Abstract
The Epstein-Barr virus (EBV)-encoded latent membrane protein-1 (LMP-1) is thought to play a role in the EBV-induced B-cell transformation and immortalization. EBV has also been implicated in certain human T-cell lymphomas; however, the phenotypic effects of the expression of this oncoprotein in T cells are not known. To learn whether LMP-1 also induces phenotypic changes in T cells, we stably expressed it in human cell lines of T and B lineages and 25 LMP-1-expressing T-cell clones and 7 B-cell clones were examined. Our results show for the first time that, in sharp contrast to B cells, LMP-1 preferentially localizes to nuclei in T cells and does not induce the phenotypic changes in these cells that it induces in B cells, does not associate with TRAF proteins, and does not arrest the cell cycle in the G2/M phase. A computer-assisted analysis revealed that LMP-1 lacks the canonical nuclear localization signal. Our results suggest that this oncoprotein may not play the same role in the lymphomagenesis of T cells as it does in B cells.
The Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus which has been linked to several benign and malignant lymphoproliferative and autoimmune disorders, e.g., endemic Burkitt's lymphoma, nonkeratinizing nasopharyngeal carcinoma, Hodgkin's disease, Sjögren's syndrome, etc. The virus induces malignant tumors in experimentally infected New World primates (reviewed in reference 42). Primary EBV infections generally occur in childhood, present with mild symptoms, and are usually self limiting. In developed countries, primary infections are usually delayed until adolescence and are the major cause of infectious mononucleosis. Once infected, the host becomes a lifelong carrier (3, 42). In vitro, EBV can infect resting human B cells and immortalizes them into continuously growing lymphoblastoid cell lines (LCLs) (reviewed in reference 26). These cells express a restricted repertoire of viral genes which include six nuclear antigens (EBNA 1, 2, 3A, 3B, 3C, and LP), three latent membrane proteins (LMPs) (LMP-1, 2A, and 2B), and two small RNAs (EBER1 and 2) (26). Of all the EBV-induced lytic and latent cycle proteins, LMP-1 is one of the two EBV-encoded proteins that can transform rodent fibroblasts to grow in an anchorage-independent fashion and is essential for the EBV-induced immortalization of B cells (23, 26).
LMP-1 is a type III integral protein of 63 kDa and possesses a short hydrophilic N terminus, six hydrophobic membrane-spanning regions, and a relatively long C-terminal cytoplasmic tail (reviewed in references 9 and 26). The protein occurs as aggregated patches in the plasma membrane of EBV-infected and -immortalized cells in association with intermediate filaments (vimentin) of cytoskeleton (10, 28). LMP-1 itself induces the expression of vimentin; however, association with vimentin is not essential for the aggregation of LMP-1 in the plasma membranes of cells (4, 9, 28). By virtue of forming aggregated patches in the plasma membranes, LMP-1 acts as a constitutively activated receptor of the tumor necrosis factor (TNF) receptor superfamily and activates mitogen-activated kinases, NF-κB, and the JAK/STAT pathway via TNF receptor-associated factors (TRAFs) (9, 10, 14, 17, 33, 35, 48, 51). The expression of LMP-1 in human cells induces phenotypic changes that are characteristic of the EBV-infected and -immortalized cells, e.g., expression of CD23, cell adhesion molecules, and epithelial growth factor receptor (33, 50). It also induces the expression of antiapoptotic genes Bcl-2, Mcl-l, and A20 in different cell types and imparts resistance to the growth inhibitory effects of TGF-β (2, 9, 51). Furthermore, LMP-1 is consistently expressed in many EBV-associated tumors.
The type II complement receptor CR2 (CD21), a 145-kDa transmembrane protein, has been convincingly shown to be the EBV receptor (26). It functions as a natural receptor for the complement component C3dg. The virus binds CD21 via its major envelope glycoprotein 350/220 and initiates the infection process. CD21 occurs predominantly on B cells; however, several studies have reported its occurrence on a variety of T cells, including immature cortical thymocytes (8, 43, 47). Recently, these thymocytes were shown to be infectible with EBV (37, 38, 52). The virus is transcriptionally active within these T cells. Furthermore, EBV genome-carrying T-cell lymphomas have been described previously (5, 6, 16, 19, 24, 31, 44, 46). The viral genome contained in these lymphoma cells is monoclonal, which suggests the occurrence of the infection before malignancy and its potential role in the lymphomagenesis. Despite this ability of EBV to infect cells of T lineage, the phenotypic expression of the EBV-encoded antigens in these cells has not been studied. We conducted this study to find out whether the EBV oncoprotein LMP-1 induces phenotypic changes in T cells as it does in B cells. To this end, we developed human T- and B-cell clones with stable expression of the transfected LMP-1 gene.
Nuclear localization of LMP-1 in T cells.
