thawed and centrifuged at 20000 g for 1 h. The fat layer and the pellet were removed and the remaining skimmed milk was filtered. In order to remove small molecules such as oligosaccharides, skimmed milk was dialysed against PBS. Human fat globule membranes were prepared by sonicating the fat layer after addition of PBS followed by ultracentrifugation at 100000 g for 1 h and recovery of the pellet as previously described [18].
Determination of the secretor character
Milk samples were diluted at 1:10 in 100 mmol/l carbonate buffer (pH 9.6), coated on to NUNC immunoplates (Roskilde, Denmark) and stored overnight at 37 °C. After blocking with 5% dried cows' milk in PBS, mAb (monoclonal antibody) LM137, which is specific for H type 1 and Leb (Lewis b histo-blood-group) antigens, was added. After incubation for 2 h at 37 °C, horseradish peroxidase conjugated anti-(goat IgG) and anti-(mouse IgG) (Uptima, Montluçon, France) at 1:2000 dilutions were added. Between each step, the plates were washed three times with PBS, 5% (v/v) Tween-20. The enzyme signals were detected using TMB (5-tetramethylbenzidine) as substrate (BD Bioscience, San Jose, CA, U.S.A.) then read at A450. Strong reactivity with the anti-H type 1/Leb mAb was detected in 11 out of 16 cases. To confirm that this did correspond to the difference between secretor- and non-secretor-mothers, genotyping for the FUT2 gene was performed. The G428A mutation is responsible for nearly all cases of non-secretor phenotype in the French population, thus it was the only mutation that was screened. Genomic DNA from total milk that contains epithelial cells was extracted from the first pellet using the Qiagen QIAmp DNA mini kit according to the manufacturer's instructions. A fragment of the FUT2 gene was amplified with the following primers; GAGGAATACCGCCACATCCCGGGGGAGTAC, forward; ATGGACCCCTACAAAGGTGCCCGGCCGGCT, reverse, and digested using AvaII. The G428A mutation eliminates this restriction site.
Inhibition of rNV VLP-binding to saliva or synthetic H type 1 antigen
Saliva from secretor individuals [secretor (active FUT2 allele), blood type O, Le positive] at a dilution of 1:1000, or H type 1 synthetic oligosaccharide bound to polyacrylamide at 50 μg/ml was coated on to Maxisorb NUNC plates, stored overnight at 37 °C and saturated with 5% dried milk in PBS. rNV VLPs were produced using a baculovirus expression system as previously described [19]. rNV VLPs were pre-incubated with serially diluted total milk samples or milk fractions (v/v) at a final concentration of 0.6 μg/ml for 60 min at room temperature and were then incubated for 1 h on the coating plate. Binding was detected using a rabbit anti-rNV VLP antiserum diluted to 1:2000 in 5% dried milk in PBS, followed by incubation with peroxidase-conjugated anti-(rabbit IgG). Both reagents were incubated for 1 h at 37 °C. Reactions were revealed by using the TMB substrate and then read at A450.
Immunohistochemical analyses
Paraffin embedded tissue sections of the gastroduodenal junction from adult secretor individuals of blood-group O type were rehydrated in graded ethanol and washed in PBS. Endogenous peroxidase was inhibited using 0.3% methanol or H2O2 for 15 min. After saturation with PBS containing 3% BSA for 30 min, sections were covered for 90 min at room temperature with a 1:1 (v/v) mixture of 5 μg/ml rNV VLPs and human milk samples at 1:10 dilution, in PBS that had been pre-incubated at 4 °C overnight. The anti-NV capsid protein 9C3 antiserum, diluted 1:1000 in PBS and 1% (w/v) BSA was added and the mixture was incubated at room temperature for 1 h. Sections were then rinsed three times with PBS and incubated with peroxidase-conjugated anti-(mouse IgG) diluted to 1:2000 in PBS, 1% BSA (Vector Labs, Burlingame, CA, U.S.A.) for 45 min, and reactions were visualized with 3-amino-9-ethylcarbazol (Vector Labs). Counterstaining was performed using Mayer's hemalun.
Western blotting
Protein concentrations were measured using bicinchoninic acid. Proteins (30 μg) were subjected to SDS/PAGE (5–10% gel). Separated proteins were electrophoretically transferred to Immobilon membranes (Millipore, Milford, MA, U.S.A.) in 25 mM Tris, 192 mM glycine, 20% methanol at 350 mA for 1 h. After transfer, membrane strips were incubated in PBS containing 3% skimmed milk for 1 h at 37 °C. Membranes were then incubated with 3 μg/ml rNV VLPs diluted in PBS containing 1% skimmed milk overnight at 4 °C. Binding was detected using a rabbit anti-rNV VLP antiserum diluted to 1:2000 in 5% dried milk in PBS, followed by peroxidase-conjugated anti-(rabbit IgG) at a dilution of 1:1000. After washing twice in PBS containing 5% (v/v) Tween-20 and once in PBS, the bound antibodies were revealed by chemiluminescence using the ECL® (enhanced chemiluminescence) Kit from Amersham (Little Chalfont, U.K.). The following primary antibodies were also used: LM137 anti-H type 1/Leb, L64 rabbit anti-BSDL (bile-salt-dependent lipase) [20], or HMFG1 and 8G7 directed against the MUC1 and MUC4 human mucins respectively [21,22]. After washes, membranes were incubated for 1 h with peroxidase-labelled anti-(mouse IgG) diluted to 1:2000 in PBS containing 1% skimmed milk and reactions were visualized as described above. Alternatively, membranes that were treated with polyclonal L64 antibody were incubated for 1 h with alkaline phosphatase-labelled anti-(rabbit IgG) and reactions were carried out as previously described [20].
