Abstract
Immunization of mice with herpes simplex virus type 1 (HSV-1) mutant viruses containing deletions in the gene for virion host shutoff (vhs) protein diminishes primary and recurrent corneal infection with wild-type HSV-1. vhs mutant viruses are severely attenuated in vivo but establish latent infections in sensory neurons. A safer HSV-1 mutant vaccine strain, Δ41Δ29, has combined vhs and replication (ICP8−) deficits and protects BALB/c mice against primary corneal infection equivalent to a vhs− strain (BGS41). Here, we tested the hypothesis that Δ41Δ29 can protect as well as BGS41 in a therapeutic setting. Because immune response induction varies with the mouse and virus strains studied, we first determined the effect of prophylactic Δ41Δ29 vaccination on primary ocular infection of NIH inbred mice with HSV-1 McKrae, a model system used to evaluate therapeutic vaccines. In a dose-dependent fashion, prophylactic Δ41Δ29 vaccination decreased postchallenge tear film virus titers and ocular disease incidence and severity while eliciting high levels of HSV-specific antibodies. Adoptive transfer studies demonstrated a dominant role for immune serum and a lesser role for immune cells in mediating prophylactic protection. Therapeutically, vaccination with Δ41Δ29 effectively reduced the incidence of UV-B-induced recurrent virus shedding in latently infected mice. Therapeutic Δ41Δ29 and BGS41 vaccination decreased corneal opacity and delayed-type hypersensitivity responses while elevating antibody titers, compared to controls. These data indicate that replication is not a prerequisite for generation of therapeutic immunity by live HSV mutant virus vaccines and raise the possibility that genetically tailored replication-defective viruses may make effective and safe therapeutic vaccines.
Herpetic stromal keratitis (HSK) is a potentially blinding corneal inflammation that accompanies herpes simplex virus (HSV) infection of the eye. Following primary ocular infection, the virus becomes latent within sensory neurons innervating the cornea. Repeated episodes of virus reactivation exact a toll on corneal clarity. Corneal morbidity occurs when virus replication triggers immune-mediated pathological changes, including stromal opacities and neovascularization (8, 26, 33, 35), and may result in blindness. Consequently, HSV is a leading viral cause of sight-threatening disease in humans (34), and a vaccine that limits herpetic infection at ocular as well as other body sites is a highly desirable objective.
The “Holy Grail” in herpesvirus vaccinology is the development of a vaccine that prevents initial virus replication and the establishment of latency or, in previously infected individuals, eliminates virus reactivation, shedding, and clinical disease. Despite much time and effort, these lofty goals have been largely unmet (reviewed in references 2, 7, and 38). Of the potential vaccine candidates, live attenuated viruses have the advantage of generating broader and more durable immune responses than inactivated or glycoprotein subunit preparations because of their capacity to express viral proteins from within infected cells to stimulate cell-mediated as well as humoral immunity.
Virion host shutoff protein (vhs), the product of the UL41 gene, is a virus structural protein that destabilizes host and viral mRNAs after infection of a cell (17, 18). vhs− viruses grow normally in vitro but are severely attenuated in vivo (40). Vaccination with vhs− virus has been shown to protect mice against primary ocular infection with HSV (11, 43). The immunogenicity of vhs− vaccine strains may be related to overaccumulation of viral proteins (17) and/or uninterrupted expression of antigen presentation-associated major histocompatibility complex (MHC) I molecules in infected cells (1, 17, 24, 25, 40-42). Viruses without vhs activity retain the capacity to replicate and to establish latency, however, making them inherently less safe than replication-incompetent strains.
As vaccines, viral mutants that neither replicate in vaccinees nor establish latency with any measurable frequency offer improved safety. Viruses that bear mutations in the UL29 gene encoding ICP8, the viral single-stranded-DNA-binding protein, synthesize the panoply of HSV gene products that are expressed independently of viral DNA replication, but infected cells produce no progeny virus (22, 27, 47). ICP8−, replication-incompetent mutants of HSV-1 elicit immune responses that protect mice against corneal challenge with HSV-1 by decreasing replication in the cornea and establishment of neuronal latency by challenge virus (11, 27). Genetic crippling of the vaccine virus's capacity to replicate, however, limits the amount of viral antigen produced in the vaccinated host.
vhs is incorporated into virions and is expressed in cells infected with ICP8-deficient viruses. In order to enhance vaccine immunogenicity while retaining the safety associated with nonreplicating live virus vaccines, we created an HSV-1 mutant lacking vhs as well as ICP8 functions (11). We have recently examined the capacity of Δ41Δ29 (a vhs− ICP8− HSV-1 strain) to protect against primary ocular challenge of BALB/c mice with the mP strain of HSV-1 (11). In all regards, the degree of protection afforded by prechallenge vaccination with Δ41Δ29 was similar to that observed in mice vaccinated with a replication-competent vhs− virus vaccine and greater than that elicited by an ICP8− virus vaccine (11). Thus, prophylaxis against primary infection has been achieved by using vhs−, ICP8−, and vhs− ICP8− mutant viruses, but the immunologic basis for protection by vhs− virus is unknown.
Therapeutic success via vaccination has been more difficult to demonstrate (16, 30-32, 44). Therapeutic vaccination with vhs− virus has been the only vaccine modality shown to decrease UV-B-induced recurrent virus shedding in mice (44). In this report, we tested the hypothesis that Δ41Δ29 can serve as well as vhs− vaccine strains in a therapeutic setting. HSV-specific antibody and delayed-type hypersensitivity (DTH) responses were also assessed, in an initial investigation of vaccine-induced immunoprotective and pathological responses, respectively.
MATERIALS AND METHODS
Viruses and cells.
Vero and S2 cells were cultured as previously described (10). S2 cells (10) stably express the ICP8 protein (single-stranded-DNA-binding protein; product of UL29) of HSV-1. The replication-incompetent, vhs− ICP8− HSV-1 mutant Δ41Δ29 (11) was propagated on S2 cells, and the replication-competent, vhs− HSV-1 mutant BGS41 (40) was propagated on Vero cells. Infected-cell lysate and supernatant stocks of Δ41Δ29 and BGS41 were prepared as previously described (11, 28). Uninfected control cell lysates and supernatants were prepared in the same manner as infected stocks. The human isolate HSV-1 McKrae strain was propagated on Vero cells and used for primary ocular infection of mice in these studies (21).
In reactivation studies, recurrent ocular virus shedding was detected by culture of tear film swab material on Vero cells, which were then monitored for virus-induced cytopathic effects 48 and 96 h later. Virus titers in eye swabs were determined by standard plaque assay (16).
Mice and vaccination studies.
Investigations with female, National Institutes of Health (NIH) inbred and BALB/c mice conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. In prophylactic vaccination studies, 6-week-old NIH inbred or BALB/c mice were inoculated subcutaneously with 106 (high dose) or 5 × 104 (low dose) PFU of Δ41Δ29 supernatant-derived virus, consistent with previous prophylaxis experiments (11). Age-matched control mice received an equal volume of supernatant from mock-infected cells. Four weeks after vaccination, mice were challenged by corneal infection as described previously (21) with 106 (NIH inbred) or 105 (BALB/c) PFU of HSV-1 McKrae strain. Infection with challenge virus was confirmed by virus-positive tear film swabs taken 3 days postinfection. Animals were subsequently evaluated for the incidence and severity of corneal opacity, the incidence of periocular lesions, preinfection antibody titers (sera collected 3 days before challenge), and day 3 tear film virus titers.