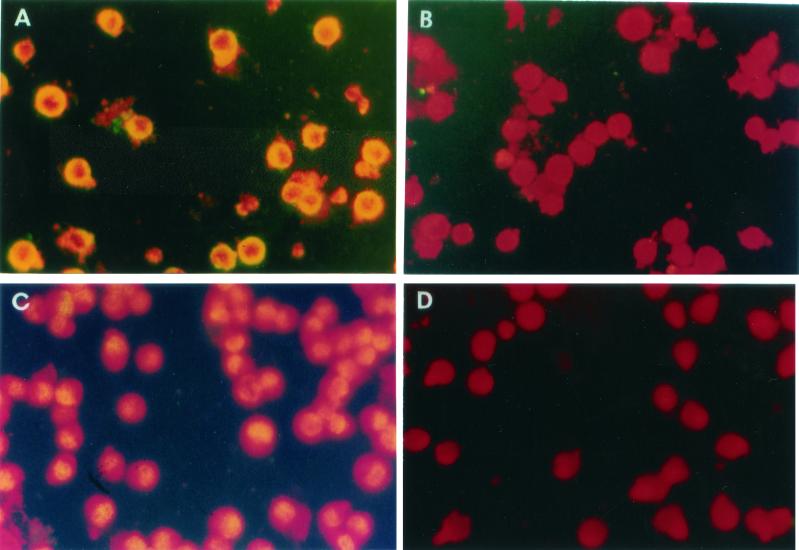
Initially, we introduced LMP-1 into the EBV genome-negative BJA-B cells (a B-cell line) (32) and CEM cells (a T-cell line) by electroporating (1) a PGEM2-based expression plasmid pIgLMP-1 (kindly provided by Nancy Raab-Traub, University of North Carolina, Chapel Hill) as described earlier (54). The control plasmid was derived from pIgLMP-1 after deleting the LMP-1 sequences. After electroporation, the transfected cells were cultured in the selection medium, cloned by limiting dilutions, and screened for expression of LMP-1 by indirect immunofluorescence. After fixation in methanol, cell smears on slides were first incubated with S12 and CS1-4 anti-LMP monoclonal antibodies, washed, and treated with anti-mouse immunoglobulin G (IgG) fluorescein isothiocyanate-conjugated antibodies. The slides were then examined under an immunofluorescence microscope (54). In sharp contrast to the B (i.e., BJA-B)-cell clones, in which LMP-1 was localized to both the plasma membrane and cytoplasm of the cells, in all T (i.e., CEM)-cell clones the location of LMP-1 was nuclear only (Fig. 1). In order to determine whether the nuclear localization of LMP-1 was specific to CEM, we expressed LMP-1 in two other T-cell lines, Jurkat and Molt-4, under similar conditions and examined the transfected cells for LMP-1 localization without cloning. As shown in Fig. 2, LMP-expressing Molt-4 cells initially showed three patterns: aggregated patches in the membrane (Fig. 2A), diffused expression in the nuclei (Fig. 2C), and a mixture of these two forms (Fig. 2B). However, an overwhelming majority of the positive cells (>95%) showed nuclear localization. Furthermore, with passage of time, the cells with membrane expression declined and disappeared. The same was observed for LMP-1 expression in Jurkat cells (data not shown). Taken together, these results clearly indicated that although LMP-l is initially also expressed on the membrane of a few T cells, subsequently, its expression occurs solely in the nuclei of these T cells.
FIG. 1.
Expression of LMP-1 in CEM and BJA-B cells. LMP-1 was detected by indirect immunofluorescence in permeabilized cells with S12 antibody and fluorescein isothiocyanate-conjugated goat anti-mouse IgG. The upper panels show LMP-expressing BJA-B cells stained with S12 (A) or control (B) antibody. The lower panels show LMP-expressing CEM cells stained with S12 (C) or control (negative) (D) antibody. Note the localization of immunofluorescence in the nucleus in CEM cells (C) and at the membranes in BJA-B cells (A).
FIG. 2.
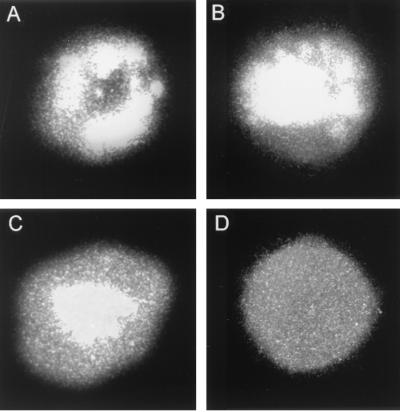
Different patterns of LMP-1 localization in Molt4 cells. LMP-expressing Molt4 cells were stained with S12 as described in the legend to Fig. 1. Shown here are three different patterns of LMP-1 localization in these cells: on the membrane (A), both on the membrane and in the nucleus (B), and in the nucleus (C). Panel D represents an LMP-expressing cell stained with a control (negative) antibody.
LMP-1 protein expressed in the transfectants has a normal molecular mass (i.e., 63 kDa).
