Abstract
Human and simian immunodeficiency virus (HIV and SIV, respectively) infections are characterized by gradual depletion of CD4+ T cells. The underlying mechanisms of CD4+ T-cell depletion and HIV and SIV persistence are not fully determined. The Nef protein is expressed early in infection and is necessary for pathogenesis. Nef can cause T-cell activation and downmodulates cell surface signaling molecules. However, the effect of Nef on the cell cycle has not been well characterized. To determine the role of Nef in the cell cycle, we investigated whether the SIV Nef protein can modulate cell proliferation and apoptosis in CD4+ Jurkat T cells. We developed a CD4+ Jurkat T-cell line that stably expresses SIV Nef under the control of an inducible promoter. Alterations in cell proliferation were determined by flow cytometry using stable intracytoplasmic fluorescent dye 5- and 6-carboxyfluorescein diacetate succinimidyl ester and bromodeoxyuridine incorporation. Apoptotic cell death was measured by annexin V and propidium iodide staining. Our results demonstrated that SIV Nef inhibited Fas-induced apoptosis in these cells and that the mechanism involved upregulation of the Bcl-2 protein. SIV Nef suppressed CD4+ T-cell proliferation by inhibiting the progression of cells into S phase of the cell cycle. Suppression involved an upregulation of cyclin-dependent kinase inhibitors p21 and p27 and the downregulation of cyclin D1 and cyclin A. In summary, inhibition of apoptosis by Nef can lead to persistence of infected cells and can support viral replication. In addition, a Nef-mediated delay in cell cycle progression may contribute to CD4+ T-cell anergy/depletion seen in HIV and SIV disease.
Human immunodeficiency virus (HIV) infection in humans and simian immunodeficiency virus (SIV) infection in rhesus macaques are characterized by a progressive depletion of CD4+ T cells in peripheral blood and lymphoid organs (13). Several mechanisms to account for the virus-induced CD4 T-cell loss, including cytolysis of infected cells and induction of apoptosis in uninfected bystander cells, have been proposed (15). It has been suggested that abnormalities in differentiation and proliferation of precursor CD4+ T cells during T-cell development and disruption in the activation and proliferation of naive CD4 T cells in primary lymphoid organs during viral infection may contribute to CD4 T-cell loss (12, 14, 36). Viral gene products, such as Env, Nef, Tat, Vpr, and Vpu, have been shown to modulate CD4 T-cell proliferation and/or apoptosis (50).
Nef, a 25- to 27-kDa multifunctional accessory protein of HIV and SIV, has been shown to play a key role in HIV and SIV pathogenesis (47, 59). While Nef is not required for viral replication in tissue culture, it is necessary for the development of high viral loads and disease progression in rhesus macaques (29). Rhesus macaques infected with SIV harboring nef mutants with deletions do not develop high viral loads and maintain stable CD4+ T-cell counts (9). Similarly, HIV-infected long-term nonprogressor individuals, who remain asymptomatic for more than 10 years, maintain low viral loads and stable CD4 T-cell counts. In some of these individuals, evaluation of the viral genomes has identified deletions in the nef gene. Taken together, these studies suggest that Nef may be involved in CD4 T-cell loss associated with HIV pathogenesis in humans (9, 30). Consistent with these observations, transgenic mice expressing HIV Nef have been shown to develop an AIDS-like disease characterized by depletion of CD4 T cells, alterations in CD4 T-cell activation, and defects in precursor CD4 T-cell differentiation and proliferation in lymphoid organs (20, 56).
Nef can have multiple independent effects on T cells in vitro (23, 46, 47): Nef downregulates the surface expression of CD4 (16, 35, 49), CD28 (58), and major histocompatibility complex class I (53) molecules from the cell surface, increases viral infectivity (7), and modulates signal transduction pathways in T cells (3, 38, 56). It is speculated that the ability of Nef to mediate CD4 T-cell depletion probably results directly from the interaction of Nef with CD4 activation and signaling molecules. SIV Nef directly interacts with the ζ subunit of CD3 and downregulates expression of the CD3-T-cell receptor (TCR) complex from the cell surface, thus disrupting TCR-initiated signaling in CD4 T cells. Additional effects of Nef on CD4 T-cell signaling involve modulation of calcium levels (56) and interaction with serine and tyrosine kinases, protein kinase C, and the Src family kinase p56lck signaling pathways (2, 19, 32, 51, 57). In spite of attempts to elucidate the molecular mechanisms of these interactions, few studies have examined the physiological consequences of these Nef interactions for CD4+ T cells.
Recent studies have demonstrated that HIV and SIV Nef proteins induce apoptotic cell death in T cells through Nef-mediated upregulation of Fas ligand on the surfaces of infected cells. This induction of apoptosis involves direct association between the Nef protein and the ITAM motif of the CD3 ζ subunit (62). In a contradictory report, Nef expression was shown to inhibit apoptosis by inhibiting activation of caspases 8 and 3 (63). In addition, Geleziunas et al. (17) reported that HIV Nef inhibits ASK-1 activation, thus blocking both Fas- and tumor necrosis factor alpha-induced apoptosis in HIV-infected and Nef-transfected cells. In light of these conflicting reports, we opted to examine the effects of SIV Nef on cell proliferation, apoptosis, and expression of cell cycle-related genes in CD4+ T cells by using the Jurkat T-cell line. Previous studies using retrovirus vectors to stably express Nef proteins in transfected or transduced T cells provided variable and sometimes contradictory results, which may be attributed to various possibilities (31). The Nef-expressing cells had different levels of Nef expression, leading to differing cytotoxic effects of Nef. Constant expression of the Nef protein could lead to the selection of particular cell phenotypes resistant to Nef cytotoxicity. This is evidenced by the emergence of mutations that inhibited the expression of the full-length Nef protein (3). Some of these problems can be overcome by developing an inducible Nef-expressing stable CD4+ T-cell line, thus providing a consistent and reliable system for studying the effects of Nef on CD4+ T-cell proliferation and apoptosis immediately following Nef expression.
Proliferation through the cell cycle is regulated by the cyclins and their catalytic subunits, the cyclin-dependent kinases (CDK). Cyclin A associates with CDK-2 and Cdc2 and is important for S-phase progression as well as for the G2-M transition (41, 42). Cyclin D, with its catalytic partners CDK-4 and CDK-6, and cyclin E, with its catalytic partner CDK2, can both phosphorylate the retinoblastoma (Rb) protein (22, 24, 28). Rb phosphorylation results in the release of bound transcription factor E2F1-3 from the E2F-Rb complex, thus promoting transcription of genes encoding proteins required for DNA replication (40). Other members of the Rb family, p107 and p130, bind to E2F4. The p130-E2F complexes are mainly found in quiescent or differentiated cells, while p107-E2F complexes are most commonly found in proliferating cells during S phase (8, 11, 43, 44). Cyclin A, along with its catalytic partner CDK-2 or cdc2, is essential for DNA replication and thus for S-phase progression. The activity of cyclins and CDK is negatively regulated by CDK inhibitors (CKIs) (37, 54). There are two main classes of CKIs. The INK4 family, which includes inhibitor p16, specifically inhibits cdk4 and cdk6, while the CIP/KIP family, which includes inhibitors p21 and p27, inhibits a wide range of cyclins and CDKs (55). The effects of Nef on cyclins and the cell cycle are not known.
