Abstract
In this work, we have identified the heat shock cognate protein (hsc70) as a receptor candidate for rotaviruses. hsc70 was shown to be present on the surface of MA104 cells, and antibodies to this protein blocked rotavirus infectivity, while not affecting the infectivity of reovirus and poliovirus. Preincubation of the hsc70 protein with the viruses also inhibited their infectivity. Triple-layered particles (mature virions), but not double-layered particles, bound hsc70 in a solid-phase assay, and this interaction was blocked by monoclonal antibodies to the virus surface proteins VP4 and VP7. Rotaviruses were shown to interact with hsc70 at a postattachment step, since antibodies to hsc70 and the protein itself did not inhibit the virus attachment to cells. We propose that the functional rotavirus receptor is a complex of several cell surface molecules that include, among others, hsc70.
Rotaviruses are formed by a triple-layered protein capsid (12). In the surface of the virus there are two proteins, VP4 and VP7, which are responsible for the initial interactions of the virus with the host cell. VP4, the viral attachment polypeptide, is cleaved by trypsin into subunits VP5 and VP8, and this cleavage is associated with the penetration of the virion into the cell (12).
Rotavirus strains can be divided, with regard to their requirements to attach to the host cell, into neuraminidase (NA)-sensitive (those requiring sialic acid) and NA-resistant strains (those that either do not require sialic acid or bind to sialic acid molecules resistant to the NA treatment). Many of the strains isolated from animals, including the rhesus rotavirus RRV, are NA sensitive (9, 16, 28, 33), while a number of animal rotaviruses and most, if not all, human rotavirus strains, including the human rotavirus Wa, are resistant to NA (9, 16, 34). Some NA-sensitive rotavirus strains have been suggested to bind ganglioside GM3 containing N-glycolyl neuraminic acid as the sialic acid moiety, which is sensitive to NA treatment (11, 47), while some human rotaviruses have been proposed to use GM1, an NA-resistant ganglioside, to attach to cells (21). In addition, it has recently been shown that rotavirus nar3, a variant of RRV that, unlike the parental virus, does not require sialic acid to bind to cells, uses integrin α2β1 as its docking molecule on the cell surface (54).
Regardless of the cell molecule employed to initially attach to the cell surface, it has been shown that both NA-sensitive and NA-resistant rotaviruses interact with integrin αvβ3 at a postattachment step (19). These findings have led to the hypothesis that rotavirus cell entry is a multistep process (1, 34).
We recently showed that an octyl-β-glucoside extract of MA104 cells, obtained under nonlytic conditions, has the ability to efficiently block rotavirus infectivity when preincubated with the virus before cell infection (20). From this detergent extract, we isolated a protein band of about 73 kDa which showed a high blocking specific activity for rotaviruses (20). Four tryptic peptides derived from this band were sequenced; two of them corresponded to α-actinin, while the other two had a 100% identity with the human heat shock cognate protein hsc70, at amino acid regions 160 to 171 and 221 to 236.
hsc70 is present on the surface of rotavirus-susceptible and nonsusceptible cells.
