Abstract
The cellular promyelocytic leukemia protein (PML) associates with the proteins of several viruses and in some cases reduces viral propagation in cell culture. To examine the role of PML in vivo, we compared immune responses and virus loads of PML-deficient and control mice infected with lymphocytic choriomeningitis virus (LCMV) and vesicular stomatitis virus (VSV). PML−/− mice exhibited accelerated primary footpad swelling reactions to very-low-dose LCMV, higher swelling peaks upon high-dose inoculation, and higher viral loads in the early phase of systemic LCMV infection. T-cell-mediated hepatitis and consequent mortality upon infection with a hepatotropic LCMV strain required 10- to 100-times-lower inocula despite normal cytotoxic T-lymphocyte reactivity in PML−/− mice. Furthermore, PML deficiency rendered mice 10 times more susceptible to lethal immunopathology upon intracerebral LCMV inoculation. Accordingly, 10-times-lower VSV inocula elicited specific neutralizing-antibody responses, a replication-based effect not observed with inactivated virus or after immunization with recombinant VSV glycoprotein. These in vivo observations corroborated our results showing more virus production in PML−/− fibroblasts. Thus, PML is a contributor to innate immunity, defining host susceptibility to viral infections and to immunopathology.
The promyelocytic leukemia protein (PML) was first described as the product of a gene that fuses to the retinoic acid receptor α gene in chromosomal translocation t(15;17), resulting in acute promyelocytic leukemia (10, 20, 30).
Structural analysis of the PML protein revealed several domains involved in protein-protein (RING finger, coiled coil domain) or protein-DNA (cysteine-rich B boxes) interactions (30, 39, 59; P. S. Freemont, I. M. Hanson, and J. Trowsdale, Letter, Cell 64:483-484, 1991). In accord with these structural properties, subsequent studies suggested that PML acts as a growth and transformation suppressor (45, 55), cell cycle regulator (28, 42), mediator of apoptosis (69), and regulator of transcription (2, 24, 34, 48, 67, 73), translation (12), and hematopoietic differentiation (68). In agreement with these in vitro studies, PML−/− mice have been shown to be prone to tumorigenesis upon exposure to different carcinogens, although the incidence of spontaneous tumors did not differ from that for controls (68). On the other hand, a murine plasmocytoma expressing a mutated PML molecule suppressed cytotoxic T-lymphocyte (CTL)-dependent rejection, apparently due to loss of expression of molecules involved in major histocompatibility complex (MHC) class I presentation (72).
Immunofluorescence and electron microscopy studies have revealed a dynamic distribution of PML: in normal fibroblasts PML has a speckled nuclear distribution, and it is associated with multiprotein complexes described as nuclear bodies, nuclear domain 10, Kr bodies, or PML oncogenic domains (26). To a lesser extent, PML bodies are also present in the cytoplasm of normal cells and are dynamically exchanged between the nucleus and cytoplasm (64). In acute promyelocytic leukemia (19, 39, 43) as well as after several viral infections in vitro (herpes simplex virus type 1, human cytalomegalovirus, adenovirus type 5, human immunodeficiency virus, and lymphocytic choriomeningitis virus [LCMV]) (9, 23, 29, 41, 66), this normal distribution is changed, resulting in a diffuse nuclear or cytoplasmic distribution of PML. By means of immunofluorescence and coimmunoprecipitation, PML has been shown to colocalize with the Z proteins of the Arenaviridae, LCMV and Lassa fever virus, offering a possible explanation for the coincidence of LCMV infection and PML redistribution (9). Other viruses such as Epstein-Barr virus or simian virus 40 also exhibited direct interaction with PML within intact nuclear bodies (38, 65). To complement these findings, PML overexpression induced partial resistance to influenza virus and vesicular stomatitis virus (VSV) in vitro: 100-fold-less virus production along with reduced mRNA and protein synthesis (15) indicated a strong contribution of PML to cellular antiviral defense strategies. A less dramatic effect was also observed when production of LCMV was analyzed in cultures of PML−/− and PML+/+ primary embryonic fibroblasts (21). Taken together with the interferon (IFN) inducibility of PML (14, 21, 44), these in vitro observations suggest a participation in IFN-mediated antiviral effects. In cell culture, PML expression also potentiates IFN-γ effects on viral transcription (21), but the impact of PML on the course and outcome of viral infection in vivo still remains elusive. Neither has a potential influence of PML on immune responses, either directly or by modulating the virus-host balance, been investigated so far.
We have therefore analyzed virus-specific cellular and humoral immune responses in mice deficient in PML protein (PML−/− mice) by using a cytopathic virus, VSV, and a noncytopathic virus, LCMV. We used different strains of LCMV due to different multiplication capacities (49, 52) and another strain in particular for its ability to cause liver disease (75). The acute phase of a primary LCMV infection, lasting approximately 2 to 3 weeks, is immunologically almost exclusively controlled by CTLs (11, 74). Intracerebral (i.c.) inoculation leads to development of lethal choriomeningitis in immunocompetent mice but not in T-cell-deficient nude mice (16, 22, 36, 46). This well-characterized CTL-mediated immunopathology offers a good model in which to assess the beneficial or detrimental effects of host resistance. In contrast, VSV, a highly cytopathogenic RNA virus, was used to study the effects of PML on B-cell responses. Intravenous (i.v.) infection of mice leads to disease only if very limited replication secondarily spreads to the central nervous system, a process that can be prevented only by an early neutralizing antibody response (6). Immunoglobulin M (IgM) levels up to day 4 after infection are T-cell independent, while a subsequent switch to IgG is strictly dependent on CD4+ T helper cells (4). Our results demonstrate an inhibitory effect of PML on virus infections in vivo and in vitro; PML reduces the extent of infection at an early stage, thereby indirectly downregulating subsequent specific immune responses to low-dose viral exposure and reducing immunopathological disease upon intermediate- to high-dose infections.
MATERIALS AND METHODS
Mice.
PML-deficient mice were generated on a 129Sv background as described previously (68). Age- and sex-matched 129Sv control mice were purchased from the Institute for Laboratory Animals (Veterinary Hospital, Zurich, Switzerland). Experiments were performed in a conventional mouse house facility, and mice were used at the ages of 6 to 15 weeks. After i.c. infection with LCMV (see Fig. 4), mice were assessed for clinical symptoms of choriomeningitis every 12 h. Animals exhibiting clear signs of disease (usually after day 5) (half-closed eyes, seizures, and coarse tremors of head and extremities after they were suspended by the tail) were euthanatized in accordance with Swiss laws for animal protection. Mice from the experiment for which results are shown in Fig. 3 were assessed every 24 h for signs of terminal liver disease, apparent between day 7 to 10 by ruffled fur, serious exsiccosis with enophthalmus, and a precomatose state. Only upon appearance of these severe signs of terminal disease were the mice sacrificed as required by local animal protection laws.
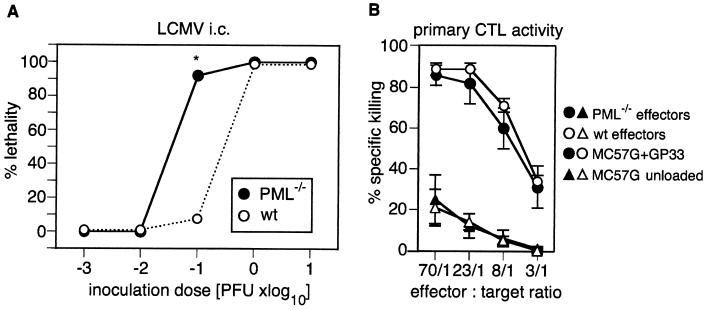
FIG. 4.
