Abstract
The relationship between orbitofrontal cortex (OFC) volumes and functional domains in treatment-resistant patients with schizophrenia or schizoaffective disorder is poorly undestood. OFC dysfunction is implicated in several of the behaviors that are abnormal in schizophrenia. However, little is known about the relationship between these behaviors and OFC volumes. Forty-nine (49) patients received magnetic resonance imaging scanning as part of a double-blind treatment study in which psychiatric symptomatology, neuropsychological function, and aggression were measured. OFC volumes were manually traced on anatomical images. Psychiatric symptomatology was measured with the Positive and Negative Syndrome Scale (PANSS). Aggression was measured with the Overt Aggression Scale (OAS) and with the PANSS. Neuropsychological function was assessed using a comprehensive test battery. Larger right OFC volumes were associated with poorer neuropsychological function. Larger left OFC gray matter volumes and larger OFC white matter volumes bilaterally were associated with greater levels of aggression. These findings are discussed in the context of potential iatrogenic effects.
Keywords: orbitofrontal cortex, schizophrenia, aggression, neuropsychological function
1. Introduction
The orbitofrontal cortex (OFC) plays a key role in several behaviors that are relevant to the psychopathology of schizophrenia. Damage to this region is associated with disinhibition, and impaired inhibition has been observed in neurocognitive measures (Garavan et al., 1999), as well as in aggression (Grafman et al., 1996)(see (Hoptman, 2003), for a review) and substance abuse (Goldstein et al., 2001; Volkow et al., 1999). An important question is whether quantitative measures of OFC volumes are correlated with these behaviors in schizophrenia.
Numerous magnetic resonance imaging (MRI) studies have shown that several regional brain volumes differ between patients with schizophrenia and healthy controls (see (Niznikiewicz et al., 2003; Pearlson and Marsh, 1999; Shenton et al., 2001) for reviews). These structures include the ventricles, subregions of the frontal and temporal lobes, as well as subcortical structures including the basal ganglia, corpus callosum, thalamus, and possibly the cerebellum. In addition, many of these studies have found that regional parenchymal volumes, such as medial temporal and inferior parietal regions, are larger in healthy controls than in patients with schizophrenia.
However, the reverse is also found, especially in the basal ganglia. The enlargement of basal ganglia volumes in patients with schizophrenia was initially described in MRI studies by DeLisi et al. (DeLisi et al., 1991) and Jernigan et al. (Jernigan et al., 1991). This relationship appears to vary with treatment history. Patients with schizophrenia who had been treated with typical antipsychotic drugs (APD) showed increases in the volume of the caudate (Chakos et al., 1994). Typical APDs were also associated with a similar, though statistically nonsignificant, enlargement of cortical volumes. Similar results for caudate volumes were found by Keshavan et al.(Keshavan et al., 1994). Overall, a substantial majority of the MRI studies on the caudate have shown increased volumes in schizophrenia (Shenton et al., 2001).
In a postmortem study in rats, chronic treatment with haloperidol was associated with increased striatal volumes (Chakos et al., 1998). This result indicates that the effect of haloperidol on striatal volumes is not entirely secondary to the pathophysiology of schizophrenia. In further support of this argument, when patients were switched to clozapine, an atypical APD, caudate volumes were reduced (Chakos et al., 1995; Corson et al., 1999; Frazier et al., 1996). Thus, the increase in caudate volumes may reflect an iatrogenic effect of typical APD treatment.
The type of APD medication administered will almost certainly vary with the duration of illness, given that the oldest of the atypicals, clozapine, was only given FDA approval in 1989. Thus, older patients have received considerable exposure to typical APDs. Consistent with this notion, Lang et al. (Lang et al., 2001) found that basal ganglia volumes were larger in patients who received at least 12 weeks of continuous typical APD treatment than in first-episode patients. Moreover, the correlation between globus pallidus volume and length of typical APD treatment approached significance (r = .56, p = .06).
The implications of these findings may extend to other regions of the brain as well, as suggested also by the data published by Chakos et al. (Chakos et al., 1994) and by Benes et al. (Benes et al., 1985a; Benes et al., 1985b), who found increased neuronal soma volumes in the striatum and medial prefrontal cortex in rats treated with haloperidol. It is therefore reasonable to suggest that such volumetric effects might be found in regions other than the basal ganglia. This issue is particularly relevant in a region such as the OFC that plays a key role in functions that are impaired in schizophrenia.
The findings of Arango et al. (Arango et al., 2003) appear to be particularly relevant in this regard. They found that patients with larger prefrontal brain volumes were more likely to benefit from clozapine treatment. However, patients with larger brain volumes showed a worsening of symptoms with haloperidol treatment. These patterns were obtained for the SANS total score and the BPRS total score, anxiety/depression score, and BPRS hostility score. In addition, larger gray matter volumes predicted greater levels of akathisia with haloperidol treatment.
Structural neuroimaging studies of the orbitofrontal region in schizophrenia are rare and have yielded inconsistent results. Szeszko et al. (Szeszko et al., 1999) studied 19 first episode patients with schizophrenia who were treated with typical APDs, and found that male patients had larger right orbitofrontal volumes than did healthy controls. On the other hand, Convit et al. (Convit et al., 2001) found reductions in orbitofrontal volumes in chronic male patients relative to healthy controls. Similarly, in cross-sectional and longitudinal studies, Pantelis et al. (Pantelis et al., 2002) found reduced gray matter (GM) volumes in orbitofrontal (and other temporolimbic) regions in high-risk individuals who went on to develop schizophrenia. Kawasaki et al. (Kawasaki et al., 2004) used a voxel-based morphometry approach and found reduced gray matter density in the OFC region in patients with schizophrenia compared to controls and in patients with schizophrenia compared to patients with schizotypal disorder. Gur et al. (Gur et al., 2000) found that OFC GM volumes were reduced in female, but not male patients. Finally, some studies have found no difference in OFC volumes between patients with schizophrenia and controls. For example, Baaré et al. (Baaré et al., 1999) found no difference in orbitofrontal volume in groups of 13 patients and 14 well-matched controls. Similarly, Rupp et al. (Rupp et al., 2005) found no difference in OFC volumes between 33 patients and 40 healthy controls, although there was a trend for gray matter reduction in this region. This variation in results may reflect sampling variation, duration of illness, treatment history, and other effects.