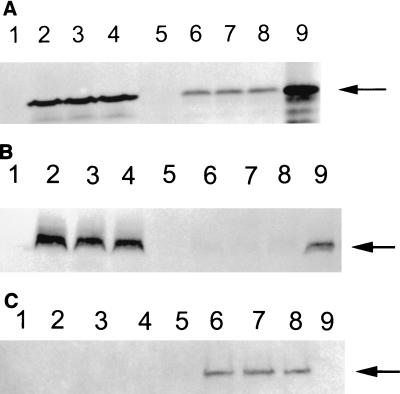
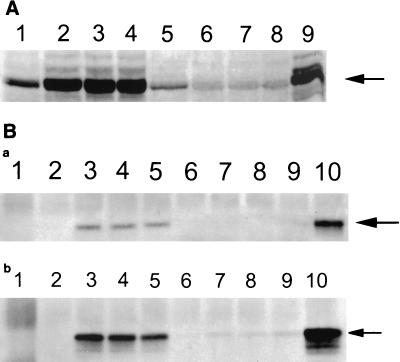
The third exon of the LMP-1 gene contains an initiation site, and an amino terminus-deleted form of LMP-1 (45 kDa) occurs naturally in EBV-infected B cells (18, 49). This truncated form of LMP-1 does not form aggregates in plasma membranes. To determine whether the unusual localization of LMP-1 in T cells was due to the preferential expression of this naturally truncated form of LMP-1 in these cells, we performed Western blotting for this protein on whole-cell lysates. Cells were lysed in 50 mM Tris-HCl (pH 6.8) and 2% sodium dodecyl sulfate (SDS) and sonicated. After clarification by centrifugation, 40 μg of the lysate protein was resolved by SDS-10% polyacrylamide gel electrophoresis (PAGE) and then transferred onto a nylon membrane. The resolved proteins on the blots were incubated with S12 antibodies and detected with alkaline phosphatase-conjugated secondary antibodies (54). As shown in Fig. 3A, the electrophoretic mobility of LMP-1 in T cells was comparable to that of B cells, suggesting that this protein is not truncated in T cells. To further confirm the localization of LMP-1 in T and B cells, we selected three LMP-1-expressing clones from each cell line (BJA-B and CEM) for further analyses of their cytoplasmic and nuclear fractions (20). These cloned cells were washed with phosphate-buffered saline and resuspended in 200 μl of the lysis buffer (10 mM Tris-HCl [pH 7.5], 150 mM KCl, 3 mM MgCl2, 1 mM CaCl2, 2% 90-kDa polyvinylpyrrolidone, 0.2% bovine serum albumin, and 0.5% Nonidet P-40). After vigorous shaking, the resuspension was gently overlaid on top of 1 ml of the same buffer without Nonidet P-40 to which sucrose was added to a final concentration of 24%. The nuclei were pelleted and resuspended in a buffer containing 10 mM Tris-HCl (pH 7.4), 10 mM NaCl, and 1.5 mM MgCl2. Both the nuclear and cytoplasmic fractions were mixed with sample loading buffer, boiled, and resolved on SDS-10% PAGE for Western blotting (54). As shown in Fig. 3B and C, the nuclear fraction in CEM clones and the cytoplasmic fraction in B-cell clones were positive for LMP-1. These data confirm that, unlike in B cells, in T cells LMP-1 localizes in the nuclei.
FIG. 3.
Detection of LMP-1 by Western blotting in CEM and BJA-B cells. (A) Whole-cell lysates (40 μg) from LMP-expressing and control vector-transfected cells were resolved by SDS-10% PAGE. After transfer onto nylon membranes, LMP-1 was detected by anti-LMP monoclonal antibody S12 followed by detection with alkaline phosphatase-conjugated anti-mouse antibodies 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium. Lanes: 1, BJA-B transfected with the control vector; 2 to 4, three different LMP-expressing BJA-B clones; 5, CEM transfected with the control vector; 6 to 8, three different LMP-expressing CEM clones; 9, an EBV-transformed B-cell line (LCL). The LMP-expressing BJA-B and CEM cells were fractionated into cytoplasmic and nuclear lysates, and each fraction was analyzed for LMP-1 expression by Western blotting. Panels B and C show LMP-1 expression in cytoplasmic and nuclear fractions, respectively. The lanes represent the clones indicated for panel A. The arrows point to LMP-1 bands.
LMP-mediated effects on transfected cells.
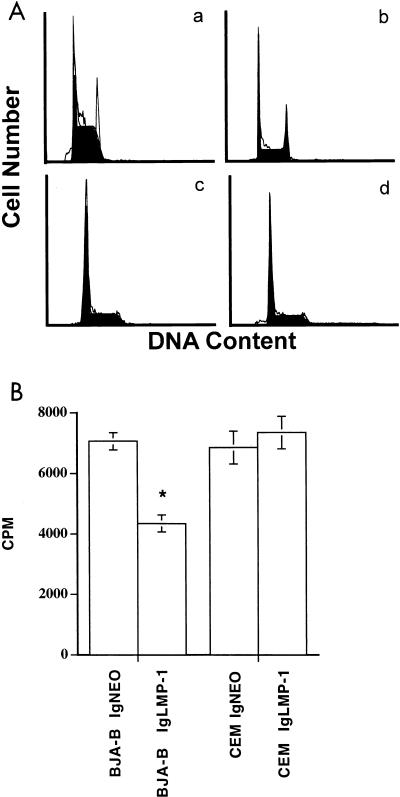
To determine whether differential localization of LMP-1 in T and B cells induced differential effects on the cell cycle, cells were incubated for 12 h in serum-free medium to synchronize them for the cell cycle. Then they were maintained in the culture medium for 24 h. One million cells were washed with phosphate-buffered saline and resuspended in 1 ml of buffer containing sodium citrate (1 mg/ml, pH 7.4), RNase (0.05 μg/ml), Igepal CA-630 (3 μl/ml), and propidium iodide (PI; 50 μg/ml). After a 30-min incubation at room temperature, cell cycles were analyzed from cellular DNA content by using Cellfit software. The LMP-expressing BJA-B, but not CEM, cells showed a significantly increased number of cells accumulated in the G2/M phase (Table 1 and Fig. 4). To further demonstrate the effects of LMP-1 on the cell cycle, we performed proliferation assays on pIgLMP-1- and pIgNEO-transfected cells. Cells (105 per well) were cultured in triplicate in RPMI 1640 supplemented with 10% fetal bovine serum and 500 μg of G418/ml for 24 h and then pulsed with 1 μCi of [3H]thymidine ([3H]TdR)/well for 16 h. The radioactivity from harvested cells was measured in a liquid scintillation counter. As shown in Fig. 5, LMP-1 significantly inhibited the rate of [3H]TdR uptake by BJA-B cells (P < 0.01) but not by CEM cells (P > 0.05). Clearly, the cell cycle arrest in the G2/M phase induced by LMP-1 resulted in the prolongation of the cell cycle and the inhibition of proliferation of BJA-B cells. These data strongly suggest that LMP-1 expressed in the nuclei of CEM cells does not affect their normal progression through the cell cycle as it does in B cells.
