Abstract
HBEpCs (human bronchial epithelial cells) contribute to airway inflammation by secreting a variety of cytokines and chemokines in response to allergens, pathogens, viruses and environmental toxins and pollutants. The potent neutrophil chemoattractant, IL-8 (interleukin-8), is a major cytokine secreted by HBEpCs. We have recently demonstrated that LPA (lysophosphatidic acid) stimulated IL-8 production in HBEpCs via protein kinase C δ dependent signal transduction. However, mechanisms of IL-8 expression and secretion are complex and involve multiple protein kinases and transcriptional factors. The present study was undertaken to investigate MAPK (mitogen-activated protein kinase) signalling in the transcriptional regulation of IL-8 expression and secretion in HBEpCs. Exposure of HBEpCs to LPA (1 μM) enhanced expression and secretion of IL-8 by 5–8-fold and stimulated threonine/tyrosine phosphorylation of ERK (extracellular-signal-regulated kinase), p38 MAPK and JNK (c-Jun N-terminal kinase). The LPA-induced secretion of IL-8 was blocked by the p38 MAPK inhibitor SB203580, by p38 MAPK siRNA (small interfering RNA), and by the JNK inhibitor JNKi II, but not by the MEK (MAPK/ERK kinase) inhibitor, PD98059. LPA enhanced the transcriptional activity of the IL-8 gene; that effect relied on activation of the transcriptional factors NF-κB (nuclear factor κB) and AP-1 (activator protein-1). Furthermore, SB203580 attenuated LPA-dependent phosphorylation of IκB (inhibitory κB), NF-κB and phospho-p38 translocation to the nucleus, NF-κB transcription and IL-8 promoter-mediated luciferase reporter activity, without affecting the JNK pathway and AP-1 transcription. Similarly, JNKi II only blocked LPA-mediated phosphorylation of JNK and c-Jun, AP-1 transcription and IL-8 promoter-mediated luciferase reporter activity, without blocking p38 MAPK-dependent NF-κB transcription. Additionally, siRNA for LPA1–3 receptors partially blocked LPA-induced IL-8 production and activation of MAPKs. The LPA1 and LPA3 receptors, as compared with LPA2, were most efficient in transducing LPA-mediated IL-8 production. These results show an independent role for p38 MAPK and JNK in LPA-induced IL-8 expression and secretion via NF-κB and AP-1 transcription respectively in HBEpCs.
Keywords: AP-1, interleukin-8, JNK, lysophosphatidic acid, NFκB, p38 MAPK
Abbreviations: AP-1, activator protein-1; BALF, bronchoalveolar lavage fluid; BEBM, bronchial epithelial cell basal medium; BEGM, bronchial epithelium growth medium; EMSA, electrophoretic mobility-shift assay; ERK, extracellular-signal-regulated kinase; HBEpCs, human bronchial epithelial cells; IL-8, interleukin-8; IκB, inhibitory κB; JNK, c-Jun N-terminal kinase; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; MAPK, mitogen-activated protein kinase; MAPKAPK2, MAPK-activated protein kinase 2; NF-κB, nuclear factor κB; PKC, protein kinase C; RT, reverse transcriptase; S1P, sphingosine 1-phosphate; siRNA, small interfering RNA
INTRODUCTION
Human airway epithelium, apart from having a barrier function, also contributes to airway inflammation and defence mechanisms through release of a number of pro- and anti-inflammatory mediators [1,2]. The airway epithelial cells, in response to environmental factors such as allergens [1], cigarette smoke [2], viruses [3] and air pollutants [4], enhance expression and secretion of a wide array of cytokines and chemokines, including IL-8 (interleukin-8), that participate in innate immunity. IL-8, a member of the CXC family of chemokines, is a major chemoattractant and activator of neutrophils at sites of acute inflammation and lung injury [5]. Increased secretion of IL-8 in the BALFs (bronchoalveolar lavage fluids) of patients with asthma, chronic obstructive lung disease, pulmonary sarcoidosis and acute respiratory distress syndrome supports a role for this chemokine in airway inflammation and innate immunity. Recent studies indicate that IL-8 is angiogenic [6] and therefore may play an important role in airway remodelling of chronic asthmatics. Although much is known about the pathophysiological actions of this powerful chemokine and about the factors that stimulate its release, the pathways involved in its gene and protein expression are not fully understood.
LPA (lysophosphatidic acid), a bioactive phospholipid, is present in human plasma, biological fluids and tissues. It mediates many cellular responses, such as proliferation, anti-apoptosis, cytoskeletal reorganization and tumour metastasis, by binding to specific GPCRs (G protein-coupled receptors) formerly known as endothelial differentiation gene receptors [7–10]. To date, four LPA receptors, LPA1–, have been cloned and shown to be present in most mammalian cells and tissues [11–13]. Recently, LPA was shown to be a ligand for PPARγ (peroxisome proliferator-activator receptor γ), suggesting participation of LPA in intracellular signalling and cell function [14]. We showed the expression of LPA1–3 in primary cultures of HBEpCs (human bronchial epithelial cells) and demonstrated that exogenously added LPA, at physiological concentrations, was a potent stimulator of IL-8 secretion and was regulated, in part, by PKC (protein kinase C) δ-dependent NF-κB (nuclear factor κB) activation [15]. Additionally, intratracheal administration of LPA to mice resulted in a significant increase in macrophage inflammatory protein-2 (an orthologue of IL-8 in mice) and the influx of neutrophils into the alveolar space indicating pro-inflammatory response of LPA in vivo [15].
In addition to activation of PKC δ in HBEpCs [15], LPA induced release of [Ca2+]i [8], and stimulated NF-κB [15] and ERK (extracellular-signal-regulated kinase) activity [7]. ERK, p38 and JNK (c-Jun N-terminal kinase), belonging to the MAPK (mitogen-activated protein kinase) family, are important components of signal transduction pathways stimulated by growth factors, heterotrimeric G protein-coupled receptors, and cytokine receptors. The p38 MAPK is activated by proinflammatory cytokines, environmental stresses, endotoxin, mitogenic stimuli, and oxidative stress [4,16,17], while JNK signalling is implicated in cellular stress, inflammation, and apoptosis [16]. LPA is pro-inflammatory in HBEpCs [15] and stimulates phosphorylation of ERK [7]; however, the role of MAPKs in LPA-induced transcriptional regulation of IL-8 expression and secretion is yet to be fully defined. Therefore, the present study was undertaken to investigate the potential role of LPA induced ERK, p38 MAPK and JNK signalling in the regulation of IL-8 gene expression and secretion in HBEpCs. Further, using siRNA (small interfering RNA), the role of LPA1–3 receptors in LPA-induced IL-8 secretion was investigated. Our results show that LPA activates ERK, p38 MAPK and JNK in HBEpCs; however, only p38 MAPK and JNK, but not ERK, are involved in LPA-induced IL-8 production. Evidence is also provided for p38 MAPK/NF-κB and JNK/AP-1 (activator protein-1) signal transduction for IL-8 secretion by LPA in HBEpCs. Down-regulation of the mRNAs for LPA1–3, by siRNAs specific to each of the three receptors, indicates that all the three receptors participate in the LPA-induced p38 MAPK/JNK signalling that regulates IL-8 secretion.
EXPERIMENTAL PROCEDURES
Materials
LPA was obtained from Avanti Polar Lipids. The p38 MAPK inhibitor SB203580, MEK inhibitor PD98059 and JNK inhibitor JNKi II were purchased from Calbiochem. Antibodies to total and phosphorylated forms of ERK1/2, phospho-p38 MAPK, phospho-JNK, β-actin, phospho-IκB (inhibitory κB) and the NF-κB p65 subunit were purchased from Santa Cruz Biotechnology. Antibodies against total MAPKAPK2 (mitogen-activated protein kinase-activated protein kinase 2) and phospho-MAPKAPK2 were purchased from Stressgen Biotechnologies and Cell Signaling respectively. Horseradish peroxidase-conjugated goat anti-rabbit, anti-mouse and Alexa Fluor 488 goat anti-rabbit and anti-mouse antibodies were purchased from Molecular Probes. BEBM (bronchial epithelial cell basal medium) and supplement kit were purchased from Cambrex Bio Science Inc. The ECL® (enhanced chemiluminescence kit) for Western blotting was obtained from Amersham Biosciences. All reagents were purchased from Bio-Rad Laboratories. Pre-cast SDS/10% PAGE gels were obtained from Invitrogen. The ELISA kit for IL-8 measurement was purchased from Biosource. Smartpool RNA duplexes for LPA1–3 receptors, p38 MAPK and c-Jun were purchased from Dharmacon Research and Santa Cruz Biotechnology respectively.