1.1. Neuropsychological function
The OFC plays a key role in tasks requiring response inhibition (Garavan et al., 1999) and tasks sensitive to reward mechanisms (Rolls, 2000). Thus, functional MRI studies have shown activation in inferior frontal regions on the Stop (Rubia et al., 2001) and Go/No Go (Liddle et al., 2001) tasks, and damage to OFC results in impaired performance on tasks such as the Iowa Gambling Task (Bechara et al., 1994).
1.2. Aggression
Damage to ventromedial regions has been associated with aggression since the original study of Phineas Gage, a worker who suffered damage to the ventromedial cortex and subsequently became belligerent and socially inappropriate (Harlow, 1868). Several studies have suggested reductions in brain volumes in aggressive individuals. For instance, frontal gray matter (GM) volumes are reduced in aggressive patients with antisocial personality disorder compared to healthy controls and patients with substance abuse (Raine et al., 2000). Similarly, reduced GM volumes were found in affectively aggressive patients with temporal lobe epilepsy (Woermann et al., 2000). In addition, Hoptman et al. (Hoptman et al., 2002; Hoptman et al., 2004) found that reduced white matter (WM) integrity in inferior frontal regions was associated with higher levels of impulsivity and aggression. However, there have been no published quantitative MR studies of regional frontal volumes in aggressive patients with schizophrenia.
1.3. Comorbid substance abuse
Abuse of at least certain substances appears to adversely affect inferior frontal regions. Thus, positron emission tomography (PET) studies have shown that OFC plays an important role in the initiation and maintenance of addiction (Volkow et al., 2002). Moreover, Lim et al. (Lim et al., 2002) found reduced white matter integrity in such regions in nonabstinent cocaine abusers relative to healthy controls. Findings such as these have lead to the proposal that OFC dysfunction plays a critical role in the neurobiology of addiction (Goldstein and Volkow, 2002).
None of these studies have examined effects of comorbidity with schizophrenia, although a recently published finding suggests that several regional brain volumes were reduced in both patients with alcohol abuse and in patients with schizophrenia (Mathalon et al., 2003). These authors also found that comorbid alcohol abuse may lead to an additional reduction in regional brain volumes over that seen in either group alone. We are aware of no other published morphometric studies of comorbid substance abuse.
In the current article, we examine relationships between OFC GM and WM volumes and functional domains including neurocognitive function, aggression, and substance abuse in chronic treatment-resistant patients with schizophrenia or schizoaffective disorder. These patients were participants in a larger study comparing treatment response to haloperidol (HAL) with that of clozapine (CLO), risperidone (RIS), and olanzapine (OLZ) (Volavka et al., 2002). Patients were recruited for this study from four sites, two in North Carolina (Dorothea Dix Hospital [DDH] and John Umstead Hospital [JUH]) and two in New York (Rockland Psychiatric Center [RPC], and Manhattan Psychiatric Center [MPC]). The study was started in June 1996 and ended in December 2000. The basic findings pertaining to neuropsychological performance (Bilder et al., 2002a; Bilder et al., 2002b) and aggression (Citrome et al., 2001) have been reported elsewhere.
We hypothesized that smaller OFC volumes would significantly correlate with neurocognitive function, particularly for tasks sensitive to frontal lobe function. We also hypothesized that smaller orbitofrontal volumes would be associated with aggression. Finally, we hypothesized that smaller orbitofrontal volumes would be associated with a comorbid diagnosis of substance abuse.
2. Materials and Methods
2.1. Subjects
Sixty-two patients received a MRI scan. These patients had shown resistance to treatment with typical APDs as defined by 1) persistence of positive symptoms despite treatment with typical APDs at dosages of at least 600mg chlorpromazine equivalents, and 2) poor level of functioning over the two years prior to study entry (Volavka et al., 2002). Nine scans were unusable due to poor scan quality or other technical problems. Of the remaining 53 scans, the OFC was not traceable in four cases because the requisite anatomical landmarks were not present. Thus, the final sample consisted of forty-nine patients. There were 6 women and 43 men. Two of the patients came from MPC, 15 from RPC, and 32 from DDH. Of the 49 patients in the study, 25 (51%) had a comorbid alcohol use diagnosis (dependence or abuse), and 29 (59%) had a comorbid substance usediagnosis. Seven of these latter patients had a cocaine use diagnosis. All patients were hospitalized and were presumed to be free of current alcohol and substance abuse.
Included subjects had a mean age (±SD) of 41.5 ± 8.2 years. Mean age of onset, defined as age at first psychiatric hospitalization, was 22.8 ± 5.11, and had a mean duration of illness of 18.6 ± 7.7 years. Of the 49 patients, 10 were treated with CLO, 8 with HAL, 18 with OLZ, and 13 with RIS. All subjects gave written informed consent to participate in the study.
2.2. Psychiatric Diagnoses
The patients were diagnosed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)(First et al., 1995). Although the principal purpose of the SCID was to confirm the diagnosis of schizophrenia or schizoaffective disorder, alcohol and other substance use disorders were also diagnosed using module E of the SCID.