Preparation of the mucin fraction and purification of BSSL and BSDL
The mucin fraction from human milk was prepared by preparative electrophoresis. Skimmed milk samples (750 μl) containing 30 mg/ml protein were loaded on to SDS/PAGE (5%) gel and allowed to migrate for 1 h at 150 V until Coomassie Blue stained bands were no longer detectable. The upper third of the gel was cut and minced in to small fragments and incubated in PBS for 48 h at 4 °C before elution. After centrifugation at 800 g for 5 min, the soluble material was dialysed against PBS and concentrated to the original volume of milk on 100 kDa cut-off Centricon membranes (Millipore). BSSL and its pancreatic counterpart BSDL were purified from human milk and human pancreatic juice respectively [20]. The esterolytic activity of these enzymes was recorded on 4-nitrophenyl caproate as described by Gjellesvik et al. [23].
RESULTS
Inhibition of rNV VLP ligand-binding by human milk
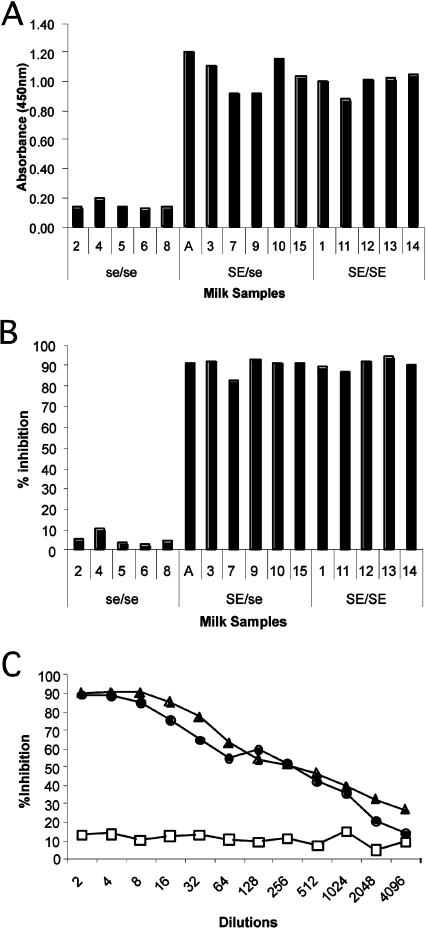
We have shown previously that human milk could inhibit rNV VLP-binding to their histo-blood-group ligand [7]. We thus sought to determine if this inhibition could be influenced by the secretor character of the mother. Since the composition of milk varies during the course of lactation, all samples used were collected at the same time-point post-partum. The mothers' secretor phenotype was determined from their milk samples (Figure 1A). A total of five non-secretors were found, all of whom were homozygous for the G428A mutation. Serially diluted total milk samples were used and as can be seen in Figure 1(B) at a dilution of 1:10, none of the five non-secretor samples inhibited rNV VLP-binding to immobilized synthetic H type 1, in contrast with the strong inhibition given by all samples from secretor mothers, irrespective of whether they were homozygotes or heterozygotes. A representative example of the strengh of the inhibition by secretor milk is shown in Figure 1(C). Inhibition (50%) was obtained at a dilution of 1:500, whereas no significant inhibition was observed even at a dilution of 1:2 in non-secretor milk. Similar results were obtained when saliva from an O type secretor individual was used, instead of synthetic H type 1, for attachment of rNV VLPs (results not shown).
Figure 1. FUT2-dependent molecules from human milk inhibit rNV VLP attachment to H type 1 histo-blood-group antigen.
(A) Milk samples from women genotyped for the FUT2 locus were coated on to ELISA plates at a dilution of 1:1000 and the presence of H type 1-reactive molecules was determined by reactivity of the anti-H type 1/Leb mAb, LM137. A total of five women were considered as non-secretors (2, 4, 5, 6 and 8) and the remaining as secretors. SE=active FUT2 alelle; se=inactive FUT2 allele. (B) Inhibition of rNV VLP attachment to polyacrylamide-conjugated H type 1 by milk samples from secretor and non-secretor women diluted to 1:10. (C) Examples of the inhibitory potency of milk samples, from 2 secretors (●, ▲) and a non-secretor (□), on rNV VLP attachment to saliva from an O type secretor individual. The inhibition assay was performed as described in the Materials and methods section. The percentage of inhibition is shown as a function of the reciprocal milk dilution.
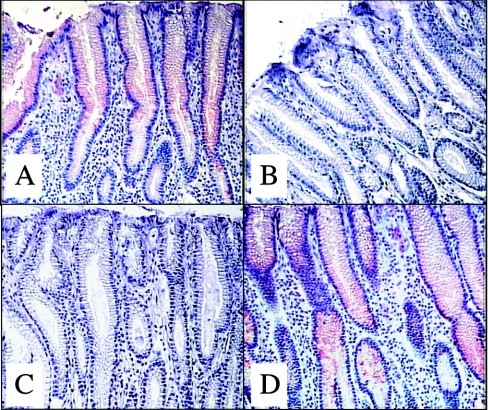
In order to determine if the inhibition by the synthetic ligand could be physiologically relevant, it was tested on gastroduodenal junction tissue sections. We have previously shown that rNV VLPs attach to the surface of epithelial cells only in secretors [7]. Thus rNV VLPs were co-incubated with milk samples on tissue sections from secretor individuals. As illustrated in Figure 2, for the pyloric area, a strong binding of recombinant capsids occurred in the presence of milk from non-secretor mothers, whereas no binding to tissue sections could be seen in the presence of milk from secretor mothers. The same results were obtained in tissue sections from the duodenum (results not shown). This indicates that human milk from secretors has the potential to block the attachment of NV to its carbohydrate ligand on epithelial cells.