Bronchial epithelial cell culture
Lung tissue was purchased from Tissue Transformation Technologies, a non-profit organization that makes available for medical research organs from donors not needed for human transplantation. Lungs were selected from male and female, non-smoking, 18–40 years old, serology-negative, healthy accident victims with no history of chronic respiratory disease, whose lungs were clear on X-ray. The anonymous human tissue obtained in this manner has been determined by the Committee on Human Research of the Johns Hopkins Bloomberg School of Public Health to be exempt from human subjects review. HBEpCs from large airways were isolated following established procedures [18,19]. In brief, following overnight digestion of the trachea at 4 °C in 0.1% (w/v) protease Sigma Type XIV (Sigma–Aldrich) in Ham's F-12 medium containing penicillin (100 units/ml, Gibco), streptomycin (100 μg/ml, Gibco), amphotericin B (2.5 μg/ml, Gibco), and gentamicin (50 μg/ml, Gibco), the protease was neutralized by the addition of 10% (v/v) fetal calf serum (Invitrogen) and the epithelial cells were freed from extraneous tissue by agitation and isolated by centrifugation. The washed P0 (passage 0) HBEpCs were then seeded, at a density of 1.5×104 cells/cm2, onto Vitrogen 100-coated (1:75 in sterile water; Cohesion) P-100 dishes in BEGM (bronchial epithelium growth medium), as described previously in [19]. Upon reaching confluence, HBEpCs were transferred to Vitrogen 100-coated 6-well plates and grown to approx. 90% confluence with BEGM. All experiments were carried out between passages one and three.
RNA isolation
Total RNA was isolated from cultured HBEpCs using TRIzol® reagent (Life Technology) according to the manufacturer's instructions. TRIzol® (1 ml) was added to each well (6-well plates), the cells were scraped and collected in RNase- and DNase- free tubes, thoroughly mixed, and the tubes were allowed to stand at 25 °C for 5 min. After the addition of 250 μl chloroform (Sigma–Aldrich), the tubes were vortexed for 15 s and further incubated for 2–3 min at 25 °C. They were then centrifuged at 15000 g for 15 min, the aqueous phase was removed and the RNA was precipitated with propan-2-ol (Sigma–Aldrich), washed with 70% ethanol, air-dried, and resuspended in 30–40 μl of DEPC (diethyl pyrocarbonate) water. RNA was quantified spectrophotometrically and samples with an absorbance of ≥1.8 measured at 260/280 nm were analysed by real time RT (reverse transcriptase)–PCR.
Real-time RT-PCR
One-step RT-PCR was performed in a Light-Cycler using the SYBR Green QuantiTech® RT-PCR Kit (Qiagen). Human IL-8 was amplified with the sense primer 5′-TTCTGCAGCTCTGTGTGAAGG-3′ and the anti-sense primer 5′-ATGAATTCTCAGCCCTCTTC-3′. 18 S rRNA sense (5′-GTAACCCGTTGAACCCCATT-3′), antisense (5′-CCATCCAATCGGTAGTAGCG-3′), was used as a housekeeping gene with which to normalize expression. Human LPA receptors were amplified with LPA1 sense primer (5′-GTAATGGTGGTTCTCTATGCTCAC-3′), antisense primer (5′-GGACAGCACACGTCTAGAAG-3′); LPA2 sense primer (5′-GTCGAGCCTGCTTTGTCTTC-3′), antisense primer (5′-CCAGAGCAGTACCACCTG-3′); and LPA3 sense primer (5′-TTAGCTGCTGCCGATTTCTT-3′), antisense (5′-ATGATGAGGAAGGCCATGAG-3′). The reaction mix consisted of 0.3 μg of total RNA (target gene) or 0.03 μg of total RNA (18 S rRNA), 10 μl of QuantiTech SYBR Green PCR, 0.2 μl of QuantiTech RT mix, 1.5 μM of target primers or 1 μM of 18 S rRNA primers, in total volume of 20 μl. For all samples, reverse transcription was carried out at 50 °C for 20 min, followed by cycling to 95 °C for 15 min, to inactivate the RT enzyme and activate the Taq polymerase. Amplicon quantity in each sample was normalized to its 18 S RNA content. The relative abundance of target mRNA in each sample was calculated as 2 raised to the negative of its threshold cycle value multiplied by 106 after being normalized to the abundance of its corresponding 18 S, [e.g., 2−(IL-8 Threshold Cycle)/2−(18S Threshold Cycle)×106]. All primers were designed by inspection of the gene of interest. Negative controls, consisting of reaction mixtures containing all components except target RNA, were included with each RT-PCR run. To verify that amplified products were derived from mRNA and did not represent genomic DNA contamination, representative PCR reaction mixtures for each gene were run in the absence of the RT enzyme after first being cycled to 95 °C for 15 min. In the absence of reverse transcription, no PCR products were observed.
Measurement of IL-8 protein
HBEpCs were pretreated with SB203580 (25 μM) or JNK inhibitor II (25 μM) for 1 h or transfected with LPA1–3 siRNA for 72 h in BEBM. Cells were then challenged with LPA (1 μM) in BEBM containing 0.1% (w/v) BSA with or without LPA for 1–24 h. Cell supernatants were collected and centrifuged to remove all cell debris (10000 g for 10 min at 4 °C). Supernatants were frozen at −80 °C until needed for analysis of IL-8 by ELISA according to manufacturer's instructions (Biosource).
Preparation of cell lysates and Western blotting
HBEpCs grown in 6-well plates (approx. 90% confluence) were stimulated with LPA (1 μM) for 15 min, rinsed with ice-cold PBS containing 1 mM Na3VO4 and lysed in RIPA buffer [20 mM Tris/HCl (pH 7.4), 1% (v/v) Triton X-100, 150 mM NaCl, 5 mM EDTA, 100 mM NaF and 1 mM Na3VO4]. Cell lysates were sonicated (3×15 s) on ice and centrifuged at 5000 g for 5 min at 4 °C. Protein concentration was determined with a BCA protein assay kit (Pierce Chemical Co.) using BSA as standard. Equal amounts of cell lysates were fractionated on SDS/10% PAGE gels (Invitrogen) and transferred to methanol soaked PVDF membranes at 300 mA for 1 h in a Bio-Rad Laboratories mini transblot apparatus. Membranes were blocked with 5% (w/v) BSA in TBS/T [25 mM Tris base (pH 7.4), 137 mM NaCl and 0.1% (v/v) Tween 20] for at least 1 h before probing with antibodies. Primary and secondary antibody incubations were performed in 5% (w/v) BSA for 1 h and membranes were washed at least six times for 30 min using TBS/T. Antibody–antigen complexes were detected using ECL® according to the manufacturer's instructions (Amersham Biosciences).
siRNA
Gene silencing of p38 MAPK, c-Jun, and LPA1–3 receptors was performed essentially as described by Elbashir et al. in [20]. GL2 luciferase siRNA duplex (target sequence 5′-CGTACGCGGAATACTTCGA-3′) was used as a nonspecific control siRNA (Dharmacon Research). Based on observable fluorescence, transfection efficiency appeared to be consistent at approx. 70% across all experiments. HBEpCs (P1) cultured on 6-well plates (50–60% confluence), were transiently transfected with siRNA (200 nM) condensed with Enhancer R and formulated with Transmessenger reagent, according to the manufacturer's instructions (Qiagen). The transfection complex was diluted in 900 μl of BEGM and added directly to cells. After 3 h, the transfection medium was replaced by complete BEBM and cells were analysed 24 h or 72 h after transfection by real time RT-PCR or Western blotting.
Luciferase reporter assay
The human IL-8/luciferase (hIL-8/Luc) reporter plasmid containing the −162 to +44 nt region of the IL-8 promoter, cloned into the poLUC reporter plasmid, was generously provided by Dr R. Brasier (University of Texas Medical Branch, Galveston, TX, U.S.A.) and Dr Jeffrey Hasday (University of Maryland Medical System, Baltimore, MD, U.S.A.). HBEpCs seeded on 24-well plates, were allowed to grow to 60–80% confluence. Cells were transiently transfected with 100 ng of IL-8, NF-κB or AP-1 reporter construct along with 1 ng of Renilla luciferase (pRL-TK, Promega) for normalization of transfection efficiency using Fugene 6 (Roche). After incubation at 37 °C in 5% CO2 overnight, cells were pretreated with vehicle, SB203580 or JNKi II for 1 h and then treated with vehicle or LPA (1 μM) for 3 h. Cells were lysed in a luciferase lysis buffer (Promega), and luciferase activity was determined using a dual luciferase assay kit (Promega) with a luminometer. Firefly luciferase activity was normalized to that of Renilla luciferase. All experiments were performed in triplicate and the results are expressed as the means±S.E.M. (n=3).
EMSA (electrophoretic mobility-shift assay)
Nuclear extracts were prepared according to the manufacturer's instructions (Active Motif). The oligonucleotide probes encoding the consensus sequences of NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) and AP-1 (5′-CGCTTGATGACTCAGCCGGAA-3′) transcription factors were purchased from Promega. End-labelling of the probe was performed using T4 kinase and [γ-32P]ATP. EMSAs were performed using nuclear extracts (3 μg) from vehicle, SB203580, JNKi II, or c-Jun siRNA pretreated cells with or without LPA treatment and binding buffer [10 mM Tris/HCL (pH 7.5), 4% (v/v) glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 50 mM NaCl, 50 μg of the nonspecific blocker poly (dI–dC), and 20000 dpm of [γ-32P]-labelled probe] at 37 °C for 30 min. The protein–DNA complexes were analysed by electrophoresis through a 4% non-denaturing polyacrylamide gel. The gels were vacuum-dried and subjected to autoradiography.