Susceptibilities of PML−/− and control mice to lethal immunopathology after i.c. inoculation with different doses of LCMV. (A) PML−/− (•) and control (wild-type [wt]) (○) mice were infected i.c. with different doses of LCMV-ARM in a volume of 30 μl of balanced salt solution on day 0. During an observation period of 34 days, lethal choriomeningitis was eventually observed between days 6 and 9. Percent lethality was calculated as the number of mice developing choriomeningitis per total number of mice tested. Results for 4 (10−3 or 10−2 PFU) or 13 (10−1, 1, or 10 PFU) mice per group from one or four pooled experiments, respectively, are shown. PFU up to 10 PFU were determined by a standard plaque assay; lower doses were calculated from serial dilutions of the minimal clearly measurable dose. (B) PML−/− (•, ▴) and control (○, ▵) mice were infected i.v. with 200 PFU of LCMV-WE on day 0; mice were sacrificed on day 8, and splenocytes were tested by a primary in vitro cytotoxicity assay as described in Materials and Methods. Specific cytotoxic activity against GP33-labeled (•, ○) or unlabeled (▴, ▵) MC57G target cells was measured as described in Materials and Methods. Each symbol represents a group of three mice. One representative experiment out of two performed is shown. Spontaneous release was always below 20%. Values are means ± standard deviations. ∗, P < 0.001.
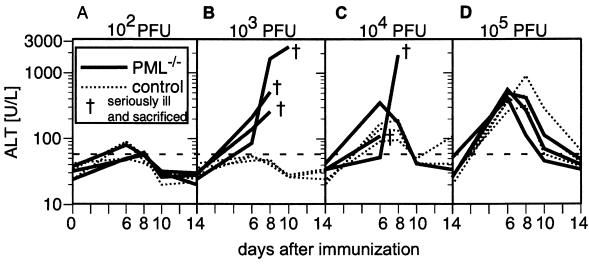
FIG. 3.
Susceptibility of PML−/− mice to cytotoxic T-cell-mediated LCMV-induced hepatitis. PML−/− mice (solid lines) or 129Sv control mice (dotted lines) were infected i.v. on day 0 with 102 (A), 103 (B), 104 (C), or 105 (D) PFU of LCMV-WE. On days 0, 6, 8, 10, and 14, blood samples were taken and ALT levels in the serum were determined as described in Materials and Methods. Seriously ill mice were sacrificed at the indicated time points (†). The horizontal dashed line indicates the threshold for pathological values. Each line represents an individual mouse.
Viruses, recombinant viral antigens, and virus detection.
VSV Indiana (VSV-IND; Mudd-Summers isolate) was originally obtained from D. Kolakovsky (University of Geneva, Geneva, Switzerland). For virus stock production, BHK21 cells were infected at a multiplicity of infection (MOI) of 0.01. After 2 h of incubation at room temperature, the initial inoculum was discarded and replaced by fresh medium. The virus was harvested after 22 h of incubation at 37°C from the second supernatant. For UV inactivation, a small volume of a high-titer VSV preparation was exposed as a thin layer in a petri dish to a UV lamp (15 W; Philips) for 3 min at a distance of 8 cm (3). The recombinant baculovirus expressing the glycoprotein of VSV (VSV-G) was a gift from D. H. L. Bishop, NERC Institute of Virology (Oxford, United Kingdom). It was derived from nuclear polyhedrosis virus and was grown at 28°C in Spodoptera frugiperda cells in Spinner cultures (50). VSV titers were analyzed by a plaque-forming assay. Tenfold serial dilutions of equal volumes of culture supernatants were incubated on Vero cell monolayers in 24-well plates for 3 h at 37°C under a 5% CO2 atmosphere. The monolayers were overlaid with 100 μl of Dulbecco's modified Eagle medium containing 1% methylcellulose and were incubated for an additional 21 h at 37°C. The overlay was flicked off, monolayers were fixed, and plaques were visualized by staining with a 0.5% crystal violet solution. Virus titers were calculated from the highest dilution at which plaques were clearly distinguishable.
LCMV strain WE (LCMV-WE) was obtained from F. Lehmann-Grube (Heinrich Pette Institute, Hamburg, Germany), and LCMV strain Armstrong (LCMV-ARM) was originally provided by M. B. A. Oldstone (The Scripps Research Institute, La Jolla, Calif.) (25). The LCMV Docile (LCMV-DOC) variant isolated from an LCMV-WE (UBC) carrier mouse was obtained from C. Pfau (Troy, N. Y.) (58). Second-passage virus derived from plaque-purified isolates was propagated on either BHK cells (LCMV-ARM), L929 fibroblasts (LCMV-WE), or MDCK cells (LCMV-DOC). Infections for virus stock production were performed at an MOI of 0.01 for 48 h. The viruses, culture supernatants, and infectious viruses in mouse spleens were titrated on MC57G cell monolayer cultures, and titers were evaluated by an immunological focus-forming assay (8). Viral titers are expressed as PFU per gram of spleen or milliliter of cell culture supernatant.
Determination of serum ALT enzyme concentrations.
Assays for concentrations of alanine aminotransferase (ALT) in plasma were carried out in the Department of Clinical Chemistry at the University Hospital Zurich, by using photometric assays on a Hitachi 747 autoanalyzer.
Assessment of the primary footpad swelling reaction.
The indicated amounts of LCMV-ARM or LCMV-DOC were injected in a volume of 30 μl of balanced salt solution into both hind footpads. The footpad thickness was measured with a spring-loaded caliper (Kroeplein, Schluchtern, Hesse, Germany) (54). The mean thickness of both hind footpads of an individual mouse at a particular time point was taken for further evaluation.
CTL assay and peptides.
Virus-specific cytotoxic T cells were assayed as described previously (77). Briefly, single-cell suspensions were prepared from the spleens of mice at day 8 after LCMV infection and were used directly in a primary in vitro 51Cr release assay. Target cells were MC57G cells coated with GP33 (10−6 M) and uncoated control cells. The immunodominant H-2Db binding LCMV peptide gp 33-41 (GP33) was purchased from Neosystem Laboratoire (Strasbourg, France).
Antibody detection.
VSV neutralizing antibodies were detected by a neutralization assay as described previously (5). IgG was determined by its resistance to reduction with 2-mercaptoethanol. The difference between total immunoglobulin and IgG represents IgM. Total immunoglobulin titers of two or more titer steps above IgG were considered IgM.
Generation of mouse primary embryonic fibroblasts.
Embryos from PML−/− or control mice at 14 days of gestation were digested with a trypsin-EDTA solution at 37°C for 30 min. Fibroblasts in suspension were separated by a centrifugation step and were grown in 150-cm2 tissue culture flasks at 37°C under a 5% CO2 atmosphere.
Statistical analysis.
