Abstract
Human immunodeficiency virus type-1 Tat has been proposed to play a role in the regulation of reverse transcription. We previously demonstrated that wild-type Tat can augment viral infectivity by suppressing the reverse transcriptase (RT) reaction at late stages of the viral life cycle in order to prevent the premature synthesis of potentially deleterious viral DNA products. Here we have performed a detailed analysis of the cell-free reverse transcription reaction to elucidate the mechanism(s) whereby Tat can affect this process. Our results show that Tat can suppress nonspecific DNA elongation while moderately affecting the specific initiation stage of reverse transcription. In addition, Tat has an RNA-annealing activity and can promote the placement of tRNA onto viral RNA. This points to a functional homology between Tat and the viral nucleocapsid (NC) protein that is known to be directly involved in this process. Experiments using a series of mutant Tat proteins revealed that the cysteine-rich and core domains of Tat are responsible for suppression of DNA elongation, while each of the cysteine-rich, core, and basic domains, as well as a glutamine-rich region in the C-terminal domain, are important for the placement of tRNA onto the viral RNA genome. These results suggest that Tat can play at least two different roles in the RT reaction, i.e., suppression of DNA polymerization and placement of tRNA onto viral RNA. We believe that the first of these activities of Tat may contribute to the overall efficiency of reverse transcription of the viral genome during a new round of infection as well as to enhanced production of infectious viral particles. We hypothesize that the second activity, illustrating functional homology between Tat and NC, suggests a potential role for NC in the displacement of Tat during viral maturation.
Reverse transcription of single-stranded RNA into double-stranded DNA is an essential step in retroviral replication and is catalyzed by the viral reverse transcriptase (RT) enzyme (47). Cellular tRNA3Lys is preferentially incorporated into human immunodeficiency virus type 1 (HIV-1) virions (25) and is used to initiate reverse transcription after binding to a complementary stretch of viral RNA termed the primer binding site (PBS) (42). The viral nucleocapsid protein (NCp7) is believed to act as a nucleic acid chaperon to facilitate the rearrangement of nucleic acids into optimally base-paired conformations (43) to promote the annealing of tRNA3Lys to the PBS (5, 10, 13, 36, 44).
HIV-1 has two RNA-binding proteins that contain basic domains and Zn finger-like structures, i.e., NCp7 and Tat (9, 14, 36, 44). The Tat protein, which is a transcriptional trans-activator of HIV-1, is essential for viral transcription. Tat binds to the transactivation response element (TAR), a stem-loop structure located at the 5′ end of genomic RNA, and is consequently involved in both transcriptional initiation and elongation (23, 26). In addition, Tat is thought to have a role in maintenance of virion infectivity (19). HIV-1 virions lacking the tat gene display decreased efficiency of reverse transcription in new rounds of infection, suggesting that Tat stimulates RT activity (17). Recently, we demonstrated that wild-type, double-exon-containing Tat, i.e., 2-exon Tat, can augment virion infectivity by suppressing the RT reaction during late stages of viral replication and by preventing the premature synthesis of potentially deleterious viral DNA products before viral maturation (27). This effect of Tat is presumably directed against the low levels of RT activity associated with Gag-Pol precursor proteins or against RT that is present in the cytoplasm as a result of low-level endogenous proteolytic cleavage of such precursors. These results suggest that Tat can help to regulate viral reverse transcription.
To elucidate the mechanism(s) by which Tat plays a role in this process, we have performed a detailed analysis of the in vitro reverse transcription reaction. Tat is thought to contain five domains, i.e., N terminus (amino acids [aa] 1 to 20), cysteine-rich (aa 21 to 40), core (aa 41 to 48), basic (aa 49 to 57), and C terminus (aa 58 to 86) (26). The cysteine-rich and core domains are highly conserved among HIV strains, and aa 1 to 48 are proposed to represent a minimal activation domain of Tat for HIV transcription (26). The basic domain together with the flanking core domain confers RNA-binding activity. The C-terminal domain contains a glutamine-rich region (aa 60 to 76) and is thought to be involved in TAR binding (2, 7). However, the role of the C-terminal domain with respect to viral transcription is not well understood (23, 34).
We previously showed that a 2-exon but not a 1-exon form of Tat markedly suppressed RT activity. However, a synthetic peptide corresponding to the second exon of Tat did not suppress RT activity, indicating that the second exon was not itself responsible for RT suppression (27). In order to define the functional Tat domain(s) responsible for RT suppression, we prepared a series of mutant Tat proteins, each one containing a deletion of a different Tat domain, and employed them in cell-free RT reactions. Our results show that wild-type Tat (Tat 86) suppressed nonspecific elongation of viral DNA rather than the specific initiation stage of reverse transcription. In contrast, mutant Tat, with a deletion in either the cysteine-rich domain, the core domain, or the C-terminal glutamine-rich region, did not suppress DNA elongation. Moreover, this wild-type Tat 86, but not the mutant proteins missing either the cysteine-rich, core, or basic domain or the glutamine-rich region, displayed RNA-annealing activity and was able to promote the placement of tRNA3Lys onto the PBS. These results suggest that the cysteine-rich domain of Tat, which contains a Zn finger-like structure (14), and the flanking RNA-binding domains can cooperate to play important roles in reverse transcription. Accordingly, we propose that Tat has at least two roles in this process, i.e., promotion of the placement of the tRNA primer onto viral RNA and suppression of DNA polymerization at later stages in the viral life cycle. These results also imply possible functional and structural homologies between Tat and NCp7 with regard to the initiation phase of reverse transcription and the role that each of these proteins may play during viral assembly.
MATERIALS AND METHODS
Reagents.
Natural tRNA3Lys (purified by high-pressure liquid chromatography from human placenta) was purchased from BIO S&T (Montreal, Quebec, Canada). HIV-1 nucleocapsid protein (NCp7) was kindly provided by B. P. Roques (Département de Pharmacochimie Moléculaire et Structurale, U266 INSERM-URA D1500 CNRS, UER des Sciences Pharmaceutiques et Biologiques, Paris, France).
Plasmid construction.