TABLE 1.
Effects of LMP-1 expression on cell cycle progression
| Cell line | % of cells in cell cycle stagea
|
G2/G1 ratio | ||
|---|---|---|---|---|
| G0/G1 | S | G2/M | ||
| BJA-B IgNEO | 29.26 | 66.52 | 3.86 | 1.80 |
| BJA-B IgLMP-1(A) | 34.39 | 46.91 | 18.70 | 1.86 |
| BJA-B IgLMP-1(B) | 32.51 | 48.81 | 18.68 | 1.85 |
| BJA-B IgLMP-1(C) | 39.36 | 47.41 | 12.63 | 1.87 |
| CEM IgNEO | 55.46 | 42.75 | 1.80 | 1.90 |
| CEM IgLMP-1(A) | 51.41 | 47.94 | 0.65 | 1.91 |
| CEM IgLMP-1(B) | 59.87 | 36.74 | 3.40 | 1.81 |
| CEM IgLMP-1(C) | 51.57 | 45.26 | 3.17 | 1.87 |
Cell cycles were synchronized by incubating cells for 12 h in serum-free medium and then for 24 h in culture medium before analysis. Cells were analyzed for cell cycle stage after staining with PI by using a flow cytometer and Cellfit software. Note the accumulation of LMP-expressing BJA-B cells, but not of LMP-expressing CEM cells, in the G2/M phase.
FIG. 4.
Cell cycle profiles of LMP-expressing cells. After staining with PI, the cell cycle profile was analyzed from the DNA content by using a flow cytometer and Cellfit software. (A) The figure shows the relative number of cells in various phases of the cell cycle for control vector-transfected BJA-B (a), LMP-expressing BJA-B (b), control vector-transfected CEM (c), and LMP-expressing CEM (d) cells. Note the accumulation of cells in the G2/M phase in LMP-expressing BJA-B cells (b). (B) Proliferation of pIgLMP-1 and control vector (IgNEO)-transfected cells. Cells (105 per well) were cultured in triplicate for 24 h and pulsed with [3H]TdR for 16 h to assess proliferation. The asterisk indicates significant difference (P < 0.01).
FIG. 5.
(A) LMP-1 induced the TRAF1 protein (arrow) in BJA-B cells but not in CEM cells. Whole-cell lysates (40 μg) from LMP-expressing and control vector-transfected cells were resolved by SDS-10% PAGE. After transfer onto nylon membranes, TRAF1 was incubated with anti-TRAF1 antibody followed by detection with alkaline phosphatase-conjugated anti-rabbit antibody. The lanes represent different clones (the same as lanes 1 to 9 of Fig. 3). (B) LMP-1 (arrows) interacted with TRAF1 in BJA-B cells but not in CEM cells. TRAF1 was immunoprecipitated from cytoplasmic fractions (a) and whole-cell lysates (b), resolved by SDS-PAGE, and electroblotted onto nylon membranes. The membranes were probed for LMP-1 with LMP-specific monoclonal antibodies (CS1-4) and alkaline phosphatase-conjugated secondary antibodies. Lane 1 represents immunoprecipitation with normal rabbit serum. Lanes 2 to 10 in panel B correspond to lanes 1 to 9 in panel A, respectively.
LMP-1 does not interact with TRAF-1, nor does it induce Bcl-2, LFA-1, and ICAM-1 expression in T cells.
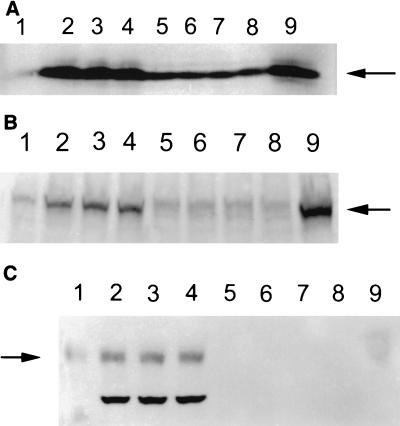
In B cells, LMP-1 is known to interact with TRAFs and induce the expression of the antiapoptotic protein Bcl-2 (2, 9). In order to see if intranuclear LMP-1 was also able to exert these effects in T cells, we first performed Western blotting to analyze the level of TRAF1 protein in LMP-1-expressing CEM and BJA-B cells. As shown in Fig. 5A, LMP-1 significantly induced TRAF1 expression in BJA-B cells but not in CEM cells. Further, we immunoprecipitated TRAF1 from the cytoplasmic fractions and whole-cell lysates of LMP-expressing CEM and BJA-B cells and determined whether LMP-1 interacts with TRAF1 protein. As shown in Fig. 5B, LMP-1 coimmunoprecipitated with TRAF1 in both cytoplasmic fractions and whole-cell lysates of B cells and not of T cells. Similarly, LMP-1 induced the expression of Bcl-2, LFA-1, and ICAM-1 in B cells but not in T cells (Fig. 6).