Differences between groups in the time of onset of the footpad swelling reaction (see Fig. 1A) and differences between virus titers in the spleens of PML−/− and 129Sv control mice (see Fig. 2) were determined by the nonparametric Mann-Whitney test. Differences between groups in the extent of footpad swelling over the entire time span of an experiment were assessed by repeated-measures analysis of variance using one or two factors (mouse genotype [Fig. 1A] or mouse genotype and experiment [Fig. 1A through C). Figure 1A, B, and C show data from only one representative experiment, while four (panels A and C) and two (panel B) similar sets of experiments were used for statistical analysis, respectively. For analysis of in vitro differences in virus production (Table 1), the respective virus yields obtained from PML−/− mouse embryonic fibroblast (MEF) supernatants (P1) were compared at six different MOIs (for VSV, 5 × 10−3 to 5; for LCMV, 5 × 10−5 to 5 × 10−2) with control supernatants from PML+/+ MEFs (P0), matched for the dose and virus. For each pair of data (P0 and P1), the ratio (P1 − P0)/P0 was calculated and tested for values greater than zero by using the nonparametric signed-rank Wilcoxon test. For evaluation of LCMV plaque size, 50 randomly chosen plaques per group were measured and analyzed in a two-sided Student t test. Percentages of lethality occurring in PML−/− and PML+/+ mice in the experiment for which results are shown in Fig. 4 were compared by using the Fisher exact test.
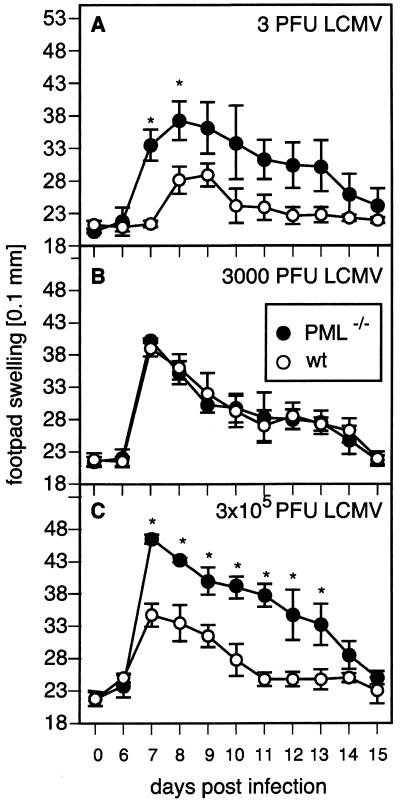
FIG. 1.
Accelerated or higher primary footpad swelling reactions in PML−/− mice. PML−/− (•) and control (wild-type [wt]) (○) mice were immunized on day 0 with 3 PFU (A), 3 × 103 PFU (B), or 3 × 105 PFU (C) of LCMV-DOC. Footpad swelling was monitored as described in Materials and Methods. Each symbol represents a group of three to four mice. One experiment representative of two to four is shown for each respective dose. Values are means for three to four mice per group ± standard deviations. Asterisks indicate a P value of <0.001 (C) or <0.005 (A).
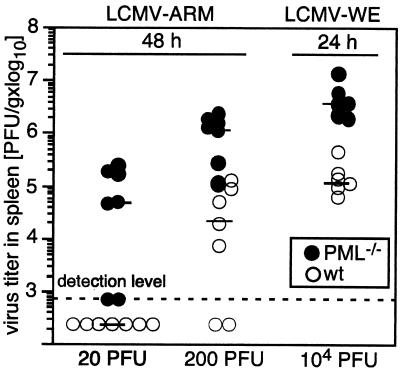
FIG. 2.
Increased LCMV titers in PML−/− mice during the early infection phase. PML−/− (•) and 129Sv control (wild-type [wt]) (○) mice were infected i.v. with 20 or 200 PFU of LCMV-ARM or with 104 PFU of LCMV-WE. Forty-eight or 24 h later, mice were sacrificed, and infectious LCMV titers were determined in their spleens as described in Materials and Methods. Each symbol represents the viral titer per gram of spleen of one individual mouse. Each horizontal bar indicates the median for the respective group and dose as indicated. Pooled data from two independent experiments for each dose are shown.
TABLE 1.
Enhanced virus production in the absence of PML in vitro
| MEF genotypea | VSV
|
LCMV-WE
|
||
|---|---|---|---|---|
| MOI | Virus productionb (PFU/ml) | MOI | Virus productionb (PFU/ml) | |
| PML−/− | 5 | 6.5 × 106 | 5 × 10−2 | 8 × 104 |
| PML+/+ | 5 | 2.5 × 106 | 5 × 10−2 | 2 × 104 |
| PML−/− | 0.5 | 2.3 × 107 | 5 × 10−3 | 3.6 × 106, 1.6 × 106, 4.5 × 106 |
| PML+/+ | 0.5 | 2.4 × 106 | 5 × 10−3 | 4 × 105, 5 × 105, 5.4 × 105 |
| PML−/− | 5 × 10−2 | 6.5 × 106 | 1 × 10−3 | 2 × 107 |
| PML+/+ | 5 × 10−2 | 1.2 × 106 | 1 × 10−3 | 9.5 × 106 |
| PML−/− | 1 × 10−2 | 4.5 × 106, 4.7 × 106 | 5 × 10−4 | 1.5 × 106 |
| PML+/+ | 1 × 10−2 | 7 × 105, 7.1 × 105 | 5 × 10−4 | 4 × 105 |
| PML−/− | 5 × 10−3 | 8 × 105 | 5 × 10−5 | 4.4 × 105 |
| PML+/+ | 5 × 10−3 | 4.2 × 105 | 5 × 10−5 | 4 × 104 |
A total of 5 × 105 PML−/− or PML+/+ control MEFs for each experimental condition were inoculated with 2.5 × 106, 2.5 × 105, 2.5 × 104, 5 × 103, or 2.5 × 103 PFU of VSV or with 2.5 × 104, 2.5 × 103, 5 × 102, 250, or 25 PFU of LCMV-WE corresponding to the indicated MOIs.
Each value represents viral titers determined after 24 (VSV) or 48 (LCMV) h by transferring pooled supernatants of eight individual cultures onto Vero (VSV) or MC57G (LCMV) cell monolayers.
RESULTS
Accelerated and increased primary footpad swelling reaction in PML−/− mice.
We used the well-established primary footpad swelling reaction after subcutaneous intrafootpad (i.f.) inoculation of LCMV as an overall readout for virus-specific CTL and T helper cell responses in PML-deficient mice (54). Low-dose i.f. infection requires local propagation before spreading to secondary lymphoid organs leads to induction of LCMV-specific CTL and T helper cells. Activated T cells then migrate to the infected footpad and mediate tissue destruction and swelling of the footpad. Higher doses directly drained via lymphatic vessels result in immediate T-cell induction and earlier swelling reactions. Therefore, monitoring of the swelling reaction during a period of 15 days recapitulates the ongoing in vivo CTL and T helper response. In this system, the correlation of maximal virus replication and the swelling reaction of the footpad gives further insight into the extent of the local infection (54). The use of a large dose range of LCMV-DOC, a virus strain exhibiting rapid propagation and distribution kinetics, allowed the analysis of a wide spectrum of virus loads generated (49, 51). Both PML−/− and PML+/+ mice were infected i.f. with 3 PFU (Fig. 1A), 3,000 PFU (Fig. 1B), or 3 × 105 PFU (Fig. 1C), and the footpad swelling reaction was monitored. After low-dose infection, control mice developed a response peaking at day 9 after inoculation whereas PML−/− mice reached the highest swelling level around days 7 to 8, significantly earlier than controls (P < 0.001). On the other hand, infection with 3 × 105 PFU of LCMV-DOC evoked a swelling reaction of similar kinetics but greater extent (P < 0.0001) in PML−/− mice (Fig. 1C). Interestingly, infection with 3,000 PFU elicited swelling reactions that were not significantly different in PML−/− and PML+/+ mice (P = 0.462). These results suggested that during the course of a low-dose infection, virus loads necessary for induction of specific cellular immune responses are generated later, when PML protein is present (Fig. 1A). Intermediate doses did not reveal a phenotype of PML-deficient mice (Fig. 1B; see also Discussion); the observation of less tissue destruction by virus-specific CTLs after high-dose infection of PML+/+ mice was most likely due to less-widespread infection and less subsequent immunopathology (Fig. 1C). Similar observations were also made with LCMV-ARM and LCMV-WE.