Immunofluorescence microscopy
HBEpCs grown in 8-well chamber slides (LabTek) to 60–70% confluency were challenged with medium containing 0.1% (w/v) BSA, or medium containing 0.1% (w/v) BSA plus LPA, for 15 min. Cells were pretreated for 1 h with SB203580 (25 μM) or JNKi II (25 μM) and then stimulated with LPA (1 μM). Cells were rinsed with pre-warmed PBS and fixed with 3.7% (v/v) formaldehyde in PBS for 20 min at 25 °C followed by permeabilization with 0.2% (v/v) Triton X-100 in PBS at 25 °C for 2 min. Cells were extensively washed with PBS, incubated with blocking buffer [1% (w/v) BSA in TBS/T] at 25 °C for 30 min and were subsequently incubated with primary antibodies against NF-κB p65 subunit (1:200), phopho-p38 (1:200) or phospho-JNK (1:200) at 25 °C for 1 h. Cells were then washed with PBS and incubated with appropriate secondary antibodies (anti-rabbit or anti-mouse, 1:200 dilution), conjugated to Alexa Fluor 488 fluorescent dye in blocking buffer for 1 h at 25 °C. After three washes with PBS, slides were mounted using aqueous mounting fluid (Lerner Laboratories) and observed under a Nikon Eclipse TE 2000-S fluorescent microscope with a 60 X objective lens, Hamamatsu digital camera and appropriate filter.
Statistical analysis
The results are expressed as the means±S.E.M. (n=3) of experiments performed in triplicate. All results were subjected to statistical analysis using ANOVA. Specific differences were tested using the Student–Newman–Keul's test. Statistical analyses were carried out with Sigma Stat software (Jandel Scientific). P values <0.05 were considered significant.
RESULTS
Real-time RT-PCR of IL-8 gene expression and secretion by LPA in HBEpCs
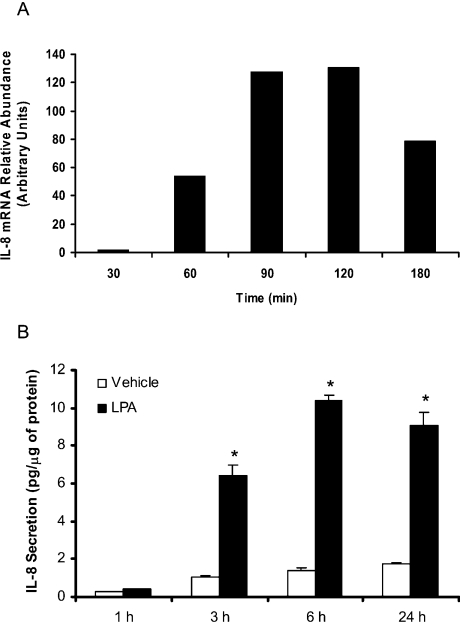
Utilizing the expression profile of cytokine genes, determined using a cDNA expression array system, we have recently shown that, among 23 different cytokine genes, only the expression of IL-8 was increased approx. 11.5-fold by LPA treatment of HBEpCs for 3 h [15]. To further investigate the effect of LPA on IL-8 gene expression, HBEpCs were exposed to LPA for 30–180 min; cells were harvested for total RNA and analysed by semi-quantitative real-time RT-PCR, using primers specific for IL-8. The quantitative real-time RT-PCR analysis showed that IL-8 mRNA, normalized to 18 S mRNA, increased by approx. 18-fold at 60 min to approx. 32-fold at 180 min and peaked at 43-fold after 120 min stimulation (Figure 1A). Under similar culture conditions, the induction of IL-8 protein was detectable from 3–24 h, with maximal protein expression at 6 h after stimulation with 1 μM LPA (Figure 1B). Interestingly, the production of IL-8 in control cells also showed an increase from 0.2 pg/μg protein to 1.8 pg/μg protein, as compared with approx. 9.6 pg/μg protein of IL-8 in LPA treated cells at 24 h. These results show a rapid up-regulation of IL-8 gene expression and secretion by LPA in HBEpCs.
Figure 1. LPA stimulates IL-8 gene expression and secretion in HBEpCs.
(A) HBEpCs (approx. 80% confluence in 35 mm dishes) were treated with BEGM containing 0.1% (w/v) BSA (vehicle) or BEGM containing 0.1% (w/v) BSA plus LPA (1 μM) for 30–180 min. IL-8 gene expression was evaluated by real-time RT-PCR as described in the Experimental procedures section. Results are representative of at least three independent experiments. (B) HBEpCs (approx. 80% confluence in 35 mm dishes) were treated with BEGM containing 0.1% (w/v) BSA (vehicle) or BEGM containing 0.1% (w/v) BSA plus LPA (1 μM) for 1–24 h. IL-8 in the medium was quantified by ELISA as described in the Experimental procedures section. The results are expressed as the means±S.D. (n=3) performed in triplicate. *, statistically different from control (P<0.05).
Role of MAPKs in LPA-induced IL-8 secretion in HBEpCs
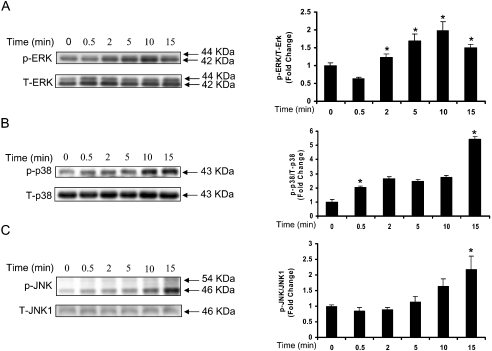
To examine the role of MAPKs in LPA-induced IL-8 production, HBEpCs were exposed to LPA (1 μM) for varying time periods (0.5–15 min) and cell lysates were analysed for enhanced phosphorylation of ERK, p38 MAPK and JNK. As shown in Figure 2, LPA (1 μM) stimulated ERK, p38 MAPK and JNK as shown by increased phosphorylation of threonine/tyrosine residues. The phosphorylation of ERK by LPA was rapid, peaked at 10 min and decreased thereafter (Figure 2A). However, the phosphorylation of p38 MAPK and JNK occurred from 5–15 min (Figures 2B and 2C) and was sustained for up to 2 h (results not shown). These results show that LPA is a potent activator of MAPKs in HBEpCs.
Figure 2. LPA stimulates phosphorylation of MAPKs in HBEpCs.
HBEpCs (approx. 80% confluence in 35 mm dishes) were treated with BEGM containing 0.1% (w/v) BSA (vehicle) or BEGM containing 0.1% (w/v) BSA plus LPA (1 μM) for 0–15 min. Cell lysates (10 μg) were subjected to SDS/PAGE and Western blotted for phospho- (p) and total-(T) ERK (A) or phospho- and the total p38 MAPK (B) or phospho- and total JNK (C). Shown are representative immunoblots of three independent experiments performed in duplicate. Fold changes in phospho-ERK/ERK or phospho-p38 MAPK/p38 MAPK or phospho-JNK/JNK were calculated from the Western blots by image analysis and results were normalized to total ERK or p38 MAPK or JNK. *, significantly different from 0 min (P<0.05).
SB203580 and p38 MAPK siRNA attenuate LPA-induced p38 MAPK and IL-8 secretion
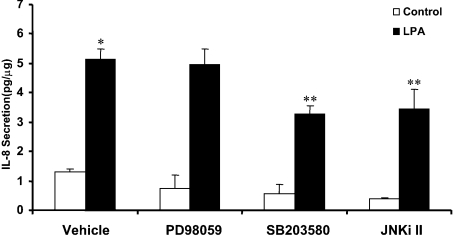
Next we examined the role of MAPKs on LPA-induced IL-8 production in HBEpCs. Pretreatment of cells with SB203580 or SP600125 (JNKi II), inhibitors of p38 MAPK and JNK respectively, partially attenuated LPA-induced IL-8 secretion. However, PD98059, an inhibitor of MEK1 [also known as MAP2K1 (MAPK kinase 1)] and ERK activation, had no significant effect on IL-8 production (Figure 3). SB203580, in a dose-dependent manner, blocked LPA-induced phosphorylation of p38 MAPK, its downstream target, MAPKAPK2, and IL-8 generation (Figures 4A and 4B). The role of p38 MAPK in IL-8 production was also evaluated using p38 MAPK siRNA. Transfection of cells with p38 MAPK siRNA (200 nM) for 72 h almost completely abolished expression of total p38 MAPK protein without altering ERK and JNK expression, as determined by Western blotting (Figure 4C). Furthermore, p38 MAPK siRNA, under similar experimental conditions, attenuated LPA-dependent IL-8 formation by approx. 45% compared with control cells (control cells: 14.7-fold stimulation with LPA; p38 MAPK siRNA cells: 8.8-fold stimulation with LPA) (Figure 4D). These results suggest the involvement of p38 MAPK in LPA-induced IL-8 production in HBEpCs.