Figure 2. Binding of rNV VLPs to human gastroduodenal tissue and inhibition by human milk.
Tissue sections from the gastroduodenal junction of a secretor (A, C and D) and a non-secretor (B) were incubated with rNV VLPs and the binding was detected as described in the Materials and methods section. In (C, D) VLPs were co-incubated with milk samples from secretor and non-secretor women respectively, at a dilution of 1:10. All pictures taken are from the pyloric area. A complete absence of VLP binding was observed in the presence of milk from a secretor, as in the tissue from a non-secretor.
Milk fractions containing inhibitory activity
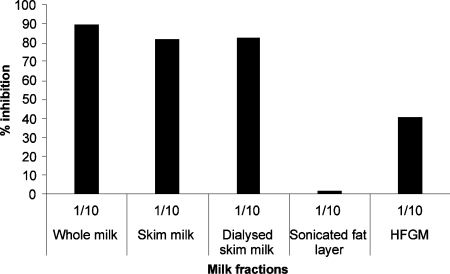
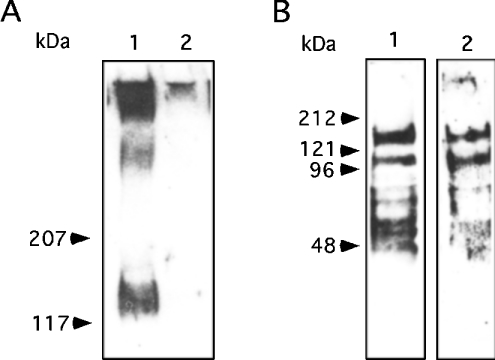
Human milk possesses large amounts of free oligosaccharides that could potentially inhibit the attachment of rNV VLPs to their carbohydrate ligands. In addition, similar carbohydrate structures can be complexed to proteins or lipids. Blocking-antibodies could also be present. In order to determine which of these components is mostly responsible for the inhibition observed above, various milk fractions were prepared. We first compared milk samples that had been boiled in the presence of β-mercaptoethanol with their untreated counterpart in the inhibition assay. No difference was observed between the two types of samples, ruling out the possibility that blocking-antibodies or milk enzyme activities were responsible for the inhibition of rNV VLP-binding to carbohydrate ligands. Fat-globule membranes showed some inhibition (Figure 3). Skimmed milk, as well as dialysed skimmed milk were nearly as potent as inhibitors as unfractionated milk. Since dialysis did not decrease inhibitory potency, it is likely that free oligosaccharides are not involved. This was confirmed by using purified oligosaccharides. Neither the pentasaccharide Fucα2Gal-β3GlcNAcβ3Galβ4Glc (LNF-I) nor the tetrasaccharide Galβ3-GlcNAcβ3Galβ4Glc (LNT) was able to inhibit ligand-binding even at concentrations as high as 22 mM. Unlike the tetrasaccharide, the fucosylated pentasaccharide is terminated by the H type 1 epitope and is characteristic of milk from secretor mothers, yet it did not inhibit binding. The inhibitory material was thus searched for in the protein fraction. Western blotting indicated that rNV VLPs could detect two sets of bands in milk samples exclusively from secretor mothers. These corresponded to a smear of very high molecular mass and to a broad band of approx. 130 kDa (Figure 4A). Most milk samples showed only one band at that level, but some samples showed two bands. Yet since larger samples were obtained from woman A whose milk showed two bands of approx. 130 kDa, most experiments were performed using this milk. Additional bands corresponding to material of lower size could be detected using an anti-H type 1/Leb mAb but they were less clearly revealed with rNV VLPs (Figure 4B).
Figure 3. Inhibition of rNV VLP attachment to saliva from a secretor individual by milk fractions from a secretor donor.
A fresh secretor milk-sample was centrifuged at 1500gfor 20 min to separate the cream from the milk. The skimmed milk was dialysed to remove free oligosaccharides and the cream was washed by centrifugation in PBS to remove soluble proteins. Washed cream was resuspended in PBS and sonicated to disrupt fat globules. The human fat globule membranes (HFGM) were pelleted by centrifugation at 100000 g for 1 h and separated from the fat layer. Each fraction corresponding to the original milk dilution at 1:10 was co-incubated with rNV VLPs in the inhibition assay as described above.
Figure 4. Detection of human milk rNV VLP ligands by Western blotting.
(A) Skimmed milk samples were run on SDS/PAGE (5% gel under reducing conditions and probed with rNV VLPs; lane 1, secretor sample number 3; lane 2, non-secretor sample number 4. (B) Skimmed milk from a secretor (sample A) was run on an SDS/PAGE 5–10% gradient gel under reducing conditions and probed either with the anti-H type 1/Leb mAb, LM137 (lane 1) or with rNV VLPs (lane 2). Positions of the molecular mass markers are shown by arrows.