A plasmid termed pQE-Histat, for the expression of wild-type Tat 86, was constructed as described previously (28). Point mutations to change amino acids at positions 22, 30, and 41 (C22G, C30G, and K41A, respectively) were introduced into tat cDNA by site-directed mutagenesis (QuikChange Site-Directed Mutagenesis Kit; Stratagene, La Jolla, Calif.). tat cDNAs that encoded mutant proteins with either 9 or 17 amino acids at the N terminus deleted, i.e., Δ2-10 and Δ2-18, respectively, were amplified from pQE-Histat by PCR using Pfu DNA polymerase (Stratagene) and the following primers: 5′-AGAggatccCATGTGGAAGCATCCAGGAAGT-3′ (sense primer for Δ2-10), 5′-AGAggatccCATGAAAACTGCTTGTACCACT-3′ (sense primer for Δ2-18), and 5′-AActgcagCCTATTCCTTCGGGCCT-3′ (antisense primer). (Lowercase letters in primer sequences represent the BamHI [ggatcc], PstI [ctgcag], KpnI [ggtacc], and SalI [gtcgac] restriction sites.) tat cDNAs that encoded mutant proteins with deletions of 16, 14, 12, 10, or 6 amino acids at the C terminus (i.e., Tat 70, Tat 72, Tat 74, Tat 76, and Tat 80, respectively) were amplified using the following primers: 5′-CGggatccCATGGAGCCAGTAGATC-3′ (sense primer), 5′-GGTTctgcagTTATGATAGAGAAACTTGATGAGT-3′ (antisense primer for Tat 70), 5′-AActgcagCCTACTTTGATAGAGAA-3′ (antisense primer for Tat 72), 5′-GGTTctgcagTTAGGTGGGTTGCTTTGATAGAGA-3′ (antisense primer for Tat 74), 5′-GGTTctgcagTTATTGGGAGGTGGGTTGCTTTGA-3′ (antisense primer for Tat 76), and 5′-GGTTctgcagTTAGTCCCCCCGGGTTTGGGAGGT-3′ (antisense primer for Tat 80). (Underlining denotes mutations in the antisense primers for introduction of a stop codon.) tat cDNAs encoding aa 1 to 20, 40 to 86, 1 to 39, 48 to 86, 1 to 49, and 59 to 86 were amplified from pQE-Histat using the following primers: 5′-CGGATAACAATTTCACACAG-3′ (sense primer for aa 1 to 20, 1 to 39, and 1 to 49), 5′-CTCggtaccAAAGCCTTAGGCATCTC-3′ (sense primer for aa 40 to 86), 5′-GGggtaccAGGAAGAAGCGGAGACAGC-3′ (sense primer for aa 48 to 86), 5′-ATGgtcgacCTCAAGACAGTCAGACT-3′ (sense primer for aa 59 to 86), 5′-CCGggtaccAGTTTTAGGCTGACTTCC-3′ (antisense primer for aa 1 to 20), 5′-GGggtaccCATGAAACAAACTTGGCAATG-3′ (antisense primer for aa 1 to 39), 5′-TGAGgtcgacCATAGGAGATGCCTAA-3′ (antisense primer for aa 1 to 49), and 5′-GTTCTGAGGTCATTACTGG-3′ (antisense primer for aa 40 to 86, 48 to 86, and 59 to 86). Then each pair of cDNAs, i.e., 1-20 and 40-86, 1-39 and 48-86, 1-49 and 59-86, was ligated during subcloning. After PCR amplification, tat cDNAs were subcloned into the pUC19 plasmid and mutations were confirmed by sequencing (dsDNA cycle sequencing system; Canadian Life Technologies, Inc., Montreal, Quebec, Canada). Next, tat cDNAs were again subcloned into pQE-31 (Qiagen, Mississauga, Ontario, Canada).
The HIV-1 proviral molecular clone BH10 was employed to generate a mutant virus with the PBS deleted, termed ΔPBS. Briefly, a sense primer containing an 18-nucleotide (nt) deletion of the PBS (5′-GGAAAATCTCTAGCAGTTGAAAGCGAAAGGGAAACC-3′, +620 to +673) and an antisense primer (5′-CCATCGATCTAATTCTCCC-3′, +837 to +819) were used to amplify an HIV sequence lacking the PBS. Then this PCR product was used as an antisense megaprimer with an additional sense primer (5′-CTGCAgttaacTGGAAGGGCTAATTCACTCCC-3′, −11 to +21) (lowercase letters represent the HpaI restriction site). After PCR amplification, the resulting fragment was digested with HpaI and BssHII and was inserted into BH10, and the mutation was confirmed by sequencing. Finally, a 911-bp fragment (BglII [+473] to PstI [+1402]) of ΔPBS was subcloned into the RNA expression vector pSP72 (Promega, Madison, Wis.) to generate pHIV-PBS(−).
Purification of recombinant proteins.
Recombinant Tat proteins were expressed in Escherichia coli M15(pREP4) and purified by using nickel-nitrilotriacetic acid resin as described previously (28). Heterodimeric HIV-1 RT (p66-p51) was also expressed in E. coli M15(pREP4) and purified by using nickel-nitrilotriacetic acid resin and ion-exchange chromatography (37). Human Staufen, a cellular RNA-binding protein that is incorporated into HIV-1 particles, was used in some experiments as a negative control and was prepared as previously described (41).
Templates and primers.
An RNA template, consisting of a 239-nt HIV-1 RNA sequence spanning the R region of the long terminal repeat (LTR) and the PBS, was in vitro transcribed from linearized pHIV-PBS (1) by using the T7-MEGA shortscript in vitro transcription kit (Ambion, Austin, Tex.). An RNA template lacking the PBS region (221 nt) was in vitro transcribed from linearized pHIV-PBS(−). The DNA primer, which is complementary to the PBS, was 5′ end labeled with [γ-32P]ATP by using T4 polynucleotide kinase. In some experiments, natural tRNA3Lys was 3′ end labeled with [5′-32P]cytidine 3′,5′-bis(phosphate) by using RNA ligase. RNA transcripts and labeled primers were electrophoretically purified prior to use in RT reactions.
Reverse transcription.
Natural tRNA3Lys or an end-labeled DNA primer was heat annealed onto the RNA template as described previously (27). In some experiments, placement of tRNA3Lys onto the RNA template was carried out by adding Tat into the reaction mixture, as described below. For elongation of negative-strand DNA, the annealed primer/template complex was incubated with RT and Tat at 37°C for 1 min in a 20-μl-reaction mixture containing 50 mM Tris-HCl (pH 7.8), 50 mM NaCl, 1 mM dithiothreitol (DTT), and 0.2 mM concentrations of deoxynucleoside triphosphates (dNTPs). To achieve a 5-nt primer extension, 1 μM concentrations of dCTP, dTTP, and dGTP and 10 μCi of [α-32P]dCTP were added to the reaction mixture instead of 0.2 mM dNTPs. Then reverse transcription was initiated by addition of MgCl2 at a final concentration of 6 mM. The reactions were allowed to proceed at 37°C for the indicated times and were stopped by adding 2-μl aliquots of the reaction mixture to 8 μl of 95% formamide. Reaction products were analyzed on 8% polyacrylamide-7 M urea gels and visualized by autoradiography.
Placement of tRNA3Lys on the RNA template by Tat.
3′-end-labeled tRNA (0.5 pmol) was incubated with the RNA template (0.5 pmol) and various concentrations of either NCp7 or Tat in a 20-μl buffer containing 50 mM Tris-HCl (pH 7.8), 200 mM NaCl, and 10 mM DTT at 37°C for 1 h unless otherwise specified. Samples were then treated with 250 μg of proteinase K/ml at 37°C for 30 min and were extracted with phenol-chloroform. After ethanol precipitation, the RNA samples were dissolved in loading buffer containing 25 mM Tris-HCl (pH 7.8), 12.5 mM EDTA, 1% sodium dodecyl sulfate, 12.5% glycerol, and 0.005% bromphenol blue and were separated on 5% polyacrylamide gels (0.5× TBE, comprising 44.5 mM Tris, 44.5 mM boric acid, and 1 mM EDTA) at 4°C. The gels were then dried, and radioactive bands were visualized with X-ray film.
RESULTS
Tat suppresses the elongation rather than the initiation stage of the RT reaction.