In the present study, we have developed a CD4+ Jurkat T-cell line that stably expresses the CD8 Nef protein under the control of an inducible promoter. We used this cell line to study the effects of Nef on cell proliferation and apoptosis. Our results demonstrated that Nef could cause a delay in cell cycle progression by modulating cell cycle regulation genes. The Nef-associated inhibition of cell proliferation could be a contributing mechanism to the viral persistence and T-cell depletion seen in HIV and SIV infection.
MATERIALS AND METHODS
Cell culture.
Jurkat E6-1 T cells, and CEMx174 cells were obtained from the AIDS Research and Reference Reagent Program (National Institutes of Health, Bethesda, Md.). Cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 100 U of penicillin-streptomycin/ml (GIBCO-BRL, Rockville, Md.).
Plasmids.
Plasmid pCMV/CD8-Nef, carrying the coding sequence for the extracellular and transmembrane domains of the human CD8 α chain fused to the SIVmac239 nef gene, was a generous gift from Earl Sawai and has previously been described (52). The use of the CD8-Nef construct facilitated the detection of Nef-expressing cells by anti-CD8 antibodies. The CD8-Nef chimera has been used by other investigators and was shown to have effects on T cells similar to those of constructs expressing wild-type Nef (3, 61).
Inducible-expression plasmid pIND/CD8-Nef was generated by cloning the coding sequence for the CD8-Nef chimera into ecdysone-inducible mammalian expression vector pIND (Invitrogen, Carlsbad, Calif.) containing a neomycin selection marker. Expression is under the control of Drosophila melanogaster minimal heat shock promoter and the ecdysone and glucocorticoid response element (E/GRE) hybrid promoter. Briefly, the CD8-Nef-coding fragment from pCMV/CD8-Nef was excised with HindIII and XbaI and cloned into the HindIII-XbaI sites of the pIND vector. Plasmid pVgRxR (Invitrogen) encodes the RxR and VgEcR ecdysone receptor subunits and contains a Zeocin selection marker.
Transfection and establishment of a Nef-inducible stable cell line.
Jurkat E6-1 T cells were transfected with 20 μg of linearized pIND-CD8-Nef plasmid by electroporation of 5 × 106 cells in 0.5 ml of growth medium without serum at 250 V and 975 μF using a Gene Pulser (Bio-Rad, Hercules, Calif.) and grown overnight. Transfected cells were plated in 96-well plates in growth media containing G-418 (1,000 μg/ml; GIBCO-BRL) for 14 days. The resulting stable clones were selected by limiting dilution and maintained in the growth media containing 250 μg of G-418/ml. Cells from stable clones were expanded and transfected by electroporation with linearized pVgRxR using conditions similar to those described for pIND-CD8-Nef transfection. Cells were grown overnight and plated in 96-well plates followed by dual selection in the presence of 250 μg of G418/ml and 200 μg of Zeocin/ml for 14 days. Double-transfected clones and clones resistant to both G-418 and Zeocin were amplified and tested for the expression of Nef following induction with ponasterone A, a synthetic analog of ecdysone. The anti-SIV Nef clone 17.2 antibody (AIDS Research and Reagents Repository Program) was used to detect Nef by Western blotting. Ecdysone-inducible Nef-expressing clone B5 was maintained in RPMI 1640 supplemented with 10% fetal calf serum, 100 U of penicillin-streptomycin/ml, 250 μg of G-418/ml, and 100 μg of Zeocin/ml. Expression of Nef was induced by addition of 10 μM ponasterone A to the cells, and cells were incubated for 12 h.
Detection of Nef protein.
To determine the amount of Nef in transfected cells, Western blot analysis was performed using ECL-plus (Amersham Pharmacia Biotech Piscataway, N.J.) and STORM imaging analysis systems. Briefly, 106 cells were lysed in NP-40 lysis buffer, followed by protein concentration determination with a bicinchoninic acid microassay (Bio-Rad, Hercules, Calif.). Proteins were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE) and blotted onto nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.). Membranes were probed with anti-SIV Nef monoclonal antibody 17.2 (AIDS Research and Reference Reagent Program). ImageQuant, version 3.0 (Molecular Dynamics, Sunnyvale, Calif.), software was used to quantitate protein levels.
Induction and analysis of apoptosis.
To determine whether Nef induced or inhibited apoptosis in Nef-expressing cells, 2 × 106 cells were incubated at 37°C for 12 h in growth media containing 2.5 μg of an anti-Fas monoclonal antibody (clone DX2; BD PharMingen, San Diego, Calif.)/ml and 2.5 μg of protein G (Sigma, St. Louis, Mo.)/ml. Cells were washed and analyzed for apoptosis by annexin V-fluorescein isothiocyanate (FITC) and propidium iodide staining using flow cytometry (BD PharMingen).
CFSE tracking assay.
To determine the effect of Nef on cell proliferation, Jurkat cells, uninduced and induced B5 cells, were labeled with 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes Inc., Eugene, Oreg.) as previously described (33). Briefly, cells were washed once in phosphate-buffered saline (PBS) and resuspended at 106 cells/ml in PBS containing 0.1% bovine serum albumin (BSA). CFSE was added to a final concentration of 10 μM, and cells were incubated for 10 min at room temperature. Cells were subsequently washed with PBS-BSA solution, suspended in RPMI 1640 medium, and cultured at 37°C for 5 days in a humidified 5% CO2 incubator. Labeled cells were harvested, washed, and analyzed by FACScan (Becton Dickinson, San Jose, Calif.) using CellQuest software (Becton Dickinson) and FlowJo software (Tree Star Inc., San Carlos, Calif.). Cell division was calculated based on the sequential halving of fluorescence intensity in daughter cells. CFSE peaks were individually gated, and the proportion of cells in each round of cell division was calculated as previously described (21). Briefly, to calculate CFSE intensity for different divisions, the geometric mean fluorescence intensity (MFI) of unlabeled cells (autofluorescence) was subtracted from the MFI of CFSE-labeled cells to give the MFI of cells after zero to five divisions. The FlowJo software was also used to calculate the MFI boundaries in order to define M0 (parental), M1 (one division), M2, M3, M4, M5, and M6 cells.
Measurement of BrdU incorporation and DNA content.