FIG. 6.
Expression of bcl-2 (A), LFA-1 (B), and ICAM-1 (C). LMP expression induces bcl-2, LFA-1, and ICAM-1 in BJA-B cells but not in CEM cells. The expression of these proteins in different clones of LMP-expressing cells was determined by Western blotting performed on 40 μg of whole-cell lysates. The lanes represent the cell clones indicated in the legend to Fig. 3. Arrows from top to bottom indicate bcl-2, LFA-1, and ICAM-1, respectively.
LMP-1 does not induce a tyrosine-phosphorylated protein in T cells.
LMP-1 is known to be phosphorylated in B cells at serine and threonine residues, and this phosphorylation is necessary for its association with the cytoskeleton and patching in the plasma membranes of these cells (30, 34). To determine whether LMP-1 was also phosphorylated on tyrosine residues in T cells, we performed comparative analysis with Far Western blots in which we immunoprecipitated LMP-1 from T and B cells and determined its reactivity with phosphotyrosine-specific antibody (54). Consistent with a previous report (34), LMP-1 was not tyrosine-phosphorylated in either T or B cells (data not shown). We further determined whether LMP-1 expression in these cells induced tyrosine phosphorylation on any protein. Therefore, Western blots prepared from the LMP-expressing and control cells were probed with anti-phosphotyrosine antibodies. As shown in Fig. 7, LMP-1 expressed in BJA-B cells (but not in CEM cells) induced a protein that reacted very strongly with phosphotyrosine-specific antibodies and migrated to approximately 40 kDa on these blots. The identity of this protein remains unknown. Interestingly, the anti-phosphotyrosine-reactive LMP-induced protein migrated to a lower level in EBV-transformed (control) LCLs, which may be due to its differential posttranslational processing.
FIG. 7.
LMP-1 expression induces a tyrosine-phosphorylated protein in B, but not in T, cells (arrow). Far Western blots were prepared from whole-cell lysates by using immunoprecipitated LMP-1. The blots were probed with anti-phosphotyrosine and alkaline phosphatase-conjugated anti-mouse IgG as described in the legend to Fig. 3. The lanes indicate the cell clones described in the legend to Fig. 3.
We have demonstrated here that the EBV oncoprotein LMP-1 predominantly localizes in the nuclei upon its forced expression in human T-cell lines. This is in contrast to its expression in B cells, in which it occurs as aggregated patches in the plasma membranes. It is by virtue of its ability to become aggregated in the plasma membrane that LMP-1 acts as a constitutively activated receptor of the TNF receptor superfamily, particularly CD40 (10, 48). Three different regions in the intracellular cytoplasmic terminus of LMP-1 interact with various TRAFs, transduce intracellular signals, and bring about the phenotypic changes seen in the EBV-immortalized B cells. Six hydrophobic membrane-spanning regions of the LMP-1 molecule impart to it the ability to form aggregated patches in the plasma membrane (9). A naturally occurring truncated version of LMP (45 kDa) is expressed from the EDL 1 promoter late in the lytic cycle (11). It has only two hydrophobic membrane-spanning regions, and it does not aggregate within the plasma membrane, transform rodent fibroblasts, or induce phenotypic changes characteristic of the EBV-immortalized B cells (11, 18, 49). From the evidence shown above, the LMP-1 expressed in T cells is the authentic full-length (63 kDa) protein and not the truncated version. Furthermore, we have used the same constructs for the expression of LMP-1 in both T and B cells, and it is highly unlikely that mutations in the expression plasmids are responsible for its differential expression features in T and B cells. Given the fact that LMP-1 does not form aggregates at the T-cell plasma membrane, it is not surprising that it does not interact with TRAFs and induce phenotypic changes as seen in the B cells. Unlike B cells, which grow in tight clumps upon LMP-1 expression, LMP-expressing T cells did not clump together (data not shown). Few investigators have reported the expression of LMP-1 in T cells (22, 40). However, either they expressed LMP-1 transiently or under inducible promoters; in any case, they did not look for the location of LMP-1 in their target cells. It is also noteworthy that LMP-1 is rarely detected in the EBV genome-containing peripheral T-cell lymphomas despite their expression of its mRNA (45). Furthermore, immature human thymocytes and a human thymocytic cell line (HPB-ALL), which can be readily infected in vitro with EBV, fail to express LMP-1 (37, 52).