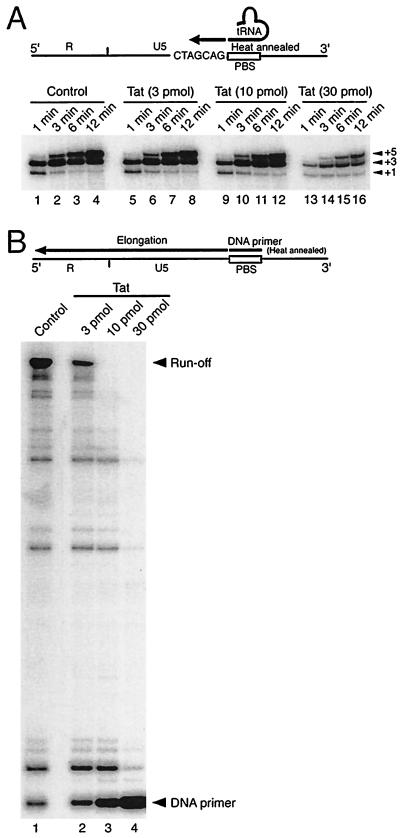
The synthesis of negative-strand DNA primed with natural tRNA3Lys in HIV-1 reverse transcription is proposed to involve both specific initiation and nonspecific elongation stages (22, 35). To investigate whether or not the initiation stage of the RT reaction was suppressed by Tat 86, we primed RT reactions with natural tRNA3Lys in the presence of low concentrations (1 μM) of dCTP, dTTP, and dGTP and 10 μCi of [α-32P]dCTP. Since the 5-nt flanking sequences at the 5′ end of the PBS contain only G, A, and C, the early products of RT reactions with pause sites at the +1, +3, and +5 positions can be detected in this system (39). As shown in Fig. 1A, early RT products up to position +3 were synthesized within 1 min; thereafter, RT products up to position +5 were rapidly generated (lanes 1 to 4). The presence of Tat 86 had no effect on these reactions at concentrations up to 10 pmol per reaction (Fig. 1A, lanes 5 to 12) and partially suppressed the reactions when used at a concentration of 30 pmol per reaction (lanes 13 to 16). In contrast, low concentrations of Tat, e.g., 10 pmol per 1 pmol of primer, strongly suppressed the synthesis of negative-strand DNA from a DNA primer, a reaction in which the initiation stage is not involved (22, 27) (Fig. 1B, lane 3). Therefore, these results suggest that wild-type Tat 86 can strongly suppress the elongation stage but not the initiation stage of reverse transcription.
FIG. 1.
Tat significantly suppresses the elongation but not the initiation stage of the RT reaction. (A) The heat-annealed tRNA3Lys (1 pmol)-template (1 pmol) complex and RT (3 pmol) were mixed with 0 (lanes 1 to 4), 3 (lanes 5 to 8), 10 (lanes 9 to 12), or 30 (lanes 13 to 16) pmol of Tat 86 in a reaction mixture containing 1 μM concentrations of dCTP, dTTP, and dGTP and 10 μCi of [α-32P]dCTP. Then reverse transcription was initiated and allowed to proceed at 37°C for 1 (lanes 1, 5, 9, and 13), 3 (lanes 2, 6, 10, and 14), 6 (lanes 3, 7, 11, and 15), and 12 (lanes 4, 8, 12, and 16) min, as described in Materials and Methods. Arrowheads and numbers on the right indicate the corresponding numbers of nucleotides extended. (B) The annealed DNA primer (2.5 pmol)-template (3.75 pmol) complex and RT (2.5 pmol) were mixed with 0 (lane 1), 7.5 (lane 2), 25 (lane 3), and 75 (lane 4) pmol of Tat 86 in a reaction mixture containing 0.2 mM concentrations of dNTPs. Note that the Tat-to-primer molar ratios were 3:1 (lane 2), 10:1 (lane 3), and 30:1 (lane 4). Next, reverse transcription was initiated and allowed to proceed at 37°C for 6 min. Reaction products were analyzed on 8% polyacrylamide-7 M urea gels and were visualized by autoradiography. The runoff and DNA primer bands indicate full-length products and unprocessed 32P-labeled DNA primer, respectively.
Identification of Tat domains involved in the suppression of viral DNA elongation.
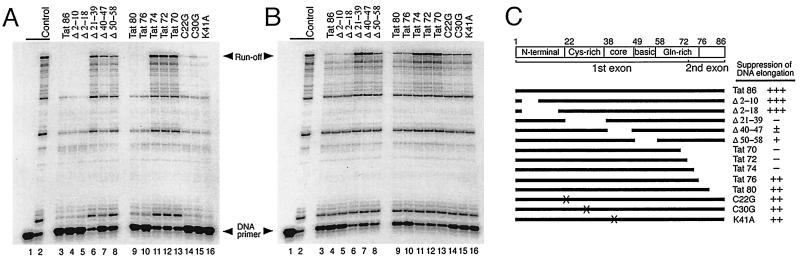
To define the Tat domain(s) involved in the suppression of DNA elongation, a series of mutant Tat proteins were prepared and employed in RT reactions primed with a DNA primer. Tat proteins were added to the RT reactions at Tat-to-primer molar ratios of 30:1 (Fig. 2A) or 10:1 (Fig. 2B). As shown in Fig. 2, Tat mutants with deletions at the N-terminal domain (Δ2-10 and Δ2-18) suppressed DNA elongation as efficiently as wild-type Tat 86, while the Tat mutants with deletions of either the cysteine-rich or the core domain (Δ21-39 and Δ40-47, respectively) did not suppress these reactions (Fig. 2A and B, lanes 3 to 7). The Tat mutant with a deletion within the basic domain (Δ50-58) partially suppressed these reactions (Fig. 2A and B, lanes 8). Additionally, Tat mutants with deletions of either 6 or 10 aa in the C-terminal domain (Tat 80 and Tat 76, respectively) partially suppressed these RT reactions, while mutants with deletions of 12, 14, or 16 aa (Tat 74, Tat 72, and Tat 70, respectively) did not (Fig. 2A and B, lanes 9 to 13). Three point mutants, i.e., C22G, C30G, and K41A, also partially suppressed the DNA elongation reactions (Fig. 2A and B, lanes 14 to 16). These results suggest that the cysteine-rich domain, the core domain, and the C-terminal glutamine-rich region are all important for the suppression of DNA elongation.
FIG. 2.
(A and B) Analysis of mutant Tat proteins for the suppression of DNA elongation reactions. RT reactions were carried out as described in the legend to Fig. 1B. The amounts of mutant Tat used were 75 pmol (A) and 25 pmol (B), i.e., the Tat-to-primer molar ratios were 30:1 (A) and 10:1 (B). Lanes 1 and 2 represent control reactions without the addition of MgCl2 and without Tat, respectively. (C) Schematic illustration of functional domains of the Tat 86 polypeptide and the mutant proteins used in this study. Numbers above the diagram represent the corresponding amino acid residues. A relative value of the amount of runoff product was determined by densitometory using NIH Image 1.61 software. The ability of mutant Tat to suppress DNA elongation is summarized on the right as follows: +++, suppression was >80% in both panels A and B; ++, suppression was >50% in panel B; +, suppression was >30% in panel B; ±, suppression was <25% in panel A and no suppression was observed in panel B; −, no suppression in either panel A or panel B.
Tat possesses RNA-annealing activity.
