Abstract
OBJECTIVE— In the Diabetes Prevention Program (DPP), metformin significantly reduced the risk of diabetes in individuals with impaired glucose tolerance. Diabetes status was assessed by oral glucose tolerance tests (OGTTs) performed while participants were still taking metformin or placebo. To determine whether the observed benefit was a transient pharmacological effect or more sustained, we performed a repeat OGTT after a short “washout” period during which medications (metformin or placebo) were withheld.
RESEARCH DESIGN AND METHODS— All participants assigned to medication who had not developed diabetes at the end of the DPP were asked to have a repeat OGTT after discontinuing the study medication for 1–2 weeks. The predesignated outcome was the odds of diabetes in metformin versus placebo comparisons during the trial and washout combined.
RESULTS— There were 1,274 participants who participated in the washout study and 529 who did not because they had already developed diabetes. Before the washout, the odds of diabetes in the metformin group was lower than that in the placebo group (odds ratio 0.66, 95% CI 0.54–0.82, P < 0.001). After the washout, diabetes was somewhat more frequently diagnosed in the metformin participants (1.49, 0.93–2.38, P = 0.098). Combining diabetes conversions during the DPP and during the washout, diabetes was diagnosed significantly less frequently in the metformin than the placebo group (0.75, 0.62–0.92, P = 0.005).
CONCLUSIONS— The primary analysis of the DPP demonstrated that metformin decreased the risk of diabetes by 31%. The washout study shows that 26% of this effect can be accounted for by a pharmacological effect of metformin that did not persist when the drug was stopped. After the washout the incidence of diabetes was still reduced by 25%.
The Diabetes Prevention Program (DPP) recently reported that the incidence of diabetes in individuals with impaired glucose tolerance (IGT) was reduced by 58% with lifestyle modifications and by 31% with metformin compared with placebo after a mean duration of 2.8 years of intervention (1). In the DPP, the diagnosis of diabetes was made on the basis of an abnormal oral glucose tolerance test (OGTT) performed annually or an elevated fasting plasma glucose (FPG) performed semiannually (1,2). Both required confirmation by repeat testing. These assessments were carried out without interruption of the lifestyle or medication regimens, except for stopping medications on the morning of the tests. At the completion of the primary study and in order to determine whether the observed effect of metformin on the development of diabetes persisted after metformin was withdrawn, we tested participants taking metformin or placebo who had not developed diabetes with a repeat OGTT after a 1- to 2-week “washout” period during which medications were withheld. We hypothesized that if the effects of metformin were transient, a 1- to 2-week washout of the drug intervention would result in a very high diabetes conversion rate among metformin participants, such that the reported difference in the overall conversion rate to diabetes compared with placebo would be greatly narrowed or obliterated. The specific questions asked were: 1) Among those who did not convert to diabetes during the DPP and before washout, do the metformin and placebo groups differ in the rate of conversion to diabetes after the washout? and 2) If the diabetes conversions during washout were combined with the conversions to diabetes during the trial, do the metformin and placebo groups differ in the proportion of participants who have converted to diabetes?
RESEARCH DESIGN AND METHODS
After the DPP announced the study results to the investigators and study participants in early August 2001, metformin and placebo participants who had not converted to diabetes during the DPP were asked to participate in the washout study. All of those who participated gave written informed consent to participate in the washout study, which had been approved by each individual institutional review board. Those consenting remained masked to their treatment assignments, were scheduled for a washout visit, and were asked to discontinue the study medication 1 week before the washout visit. The period of 1 week was chosen because it was thought that the pharmacological effects of metformin would be gone by that point and, if the drug was indeed having substantial sustained effects in delaying diabetes, a more prolonged withdrawal period might be considered to be adverse to the well-being of the participants. Because of difficulties in scheduling a large number of tests in a relatively short time, the actual time of drug withdrawal ranged from 1 to 2 weeks. The visit consisted of an OGTT conducted according to the DPP protocol (2). After the OGTT, participants were asked to remain off the medication until they were notified whether a diabetes confirmation visit was needed. As in the full DPP, diabetes conversions were verified by a confirmatory OGTT within 6 weeks (2).
The final analysis included data from all conversions to diabetes during the DPP (defined as through 31 July 2001) as well as from the washout visits for all participants assigned to metformin or placebo. However, the analysis excluded data from 17 (10 metformin and 7 placebo) participants who died during the DPP without having converted to diabetes and 335 (170 metformin and 165 placebo) participants who were inactive or refused participation in the washout study and had not converted to diabetes before washout. Six participants with diabetic OGTTs did not have confirmatory OGTTs, and the analysis was performed twice, once including these participants as not having converted to diabetes, and again as having converted to diabetes.