Cells were seeded at 106 cells/ml in T-25 flasks, cultured for 1 to 5 days, and incubated with 10 μM bromodeoxyuridine (BrdU; Sigma) for the final 1 h of the culture period. Cells were then harvested, washed twice in PBS-BSA solution, fixed, and permeabilized with a BrdU Flow kit (BD PharMingen) according to manufacturer's instructions. DNA of fixed and permeabilized cells was digested by incubating them with 10 U of DNase I (Boehringer GmbH, Mannheim, Germany)/ml for 2 h at 37°C. Finally the BrdU-treated cells were intracellularly stained with an anti-BrdU-FITC antibody and 7-aminoactinomycin D (7-AAD) and analyzed by flow cytometry.
Immunoblot analysis.
Cells were lysed in radioimmunoprecipitation buffer (50 mM Tris-HCl [pH 7.4], 0.1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, 1 mM Na3VO4, 1 mM NaF), and equal amounts of protein were separated by SDS-PAGE, transferred onto nitrocellulose membranes (Schleicher & Schuell), and blocked with 5% nonfat dry milk in PBS-0.1% Tween prior to the addition of specific antibodies. The following antibodies were used: cyclin E antibody (generous gift from G. Lozano); antibodies to cyclin A, cyclin D1, cyclin D2, cyclin D3, p21, p27, p107, p130, E2F1, E2F4, Bcl-2, Bax, Cdk2, and actin (Santa Cruz Biotechnology, Santa Cruz, Calif.); and Rb and hyperphosphorylated Rb monoclonal antibodies (BD PharMingen). After membranes were washed, immunodetection was accomplished by incubating them with a horseradish peroxidase-conjugated antibody against mouse immunoglobulin G (IgG) or rabbit IgG (1:10,000) followed by enhanced chemiluminescence (Amersham Pharmacia Biotech) in accordance with the manufacturer's recommendations. For control, membranes were stripped in 100 mM 2-mercaptoethanol-62.5 mM Tris-HCl (pH 6.8)-2% SDS for 30 min at 55°C and reblotted with actin monoclonal antibodies.
In vitro kinase assays.
In vitro kinase assays were performed as previously described (10). Briefly, cells were lysed in NP-40 lysis buffer and the extracts were precleared and incubated with specific antibodies. The antibody-protein complexes were isolated on protein A/G beads, washed, and resuspended in kinase buffer (1 mg of histone/ml, 1 mM ATP, 1 μCi of 32P/μl, 20 mM HEPES [pH 7.0], 80 mM glycerolphosphate, 20 mM EGTA, 50 mM MgCl2, 5 mM MnCl2, 1 mM dithiothreitol, 2.5 mM phenylmethylsulfonyl fluoride, 10 μM cyclic AMP [cAMP] protein kinase inhibitor). The kinase reaction was terminated by addition of gel loading buffer, and the proteins were separated by SDS-10% PAGE. The gel was fixed, dried, and autoradiographed.
RESULTS
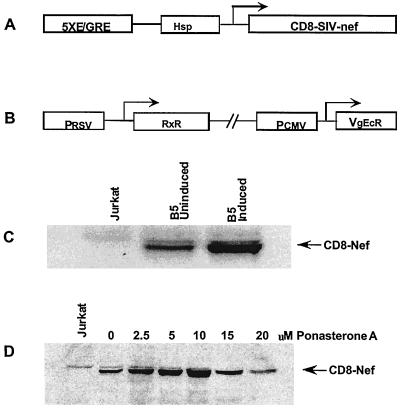
Ecdysone-inducible expression of CD8-Nef is dose dependent.
Stable high-level expression of the SIV Nef protein has been difficult to achieve partly due to the ability of Nef to modulate apoptosis and cellular proliferation of Nef-expressing cells. Loss of Nef expression, selection of truncated Nef proteins, and relative differences in Nef protein concentrations may be responsible for the conflicting results on the effects of Nef on cell proliferation and apoptosis (3, 31, 60). To overcome these difficulties, we used ecdysone-inducible expression vector pIND and subcloned a CD8-Nef chimera coding sequence downstream of the E/GRE and the Drosophila minimal heat shock protein promoters (Fig. 1A). This plasmid was used in conjunction with regulatory plasmid pVgRxR encoding ecdysone receptor subunits. The ecdysone receptor subunits are constitutively expressed from pVgRxR. In the absence of the inducer, RxR and VgEcR do not interact with the E/GRE, and thus only basal expression from the minimal promoter occurs. Induction with ponasterone A results in the association of the receptor subunits and activation of the enhancer elements, leading to the enhanced expression of CD8-Nef. Jurkat cells were cotransfected with pIND-CD8-Nef and pVgRxR plasmids. Stable clones, both G-418 and Zeocin resistant, were selected and screened for Nef expression as described in Materials and Methods. Several dually resistant clones expressing different levels of Nef were identified. There was a low basal level of Nef expression in the absence of ponasterone A and fivefold-higher Nef expression following induction with ponasterone A. We selected clone B5, which showed a fivefold increase in Nef expression upon induction but which has a minimal basal expression in uninduced cells. Using 2.5 to 20 μM ponasterone A for Nef induction, we observed an increase in Nef expression with increasing concentration of ponasterone A; expression peaked at 10 μM but declined at 15 and 20 μM ponasterone A (Fig. 1C). We used clone B5 and 10 μM ponasterone A for Nef induction in all the experiments presented in this report. Nef expression in both induced and uninduced B5 cells was considerably lower than that seen in SIVmac251-infected T cells of the CEMx174 cell line (data not shown).
FIG. 1.
Inducible stable expression of CD8-Nef in Jurkat cells. (A) Schematic diagram of the pIND-CD8-Nef construct. The coding sequence for the CD8-Nef chimera was cloned into the pIND vector downstream of the coding sequence for the hybrid E/GRE and minimal Hsp promoters. (B) Schematic diagram of the pVgRxR regulatory plasmid. This plasmid encodes Drosopila ecdysone hormone receptor subunits RxR and EcR under the control of Rous sarcoma virus (RSV) and cytomegalovirus (CMV) promoters, respectively. (C) Generation of the B5 stable cell line. Jurkat cells were stably transfected with pIND-CD8-Nef and pVgRxR plasmids. Clones resistant to both G-418 and Zeocin were screened for Nef expression by immunoblotting using an anti-Nef antibody. Clone B5 was selected and showed a fivefold increase in Nef expression following induction. There was a basal level of Nef expression in the absence of ponasterone A. (D) Induction of CD8-Nef expression is dependent on ponasterone A concentrations. B5 cells were treated with increasing concentrations of ponasterone A. Cell lysates containing equal amounts of proteins were immunoblotted with an anti-Nef antibody. Nef expression peaked at 10 μM ponasterone A but declined at 15 and 20 μM ponasterone A.