For importation into the nucleus, macromolecules of >50 kDa must be actively translocated across the nuclear pore complex (reviewed in references 22 and 39). LMP-1, being 63 kDa, cannot passively diffuse from the cytoplasm into the nucleus through the nuclear pore complex. The first step in this nuclear import process is the recognition and binding of importin α (or karyopherin α) to the so-called nuclear localization signal (NLS) of the protein (36). It is known that NLS contributes to shuttle protein from the cytoplasm to the nucleus as has been shown, for example, for the simian virus 40 T antigen, nucleoplasmin, and c-myc (10). The canonical NLS is comprised of one (monopartite) or two (bipartite) clusters of conserved basic amino acids. To determine whether the NLS may play a role in LMP-1 localization in the nuclei, we searched for the presence of canonical mono- and bipartite NLSs (PKKKRKV, KRPAATKKAGQAKKK, and PAAKRVKLD) (10) by using the BCM search launcher. LMP-1 was found to be negative for the presence of such a prototypical NLS when the six frame translation products of its cDNA sequence were searched (data not shown). However, this does not rule out the possibility of the nuclear import of LMP-1 by importin α as the latter protein may interact with a cargo protein in an NLS-independent fashion (41). Furthermore, the consensus sequence for the NLS is now considered to be much broader than has been hitherto recognized (53). Thus, LMP-1 may possess such a noncanonical NLS. In any event, a noncanonical NLS-based nuclear import of LMP-1 in T cells would imply that this NLS may be masked in B cells, probably due to differential posttranslational modifications.
It has been shown that LMP-1 associates with vimentin, which constitutes intermediate filaments of the cytoskeleton and connects the plasma membrane to the nuclear membrane (4, 29). LMP-1 expression causes vimentin to colocalize into patches in the plasma membrane and hence leads to abnormality in the arrangement of the cytoskeleton. The treatment of cells with a vimentin-depolymerizing agent causes vimentin to aggregate as patches in the perinuclear region (6). If these cells are also expressing LMP-1, it colocalizes into the perinuclear region along with vimentin (29). Cytoskeleton-associated proteins (e.g., Vif of human immunodeficiency virus type 1) are known to be nucleophilic as they glide along these filaments into the nucleus (21). Such proteins help transport viral cores to the nuclei of the infected cells. The human T cells used in this study are known to express vimentin, which may be playing a role in the nuclear localization of LMP-1 in these cells.
LMP-1 is expressed both in the viral lytic and latent cycles. It is also incorporated into virions. The protein is essential for in vitro immortalization of B cells and induces phenotypic changes in these cells which are characteristic of their immortalized phenotype. It imparts resistance to apoptosis following serum withdrawal, p53 activation, and the growth inhibitory effects of TGF-β (2, 13). Furthermore, it also inhibits differentiation of epithelial cells (7). Thus, LMP-1 acts as a classical oncoprotein and has been considered to play a role in the development of EBV-associated malignancies. On the other hand, LMP-1 is also cytotoxic and immunogenic (12, 15, 25) and there is no direct proof for its role in the in vivo process of lymphomagenesis. The transgenic mice which express LMP-1 in their B cells under the Ig promoter, however, develop tumors when they are of 12 months of age or older (27). The present data would suggest that LMP-1 may not be contributing to the development of EBV genome-containing T-cell lymphomas. A similar conclusion was drawn earlier based upon the rarity of the detection of the LMP-1 protein in peripheral T-cell lymphomas (38, 45). Unfortunately, we were unable to find or obtain cells of T-cell lymphomas for our present study. In any event, the present data demonstrate novel features of LMP-1 expression which appear to be target cell dependent. Chief among these are (i) noninduction of certain cellular changes that are found associated with LMP-1 expression in B cells and (ii) preferential localization of LMP-1 in the nuclei of T cells.
Acknowledgments
We thank the Canadian Institutes of Health Research (formally the Medical Research Council of Canada) for support.
We thank Nancy Raab-Traub of the University of North Carolina—Chapel Hill for the pIgLMP vector, Elliot Kieff of the Harvard Medical School (Boston, Mass.) for the S12 antibodies, and Sylvie Julien and Micheline Patenaude for secretarial assistance.
REFERENCES
- 1.Ahmad, A., A. Ladha, E. A. Cohen, and J. Menezes. 1993. Stable expression of the transfected HIV-1 env gene in a human B cell line: characterization of gp120-expressing clones and immunobiological studies. Virology 192:447-457. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis, L., N. Yaseen, and S. Sharma. 1995. Latent membrane protein-1 induces cyclin D2 expression, pRb hyperphosphorylation, and loss of TGF-beta 1-mediated growth inhibition in EBV-positive B cells. J. Immunol. 155:1047-1056. [PubMed] [Google Scholar]
- 3.Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. [DOI] [PubMed] [Google Scholar]