The odds ratios for conversion to diabetes at washout for metformin versus placebo were estimated using the Mantel-Haenszel adjustment for the year of randomization (3). The same methods were used for the odds of conversion before washout and for the overall conversion over the period combining both DPP and washout periods. Also examined was the heterogeneity of the odds ratios across strata based on age and BMI.
RESULTS
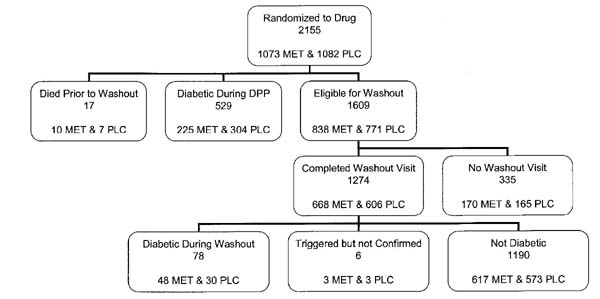
Altogether, 79.2% of eligible nondiabetic participants who were assigned to medication therapy (79.7% of eligible metformin-treated and 78.6% of placebo-treated participants) completed a washout visit (Fig. 1). The overall analysis consisted of 1,803 participants (893 metformin and 910 placebo), 1,274 of whom participated in the washout and 529 of whom had already developed diabetes. The duration of time from the last medication dose to the OGTT (i.e., the washout period) averaged 11 days in both treatment groups. Table 1 shows the diabetes status of the washout cohort by treatment group at the start of the washout visits and after the washout visits. The percent with diabetes increased by 5.4% (from 25.2 to 30.6%) in the metformin group and by 3.3% (from 33.4 to 36.7%) in the placebo group. Not all individuals who participated in the washout study were taking their assigned medications at the time of the washout study. A total of 9 of 48 (19%) participants assigned to metformin and 3 of 30 (10%) participants assigned to placebo who converted to diabetes during the washout period had not taken coded medication for >90 days before the washout OGTT; their data are included in the figures indicated above, in accordance with an intention-to-treat analysis.
Figure 1.
Schema showing distribution of the 2,155 participants who had been randomized to drug, metformin (MET), or placebo (PLC). Of the 1,274 participants completing the washout study, 78 were found to meet criteria for diabetes on the washout OGTT.
Table 1.
Number and percent with diabetes prior to and including washout
| Diabetes prior to washout |
Diabetes including washout |
|||
|---|---|---|---|---|
| Treatment | No diabetes | Diabetes | No diabetes | Diabetes |
| Metformin | 668 (74.8) | 225 (25.2) | 620 (69.4) | 273 (30.6) |
| Placebo | 606 (66.6) | 304 (33.4) | 576 (63.3) | 334 (36.7) |
Data are n (%).
These results do not take into account differing durations of follow-up by treatment group. The usual lifetable analysis is inappropriate because the categorization of diabetes during the washout study is not simply one additional data point, and because the washout assessments were performed under different conditions. We therefore stratified in semiannual groupings by date of randomization and computed date-adjusted odds ratio of diabetes in the metformin versus placebo groups. Results of this analysis showed no significant heterogeneity in the washout-diabetes odds ratio by randomization grouping. Diabetes was diagnosed less frequently before the washout in metformin participants (odds ratio 0.66, 95% CI 0.54–0.82, P < 0.001) (Table 2). Although the odds of diabetes increased by 50% in the metformin compared with the placebo group during the washout, the difference did not reach statistical significance (P = 0.098). When the diabetes conversions during the DPP plus the washout period were combined, the odds of diabetes were still reduced by 25% in the metformin compared with the placebo group (0.75, 0.62–0.92, P = 0.005). This last analysis, the primary analysis of the washout study, demonstrates that a transient pharmacological effect did not explain the overall difference between metformin and placebo, despite the 1.5-fold increase in the development of diabetes in the metformin group compared with the placebo group during the washout period. Analyses were repeated adjusting for age and BMI, with no appreciable change in results (data not shown). Finally, results were nearly identical when the six participants with a triggering abnormal OGTT at the washout visit but without a confirmation visit were included as “diabetic.”
Table 2.