Increased virus titers in organs of PML−/− mice early after LCMV-infection.
To determine whether virus amplification was accelerated in vivo in the absence of PML, we analyzed early viral titers in the spleen, an organ that parallels virus replication in most major organs (47). PML−/− or control mice were infected i.v. with low doses of LCMV-ARM (20 or 200 PFU) or an intermediate dose of LCMV-WE (104 PFU), and viral titers were measured in the spleen as described in Materials and Methods. After 48 h, when virus was first detectable (Fig. 2, LCMV-ARM), or after 24 h, when amplification of the higher viral inoculum could first be detected (Fig. 2, LCMV-WE, and data not shown), mice were sacrificed and viral titers were determined. Five of seven PML−/− mice infected with 20 PFU of LCMV-ARM produced detectable amounts of virus within 48 h after infection, while no virus was found in the spleens of similarly infected control mice (Fig. 2). When mice were infected with 200 PFU of LCMV-ARM, only five of seven control mice exhibited detectable viral titers (Fig. 2). All PML-deficient mice had titers clearly above the detection level, with a median value more than 50 times higher than that of the control group (Fig. 2, center). Similarly, when mice were infected i.v. with the hepatotropic LCMV-WE at 104 PFU, 10- to 50-times-higher viral titers were found in the spleens of PML−/− mice 24 h after infection (Fig. 2). In all these experiments, viral titers in PML-deficient mice were significantly higher than those in control mice (P = 0.026 for LCMV-ARM at 20 PFU; P < 0.001 for LCMV-ARM at 200 PFU and LCMV-WE at 104 PFU).
Along with the findings of accelerated or more-extensive primary footpad swelling reactions in PML-deficient mice, these results confirmed that PML either suppresses or slows down virus multiplication, depending on the infectious dose used.
PML-deficient mice are more susceptible to cytotoxic T-cell-mediated hepatitis in response to LCMV-WE infection.
Cytotoxic CD8+ T-cell-mediated hepatitis in mice caused by infection with the hepatotropic LCMV-WE (35, 37, 46, 71) correlates with elevated levels of ALT in the serum (75). To determine whether PML influenced this immunopathologic disease, which depends on critical parameters of virus-host balance such as infectious dose and immune competence of the host (75), PML−/− and control mice were infected i.v. with LCMV-WE in 10-fold dilution steps ranging from 102 to 105 PFU. Serum samples were taken at the indicated time points and analyzed for ALT activity as described in Materials and Methods. Mice exhibiting signs of severe disease were sacrificed. Infection with 102 PFU of LCMV-WE i.v. resulted only in a moderate, temporary increase in ALT levels in either genotype (Fig. 3A). Exposure to 103 (Fig. 3B) or 104 (Fig. 3C) PFU of LCMV-WE i.v. caused terminal immunopathology-induced hepatitis: five of six PML−/− mice displayed extreme ALT levels, while one mouse had to be sacrificed in the period between two measurements. Sera from control mice exhibited background ALT activity after infection with 103 PFU (Fig. 3B) and only moderately increased levels after infection with 104 PFU (Fig. 3C). However, at 105 PFU, both PML−/− and control mice exhibited similar, typical ALT time courses peaking around days 6 to 8 after infection (75) and decreasing thereafter to normal levels around days 10 to 14. The threshold for relevant liver tissue destruction as determined by ALT levels was 10- to 100-fold lower in the absence of PML. This was apparently due to more-extensive viral propagation (compare Fig. 2, right panel) and consequent CTL-mediated lysis of a higher number of infected hepatocytes. At the highest dose of infection, the two experimental groups exhibited similar kinetics of liver pathology, and both survived. At first sight this result appears contradictory, since PML−/− mice died from lower infectious doses, but this outcome may readily be explained by the different levels of balance between LCMV and the host's immune system (53) (see Discussion). In control mice infected with high doses (104 to 105 PFU), ALT levels went up but were resolved without any lethality. This difference in the outcome of infection was not due to differences in T-cell responsiveness between PML−/− and control mice, as will be shown (see Fig. 4B). All mice surviving the observation period of 15 days had cleared the virus (data not shown).
PML-deficient mice are more susceptible to lethal CTL-mediated immunopathology after i.c. inoculation with LCMV.
The role of PML in virus-host relationships was further tested with the well-established model of i.c. infection with LCMV, which causes a CTL-mediated choriomeningitis (16). PML−/− and control mice were infected i.c. with LCMV-ARM in 10-fold dilution steps ranging from 10−3 to 10 PFU. Mice were monitored twice a day for signs of disease until day 34 after infection. Clinical symptoms of choriomeningitis were observed between days 6 and 9, and the respective mice were sacrificed. With the lowest doses of 10−3 and 10−2 PFU, all mice survived regardless of genotype, while all mice receiving a dose of 1 or 10 PFU eventually died. Inoculation with 10−1 PFU of LCMV-ARM led to lethal choriomeningitis in 12 of 13 PML−/− mice but only in 1 of 13 control mice (P < 0.001) in a total of four separate experiments (Fig. 4A). Comparable observations were made with i.c. LCMV-WE infection (data not shown).
Since a productive i.c. infection leads to a specific CTL response and lethal immunopathology, these data indicated that the in vivo threshold for a productive infection with LCMV was decreased by a factor of about 10 in the absence of PML. The lethal i.c. inoculum in PML+/+ mice, 1 PFU, was identical to the minimal unit of virus needed to infect the MC57G cell monolayers (PML+/+) used to quantify virus stocks (see Materials and Methods). This indicates that 1 PFU contains at least 10 potentially infectious viral particles, although this is not detectable in PML-competent animals or tissue cultures. A direct effect of PML on CTL responsiveness as the underlying reason for the above results seemed unlikely, because PML−/− and control mice generated comparable CTL responses on day 8 after i.v. infection with 200 PFU of LCMV-WE (Fig. 4B) or LCMV-ARM (data not shown).
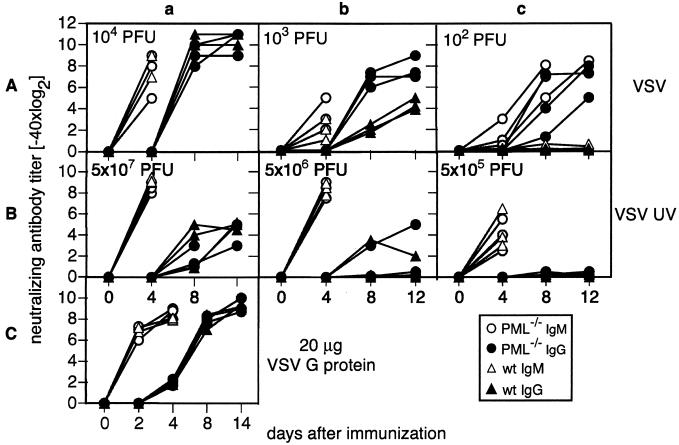
Enhanced antibody responses to low doses of VSV in PML−/− mice are due to higher viral replication rather than to enhanced responsiveness of PML−/− mice.