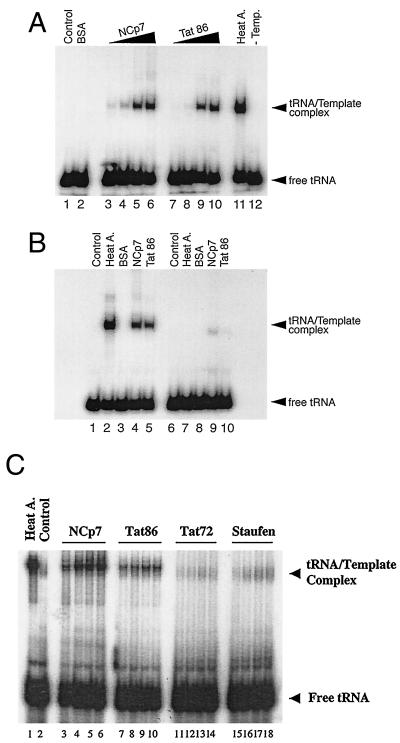
NCp7 is proposed to have a nucleic acid chaperon activity (43) and plays an important role in the annealing of tRNA primer onto the viral RNA genome (5, 10, 13, 36, 44). In addition, the basic domain and the Zn finger motifs of NCp7 are essential for this RNA-annealing reaction (10, 36, 44). Since both NCp7 and Tat have basic domains and Zn finger-like structures (9, 14, 36, 44), we asked whether functional homology might exist between these two proteins with regard to the placement of tRNA3Lys on the PBS. To this end, we employed an in vitro RNA-annealing system using an end-labeled natural tRNA3Lys and a viral RNA template. Figure 3A shows that Tat 86 was able to promote tRNA-annealing reactions in a dose-dependent manner, similarly to NCp7, although with moderately less efficiency than the latter protein (Fig. 3A; compare lanes 7 to 10 with lanes 3 to 6 and particularly lane 4 with lane 8).
FIG. 3.
Analysis of Tat-promoting RNA annealing reactions. (A) 32P-labeled tRNA (0.5 pmol) and RNA template (0.5 pmol) were incubated with 0 (lane 1), 5 (lanes 3 and 7), 10 (lanes 4 and 8), 20 (lanes 5 and 9) and 40 (lanes 6 and 10) pmol of either Tat 86 (lanes 7 to 10) or NCp7 (lanes 3 to 6) at 37°C for 1 h. Lanes 2 and 11 represent control reactions performed with bovine serum albumin (BSA) (500 ng per reaction) and by heat annealing, respectively. The reaction shown in lane 12 contained tRNA and Tat (40 pmol) but not RNA template. (B) 32P-labeled tRNA was mixed either with PBS(+) (lanes 1 to 5) or with PBS(−) (lanes 6 to 10) RNA template. RNAs were incubated with either BSA (500 ng) (lanes 3 and 8), NCp7 (40 pmol) (lanes 4 and 9), or Tat (40 pmol) (lanes 5 and 10), or without proteins (lanes 1 and 6), at 37°C for 1 h. Lanes 2 and 7 represent control reactions performed by heat annealing. (C) Experiment to study the kinetics of tRNA3Lys placement in the presence of various proteins including human Staufen as a negative control. A protein concentration of 15 pmol was used in each case. Lane 1, reaction performed by heat annealing; lane 2, negative control conducted in the absence of any additional proteins. Reactions were performed for 5 min (lanes 3, 7, 11, and 15), 10 min (lanes 4, 8, 12, and 16), 15 min (lanes 5, 9, 13, and 17), or 30 min (lanes 6, 10, 14, and 18). After the reactions, samples were treated with proteinase K, extracted with phenol-chloroform, and precipitated with ethanol, as described in Materials and Methods. Then samples were dissolved in loading buffer and separated on 5% polyacrylamide gels at 4°C. Gels were dried, and the bands were visualized on X-ray film.
Next, we tested the specificity of this tRNA-annealing activity of Tat through use of PBS(+) and PBS(−) RNA templates. As shown in Fig. 3B, Tat promoted the placement of tRNA onto the PBS(+) but not onto the PBS(−) RNA template (lanes 5 and 10), indicating that this placement reaction was specific. We further assessed the abilities of NCp7 and Tat to participate in tRNA3Lys placement reactions by performing time course experiments. The results show that the Tat 86 protein displayed marginally slower kinetics than did NCp7 in the annealing of tRNA3Lys onto the PBS, when used at a concentration of 15 pmol (Fig. 3C). As a control, a form of Tat that contains only the amino acids of the first exon of wild-type Tat, i.e., Tat 72, was employed and failed to place tRNA3Lys onto the viral RNA template. Furthermore, the human cellular RNA-binding protein Staufen was also unable to participate efficiently in the placement reaction. This further highlights the specificity of the double-exon form of Tat in this process.
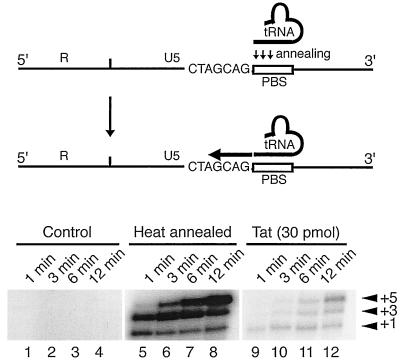
In addition, the ability of Tat-annealed tRNA3Lys to initiate RT reactions was examined using 5-nt-primer extension reactions. Figure 4 shows that initiation of reverse transcription from Tat-annealed tRNA3Lys occurred somewhat less efficiently than that from heat-annealed tRNA3Lys (compare lanes 9 to 12 with lanes 5 to 8) but nonetheless illustrates the functional activity of the Tat-annealed tRNA primer. Note that we are not proposing a role for Tat in initiation of reverse transcription in the same fashion as that of NCp (see below).
FIG. 4.
Initiation of the RT reaction from Tat-annealed tRNA3Lys. tRNA (1 pmol) and the RNA template (1 pmol) were incubated either with Tat (30 pmol) (lanes 9 to 12) or with bovine serum albumin (BSA) (500 ng) (lanes 1 to 4) at 37°C for 30 min. As a control reaction, tRNA and the RNA template were heat annealed (lanes 5 to 8). Then reverse transcription was initiated in a reaction mixture containing RT (3 pmol), MgCl2 (6 mM), 1 μM concentrations of dCTP, dTTP, and dGTP, and 10 μCi of [α-32P]dCTP. The reaction was allowed to proceed at 37°C for 1 (lanes 1, 5, and 9), 3 (lanes 2, 6, and 10), 6 (3, 7, 11, and 15) and 12 (lanes 4, 8, 12, and 16) min. Arrowheads and numbers on the right indicate the corresponding numbers of nucleotides extended.
Conditions affecting the RNA-annealing activity of Tat.
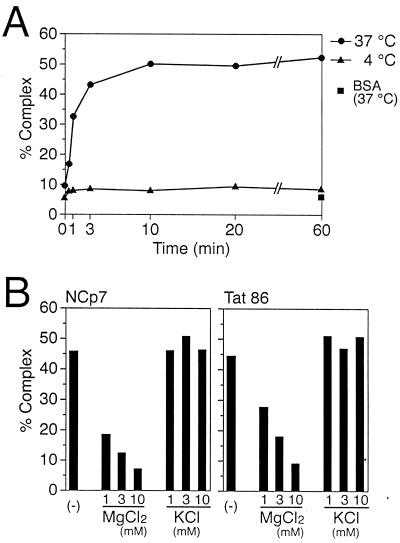
Next, we varied the conditions of these Tat-promoting RNA-annealing reactions to further delineate this activity. Figure 5A shows that the annealing of tRNA3Lys to viral genomic RNA was efficiently promoted by Tat at 37°C, as indicated by the increased ratio of tRNA-RNA template complex to free tRNA during 3 min. The annealing reaction was rapidly promoted and was saturated within 10 min. In contrast, this annealing was very inefficient at 4°C (Fig. 5A) We also examined the effect of salt concentrations, and as shown in Fig. 5B, we found that Tat-promoted RNA-annealing reactions were suppressed by Mg2+. In contrast, neither Na+ nor K+ exerted a significant effect at concentrations as high as 200 and 10 mM, respectively (data not shown and Fig. 5B). We also examined the effect of salt concentrations on NCp7-promoted RNA-annealing activity in our system and found that Mg2+ and K+ had similar effects on both NCp7 and Tat (Fig. 5B; compare right and left panels), except that NCp7 generated very strong RNA aggregation at low concentrations of Na+ (data not shown). Thus, the RNA-annealing properties of Tat and NCp7 appear to be similar.