BSSL and mucins from secretor mothers are inhibitory
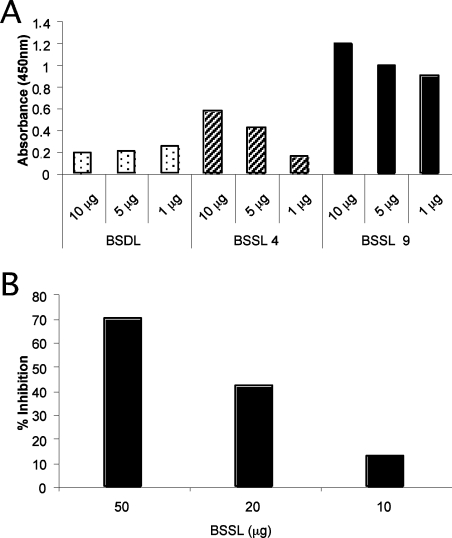
BSSL corresponds to a major milk protein. It can be found in a heavily glycosylated form and has a molecular mass of approx. 130 kDa. It therefore appeared to be a good candidate for the inhibition of rNV ligand-binding. In addition, Western blotting of our human milk samples using an antiserum raised against a BSSL isoform purified from pancreatic juice (BSDL) revealed bands that co-migrated with those detected by rNV VLPs (as described below). We thus purified BSSL from the milk of a secretor and a non-secretor mother. The purification was performed by affinity chromatography of immobilized antibodies as shown in Figure 5(A). The milk fraction from woman A, which contained the lipase activity, yielded two bands specifically recognized by the anti-BSDL antiserum, as well as by rNV VLPs (Figures 5B, 5C and 5D). The binding of rNV VLPs to purified BSSL from the secretor mother was compared with that of the non-secretor mother and to BSDL purified from the pancreatic juice of an O type secretor individual. A strong binding occurred to BSSL from the secretor mother whereas binding was much lower to BSSL from the non-secretor mother. More surprisingly, no binding was observed to purified BSDL from an O type secretor individual, suggesting a difference in glycosylation between the pancreatic and milk isoforms of the lipase that can be detected by the virus capsids (Figure 6A). The potency of BSSL was then tested in the inhibition assay. BSSL from the secretor mother was indeed able to inhibit the binding of rNV VLPs to either salivary mucins from an O type secretor or to synthetic H type 1 antigen. Final amounts of 30 or 2 μg of BSSL were necessary to produce 50% inhibition of rNV VLP-binding to salivary mucins (Figure 6B) and to synthetic H type 1 antigen respectively (results not shown).
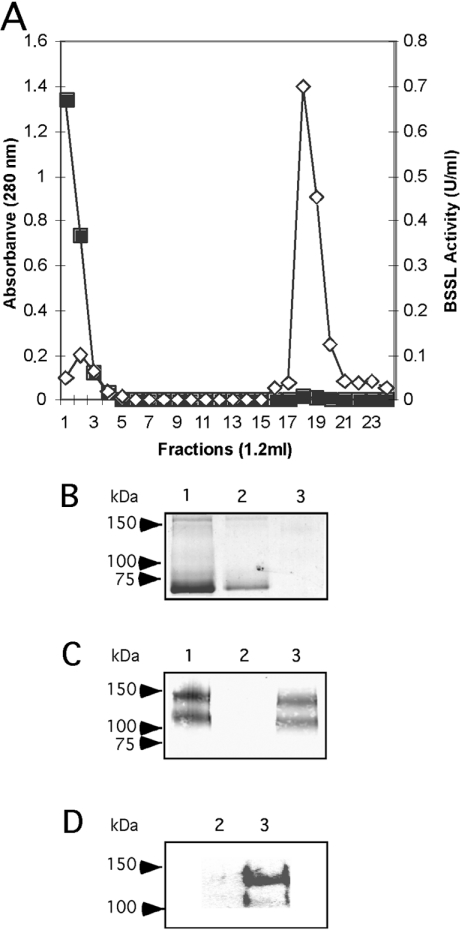
Figure 5. Purification of BSSL from the milk of secretor woman A.
(A) Total proteins were loaded on to an immobilized anti-BSDL pAbL64 (polyclonal antibody L64) column equilibrated with PBS and incubated overnight at 4 °C. Unbound fractions were eluted and the column was washed with 0.02 M Tris/HCl and 0.5 M NaCl, pH 7.4 until zero absorbance. Bound fractions were eluted with 0.1 M glycine, pH 2.5. Elution of material was recorded by the absorbance at 280 nm (■). Enzyme activity (◇) was determined with 4-nitrophenyl hexanoate as substrate. (B) Coomassie Blue stained SDS/PAGE gels and (C, D) Western blotting of fractions eluted from the immobilized pAbL64 column, (lane 1, 1 μg of starting material), (lane 2, 1 μg of unbound material), (lane 3, 1 μg of bound material). Immunodetection was performed with mAb pAbL64 (C) or rNV VLPs (D).
Figure 6. Binding of rNV VLPs to purified BSDL and BSSL.
(A) Purified glycoproteins (1, 5 or 10 μg) from secretor donors (BSDL from the pancreatic juice of an O type secretor patient and BSSL from mother number 9) or from a non-secretor milk donor (mother number 4) were coated on to ELISA plates and the binding of rNV VLPs was tested. (B) Inhibition of rNV VLP attachment to the saliva of a secretor individual by purified BSSL from secretor A.