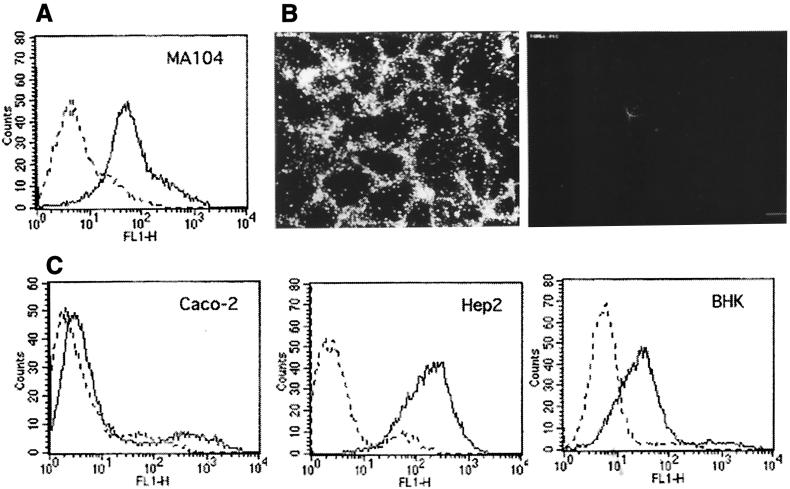
Even though hsc70 does not have an export signal sequence, it has been shown that it can be exposed on the surface of various cell types (see below). In this work, the presence of this protein on the surface of MA104 cells was demonstrated by flow cytometry (Fig. 1A) and by immunofluorescence (Fig. 1B), where nonpermeabilized cells showed the ringlike pattern of staining that has been previously observed for human 5838 Ewing's sarcoma cells expressing hsc70 on their surface (37). In addition, we analyzed by flow cytometry the presence of this protein on the surface of various cell lines which differ in their susceptibility to the virus. We assayed Caco-2 cells, which are infected as efficiently as MA104 cells by rotaviruses RRV, nar3, and Wa; Hep2 cells, which are not infected by human rotavirus Wa and are about 10- and 1,000-fold less susceptible than MA104 for rotaviruses nar3 and RRV, respectively; and BHK cells, which are about 10,000-fold less susceptible to infection by all three viruses (data not shown). Hsc70 was detected on the surface of these three cell lines, being more abundant in Hep2 and BHK cells than in Caco-2 cells (Fig. 1C).
FIG. 1.
hsc70 is present on the surface of cells. (A) Flow cytometry analysis of hsc70 surface expression in MA104 cells. MAb MA3-014, specific for hsc70 (solid line), was used at a 1:25 dilution. The control antibody of isotype immunoglobulin M (dashed line) was used at 20 μg/ml. The assay was performed as described previously (29). (B) Immunofluorescence of nonpermeabilized MA104 cells incubated with a MAb to hsc70. MA104 cells were fixed with paraformaldehyde and were incubated with a 1:25 dilution of MAb MA3-014 (left panel) or control immunoglobulin M (10 μg/ml) (right panel) for 90 min at room temperature, followed by staining with a goat anti-mouse immunoglobulin M coupled to fluorescein isothiocyanate, as described earlier (29). (C) Flow cytometry analysis of the presence of hsc70 on the surface of Caco-2, Hep2, and BHK cells, using the same hsc70 and control antibody described above for MA104 cells.
Antibodies to hsc70 block rotavirus infection.
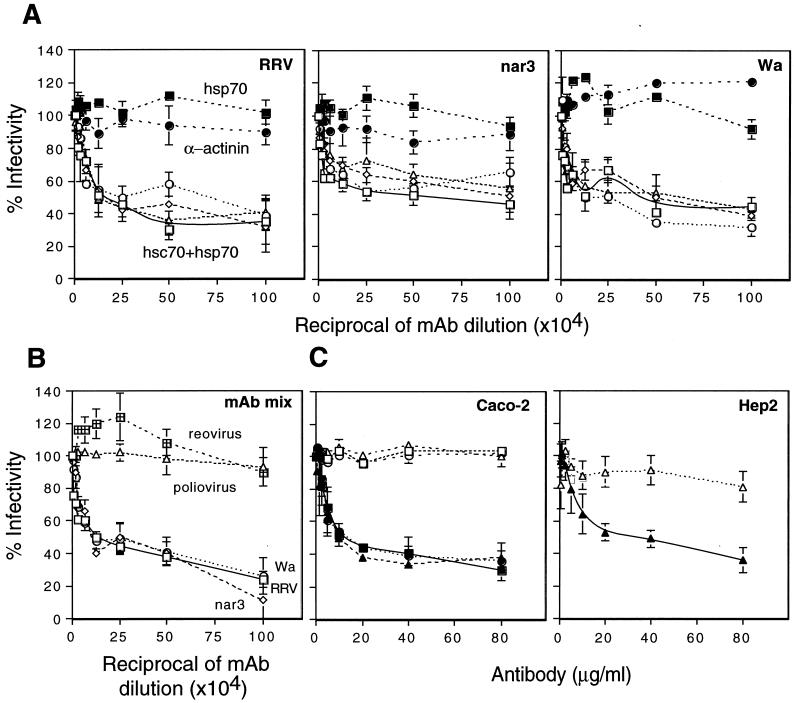
Given these findings, monoclonal antibodies (MAbs) to hsc70 were evaluated for their ability to block rotavirus infectivity when preincubated with MA104 cells before virus infection. An anti-α-actinin MAb was found not to affect rotavirus infectivity, while MAbs either specific for hsc70 or which cross-react with both hsc70 and hsp70 blocked the infectivity of all three viruses by about 50 to 60%, depending on the MAb and the virus strain (Fig. 2A). On the other hand, one MAb specific for hsp70 (MAb MA3-009) did not show rotavirus-blocking activity. In addition, a mixture of MAbs to hsc70 inhibited the infectivity of all three rotavirus strains by about 80%, while not affecting the infectivity of poliovirus and reovirus, two other nonenveloped viruses (Fig. 2B). The MAbs and a hyperimmune serum to hsc70, prepared by immunization of rabbits with a recombinant purified human hsc70 protein (see below), did not inhibit rotavirus infectivity if incubated with the cells after the viruses had been adsorbed at 37°C for 1 h (not shown). The rabbit polyclonal antibodies to hsc70 were used to test if this protein was involved in the infection by rotaviruses of cells other than MA104. These antibodies inhibited the infectivity of rotaviruses Wa, nar3, and RRV in Caco-2 cells as efficiently as in MA104 cells (Fig. 2C) and also blocked the infectivity of nar3 in Hep2 cells (Fig. 2C). The inhibition of the infectivity of rotaviruses RRV and Wa in Hep2 cells and of all three strains in BHK cells could not be evaluated reliably since only a few cells were infected. These findings suggest that hsc70 is involved in rotavirus infection of cells other than MA104 but also indicate that hsc70 by itself is not the protein that determines the tropism of rotaviruses.
FIG. 2.
Rotavirus infectivity is inhibited by antibodies to hsc70. MAbs to hsc70 (A) or a mixture of MAbs to hsc70 (B) was added to monolayers of MA104 cells for 90 min at 37°C. After incubation with antibody, the cells were washed, and then RRV, nar3, or Wa viruses (or reovirus and poliovirus in panel B) were adsorbed for 45 min at 4°C, the viral inoculum was removed, and the cultures were maintained for 14 h at 37°C. Cells were then fixed and immunostained as described earlier (20). Data are expressed as percentage of the virus infectivity obtained when the cells were preincubated with minimal essential medium as control. The average number of foci counted, representing 100% infectivity, was 107, 173, and 100 in panel A and 189, 129, and 112 in panel B for RRV, nar3, and Wa, respectively. The bars represent the standard error of at least three independent experiments performed in duplicate. The antibodies used in panel A were MA3-001 (open triangles), MA3-006 (open squares), MA3-007 (open circles), MA3-008 (open diamonds) (hsc70 + hsp70), MA3-009 (closed squares) (hsp70), and a MAb to α-actinin (closed circles). The mixture of antibodies used in panel B contained MAbs MA3-001, MA3-006, MA3-007, MA3-008, and MA3-014. All these antibodies were from Affinity Bioreagents Inc. (C) Rotavirus infectivity in Caco-2 and Hep2 cells is inhibited by antibodies to hsc70. Rabbit preimmune (open symbols) or hyperimmune (closed symbols) purified polyclonal antibodies to hsc70 were added to monolayers of Caco-2 and Hep2 cells, followed by addition of the viruses using the same incubation conditions described above. The average number of foci counted representing 100% infectivity was 118, 113, and 99 for rotaviruses RRV, nar3, and Wa, respectively, in Caco-2 cells and 80 for nar3 in Hep2 cells. The bars represent the standard error of at least two independent experiments performed in duplicate.
hsc70 binds to rotaviruses and inhibits their infectivity.