2.3. Exposure to Typical APDs
We did not have access to the complete lifetime history of APD treatment. As a proxy, we used duration of illness, defined as the age at first hospitalization, to estimate the number of years patients had been treated with typical APDs. We think that this measure provides a reasonable estimate of exposure to typical APDs because of the atypical APDs, only CLO and RIS were readily available prior to the start of this study; OLZ only received FDA approval during the course of this study. Thus, it is unlikely that patients in this study received substantial exposure to atypical APDs.
2.4. MRI
2.4.1. Acquisition
Patients were scanned at the 1.5T Siemens Vision system (Erlangen, Germany) at the Nathan Kline Institute and the 1.5T GE Signa system (Milwaukee, WI) located at the DDH. For each subject a magnetization prepared gradient echo (MPRAGE; on Siemens scanners) or spoiled gradient recall (SPGR; on GE scanners) scan was collected in the axial plane parallel to the plane connecting the anterior and posterior commissures (AC-PC plane). These scans were used for anatomical measurements. For scans collected at Nathan Kline Institute (NKI, which is on the grounds of RPC), the scan parameters were TR=13.5 ms, TE=7 ms, FOV=240 mm, matrix = 256x256, 124 slices, slice thickness = 1.5 mm, no gap. For scans collected at DDH, the scan parameters were TR = 15 ms, TE = 2.2 ms, FOV=240 mm, matrix=256 × 256, 124 slices; slice thickness = 1.5 mm, no gap, 1 NEX. Although pixel dimensions varied slightly by site, volumes are reported in mm3, which allows direct comparisons of data across sites. Moreover, site differences were explicitly examined in separate Analyses of Variance (ANOVAs) in which each volumetric measurement of interest was a dependent variable and Study Site (DDH, MPC, RPC) was a Between-Subjects factor. No effects of study site were found.
2.4.2. Image processing
For the OFC segmentation T1 only single channel segmentation was used. In a first step, the raw T1 images were registered to the Talairach space (Talairach and Tournoux, 1988) using KU Leuven’s extension of the Statistical Parametric Mapping (SPM) program. We used a rigid body registration (translation, rotation with 6 parameters) which only aligns brains to Talairach space but does not change size and shape.
Second, in the segmentation step, the registered T1 scans were used as a single input channel to create a color mapped segmentation file that matched the T1 grayscale dimensions. These images were segmented using an expectation maximization segmentation (ems) algorithm (available at http://bilbo.esat.kuleuven.ac.be/web-pages/downloads/ems/ems.html/), which runs in the SPM/Matlab environment. It should be emphasized that the original high-resolution T1 scans were used for the actual volumetric measurements. For tissue segmentation, the atlas was registered to each individual subject by a fully 3D affine transformation (translation, rotation, scaling, shear) with 12 parameters.
Third, total intracranial volumes (ICV) was defined as the sum of gray matter (GM) + white matter (WM) + cerebrospinal fluid (CSF). In addition, total brain parenchymal measurements, as well as total GM, WM, and CSF volume measurements were obtained using IRIS software (available at http://midag.cs.unc.edu).
Finally, IRIS software was used to delineate and compute measures of the left and right OFC volumes.
2.5. Morphometric Criteria
All measurements were done by loading the grayscale image into IRIS and then superimposing the segmented image. IRIS is an interactive image processing tool, used for drawing and painting in all the orthogonal slices within a volume just as on the surface of a segmented structure. This approach aided in the definition of borders for the regions of interest (ROIs) discussed below. AVS software was then used to combine these delineations with the segmentation mask to yield the GM, WM, and CSF compartmental volumes for the OFC regions of interest.
2.5.1. Hemispheric measures
Separate GM, WM, and CSF hemispheric volumes were generated automatically using AVS software.
2.5.2. Orbitofrontal cortex
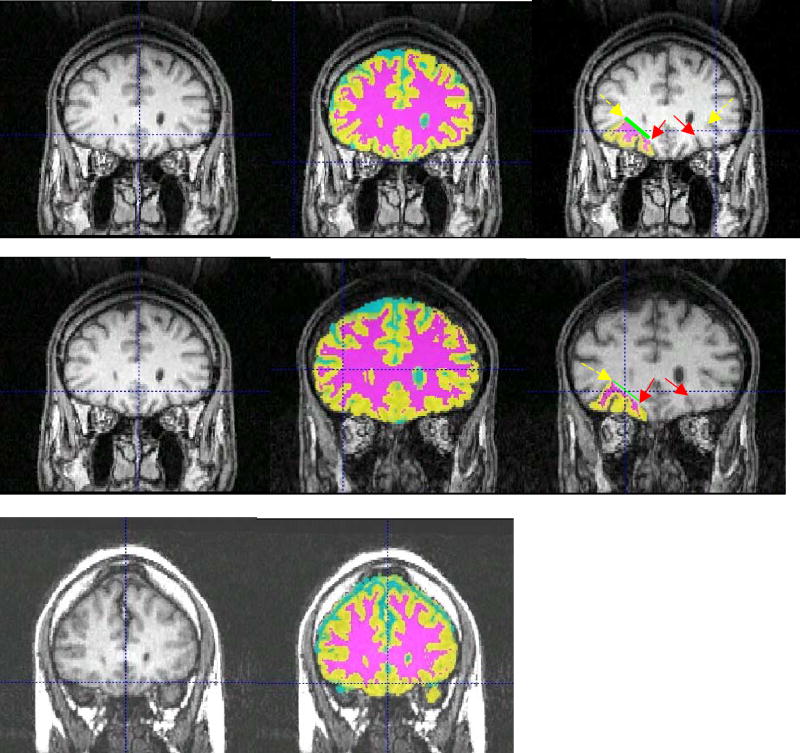
The OFC was measured using the criteria of Szeszko et al. (Szeszko et al., 1999). ROIs were drawn on contiguous slices. The posterior border was the first slice on which the olfactory sulcus appeared. This sulcus also served as the medial border of the OFC. The lateral border was the inferior fundus of the circular sulcus (posterior slices) or the anterior horizontal ramus of the central sulcus. As expected from the literature (Ono et al., 1990), this ramus was not always present, and when this was the case the ROI was not drawn (11.3% of cases). This percentage is in line with the percentage from healthy subjects. Thus, 47 left and 47 right OFCs were traceable out of 53 (usable scans) x 2 (hemispheres) = 106 possible regions. The anterior boundary was the slice on which the gray matter of the OFC no longer appears. The superior border is the line connecting the deepest points of the lateral and medial borders. The inferior border was the gray matter of the tissue between the lateral and medial borders. Representative cases are presented in Figure 1. A single rater (EW), blind to patient identity, performed these measurements. Interrater reliability (intraclass correlation) was established on ten of the cases by two trained raters (EW and MJH) and was 0.92 for the left OFC and 0.93 for the right OFC.