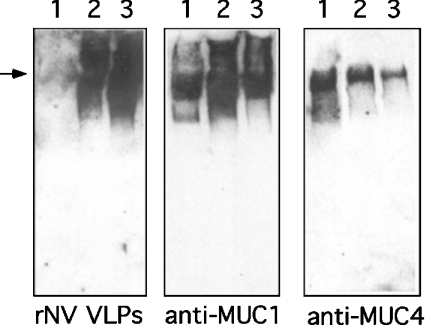
The diffuse high molecular mass band stained by rNV VLPs on Western-blots suggested recognition of a mucin component. This high molecular mass fraction was isolated by preparative electrophoresis. Western-blots from 5% polyacrylamide gels showed that rNV VLPs bind equally well to the mucin preparation as to the high molecular mass fraction from the total milk of a secretor mother. They do not bind to similar material from the milk sample of a non-secretor donor. Yet, irrespective of the donor's secretor character, milk and high molecular mass preparations contained both MUC1 and MUC4 mucins (Figure 7). The intensity of staining is weaker when using the anti-MUC4 mAb than the anti-MUC1 mAb but it should be noted that the anti-MUC4 mAb was raised against an unglycosylated synthetic peptide [22] and might not optimally recognize the native glycosylated milk mucin. Similar to that which we observed using purified BSSL, the mucin fraction from the milk of secretor mothers was able to strongly inhibit the binding of rNV VLPs whereas the mucin fraction from a non-secretor woman was not (results not shown). In order to determine the relative importance of BSSL versus the mucins, we compared the inhibitory capacity of milk from a secretor woman with that of the same BSSL-depleted milk. BSSL depletion was performed by loading 0.5 ml of skimmed milk on to the anti-BSSL pAb64 column. This corresponded to 12 mg of total protein with 290 units/mg of BSSL specific activity. The unretained fraction was collected and 12 mg of protein was recovered containing 9 units/mg of BSSL specific activity, indicating that most of the BSSL had been removed by the immobilized antibodies. This was further confirmed by Western blotting. The ratio of dilutions for the two samples (BSSL-depleted/total) required to reach 50% inhibition in the rNV VLP binding assay was then determined. The ratio was 0.40±0.07 from four independant experiments performed in triplicate, showing that BSSL contributes 60% of the total inhibitory activity, whereas the remaining 40% corresponds to the contribution from the mucins and possibly from some other minor components.
Figure 7. Binding of rNV VLPs to human milk mucins.
Skimmed milk samples from non-secretor (lane 1) or secretor (lane 2) donors and the mucin fraction from milk of a secretor donor prepared as described in the Materials and methods section (lane 3) were run on SDS/PAGE (5%) gel with a 4% stacking gel. After transfer, membranes were probed with either rNV VLPs, an anti-MUC1 mAb or an anti-MUC4 mAb. The part of the gels shown is above the 200 kDa marker and the arrow indicates the limit of the stacking gel.
DISCUSSION
Recent studies have provided evidence that fucosylated oligosaccharides from human milk confer protection against diarrhoea to breast-fed children [24]. More specifically, the presence of 2-linked fucose residues is associated with protection against both Campylobacter and calicivirus diarrhoea [25]. Campylobacter as well as various calicivirus strains have been shown to attach to 2-linked fucosylated glycoconjugates of the digestive tract [6,7,26–28] and non-secretor individuals are resistant to NV infection, indicating that the 2-linked fucosylated ligands act as receptors [10,11]. Thus it is likely that the protection conferred by breast-feeding results from the presence of decoy receptors in milk. In previous work, we have shown that binding of rNV VLPs to epithelial cells was inhibited by the milk from a secretor woman, indicating that NV decoy receptors were present in human milk [7]. Recently, Jiang et al. [29] confirmed this observation in NV and other strains of noroviruses. We have now extended these findings by characterizing the decoy receptors.
Fucosylated oligosaccharides in human milk are synthesized by the products of the FUT2 and FUT3 genes that are responsible for the secretor and Le blood types. These two fucosyltransferases catalyse the transfer of fucose residues on to positions two, three or four. The presence of fucosylated oligosaccharides in human milk depends upon genetic polymorphism at the FUT2 and FUT3 loci and milk from non-secretor women who lack the FUT2 enzyme is almost entirely devoid of 2-linked fucosylated oligosaccharides [16]. Since blocking of rNV VLP-binding to its ligand is tightly controlled by the FUT2 locus, it was tempting to assume that the NV decoy receptors in human milk were 2-linked fucosylated oligosaccharides. However, we observed that dialysis did not decrease the blocking activity and that purified LNF-I (Fucα2Galβ3GlcNAcβ3Galβ4Glc) was not inhibitory. In a previous study, Jiang et al. [29] observed that total oligosaccharides had no inhibitory activity. Thus the milk's oligosaccharide fraction does not contain the decoy receptors. In a previous study, we observed that a synthetic H type 1 trisaccharide could block the binding of rNV VLPs to saliva from a secretor individual [7]. This could be due to a structural difference between the synthetic trisaccharide and the milk oligosaccharides. The synthetic trisaccharide was coupled to a short aliphatic chain that might increase its affinity by contributing a non-specific hydrophobic interaction with the viral capsid protein. The affinity of the monomeric milk oligosacharides for their binding site is probably too low to bring about detectable inhibition in our assay conditions. Western blotting indicated that rNV VLPs recognize high molecular mass glycoproteins which turned out to correspond to BSSL and mucins. Purified BSSL from secretor mothers was inhibitory, as was a preparation of mucins that contained a mixture of MUC1 and MUC4. The degree of fucosylation of milk glycoproteins is likely to parallel that of free oligosaccharides, since both depend upon the presence and amount of the FUT2 and FUT3 enzymes. Thus, in the association recently noted between protection against calicivirus diarrhoea and the level of 2-linked fucosylated oligosaccharides [25], in all likelihood, these oligosaccharides were only a marker of the degree of fucosylation of decoy receptors that correspond to the above mentioned high molecular mass glycoproteins.