Figure 3. Effect of MAPK inhibitors on LPA-induced IL-8 secretion.
HBEpCs (approx. 80% confluence in 35 mm dishes) were pretreated with BEGM (vehicle) or PD89059 (25 μM) or SB 203580 (25 μM) or JNKi II (25 μM) for 1 h. The cells were then challenged with BEGM containing 0.1% (w/v) BSA (vehicle) or BEGM containing 0.1% (w/v) BSA plus LPA (1 μM) for 2 h. IL-8 secreted into medium was quantified by ELISA as described in the Experimental procedures section. The results are expressed as the means±S.D. (n=3) performed in triplicate. *, significantly different from control (P<0.05), **, significantly different from cells treated with LPA alone (P<0.05).
Figure 4. p38 MAPK siRNA and SB-203580 attenuate LPA-induced IL-8 secretion in HBEpCs.
HBEpCs (approx. 80% confluence in 35 mm dishes) were pretreated with various concentrations of SB203580 for 1 h prior to LPA (1 μM) challenge for either 15 min or 2 h. (A) Cell lysates (10 μg) from 15 min treatment were subjected to SDS/PAGE and Western blotted with phospho- and pan p38 MAPK and MAPKAPK-2 antibodies. (B) The medium was collected after 2 h LPA exposure and analysed for IL-8 by ELISA as described in the Experimental procedures section. Values are means of three independent experiments in triplicate. (C) HBEpCs (approx. 50% confluence in 35 mm dishes) were transfected with control siRNA or p38 MAPK siRNA (100 nM) for 72 h as described in the Experimental procedures section. Cell lysates (10 μg) were subjected to SDS/PAGE and Western blotted with p38 MAPK or β-actin or ERK or JNK1 antibodies. Shown is a representative blot of three independent experiments. (D) HBEpCs (approx. 50% confluence in 35 mm dishes) were transfected with control siRNA or p38 MAPK siRNA (100 nM) for 72 hours prior to LPA (1 μM) challenge for 2 h. The results are expressed as the means±S.D. (n=3) performed in triplicate. *, significantly different from control (P<0.05), **, significantly different from LPA treatment (P<0.05).
JNKi II inhibits LPA-induced phosphorylation of JNK and c-Jun, and IL-8 secretion in HBEpCs
To examine further the role of JNK in LPA-mediated IL-8 production, HBEpCs were treated with the JNK inhibitor JNKi II for 1 h prior to LPA challenge for varying time periods. As shown in Figure 5(A), JNKi II, in a dose-dependent fashion, reduced LPA-induced phosphorylation of JNK with optimal inhibition observed at a JNKi II concentration of 25 μM. The LPA-induced phosphorylation of c-Jun was blocked by JNKi II confirming the efficacy of the inhibitor (Figure 5B). Under identical incubation conditions, JNKi II (3–40 μM) attenuated LPA (1 μM) mediated IL-8 production with maximal reduction achieved at a JNKi II concentration of 25 μM (Figure 5D). Attenuation of IL-8 production (pg/ml) at JNKi II concentrations of 25 and 40 μM was statistically significant (P<0.05).
Figure 5. Effect of JNKi II on LPA-induced phosphorylation of JNK and c-Jun and IL-8 secretion in HBEpCs.
HBEpCs (approx. 80% confluence in 35 mm dishes) were pretreated with various concentration of JNKi II for 1 h prior to LPA (1 μM) challenge for either 15 min or 2 h. (A) Cell lysates (10 μg) from 15 min treatment were subjected to SDS/PAGE and Western blotted with phosph-JNK1/2 or total JNK1 antibodies. (B) and (C) HBEpCs (approx. 80% confluence in 35 mm dishes) were pretreated with either JNKi II (25 μM) (B) or SB203580 (25 μM) (C) for 1 h prior to challenge with BEGM containing 0.1% (w/v) BSA (vehicle) or BEGM containing 0.1% BSA plus LPA (1 μM) for 15 min. Cell lysates (10 μg) were subjected to SDS/PAGE and Western blotted with phospho-c-Jun or total c-Jun antibodies. (D) Cells were exposed to LPA for 2 h and medium was collected and analysed for IL-8 by ELISA as described in the Experimental procedures section. Values are means of two independent experiments performed in triplicate.
Transcriptional activation of IL-8 expression requires p38 MAPK and JNK in HBEpCs
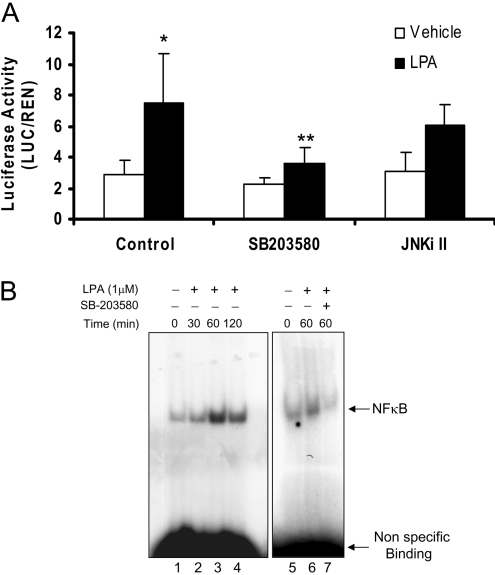
Transcription factors NF-κB and AP-1 play a major role in the regulation of IL-8 expression in airway epithelial cells [21,22]. To test the involvement of p38 MAPK and JNK in IL-8 gene expression, the luciferase reporter gene, under the control of the −162 to +44 nt region of the IL-8 promoter, was transfected in HBEpCs [23,24]. As shown in Table 1, incubation with LPA (1 μM) for 3–6 h resulted in an approx. 15-fold induction of IL-8 promoter-mediated luciferase activity. Pretreatment of cells for 1 h with SB203580 (25 μM) or JNKi II (10 μM) abolished the IL-8 promoter-mediated luciferase activity. These results suggest that p38 MAPK and JNK are involved in LPA-induced regulation of IL-8 gene expression at the transcriptional level.
Table 1. Requirement of p38 MAPK and JNK for transcription from the IL-8 promoter.
HBEpCs (approx. 60% confluence in 24 well plates) were transiently transfected with 100 ng of IL-8 promoter construct along with 1 ng of pRL-TK reference plasmid. After overnight incubation, cells were pretreated with SB203580 or JNKi II for 1 h prior to LPA (1 μM) challenge for 3 h. Luciferase activity was determined as described in the Experimental procedures section. The results are expressed as the means±S.D.(n=3) performed in triplicate.
| IL-8 | ||
|---|---|---|
| Treatment | (Luciferase activity) | (% of control) |
| Vehicle | 0.58+0.18 | 100 |
| LPA (1 μM) | 8.51+0.63 | 1516 |
| SB203580 (25 μM) | 0.38+0.22 | 67 |
| SB203580+LPA (1 μM) | 3.34+0.45 | 594 |
| JNKi II (25 μM) | 1.00+0.50 | 179 |
| JNKi II+LPA (1 μM) | 2.02+0.15 | 360 |
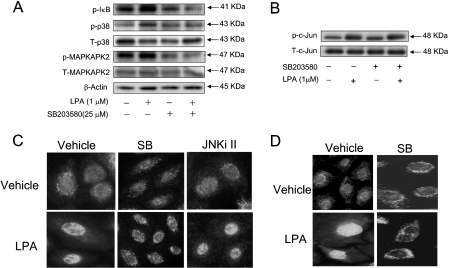
p38 MAPK, but not JNK, regulates LPA-induced NF-κB activation and transcription in HBEpCs
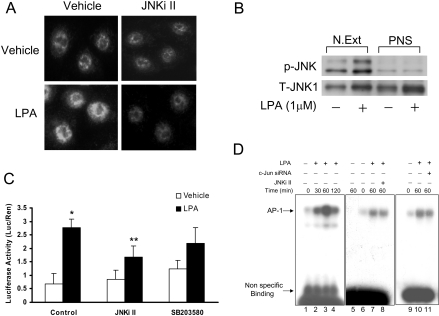
To determine whether LPA-induced p38 MAPK and/or JNK signalling is required for NF-κB activation, HBEpCs were pretreated with either SB203580 (25 μM) or JNKi II (25 μM) prior to LPA treatment. As shown in Figure 6(A), SB203580 attenuated LPA-mediated phosphorylation of p38 MAPK and its downstream target, MAPKAPK2, as well as that of IκB. Furthermore, SB203580, significantly blocked LPA-dependent translocation of NF-κB to the nucleus. However, under identical incubation conditions, JNKi II had no effect on LPA-induced IκB phosphorylation and NF-κB translocation (Figures 5C and 6C). Additionally, SB203580 blocked LPA-induced translocation of phosho-p38 MAPK to the nucleus (Figure 6D). The role of p38 MAPK and JNK in NF-κB activation was tested using direct measurement of an NF-κB-driven luciferase reporter assay and supershift of the NF-κB–DNA binding complex. As shown in Figure 7(A), LPA increased NF-κB-driven luciferase activity (approx. 4-fold) and this increase was almost completely blocked by pretreatment with SB203580, but was not significantly attenuated by JNKi II. Similarly, the LPA-driven supershift of the DNA binding complex was prevented by SB203580 (Figure 7B). Together, these results suggest that LPA-induced activation and transcription of NF-κB is driven by the p38 MAPK signalling cascade without the participation of the JNK pathway.