Mantel-Haenszel randomization date–adjusted odds ratio of diabetes for metformin versus placebo
| Diabetes diagnosis | Odds ratio | 95% CI | P |
|---|---|---|---|
| Prior to washout | 0.66 | 0.54–0.82 | <0.001 |
| At washout | 1.49 | 0.93–2.38 | 0.098 |
| Overall | 0.75 | 0.62–0.92 | 0.005 |
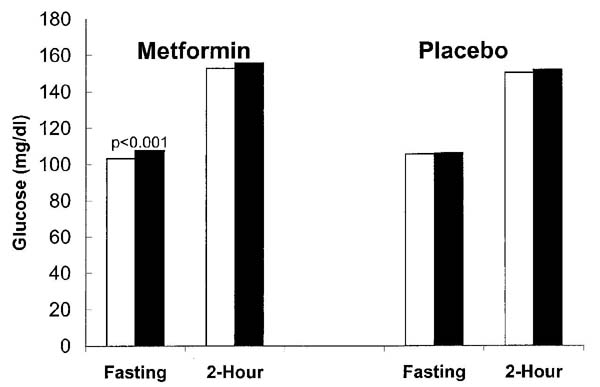
Figure 2 compares OGTT data from the most recent annual visit, when both fasting and 2-h glucose levels were measured before the washout, with data from the washout period, when fasting and 2-h measures were measured among individuals who had not developed diabetes before the washout study. At the washout visit, metformin participants had a significant 4.5-mg/dl increase in fasting glucose levels (P < 0.001) and a nonsignificant 2.9-mg/dl increase in 2-h glucose levels, whereas placebo participants had nonsignificant increases in fasting glucose (0.4 mg/dl) and 2-h glucose (1.5 mg/dl).
Figure 2.
Comparison of fasting and 2-h glucose levels during OGTTs before and during the washout study in participants who had not developed diabetes who were taking metformin or placebo. The participants taking metformin had a modest increase of 4.5 mg/dl in the fasting glucose level (P < 0.001) after being off medication for a mean of 11 days. Changes at 2 h in the metformin group and at both times in the placebo group were not significant. □, prewashout; ■, washout.
CONCLUSIONS
The primary analysis of the DPP demonstrated that the development of diabetes was decreased by 58% as a result of lifestyle intervention and by 31% as a result of metformin. In this study, when the intervention medication (metformin or placebo) was withheld for an average of 11 days before a washout OGTT was performed, it was found that approximately one-quarter of this effect could be accounted for by a pharmacological effect of metformin that did not persist when the drug was withdrawn. Nevertheless, after the washout, there was a significant reduction (by 24.9%) in the incidence of diabetes. Thus, the metformin benefit did not represent a “masking” of the development of diabetes by this medication.
How long the effects of metformin on the underlying pathophysiology of diabetes might last is unknown, since the washout period rarely exceeded 14 days. In a small study of participants with IGT, with 20 subjects on metformin (500 mg twice daily) and 20 on placebo, Lehtovirta et al. (4) reported that treatment with metformin resulted in an improvement in glucose tolerance that lasted for up to 6 months after cessation of metformin.
The mechanism whereby metformin lowers glucose levels is still not entirely clear. One possibility is an alteration in glycemia through metformin affects the liver. Another possibility is related to the weight loss observed with metformin. Although there may be a small effect of metformin to improve insulin action on glucose utilization, most of the reduction of glycemia appears to reflect a decrease in endogenous glucose production (5-8). Our results comparing the effects of withdrawal of metformin and placebo during this washout study fit this model of metformin action, since we found a greater increase in fasting glucose than in 2-h glucose levels after withdrawal of metformin, with little change after withdrawal of placebo.
In summary, it appears that approximately one-quarter of the beneficial effect of metformin to prevent type 2 diabetes in the DPP was attributable to a pharmacological effect that did not persist when the drug was withdrawn. However, the overall effect of metformin in preventing diabetes remained a substantial 25% after withdrawal, concordant with the conclusion of the DPP that metformin reduces the conversion to diabetes in individuals with IGT.
Acknowledgments
This study was supported by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Child Health and Human Development, the National Institute on Aging, the National Center on Minority Health and Health Disparities (NCMHD), the National Center for Research Resources General Clinical Research Center Program, and the Office of Research on Women's Health; the Indian Health Service; the Centers for Disease Control and Prevention; the American Diabetes Association; Bristol-Myers Squibb; and Parke-Davis.
We thank the thousands of volunteers in this program for their devotion to the goal of diabetes prevention. LifeScan, Health O Meter, Hoechst Marion Roussel, Lipha Pharmaceuticals, Merck-Medco Managed Care, Merck and Co., Nike Sports Marketing, Slim Fast Foods, and Quaker Oats donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices, Matthews Media Group, and the Henry M. Jackson Foundation provided support services under subcontract with the DPP Coordinating Center.
Abbreviations
- DPP
Diabetes Prevention Program
- FPG
fasting plasma glucose
- IGT
impaired glucose tolerance
- OGTT
oral glucose tolerance test
Footnotes
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Diabetes Prevention Program Research Group Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 4.Lehtovirta M, Forsén B, Gullström M, Häggblom M, Eriksson JG, Taskinen M-R, Groop L. Metabolic effects of metformin in patients with impaired glucose tolerance. Diabet Med. 2001;18:578–583. doi: 10.1046/j.1464-5491.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- 5.Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059–4067. doi: 10.1210/jcem.81.11.8923861. [DOI] [PubMed] [Google Scholar]
- 6.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 7.Hundal RS, Krssak M, DuFour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]