FIG. 5.
(A) Kinetics of the Tat-promoting RNA-annealing reaction. A 20 μl-reaction mixture containing tRNA (0.5 pmol), the RNA template (0.5 pmol), and Tat (40 pmol) was incubated at 37°C (circles) or at 4°C (triangles). Reactions were allowed to proceed for 0, 0.5, 1, 3, 10, 20, and 60 min and were terminated by addition of 180 μl of stop solution containing 50 mM Tris-HCl (pH 7.8), 200 mM NaCl, 1% sodium dodecyl sulfate, and 50 μg of proteinase K. As controls, RNAs were incubated with bovine serum albumin (BSA) (500 ng) at 37°C for 60 min (squares). (B) Effect of salt concentrations on RNA-annealing activity. The indicated concentrations of either MgCl2 or KCl were present in reaction mixtures during the RNA-annealing reactions, as described in Materials and Methods. Left and right panels show effects of salt concentrations on NCp7- and Tat-promoted RNA-annealing reactions, respectively; 40 pmol of either of these proteins was used. After completion of the reactions, samples were analyzed as described in the legend to Fig. 3. Then the percentage of annealed tRNA was determined by densitometory using NIH Image 1.61 software.
Analysis of Tat domains involved in the RNA-annealing reaction.
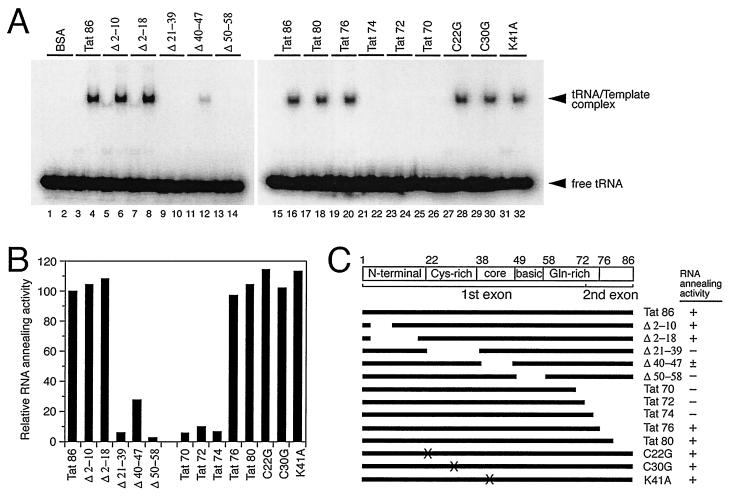
The various mutant Tat proteins described above were also employed in the in vitro RNA-annealing system in order to define the domain(s) involved. The results show that Δ2-10, Δ2-18, Tat 80, Tat 76, C22G, C30G, K41A, and wild-type Tat 86 were all able to promote the placement of tRNA onto the PBS, while Δ21-39, Δ50-58, Tat 74, Tat 72, and Tat 70 could not (Fig. 6A and B). Additionally, Δ40-47 showed some RNA-annealing activity (Fig. 6A and B). In each case, the placement of tRNA could be detected only with the PBS(+) RNA template, not with the PBS(−) RNA template (Fig. 6A; compare even-numbered with odd-numbered lanes), indicating that tRNA had been specifically annealed in each instance. These results suggest that the RNA-annealing activity of Tat may reside within its cysteine-rich and flanking RNA-binding domains. The fact that Tat, like NCp7, possesses tRNA3Lys-annealing activity is further evidence of the extent of functional redundancy within the HIV genome and the viral proteins that it encodes.
FIG. 6.
Analysis of the RNA-annealing activities of mutant Tat proteins. (A) 32P-labeled tRNA was mixed either with PBS(+) (even-numbered lanes) or PBS(−) (odd-numbered lanes) RNA templates. Then RNAs were incubated with the indicated mutant Tat proteins (30 pmol) at 37°C for 1 h as described in Materials and Methods. (B) The percentage of annealed tRNA was determined by densitometry using NIH Image 1.61 software. Then the relative RNA-annealing activity was calculated by comparison with the percentage of annealed tRNA obtained from Tat 86 samples, which was defined as 100% (i.e., results of panel A). (C) Schematic illustration of functional domains of Tat 86 polypeptide and mutant proteins used in this study. Numbers above the diagram indicate the corresponding amino acid residues. The RNA-annealing activity of mutant Tat is summarized on the right: +, approximately the same activity as Tat 86; ±, activity was >25% of activity seen with Tat 86; −, activity was <10% of activity seen with Tat 86.
DISCUSSION
These findings help to reconcile the results in both this paper and our previous publication on Tat-mediated suppression of RT activity (27) with earlier studies that suggested that Tat can promote rather than inhibit reverse transcription (4). Notably, the result that Tat can promote the annealing of tRNA3Lys onto the PBS is consistent with a positive role for Tat in this regard, while the finding that Tat negatively impacts the elongation but not the initiation stage of reverse transcription is further evidence that the negative regulatory role of Tat on RT is temporally dependent. We believe that the reaction placing tRNA3Lys onto the PBS and the potential for Tat to thereby help direct the initiation of reverse transcription are important steps for ensuring the efficiency of RT reactions during a new round of infection. However, this role of Tat must be played prior to viral assembly, during the preceding infectious cycle. At the same time, it is important for the virus that antisense and other inappropriate viral products, which might interfere with RNA dimerization and/or packaging as well as translation of viral mRNA, not be generated prematurely by RT from viral RNA in the cytoplasm; hence, Tat must also play the suppressive role described.
We have previously demonstrated that wild-type Tat, i.e., 2-exon Tat, can augment virion infectivity by suppressing the RT reaction to prevent the synthesis in the cytosol of potentially deleterious viral DNA products before viral maturation at late stages of viral replication (27). This role is presumably directed against either low levels of RT activity present within the Gag-Pol precursor and/or low levels of mature RT that is cleaved by endogenous enzymes from its precursor. To further study the role of Tat in reverse transcription, we prepared a series of mutant Tat proteins and employed them in a series of cell-free RT reactions. Our results show that Tat can strongly suppress DNA elongation (Fig. 1B) (27) while only modestly impacting on the initiation stage of reverse transcription (Fig. 1A). Furthermore, we have shown that Tat can specifically promote the annealing of tRNA onto the PBS, a step necessary for reverse transcription (Fig. 3). These results, therefore, suggest that Tat plays at least two seemingly opposed roles in the RT reaction, i.e., placement of tRNA onto viral RNA and suppression of DNA elongation. Since Tat is apparently not incorporated into virions (27, 49), these activities of Tat presumably take place within the cytosol of infected cells, and we propose that they contribute to the efficiency of reverse transcription during the next round of infection (17).