Figure 6. p38 MAPK activation and NFκB transcription in HBEpCs.
HBEpCs (approx. 80% confluence in 35 mm dishes) were pretreated with SB 203580 (25 μM) for 1 h prior to exposure to BEGM containing 0.1% (w/v) BSA (vehicle) or BEGM containing 0.1% (w/v) BSA plus LPA (1 μM) for 15 min. (A) Cell lysates (10 μg) were subjected to SDS/PAGE and Western blotted with phospho-IκB or phospho-p38 MAPK or phospho-MAPKAPK-2 or pan p38 MAPK or MAPKAPK-2 or β-actin antibodies. (B) as in A, cells were pretreated with JNKi II (25 μM) for 1 h prior to challenge with LPA and cell lysates (10 μg protein) were subjected to SDS/PAGE and Western blotted with phospho-IκB and β-actin antibodies. (C) and (D) HBEpCs were grown on 8-well chamber slides to approx. 70% confluence and pretreated with SB203580 (25 μM; SB) or JNKi II (25 μM) for 1 h prior to challenge with LPA (1 μM) for 15 min. Cells were then subjected to immuno-staining for NF-κB (p65) (C) or p-p38 MAPK (D) and examined by fluorescence microscopy as described in the Experimental procedures section. The immunofluorescent image is representative of two independent experiments.
Figure 7. Effect of MAPK inhibitors on LPA-induced NF-κB activation.
A, HBEpCs (approx. 60% confluence in 24-well plates) were transiently transfected with 100 ng of NF-κB reporter construct along with 1 ng of pRL-TK reference plasmid. After overnight incubation, cells were pretreated with SB203580 (25 μM) or JNKi II (25 μM) for 1 h prior to LPA challenge (1 μM) for 3 h. Luciferase activity was measured using a commercially available kit. The results are expressed as the means±S.D. (n=3). * significantly different from vehicle controls (p < 0.05), **, significantly different from LPA treatment alone (p < 0.05). B, Nuclear extracts isolated from control (lanes1, 5) and LPA-stimulated HBEpCs from different times (lanes 2–4) were incubated with 32P end-labelled NF-κB. Lanes 6–7 contain extracts from cells pre-incubated with vehicle or SB203580 prior to LPA challenge for 60 min. Shown is a representative gel shift assay of three independent experiments.
JNKi II attenuates nuclear translocation of p-JNK, phosphorylation of c-Jun and transcription of AP-1
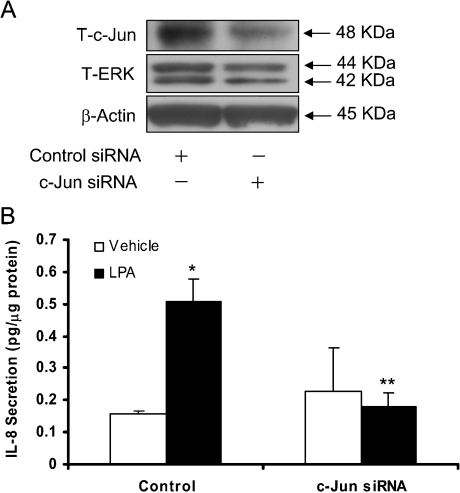
As LPA-induced activation of JNK is not involved in the transcription of NF-κB, we investigated whether JNK signals through activation of the transcriptional factor, AP-1. Stimulation of HBEpCs with LPA (1 μM) for 15 min resulted in a significantly increase in nuclear staining of phospho-JNK, which was effectively blocked by JNKi II (Figure 8A). Consistent with enhanced nuclear localization of phospho-JNK by immunofluorescence, Western blotting of nuclear extracts revealed increased immunostaining for JNK1 and phospho-JNK in the nuclear fraction, while the post-nuclear supernatant exhibited negligible phospho-JNK (Figure 8B). To determine the role of JNK in LPA-induced AP-1 transcription, HBEpCs were transfected with a cDNA encoding an AP-1 consensus binding sequence linked to a minimal promoter and luciferase (AP-1-CMV/Luc). Treatment of cells with LPA (1 μM) for 6 h induced an approx. 2.5-fold increase in luciferase activity, which was blocked by JNKi II but not SB203580 (Figure 8C). Furthermore, to determine the role of JNK in AP-1 transactivation by LPA, HBEpCs were treated with vehicle or LPA (1 μM) for various time periods and nuclear extracts were prepared for EMSAs. As shown in Figure 8(D), LPA caused an increase in the binding of nuclear proteins to an oligonucleotide encoding an AP-1 consensus binding sequence, which was partially attenuated by JNKi II. To further confirm the role of JNK in LPA-induced AP-1 transcription, we studied the effect of c-Jun siRNA on IL-8 secretion. In HBEpCs transfected with c-Jun siRNA for 72 h, c-Jun protein expression was down-regulated by approx. 85%, as determined by Western blotting (Figure 9A). Furthermore, c-Jun siRNA transfection blocked approx. 63% of IL-8 production in response to LPA (1 μM) (Figure 9B). Together, these results indicate that LPA-mediated activation of c-Jun in part regulates AP-1 transcription and IL-8 gene expression.
Figure 8. JNKi II and c-Jun siRNA block p-JNK nuclear translocation, and LPA-induced AP-1 activation.
A, HBEpCs were grown on 8-well chamber slides to approx. 70% confluence and pretreated JNKi II (25 μM) for 1 h prior to challenge with vehicle or LPA (1 μM) for 15 min. Cells were then subjected to immuno-staining with anti-phospho-JNK antibody and examined by fluorescence microscopy as described in the Experimental procedures section. The immunofluorescent image is representative of three independent experiments. (B) Nuclear extracts (N. Ext) and post-nuclear supernatant (PNS) from control (vehicle) and LPA stimulated cells (15 min) were Western blotted with anti-phospho-specific-JNK- and anti-total-JNK1- antibodies. (C) HBEpCs (approx. 60% confluence in 24-well plates) were transiently transfected with 100 ng of AP-1 reporter construct along with 1 ng of pRL-TK reference plasmid. After overnight incubation, cells pretreated with JNKi II or SB203580 for 1 h prior to LPA challenge (1 μM) for 3 h. Luciferase activity was measured using a commercially available kit. The results are expressed as the means±S.D. (n=3). * significantly different from controls (P<0.05), **, significantly different from LPA treatment (P<0.05). (D) Nuclear extracts isolated from control (lanes 1, 6, 9) and LPA-stimulated for different times (lanes 2–4) were incubated with the 32P end-labelled AP-1. Lanes 8 and 11 contain extracts from cells pretreated with JNKi II and c-Jun siRNA respectively prior to LPA challenge. Nuclear extract in lane 5 was incubated with 100-fold molar excess of unlabelled double-stranded AP-1. Shown is a representative of three independent experiments.
Figure 9. Effect of c-Jun siRNA on LPA-induced IL-8 secretion.
HBEpCs (approx. 50% confluence in 35 mm dishes) were transfected with control siRNA or c-Jun siRNA (100 nM) for 72 h and then challenged with BEGM containing 0.1% (w/v) BSA (vehicle) or BEGM containing 0.1% (w/v) BSA plus LPA (1 μM) for 2 h. (A) Cell lysates (10 μg protein) were subjected to SDS/PAGE and Western blotted for c-Jun or ERK or β-actin. (B) Cells were treated with vehicle or LPA for 2 h and IL-8 secreted into medium was quantified by ELISA as described in the Experimental procedures section. The results are expressed as the means±S.D. (n=3) performed in triplicate. *, significantly different from controls (P<0.05), **, significantly different from LPA treatment (P<0.05).
LPA-induced IL-8 secretion via LPA1–3 receptors
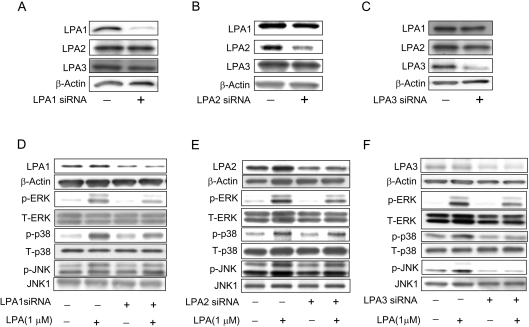
To investigate the role of LPA1–3Rs in mediating LPA-induced IL-8 production, we used siRNAs for the three receptors to knock out individual receptor expression. As shown in Figures 10(A), 10(B) and 10(C), each of the siRNAs specifically down-regulated its receptor expression without significantly altering the expression of the other two receptors. Furthermore, attenuation of LPA1, LPA2 or LPA3 receptors, by respective siRNAs, attenuated LPA-induced phosphorylation of ERK, p38 MAPK and JNK (Figures 10D, 10E and 10F). Having established the specificity of the LPA1–3 siRNAs, we then determined which of these three receptors are involved in LPA-induced IL-8 production. HBEpCs were transfected with LPA1 or LPA2 or LPA3 siRNA for 72 h, followed by exposure to either vehicle or LPA (1 μM) for 3 h and IL-8 levels were determined by ELISA. As shown in Table 2, siRNAs for LPA1–3 blocked LPA-mediated IL-8 secretion to varying degrees. While LPA1 and LPA3 siRNA blocked LPA-induced IL-8 production by approx. 60%, inhibition by LPA2 siRNA was approx. 40% (Table 2). These results suggest that LPA-dependent stimulation of IL-8 secretion in HBEpCs involve LPA1–3 receptors with varying degrees of efficacy.