In therapeutic vaccination studies, the UV-inducible model of recurrent HSK was used (21). Six-week-old NIH inbred mice (Harlan Olac Ltd., Oxford, England) were infected on the scarified right cornea with 106 PFU of HSV-1 McKrae. At the same time, each mouse received an intraperitoneal injection of 1 ml of pooled human serum (Chemicon, Temecula, Calif.; anti-HSV reactivity with an effective dose for 50% viral neutralization of 1:800) in order to protect corneas from damage during primary infection. Primary infection was confirmed by virus-positive tear film swabs taken 3 days postinfection. Four months after primary infection, latently infected mice received an intraperitoneal inoculation of 2 × 106 PFU of Δ41Δ29 or BGS41, consistent with previous therapeutic vaccine experiments (45). Control, latently infected mice received an equivalent volume of mock-infected cell lysate (control vaccine). Sera were collected 2 weeks postvaccination for determination of HSV-specific antibody titers.
Five months postinfection (4 weeks postvaccination), the eyes of latently infected and mock-infected (UV-B control) mice were exposed to 250 mJ of UV-B light cm2 using a TM20 Chromato-Vu transilluminator (UVP, Inc., San Gabriel, Calif.), which emits UV-B at a peak wavelength of 302 nm. Before (day 0, a control for spontaneous shedders) and on days 1 to 7 post-UV-B irradiation, mouse eyes were swabbed with surgical spears (Weckcel; Xomed-Treace, Jacksonville, Fla.) saturated with tissue culture medium, and swab material was cultured on Vero cells for 96 h to detect recurrent virus shedding or reactivation. Typical reactivation rates in unvaccinated NIH inbred mice vary from 50 to 70% (21). Therapeutically vaccinated mice were subsequently evaluated for recurrent virus shedding on days 1 to 7, tear film virus titers on peak shedding days (days 4 to 6), the incidence and severity of recurrent HSK, and HSV-specific antibody titers (sera collected 2 weeks postvaccination) and DTH responses.
Adoptive transfer experiments.
In adoptive transfer experiments, 8-week-old NIH inbred female donor mice were vaccinated intraperitoneally with 2 × 106 PFU of Δ41Δ29. Four weeks after vaccination, immune serum was isolated, and a single-cell suspension was prepared from combined spleens and lymph nodes (axillary and inguinal). Cells were divided into the following four treatment groups: one that was treated with complement only (undepleted) (Pel-Freez, Brown Deer, Wis.); a second that was treated with anti-CD4 antibody (clone 172.4 hybridoma supernatant) as previously described (12) plus complement; a third treated with anti-CD8 antibody (clone 53-6.72; BD Pharmingen, San Diego, Calif.) plus complement; and a fourth group of cells that was passed over a nylon wool column to deplete B cells. Serum and complement-treated cells were also prepared from naïve mice. All antibody plus complement depletions were carried out twice. Antibody plus complement treatments resulted in 90 to 97% deletion of relevant cells, as determined by fluorescence-activated cell sorting (FACS) analysis.
Cells (107/mouse, intravenously) or pooled serum (1 ml intraperitoneally, log10 enzyme-linked immunosorbent assay [ELISA] titer ± standard error of the mean [SEM] = 1.79 ± 0.20 ng/ml for naïve serum and 5.22 ± 0.06 ng/ml for immune serum) from donor mice were administered to 10-week-old female NIH inbred recipient mice 1 day before primary ocular infection with 106 PFU of HSV-1 McKrae strain. Groups of recipient mice consisted of those receiving (i) undepleted naïve cells (naïve), (ii) undepleted cells from vaccinated mice (vaccinated), (iii) CD4-depleted cells from vaccinated mice (vaccinated/CD4−), (iv) CD8-depleted cells from vaccinated mice (vaccinated/CD8−), (v) nylon wool-enriched T cells from vaccinated mice (vaccinated/T cell enriched), (vi) serum from vaccinated mice (immune serum), and (vii) serum from naïve mice (naïve serum). After primary infection, recipient mice were monitored as in previous vaccine experiments.
Clinical disease.
The incidence and severity of herpetic stromal keratitis were monitored on the indicated days after primary infection (prophylactic vaccination and adoptive transfer studies) or UV-B-stimulated virus reactivation (therapeutic vaccination studies). Clinical eye disease was scored by a masked observer using a binocular dissecting microscope. Herpetic stromal keratitis (corneal opacity) was rated on a scale of 0 to 4, where 0 indicates clear stroma, 1 indicates mild stromal opacification, 2 indicates moderate opacity with discernible iris features, 3 indicates dense opacity with loss of defined iris detail except pupil margins, and 4 indicates total opacity with no posterior view. Animals with corneal opacity scores of ≥1 were deemed positive for incidence determinations. All grossly detectable lid lesions were assessed as positive for determination of periocular disease incidence. Evidence of encephalitis included hunched back, ruffled hair, and obvious neurologic abnormalities.
Assays of antibody titers.
Serum was collected from mice 4 weeks after prophylactic vaccination or 2 weeks after therapeutic vaccination and examined for HSV-specific antibody content as previously described (11, 27). Briefly, for ELISA, serial fourfold dilutions of mouse serum were incubated for 2 h in duplicate wells of a 96-well plate coated with purified HSV-1 glycoprotein. Biotinylated goat anti-mouse immunoglobulin G (IgG) was subsequently used in a colorimetric assay to determine specific IgG amounts based on comparison to a standard curve generated as previously described (11). For neutralization studies, serial twofold dilutions of serum in medium or medium alone were incubated with an equal volume of HSV-1 at 103 PFU/ml and incubated for 2 h at 4°C. Two hundred microliters of the mixture was then adsorbed to Vero cell monolayers, and a standard plaque assay was carried out in duplicate. The neutralizing antibody titer was defined as the serum dilution yielding ≥50% reduction in the number of plaques.
DTH responses.
As previously described, 5 × 106 PFU (before inactivation) of UV-inactivated HSV-1 McKrae strain in 30 μl of medium was injected into the right rear footpad of mice (16). The left rear footpad was injected with the same amount of virus-free tissue culture medium. Footpad swelling was measured with a micrometer (Mitutoyo Manufacturing, Tokyo, Japan) immediately prior to and 24 h after injection. HSV-specific footpad swelling was determined by the formula [(right footpad swelling at 24 h − right footpad swelling before injection) − (left footpad swelling at 24 h after injection − left footpad swelling before injection)]. DTH responses were determined 2 weeks after UV-B irradiation.
Statistical analyses.
All statistics were performed with the aid of SigmaStat for Windows version 2.0 (Jandel, Corte Madera, Calif.). Student's t test and the Mann-Whitney rank sum test were used as appropriate to analyze opacity, antibody and virus titers, and the number of virus shedding days. Geometric means were used in analysis of antibody and virus titers. A Fisher's exact or chi square test was used to examine the incidence of HSK lesions and periocular disease, percentage of mice shedding virus/day, percent virus-positive swabs, and reactivation rates.