SIV Nef inhibits Fas-induced apoptosis in Nef-expressing Jurkat cells.
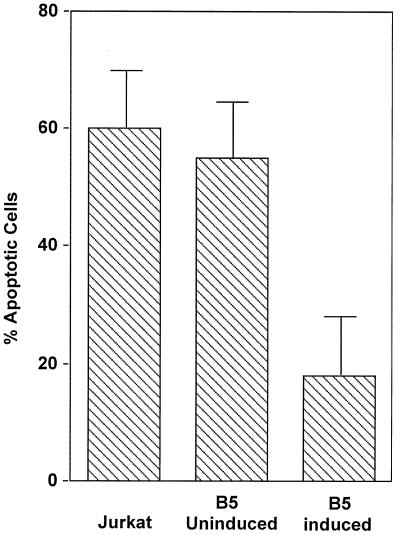
We sought to determine whether Nef activated or inhibited Fas-induced apoptosis in Jurkat T cells. Cells were incubated for 12 h with an anti-Fas antibody cross-linked with protein G. Apoptosis was analyzed by annexin V-FITC and propidium iodide staining. Apoptotic cells were detected by flow cytometry (FACScan; Becton Dickinson). As shown in Fig. 2, we observed a high percentage of apoptotic cells in control Jurkat cells (60%) and B5 uninduced (55%) cells, compared to that in B5 induced cells (18%), following the induction of apoptosis. The threefold decrease in the percentage of apoptotic cells indicated that Nef expression led to inhibition of Fas-induced apoptosis in Jurkat cells. This finding is in agreement with two recent reports (17, 63) that Nef inhibited Fas-mediated apoptosis.
FIG. 2.
Analysis of Fas-induced apoptosis. Jurkat and B5 uninduced and induced cells were treated for 6 h with anti-human Fas antibody DX2 (2 μg/ml), and protein G (2 μg/ml) to induce apoptosis. Cells were stained with annexin V-FITC and propidium iodide and analyzed by flow cytometry. The results are averages of three independent experiments.
SIV Nef suppresses cell proliferation in Jurkat T cells.
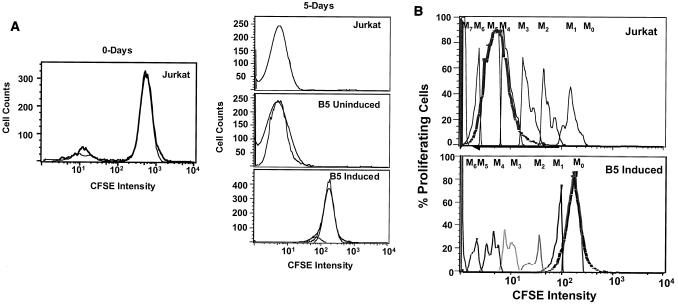
We examined the effect of Nef expression on cellular proliferation by a CFSE tracking assay. CFSE is a membrane-permeable fluorescent dye containing two acetate moieties and a succinimidyl ester functional group. In the native form, CSFE is nonfluorescent. However, after diffusion into the cell, endogenous esterases remove the acetate groups, rendering the molecule highly fluorescent and unable to diffuse out of the cell. In addition, CFSE forms protein-dye adducts, with low turnover of cytoskeletal proteins, which are responsible for the long-lasting staining afforded by CFSE. When a cell undergoes one cycle of cell division, the stain is halved in each of the daughter cells. This decrease in fluorescence can be detected by flow cytometry. This assay also allows the visualization of 8 to 10 discrete cycles of cell division and permits estimation of progenitor cell numbers, defined as the number of cells giving rise to the proliferating population. CFSE-labeled Jurkat cells and B5 uninduced and induced cells were placed in culture for 5 days and then were examined for CFSE intensity by flow cytometry (Fig. 3). The CFSE profiles indicated a 2-log-unit reduction in CFSE intensity from 103 to 101 in Jurkat and uninduced B5 cells. In contrast, the B5 induced cells were brightly stained, with a very small reduction (0.5 log unit) in CFSE intensity compared to Jurkat and B5 uninduced cells (Fig. 3A). These data indicated that cell division and cellular proliferation occurred in Jurkat and uninduced B5 cells. On the other hand, the small reduction in CFSE intensity observed in induced B5 cells indicated that cell division and cellular proliferation were constrained in Nef-expressing B5 induced cells. Further analysis of CFSE intensity profiles using Flowjo software indicated that Nef expression profoundly affected the distribution of cells within the cell cycle. The histogram presentation of the data in Fig. 3B shows the CFSE intensity of cells after 5 days of culture compared to that of freshly labeled cells at day 0 and the different cell divisions. We recorded eight discrete generations of cell division. For each assay, there were various proportions of proliferating cells in each division cycle. In Jurkat and uninduced B5 cultures, the proportion in each division cycle diminished from M7 to M0, with the highest proportion of cells having undergone seven cell division cycles. In induced B5 cells, the profile was reversed: a higher proportion of cells were at M0, and only a small proportion of cells had undergone cell division. Thus cell division was suppressed in the Nef-expressing induced B5 cell line.
FIG. 3.
Analysis of the effects of Nef on cell proliferation. Jurkat, B5 uninduced, and B5 induced cells were labeled with CFSE and cultured for 5 days. Cells were harvested, and fluorescence intensity and cell counts were analyzed by flow cytometry. Proliferation and cell division were characterized by sequential reduction in CFSE fluorescence intensity, generating a series of shifted equally spaced peaks on a logarithmic scale. (A) Comparison of CFSE intensity profiles of freshly labeled Jurkat cells (left) and cells after 5 days of culture (right). (B) Histograms showing different discrete cycles of cell division. Shown are cell division cycles of Jurkat and B5 uninduced cells (top) and B5 induced cells (bottom) after 5 days of culture.
SIV Nef slows the progression of cells into S phase of the cell cycle.