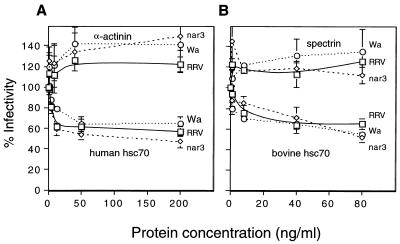
A recombinant human hsc70 protein containing a COOH-terminal histidine tail was produced in bacteria and purified by nickel column affinity. This protein, as well as a natural bovine hsc70 protein (StressGen Biotechnologies), was able to block rotavirus infectivity in a dose-dependent manner when preincubated with the viruses before infection. At a concentration of 50 ng/ml, they inhibited the infectivity of the viruses by about 40 to 50% (Fig. 3A and B). In contrast, α-actinin (Fig. 3A), spectrin (Fig. 3B), and bovine serum albumin (not shown) had no effect on the infectivity of the viruses.
FIG. 3.
Rotavirus infectivity is blocked by protein hsc70. The indicated concentrations of either recombinant human hsc70, bovine hsc70, α-actinin, or spectrin were incubated with the viruses for 90 min at 37°C. The virus-protein mixtures were added to MA104 cells for 45 min at 4°C. The cells were washed and the cultures were maintained for 14 h at 37°C. Cells were then fixed and immunostained as described earlier (20). Data are expressed as the percentage of the virus infectivity obtained when the viruses were preincubated with minimal essential medium as control. The average number of foci counted, representing 100% infectivity, was 134, 127, and 116 in panel A and 114, 94, and 118 in panel B for RRV, nar3, and Wa, respectively. The bars represent the standard error of at least three independent experiments performed in duplicate.
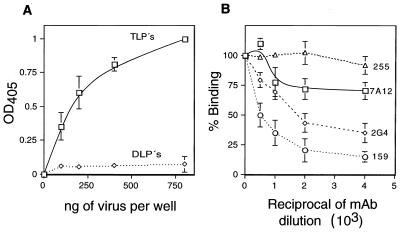
To confirm that rotaviruses bind directly and specifically to hsc70, purified triple-layered particles (TLPs) of RRV or virus particles lacking the surface proteins (double-layered particles, DLPs) were tested for their ability to bind the recombinant human hsc70 protein in an enzyme-linked immunosorbent assay. The TLPs bound to hsc70 in a dose-dependent manner, while the DLPs failed to bind this protein at the concentrations assayed (Fig. 4A). The binding of TLPs to hsc70 was shown to be specific, since it was efficiently competed by MAbs 159 to VP7 (P = 0.02) and 2G4 to VP5 (P = 0.009) but not by MAbs 7A12 to VP8 (P = 0.14) and 255/60 to VP6 (Fig. 4B).
FIG. 4.
Binding of rotavirus RRV to hsc70. (A) The indicated amounts of purified RRV TLPs and DLPs were added to microtiter plates to which 500 ng of nickel column affinity-purified recombinant human hsc70/well had been previously adsorbed. The bound virus was detected by incubation with a rabbit hyperimmune serum to rotavirus, followed by an alkaline phosphatase-conjugated secondary antibody, as described earlier (55). The phosphatase activity was detected with the Sigma 104 substrate, reading the optical density at 405 nm (OD405). (B) Binding of RRV to hsc70 in the presence of antirotavirus MAbs. Purified RRV TLPs (300 ng) were preincubated for 1 h at room temperature with the indicated dilutions of MAbs (ascites fluid). After incubation with the MAbs, the virus-antibody mixture was added to the hsc70-coated wells and the bound virus was detected as described above. The bars represent the standard deviation of the mean of two independent experiments performed in duplicate. The MAbs used were 255 to VP6, 7A12 to VP8, 2G4 to VP5, and 159 to VP7. By Student's t test, the P values for antibodies 2G4 and 159 showed that the blocking of binding for the two highest concentrations of these antibodies was significantly different from the control MAb 255 to VP6 (P = 0.02 and 0.05 for 2G4; P = 0.009 and 0.04 for 159). In the case of antibody 7A12, the P values were 0.14 and 0.07.
Neither antibodies to hsc70 nor the hsc70 protein blocks the binding of rotaviruses to cells.