BSSL is one of the major glycoproteins from human milk, which is absent from cows' milk. This lipase is not expressed in the newborn immature pancreas and it is thought that its presence in milk compensates for this lack of pancreatic enzyme [30]. It can participate in the degradation of a large spectrum of lipids and thus may have an essential role in nutrition [31] but also in protection in the newborn [32]. Regardless, the blocking activity of BSSL from secretor milk strongly suggests that it could have a dual function. The first, corresponding to the catalytic domain of the lipase is located at the N-terminus of the protein. The second, involving innate protection against certain infectious diseases, would correspond to the C-terminal mucin-like domain that could act as a decoy receptor depending upon its glycosylation status. A protective role against Giardia lamblia trophozoites has previously been attributed to BSSL [33,34]. This pathogen provokes severe diarrhoea in the newborn [35] and its toxic effect is counteracted by BSSL upon the release of cis-unsaturated fatty acids from milk triacylglycerol [34]. However, the inhibitory effect of BSSL against attachment of rNV VLPs to their carbohydrate ligands cannot be attributed to a similar mechanism since its catalytic lipase activity is destroyed by heating [32] whereas boiling milk samples did not affect the inhibitory activity. However, BSSL, like BSDL its pancreatic isoform, possesses a mucin-type C terminal domain with 16 tandem repeats rich in potential O-glycosylation sites. By the ELISA method, both BSSL and BSDL from O type secretor individuals reacted with the anti-H type 1/Leb mAb, whereas those from non-secretor individuals reacted with an anti-Lea (Lewis a histo-blood-group-antigen) mAb, indicating that the two isoforms from the mammary gland and the pancreas respectively, carried histo-blood-group carbohydrates as previously reported (results not shown). However, only the milk isoform could be recognized by rNV VLPs and recognition was dependent upon the presence of 2-linked fucose residues since BSSL from a non-secretor woman was not recognized. The total carbohydrate content of BSSL ranges from 19 to 26% of the glycoprotein mass, whereas that of BSDL has been estimated at approx. 7% [36,37]. The lipase possesses a single site of N-glycosylation and its N-linked sugar chain corresponds to about 10% of the total oligosaccharides in the milk isoform [38]. Consistent with its greater degree of glycosylation, we noted, as have previous authors, that BSSL from most individuals displayed a higher apparent molecular mass by gel electrophoresis than did BSDL. Thus the presence of complex O-glycans on the milk isoform could explain why rNV VLPs could bind to BSSL and not to BSDL. In one individual, we observed two distinct forms of BSSL with apparent molecular masses of approx. 130 and 110 kDa. Such variants have already been described but their significance is still unknown. Given the difference in size between the two forms of BSSL in that individual's milk, it is possible that they result from a polymorphism of the number of tandem repeats of the C-terminal domain [39]. This type of polymorphism is well described for mucins, particularly for MUC1, and its existence for BSSL warrants further study regarding the inhibitory potency of the variants. Changes in BSSL glycosylation have been observed during lactation [38]. It will thus be interesting to determine whether the inhibitory activity of BSSL on rNV VLP binding, as observed here, varies during the course of lactation.
MUC1 has for a long time been known as a component of human fat globule membranes and as a soluble mucin. Its presence in fat globule membranes could explain the inhibition of rNV VLP attachment by the fat globule membrane fraction. A second mucin, distinguished by its higher mass, was called MUCX [40]. Although we were not able to clearly distinguish the two types of mucins by gel electrophoresis based on their size and using a specific antibody, we could show that our mucin preparations contained MUC1 as expected, regardless of the secretor character of the donor. Since lactating rat mammary gland has recently been shown to express MUC4 [41], which like MUC1 can be either membrane bound or soluble, we used a recently developed anti-human MUC4 mAb to assay the presence of MUC4 in human milk [22]. A clear reactivity was observed in all samples irrespective of the secretor character. MUC4 is a much larger mucin than MUC1 [42]. Thus the mucin previously termed MUCX, which was larger than MUC1, probably corresponds to MUC4. Our preparations of high molecular mass material containing the two mucins strongly blocked the attachment of rNV VLPs to their carbohydrate ligands, indicating that these mucins can act as decoy receptors, in accordance with their glycosylation state controlled by polymorphism in the fucosyltransferase genes. MUC1 from milk binds to rotavirus and to enteropathogenic strains of Escherichia coli and for this reason is already considered as a component of innate immunity from milk [43,44]. Our inability to separate MUC1 from MUC4 did not allow us to determine which of them is the best inhibitor but our results raise the possibility that MUC4 could also be a decoy receptor for some pathogens.
In conclusion, the results presented here show that human milk blocks the binding of NV capsids to their receptor and that the blocking results from the interaction with 2-linked fucose residues complexed on high molecular mass glycoproteins that possess mucin-type tandem repeats. This repetitive structure in the core peptide, allowing a multimeric presentation of the glycan, probably increases the avidity of the interaction. Thus it should be possible to design artificial decoy receptors against noroviruses based on repeatedly complexed 2-linked fucosylated oligosaccharides mimicking the mucin-type arrangement. Such artificial decoy receptors could be included in milk formulations.
Acknowledgments
This work was supported by INSERM and by a grant (02/2) from the Direction de la Recherche Clinique from Nantes University Hospital. S.M. was supported by a grant from Danone. The authors thank Dr R. F. Fraser (Blood Transfusion Centre, Carluke, Scotland, U.K.), J. Burchell (Breast Cancer Biology Group, Guy's Hospital, London, U.K.) and S. K. Batra (Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, NE, U.S.A.) for their generous gifts of antibodies LM137, HFGM-1 and 8G7. We also thank Dr G. Strecker (Université de Sciences et Technologies de Lille, France) for his gift of purified milk LND and LNF-1 and Mme C. Delalande (Nantes University Hospital, France) for her help with the collection of milk samples.