Figure 1.
For each panel, let is raw image, middle is segmented image overlaid on raw image, and right is segmented OFC overlaid on raw image.. Left side of image is right side of brain, superior is up. For segmented images: Cyan = CSF, Yellow = Gray Matter, Magenta = White Matter. The medial borders of the OFC (deepest aspect of orbital sulcus) are indicated with red (solid) arrow, whereas the lateral borders (deepest aspect of anterior horizontal ramus [AHR] of the central suclus) are indicated with yellow (dashed) arrow. The medial boundary of the white matter is indicated by the green (thick) line. Top row: image on which both AHRs are visible. Middle row: case in which one AHR is visible. Bottom row: case in which neither AHR is visible (no OFCs were drawn).
2.6. Psychiatric Symptomatology
Psychiatric symptomatology was rated at baseline and throughout the study using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1986). Raters were trained to an interrater reliability of ICC ≥ .93 with the criterion rater (J-PL). For more details on PANSS administration and ratings, see Volavka et al. (Volavka et al., 2002).
2.7. Neurocognitive Function
Neurocognitive function was assessed at baseline using the battery described in Bilder et al (Bilder et al., 2002a). Correlations between the Global Factor, as well as the four obtained Factors, and the OFC volumetric data were computed.
2.8. Aggression Data
Individual incidents of aggression were recorded using the Overt Aggression Scale (OAS) that provides subscales for 5 items: Verbal Aggression, Physical Aggression against Objects, Physical Aggression Against Self, Physical Aggression against Other People, and Intervention (Yudofsky et al., 1986). During the double-blind study, the research subjects and their ward documentation, such as progress notes and p.r.n. medications, were monitored daily by research staff. Events that could qualify as incidents of aggression were detected by reviewing the documentation (ward journals, chart progress notes and incident reports) and by staff and patient verbal reports. After the initial detection of an event, verification and detailed description were obtained by research personnel interviews of the nursing staff that reported or documented the incident. If these investigations confirmed that an incident of overt aggression had occurred, the research staff then filled in the OAS form using the information obtained in the interviews. Data were missing for two subjects. Twenty-one patients (45%) exhibited at least one aggressive incident during the study period.
For each aggressive incident, a severity score was computed by summing weighted scores of all subscales that applied (for example, verbal aggression and physical aggression against others frequently coincided in the same incident, and thus the scores on these subscales were summed). The weights were assigned using an algorithm developed by the OAS authors (for example, verbal aggression has a lower weight than physical assault)(Silver and Yudofsky, 1991). The weighted scores for individual subscales range from 1 (lowest severity of verbal aggression like angry shouting) to 6 (physical aggression against self or other people, resulting in serious injuries). The Total Aggression Severity score (TAS) was obtained by summing these weighted scores for individual incidents over time (if there were multiple incidents) for each individual patient. Thus, the TAS reflects the seriousness and the number of incidents during the period of randomized treatment. These scores were available both during the pretreatment phase of the study, and for the 14-week study period. TAS scores were log-transformed to normalize distributions. These methods are described in more detail elsewhere (Volavka et al., 2004).
The PANSS hostility item was also used as a measure of aggression in our population. We have used this measure in other studies to examine relationships between medication effects and aggression (Citrome et al., 2001; Citrome et al., 2004; Czobor et al., 1995).
2.9. Extrapyramidal Symptoms
Extrapyramidal symptoms were measured with the Extrapyramidal Symptom Rating Scale (ESRS)(Chouinard et al., 1980), on which raters achieved high interrater reliability (ICCs ≥ 0.86). Because of previous findings relating akathisia to variation in prefrontal brain volumes (Arango et al., 2003), particular attention was focused on this item of the scale. Thus, we examined the relationship between akathisia and OFC volume, and we also examined the extent to which relationships between OFC volumes and the other measures were influenced by akathisia.
2.10. Statistical Analyses
The p = 0.05 level (two-sided, comparisonwise) was adopted for all statistical analyses. Relationship between measures of neuropsychological function and orbitofrontal volumes was investigated by general linear model analysis (GLM). Neuropsychological variables served as dependent variables in the GLM analysis; orbitofrontal volumes were used as independent variables. Intracranial volume, and age were aplied as covariates. A separate analysis was conducted for each neuropsychological measure and each volumetric measure of interest. The GLM analyses tested the null-hypothesis of no association. Analyses analogous to those for the neuropsychological measures were performed for the measures of aggression (i.e., log-transformed value of total aggression score and rating of hostility). The independent variables (orbitofrontal morphometric variables) and covariates were identical to those applied in the analyses of neuropsychological variables. Relationship between past history of substance use and orbitofrontal morphometric variables observed during the study was investigated by hierarchical linear model (HLM) analysis. Substance abuse history (yes/no) was applied as independent variable; morphometric measures were used as dependent variables (in separate analyses). Similar to the GLM, intracranial volume, age, and study site were applied as covariates in the HLM model. In addition to the principal analyses described above, associations of regional orbitofrontal morphometric measures with potentially important demographic variables (age, duration of illness, total head size) and clinical symptoms reflecting long term exposure to APD treatment (total ESRS scores and akathisia) were investigated with bivariate correlation analysis. The ESRS and akathisia correlations were computed controlling for age.