The influence of PML on virus-specific humoral immune responses was assessed in the well-established murine VSV infection model (6). We infected PML−/− mice and 129Sv control mice i.v. with different doses of replicating VSV-IND (Fig. 5A). At the indicated time points, serum samples were collected, and neutralizing-antibody titers were determined as described in Materials and Methods. After infection with 104 PFU of VSV, PML−/− and control mice generated comparable neutralizing IgM and IgG titers (Fig. 5Aa). Infection with 103 PFU led to comparable IgM responses independent of the mouse genotype, but IgG titers were increased in PML−/− mice compared to control mice (Fig. 5Ab). After inoculation with 102 PFU of VSV, specific neutralizing IgM and IgG could be detected only in PML−/− mice, while control mice remained unresponsive (Fig. 5Ac). This effect could have been either the consequence of a higher humoral immune responsiveness of PML−/− mice or the result of higher antigen levels due to better virus multiplication in PML−/− mice. Due to very poor extraneuronal replication in the murine host, VSV stays below detectable levels under the experimental conditions used here (i.v. infection with 102 to 104 PFU). Infectious VSV can be measured only for the first 24 to 48 h upon inoculation of 2 × 106 or more PFU i.v. and does not allow discrimination between the initial inoculum and progeny virus particles (A. F. Ochsenbein, unpublished data). Accordingly, we had to use the intensity of the immune response as a sensitive but only indirect readout of viral antigen loads to differentiate between those two possible mechanisms for the above observations: PML−/− and control mice were immunized with different doses of UV-inactivated and therefore nonreplicating VSV. Independent of the mouse genotype, 5 × 107 PFU equivalents of UV-inactivated VSV led to equal neutralizing IgM and IgG titers (Fig. 5Ba). An intermediate dose (5 × 106 PFU of UV-inactivated VSV [Fig. 5Bb]) still evoked comparably high IgM levels in all mice, while only one of three mice in every group exhibited a T-cell-dependent isotype switch to IgG. With 5 × 105 PFU equivalents, only T-cell-independent IgM could be detected in PML-competent and -deficient mice (Fig. 5Bc). To additionally quantify antibody responses to inert antigens, we immunized PML−/− and control mice with 20 μg of recombinant VSV-G (Fig. 5C). Serum samples were collected at the indicated time points, and VSV-specific neutralizing IgM and IgG responses were determined. PML−/− and PML+/+ mice exhibited identical VSV neutralizing-antibody responses after immunization with VSV-G (Fig. 5C), corroborating that B cells and CD4+ T helper cells respond equally well in PML−/− and control mice (Fig. 5Ba through c and 5C). Therefore, the higher IgM and IgG levels of PML−/− mice are very likely the result of better virus propagation and not the result of differences in immune responsiveness (Fig. 5Ab and c).
FIG. 5.
VSV neutralizing-antibody responses of PML−/− or 129Sv control mice. PML−/− (circles) and control (wild-type [wt]) (triangles) mice were inoculated i.v. on day 0 with either live VSV (A) at 104 (a), 103 (b), or 100 (c) PFU, UV-inactivated VSV (B) at 5 × 107 (a), 5 × 106 (b), or 5 × 105 (c) PFU equivalents, or VSV-G (20 μg) (C). Serum samples were taken at the indicated time points, and neutralizing IgM (open symbols) and IgG (solid symbols) titers were determined by a neutralization assay. Each symbol represents an individual mouse. Results from one representative experiment of two are shown.
Replication of VSV and LCMV in PML−/− MEFs.
The above experiments indicated enhanced propagation of VSV and LCMV in PML−/− mice. We therefore investigated PML-dependent effects on virus production in vitro. MEFs were generated from PML−/− and control mice as described in Materials and Methods. The two cell lines exhibited comparable cell sizes and cell cycle kinetics (data not shown). A total of 5 × 105 MEFs of either genotype per well were seeded and grown overnight in 24-well tissue culture plates. Thereafter, VSV infection was performed by using decreasing MOIs ranging from 5 to 5 × 10−3. Twenty-four hours later, the supernatants of eight individual cultures infected at the same MOI were collected and pooled; VSV production was determined by a standard VSV plaque assay as described in Materials and Methods. Virus yields obtained from supernatants of PML−/− MEFs infected at an MOI of 5 to 5 × 10−3 exceeded those from controls by a factor of 1.9 to 9 (P = 0.014) (Table 1). A similar experimental setup was used to establish LCMV production in vitro: 5 × 105 MEFs per well of the PML−/− or control genotype were seeded in 24-well plates, infected simultaneously with LCMV-WE at MOIs ranging from 5 × 10−2 to 5 × 10−5, and cultured for 48 h. Amounts of infectious viral progeny were determined in the culture supernatants by using a standard LCMV plaque assay (see Materials and Methods). At MOIs from 5 × 10−2 to 5 × 10−5, PML−/− fibroblasts produced 2 to >10 times more virus than control fibroblasts (P = 0.014) (Table 1). To further address PML effects on LCMV replication in tissue culture, we analyzed infectious focus formation in PML−/− and control MEFs by plating 2.8 × 105 MEFs simultaneously with LCMV-WE or LCMV-ARM (MOI = 5 × 10−4). Four to five hours later, we supplemented the cultures with an overlay of methylcellulose and medium. At 48 h after inoculation, infected cells were visualized by an intracellular LCMV-specific stain. The diameter of plaques formed by LCMV-WE on PML−/− fibroblasts was 802 ± 120 μm, and that on PML+/+ fibroblasts was 448 ± 90 μm. With LCMV-ARM we measured 732 ± 170 μm (PML−/−) and 374 ± 90 μm (PML+/+) for the respective plaque diameters. Therefore, plaques formed on PML−/− fibroblasts had approximately twice the diameter of plaques in control cells (P < 0.0001 for LCMV-WE; P < 0.0001 for LCMV-ARM) (data correspond to one experiment representative of two).
DISCUSSION
In cell culture, PML expression has negative effects on the propagation of certain RNA viruses (VSV, influenza virus, LCMV) (15, 21; also this report); however, it was not clear that these effects could impact viral disease. Our results show distinct antiviral effects of PML in vivo. Inoculations with limiting doses of VSV i.v. and LCMV i.c. suggest that PML can, under certain conditions, suppress productive viral infection in vivo to below the minimal levels required for induction of a specific immune response (Fig. 4A and 5Ac). This was observed for control animals where lethal immunopathology was prevented (Fig. 4A) or a specific immune response was absent (Fig. 5Ac). Ten- to 100-fold-lower doses of hepatotropic LCMV-WE administered i.v. caused terminal T-cell-mediated immunopathology in PML-deficient animals (Fig. 3), and infection with high-dose LCMV-DOC resulted in stronger T-cell-mediated swelling of the footpad (Fig. 1C). These observations, together with larger LCMV plaque sizes in PML−/− fibroblasts, increased virus production, and a lower minimal MOI required for infection in PML−/− cell culture, suggest a mechanism of higher viral burden with secondary effects on antigen-driven immune responses.