The results of our experiments that employed mutant Tat preparations with deletions in one of five functional domains suggest that the cysteine-rich domain, the core domain, and a glutamine-rich region in the C-terminal domain cooperate to suppress DNA elongation (Fig. 2), while the cysteine-rich and flanking RNA-binding domains seem important for the placement of tRNA onto the PBS (Fig. 6). Tat mutants containing only a single amino acid substitution in the cysteine-rich (C22G or C30G) or core (K41A) domain partially lost the ability to suppress DNA elongation (Fig. 2B), suggesting that minor structural alterations of these domains as well as large deletions (Δ21-39 and Δ40-47) can affect the suppressive activity. In contrast, these point mutants had no effect on RNA-annealing activity, whereas mutants lacking entire domains (Δ21-39 and Δ40-47) lost this activity almost completely (Fig. 6). These results suggest that the cysteine-rich and core domains may contribute to these two activities by different mechanisms. In addition, the mutant Tat that lacks the basic domain was partially able to suppress DNA elongation (Fig. 2), suggesting that TAR-Tat interactions are not essential for this effect. This result is consistent with our previous work showing the TAR-independent suppression of RT activity by wild-type Tat (27).
Our experiments that used a series of C-terminal deletion mutants revealed that the 10-aa deletion (Tat 76), but not the 12-aa deletion (Tat 74), strongly suppressed DNA elongation (Fig. 2). Since a portion of the C-terminal domain, namely, the glutamine-rich region (aa 60 to 76), is thought to be involved in binding to TAR (2, 7), the RNA-binding activity of this region may also be important for the effect of Tat on reverse transcription. In addition, both Tat 76 and Tat 80 partially lost the ability to suppress DNA elongation in comparison with wild-type Tat 86 (Fig. 2B); this suggests that the C-terminal domain may also play an auxiliary role in stabilizing RT (7). Although it has been suggested that the N-terminal domain of Tat is critical for the modulation of HIV reverse transcription in vivo (49), we found no functional differences with regard to in vitro RT reactions between Tat mutants with deletions of the N-terminal domain (Δ2-10 and Δ2-18) and wild-type Tat 86 (Fig. 2 and 6). These results point to the possibility that the N-terminal domain of Tat may not have a direct effect on RT reactions but might indirectly modulate certain steps of reverse transcription, e.g., by interacting with cellular proteins that are absent in cell-free RT reaction systems.
NCp7 has been shown to play an important role in the annealing of the tRNA primer to the PBS (9, 43), and we are not suggesting that this activity is not key. However, since NCp7 and Tat possess structural similarities, e.g., both have basic domains and Zn finger-like structures (9, 14, 36, 44), we decided to examine the possibility that these two proteins might possess functional homology with regard to RT reactions. It has been proposed that either the basic domain (10, 36) or the Zn finger-structure of NCp7 (44) is essential for its RNA-annealing activity. On the other hand, the cysteine-rich domain of Tat is thought to form a Zn finger-like structure (14), and we show here that the cysteine-rich and basic domains of Tat are essential for its RNA-annealing activity (Fig. 6). Thus, NCp7 and Tat may share a common structural basis with regard to the placement of the tRNA primer onto the PBS. In contrast, NCp7 can enhance DNA elongation by transiently eliminating secondary structure in the RNA template (24, 50), while Tat suppresses such elongation (Fig. 1 and 2). The tight association between NCp7 and viral RNA may keep the RNA-NCp7 complex accessible to the viral RT and integrase enzymes while simultaneously protecting it from attack by nucleases (9). In contrast, the qualitatively different nature of the association between Tat and viral RNA must, at some level, prevent efficient interactions with RT.
Although Tat is apparently not incorporated into virions (27, 49), it may nonetheless assist precursor Gag polyproteins in the recruitment of tRNA onto viral RNA at late stages in viral replication (5, 13). The RNA-annealing activity of NCp7 has been attributed to its nucleic acid chaperon activity (43). Yet other proteins besides NCp7 (18, 48), such as the Gag-like protein of the yeast Ty1 retrotransposon (8), the hepatitis delta antigen (21), the heterogeneous nuclear ribonucleoprotein A1 (4, 18), and prion proteins (15, 16) can all act as nucleic acid chaperons. The common feature of these proteins is an ability to bind RNA with broad specificity and to catalyze the rearrangement of nucleic acids by the transient destabilization of base pairs followed by the repairing of nucleic acids into alternative combinations (43). Since Tat plays a role in viral gene transcription by specifically binding to TAR (7, 11), it is difficult to imagine that it can also act as an RNA chaperon protein and bind RNA with broad specificity. However, Tat can also stimulate HIV transcription in a TAR-independent manner (30, 32, 46, 51) and is even thought to bind DNA sequences to activate HIV (3) and cytomegalovirus (33) transcription. These results indicate that Tat potentially acts by binding not only to TAR but to other nucleic acid sequences as well. Further studies on the ability of Tat to act as a nucleic acid binding protein should be performed to unravel the role of this multifunctional protein (23, 45) and its potential as a novel target for antiviral drug discovery.
In this context, the subject of specificity is of key importance. Our results show that an unrelated RNA-binding protein, i.e., human Staufen, was unable to efficiently place tRNA3Lys onto the PBS (Fig. 3C). Furthermore, a series of derivative Tat proteins that all contain RNA-binding domains, i.e., Tat 74, Tat 72, Tat 70, Δ21-39, and Δ40-47, showed no ability to suppress DNA elongation reactions (Fig. 2A and B, lanes 6 to 7 and 11 to 13). Thus, other functional regions of Tat, in addition to its RNA-binding domain, must be involved in regulation of RT activity.
We recognize that additional research will be necessary to clarify the relationships between the Tat and NC proteins that are discussed here. In particular, it will be important to demonstrate that Tat and NC can potentially displace each other under suitable conditions in our tRNA placement assays and to assess the functional stability of initiation complexes that are generated by use of NCp versus Tat. It will also be important to assess whether NC can compensate in our assays for the loss of function associated with mutated Tat. Finally, the introduction of a lethal protease (PR) mutation into the pMIex construct could provide evidence as to whether postintegrational RT activity is attributable to the RT domain within the Gag-Pol polyprotein or results from endogenous, proteolyzed RT enzyme. These studies are relevant but are beyond the scope of the present paper and represent works in progress.
A compelling issue is how Tat can play the role of tRNA placement, attributed to it in this paper, and what the relationship is between NC and Tat with regard to this activity. In the case of HIV-1, enrichment of tRNA3Lys within virions is mediated by Gag-Pol precursor (5, 25, 31, 40) together with tRNALys synthetase (6), while placement of tRNA3Lys onto viral RNA is facilitated by the NC protein in concert with RNA sequences that flank the PBS (20). tRNA3Lys is thought to first be enriched within virions; then, during Gag processing and viral maturation, the nucleic acid chaperon activity of NC anneals tRNA3Lys onto the PBS. Interestingly, tRNA species other than tRNA3Lys, such as tRNAHis (38) and tRNAMet (29), although not enriched within HIV-1 particles, can be successfully used as primers, provided that the PBS and proximal RNA sequences are changed so as to be complementary to these alternate tRNAs. This suggests that primer placement can occur through a mechanism(s) that does not enrich relevant tRNA species and that this process might be initiated prior to virus assembly. Tat is a possible candidate molecule to perform such tRNA primer annealing while NC is still buried within Gag precursors. Conceivably, the ability of Tat to play this role is part of the redundancy of viral protein function that has long been understood to be a hallmark of HIV-1. For example, situations may arise in which mutated forms of NC, in the context of Gag and/or Gag-Pol, may be impaired in the ability to efficiently promote tRNA3Lys annealing yet may still be able to participate in initiation of reverse transcription; under such circumstances, Tat may be able to fill this breach. Finally, although Tat has not been shown to be present within virions, despite the ability of Tat to permeate cell plasma membranes (12), extracellular Tat may be able to enter newly infected cells and exert effects on reverse transcription.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research.