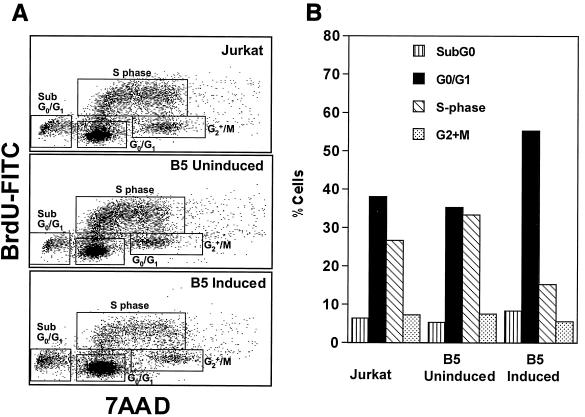
Since cell proliferation and cell division were suppressed or inhibited in Nef-expressing cells, we sought to identify the stage of cell cycle arrest in these cells. We determined the cell cycle profiles by measuring DNA synthesis in S phase (BrdU labeling) and DNA content (7-AAD labeling). Using this approach, we were able to identify and discriminate the proportion of cells at sub-G0/G1, G0/G1, S, and G2 plus M phases of the cell cycle (Fig. 4A). As shown in Fig. 4B, percentages of the Jurkat cells in the different stages of the cell cycle were as follows: sub-G0/G1, 6.5%; G0/G1, 38.1%; S, 26.8%; G2 plus M, 7.21%. However, in the B5 induced cell line, the proportions were different: sub-G0/G1, 8.23%; G0/G1, 55.4%; S, 15.2%; G2 plus M, 5.51%. Thus, the percentage of cells in G0/G1 phase in Nef-expressing cells increased to 55.4%, a 1.5-fold increase, while the proportion of cells in S phase decreased to 15.2%, a 2-fold decrease, compared to Jurkat and uninduced B5 cells. We did not observe any significant change in the proportion of cells in other phases, such as sub-G0/G1 and G2 plus M, in either Jurkat or Nef-expressing cells. These data suggested that a block or delay in the progression of cells into the S phase may be involved in the suppression of cell proliferation observed in the Nef-expressing B5 cells.
FIG. 4.
BrdU incorporation and DNA content analysis. Cells were cultured with 10 μM BrdU for the final 1 h of the culture. Cell-incorporated BrdU (measured with FITC-conjugated anti-BrdU antibody) and total DNA content (measured with 7-AAD) were analyzed by flow cytometry. The proportions of cells at sub-G0/G1, G0/G1, S, and G2 plus M phases of the cell cycle were identified. (A) Dot plots showing percentages of cells in different phases of the cell cycle for Jurkat, B5 uninduced, and B5 induced cells. The data are representative of four independent experiments. (B) Bar graph showing the percentages of cells at various phases of the cell cycle.
The level of Bcl-2 protein is upregulated in Nef-expressing cells.
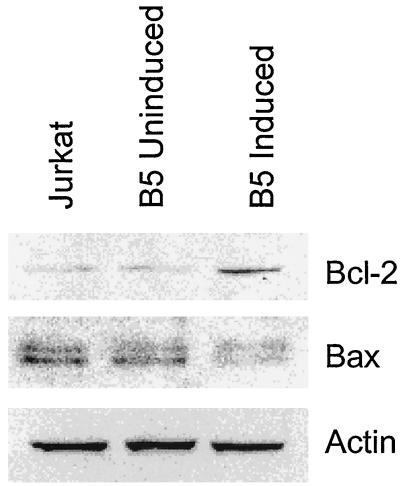
Since the early apoptotic changes are regulated by the Bcl-2 protein family, we used immunoblot analysis to determine whether the effects of Nef expression on cell apoptosis could be associated with changes in the levels of antiapoptotic (Bcl-2) and proapoptotic (Bax) proteins. We found a threefold upregulation in the Bcl-2 protein level in induced B5 cells (Fig. 5). The levels of Bcl-XL, another member of the Bcl-2 family, did not change (data not shown). The level of Bax protein expression decreased threefold upon induction of Nef (Fig. 5). Thus, Nef-mediated inhibition of apoptosis in Jurkat cells is associated with deregulation of the expression of pro- and antiapoptotic proteins.
FIG. 5.
Bcl-2 and Bax levels in Jurkat and B5 transfected Nef-expressing cells. Exponentially growing Jurkat, B5 uninduced, and B5 induced cells were subjected to Western blot analysis using 20 μg of protein for each cell line. Samples were analyzed on a SDS-10% PAGE gel and blotted to the membrane. Equal loading of the samples was determined by reblotting with actin antibodies.
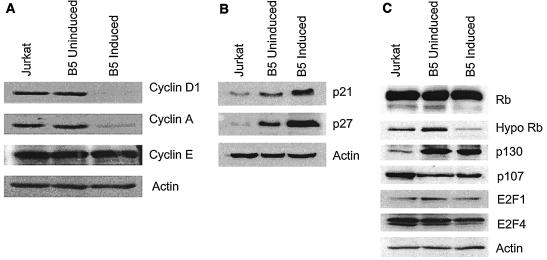
The levels of cyclin D1 and cyclin A but not of CDK-2, -4, or -6 are downregulated during Nef-mediated suppression of G1/S phase transition.
We sought to determine which of the cyclins were involved in Nef-mediated suppression of cell cycle progression of CD4+ T cells. In T cells, cyclin D1 is rapidly upregulated after entry into the G1, cyclin E is synthesized at late G1, and cyclin A is expressed during S phase. Levels of cyclin D1 were decreased 7-fold, while levels of cyclin A were decreased 10-fold, in the Nef-expressing cells. There was no change in cyclin D2 (data not shown), cyclin D3 (data not shown), and cyclin E expression levels (Fig. 6A). These data suggested that downregulation of cyclins D and A may be involved in the slow progression of cells into S phase. Immunoblot analysis of CDK-4, CDK-6, and CDK-2 showed no changes in their levels in Jurkat and B5 uninduced and induced cells (data not shown). It is likely that Nef-mediated suppression of G1/S phase progression did not involve changes in CDK levels.
FIG. 6.
Expression of cell cycle regulatory proteins in Jurkat and Nef-expressing cells. (A) Analysis of cyclin expression. Exponentially growing Jurkat, B5 uninduced, and B5 induced cells were subjected to Western blot analysis using 20 μg of protein for each cell line. The cyclin D1, cyclin A, and cyclin E proteins were detected by Western blot analysis. Membranes were reblotted with actin antibodies to ensure equal loading of the samples. (B) Analysis of CKI expression. Cells were subjected to Western blot analysis using 20 μg of protein for each cell line. Samples were analyzed on an SDS-12 (p21) or 10% (p27) PAGE gel. (C) Analysis of Rb family and E2F expression. Cells were subjected to Western blot analysis using 40 μg of protein. Samples were analyzed on an SDS-8 (Rb, hyperphosphorylated Rb, p130, and p107) or 10% (E2F1 and E2F4) PAGE gel.
Expression of CKIs is upregulated in Nef-expressing Jurkat T cells.
To determine whether CKIs p21 and p27 were involved in the regulation of the G1/S phase transition in Jurkat and B5 uninduced and induced cells, we determined levels of these CKIs in Jurkat and uninduced and induced B5 cells by immunoblot analysis (Fig. 6B). Western blot analysis demonstrated that p21 and p27 were detected in both Jurkat and B5 cells and that the levels of these proteins in induced B5 cells were increased. There was a 5-fold increase in p21 levels and a 20-fold increase in p27 levels in B5 induced cells compared to levels in Jurkat T cells (Fig. 6B). There was a modest increase in the levels of these proteins in the uninduced B5 cells, indicating that the basal level of Nef was sufficient to cause this change. Our results strongly suggested that an upregulation of the CKIs may be involved in the Nef-mediated suppression of cell cycle progression.
p130 levels are elevated in Nef-expressing Jurkat cells.