The virus dose range within which in vivo effects of PML could be observed varies with the experimental setup. LCMV footpad inoculation revealed a clear PML-dependent phenotype at low and very high virus doses; VSV immunization did so at low doses. In PML-deficient animals, LCMV-WE-mediated terminal immunopathologic hepatitis was observed at 103 to 104 PFU given i.v.—a dose controlled by normal mice without serious signs of clinical disease. Higher and lower doses of virus elicited a course of infection similar to that in wild-type mice (Fig. 3). Different explanations that are not mutually exclusive may apply. We favor the idea that the specific immune system has evolved to fight intermediate doses of pathogens, with low doses being partially (Fig. 1A) or completely (Fig. 5Ac) controlled by innate defenses (56) such as the PML effects described here and with high doses exhausting the adaptive immune response capacity, thereby eliminating immunopathology. Intermediate doses of a pathogen elicit adaptive B- and T-cell responses that remain constant in intensity over a broad range of doses (54, 75); thus, moderate changes in antigen load within the intermediate range will not affect the outcome (see, e.g., Fig. 1B and 3A). Only when the pathogen dose reaches a critical upper threshold, i.e., when adaptive immunity cannot control the infection without considerable pathogen- or immune-mediated harm to the host, do even moderate differences in pathogen load (including differences due to PML expression) become critical (Fig. 1C and 3B and C). Lethality upon LCMV-WE challenge is due to an immunopathology that is influenced by H-2 haplotype (52, 75), specific T-cell precursor frequency (27, 57), or viral determinants such as tropism, mutations (60), and virus dissemination (58). The outcome of infection is determined by a continuous spectrum of the host response, from virus clearance (where the host dominates) to life-threatening immunopathology (where the virus and the host immune response are in balance) to the extreme of high-zone immune tolerance or T-cell exhaustion, in which noncytopathic viruses can dominate, persist, and exhaust the T-cell response (52, 53, 76). Thus, intermediate doses of LCMV-WE (where the virus is in balance with the PML-deficient immune system) evoked terminal hepatitis in PML−/− mice, while their littermates receiving the highest inoculum (where the virus dominates) survived despite transiently elevated ALT levels. Conversely, a switch to noncytolytic effector mechanisms, such as the IFN-γ or tumor necrosis factor alpha mechanisms used to control hepatitis B virus (HBV) infection (31-33), could have accounted for the survival of the latter group of mice.
Do these two extremes in the dose of pathogen challenge reflect a relevant natural scenario? The low-dose infection represents the most likely and most frequent situation in nature (17, 18, 40, 70). High-dose infection with viruses is probably very rare in nature but can occur during transplacental transmission, neonatal infections during birth, blood transfusions, or organ transplants, or occasionally upon encounter with arthropod-borne diseases. Further, our experiments with high-dose LCMV given i.f. and particularly with intermediate doses of LCMV-WE given i.v. may imitate infections with noncytolytic viruses such as HBV, HCV, and possibly human immunodeficiency virus in humans, not primarily with respect to the initial inoculum (except for HCV when it is acquired by transfusion) but with regard to the number of infected cells critically determining immunopathology.
A study in which recurrent model tumors emerged as a consequence of a dominant-negative mutation of PML suggested that PML might be required for expression of functional MHC class I-peptide complexes (see the introduction) (72). Our data indicate that MHC class I and II expression and peptide processing as monitored by the resulting T-cell response and immunoglobulin class switch in vivo and in vitro, as well as class I and II levels assessed by fluorescence-activated cell sorter analysis, were normal when disruption of exon 2 (this study) was used to completely eliminate PML expression (68).
As an IFN-inducible gene, PML could mediate antiviral effects through a variety of intracellular pathways: PML is constitutively expressed, unlike other mediators of antiviral IFN activity such as Mx proteins, P1/eIF-2 protein kinase, and 2′,5′-oligoadenylate synthetase (1, 61, 62). This would allow for immediate antiviral activity of this molecule, an effect which could be potentiated upon upregulation through IFN. Alternatively, PML could indirectly participate in the still poorly defined IFN-mediated upregulation of known or yet unknown IFN-regulated proteins with antiviral activity. Future studies with extensive and detailed molecular work will be needed to dissect this large array of potentially redundant and parallel pathways. (Since the mouse strain used in the present experiments is Mx deficient [63] and VSV susceptibility to 2′,5′-oligoadenylate synthetase activity seems negligible [13], these mediators of antiviral IFN activity are probably not of great importance.) A mechanism by which PML could reduce virus multiplication has been suggested by previous work with LCMV. Arenaviruses have a small zinc-binding protein (Z) that binds the N-terminal region of PML and can alter the distribution of PML bodies in cultured cells (9). It is likely that PML interferes with the essential functions of the viral Z protein. Although cellular nuclear factors are essential for arenavirus replication (7), PML is a nuclear factor that works against virus multiplication. Since PML-deficient animals and tissue cultures are 10-fold more susceptible to VSV and LCMV, and probably to other viruses, PML-deficient systems can offer a very sensitive readout for viral infection in experimental or diagnostic work. In addition, antiviral therapies that enhance PML activities could conceivably be developed.
The present study demonstrates that PML acts as a cellular mediator of antiviral activity by raising the minimal viral dose needed for infection and damping viral propagation. These effects in turn influence the specific immune response, which is either completely absent or slowed down, leading to diminished and delayed immunopathology at the site of viral infection. Therefore, individual differences in expression of PML and other factors with similar effects may account for different susceptibilities and outcomes for viral diseases within an outbred population.
Acknowledgments
This work was supported by the Swiss National Science Foundation, the Canton of Zurich, and the Wellcome Trust, United Kingdom.
We thank Juan Carlos de la Torre, Doron Merkler, Gennadii Bocharov, Karin Senn, and Kathy McCoy for helpful discussions.