Figure 10. Effect of LPA receptor siRNA on LPA-induced phosphorylation of MAPKs.
HBEpCs (approx. 50% confluence in 35 mm dishes) were transfected with control siRNA or LPA1–3 receptor siRNA (200 nM) for 72 h and then harvested (A, B, C) or challenged with BEGM with 0.1% (w/v) BSA (vehicle) or BEGM with 0.1% (w/v) BSA plus LPA (1 μM) for 15 min (D, E, F). Cell lysates were subjected to SDS/PAGE and Western blotted with LPA1, LPA2 or LPA3 or β-actin antibodies (A, B, C) or anti-phospho-specific- and pan-antibodies for ERK, p38 MAPK, JNK (D, E, F).
Table 2. Effect of LPA1–3 siRNA on LPA-induced IL-8 secretion.
HBEpCs (approx. 50% confluence in 6-well plates) were transfected with control siRNA or LPA1–3 receptor siRNAs (200 nM) for 72 h prior to challenge with BEGM containing 0.1% (w/v) BSA (vehicle) or BEGM containing 0.1% (w/v) BSA plus LPA (1 μM) for 2 h. Media were collected and IL-8 concentrations were analysed by ELISA as described in the Experimental procedures section. The results are expressed as the means±S.D.(n=3) performed in triplicate. *, significantly different from controls (P<0.05), **, significantly different from LPA treatment alone (P<0.05).
| IL-8 (pg/μg protein) | ||||
|---|---|---|---|---|
| Treatment | (−) LPA | (+) LPA (1 μM) | Δ IL-8 | % Over LPA stimulation |
| Vehicle | 0.212±0.003 | 1.315±0.08* | 1.103 | 100 |
| LPA1 siRNA | 0.126±0.037 | 0.560±0.034** | 0.434 | 38 |
| LPA2 siRNA | 0.267±0.142 | 0.933±0.057** | 0.673 | 61 |
| LPA3 siRNA | 0.110±0.009 | 0.545±0.054** | 0.435 | 39 |
DISCUSSION
Recent evidence suggests that the bioactive lipids, S1P (sphingosine 1-phosphate) and LPA, stimulate production of IL-8, a chemoattractant for neutrophils in the lung [15,25]. In airway epithelial cells, both S1P and LPA stimulated IL-8 secretion was dependent on PKC- and PLD (phospholipase D)- dependent signal transduction [15,26,27]. In the present study, we have further characterized the role of MAPKs in LPA-mediated IL-8 production in HBEpCs. Our results show that LPA-induced expression and secretion of IL-8 is regulated by p38 MAPK and JNK, but not ERK. Furthermore, the novel finding of the present study is the role that p38 MAPK activation plays in regulating NF-κB transcription, compared with the role of JNK signalling via AP-1, which enhances LPA-induced IL-8 expression and secretion. Additionally, using siRNAs against the three LPA receptors, we demonstrate that LPA signals, leading to MAPK activation and IL-8 formation in HBEpCs, are transduced by LPA receptors 1–3, possibly with differing efficiencies.
IL-8, a CXC cytokine, plays an important role in the pathogenesis of autoimmune and inflammatory diseases [1–6] and is implicated in ovarian cancer progression and prognosis [28]. Expression and secretion of IL-8 is increased in the airways of patients with asthma [25], cystic fibrosis [29] and in the BALF of infants developing bronchopulmonary dysplasia [30]. Furthermore, environmental factors such as viruses [31], cigarette smoke [2], ozone [31] and air pollutants increase IL-8 production in airway epithelial cells. Although both S1P and LPA stimulated IL-8 expression and secretion in the epithelial cell line Beas-2B [32], in primary cultures of HBEpCs, LPA was a better inducer than S1P [15]. Furthermore, LPA was a potent stimulator of induced IL-6 and IL-8 production in ovarian cancer cells [28]. As IL-8 production by LPA plays an important role in the pathogenesis of airway diseases and in promoting neovascularization, it is critical to define mechanisms underlying the transcriptional and translational regulation of IL-8 expression and secretion.
The MAPKs have been shown to regulate IL-8 expression and secretion in a number of cells types, including lung epithelial cells [33,34]. It appears that MAPKs primarily regulate IL-8 through activation of NF-κB, AP-1 and C/EBPβ (CAAT/enhancer binding protein β, formerly nuclear factor of IL-6) and/or posttranscriptional mechanisms such as mRNA stabilization. However, the role of ERK, p38 MAPK and JNK in IL-8 regulation seems to be cell-type-dependent and stimulus-specific. Activation of ERK seemed to be required for IL-8 mRNA and protein expression in A549 lung epithelial cells, 16HBE14o−, THP-1 and squamous carcinoma cell lines [16,35,36]. In the present study, we demonstrated that LPA induced phosphorylation of all three MAPKs. However, inhibition of the ERK signalling pathway by the pharmacological inhibitor of MEK1, PD98059, failed to block LPA-induced IL-8 secretion in HBEpCs. In contrast to ERK, blocking p38 MAPK or JNK activation with chemical inhibitors or siRNA partially prevented LPA-induced IL-8 production, suggesting a role for these two MAPKs in regulating its expression and secretion. The IL-8 promoter region contains binding sites for NF-κB, C/EBP and AP-1 [21,22]. We found that inhibition of p38 MAPK, but not JNK, attenuated LPA-induced IκB phosphorylation, NF-κB activation and NF-κB binding to DNA, but had no effect on c-Jun phosphorylation and AP-1 transactivation (Figure 5C). Interestingly, LPA challenge of HBEpCs also resulted in the translocation of phosphorylated p38 MAPK to the nucleus. However, it is unclear if both the NF-κB and the phospho-p38 MAPK translocated to the nucleus are co-localized and if they are translocated from the cytoplasm together or separately. It has been reported that high concentrations of SB203580 could potentially reduce signalling via JNK cascades [37]. Our results show that SB203580 had no effect on c-Jun phosphorylation by LPA, indicating specificity of this chemical inhibitor for p38 MAPK activation with no effect on JNK. In contrast to primary HBEpCs, an earlier study in the 16HBE14o− bronchial epithelial cell line showed that inhibition of JNK, but not p38 MAPK, decreased TNF-α induced transcription from the IL-8 promoter [23].
In addition to p38 MAPK, we found a role for JNK in LPA-induced IL-8 expression and secretion. JNK enhances the transcriptional activity of AP-1 by the phosphorylation of c-Jun on Ser63 and Ser73 of its transactivation domain [16]. Inhibition of JNK signalling by JNKi II blocked phosphorylation of c-Jun, translocation of phosphorylated c-Jun to the nucleus and AP-1 binding to DNA. Furthermore, c-Jun siRNA also attenuated LPA-induced AP-1 transactivation and IL-8 production, confirming roles for JNK, c-Jun and AP-1 in IL-8 gene expression. Pretreatment of HBEpCs with JNKi II failed to block LPA-induced IκB phosphorylation and nuclear translocation of NFκB. These results clearly show that, in primary cultures of HBEpCs, LPA-induced IL-8 gene expression and protein secretion utilize the p38 MAPK and JNK signalling pathways that specifically activate the NF-κB and AP-1 transcriptional factors respectively. Furthermore, we have demonstrated that there was no cross-talk between the p38 MAPK and JNK signalling cascades in NF-κB or AP-1 DNA binding.
In addition to MAPKs, activation of PKC by agonists or phorbol ester seems to regulate IL-8 secretion [38]. Among various PKC isoforms investigated, PKC δ enhanced TNF-α and phorbol ester mediated transcription of NF-κB and IL-8 luciferase promoter activity in the 16HBE14o− bronchial epithelial cell line [38]. Recent studies in primary cultures of HBEpCs demonstrated that LPA is a potent stimulator of IL-8 expression and secretion that involved PKC δ and λ and NF-κB signalling pathways [15]. Furthermore, the LPA-induced IL-8 production was regulated by lipid phosphatase-1 via calcium release and NF-κB activation in HBEpCs [8]. These results point to a novel mechanism of LPA metabolism and signalling downstream to LPA receptor activation in IL-8 release and innate immune responses in the airway. While most of the published data strongly support transcriptional regulation of IL-8 expression and secretion, in intestinal epithelial cells TNF-α and prostaglandin E2-induced IL-8 secretion occurred via a post-transcriptional mechanism of stabilization of the IL-8 mRNA by ERK, p38 MAPK and cAMP signal transduction [17,39].