REFERENCES
- 1.Arts, E. J., X. Li, Z. Gu, L. Kleiman, M. A. Parniak, and M. A. Wainberg. 1994. Comparison of deoxyoligonucleotide and tRNA3Lys as primers in an endogenous human immunodeficiency virus-1 in vitro reverse transcription/template-switching reaction. J. Biol. Chem. 269:14672-14680. [PubMed] [Google Scholar]
- 2.Bayer, P., M. Kraft, A. Ejchart, M. Westendorp, R. Frank, and P. Rösch. 1995. Structural studies of HIV-1 Tat protein. J. Mol. Biol. 247:529-535. [DOI] [PubMed] [Google Scholar]
- 3.Berkhout, B., A. Gatignol, A. B. Ranson, and K.-T. Jeang. 1990. TAR-independent activation of the HIV-1 LTR: evidence that tat requires specific regions of the promoter. Cell 62:757-767. [DOI] [PubMed] [Google Scholar]
- 4.Bertrand, E. L., and J. J. Rossi. 1994. Facilitation of hammerhead ribozyme catalysis by the nucleocapsid protein of HIV-1 and the heterogeneous nuclear ribonucleoprotein A1. EMBO J. 13:2904-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cen, S., Y. Huang, A. Khorchid, J.-L. Darlix, M. A. Wainberg, and L. Kleiman. 1999. The role of Pr55gag in the annealing of tRNA3Lys to human immunodeficiency virus type 1 genomic RNA. J. Virol. 73:4485-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cen, S., A. Khorchid, H. Javanbakht, J. Gabor, T. Stello, K. Shiba, K. Musier-Forsyth, and L. Kleiman. 2001. Incorporation of lysyl-tRNA synthetase into human immunodeficiency virus type 1. J. Virol. 75:5043-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churcher, M. J., C. Lamont, F. Hamy, C. Dingwall, S. M. Green, A. D. Lowe, P. J. G. Bulter, M. J. Gait, and J. Karn. 1993. High affinity binding of TAR RNA by the human immunodeficiency virus type-1 Tat protein requires base-pairs in the RNA stem and amino acid residues flanking the basic region. J. Mol. Biol. 230:90-110. [DOI] [PubMed] [Google Scholar]
- 8.Cristofari, G., D. Ficheux, and J.-L. Darlix. 2000. The Gag-like protein of the yeast Ty1 retrotransposon contains a nucleic acid chaperone domain analogous to retroviral nucleocapsid proteins. J. Biol. Chem. 275:19210-19217. [DOI] [PubMed] [Google Scholar]
- 9.Darlix, J.-L., M. Lapadat-Taposky, H. de Rocquigny, and B. P. Roques. 1995. First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. J. Mol. Biol. 254:523-537. [DOI] [PubMed] [Google Scholar]
- 10.De Rocquigny, H., C. Gabus, A. Vincent, M.-C. Fournié-Zaluski, B. Roques, and J.-L. Darlix. 1992. Viral RNA annealing activities of human immunodeficiency virus type 1 nucleocapsid protein require only peptide domains outside the zinc fingers. Proc. Natl. Acad. Sci. USA 89:6472-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingwall, C., I. Ernberg, M. J. Gait, S. M. Green, S. Heaphy, J. Karn, A. D. Lowe, M. Singh, and M. A. Skinner. 1990. HIV-1 Tat protein stimulates transcription by binding to a U-rich bulge in the stem of the TAR RNA structure. EMBO J. 9:4145-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensoli, B., L. Buonaguro, G. Barillari, V. Fiorelli, R. Gendelman, R. A. Morgan, P. Wingfield, and R. C. Gallo. 1993. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, Y.-X., S. Campbell, D. Harvin, B. Ehresmann, C. Ehresmann, and A. Rein. 1999. The human immunodeficiency virus type 1 Gag polyprotein has nucleic acid chaperone activity: possible role in dimerization of genomic RNA and placement of tRNA on the primer binding site. J. Virol. 73:4251-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankel, A. D., D. S. Bredt, and C. O. Pabo. 1988. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science 240:70-73. [DOI] [PubMed] [Google Scholar]
- 15.Gabus, C., S. Auxilien, C. Péchoux, D. Dormont, W. Swietnicki, M. Morillas, W. Surewicz, P. Nandi, and J.-L. Darlix. 2001. The prion protein has DNA strand transfer properties similar to retroviral nucleocapsid protein. J. Mol. Biol. 307:1011-1021. [DOI] [PubMed] [Google Scholar]
- 16.Gabus, C., E. Derrington, P. Leblanc, J. Chnaiderman, D. Dormont, W. Swietnicki, M. Morillas, W. K. Surewicz, D. Marc, P. Nandi, and J.-L. Darlix. 2001. The prion protein has RNA binding and chaperoning properties characteristic of nucleocapsid protein NCp7 of HIV-1. J. Biol. Chem. 276:19301-19309. [DOI] [PubMed] [Google Scholar]
- 17.Harrich, D., C. Ulich, L. F. García-Martínez, and R. B. Gaynor. 1997. Tat is required for efficient HIV-1 reverse transcription. EMBO J. 16:1224-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herschlag, D., M. Khosla, Z. Tsuchihashi, and R. L. Karpel. 1994. An RNA chaperone activity of non-specific RNA binding proteins in hammerhead ribozyme catalysis. EMBO J. 13:2913-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, L.-M., A. Joshi, R. Willey, J. Orenstein, and K.-T. Jeang. 1994. Human immunodeficiency viruses regulated by alternative trans-activators: genetic evidence for a novel non-transcriptional function of Tat in virion infectivity. EMBO J. 13:2886-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, Y., A. Khorchid, J. Gabor, J. Wang, X. Li, J. L. Darlix, M. A. Wainberg, and L. Kleiman. 1998. The role of nucleocapsid and U5 stem/A-rich loop sequences in tRNA3Lys genomic placement and initiation of reverse transcription in human immunodeficiency virus type 1. J. Virol. 72:3907-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, Z.-S., and H.-N. Wu. 1998. Identification and characterization of the RNA chaperone activity of hepatitis delta antigen peptides. J. Biol. Chem. 273:26455-26461. [DOI] [PubMed] [Google Scholar]
- 22.Isel, C., J. M. Lanchy, S. F. Le Grice, C. Ehresmann, B. Ehresmann, and R. Marquet. 1996. Specific initiation and switch to elongation of human immunodeficiency virus type 1 reverse transcription require the post-transcriptional modification of primer tRNA3Lys. EMBO J. 15:917-924. [PMC free article] [PubMed] [Google Scholar]
- 23.Jeang, K.-T., H. Xiao, and E. A. Rich. 1999. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J. Biol. Chem. 274:28837-28840. [DOI] [PubMed] [Google Scholar]
- 24.Ji, X., G. J. Klarmann, and B. D. Preston. 1996. Effect of human immunodeficiency virus type 1 (HIV-1) nucleocapsid protein on HIV-1 reverse transcriptase activity in vitro. Biochemistry 35:132-143. [DOI] [PubMed] [Google Scholar]
- 25.Jiang, M., J. Mak, A. Ladha, E. Cohen, M. Klein, B. Rovinski, and L. Kleiman. 1993. Identification of tRNAs incorporated into wild-type and mutant human immunodeficiency virus type 1. J. Virol. 67:3246-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, K. A., and B. M. Peterlin. 1994. Control of RNA initiation and elongation at the HIV-1 promoter. Annu. Rev. Biochem. 63:717-743. [DOI] [PubMed] [Google Scholar]