CKIs p21 and p27 inhibit the phosphorylation of cyclin/CDK substrates including the tumor suppressor Rb. Since these CKIs were upregulated in Nef-expressing cells, we examined the status of Rb family members by Western blot analysis. Rb migrated as two species, a hyperphosphorylated species of approximately 117 kDa and a hypophosphorylated form (pRb) of approximately 110 kDa, in Jurkat cells. In Jurkat cells, both forms were present. Surprisingly, in B5 induced cells, the hypophosphorylated Rb was two- to threefold less abundant but there was no change in overall levels of Rb (Fig. 6C). The decrease in hypophosphorylated Rb was observed in several independent experiments. We also examined p107 and p130 levels. The p130 protein is expressed at high levels during quiescence, while p107 is associated with proliferation (8, 43, 44). We found a fivefold increase in the levels of p130 and a twofold decrease in the levels of p107 between control and uninduced cells, suggesting that these proteins are highly sensitive to the presence of Nef (Fig. 6C). We observed no difference in levels of the transcription factor E2F1, which interacts with pRb, but a slight twofold decrease in levels of E2F4, the protein that interacts with p107 and p130 (Fig. 6C).
DISCUSSION
Our studies demonstrate that induction of SIV Nef expression in stably transfected Jurkat T cells inhibited Fas-induced apoptosis and suppressed cell proliferation. These results suggest that SIV Nef has a role to play in inducing CD4+ T-cell anergy. The Bcl-2 family members modulate the cellular apoptotic response. Antiapoptotic proteins such as Bcl-2 and Bcl-XL inhibit apoptosis, while proapoptotic proteins such as Bax promote this process. Overexpression of Bcl-2 has been shown to result in inhibition of anti-Fas- and tumor necrosis factor alpha-mediated apoptosis in T cells (27). We found that the expression of SIV Nef resulted in a threefold increase in the Bcl-2 protein. A decline in Bax expression was seen, but Bcl-XL levels were unchanged. Previous studies have reported that Nef inhibits caspase 3 and 8 activation (63), events that would be affected by Bcl-2. The increase in Bcl-2 is consistent with the decrease in the apoptotic response in SIV Nef-expressing cells and suggests that inhibition of Fas-mediated apoptosis may depend on the elevation of this antiapoptotic protein. The ability of Nef to mediate the upregulation of Bcl-2 and thus to inhibit apoptosis may have profound implications in primary as well as chronic viral infections by facilitating survival of infected cells and viral persistence. Nef is expressed early in the life cycle of HIV and accumulates in the cytoplasm of infected cells. The ability of Nef to inhibit apoptosis could enable the virus to complete the replication cycle in infected cells and could promote the persistence of the virally infected cells. Another regulatory protein of HIV, Vpr, has been shown to prevent the proliferation of infected cells by inhibiting the progression of cells through the G2/M checkpoint, which in turn supports increased virus production (18). Thus, the virus seems to modulate the different stages of the cell cycle of T cells through its regulatory proteins for increased virus production and persistence of infected cells.
We have also demonstrated that SIV Nef suppresses cell proliferation by slowing the progression of cells through the cell cycle. The retardation of cells in the G1 stage is accompanied by a downregulation of cyclins D and A as well as cyclin A-dependent kinase activity (data not shown). However, the levels of cyclin E remained constant. While CDK activity is regulated by interaction with the cyclin regulatory subunit, it is also regulated by interaction with CKIs. Induction of SIV Nef expression resulted in an increase of CKIs p27 and p21. Our analysis of CDK-2 activity upon SIV Nef induction revealed only a slight decrease (data not shown). Moreover, a significant decrease in cyclin A and D levels as well as a significant induction of CKIs was observed. Since the levels of cyclin E are not altered on SIV Nef induction, we postulate that the majority of CDK-2 activity is cyclin E dependent. It is also possible that SIV Nef mediates alterations of other proteins, such as phosphatases, that are instrumental in the activation of CDK activity and that these modifications may counteract the effect of CDK inhibitors and permit the CDK-2 to remain active. However, flow-cytometric analysis demonstrated that the combined dysregulation of cyclins and CKIs resulted in G1 growth arrest. Since cyclin D levels were decreased, we analyzed cyclin D substrate Rb as well as Rb-related proteins p107 and p130. Hyperphosphorylation of the Rb protein results in the release of the E2F transcription factors and subsequent expression of growth-promoting genes. Rb relative p130 is associated with quiescence and growth arrest, while p107 is most abundant during S phase. Expression of basal levels of Nef led to significantly increased p130 levels and a decrease in p107 levels. However, while the level of Rb was unchanged, there was an increase in phosphorylated Rb, a change that is usually associated with proliferation. This suggests that the levels of Nef present in uninduced cells were sufficient to change the expression pattern of these proteins, but these changes did not appear to be the cause of the retardation of the cell cycle progression observed on Nef induction. This also suggests that, while cyclin D levels decreased on the Nef induction, the observed reduction in S phase was not due to a decreased cyclin D-dependent kinase activity. These results also argue that a deregulation of the Rb/E2F pathway is not the primary cause for the retardation of proliferation. We observed a 10-fold decrease in cyclin A, a cyclin that is essential for S phase. Cyclin A interacts with CDK-2 in early S phase and with cdc2 in late S phase and phosphorylates multiple substrates including proteins that are required for DNA replication. We propose that a 10-fold decline in cyclin A levels along with an increase of CKIs p21 and p27 would inhibit cyclin A activity and DNA replication.
The molecular mechanisms governing Nef-induced suppression of cell proliferation and apoptosis may involve multiple molecular interactions. SIV Nef may interact with multiple signaling pathways, and distinct pathways may govern the combined effect of an inhibition of apoptosis and proliferation. Several lines of evidence implicate SIV Nef in the modulation of signaling pathways of T cells such as those associated with PAK and protein kinase C (32, 57), activation of NFAT1 (34), and modulation of calcium signaling (3, 56). These pathways are also involved in the regulation of cyclin D abundance and turnover in proliferating T cells. The mechanism, biological consequences, and role of these effects remain speculative. We speculate that SIV Nef may be involved in the modulation of proliferation and apoptosis through the same signaling pathways.