3. Results
Whole brain and regional gray and white matter volumes are presented in Table 1. The total volumes for OFC correspond well to the results of prior studies using these methods (Szeszko et al., 1999).
Table 1.
Global and regional brain volumes in patients with schizophrenia and schizoaffective disorder
| Lefta |
Righta |
|||
|---|---|---|---|---|
| Region | M | SD | M | SD |
| ICV | 604.6 | 56.9 | 600.4 | 57.4 |
| Gray Matter | 305.6 | 31.8 | 302.6 | 31.0 |
| White Matter | 214.4 | 20.1 | 214.8 | 19.8 |
| CSF | 84.6 | 13.9 | 83.0 | 14.6 |
| OFC | ||||
| Gray Matter | 4.81 | 1.37 | 4.77 | 1.22 |
| White Matter | 2.17 | 0.68 | 1.94 | 0.62 |
Note: M = mean, SD = standard deviation, ICV = intracranial volume, OFC = orbitofrontal cortex, CSF = cerebrospinal fluid,
Units in cc3.
3.1. OFC Volumes
3.1.1. Correlations with Age
Head size was inversely correlated with age of onset (r < −0.32, P < 0.02). In addition, left OFC GM volumes were highly correlated with ICV (r = 0.49, P = 0.0004). Thus, in the remaining correlational analyses, head size was treated as a covariate. Corrected for head size, regional cerebral volumes did not correlate significantly with age. However, total right hemisphere GM volume was inversely related to age of onset (r = −0.28, P = 0.05).
3.1.2. Neurocognitive Function
Relations between neuropsychological functions were evaluated using GLM analyses. In general, smaller OFC volumes, corrected for age and ICV, were associated with better neurocognitive performance. Right OFC WM volume was inversely associated with performance on the Global scale at baseline (F[1,43] = 4.49, r = −0.31, P = 0.04). Right OFC GM volume was inversely associated with the Attention and Processing Speed factor at baseline (F[1,41] = 12.06, r = −0.48, P = 0.001). In addition, right OFC WM volume was negatively associated with baseline Attention and Processing Speed factor scores (F[1,41] = 17.68, r = 0.55, P = 0.0001). Finally, a larger leftward asymmetry in OFC WM volumes was associated with better baseline Attention and Processing Speed factor scores (F[1,39] = 4.09, r = 0.31, P = 0.05). OFC volumes were unrelated to scores on the Executive Dysfunction and Perceptual Organization, Declarative Learning and Memory and Simple Motor factors.
Additional analyses examined the relationships between specific neuropsychological variables and OFC volumes. These additional exploratory analyses were conducted at the individual test level without Bonferroni correction, to determine whether meaningful patterns might exist that were missed at the scale and global levels. Percent perseverative errors on the Wisconsin Card Sorting test were associated with larger right OFC WM volumes (F[1,38] = 7.03, r = 0.40, P = 0.01) and with a larger rightward asymmetry in OFC WM volumes (F[1,36] = 5.74, r = −0.37, P = 0.02). In addition, errors on the Trails B were associated with larger right OFC GM volumes (F [1,27] = 6.25, r= .45, P = 0.02) and larger left OFC WM volumes (F[1,24] = 8.26, r = 0.51, P = 0.008). In addition, the longest span achieved on the letter number span was associated with larger leftward asymmetry in OFC WM volumes (F[1,25] = 4.67, r = 0.40, P = 0.04).
3.1.3. Aggression
The association between TAS and regional brain volumes were analyzed using GLM analyses. Age and ICV were covariates in these analyses. Type III Sums of Squares were used for statistical tests. Higher log-transformed TAS scores were associated with larger left OFC GM volumes (F[1,43] = 8.04, r = 0.48, P = 0.007) and larger left and right OFC WM volumes (F[1,43]s > 5.43, rs > .33, Ps < 0.02). Finally, higher scores on the PANSS Hostility item were associated with larger leftward asymmetry in OFC WM volumes (F[1,41] = 4.42, p = .04).
3.1.4. Alcohol and Cocaine Abuse
Similar analyses to those described above in the Aggression section were performed on substance abuse data. Patients with any alcohol use disorder had larger left than right OFC WM volumes (F[1,45] = 5.07, P = 0.03). In addition, patients with comorbid cocaine use disorders had larger left than right OFC WM volumes, (F[1,46] = 4.39, p = .04).
3.1.5. Extrapyramidal Symptoms
Patients had a mean baseline akathisia score of 0.66 ± 1.12 on the ESRS, and a mean baseline total ESRS score of 34.7 ± 19.1. Higher baseline akathisia scores were correlated with larger left OFC WM volumes, r = 0.30, P = 0.05. In addition, higher baseline total ESRS scores were correlated with larger left hemisphere volumes, r = 0.30, P = 0.05.
3.1.6. Duration of Illness
Additional analyses were conducted with duration of illness (years) as a covariate. In none of the cases described above did the results change after controlling for this variable.
Additional analyses were conducted with gender as a covariate.. The results using this covariate were essentially unchanged from those discussed above
4. Discussion
The primary findings of this study were that, corrected for age and head size, smaller OFC volumes were associated with better neuropsychological performance and lower levels of aggression. This pattern was obtained for neurocognitive function and aggressive behavior. In addition, comorbid alcohol use disorders were associated with larger left than right white matter volumes. Although the relationships between OFC volumes and our functional measures appear counterintuitive, it should be noted that larger volumes do not necessarily reflect better neural function.