Although LPA signals via LPA1– receptors in many cell types, only a few studies have addressed the relative contribution of each of the LPA receptors in cell function. In the present study, we successfully employed siRNAs for the LPA1–3 receptors to identify which of these reporters were involved in LPA-induced IL-8 secretion in HBEpCs. Our results show that all the three LPA receptors were expressed in HBEpCs and were effective in linking LPA to IL-8 production in the order of LPA1=LPA3>LPA2. Interestingly, LPA2 mRNA expression was much lower compared with that of either LPA1 or LPA3 in HBEpCs. However, in ovarian cancer cell lines, over-expressed LPA2 receptor, compared with the LPA1 and LPA3 receptors, was the most effective receptor in LPA-induced IL-6 and IL-8 production [40]. The exact coupling between LPA1–3 receptors and heterotrimeric G-proteins in LPA-induced IL-8 secretion is unclear. However, use of genetically modified LPA1–3 receptor mice in combination with siRNAs, should provide a better understanding of the coupling of LPA receptors to various heterotrimeric G-proteins.
The physiological implication of LPA as lipid mediator in neutrophil diapedesis during innate immunity is yet to be fully defined and characterized. Instillation of LPA (1–10 μg/ml) into guinea pig airways for 4 h resulted in an increased infiltration of eosinophils and neutrophils into the alveolar space, which was attenuated by Y-27632, an inhibitor of Rho kinase [15,41]. In C57BL/6J mice, intratracheal instillation of LPA (10 μM) enhanced MIP-2 (the murine homologue of human IL-8) and neutrophil influx in the BALF after 3 and 6 h of LPA instillation [15]. These results indicate a correlation between LPA accumulation and infiltration of inflammatory cells at sites of injury or inflammation in the airway. Furthermore, LPA levels in BALF from allergen challenged asthmatics, compared with non-allergen challenged subjects, were significantly higher and correlated with higher eosinophil counts and IL-8 levels in the BALF [15]. At present, the cellular source of LPA and its mechanism of generation and accumulation in the BALF after allergen challenge are unknown. It is tempting to speculate that the accumulated LPA in the BALF could be derived either from any of the infiltrating inflammatory cells or by increased influx of plasma and plasma components into the alveolar space due to loss of epithelial barrier function. Regarding the mechanisms of generation of LPA, at least two major pathways need to be evaluated. One mechanism would involve the role of PLD1/PLD2 in the generation of phosphatidic acid that is subsequently hydrolysed to LPA by phosphatidic acid specific phospholipase A1/A2 [42–44], and recently two such phospholipases have been cloned in mammalian cells [45,46]. The second pathway would involve the action of lysophospholipase D, also known as autotoxin or ENPP2 (ectonucleotide pyrophosphatase/phosphodiesterase 2), on LPC (lysophosphatidylcholine) resulting in production of LPA and free choline. Recently, the gene encoding lysophospholipase D was cloned and partially characterized [47]. It is interesting to point out that BALF from allergen challenged asthmatics had very high levels of LPC [48] and it needs to be established if lysophospholipase D expression or activity is enhanced in the BALF or in any of the infiltrating cells.
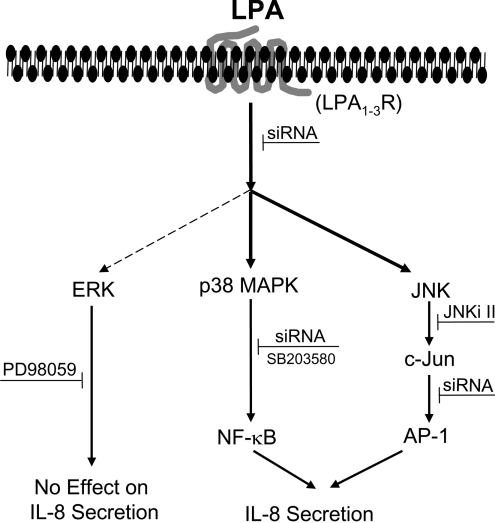
In summary, we have shown that LPA-induced activation of p38 MAP kinase and JNK signalling, but not ERK, regulates IL-8 expression and secretion in primary HBEpCs (Figure 11). Our results provide a definitive link between LPA-induced p38 MAPK/NF-κB and JNK/AP-1 transactivation in the transcriptional regulation of IL-8 formation. Our results also show that HBEpCs express LPA1–3 receptors and that blocking LPA1–3 using siRNA attenuated LPA-dependent intracellular signalling of MAPKs and IL-8 secretion.
Figure 11. Proposed MAPK signalling involved in LPA-induced IL-8 secretion.
Ligation of LPA to its G-protein-coupled receptors LPA1–3 results in activation of ERK, p38 MAPK and JNK pathways. LPA-induced activation of p38 MAPK and JNK (heavy arrows), but not ERK (dashed arrow) regulates NF-κB and AP-1 transactivation and IL-8 secretion.
Acknowledgments
This work was supported by the National Institutes of Health Grants HL 71152 to V.N.
References
- 1.Matsukura S., Kokubu F., Noda H., Tokunaga H., Adach I. M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J. Allergy Clin. Immunol. 1996;98:1080–1087. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 2.Mio T., Romberger D. J., Thompson A. B., Robbins R. A., Heires A., Rennard S. I. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 1997;155:1770–1776. doi: 10.1164/ajrccm.155.5.9154890. [DOI] [PubMed] [Google Scholar]
- 3.Johnston S. I., Papi A., Bates P. J., Mastronarde J. G., Monick M. M., Hunninghake G. W. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J. Immunol. 1998;160:6172–6181. [PubMed] [Google Scholar]
- 4.Hashimoto S., Gon Y., Takeshita I., Matsumoto K., Jibiki I., Takizawa H., Kudoh S., Horie T. Diesel exhaust particles activates p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-acetylcysteine attenuates p38 MAP kinase activation. Am. J. Respir. Crit. Care Med. 2000;161:280–285. doi: 10.1164/ajrccm.161.1.9904110. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal A., Baker C. S., Evans T. W., Haslam P. L. G-CSF and IL-8 but not GM-CSF correlates with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur. Respir. J. 2000;15:895–901. doi: 10.1034/j.1399-3003.2000.15e14.x. [DOI] [PubMed] [Google Scholar]
- 6.Koch A. E., Polverini P. J., Kunkel E. J., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science (Washington, D.C.) 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Cummings R., Zhao Y., Kazlauskas A., Sham J. K. S., Morris A., Georas S., Brindley D. N., Natarajan V. Involvement of phospholipase D2 in lysophosphatidate-induced transactivation of platelet-derived growth factor receptor-beta in human bronchial epithelial cells. J. Biol. Chem. 2003;278:39931–39940. doi: 10.1074/jbc.M302896200. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y., Usatyuk P. V., Cummings R., Saatian B., He D., Watkins T., Morris A., Spannhake E. W., Brindley D. N., Natarajan V. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid-induced calcium release, NF-κB activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem. J. 2005;385:493–502. doi: 10.1042/BJ20041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano T., Baker D. L., Virag Wada T. A., Yatomi Y., Kobayashi T. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine-1 phosphate generation in blood. J. Biol. Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 10.Aoki J., Taira A., Takanezawa Y., Kishi Y., Hama K., Kishimoto T. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem. 2002;277:39696–39702. doi: 10.1074/jbc.M206812200. [DOI] [PubMed] [Google Scholar]
- 11.An S., Bleu T., Hallmark O. G., Goetzl E. J. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- 12.Bandoh K., Aoki J., Hosono H., Kobayashi S., Kobayashi T., Murakami-Murofushi K., Tsujimoto M., Arai H., Inoue K. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J. Biol. Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi K., Ishii S., Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the EDG family. J. Biol. Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C., Baker D. L., Yasuda S., Makarova N., Balazs L., Johnson L. R., Marathe G. K., McIntyre T. M., Xu Y., Prestwich G. D., et al. Lysophosphatidic acid induces neointima formation through PPARγ activation. J. Exp. Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings R., Zhao Y., Jacoby D., Spannhake E. W., Ohba M., Garcia J. G. N., Watkins T., He D., Saatian B., Natarajan V. Protein Kinase C δ mediates lysophosphatidic acid-induced NF-κB activation and interleukine-8 secretion in human bronchial epithelial cells. J. Biol. Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- 16.Raingeaud J., Gupta S., Rogers J. S., Dickens M., Han J., Ulevitch R. J., Davis R. J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 17.Jijon H. B., Panenka W. J., Madsen K. L., Parsons H. G. MAP kinases contribute to IL-8 secretion by intestinal epithelial cells via a posttranscriptional mechanism. Am. J. Physiol. Cell Physiol. 2002;283:31–41. doi: 10.1152/ajpcell.00113.2001. [DOI] [PubMed] [Google Scholar]