RESULTS
Prophylactic immunization studies.
Research indicates that host and viral genetic factors may influence immune responses and the clinical outcome of ocular HSV infection (9, 13). Our model of UV-B-induced reactivation requires use of the NIH mouse strain and the McKrae strain of HSV-1. HSV-1 McKrae strain is a highly virulent human isolate and represents a rigorous challenge to existing immunity (45). Thus, although it was previously shown to be effective as a prophylactic vaccine in BALB/c mice challenged with HSV-1 mP strain (11), we examined the capacity of Δ41Δ29 (vhs− ICP8−) virus to protect naïve NIH inbred mice from challenge with the HSV-1 McKrae strain.
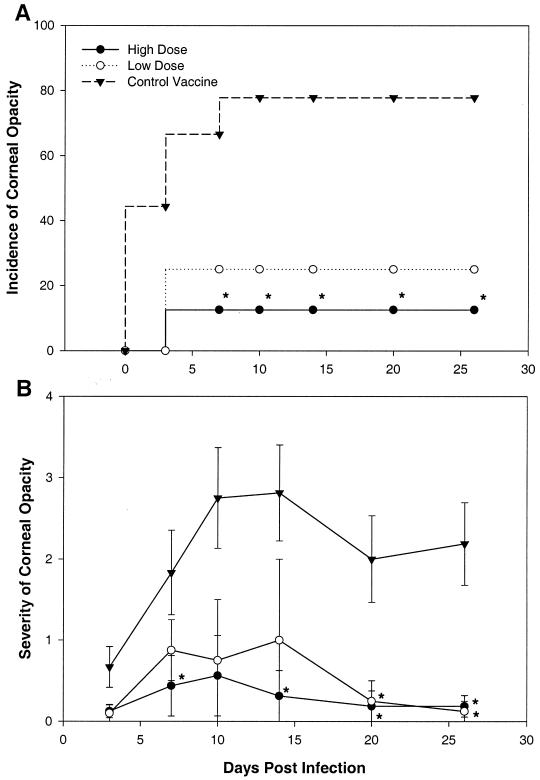
Accordingly, mouse corneas were infected with 106 PFU of McKrae 4 weeks after subcutaneous vaccination with 106 (high dose) or 5 × 104 (low dose) PFU of the live but replication-incompetent Δ41Δ29 vaccine strain. An equal number of mice were inoculated with uninfected cell supernatant (control vaccine). BALB/c mice were vaccinated and infected in parallel with NIH inbred mice. As demonstrated in Fig. 1A and B, high-dose prophylactic immunization with Δ41Δ29 greatly reduced both the incidence and severity of corneal opacity in NIH mice from 7 to 26 days postinfection. Low-dose vaccination had a significant effect on disease severity only on days 20 through 26, suggesting dose dependence of the response to vaccination with Δ41Δ29.
FIG. 1.
Incidence and severity of corneal opacity after viral challenge of prophylactically vaccinated mice. High or low doses of live HSV-1 mutant Δ41Δ29 (vhs− ICP8−) or control vaccine were administered to NIH inbred mice 4 weeks before corneal infection with 106 PFU of HSV-1 McKrae. Postchallenge corneal opacity was scored in a masked fashion at the indicated time points, and an opacity score of ≥1 was considered positive for incidence determinations. (A) Incidence determination, reported as a percentage. ∗, P = 0.015 to 0.05. (B) Severity determinations, reported as mean opacity scores ± SEM. ∗, P = 0.005 to 0.05, n = 5 to 9 mice per group.
HSV-specific antibody titers in serum collected 4 weeks after vaccination showed an elevated response only in mice receiving the higher vaccine dose (Table 1). After corneal challenge, the incidence of periocular lesions was also diminished in animals receiving the higher vaccine dose (Table 1). Protection from acute herpetic keratitis in the high-dose group accompanied a significant decrease in day 3 viral tear film titers (Table 1). In BALB/c mice, we found that high-dose Δ41Δ29 vaccination also protected animals from HSV-1 McKrae infection of the cornea while stimulating elevated levels of HSV-specific antibodies (data not shown). Therefore, prechallenge vaccination with Δ41Δ29 was as protective against primary corneal disease in NIH inbred mice as it was in BALB/c mice here and in previous studies (11).
TABLE 1.
Antibody and virus titers and periocular disease in prophylactically vaccinated mice
| Immunization group | Serum antibody titera (log10 ng/ml ± SEM) | Tear film virus titerb (log10 PFU/ml ± SEM) | Periocular diseasec (% positive) |
|---|---|---|---|
| High-dose Δ41Δ29 | 3.20 ± 0.20 (P < 0.0001)d | 1.49 ± 0.54 (P = 0.015, 0.047)d | 0 (P < 0.001, 0.018)d |
| Low-dose Δ41Δ29 | 0.75 ± 0.08 (P < 0.0001) | 2.98 ± 0.28 | 25 |
| Control vaccine | 0.70 ± 0.10 (P < 0.0001) | 3.13 ± 0.25 | 100 |
Titer of HSV-specific IgG determined by ELISA 4 weeks after vaccination and just prior to ocular challenge (n = 5 to 9 per group).
Virus titer in tear film samples collected 3 days after ocular challenge with 106 PFU of HSV-1 McKrae (n = 5 to 9 per group).
Cumulative percentage of mice exhibiting periocular lesions at 20 days postinfection (n = 5 to 9 per group).
P versus control vaccine (first value) and low-dose (second value) groups.
Adoptive transfer studies.
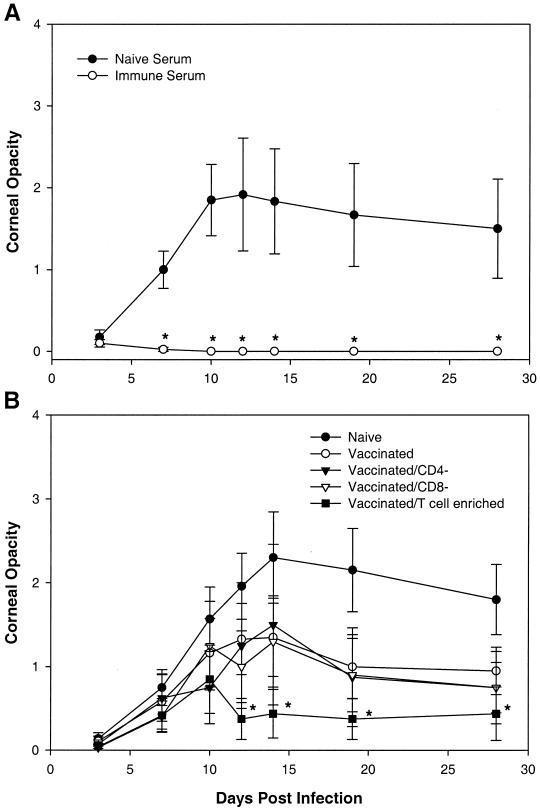
To assess the role of serum antibodies and immune cells in protection resulting from prophylactic Δ41Δ29 vaccination, an adoptive transfer experiment was performed. In this study, cells or serum from NIH inbred donor mice vaccinated 4 weeks previously with Δ41Δ29 were administered to naïve recipient mice 1 day before ocular HSV infection. Mice receiving serum from Δ41Δ29-vaccinated donors (immune serum) were completely protected against periocular disease, encephalitis, and death after corneal challenge infection compared with mice injected with naïve donor serum (Table 2). Serum from Δ41Δ29-vaccinated donors also greatly decreased the incidence (Table 2) and severity (Fig. 2A) of corneal opacification in infected recipient mice.