Nef may be involved in the induction of anergy through several mechanisms. Nef has been shown to disrupt normal TCR-initiated signaling by interfering with the CD3-TCR complex (26). SIV Nef interacts directly with the ζ chain of CD3 and downregulates TCR-CD3 from the cell surface (4, 25, 58). Nef downmodulates CD28, a major costimulatory molecule that mediates effective T-cell activation (56). Lack of CD28 ligation during T-cell activation results in the induction of anergy (1, 5, 6). Since both the ζ chain and CD28 are involved in the pathways involved in the induction of anergy, it is most likely that the ability of Nef to induce anergy is mediated through Nef functions that involve these cellular molecules.
In our study, SIV Nef-expressing cells showed molecular and biochemical characteristics identical to those observed in anergized T-cell clones. The T cells undergo anergy when the TCR is engaged by an antigen in the absence of a costimulatory signal, usually provided by the CD28-B7 interaction or interleukin-2. Anergized T cells remain viable but are incapable of proliferating in response to stimulation. Specifically, anergy is characterized by lack of activation of lck, ZAP-70, Ras, ERK, JNK, AP-1, and NFAT (1, 39, 45, 48). Anergizing stimuli appear to activate protein tyrosine kinase Fyn and increase intracellular levels of calcium and cAMP. The cAMP upregulation provides a link between the induction of anergy and cell cycle regulatory mechanisms. Elevated levels of cAMP induce an upregulation of CKI p27, which in turn sequesters cyclin D1-CDK-4 and cyclin E-CDK-2 complexes and thus inhibits progression of T cells through the G1/S phase checkpoint control of the cell cycle. In addition, costimulation through the CD28 pathway can prevent p27 accumulation by promoting p27 downregulation by protein ubiquitination and degradation.
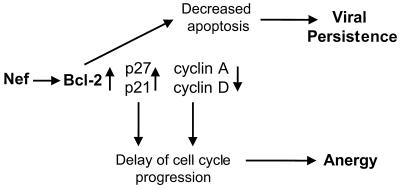
In conclusion, our results showed that Nef may suppress proliferation of CD4+ T cells through the modulation of cell cycle-associated gene expression. A potential model for the mechanism of Nef-induced cell cycle arrest has been developed (Fig. 7). Nef can increase Bcl-2 expression and inhibit Fas-mediated apoptosis, which can result in persistence of Nef-expressing cells. The decrease in cyclins D and A and the increase in CKIs p21 and p27 can lead to a cell cycle arrest at G1 stage. This may contribute to T-cell anergy. Thus, Nef can play an important role in the persistence of virally infected cells as well as in CD4+ T-cell depletion.
FIG. 7.
Model for Nef-mediated induction of T-cell anergy and viral persistence.
Acknowledgments
We thank Earl Sawai for pCMV/CD8-Nef plasmid, Elizabeth Reay and Carol Oxford for technical support, and the AIDS Research and Reference Reagent Program at NIAID for the reagents.
This work was supported by NIH grants DK-43183 and AI-43274 and a University of California, Davis, Health System award.
REFERENCES
- 1.Appleman, L. J., D. Tzachanis, T. Grader-Beck, A. A. van Puijenbroek, and V. A. Boussiotis. 2001. Helper T cell anergy: from biochemistry to cancer pathophysiology and therapeutics. J. Mol. Med. 78:673-683. [DOI] [PubMed] [Google Scholar]
- 2.Baur, A. S., G. Sass, B. Laffert, D. Willbold, C. Cheng-Mayer, and B. M. Peterlin. 1997. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity 6:283-291. [DOI] [PubMed] [Google Scholar]
- 3.Baur, A. S., E. T. Sawai, P. Dazin, W. J. Fantl, C. Cheng-Mayer, and B. M. Peterlin. 1994. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity 1:373-384. [DOI] [PubMed] [Google Scholar]
- 4.Bell, I., C. Ashman, J. Maughan, E. Hooker, F. Cook, and T. A. Reinhart. 1998. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 79:2717-2727. [DOI] [PubMed] [Google Scholar]
- 5.Boussiotis, V. A., D. L. Barber, B. J. Lee, J. G. Gribben, G. J. Freeman, and L. M. Nadler. 1996. Differential association of protein tyrosine kinases with the T cell receptor is linked to the induction of anergy and its prevention by B7 family-mediated costimulation. J. Exp. Med. 184:365-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boussiotis, V. A., G. J. Freeman, J. G. Gribben, and L. M. Nadler. 1996. The role of B7-1/B7-2:CD28/CLTA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol. Rev. 153:5-26. [DOI] [PubMed] [Google Scholar]
- 7.Chowers, M. Y., M. W. Pandori, C. A. Spina, D. D. Richman, and J. C. Guatelli. 1995. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212:451-457. [DOI] [PubMed] [Google Scholar]
- 8.Claudio, P. P., C. M. Howard, A. Baldi, A. De Luca, Y. Fu, G. Condorelli, Y. Sun, N. Colburn, B. Calabretta, and A. Giordano. 1994. p130/pRb2 has growth suppressive properties similar to yet distinctive from those of retinoblastoma family members pRb and p107. Cancer Res. 54:5556-5560. [PubMed] [Google Scholar]
- 9.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 10.Devoto, S. H., M. Mudryj, J. Pines, T. Hunter, and J. R. Nevins. 1992. A cyclin A-protein kinase complex possesses sequence-specific DNA binding activity: p33cdk2 is a component of the E2F-cyclin A complex. Cell 68:167-176. [DOI] [PubMed] [Google Scholar]
- 11.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 12.Estaquier, J., T. Idziorek, F. de Bels, F. Barre-Sinoussi, B. Hurtrel, A. M. Aubertin, A. Venet, M. Mehtali, E. Muchmore, P. Michel, et al. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. USA 91:9431-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fauci, A. S. 1996. Host factors and the pathogenesis of HIV-induced disease. Nature 384:529-534. [DOI] [PubMed] [Google Scholar]
- 14.Finkel, T. H., and N. K. Banda. 1994. Indirect mechanisms of HIV pathogenesis: how does HIV kill T cells? Curr. Opin. Immunol. 6:605-615. [DOI] [PubMed] [Google Scholar]
- 15.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 16.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 17.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 18.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 19.Greenway, A., A. Azad, J. Mills, and D. McPhee. 1996. Human immunodeficiency virus type 1 Nef binds directly to Lck and mitogen-activated protein kinase, inhibiting kinase activity. J. Virol. 70:6701-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 21.Hasbold, J., A. B. Lyons, M. R. Kehry, and P. D. Hodgkin. 1998. Cell division number regulates IgG1 and IgE switching of B cells following stimulation by CD40 ligand and IL-4. Eur. J. Immunol. 28:1040-1051. [DOI] [PubMed] [Google Scholar]
- 22.Hatakeyama, M., J. A. Brill, G. R. Fink, and R. A. Weinberg. 1994. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes Dev. 8:1759-1771. [DOI] [PubMed] [Google Scholar]
- 23.Herna Remkema, G., and K. Saksela. 2000. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front. Biosci. 5:D268-D283. [DOI] [PubMed] [Google Scholar]