REFERENCES
- 1.Aebi, M., J. Fah, N. Hurt, C. E. Samuel, D. Thomis, L. Bazzigher, J. Pavlovic, O. Haller, and P. Staeheli. 1989. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol. Cell. Biol. 9:5062-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcalay, M., L. Tomassoni, E. Colombo, S. Stoldt, F. Grignani, M. Fagioli, L. Szekely, K. Helin, and P. G. Pelicci. 1998. The promyelocytic leukemia gene product (PML) forms stable complexes with the retinoblastoma protein. Mol. Cell. Biol. 18:1084-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., H. Hengartner, and R. M. Zinkernagel. 1994. Immunization with recombinant protein: conditions for cytotoxic T cell and/or antibody induction. Med. Microbiol. Immunol. (Berlin) 183:315-324. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., H. Hengartner, and R. M. Zinkernagel. 1995. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur. J. Immunol. 25:3445-3451. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 1996. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J. Exp. Med. 183:2259-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmann, M. F., and R. M. Zinkernagel. 1997. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15:235-270. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee, S. N., M. Buchmeier, and W. E. Rawls. 1975. Requirement of cell nucleus for the replication of an arenavirus. Intervirology 6:190-196. [DOI] [PubMed] [Google Scholar]
- 8.Battegay, M., S. Cooper, A. Althage, J. Baenziger, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33:191-198. (Errata, 35:115, 1991 and 38:263, 1992.) [DOI] [PubMed]
- 9.Borden, K. L., E. J. Campbell Dwyer, and M. S. Salvato. 1998. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J. Virol. 72:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrow, J., A. D. Goddard, D. Sheer, and E. Solomon. 1990. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science 249:1577-1580. [DOI] [PubMed] [Google Scholar]
- 11.Buchmeier, M. J., R. M. Welsh, F. J. Dutko, and M. B. A. Oldstone. 1980. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv. Immunol. 30:275-331. [DOI] [PubMed] [Google Scholar]
- 12.Campbell Dwyer, E. J., H. Lai, R. C. MacDonald, M. S. Salvato, and K. L. Borden. 2000. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner. J. Virol. 74:3293-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chebath, J., P. Benech, M. Revel, and M. Vigneron. 1987. Constitutive expression of (2′-5′) oligo A synthetase confers resistance to picornavirus infection. Nature 330:587-588. [DOI] [PubMed] [Google Scholar]
- 14.Chelbi-Alix, M. K., L. Pelicano, F. Quignon, M. H. Koken, L. Venturini, M. Stadler, J. Pavlovic, L. Degos, and H. de The. 1995. Induction of the PML protein by interferons in normal and APL cells. Leukemia 9:2027-2033. [PubMed] [Google Scholar]
- 15.Chelbi-Alix, M. K., F. Quignon, L. Pelicano, M. H. Koken, and H. de The. 1998. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J. Virol. 72:1043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole, G. A., N. Nathanson, and R. A. Prendergast. 1972. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature 238:335-337. [DOI] [PubMed] [Google Scholar]
- 17.Couch, R. B., V. Knight, R. G. Douglas, Jr., and S. H. B. Black. 1969. The minimal infectious dose of adenovirus type 4; the case for natural transmission by viral aerosol. Trans. Am. Clin. Climatol. Assoc. 80:205-211. [PMC free article] [PubMed] [Google Scholar]
- 18.D'Alessio, D. J., C. K. Meschievitz, J. A. Peterson, C. R. Dick, and E. C. Dick. 1984. Short-duration exposure and the transmission of rhinoviral colds. J. Infect. Dis. 150:189-194. [DOI] [PubMed] [Google Scholar]
- 19.Daniel, M. T., M. Koken, O. Romagne, S. Barbey, A. Bazarbachi, M. Stadler, M. C. Guillemin, L. Degos, C. Chomienne, and H. de The. 1993. PML protein expression in hematopoietic and acute promyelocytic leukemia cells. Blood 82:1858-1867. [PubMed] [Google Scholar]
- 20.de The, H., C. Chomienne, M. Lanotte, L. Degos, and A. Dejean. 1990. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature 347:558-561. [DOI] [PubMed] [Google Scholar]
- 21.Djavani, M., J. Rodas, I. S. Lukashevich, D. Horejsh, P. P. Pandolfi, K. L. Borden, and M. S. Salvato. 2001. Role of the promyelocytic leukemia protein PML in the interferon sensitivity of lymphocytic choriomeningitis virus. J. Virol. 75:6204-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doherty, P. C., and R. M. Zinkernagel. 1974. T-cell-mediated immunopathology in viral infections. Transplant. Rev. 19:89-120. [DOI] [PubMed] [Google Scholar]
- 23.Doucas, V., A. M. Ishov, A. Romo, H. Juguilon, M. D. Weitzman, R. M. Evans, and G. G. Maul. 1996. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 10:196-207. [DOI] [PubMed] [Google Scholar]
- 24.Doucas, V., M. Tini, D. A. Egan, and R. M. Evans. 1999. Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc. Natl. Acad. Sci. USA 96:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutko, F. J., and M. B. Oldstone. 1983. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J. Gen. Virol. 64:1689-1698. [DOI] [PubMed] [Google Scholar]
- 26.Dyck, J. A., G. G. Maul, W. H. Miller, J. D. Chen, A. Kakizuka, and R. M. Evans. 1994. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell 76:333-343. [DOI] [PubMed] [Google Scholar]
- 27.Ehl, S., P. Klenerman, R. M. Zinkernagel, and G. Bocharov. 1998. The impact of variation in the number of CD8+ T-cell precursors on the outcome of virus infection. Cell. Immunol. 189:67-73. [DOI] [PubMed] [Google Scholar]
- 28.Everett, R. D., P. Lomonte, T. Sternsdorf, R. van Driel, and A. Orr. 1999. Cell cycle regulation of PML modification and ND10 composition. J. Cell Sci. 112:4581-4588. [DOI] [PubMed] [Google Scholar]
- 29.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 13:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goddard, A. D., J. Borrow, P. S. Freemont, and E. Solomon. 1991. Characterization of a zinc finger gene disrupted by t(15;17) in acute promyelocytic leukemia. Science 254:1371-1373. [DOI] [PubMed] [Google Scholar]
- 31.Guidotti, L. G., K. Ando, M. V. Hobbs, T. Ishikawa, L. Runkel, R. D. Schreiber, and F. V. Chisari. 1994. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl. Acad. Sci. USA 91:3764-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 33.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 34.Guiochon-Mantel, A., J. F. Savouret, F. Quignon, K. Delabre, E. Milgrom, and H. De The. 1995. Effect of PML and PML-RAR on the transactivation properties and subcellular distribution of steroid hormone receptors. Mol. Endocrinol. 9:1791-1803. [DOI] [PubMed] [Google Scholar]
- 35.Hoffsten, P. E., M. B. Oldstone, and F. J. Dixon. 1977. Immunopathology of adoptive immunization in mice chronically infected with lymphocytic choriomeningitis virus. Clin. Immunol. Immunopathol. 7:44-52. [DOI] [PubMed] [Google Scholar]
- 36.Hotchin, J. 1962. The biology of lymphocytic choriomeningitis infection: virus induced immune disease. Cold Spring Harbor Symp. Quant. Biol. 27:479-499. [DOI] [PubMed] [Google Scholar]
- 37.Hotchin, J. 1971. Persistent and slow virus infection. Monogr. Virol. 3:1. [Google Scholar]
- 38.Ishov, A. M., and G. G. Maul. 1996. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J. Cell Biol. 134:815-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kastner, P., A. Perez, Y. Lutz, C. Rochette-Egly, M. P. Gaub, B. Durand, M. Lanotte, R. Berger, and P. Chambon. 1992. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J. 11:629-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz, M., and S. A. Plotkin. 1967. Minimal infective dose of attenuated poliovirus for man. Am. J. Public Health Nations Health 57:1837-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly, C., R. Van Driel, and G. W. Wilkinson. 1995. Disruption of PML-associated nuclear bodies during human cytomegalovirus infection. J. Gen. Virol. 76:2887-2893. [DOI] [PubMed] [Google Scholar]