TABLE 2.
Virus titers and disease parameters in mice receiving prophylactic passive transfer of seruma
| Serum | Tear film virus titer (log10 PFU/ml ± SEM) | Cumulative % of eyes
|
Cumulative % of mice
|
||
|---|---|---|---|---|---|
| Periocular disease | Corneal opacity | Encephalitis | Mortality | ||
| Naïve | 4.21 ± 0.18 | 85 | 65 | 70 | 70 |
| Immune | 3.27 ± 0.20 (P = 0.003) | 0 (P < 0.001) | 0 (P < 0.001) | 0 (P = 0.002) | 0 (P = 0.002) |
Donor mice were vaccinated with Δ41Δ29 as described in the text 4 weeks before passive transfer. One day before primary corneal infection with HSV-1, recipient mice were given an intraperitoneal injection of 1 ml of pooled serum from either naïve or vaccinated donor mice (n = 10 mice and 20 eyes per recipient group). The anti-HSV ELISA titer was 1.79 ± 0.02 log10 ng/ml for naïve serum and 5.22 ± 0.06 log10 ng/ml for immune serum. Tear film samples were collected 3 days after ocular challenge with 106 PFU of HSV-1 McKrae. The cumulative percentage of eyes exhibiting periocular lesions or a corneal opacity score of ≥1 at 20 days postinfection is shown, and the cumulative percentage of mice exhibiting signs of encephalitis or mortality at 20 days postinfection is shown.
FIG. 2.
Corneal opacity after primary ocular HSV infection of mice receiving adoptive transfers of serum or cells from Δ41Δ29-vaccinated donors. Donor mice were vaccinated with Δ41Δ29 as described in Materials and Methods 4 weeks before harvest of serum and cells. Serum (1 ml, intraperitoneally) (A) or spleen and lymph node cells (107/mouse intravenously) (B) from naïve and Δ41Δ29-vaccinated donor mice were administered to NIH inbred recipient mice 1 day before primary ocular infection with 106 PFU of HSV-1 McKrae strain. ∗, P < 0.001 to 0.042, n = 6 to 20 eyes (A) or 4 to 10 eyes (B) per recipient group.
As with mice receiving direct prophylactic vaccination (Table 1), recipients of serum from Δ41Δ29-immunized mice exhibited decreased day 3 tear film virus titers (Table 2). In contrast to immune serum, the protective effects of immune cells from Δ41Δ29-vaccinated animals were limited to corneal disease. While all spleen or lymph node cell preparations (vaccinated, vaccinated/CD4−, vaccinated/CD8−) from immunized mice tended to reduce disease intensity compared to the naïve cell control, only the vaccinated/T-cell-enriched group had a significant effect (Fig. 2B, days 12 to 28; P = 0.007 to 0.033). Thus, our adoptive transfer studies demonstrated a dominant role for immune serum and a lesser role for immune cells in mediating prophylactic protection by Δ41Δ29 vaccination.
Therapeutic immunization studies.
In previous work, immunization of latently infected mice with a replication-competent, vhs-defective virus decreased UV-B radiation-induced ocular HSV shedding (44). These findings prompted us to examine the performance of Δ41Δ29 as a therapeutic vaccine in the same experimental setting. Toward that goal, mice with latent ocular HSV infections were vaccinated with Δ41Δ29 (vhs− ICP8−), BGS41 (vhs−), or control vaccine 4 months after primary eye infection with 106 PFU of HSV-1 McKrae. Two weeks after vaccination, HSV-specific serum antibody titers were assessed as a marker of immunity. Four weeks after vaccination, the corneas of all latently infected eyes were exposed to 250 mJ of UV-B irradiation cm−2 to induce recurrent virus shedding. The eyes of uninfected animals were also irradiated to provide a control for transient corneal clouding associated with UV-B exposure alone (UV control). Mice were subsequently monitored for recurrent virus shedding, corneal pathology, and DTH responses.
Antibody responses.
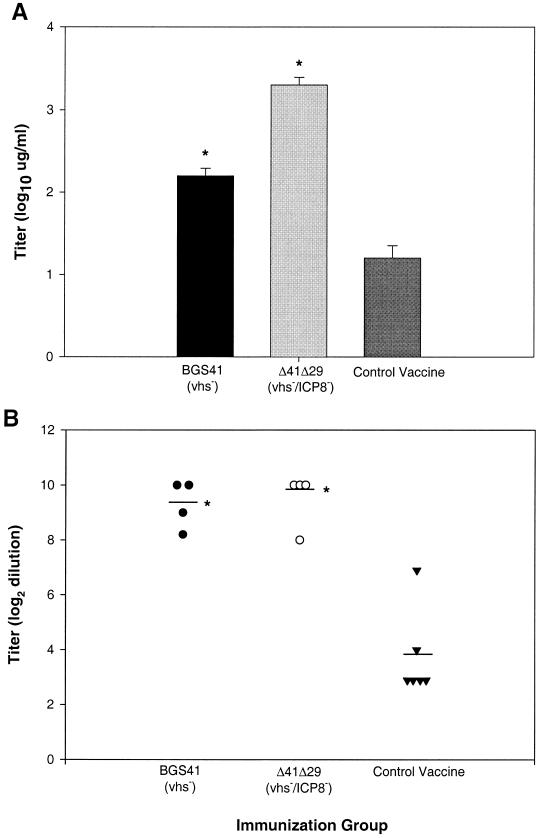
As one measure of immune induction, we determined the titer of HSV-specific IgG antibody in individual serum samples from mice 2 weeks after therapeutic vaccination. As demonstrated in Fig. 3A and B, therapeutic vaccination with Δ41Δ29 or BGS41 enhanced HSV-specific serum antibody levels, as determined by ELISA and virus neutralization. In one of two experiments, the ELISA antibody response in Δ41Δ29-treated mice was greater than that of BGS41 vaccinees. Nevertheless, protective virus neutralization titers were similar in both groups.
FIG. 3.
HSV-specific antibody titers in serum of therapeutically vaccinated mice. Four months after primary corneal infection, mice with latent ocular HSV infections were vaccinated with HSV-1 mutants or control vaccine. Two weeks after vaccination, HSV-specific antibody levels in serum were determined by (A) ELISA and (B) virus neutralization assay. Results are shown for one of two experiments with similar results and reported as mean log titer ± SEM for ELISA (n = 12) and virus neutralization (n = 4 to 6). ∗, P < 0.001 to 0.003 compared to control vaccine.
Recurrent virus shedding.