- 24.Hinds, P. W., S. Mittnacht, V. Dulic, A. Arnold, S. I. Reed, and R. A. Weinberg. 1992. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell 70:993-1006. [DOI] [PubMed] [Google Scholar]
- 25.Howe, A. Y., J. U. Jung, and R. C. Desrosiers. 1998. Zeta chain of the T-cell receptor interacts with Nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J. Virol. 72:9827-9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iafrate, A. J., S. Bronson, and J. Skowronski. 1997. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 16:673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh, N., Y. Tsujimoto, and S. Nagata. 1993. Effect of bcl-2 on Fas antigen-mediated cell death. J. Immunol. 151:621-627. [PubMed] [Google Scholar]
- 28.Kato, J., H. Matsushime, S. W. Hiebert, M. E. Ewen, and C. J. Sherr. 1993. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 7:331-342. [DOI] [PubMed] [Google Scholar]
- 29.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 30.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 31.Liu, X., J. A. Schrager, G. D. Lange, and J. W. Marsh. 2001. HIV NEF-mediated cellular phenotypes are differentially expressed as a function of intracellular NEF concentrations. J. Biol. Chem. 276:32763-32770. [DOI] [PubMed] [Google Scholar]
- 32.Lu, X., X. Wu, A. Plemenitas, H. Yu, E. T. Sawai, A. Abo, and B. M. Peterlin. 1996. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr. Biol. 6:1677-1684. [DOI] [PubMed] [Google Scholar]
- 33.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 34.Manninen, A., G. H. Renkema, and K. Saksela. 2000. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J. Biol. Chem. 275:16513-16517. [DOI] [PubMed] [Google Scholar]
- 35.Mariani, R., and J. Skowronski. 1993. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc. Natl. Acad. Sci. USA 90:5549-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyaard, L., S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. Keet, and F. Miedema. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217-219. [DOI] [PubMed] [Google Scholar]
- 37.Morgan, D. O. 1995. Principles of CDK regulation. Nature 374:131-134. [DOI] [PubMed] [Google Scholar]
- 38.Niederman, T. M., J. V. Garcia, W. R. Hastings, S. Luria, and L. Ratner. 1992. Human immunodeficiency virus type 1 Nef protein inhibits NF-κB induction in human T cells. J. Virol. 66:6213-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunes, J. A., Y. Collette, A. Truneh, D. Olive, and D. A. Cantrell. 1994. The role of p21ras in CD28 signal transduction: triggering of CD28 with antibodies, but not the ligand B7-1, activates p21ras. J. Exp. Med. 180:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohtani, K., J. DeGregori, and J. R. Nevins. 1995. Regulation of the cyclin E gene by transcription factor E2F1. Proc. Natl. Acad. Sci. USA 92:12146-12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagano, M., G. Draetta, and P. Jansen-Durr. 1992. Association of cdk2 kinase with the transcription factor E2F during S phase. Science 255:1144-1147. [DOI] [PubMed] [Google Scholar]
- 42.Pagano, M., R. Pepperkok, F. Verde, W. Ansorge, and G. Draetta. 1992. Cyclin A is required at two points in the human cell cycle. EMBO J. 11:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paggi, M. G., F. Bonetto, A. Severino, A. Baldi, T. Battista, F. Bucci, A. Felsani, D. Lombardi, and A. Giordano. 2001. The retinoblastoma-related Rb2/p130 gene is an effector downstream of AP-2 during neural differentiation. Oncogene 20:2570-2578. [DOI] [PubMed] [Google Scholar]
- 44.Paggi, M. G., and A. Giordano. 2001. Who is the boss in the retinoblastoma family? The point of view of Rb2/p130, the little brother. Cancer Res. 61:4651-4654. [PubMed] [Google Scholar]
- 45.Peeper, D. S., T. M. Upton, M. H. Ladha, E. Neuman, J. Zalvide, R. Bernards, J. A. DeCaprio, and M. E. Ewen. 1997. Ras signalling linked to the cell-cycle machinery by the retinoblastoma protein. Nature 386:177-181. [DOI] [PubMed] [Google Scholar]
- 46.Piguet, V., O. Schwartz, S. Le Gall, and D. Trono. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 168:51-63. [DOI] [PubMed] [Google Scholar]
- 47.Piguet, V., and D. Trono. 1999. The Nef protein of primate lentiviruses. Rev. Med. Virol. 9:111-120. [DOI] [PubMed] [Google Scholar]
- 48.Raab, M., Y. C. Cai, S. C. Bunnell, S. D. Heyeck, L. J. Berg, and C. E. Rudd. 1995. p56Lck and p59Fyn regulate CD28 binding to phosphatidylinositol 3-kinase, growth factor receptor-bound protein GRB-2, and T cell-specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proc. Natl. Acad. Sci. USA 92:8891-8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhee, S. S., and J. W. Marsh. 1994. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 68:5156-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross, T. M. 2001. Using death to one's advantage: HIV modulation of apoptosis. Leukemia 15:332-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawai, E. T., A. Baur, H. Struble, B. M. Peterlin, J. A. Levy, and C. Cheng-Mayer. 1994. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc. Natl. Acad. Sci. USA 91:1539-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawai, E. T., A. S. Baur, B. M. Peterlin, J. A. Levy, and C. Cheng-Mayer. 1995. A conserved domain and membrane targeting of Nef from HIV and SIV are required for association with a cellular serine kinase activity. J. Biol. Chem. 270:15307-15314. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 54.Sherr, C. J. 1994. G1 phase progression: cycling on cue. Cell 79:551-555. [DOI] [PubMed] [Google Scholar]
- 55.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 56.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith, B. L., B. W. Krushelnycky, D. Mochly-Rosen, and P. Berg. 1996. The HIV nef protein associates with protein kinase C theta. J. Biol. Chem. 271:16753-16757. [DOI] [PubMed] [Google Scholar]
- 58.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trono, D. 1995. HIV accessory proteins: leading roles for the supporting cast. Cell 82:189-192. [DOI] [PubMed] [Google Scholar]
- 60.Walk, S. F., M. Alexander, B. Maier, M. L. Hammarskjold, D. M. Rekosh, and K. S. Ravichandran. 2001. Design and use of an inducibly activated human immunodeficiency virus type 1 Nef to study immune modulation. J. Virol. 75:834-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 62.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J. Exp. Med. 189:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon, K., J. G. Jeong, and S. Kim. 2001. Stable expression of human immunodeficiency virus type 1 Nef confers resistance against Fas-mediated apoptosis. AIDS Res. Hum. Retroviruses 17:99-104. [DOI] [PubMed] [Google Scholar]