- 42.Koken, M. H., G. Linares-Cruz, F. Quignon, A. Viron, M. K. Chelbi-Alix, J. Sobczak-Thepot, L. Juhlin, L. Degos, F. Calvo, and H. de The. 1995. The PML growth-suppressor has an altered expression in human oncogenesis. Oncogene 10:1315-1324. [PubMed] [Google Scholar]
- 43.Koken, M. H. M., F. Puvion-Dutilleul, M. C. Guillemin, A. Viron, G. Linares-Cruz, N. Stuurman, L. de Jong, C. Szostecki, F. Calvo, C. Chomienne, L. Degos, E. Puvion, and H. de Thé. 1994. The t(15;17) translocation alters a nuclear body in a RA-reversible fashion. EMBO J. 13:1073-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavau, C., A. Marchio, M. Fagioli, J. Jansen, B. Falini, P. Lebon, F. Grosveld, P. P. Pandolfi, P. G. Pelicci, and A. Dejean. 1995. The acute promyelocytic leukaemia-associated PML gene is induced by interferon. Oncogene 11:871-876. [PubMed] [Google Scholar]
- 45.Le, X. F., P. Yang, and K. S. Chang. 1996. Analysis of the growth and transformation suppressor domains of promyelocytic leukemia gene, PML. J. Biol. Chem. 271:130-135. [DOI] [PubMed] [Google Scholar]
- 46.Lehmann-Grube, F. 1971. Lymphocytic choriomeningitis virus. Virol. Monogr. 10:1-173. [Google Scholar]
- 47.Lehmann-Grube, F., I. Krenz, T. Krahnert, R. Schwachenwald, D. Moskophidis, J. Lohler, and C. J. Villeda Posada. 1987. Mechanism of recovery from acute virus infection. IV. Questionable role of mononuclear phagocytes in the clearance of lymphocytic choriomeningitis virus from spleens of mice. J. Immunol. 138:2282-2289. [PubMed] [Google Scholar]
- 48.Li, H., C. Leo, J. Zhu, X. Wu, J. O'Neil, E. J. Park, and J. D. Chen. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matloubian, M., S. R. Kolhekar, T. Somasundaram, and R. Ahmed. 1993. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J. Virol. 67:7340-7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuura, Y., R. D. Possee, H. A. Overton, and D. H. Bishop. 1987. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J. Gen. Virol. 68:1233-1250. [DOI] [PubMed] [Google Scholar]
- 51.Moskophidis, D., M. Battegay, M.-A. Bruendler, E. Laine, I. Gresser, and R. M. Zinkernagel. 1994. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J. Virol. 68:1951-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moskophidis, D., F. Lechner, H. Hengartner, and R. M. Zinkernagel. 1994. MHC class I and non-MHC-linked capacity for generating an anti-viral CTL response determines susceptibility to CTL exhaustion and establishment of virus persistence in mice. J. Immunol. 152:4976-4983. [PubMed] [Google Scholar]
- 53.Moskophidis, D., F. Lechner, H. P. Pircher, and R. M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362:758-761. [DOI] [PubMed] [Google Scholar]
- 54.Moskophidis, D., and F. Lehmann-Grube. 1989. Virus-induced delayed-type hypersensitivity reaction is sequentially mediated by CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 86:3291-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mu, Z. M., K. V. Chin, J. H. Liu, G. Lozano, and K. S. Chang. 1994. PML, a growth suppressor disrupted in acute promyelocytic leukemia. Mol. Cell. Biol. 14:6858-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ochsenbein, A. F., and R. M. Zinkernagel. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21:624-630. [DOI] [PubMed] [Google Scholar]
- 57.Oehen, S., H. Hengartner, and R. M. Zinkernagel. 1991. Vaccination for disease. Science 251:195-198. [DOI] [PubMed] [Google Scholar]
- 58.Pfau, C. J., J. K. Valenti, D. C. Pevear, and K. D. Hunt. 1982. Lymphocytic choriomeningitis virus killer T cells are lethal only in weakly disseminated murine infections. J. Exp. Med. 156:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reddy, B. A., L. D. Etkin, and P. S. Freemont. 1992. A novel zinc finger coiled-coil domain in a family of nuclear proteins. Trends Biochem. Sci. 17:344-345. [DOI] [PubMed] [Google Scholar]
- 60.Salvato, M., P. Borrow, E. Shimomaye, and M. B. Oldstone. 1991. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J. Virol. 65:1863-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuel, C. E. 1979. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc. Natl. Acad. Sci. USA 76:600-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samuel, C. E. 1988. Mechanisms of the antiviral action of interferons. Prog. Nucleic Acid Res. Mol. Biol. 35:27-72. [DOI] [PubMed] [Google Scholar]
- 63.Staeheli, P., and O. Haller. 1987. Interferon-induced Mx protein: a mediator of cellular resistance to influenza virus. Interferon 8:1-23. [PubMed] [Google Scholar]
- 64.Stuurman, N., A. Floore, E. Middelkoop, R. van Driel, and L. de Jong. 1997. PML shuttles between nuclear bodies and the cytoplasm. Cell Mol. Biol. Lett. 2:137-150. [Google Scholar]
- 65.Szekely, L., K. Pokrovskaja, W. Q. Jiang, H. de The, N. Ringertz, and G. Klein. 1996. The Epstein-Barr virus-encoded nuclear antigen EBNA-5 accumulates in PML-containing bodies. J. Virol. 70:2562-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turelli, P., V. Doucas, E. Craig, B. Mangeat, N. Klages, R. Evans, G. Kalpana, and D. Trono. 2001. Cytoplasmic recruitment of INI1 and PML on incoming HIV preintegration complexes: interference with early steps of viral replication. Mol. Cell 7:1245-1254. [DOI] [PubMed] [Google Scholar]
- 67.Vallian, S., K. V. Chin, and K. S. Chang. 1998. The promyelocytic leukemia protein interacts with Sp1 and inhibits its transactivation of the epidermal growth factor receptor promoter. Mol. Cell. Biol. 18:7147-7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang, Z. G., L. Delva, M. Gaboli, R. Rivi, M. Giorgio, C. Cordon-Cardo, F. Grosveld, and P. P. Pandolfi. 1998. Role of PML in cell growth and the retinoic acid pathway. Science 279:1547-1551. [DOI] [PubMed] [Google Scholar]
- 69.Wang, Z. G., D. Ruggero, S. Ronchetti, S. Zhong, M. Gaboli, R. Rivi, and P. P. Pandolfi. 1998. PML is essential for multiple apoptotic pathways. Nat. Genet. 20:266-272. [DOI] [PubMed] [Google Scholar]
- 70.Ward, R. L., D. I. Bernstein, E. C. Young, J. R. Sherwood, D. R. Knowlton, and G. M. Schiff. 1986. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J. Infect. Dis. 154:871-880. [DOI] [PubMed] [Google Scholar]
- 71.Wilsnack, R. W., and W. P. Rowe. 1964. Immunofluorescent studies of the histopathogenesis of lymphocytic choriomeningitis virus infection. J. Exp. Med. 120:829.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng, P., Y. Guo, Q. Niu, D. E. Levy, J. A. Dyck, S. Lu, L. A. Sheiman, and Y. Liu. 1998. Proto-oncogene PML controls genes devoted to MHC class I antigen presentation. Nature 396:373-376. [DOI] [PubMed] [Google Scholar]
- 73.Zhong, S., L. Delva, C. Rachez, C. Cenciarelli, D. Gandini, H. Zhang, S. Kalantry, L. P. Freedman, and P. P. Pandolfi. 1999. A RA-dependent, tumour-growth suppressive transcription complex is the target of the PML-RARα and T18 oncoproteins. Nat. Genet. 23:287-295. [DOI] [PubMed] [Google Scholar]
- 74.Zinkernagel, R. M., and P. C. Doherty. 1979. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv. Immunol. 27:51-177. [DOI] [PubMed] [Google Scholar]
- 75.Zinkernagel, R. M., E. Haenseler, T. Leist, A. Cerny, H. Hengartner, and A. Althage. 1986. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J. Exp. Med. 164:1075-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zinkernagel, R. M., and H. Hengartner. 1997. Antiviral immunity. Immunol. Today 18:258-260. [DOI] [PubMed] [Google Scholar]
- 77.Zinkernagel, R. M., T. Leist, H. Hengartner, and A. Althage. 1985. Susceptibility to lymphocytic choriomeningitis virus isolates correlates directly with early and high cytotoxic T cell activity, as well as with footpad swelling reaction, and all three are regulated by H-2D. J. Exp. Med. 162:2125-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]