Increased volumes could reflect reduced neural density, increased neuronal size, or could reflect edema or other pathophysiological processes. Treatment with typical APDs appears to increase caudate volumes. The effects of long-term treatment with typical APDs on other brain regions are not well studied. However, one might speculate that the current results could be explained if such treatment has a similar effect on OFC volumes. It should be pointed out that we have obtained similar effects to the ones reported here in our examination of caudate volumes (Hoptman et al., submitted). In this light, it should be noted that although Chakos et al. (Chakos et al., 1994) did not find a significant increase in cortical volume in their study, the cortical volume did increase by 3.2% in their patients, suggesting that the increased striatal volume associated with typical APD treatment may not be specific to that region.
The mean duration of illness, and, presumably, treatment with APDs, of patients in our sample was 18.6 years. The fact that these patients did not, by definition, respond well to such treatment, suggests that the deleterious effects of such treatments may be pronounced in our sample. It is thus relevant that higher levels of akathisia were associated with larger left OFC WM volumes. As noted in the Introduction, the duration of typical APD treatment may be related to increases in volume of the basal ganglia (Lang et al., 2001), suggesting that the effects of such treatment may be cumulative. On the other hand, duration of illness did not account for the relationships between orbitofrontal volumes and either neuropsychological function or aggression. It is possible that long-term treatment with typical APDs had a nonlinear relationship with OFC volumes. The effects may plateau after a certain period of exposure. In other words, after a certain period of exposure to typical neuroleptics, a level of iatrogenic effects may be reached that does not progress further. As discussed by Jeste et al. (Jeste et al., 1998), most studies of the effects of typical APDs do not find a correlation between changes in volume and duration of illness. Chakos et al. (Chakos et al., 1994) suggested that this lack of correlation in the caudate may reflect reduced neuronal plasticity in older patients.
In this context, the patterns of relationships between OFC volumes, neuropsychological function, and aggression, are consistent with literature indicating a key role for that region in certain aspects of psychiatric symptomatology, neurocognitive function, and aggression.
With respect to the neurocognitive data, only the Attentional and Processing Speed Factor was related to OFC volumes. Of the specific tests administered, only the percentage of perseverative errors on the WCST, the number of errors on Trails B, and the span for the Letter Number span task were significantly related to OFC volumes. This pattern of findings suggests that variations in OFC volumes have relevance only for tasks that require cognitive abilities in which this region plays an important role. The performance of each of these tasks has in the past been shown to be dependent on the integrity of prefrontal regions. Similarly, the relationships between OFC volumes and aggression are consistent with a key role of the OFC in the modulation of such behavior (Grafman et al., 1996; Hoptman, 2003).
The current findings have implications for the interpretation of studies in which the relationship between neuropsychological and behavioral function and brain volumes are studied. Thus, for volumetric studies of schizophrenia, it is important to know the patients’ treatment history. Unfortunately, the participants in our study have a long duration of illness with many hospitalizations, but also many periods in which they were out of the hospital. As a result, their complete medical records were not available to us. Nonetheless, the issue is worthy of further attention.
The mechanism underlying the relationship between increased volume and poor neuropsychological and behavioral function is unclear. Although the basal ganglia volumetric data could possibly reflect a change in MRI signal properties with typical APD treatment that would artifactually affect results, this explanation seems highly unlikely given that the same effects have been found in post mortem tissue (Heckers et al., 1991).
Typical APDs are potent D2 receptor blockers. It has been hypothesized that the increased caudate volumes seen with typical APD treatment are the result of compensatory neural mechanisms related to blockade of the D2 receptors. This hypothesis is consistent with the observation that haloperidol increased the size of axon terminals in the striatum (Benes et al., 1985b) and increased the mean neuronal size in the medial prefrontal cortex (Benes et al., 1985a) of rats with no change in neuronal density. The argument has been raised that D2 blockade cannot completely explain haloperidol-related increases in striatal size because such effects are not seen, with equivalent D2 blockade, following RIS treatment (Lang et al., 2001). However, RIS’s effects on 5-HT2 receptors may partially compensate for its effects on D2 receptors.
It is also possible that these volumetric effects may be unrelated to treatment. The notion has been advanced that hyperactivity in the OFC may induce hypertrophy. Still another possibility is that aberrant cortical pruning might account for these effects (Swayze et al., 1992).
A recent study (Molina et al., 2004) using atlas based regions of interest has found that atrophy in the OFC region is associated with greater improvement in positive symptoms in patients with schizophrenia after 20–24 weeks of treatment with olanzapine. The authors suggested several possible mechanisms for this finding, including the speculation that N-methyl-D-aspartate (NMDA) receptor hypofunction may contribute both to regional atrophy and to positive symptoms. This suggestion was based on findings that the NMDA antagonist ketamine increases frontal metabolic activity (Vollenweider et al., 1997) and that a positive correlation between positive symptoms and orbital metabolism has been observed (Silbersweig et al., 1995). In addition, NMDA function plays a role in neuronal migration (Komuro and Rakic, 1993). If the receptors are hypofunctional, Molina et al. (2004) suggest, this might lead to gray matter deficits. Molina et al.’s interpretation cannot be ruled out from the present data. It should be noted that the patients in this study had a far lower duration of illness than the patients in the current study. It is therefore likely that their exposure to typical antipsychotics was considerable lower than the patients in our study.
The study has some important limitations. Firstly, a large number of tests were conducted, and we did not correct for multiplicity. We think that this is justified in light of the consistency of results across different domains and because of the exploratory nature of the analyses. Second, the subjects who received the MRI may not be representative of treatment-resistant schizophrenia patients, or even of the small sample from which they were selected. There were too few females to permit analyses of possible gender effects. We also were unable to evaluate the full medication history of the participants in the study. Finally, because we did not have a control group, we are unable to evaluate the specificity of these findings to schizophrenia. It is possible, for instance, that similar relationships to those we obtained here might be found in healthy controls.