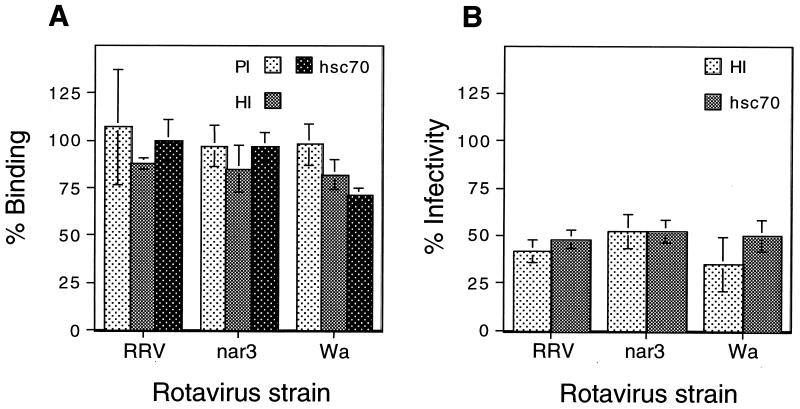
The hsc70 polyclonal serum blocked the infectivity of the viruses in MA104 cells to an extent similar to that achieved with the MAbs to this protein; however, it did not significantly block the binding of rotaviruses to cells (P = 0.29, 0.50, and 0.47 for rotaviruses Wa, nar3, and RRV, respectively; Fig. 5A). Similarly, despite its ability to block the infectivity of rotaviruses, the hsc70 protein did not significantly affect the binding of the viruses to cells (P = 0.11, 0.74, and 0.50 for rotaviruses Wa, nar3, and RRV, respectively; Fig. 5A). These results suggest that rotaviruses interact with hsc70 on the cell surface at a postattachment step. This observation is supported by the fact that if antibodies to hsc70 or the hsc70 protein is added after the viruses have been adsorbed to cells at 4°C, conditions where the virus particles attach to the cell surface but do not enter the cell, they are still able to efficiently inhibit infectivity (Fig. 5B).
FIG. 5.
Rotaviruses interact with hsc70 at a postattachment step. (A) MA104 cells in suspension were preincubated with 80 μg of preimmune (PI) or hyperimmune (HI) sera/ml to human hsc70 (partially purified by ammonium sulfate precipitation) during 1 h at 4°C. The excess, unbound antibody was removed, and then 300 ng of either RRV-, nar3-, or Wa-purified TLPs was added and the mixture was further incubated for 1 h at 4°C. To assay the binding blocking activity of the hsc70 protein (hsc70), 300 ng of the corresponding virus was incubated with the recombinant human hsc70 (10 μg/ml) for 1 h at room temperature, and then the virus-protein mixture was adsorbed to cells as described above. The amount of virus bound to cells was determined by an enzyme-linked immunosorbent assay as described earlier (55). By Student's t test, the P values for the hyperimmune serum and the hsc70 protein showed that their blocking activity for the binding of all three viruses was not significantly different from that of the control preimmune serum. The P values ranged from 0.11 for hsc70 incubated with rotavirus Wa to 0.74 for hsc70 incubated with virus nar3. (B) MA104 cells were incubated in 96-well plates with rotaviruses for 1 h at 4°C, and then either a hyperimmune (HI) serum to hsc70 (80 μg/ml) or the protein itself (hsc70) was added for 1 h at 4°C h. The cells were then further incubated for 14 h at 37°C and were immunostained for the virus as described earlier (20). Data are expressed as the percentage of the virus binding or infectivity when the cells were preincubated with minimal essential medium as control. The average numbers of foci representing 100% infectivity were 109, 119, and 145 for RRV, nar3, and Wa, respectively. The bars represent the standard deviation of the mean of at least two independent experiments performed in duplicate.
hsc70 is a constitutive member of the heat shock-induced hsp70 protein family that functions in normal cellular physiology. The proteins in this family are highly conserved nucleocytoplasmic ATPases which have been associated to a number of functions, including protein folding, translocation across biological membranes, and assembly and disassembly of oligomeric complexes. In response to different stress conditions, these proteins prevent the formation of protein aggregates by stabilizing unfolded intermediates which are subsequently refolded to the native state or degraded (23, 32, 36). In particular, hsc70 has been shown to favor protein transport across organellar membranes, bind nascent polypeptides, and dissociate clathrin from clathrin coats (40).