- 18.Saatian B., Yu X. Y., Lane A. P., Doyle T., Casolaro V., Spannhake E. W. Expression of genes for B7-H3 and other T cell ligands by nasal epithelial cells during differentiation and activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L217–L225. doi: 10.1152/ajplung.00132.2003. [DOI] [PubMed] [Google Scholar]
- 19.Bernacki S. H., Nelson A. L., Abdullah L., Sheehan J. K., Harris A., Davis C. W., Randell S. H. Mucin gene expression during differentiation of human airway epithelia in vitro. Am. J. Respir. Cell Mol. Biol. 1999;20:595–604. doi: 10.1165/ajrcmb.20.4.3442. [DOI] [PubMed] [Google Scholar]
- 20.Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature (London) 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 21.Mastronarde J. G., Monick M. M., Mukaida N., Matsushima K., Hunninghake G. W. Activator protein-1 is the preferred transcription factor for cooperative interaction with nuclear factor-κB in respiratory syncytial virus-induced interleukin-8 gene expression in airway epithelium. J. Infect. Dis. 1998;177:1275–1281. doi: 10.1086/515279. [DOI] [PubMed] [Google Scholar]
- 22.Roebuck K. A. Regulation of interleukin-8 gene expression. J. Interferon Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- 23.Li J., Kartha S., Iasvovskaia S., Tan A., Bhat R. K., Manaligod J. M., Page K., Brasier A. R., Hershenson M. B. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am. J. Physiology. Lung Cell. Mol. Physiol. 2002;283:L690–L699. doi: 10.1152/ajplung.00060.2002. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura H., Yoshimura K., Jaffe H. A., Crystal R. G. Interleukin-8 gene expression in human bronchial epithelial cells. J. Biol. Chem. 1991;266:19611–19617. [PubMed] [Google Scholar]
- 25.Wang L., Cummings R., Usatyuk P., Morris A., Irani K., Natarajan V. Involvement of phospholipases D1 and D2 in sphingosine 1-phosphate-induced ERK (extracellular-signal-regulated kinase) activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem. J. 2002;367:751–760. doi: 10.1042/BJ20020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L566–L577. doi: 10.1152/ajplung.00233.2002. [DOI] [PubMed] [Google Scholar]
- 27.Toews M. L., Ediger T. L., Romberger D. J., Rennard S. I. Lysophosphatidic acid in airway function and disease. Biochim. Biophys. Acta. 2002;1582:240–250. doi: 10.1016/s1388-1981(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 28.Kassim S. K., El-Salahy E. M., Fayed S. T., Helal S. A., Helal T., Azzam Eel-D., Khalifa A. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin. Biochem. 2004;37:363–369. doi: 10.1016/j.clinbiochem.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Joseph T., Look D., Ferkol T. NF-κB activation and sustained IL-8 gene expression in primary cultures of cystic fibrosis airway epithelial cells stimulated with Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L471–L479. doi: 10.1152/ajplung.00066.2004. [DOI] [PubMed] [Google Scholar]
- 30.Tullus K., Noack G. W., Burman L. G., Nilsson R., Wretlind B., Brauner A. Elevated cytokine levels in tracheobronchial aspirate fluids from ventilator treated neonates with bronchopulmonary dysplasia. Eur. J. Pediatr. 1996;155:112–116. doi: 10.1007/BF02075762. [DOI] [PubMed] [Google Scholar]
- 31.Spannhake E. W., Reddy S. P., Jacoby D. B., Yu X. Y., Saatian B., Tian J. Synergism between rhinovirus infection and oxidant pollutant exposure enhances airway epithelial cell cytokine production. Environ. Health Perspect. 2002;110:665–670. doi: 10.1289/ehp.02110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings R. J., Parinandi N. L., Zaiman A., Wang L., Usatyuk P. V., Garcia J. G., Natarajan V. Phospholipase D activation by sphingosine 1-phosphate regulates interleukin-8 secretion in human bronchial epithelial cells. J. Biol. Chem. 2002;277:30227–30235. doi: 10.1074/jbc.M111078200. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto S., Matsumoto K., Gon Y., Nakayama T., Takeshita I., Horie T. Hyperosmolarity-induced IL-8 expression in human bronchial epithelial cells through p38 MAP kinase. Am. J. Respir. Crit. Care Med. 1999;159:634–640. doi: 10.1164/ajrccm.159.2.9712090. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto K., Hashimoto S., Gon Y., Nakayama T., Horie T. Proinflamatory cytokine-induced and chemical mediator-induced IL-8 expression in bronchial epithelial cells through p38 MAP kinase-dependent pathway. J. Allergy Clin. Immunol. 1998;101:825–831. doi: 10.1016/S0091-6749(98)70311-2. [DOI] [PubMed] [Google Scholar]
- 35.Liu R., O'Connell M., Johnson K., Pritzker K., Mackman N., Terkeltaub R. Extracellular signal-regulated kinase 1/extracellular signal-regulated kinase 2 mitogen-activated protein kinase signalling and activation of activator protein 1 and nuclear factor-κB transcription factors play central roles in interleukin-8 expression stimulated by monosodium urate monohydrate and calcium pyrophosphate crystals in monocytic cells. Arthritis Rheum. 2000;43:1145–1155. doi: 10.1002/1529-0131(200005)43:5<1145::AID-ANR25>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 36.Bancroft C. C., Chen Z., Dong G., Sunwoo J. B., Yeh N., Park C., Van Waes C. Coexpression of proangiogenic factors IL-8 and VEGF by human head and neck squamous cell carcinoma involves coactivation by MEK-MAPK and IKK-NF-κB signal pathways. Clin. Cancer Res. 2001;7:435–442. [PubMed] [Google Scholar]
- 37.Nick J. A., Young S. K., Arndt P. G., Lieber J. G., Suratt B. T., Poch K. R., Avdi N. J., Malcolm K. C., Taube C., Henson P. M., Worthen G. S. Selective suppression of neutrophil accumulation in ongoing pulmonary inflammation by systemic inhibition of p38 mitogen-activated protein kinase. J. Immunol. 2002;169:5260–5269. doi: 10.4049/jimmunol.169.9.5260. [DOI] [PubMed] [Google Scholar]
- 38.Page K., Li J., Zhou L., Iasvoyskaia S., Corbit K. C., Soh J. W., Weinstein I. B., Brasier A. R., Lin A., Hershenson M. B. Regulation of airway epithelial cell NF-κB-dependent gene expression by protein kinase Cδ. J. Immunol. 2003;170:5681–5689. doi: 10.4049/jimmunol.170.11.5681. [DOI] [PubMed] [Google Scholar]
- 39.Yu Y., Chadee K. Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. J. Immunol. 1998;161:3746–3752. [PubMed] [Google Scholar]
- 40.Fang X., Yu S., Bast R. B., Liu S., Xu H. J., Hu S. X., LaPushin R., Claret F. X., Aggarwal B. A., Lu Y., Mills G. B. Mechanism for lysophosphstidic acid-induced cytokine production in ovarian cancer cells. J. Biol. Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto T., Yamashita M., Ohata H., Momose K. Lysophosphatidic acid enhances in vivo infiltration and activation of guinea pig eosinophils and neutrophils via a Rho/Rho-associated protein kinase-mediated pathway. J. Pharmacol. Sci. 2003;91:8–14. doi: 10.1254/jphs.91.8. [DOI] [PubMed] [Google Scholar]
- 42.Gaits F., Fourcade O., le Balle F., Gueguen G., Gaige B., Gassama-Diagne A., Fauvel J., Salles J. P., Mauco G., Simon M. F., Chap H. Lysophosphatidic acid as a phospholipid mediator: pathways of synthesis. FEBS Lett. 1997;410:54–58. doi: 10.1016/s0014-5793(97)00411-0. [DOI] [PubMed] [Google Scholar]
- 43.Luquain C., Singh A., Wang L., Natarajan V., Andrew A. J. Role of phospholipase D in agonist-stimulated lysophosphatidic acid synthesis by ovarian cancer cells. J. Lipid Res. 2003;44:1963–1975. doi: 10.1194/jlr.M300188-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Aoki J. Mechanism of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004;15:477–489. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Sonoda H., Aoki J., Hiramatsu T., Ishida M., Bandoh K., Nagai Y., Taguchi R., Inoue K., Arai H. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J. Biol. Chem. 2002;277:34254–34263. doi: 10.1074/jbc.M201659200. [DOI] [PubMed] [Google Scholar]
- 46.Hiramatsu T., Sonoda H., Takanezawa Y., Morikawa R., Ishida M., Kasahara K., Sanai Y., Taguchi R., Aoki J., Arai H. Biochemical and molecular characterization of two phosphatidic acid-selective phospholipase A1s, mPA-PLA1α and mPA-PLA1β. J. Biol. Chem. 2003;278:49438–49447. doi: 10.1074/jbc.M213018200. [DOI] [PubMed] [Google Scholar]
- 47.Murata J., Lee H. Y., Clair T., Krutzsch H. C., Arestad A. A., Sobel M. E., Liotta L. A., Stracke M. L. cDNA cloning of the human tumor motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterases. J. Biol. Chem. 1994;269:30479–30484. [PubMed] [Google Scholar]
- 48.Chilton F. H., Averill F. J., Hubbard W. C., Fonteh A. N., Triggiani M., Liu M. C. Induced generation of lyso-phospholipids in human airways. J. Exp. Med. 1996;183:2235–2245. doi: 10.1084/jem.183.5.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]