- 27.Kameoka, M., L. Rong, M. Götte, C. Liang, R. S. Russell, and M. A. Wainberg. 2001. Role for human immunodeficiency virus type 1 Tat protein in suppression of viral reverse transcriptase activity during late stages of viral replication. J. Virol. 75:2675-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kameoka, M., Y. Tanaka, K. Ota, A. Itaya, K. Yamamoto, and K. Yoshihara. 1999. HIV-1 Tat protein is poly(ADP-ribosyl)ated in vitro. Biochem. Biophys. Res. Commun. 261:90-94. [DOI] [PubMed] [Google Scholar]
- 29.Kang, S. M., and C. D. Morrow. 1999. Genetic analysis of a unique human immunodeficiency virus type 1 (HIV-1) with a primer binding site complementary to tRNAMet supports a role for U5-PBS stem-loop RNA structures in initiation of HIV-1 reverse transcription. J. Virol. 73:1818-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashanchi, F., E. T. Agbottah, C. A. Pise-Masison, R. Mahieux, J. Duvall, A. Kumar, and J. N. Brady. 2000. Cell cycle-regulated transcription by the human immunodeficiency virus type 1 Tat transactivator. J. Virol. 74:652-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khorchid, A., H. Javanbakht, S. Wise, R. Halwani, M. A. Parniak, M. A. Wainberg, and L. Kleiman. 2000. Sequences within Pr160gag-pol affecting the selective packaging of primer tRNA3Lys into HIV-1. J. Mol. Biol. 299:17-26. [DOI] [PubMed] [Google Scholar]
- 32.Kim, Y.-S., and A. T. Panganiban. 1993. The full-length Tat protein is required for TAR-independent, posttranscriptional trans-activation of human immunodeficiency virus type 1 env gene expression. J. Virol. 67:3739-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim, Y.-S., and R. Risser. 1993. TAR-independent transactivation of the murine cytomegalovirus major immediate-early promoter by the Tat protein. J. Virol. 67:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuppuswamy, M., T. Subramanian, A. Srinivasan, and G. Chinnadurai. 1989. Multiple functional domains of Tat, the trans-activator of HIV-1, defined by mutational analysis. Nucleic Acids Res. 17:3551-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanchy, J. M., C. Ehresmann, S. F. Le Grice, B. Ehresmann, and R. Marquet. 1996. Binding and kinetic properties of HIV-1 reverse transcriptase markedly differ during initiation and elongation of reverse transcription. EMBO J. 15:7178-7187. [PMC free article] [PubMed] [Google Scholar]
- 36.Lapadat-Tapolsky, M., C. Pernelle, C. Borie, and J.-L. Darlix. 1995. Analysis of the nucleic acid annealing activities of nucleocapsid protein from HIV-1. Nucleic Acids Res. 23:2434-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Grice, S. F. J., and F. Grüninger-Leitch. 1990. Rapid purification of homodimer and heterodimer HIV-1 reverse transcriptase by metal chelate affinity chromatography. Eur. J. Biochem. 187:307-314. [DOI] [PubMed] [Google Scholar]
- 38.Li, Y., Z. Zhang, J. K. Wakefield, S. M. Kang, and C. D. Morrow. 1997. Nucleotide substitutions within U5 are critical for efficient reverse transcription of human immunodeficiency virus type 1 with a primer binding site complementary to tRNAHis. J. Virol. 71:6315-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang, C., L. Rong, M. Götte, X. Li, Y. Quan, L. Kleiman, and M. A. Wainberg. 1998. Mechanistic studies of early pausing events during initiation of HIV-1 reverse transcription. J. Biol. Chem. 273:21309-21315. [DOI] [PubMed] [Google Scholar]
- 40.Mak, J., A. Khorchid, Q. Cao, Y. Huang, I. Lowy, M. A. Parniak, V. R. Prasad, M. A. Wainberg, and L. Kleiman. 1997. Effects of mutations in Pr160gag-pol upon tRNA3Lys and Pr160gag-pol incorporation into HIV-1. J. Mol. Biol. 265:419-431. [DOI] [PubMed] [Google Scholar]
- 41.Mouland, A. J., J. Mercier, M. Luo, L. Bernier, L. DesGroseillers, and E. A. Cohen. 2000. The double-stranded RNA-binding protein Staufen is incorporated in human immunodeficiency virus type 1: evidence for a role in genomic RNA encapsidation. J. Virol. 74:5441-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratner, L., W. Haseltine, R. Patarca, K. J. Livak, B. Starcich, S. F. Josephs, E. R. Doran, J. A. Rafalski, E. A. Whitehorn, K. Baumeister, L. Ivanoff, S. R. Petteway, Jr., M. L. Pearson, J. A. Lautenberger, T. S. Papas, J. Ghrayeb, N. T. Chang, R. C. Gallo, and F. Wong-Staal. 1985. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 313:277-284. [DOI] [PubMed] [Google Scholar]
- 43.Rein, A., L. E. Henderson, and J. G. Levin. 1998. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem. Sci. 23:297-301. [DOI] [PubMed] [Google Scholar]
- 44.Remy, E., H. de Rocquigny, P. Petitjean, D. Muriaux, V. Theilleux, J. Paoletti, and B. P. Roques. 1998. The annealing of tRNA3Lys to human immunodeficiency virus type 1 primer binding site is critically dependent on the NCp7 zinc finger structure. J. Biol. Chem. 273:4819-4822. [DOI] [PubMed] [Google Scholar]
- 45.Rubartelli, A., A. Poggi, R. Sitia, and M. R. Zocchi. 1998. HIV-1 Tat: a polypeptide for all seasons. Immunol. Today 19:543-545. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, J. P., R. Pomerantz, O. Bagasra, M. Chowdhury, J. Rappaport, K. Khalili, and S. Amini. 1992. TAR-independent transactivation by Tat in cells derived from the CNS: a novel mechanism of HIV-1 gene regulation. EMBO J. 11:3395-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Telesnitsky, A., and S. P. Goff. 1997. Reverse transcriptase and generation of retroviral DNA, p. 121-160. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 48.Tsuchihashi, Z., M. Khosla, and D. Herschlag. 1993. Protein enhancement of hammerhead ribozyme catalysis. Science 262:99-102. [DOI] [PubMed] [Google Scholar]
- 49.Ulich, C., A. Dunne, E. Parry, C. W. Hooker, R. B. Gaynor, and D. Harrich. 1999. Functional domains of Tat required for efficient human immunodeficiency virus type 1 reverse transcription. J. Virol. 73:2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, W., L. E. Henderson, T. D. Copeland, R. J. Gorelick, W. J. Bosche, A. Rein, and J. G. Levin. 1996. Human immunodeficiency virus type 1 nucleocapsid protein reduces reverse transcriptase pausing at a secondary structure near the murine leukemia virus polypurine tract. J. Virol. 70:7132-7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, L., G. F. Morris, J. M. Lockyer, M. Lu, Z. Wang, and C. B. Morris. 1997. Distinct transcriptional pathways of TAR-dependent and TAR-independent human immunodeficiency virus type-1 transactivation by Tat. Virology 235:48-64. [DOI] [PubMed] [Google Scholar]