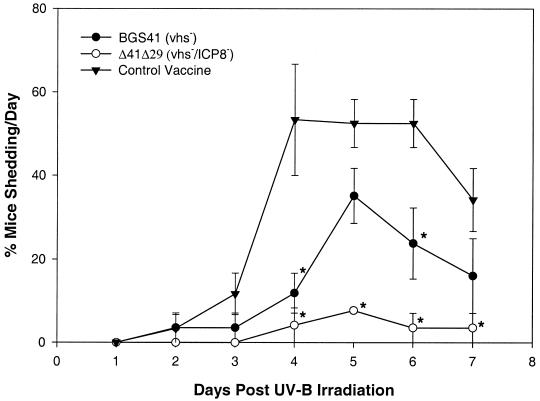
After UV irradiation, three measures of the incidence of recurrent virus shedding were assessed: percentage of mice shedding virus per day (Fig. 4), percent virus-positive swabs for all 7 days, and percentage of mice per group that shed virus at any time during the 7-day period (reactivation rate) (Table 3). In two independent experiments (combined for data presentation), it was observed that immunization with BGS41 resulted in significant reductions in the proportion of mice shedding virus on days 4 and 6 (Fig. 4) and percentage of virus-positive eye swabs (Table 3). There was no significant decline in reactivation rate in the BGS41 group, although the rate was reduced compared with control mice. Δ41Δ29 vaccination decreased the percentage of mice shedding virus per day on days 4 through 7 (Fig. 4), the percent virus-positive eye swabs, and the reactivation rate (Table 3) compared to control vaccinated mice.
FIG. 4.
Time course of virus shedding after ocular UV-B irradiation of therapeutically vaccinated mice. Mice with latent ocular HSV infections were vaccinated with HSV-1 mutants or control vaccine as described for Fig. 3, and their eyes were UV-B irradiated 4 weeks later. Recurrent virus shedding was detected in tear film swabs taken 1 to 7 days after irradiation. Values are the percentage of mice shedding virus on each day ± the range in two pooled experiments with similar results (n = 26 to 27 mice per group). ∗, P < 0.001 to 0.047 compared to control vaccine.
TABLE 3.
Recurrent virus shedding in therapeutically vaccinated micea
| Immunization group | % Virus-positive mice | % Virus-positive swabs | Mean no. of days of virus shedding ± SEM |
|---|---|---|---|
| BGS41 (vhs−) | 35 ± 7 (P = 0.038)* | 13 ± 3 (P = 0.001)*† | 2.7 ± 0.4 |
| Δ41Δ29 (vhs− ICP8−) | 8 ± 1 (P < 0.001)† | 3 ± 1 (P < 0.001)† | 2.5 ± 0.5 |
| Control vaccine | 60 ± 7 | 29 ± 4 | 3.2 ± 0.3 |
The percentage of mice shedding virus (26 to 27 mice per group) and the percentage of virus-positive eye swabs (182 to 189 eye swabs per group) were determined for the 7-day period following UV-B irradiation. Values are combined data from two experiments with equivalent results and are reported as percent ± range. The data for days shedding virus are for two combined experiments. *, P versus Δ41Δ29; †, P versus control vaccine.
When we examined titers of virus in the tears from shedding mice, no significant differences were found between the vaccinated and control groups on any day after reactivation (data not shown). Likewise, there were no differences in the number of days of virus shedding between any two groups (Table 3). Thus, therapeutic vaccination of previously infected mice with Δ41/Δ29 and BGS41 decreased the incidence of induced virus shedding, but once stimulated to shed virus, virus titers and the number of days of shedding the virus were similar to the values for control eyes. Of note, in these experiments, Δ41Δ29 proved at least as effective as replication-competent BGS41 in its protective effects.
Recurrent herpetic stromal keratitis.
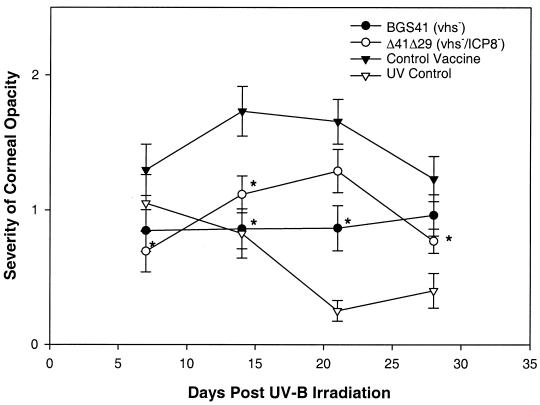
Because herpetic stromal keratitis is thought to be an immune-mediated disease triggered by virus infection or reactivation, therapeutic immunization has the potential to elicit immune responses that exacerbate corneal disease. Accordingly, we examined post-UV corneal opacity in latently infected, vaccinated mice. We found that the incidence of opacity among shedders was not different between the groups (data not shown). However, the severity of corneal opacity in mice vaccinated with the HSV mutants was lower than that of the control vaccine group at all time points, with significant reductions occurring on days 7, 14, and 28 or days 14 and 21 for Δ41Δ29 and BGS41, respectively (Fig. 5). Thus, instead of making corneal disease worse, therapeutic vaccination with both HSV-1 mutants decreased corneal pathology in mice treated to stimulate virus reactivation.
FIG. 5.
Severity of corneal opacity in therapeutically vaccinated mice after ocular UV-B irradiation. Latently infected mice were vaccinated and irradiated as described for Fig. 4. Eyes of uninfected mice were also irradiated to control for transient corneal clouding associated with UV-B exposure (UV control). Post-UV-B corneal opacity was scored in a masked fashion at the indicated time points. Results are combined data from two experiments with equivalent results and are reported as mean opacity score ± SEM for 26 to 27 mice per group. ∗, P < 0.001 to 0.045 compared to control vaccine.
DTH responses.
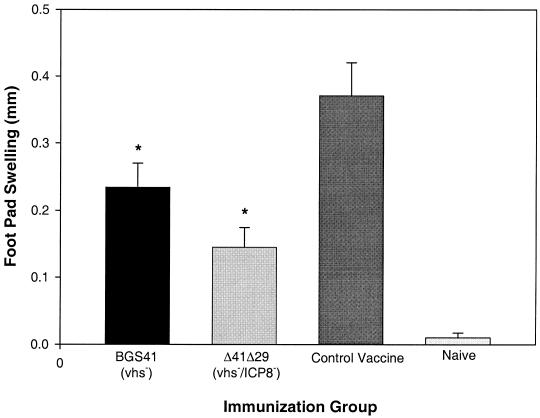
DTH activity is a T-cell-mediated immune response thought to play a role in the immunopathogenesis of herpetic stromal keratitis that accompanies primary infection (20). In addition, numerous studies in our laboratory have demonstrated a correlation between the magnitude of the DTH response and the severity of recurrent HSK (T. L. Keadle, unpublished observations). We therefore examined the effect of therapeutic vaccination on DTH responses to HSV antigens 2 weeks after UV-B irradiation. The data in Fig. 6 indicate that, while maintaining a significant DTH response compared to naïve animals, Δ41Δ29- and BGS41-vaccinated mice had lower responses compared to the control vaccinated group. Our observations of low DTH responses and decreased corneal opacity suggest that therapeutic vaccination with Δ41Δ29 favored protective rather than pathological immune responses.
FIG. 6.