References
- 1.Fankhauser R. L., Noel J. S., Monroe S. S., Ando T., Glass R. I. Molecular epidemiology of Norwalk-like viruses in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 1998;178:1571–1578. doi: 10.1086/314525. [DOI] [PubMed] [Google Scholar]
- 2.Lopman B. A., Reacher M. H., van Duijnhoven Y., Hanon F.-X., Brown D. W., Koopmans M. Viral gastroenteritis outbreaks in Europe, 1995–2000. Emerg. Infect. Dis. 2003;9:90–96. doi: 10.3201/eid0901.020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bon F., Fascia P., Dauvergne M., Tenenbaum D., Planson H., Petion A. M., Pothier P., Kohli E. Prevalence of group A rotavirus, human calicivirus, astrovirus and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J. Clin. Microbiol. 1999;37:3055–3058. doi: 10.1128/jcm.37.9.3055-3058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang X.-L., Honma S., Nakata S., Vesikari T. Human caliciviruses in acute gastroenteritis of young children in the community. J. Infect. Dis. 2000;81:S288–S294. doi: 10.1086/315590. [DOI] [PubMed] [Google Scholar]
- 5.Parashar U. D., Li J. F., Cama R., DeZalia M., Monroe S. S., Taylor D. N., Figueroa D., Gilman R. H., Glass R. I. Human caliciviruses as a cause of severe gastroenteritis in peruvian children. J. Infect. Dis. 2004;190:1088–1092. doi: 10.1086/423324. [DOI] [PubMed] [Google Scholar]
- 6.Harrington P. R., Lindensmith L., Yount B., Moe C. L., Baric R. S. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J. Virol. 2002;76:12325–12343. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marionneau S., Ruvöen-Clouet N., Le Moullac-Vaidye B., Clement M., Cailleau-Thomas A., Riuz-Palacios G., Huang P. W., Jiang X., Le Pendu J. Norwalk virus binds to H types 1/3 histo-blood group antigens on gastro-duodenal epithelial cells of “secretor” individuals. Gastroenterology. 2002;122:1967–1977. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutson A. M., Atmar R. L., Graham D. Y., Estes M. K. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 2002;185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 9.Marionneau S., Cailleau-Thomas A., Rocher J., Le Moullac-Vaidye B., Ruvoën-Clouet N., Clément M., Le Pendu J. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie. 2001;83:565–573. doi: 10.1016/s0300-9084(01)01321-9. [DOI] [PubMed] [Google Scholar]
- 10.Lindesmith L., Moe C. L., Marionneau S., Ruvoën-Clouet N., Jiang X., Lindblad L., Stewart P. A., Le Pendu J., Baric R. S. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 11.Hutson A. M., Airaud F., Le Pendu J., Estes M. K., Atmar R. L. Norwalk virus infection associates with secretor status genotyped from sera. J. Med. Virol. 2005;77:116–120. doi: 10.1002/jmv.20423. [DOI] [PubMed] [Google Scholar]
- 12.Hamosh M. Protective function of proteins and lipids in human milk. Biol. Neonate. 1998;74:163–176. doi: 10.1159/000014021. [DOI] [PubMed] [Google Scholar]
- 13.Bernt K., Walker W. A. Human milk and the response of intestinal epithelium to infection. Adv. Exp. Med. Biol. 2001;501:11–30. doi: 10.1007/978-1-4615-1371-1_2. [DOI] [PubMed] [Google Scholar]
- 14.Brand Miller J., Bull S., Miller J., McVeagh P. The oligosaccharide composition of human milk: Temporal and individual variations in monosaccharide components. J. Pediatr. Gastroenterol. Nutr. 1994;19:371–376. doi: 10.1097/00005176-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Thurl S., Henker J., Siegel M., Tovar K., Sawatzki G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj. J. 1997;14:795–809. doi: 10.1023/a:1018529703106. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi P., Warren C. D., Altaye M., Morrow A. L., Ruiz-Palacios G. M., Pickering L. K., Newburg D. S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology. 2001;11:365–372. doi: 10.1093/glycob/11.5.365. [DOI] [PubMed] [Google Scholar]
- 17.Le Pendu J. Histo-blood group antigens and milk oligosaccharides. Adv. Exp. Med. Biol. 2004;554:135–143. doi: 10.1007/978-1-4757-4242-8_13. [DOI] [PubMed] [Google Scholar]
- 18.Patton S., Huston G. E. A method for isolation of milk fat globules. Lipids. 1986;21:170–174. doi: 10.1007/BF02534441. [DOI] [PubMed] [Google Scholar]
- 19.Jiang X., Wang M., Graham D. Y., Estes M. K. Expresion, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abouakil N., Rogalska E., Bonicel J., Lombardo D. Purification of pancreatic carboxylic-ester hydrolase by immunoaffinity and its application to the human bile-salt-stimulated lipase. Biochim. Biophys. Acta. 1988;961:299–308. doi: 10.1016/0005-2760(88)90077-x. [DOI] [PubMed] [Google Scholar]
- 21.Burchell J., Gendler S., Taylor-Papadimitriou J., Girling A., Lewis A., Millis R., Lamport D. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of human milk mucin. Cancer Res. 1987;47:5476–5482. [PubMed] [Google Scholar]
- 22.Moniaux N., Varshney G. C., Chauhan S. C., Copin M. C., Jain M., Wittel U. A., Andrianifahanana M., Aubert J. P., Batra K. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cell lines in humans. J. Histochem. Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 23.Gjellesvik D. R., Lombardo D., Walther B. T. Pancreatic bile salt dependent lipase from cod (Gadus morhua): purification and properties. Biochim. Biophys. Acta. 1992;1124:123–134. doi: 10.1016/0005-2760(92)90088-d. [DOI] [PubMed] [Google Scholar]