- 4.Birkenbach, M., D. Liebowitz, F. Wang, J. Sample, and E. Kieff. 1989. Epstein-Barr virus latent infection membrane protein increases vimentin expression in human B-cell lines. J. Virol. 63:4079-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonagura, V. R., B. Z. Katz, B. C. Edwards, D. J. Valaces, P. Nisen, E. Gloster, R. Mir, and P. Lanzkowsky. 1990. Severe chronic EBV infection associated with specific EBV immunodeficiency and an EBNA+ T-cell lymphoma containing linear, EBV DNA. Clin. Immunol. Immunopathol. 57:32-44. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C.-L., R. H. Sadler, D. M. Walling, I.-J. Su, H.-C. Hsieh, and N. Raab-Traub. 1993. Epstein-Barr virus (EBV) gene expression in EBV-positive peripheral T-cell lymphomas. J. Virol. 67:6303-6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson, C. W., A. B. Rickinson, and L. S. Young. 1990. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature 344:777-780. [DOI] [PubMed] [Google Scholar]
- 8.Delibrias, C. C., E. Fisher, G. Bismuth, and M. Katzatchkine. 1992. Expression, molecular association, and functions of C3 complement receptors CR1 (CD35) and CR2 (CD21) on the human T cell line HPB-ALL. J. Immunol. 149:768-774. [PubMed] [Google Scholar]
- 9.Eliopoulos, A. G., and A. B. Rickinson. 1998. Epstein-Barr virus: LMP1 masquerades as an active receptor. Curr. Biol. 8:R196-R201. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos, A. G., S. M. S. Blake, J. E. Floettmann, M. Rowe, and L. S. Young. 1999. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J. Virol. 73:1023-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson, K. D., and J. M. Martin. 1997. Early detection of the lytic LMP-1 protein in EBV-infected B-cells suggests its presence in the virion. Virology 234:1-13. [DOI] [PubMed] [Google Scholar]
- 12.Floettmann, J. E., K. Ward, A. B. Rickinson, and M. Row. 1996. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 analyzed using tetracycline-regulated expression in B cell lines. Virology 223:29-40. [DOI] [PubMed] [Google Scholar]
- 13.Fries, K. L., W. E. Miller, and N. Raab-Traub. 1996. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J. Virol. 70:8653-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gires, O., F. Kohlhuber, E. Kilger, M. Baumann, A. Kieser, C. Kaiser, R. Zeidler, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammerschmidt, M., B. Sudgen, and V. R. Baichwal. 1989. The transforming domain alone of the latent membrane protein of Epstein-Barr virus is toxic to cells when expressed at high levels. J. Virol. 63:2469-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harabuchi, Y., N. Yamanaka, A. Kataura, S. Imai, T. Kinoshita, F. Mizuno, and F. Osato. 1990. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet 335:128-130. [DOI] [PubMed] [Google Scholar]
- 17.Hatzivassiliou, E., W. E. Miller, N. Raab-Traub, E. Kieff, and G. Mosialos. 1998. A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-kappa B, and stress-activated protein kinase. J. Immunol. 160:1116-1121. [PubMed] [Google Scholar]
- 18.Hudson, G. S., P. J. Farrell, and B. J. Barrell. 1985. Two related but differentially expressed potential membrane proteins encoded by the EcoRI Dhet region of Epstein-Barr virus B95-8. J. Virol. 53:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, J. F., S. Shurin, C. Abramowsky, R. R. Tubbs, C. G. Sciotto, R. Wahl, J. Sands, D. Gottman, B. Z. Katz, and J. Sklar. 1988. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N. Engl. J. Med. 318:733-741. [DOI] [PubMed] [Google Scholar]
- 20.Kalland, K.-H., A. M. Szilvay, K. A. Brokstad, W. Sactrevik, and G. Haukenes. 1994. The human immunodeficiency virus type 1 Rev protein shuttles between the cytoplasm and nuclear compartments. Mol. Cell. Biol. 14:7436-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karczewski, M. D., and K. Strebel. 1996. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein. J. Virol. 70:494-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawanishi, M. 1997. Expression of Epstein-Barr virus latent membrane protein 1 protects Jurkat T cells from apoptosis induced by serum deprivation. Virology 228:244-250. [DOI] [PubMed] [Google Scholar]
- 23.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 90:9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan, G., A. J. Horton, and G. Slavin. 1993. Epstein-Barr virus in angioimmunoblastic T-cell lymphomas. Histopathology 22:145-149. [DOI] [PubMed] [Google Scholar]
- 25.Khanna, R., S. R. Burrows, and D. J. Moss. 1995. Immune regulation in Epstein-Barr virus-associated diseases. Microbiol. Rev. 59:387-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2348-2396. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 27.Kulwichit, W., H. R. Ewards, E. M. Davenport, J. F. Baskar, V. Godfrey, and N. Raab-Traub. 1998. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc. Natl. Acad. Sci USA 95:11963-11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebowitz, D., and E. Kieff. 1989. Epstein-Barr virus latent membrane protein: induction of B-cell activation antigens and membrane patch formation does not require vimentin. J. Virol. 63:4051-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebowitz, D., R. Kopan, E. Fuchs, J. Sample, and E. Kieff. 1987. An Epstein-Barr virus transforming protein associates with vimentin in lymphocytes. Mol. Cell. Biol. 7:2299-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mann, K. P., and D. Thorley-Lawson. 1987. Posttranslational processing of the Epstein-Barr virus-encoded p63/LMP protein. J. Virol. 61:2100-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuire, L. J., D. P. Huang, R. Teoh, M. Arnold, K. Wong, and J. C. Lee. 1988. Epstein-Barr virus genome in thymoma and thymic lymphoid hyperplasia. Am. J. Pathol. 131:385-390. [PMC free article] [PubMed] [Google Scholar]