HSV-specific DTH responses in therapeutically vaccinated mice. Latently infected mice were vaccinated and irradiated as described for Fig. 4, and DTH testing was performed on mouse footpads 2 weeks later. Naïve mice served as negative controls. Results are combined data from two experiments with equivalent results and are reported as mean HSV-specific footpad swelling ± SEM for 18 to 19 mice per group. ∗, P = 0.002 to 0.05 compared to control vaccine. DTH responses in all vaccine groups exceeded values for naïve mice (P < 0.001).
DISCUSSION
Because recurrent HSK has the greatest potential to cause visual impairment, vaccines effective in a therapeutic setting are highly desirable. In contrast to vaccination of naïve animals, only a handful of agents have been used successfully in treating mice harboring latent ocular HSV infection (16, 30-32, 44). The nature of reactivation events and the role of the primed immune system in controlling it remain somewhat enigmatic, and this contributes to difficulties in designing an effective therapeutic vaccine regimen. Further compounding the problem are diseases such as herpetic keratitis, in which immunopathologic elements are key to lesion development. As a consequence, any vaccine that decreases recurrent virus shedding without exacerbating recurrent herpetic stromal keratitis is of value.
In this work, we demonstrated that vaccination with vhs− ICP8− virus protects mice from UV-B-induced recurrent virus shedding and corneal disease as well as primary ocular challenge with HSV. These findings are in accord with earlier studies in which prophylactic immunization with vhs−, ICP8−, or vhs− ICP8− mutant HSV-1 strains reduced the effects of wild-type HSV corneal infection (11, 27, 43). They also support the observation that vaccination with vhs− viruses decreases the incidence of virus shedding in previously infected mice (44).
Data presented here for therapeutic vaccination with Δ41Δ29 indicate that protection by the replication-incompetent double mutant virus is on a par with that of the replication-competent BGS41 vaccine strain, as was previously seen for prophylactic immunization (11). Accordingly, compared to control vaccinated mice, parameters associated with the incidence of recurrent virus shedding (Fig. 4, Table 3) were reduced in mice given Δ41Δ29 4 weeks prior to stimulation of virus reactivation by UV-B light. By all measurements, reductions in incidence of virus shedding in Δ41Δ29-vaccinated mice were equivalent to or even greater than those in BGS41-vaccinated mice, and these results were consistent in two independent experiments. Reductions in postreactivation corneal opacity (Fig. 5) were also comparable, indicating that, at a minimum, therapeutic vaccination did not adversely affect corneal pathology in animals with recurrent HSK. Thus, it appears that the capacity to replicate is not a prerequisite for vaccine efficacy. Even so, a vhs− ICP8− virus was shown to be more effective than a replication-defective, ICP8− virus in controlling primary HSV infection of the eye (11), suggesting that deletion of vhs function significantly enhances immune induction by replication-incompetent vaccine viruses.
The observation that Δ41Δ29 was reproducibly more effective than BGS41 in decreasing the incidence of recurrent virus shedding may be due to a higher particle-to-PFU ratio, although UV-inactivated virus as a vaccine is significantly less effective than live replication-defective virus in prophylactic protection. Alternatively, replication-defective virus may induce a greater proportion of protective, nonpathological responses. Distinguishing between these or other possibilities will require further experimentation.
Reduced incidence of virus shedding had previously been reported for mice therapeutically vaccinated with vhs− virus (44), and our results extend those findings to Δ41Δ29, a safer, replication-defective relative of the vhs-deficient vaccine. Protection from renewed virus shedding and corneal disease has also been demonstrated in the rabbit eye model of spontaneous ocular HSV recurrence after glycoprotein vaccine therapy (30-32). The clinical usefulness of these subunit vaccines may be limited, however, because of the necessity for powerful adjuvants that have adverse side effects, the limited duration and types of immune responses elicited, and the apparent requirement for periocular vaccination, a route unlikely to be clinically practical.
In this work, vaccine therapy affected post-UV-B corneal opacity in latently infected mice (Fig. 5). Fewer eyes with reactivated virus in the Δ41Δ29 and BGS41 vaccine groups is a possible cause of the reduction in corneal opacity. However, a similar pattern of reduced opacity in Δ41Δ29- and BGS41-treated mice can be detected when only reactivated (virus shedding) eyes are considered (data not shown). Lowered DTH responses in Δ41Δ29- and BGS41-vaccinated mice support the idea that vaccine-induced decreases in opacity result from alterations of the immune response and not the relative proportion of virus-shedding eyes per group. Finally, because corneal opacity can occur in nonreactivated (non-virus-shedding) eyes in association with viral recurrence in deep corneal layers (21), the data reported for all UV-irradiated eyes (reactivated and nonreactivated) in Fig. 5 may accurately reflect the population effect of vhs− vaccines.
Clues to the mechanism of protection afforded by Δ41Δ29 immunization are provided by adoptive transfer results in the primary infection model. From these results, it is clear that HSV-specific antibodies play a major role in vaccine-induced protection (Table 2, Fig. 2A). Indeed, it is known that antibodies can preserve corneal clarity during primary ocular infection with HSV, and passively transferred anti-HSV antibody can inhibit spread of virus within the nervous system. (19, 21, 29, 37). Thus, Δ41Δ29-stimulated anti-HSV antibodies are likely an important factor in reducing disease manifestations such as opacity, encephalitis, and death after primary infection in prophylactically vaccinated mice as well as recipients of immune serum (Tables 1 and 2, Fig. 1 and 2).
Reductions in viral tear film titers by passively transferred Δ41Δ29-immune serum were surprising, since previous work had shown no effect of replication-defective virus-immune serum on viral tear film titers (29). Because ICP8− virus was not compared to vhs− ICP8− virus in our current experiments, we cannot say whether the apparent difference in the two serum passive transfer results is due to quantitative or qualitative differences in the sera. While T-cell responses have been suggested to be important in controlling the extent and incidence of periocular disease (3, 14), in passive transfer experiments we found that immune serum was also capable of preventing periocular disease, possibly in conjunction with recipient T-cell activity.
A role for vaccine-induced T cells in reducing corneal opacity is suggested by the observation that naïve mice given the T-cell-enriched preparation from vaccinated donors (Fig. 2B) before primary infection developed less corneal disease. The significant decrease in opacity was observed only in nylon wool-treated cells, probably reflecting the larger proportion of T cells in this group (vaccinated/T-cell enriched). Protection against corneal opacity by T cells during primary infection is of interest because of their association with immunopathology (8, 26, 33, 35), and further studies are needed to define the mechanism of this protection. Δ41Δ29-induced T cells could protect against opacity by providing help for antibody production and activity, by creating an immunosuppressive cytokine environment within the cornea, or by rapidly removing virus-infected cells through cytolysis.
An understanding of the protective mechanisms elicited by therapeutic administration of Δ41Δ29 is complicated by the still undefined aspects of virus reactivation and immunomodulation in latently infected hosts. Nevertheless, our findings are consistent with the possibility that both antibody responses and cell-mediated immunity play roles in protection against recurrent virus shedding and disease in mice vaccinated with vhs− viruses. Accordingly, Δ41Δ29 and BGS41 vaccination of previously infected mice resulted in induction of robust anti-HSV antibody responses (Fig. 3) compared to control vaccinated animals. In therapeutically vaccinated animals, this antibody might limit virus trafficking between neurons or between neurons and the cornea. Indeed, we observed that Δ41Δ29-vaccinated mice had a decreased incidence of shedding (reactivation events), but those mice that shed did so for the same number of days and with the same titer as control vaccinated mice. This observation suggests that antibody may effectively inhibit reactivation in the nervous system but not replication once virus reaches the periphery.