- 24.Newburg D. S., Ruiz-Palacios G., Altaye M., Chaturvedi P., Meinzen-Derr J., Guerrero M., Morrow A. L. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhoea in breastfed infants. Glycobiology. 2004;14:253–263. doi: 10.1093/glycob/cwh020. [DOI] [PubMed] [Google Scholar]
- 25.Morrow A., Ruiz-Palacios G., Altaye M., Jiang X., Guerrero M., Meinzen-Derr J., Farkas T., Chatuverdi P., Pickering L. K., Newburg D. S. Human milk oligosaccharides are associated with protection against diarrhoea in breast-fed infants. J. Pediatr. 2004;145:297–303. doi: 10.1016/j.jpeds.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Palacios G. M., Cervantes L. E., Ramos P., Chavez-Munguia B., Newburg D. S. Campylobacter jejuni binds intestinal H(O) antigen and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003;278:14112–14120. doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- 27.Huang P. W., Farkas T., Marionneau S., Zhong W. M., Ruvoën-Clouet N., Morrow A., Pickering L. K., Newburg D. S., Le Pendu J., Jiang X. Norwalk-like viruses bind to ABO, Lewis and secretor histo-blood group antigens but different strains bind to distinct antigens. J. Infect. Dis. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 28.Hutson A. M., Atmar R. L., Marcus D. M., Estes M. K. Norwalk virus-like particles hemagglutination by binding to H histo-blood group antigens. J. Virol. 2003;77:405–415. doi: 10.1128/JVI.77.1.405-415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X., Huang P., Zhong W., Tan M., Farkas T., Morrow A. L., Newburg D. S., Ruiz-Palacios G., Pickering L. K. Human milk contains elements that block binding of noroviruses to human histo-blood group antigens in saliva. J. Infect. Dis. 2004;190:1850–1859. doi: 10.1086/425159. [DOI] [PubMed] [Google Scholar]
- 30.Sbarra V., Mas E., Henderson T. R., Walther B. T. Digestive lipases of the newborn ferret: compensatory role of milk bile-salt dependent lipase. Pediatr. Res. 1996;40:263–268. doi: 10.1203/00006450-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Lombardo D. Bile salt-dependent lipase: its pathophysiological implications. Biochim. Biophys. Acta. 2001;1533:1–28. doi: 10.1016/s1388-1981(01)00130-5. [DOI] [PubMed] [Google Scholar]
- 32.Gillin F. D., Reiner D. S., Wang C. S. Human milk kills parasitic intestinal protozoa. Science (Washington, D.C.) 1983;221:1290–1292. doi: 10.1126/science.6310751. [DOI] [PubMed] [Google Scholar]
- 33.Reiner D. S., Wang C. S., Gillin F. D. Human milk kills Giardia lamblia by generating toxic lipolytic products. J. Infect. Dis. 1986;154:825–832. doi: 10.1093/infdis/154.5.825. [DOI] [PubMed] [Google Scholar]
- 34.Hernell O., Ward H., Blackberg L., Pereira M. Killing of Giardia lamblia by human milk lipases: an effect mediated by lipolysis of milk lipids. J. Infect. Dis. 1986;153:715–720. doi: 10.1093/infdis/153.4.715. [DOI] [PubMed] [Google Scholar]
- 35.Ali S. A., Hill D. R. Giardia intestinalis. Curr. Opin. Infect. Dis. 2003;16:453–460. doi: 10.1097/00001432-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Mas E., Abouakil N., Roudani S., Miralles F., Guy-Crotte O., Figarella C., Escribano M. J., Lombardo D. Human fetoacinar pancreatic protein: an oncofetal glycoform of the normally secreted pancreatic bile-salt-dependent lipase. Biochem. J. 1993;289:609–615. doi: 10.1042/bj2890609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mas E., Sadoulet M. O., El Battari A., Lombardo D. Glycosylation of bile-salt-dependent lipase (cholesterol esterase) Methods Enzymol. 1997;284:340–353. doi: 10.1016/s0076-6879(97)84022-0. [DOI] [PubMed] [Google Scholar]
- 38.Landberg E., Huang Y., Strömqvist M., Mechref Y., Hansson L., Lundblad A., Novotny M. V., Pahlsson P. Changes in glycosylation of human bile-salt-stimulated lipase during lactation. Arch. Biochem. Biophys. 2000;377:246–254. doi: 10.1006/abbi.2000.1778. [DOI] [PubMed] [Google Scholar]
- 39.Lindquist S., Blackberg L., Hernell O. Human bile salt-stimulated lipase has a high frequency of size variation due to a hypervariable region in exon 11. Eur. J. Biochem. 2002;269:759–767. doi: 10.1046/j.0014-2956.2001.02666.x. [DOI] [PubMed] [Google Scholar]
- 40.Patton S. MUC1 and MUC-X, epithelial mucins of breast and milk. Adv. Exp. Med. Biol. 2001;501:35–45. doi: 10.1007/978-1-4615-1371-1_4. [DOI] [PubMed] [Google Scholar]
- 41.Price-Schiavi S. A., Andrechek E., Idris N., Li P., Rong M., Zhang J., Carraway C. A., Muller W. J., Carraway K. L. Expression, location and interactions of ErB2 and its intramembrane ligand Muc4 (sialomucin complex) in rat mammary gland during pregnancy. J. Cell. Physiol. 2004;203:44–53. doi: 10.1002/jcp.20200. [DOI] [PubMed] [Google Scholar]
- 42.Moniaux N., Escande F., Porchet N., Aubert J. P., Batra S. K. Structural organization and classification of the human mucin genes. Front. Biosci. 2001;6:D1192–D1206. doi: 10.2741/moniaux. [DOI] [PubMed] [Google Scholar]
- 43.Yolken R. H., Peterson J. A., Vonderfecht S. L., Fouts E. T., Midthun K., Newburg D. S. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J. Clin. Invest. 1992;90:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroten H., Hanisch F. G., Plogmann R., Hacker J., Uhlenbruck G., Nobis-Bosch R., Wahn V. Inhibition of adhesion of S-fimbriated Escherichia coli to buccal epithelial cells by human fat globule membrane components: a novel aspect of the protective function of mucins in the nonimmunoglobulin fraction. Infect. Immun. 1992;60:2893–2899. doi: 10.1128/iai.60.7.2893-2899.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]