In conclusion, in this sample of patients with treatment-resistant schizophrenia, larger OFC volumes were associated with poorer neuropsychological performance and aggression. Moreover, larger left than right OFC volumes were associated with comorbid substance use disorders. It seems possible that these results may be attributed to the iatrogenic effects of long-term ineffective treatment with typical APDs in this population, although it is also possible that they may related to the pathophysiology of schizophrenia.
Table 2.
Relationships between neuropsychological functions, aggression, and orbitofrontal cortex volumes
|
Left |
Right |
Asymmetry1 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | WM | GM | WM | GM | WM | |||||||
| Domain | t | P | t | P | t | P | t | P | t | P | t | P |
| Global | −2.12 | 0.04 | ||||||||||
| Attention/Processing | ||||||||||||
| 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Speed | ||||||||||||
| Variable | ||||||||||||
| TMT B Errors2 | 2.50 | 0.02 | 2.87 | 0.008 | ||||||||
| WCST Persev2 | 2.65 | 0.011 | −2.40 | 0.02 | ||||||||
| LNS Longest | 2.16 | 0.04 | ||||||||||
| Aggression | ||||||||||||
| TAS | 2.84 | 0.007 | 2.40 | 0.058 | ||||||||
| PANSS Hostility | −2.1 | 0.04 | ||||||||||
Note:
Positive = right > left, negative = left > right,
Higher scores indicate poorer performance, GM = gray matter, WM = white matter. TMT = Trail Making Test, WCST Persev = Wisconsin Card Sorting Test Percent Perseverative Errors, LNS = Letter Number Span, TAS =Total Aggression Score (log-transformed), PANSS = Positive and Negative Syndrome Scale.
Acknowledgments
NIMH grant (R10 MH53550) provided the principal support for this project. Additional support was provided by the UNC-Mental Health and Neuroscience Clinical Research Center (MH MH33127) and the Foundation of Hope, Raleigh North Carolina. We thank Janssen Pharmaceutica Research Foundation, Eli Lilly and Company, Novartis Pharmaceuticals Corporation, and Merck and Co., Inc. for their generous gifts of medications. Eli Lilly and Company contributed supplemental funding. However, overall experimental design, data acquisition, statistical analyses, and interpretation of the results were implemented without any input from any of the pharmaceutical companies. E.M. Weiss was supported by the Austrian Fonds zur Foerderung wissenschaftlicher Forschung (FWF: J2227-B05). MJH received additional support from R01 MH064783).
References
- Arango C, Breier A, McMahon R, Carpenter WT, Jr, Buchanan RW. The relationship of clozapine and haloperidol treatment response to prefrontal, hippocampal, and caudate brain volumes. American Journal of Psychiatry. 2003;160:1421–1427. doi: 10.1176/appi.ajp.160.8.1421. [DOI] [PubMed] [Google Scholar]
- Baaré WFC, Pol HEH, Hijman R, Mali WP, Th Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: Relation to cognitive function and symptomatology. Biological Psychiatry. 1999;45:1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Benes FM, Paskevich PA, Davidson J, Domesick VB. Synaptic rearrangements in medial prefrontal cortex of haloperidol-treated rats. Brain Research. 1985a;348:15–20. doi: 10.1016/0006-8993(85)90353-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Paskevich PA, Davidson J, Domesick VB. The effects of haloperidol on synaptic patterns in the rat striatum. Brain Research. 1985b;329:265–274. doi: 10.1016/0006-8993(85)90532-3. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horwitz TL, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol on treatment-resistant patients with schizophrenia and schizoaffective disorder. American Journal of Psychiatry. 2002a;159:1018–1028. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, Goldman RS, Hoptman MJ, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Lieberman JA. Neurocognitive correlates of the COMT Val158Met polymorphism in chronic schizophrenia. Biological Psychiatry. 2002b;52:701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Chakos M, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA. Striatal enlargement in rats chronically treated with neuroleptic. Biological Psychiatry. 1998;44:675–684. doi: 10.1016/s0006-3223(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. American Journal of Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Ross-Chouinard A, Annable L, Jones B. Extrapyramidal Symptom Rating Scale. Canadian Journal of Neurological Sciences. 1980;7:233. Ref Type: Abstract. [Google Scholar]
- Citrome L, Casey DE, Daniel DG, Wozniak P, Kochan LD, Tracy KD. Adjunctive divalproex and hostility among patients with schizophrenia receiving olanzapine or risperidone. Psychiatric Services. 2004;55:290–294. doi: 10.1176/appi.ps.55.3.290. [DOI] [PubMed] [Google Scholar]
- Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer JP, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatric Services. 2001;52:1510–1514. doi: 10.1176/appi.ps.52.11.1510. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, de Leon MJ, Patalinjug M, Kandil E, Caraos C, Scherer A, Saint Louis LA, Cancro R. Volumetric analysis of pre-frontal regions: Findings in aging and schizophrenia. Psychiatry Research: Neuroimaging. 2001;107:61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia voilume over 2 years in patients with schizophrenia: Typical versus atypical neuroleptics. Am J Psychiat. 1999;156:1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. Journal of Clinical Psychopharmacology. 1995;15:243–249. doi: 10.1097/00004714-199508000-00002. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Hoff AL, Schwartz JE, Shields GW, Halthore SN, Gupta S, Henn FA, Anand AK. Brain morphology in first-episode schizophrenia-like psychotic patients: A quantitative magnetic resonance imaging study. Biological Psychiatry. 1991;28:159–175. doi: 10.1016/0006-3223(91)90044-m. [DOI] [PubMed] [Google Scholar]
- First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (1995). Structured Clinical Interview for DSM-IV Axis-I Disorders: SCID Clinician Version, Biometrics Research Department, New York State Psychiatric Institute, Department of Psychiatry, Columbia University, New York.
- Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL. Childhood-onset schizophrenia: Brain MRI after 2 years of clozapine maintenance treatment. American Journal of Psychiatry. 1996;153:546–566. doi: 10.1176/ajp.153.4.564. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: Involvement in response inhibition. NeuroReport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: A report of the Vietnam Head Injury study. Neurology. 1996;46:1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Lattuada E, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Archives of General Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Publs Mass Med Soc. 1868;2:329–348. [Google Scholar]
- Heckers S, Heinsen H, Heinsen Y, Beckmann H. Cortex, white matter, and basal ganglia in schizophrenia: A volumetric postmortem study. Biological Psychiatry. 1991;29:556–566. doi: 10.1016/0006-3223(91)90091-y. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ. Neuroimaging studies of violence and antisocial behavior. Journal of Psychiatric Practice. 2003;9:265–278. doi: 10.1097/00131746-200307000-00002. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Ardekani BA, Butler PD, Nierenberg J, Javitt DC, Lim KO. DTI and impulsivity in schizophrenia: A first voxelwise correlational study. Biological Psychiatry. 2004;15:2467–2470. doi: 10.1097/00001756-200411150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter, aggression and impulsivity in men with schizophrenia. Biological Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Archives of General Psychiatry. 1991;48:881–890. doi: 10.1001/archpsyc.1991.01810340013002. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Lohr JB, Eastham JH, Rockwell E, Caligiuri MP. Adverse neurobiological effects of long-term use of neuroleptics: Human and animal studies. Journal of Psychiatric Research. 1998;32:201–214. doi: 10.1016/s0022-3956(97)00018-6. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Nohara S, Hagino H, Takahashi T, Matsui M, Yamashita I, Chitnis XA, McGuire PK, Seto H, Kurachi M. Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. European Archive of Psychiatry and Clinical Neuroscience. 2004;254:406–414. doi: 10.1007/s00406-004-0522-1. [DOI] [PubMed] [Google Scholar]
- Kay, S. R., Opler, L. A., & Fiszbein, A. (1986). Positive and Negative Syndrome Scale (PANSS), Bronx Psychiatric Center, N.Y.
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Lang DJ, Kopala LC, Vandrope RA, Rui Q, Smith GN, Goghari VM, Honer WG. An MRI study of basal ganglia volumes in first-episode schizophrenia patients treated with risperidone. American Journal of Psychiatry. 2001;158:625–631. doi: 10.1176/appi.ajp.158.4.625. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibtion. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biological Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV. Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry. 2003;60:245–252. doi: 10.1001/archpsyc.60.3.245. [DOI] [PubMed] [Google Scholar]
- Molina V, Sanz J, Benito C, Palomo T. Direct association between orbitofrontal atrophy and the response of psychotic symptoms to olanzapine in schizpohrenia. International Clinical Psychopharmacology. 2004;19:221–228. doi: 10.1097/01.yic.0000125753.01426.d7. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, Kubicki M, Shenton ME. Recent structural and functional imaging findings in schizophrenia. Current Opinion in Psychiatry. 2003;16:123–147. [Google Scholar]
- Ono, M., Kubik, S., & Abernathey, C. D. (1990). Atlas of the cerebral sulci, Thieme Medical Publishers, New York.
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: A cross-sectional and longitudinal MRI comparison. Lancet. 2002;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Marsh L. Structural brain imaging in schizophrenia: A selective review. Biological Psychiatry. 1999;46:627–649. doi: 10.1016/s0006-3223(99)00071-2. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophrenia Research. 2001;52:47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Kemmler G, Kremser C, Bilder RM, Mechtcheriakov S, Szeszko PR, Walch T, Scholtz AW, Klimbacher M, Maier C, Albrecht G, Lechner-Schoner T, Felber S, Hinterhuber H. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophrenia Research. 2005;74:149–161. doi: 10.1016/j.schres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Silver JM, Yudofsky SC. The overt aggression scale: Overview and guiding principles. Journal of Neuropsychiatry and Clinical Neurosciences. 1991;3:S22–S29. [PubMed] [Google Scholar]
- Swayze VWI, Andreasen NC, Alliger RJ, Yuh WTC, Erhardt JC. Subcortical and temporal structures in affective disorder and schizophrenia: A magnetic resonance imaging study. Biological Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir J, Ma J, Ashtari M, Wu H, Lieberman JA. Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Research: Neuroimaging. 1999;90:1–15. doi: 10.1016/s0925-4927(99)00002-5. [DOI] [PubMed] [Google Scholar]
- Talairach, J. & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging, Thieme Medical Publishers, Inc., New York.
- Volavka J, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Lieberman JA. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. Journal of Clinical Psychopharmacology. 2004;24:225–228. doi: 10.1097/01.jcp.0000117424.05703.29. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia and schizoaffective disorder. American Journal of Psychiatry. 2002;159:255–262. doi: 10.1176/appi.ajp.159.2.255. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: Implications in addiction. American Journal of Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G, Goldstein R. Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insight from imaging studies. Neurobiology of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- Woermann FG, van Elst LT, Koepp MJ, Free SL, Thompson PJ, Trimble MR, Duncan JS. Reduction of frontal neocortical grey matter associated with affective aggression in patients with temporal lobe epilepsy: An objective voxel by voxel analysis of automatically segmented MRI. Journal of Neurology, Neurosurgery and Psychiatry. 2000;68:162–169. doi: 10.1136/jnnp.68.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D. The Overt Aggression Scale for the objective rating of verbal and physical aggression. American Journal of Psychiatry. 1986;143:35–39. doi: 10.1176/ajp.143.1.35. [DOI] [PubMed] [Google Scholar]