As in other systems, antibodies are unlikely to be the sole cause of reductions in the numbers of virus-shedding mice (5, 39) or mice with HSV-related pathology, and a number of studies have linked immunization-stimulated cell-mediated immunity with protection from HSV infection (3, 6, 14, 15, 36, 46). With regard to therapeutic Δ41Δ29 vaccination, elevated antibody titers suggest that helper T-cell-dependent responses are increased in comparison to control vaccinated animals (Fig. 3). While T-cell-mediated immune responses are aimed at controlling viral replication, T-cell-mediated DTH responses may have deleterious effects on corneal clarity and vision during primary (20) and recurrent (T. L. Keadle, unpublished observation) infection. Thus, lowered DTH responses in vhs− virus vaccinees (Fig. 6) could contribute to decreased opacity after UV-B exposure and indicate that T-cell responses are favorably altered by these vaccine strains.
One of the principal effects of therapeutic vaccination with the vhs-deficient viruses used in this study appears to be on viral reactivation events, with a resulting decrease in the incidence of inducible virus shedding. Several lines of evidence suggest that cell-mediated immunity responses, including cytotoxic T-cell (CTL) activity and gamma interferon production, play a central role in controlling recurrent HSV infections (2, 7, 38). There are also data to support the notion that CD8+ T cells can block HSV-1 reactivation from latency in sensory neurons (23).
While CTL responses were not examined in this work, ICP8− HSV strains have been shown to elicit strong virus-specific CTL activity (4). vhs-deficient viruses, in addition to causing overexpression of viral proteins, should support continuous expression of cellular MHC I molecules important for recognition and lysis of infected cells by MHC I-restricted CTL (17, 42). It follows, then, that vhs− or vhs− ICP8− mutant vaccine strains such as BGS41 and Δ41Δ29 could generate CTL responses comparable to or better than those of wild-type viruses, which in turn may be responsible for suppression of virus shedding in latently infected mice. Examination of CTL activity and immunohistochemical analysis of CD8+/CTL and cytokine presence in trigeminal ganglia of vaccinated mice, along with quantitation of latency-associated transcript-positive (LAT+) neurons, may provide support for these suppositions.
In summary, we have examined the capacity of Δ41Δ29, a replication-defective, vhs-deficient virus, to control primary and recurrent ocular HSV infection in mice. The vaccine was effective in both regards. However, until prophylactic vaccination becomes universally applied and protective, therapeutic vaccines may hold the most potential for future benefit to infected individuals. Thus far, vaccine successes in animal models have not been fully realized in humans (2, 7, 38). Success may await the development of safe vaccines tailored to magnify species-specific protective immune responses (7). Customized vhs− and replication-defective HSV mutants offer hope in this regard.
Acknowledgments
We thank Robert Reass and Stacey Plambeck for technical assistance.
This work was supported by National Eye Institute grants EY118850 and EY02687 and a departmental RPB unrestricted grant to P.M.S. and by Public Health Service award CA75052 to L.A.M.
REFERENCES
- 1.Banks, T. A., S. Nair, and B. T. Rouse. 1993. Recognition by and in vitro induction of cytotoxic T lymphocytes against predicted epitopes of the immediate-early protein ICP27 of herpes simplex virus. J. Virol. 67:613-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein, D. I., and L. R. Stanberry. 1999. Herpes simplex virus vaccines. Vaccine 17:1681-1689. [DOI] [PubMed] [Google Scholar]
- 3.Brandt, C. R. 1992. Susceptibility of +/+, +/nu and nu/nu BALB/c mice to ocular herpes simplex virus infection. Ophthalm. Res. 24:332-337. [DOI] [PubMed] [Google Scholar]
- 4.Brehm, M. A., R. H. Bonneau, D. M. Knipe, and S. S. Tevethia. 1997. Immunization with a replication-deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+ cytotoxic T-lymphocyte response and confers a level of protection comparable to that of wild-type HSV-1. J. Virol. 71:3534-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke, R. L., C. Goldbeck, P. Ng, L. Stanberry, G. Ott, and G. Van Nest. 1994. The influence of adjuvant on the therapeutic efficacy of a recombinant genital herpes vaccine. J. Infect. Dis. 170:1110-1119. [DOI] [PubMed] [Google Scholar]
- 6.Chan, W. L., M. L. Lukig, and F. Y. Liew. 1985. Helper T cells induced by an immunopurified herpes simplex virus type 1 (HSV-1) 115 kilodalton glycoprotein (gB) protect mice against HSV-1 infection. J. Exp. Med. 162:1304-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande, S. P., U. Kumaraguru, and B. T. Rouse. 2000. Why do we lack an effective vaccine against herpes simplex virus infections? Microbes Infect. 2:973-978. [DOI] [PubMed] [Google Scholar]
- 8.Doymaz, M. Z., and B. T. Rouse. 1992. Immunopathology of herpes simplex virus infection. Curr. Top. Microbiol. Immunol. 179:121-128. [DOI] [PubMed] [Google Scholar]
- 9.Foster, C. S., Y. Tsai, J. G. Monroe, R. Campbell, M. Cestari, R. Wetzig, D. Knipe, and M. I. Greene. 1986. Genetic studies on murine susceptibility to herpes simplex keratitis. Clin. Immunol. Immunopathol. 40:313-325. [DOI] [PubMed] [Google Scholar]
- 10.Gao, M., and D. M. Knipe. 1989. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J. Virol. 63:5258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiss, B. J., T. J. Smith, D. A. Leib, and L. A. Morrison. 2000. Disruption of virion host shutoff activity improves the immunogenicity and protective capacity of a replication-incompetent herpes simplex virus type 1 vaccine strain. J. Virol. 74:11137-11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haskova, Z., N. Usui., T. A. Ferguson, J. S. Pepose, and P. M. Stuart. 2000. CD4+ T cells are critical in corneal but not skin allograft rejection. Transplantation 69:483-488. [DOI] [PubMed] [Google Scholar]
- 13.Hendricks, R. L., and T. M. Tumpey. 1990. Contribution of virus and immune factors to herpes simplex virus type 1-induced corneal pathology. Investig. Ophthalmol. Vis. Sci. 31:1929-1939. [PubMed] [Google Scholar]
- 14.Hendricks, R. L., and T. M. Tumpey. 1991. Concurrent regeneration of T lymphocytes and susceptibility to HSV-1 corneal stromal disease. Curr. Eye Res. 10:47-53. [DOI] [PubMed] [Google Scholar]
- 15.Hendricks, R. L., T. M. Tumpey, and A. Finnegan. 1992. IFN-γ and IL-2 are protective in the skin but pathologic in corneas of HSV-1 infected mice. J. Immunol. 149:3023-3028. [PubMed] [Google Scholar]