- 32.Menezes, J., W. Leibold, G. Klein, and G. Clements. 1975. Establishment and characterization of an Epstein-Barr virus (EBV) negative lymphoblastoid B cell line from an exceptional, EBV-genome negative African Burkitts lymphoma. Biomedicine 22:276-284. [PubMed] [Google Scholar]
- 33.Miller, W. E., G. Mosialos, E. Kieff, and N. Raab-Traub. 1997. Epstein-Barr virus LMP1 induction of the epidermal growth factor receptor is mediated through a TRAF signaling pathway distinct from NF-κB activation. J. Virol. 71:586-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moorthy, R. K., and D. A. Thorley-Lawson. 1993. All three domains of the Epstein-Barr virus-encoded latent membrane protein LMP-1 are required for transformation of rat-1 fibroblasts. J. Virol. 67:2637-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosialos, G., M. Birkenbach, R. Yalamanchili, T. Van Arsdale, C. Ware, and E. Kieff. 1995. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 80:389-399. [DOI] [PubMed] [Google Scholar]
- 36.Nigg, E. A. 1997. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature 386:779-787. [DOI] [PubMed] [Google Scholar]
- 37.Paterson, R. L. K., E. W. Kelleher, J. E. Streib, T. D. Amankonah, J. W. Xu, J. F. Jones, and E. W. Gelfand. 1995. Activation of human thymocytes after infection by EBV. J. Immunol. 154:1440-1449. [PubMed] [Google Scholar]
- 38.Paterson, R. L. K., C. Kelleher, T. D. Amankonah, J. E. Streib, J. W. Xu, J. F. Jones, and E. W. Gelfand. 1995. Model of Epstein-Barr virus infection of human thymocytes: expression of viral genome and impact on cellular receptor expression in the T-lymphoblastic cell line, HPB-ALL. Blood 85:456-464. [PubMed] [Google Scholar]
- 39.Pemberton, L. F., G. Blobel, and J. S. Rosenblum. 1998. Transport routes through the nuclear pore complex. Curr. Opin. Cell Biol. 10:392-399. [DOI] [PubMed] [Google Scholar]
- 40.Peng, M., and E. Lundgren. 1993. Transient expression of the Epstein-Barr virus LMP1 gene in B-cell chronic lymphocytic leukemia cells, T cells, and hematopoietic cell lines: cell-type-independent-induction of CD23, CD21, and ICAM-1. Leukemia 7:104-112. [PubMed] [Google Scholar]
- 41.Popov, S., M. Rexach, G. Zybarth, N. Reiling, M. A. Lee, L. Ratner, C. M. Lane, M. S. Moore, G. Blobel, and M. Burkinsky. 1998. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 43.Sinha, S. K., S. C. Todd, J. A. Hedrick, C. L. Speiser, J. D. Lambris, and C. D. Tsoukas. 1993. Characterization of the EBV/C3d receptor on the human Jurkat T cell line: evidence for a novel transcript. J. Immunol. 150:5311-5320. [PubMed] [Google Scholar]
- 44.Suzushima, H., N. Asou, T. Fujimoto, S. Nishimura, T. Okubu, H. Yamasaki, M. Osato, M. Matsuoka, A. Tsukamoto, K. Takai, F. Kawano, and K. Takatsuki. 1995. Lack of the expression of EBNA-2 and LMP-1 in T-cell neoplasms possessing Epstein-Barr virus. Blood 85:480-486. [PubMed] [Google Scholar]
- 45.Suzushima, H., S. Matsushita, R. Nakamura, S. Nishimura, T. Okubo, N. Asou, K. Kawa-Ha, K. Hirai, and K. Takatsuki. 1992. Detection of Epstein-Barr viral DNA in aggressive CD8+ T cell leukaemic cells. Br. J. Haematol. 82:774-776. [DOI] [PubMed] [Google Scholar]
- 46.Tokunaga, M., S. Imai, Y. Uemura, T. Tokudome, T. Osato, and E. Sato. 1993. Epstein-Barr virus in adult T-cell leukemia/lymphoma. Am. J. Pathol. 143:1263-1268. [PMC free article] [PubMed] [Google Scholar]
- 47.Tsoukas, C. D., and J. D. Lambris. 1988. Expression of CR2/EBV receptors on human thymocytes detected by monoclonal antibodies. Eur. J. Immunol. 18:1299-1302. [DOI] [PubMed] [Google Scholar]
- 48.Uchida, J., T. Yasui, Y. Takaoka-Shichijo, M. Muraoka, W. Kulwichit, N. Raab-Traub, and H. Kikutani. 1999. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 286:300-303. [DOI] [PubMed] [Google Scholar]
- 49.Wang, D., D. Liebowitz, and E. Kieff. 1998. The truncated form of the Epstein-Barr virus latent-infection membrane protein expressed in virus replication does not transform rodent fibroblasts. J. Virol. 62:2337-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Leibowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, S., M. Rowe, and E. Lundgren. 1996. Expression of the Epstein Barr virus transforming protein LMP1 causes a rapid and transient stimulation of the Bcl-2 homologue Mcl-1 levels in B-cell lines. Cancer Res. 56:4610-4613. [PubMed] [Google Scholar]
- 52.Watry, D., J. A. Hedrick, S. Siervo, G. Rhodes, J. J. Lamberti, J. D. Lambris, and C. D. Tsoukas. 1991. Infection of human thymocytes by Epstein-Barr virus. J. Exp. Med. 173:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welch, K., J. Franke, M. Kohler, and I. G. Macara. 1999. RanBP3 contains an unusual nuclear localization signal that is imported preferentially by importin-alpha3. Mol. Cell. Biol. 19:8400-8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu, J., A. Ahmad, M. D'Addario, L. Knafo, J. F. Jones, U. Prasad, R. Dolcetti, E. Vaccher, and J. Menezes. 2000. Analysis and significance of anti-latent membrane protein-1 antibodies in the sera of patients with EBV-associated diseases. J. Immunol. 164:2815-2822. [DOI] [PubMed] [Google Scholar]