- 16.Keadle, T. L., K. A. Laycock, J. K. Miller, K. K. Hook, E. D. Fenoglio, M. Francotte, M. Slaoui, P. M. Stuart, and J. S. Pepose. 1997. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J. Infect. Dis. 176:331-338. [DOI] [PubMed] [Google Scholar]
- 17.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA 84:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwong, A. D., and N. Frenkel. 1989. The herpes simplex virus virion host shut off function. J. Virol. 63:4834-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lausch, R. N., C. Monteiro, W. R. Kleinschrodt, and J. E. Oakes. 1987. Superiority of antibody versus delayed hypersensitivity in clearance of HSV-1 from the eye. Investig. Ophthalmol. Vis. Sci. 28:565-570. [PubMed] [Google Scholar]
- 20.Lausch, R. N., W. R. Kleinschrodt, C. Monteiro, S. G. Kayes, and J. E. Oakes. 1985. Resolution of HSV corneal infection in the absence of delayed-type-hypersensitivity. Investig. Ophthalmol Vis. Sci. 26:1509-1515. [PubMed] [Google Scholar]
- 21.Laycock, K. A., S. F. Lee, R. H. Brady, and J. S. Pepose. 1991. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Investig. Ophthalmol. Vis. Sci. 32:2741-2746. [PubMed] [Google Scholar]
- 22.Littler, E., D. Purifoy, A. Minson, and K. L. Powell. 1983. Herpes simplex virus nonstructural proteins. III. Function of the major DNA-binding protein. J. Virol. 64:983-995. [DOI] [PubMed] [Google Scholar]
- 23.Liu, T., K. M. Khanna, X. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin, S., R. J. Courtney, G. Fowler, and B. T. Rouse. 1988. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize virus nonstructural proteins. J. Virol. 62:2265-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, S., X. Zhu, S. J. Silverstein, R. J. Courtney, F. Yao, F. J. Jenkins, and B. T. Rouse. 1990. Murine cytotoxic T lymphocytes specific for herpes simplex virus type 1 recognize the immediate early protein ICP4 but not ICP0. J Gen. Virol. 71:2391-2399. [DOI] [PubMed] [Google Scholar]
- 26.Mercadal, C. M., D. M. Bouley, D. DeStephano, and B. T. Rouse. 1993. Herpetic stromal keratitis in the reconstituted scid mouse model. J. Virol. 67:3404-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison, L. A,., and D. M. Knipe. 1994. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J. Virol. 68:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison, L. A., and D. M. Knipe. 1996. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 320:402-413. [DOI] [PubMed] [Google Scholar]
- 29.Morrison, L. A., and D. M. Knipe. 1997. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology 239:315-326. [DOI] [PubMed] [Google Scholar]
- 30.Nesburn, A. B., R. L. Burke, H. Ghiasi, S. M. Slanina, and S. L. Wechsler. 1998. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Investig. Ophthalmol. Vis. Sci. 39:1163-1170. [PubMed] [Google Scholar]
- 31.Nesburn, A. B., R. L. Burke, H. Ghiasi, S. Slanina, S. Bahri, and S. L. Wechsler. 1994. Vaccine therapy for ocular herpes simplex virus (HSV) infection: periocular vaccination reduces spontaneous ocular HSV type 1 shedding in latently infected rabbits. J. Virol. 68:5084-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nesburn, A. B., R. L. Burke, H. Ghiasi, S. M. Slanina, and S. L. Wechsler. 1998. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology 252:200-209. [DOI] [PubMed] [Google Scholar]
- 33.Niemialtowski, M. G., and B. T. Rouse. 1992. Predominance of Th1 cells in ocular tissue during herpetic stromal keratitis. J. Immunol. 149:3035-3041. [PubMed] [Google Scholar]
- 34.Pepose, J. S., D. A. Leib, P. M. Stuart, and D. L. Easty. 1996. Herpes simplex virus diseases: anterior segment of the eye, p. 905-932. In J. S. Pepose, G. A. N. Holland, and K. R. Wilhelmus (ed.), Ocular infection and immunity. Mosby, St. Louis, Mo.
- 35.Russell, R. G., M. P. Nasisse, and B. T. Rouse. 1984. Role of T lymphocytes in the pathogenesis of herpetic stromal keratitis. Investig. Ophthalmol. Vis. Sci. 25:938-942. [PubMed] [Google Scholar]
- 36.Schrier, R. D., L. I. Pizer, and J. W. Moorhead. 1983. Type-specific delayed hypersensitivity and protective immunity induced by isolated herpes simples virus glycoprotein. J. Immunol. 130:1413-1418. [PubMed] [Google Scholar]
- 37.Shimeld, C., T. J. Hill, W. A. Blyth, and D. L. Easty. 1990. Passive immunization protects the mouse eye from damage after herpes simplex virus infection by limiting spread of virus in the nervous system. J. Gen. Virol. 71:681-687. [DOI] [PubMed] [Google Scholar]
- 38.Stanberry, L. R., A. L. Cunningham, A. Mindel, L. L. Scott, S. L. Spruance, F. Y. Aoki, and C. J. Lacey. 2000. Prospects for control of herpes simplex virus disease through immunization. Clin. Infect. Dis. 30:549-566. [DOI] [PubMed] [Google Scholar]
- 39.Straus, S. S., L. Corey, R. Burke, B. Savarese, G. Barnum, P. R. Krause, R. G. Khost, J. L. Meier, R. Sekulovich, S. F. Adair, and C. L. Dekker. 1994. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 343:1460-1463. [DOI] [PubMed] [Google Scholar]
- 40.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tigges, M. A., D. Koelle, K. Hartog, R. E. Sekulovich, L. Corey, and R. L. Burke. 1992. Human CD8+ herpes simplex virus-specific cytotoxic T-lymphocyte clones recognize diverse virion protein antigens. J. Virol. 66:1622-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tigges, M. A., S. Leng, D. C. Johnson, and R. L. Burke. 1996. Human herpes simplex virus specific CD8+ CTL clones recognize HSV-2 infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J. Immunol. 156:3901-3910. [PubMed] [Google Scholar]
- 43.Walker, J., and D. A. Leib. 1998. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine 16:1-5. [DOI] [PubMed] [Google Scholar]
- 44.Walker, J., K. A. Laycock, J. S. Pepose, and D. A. Leib. 1998. Post exposure vaccination with a virion host shutoff defective mutant reduces UV-B radiation-induced ocular herpes simplex virus shedding in mice. Vaccine 16:6-8. [DOI] [PubMed] [Google Scholar]
- 45.Wander, A. H., Y. M. Centifanto, and H. E. Kaufman. 1980. Strain specificity of clinical isolates of herpes simplex virus. Arch. Ophthalmol. 98:1458-1461. [DOI] [PubMed] [Google Scholar]
- 46.Watari, E., B. Dietzschold, G. Szokan, and E. Heber-Katz. 1987. A synthetic peptide induces long-term protection from lethal infection with herpes simplex virus 2. J. Exp. Med. 165:459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, C. A., N. J. Nelson, D. J. McGeoch, and M. D. Challberg. 1988. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J. Virol. 62:435-443. [DOI] [PMC free article] [PubMed] [Google Scholar]