It is well documented that infection of animal cells by viruses often results in alterations of the cellular stress response, which is characterized by elevation and relocalization of heat shock proteins (26). In most cases the induced stress proteins have been shown to be members of the hsp60, hsp70, and hsp90 families, depending on the type of virus and host cell (26). In some cases, a direct interaction between the stress proteins and viral polypeptides has been documented, and stress proteins have been described to be present in the mature virions of rabies (48) and human immunodeficiency (52) viruses. In particular, hsp70 and hsc70 have been reported to interact with capsid proteins from poliovirus (30), vesicular stomatitis virus (22), Sindbis virus (39), vaccinia virus (27), simian virus 40 (50), adenovirus (51), hepatitis B virus (44), reovirus (56), and polyomavirus (10). These interactions have been shown to facilitate different stages of the viruses' life cycle, including the transport into the nucleus of adenoviral DNA (51) and polyomavirus proteins (10); the activation of the polymerase of hepatitis B virus (42); the enhancement of virus gene expression in measles and canine distemper viruses (41, 43); and, in general, the packaging of the virus particles. In the case of adenovirus, it has been shown that the induction of hsp70 and hsp40 is essential for the replication of the virus (18).
Despite their typical nucleocytoplasmic residence and the fact they do not contain obvious endoplasmic reticulum-Golgi targeting signal sequences, hsc70 and other heat shock proteins have been reported to be present on the surface of tumor cell lines (5, 15, 37, 38) and in cells infected with viruses (7, 8), as well as in mammalian spermatogenic cells (6, 35), epidermal cells (46), and monocytes and B cells (31). The type of association that the heat shock proteins establish with the cell surface is not known; however, it has been recently shown that hsc70, hsp70, and other stress proteins interact with specific receptors in antigen-presenting cells and are internalized by receptor-mediated endocytosis (3, 4). hsc70 is also known to interact with lipids, and it has been shown that this protein is able to form cation channels in acidic phospholipid membranes (2).
With regard to the interaction of stress proteins with viral polypeptides at the cell surface level, an hsp60-like protein located on the surface of freshly isolated human monocytes, as well as in established monocytic and T-cell lines, has been shown to interact with the human immunodeficiency virus gp41 protein, and it has been suggested that this interaction might facilitate the virus infection (52). Also, hsc70 has been described as an enhancement factor on the surface of murine fibroblasts and in a human T-cell line for the syncytium formation induced by human T-cell lymphotropic virus type 1 (13, 49). In this work, we have shown that hsc70 seems to be involved in the cell entry of rotaviruses. To our knowledge, this is the first report to provide evidence to support the role of a stress protein during the early steps of a viral replication cycle.
Similar to what was found with integrin αvβ3, rotaviruses seem to interact with hsc70 at a postattachment step. This observation is supported by the fact that (i) antibodies to hsc70 which block the infectivity of the viruses when preincubated with the cell before infection do not block their cell attachment; (ii) preincubation of the viruses with a recombinant human hsc70 protein inhibits the virus infectivity without notoriously affecting the binding of the viruses to cells; and (iii) when antibodies to hsc70, or the protein itself, are added to cells after the virus has been allowed to adsorb to the cell surface at 4°C, they still efficiently inhibit virus infectivity. These findings indicate that the interaction of rotaviruses with hsc70 may represent a late and common interaction of the viruses with the cell, after their initial attachment to other cell surface molecules. Given the major conformational changes that viral surface proteins have been shown to undergo during the cell entry of several enveloped and nonenveloped viruses (14, 17, 24, 45), it is reasonable to hypothesize that the rotavirus outer layer proteins, VP4 and VP7, also undergo conformational rearrangements during one or more of the multiple contacts that are proposed to take place between the virus particles and cellular molecules on the cell surface or during the cell entry and/or uncoating of the viruses. In this scenario, it is tempting to speculate that a protein with chaperone activity, like hsc70, could have a pivotal role to help in these processes (19, 25).
It has been shown that rotavirus-nonsusceptible cells stably transfected with the genes for α2, α4, or αv and β3 integrin subunits are only a few times more susceptible than the parental cell lines.
This limited increase in susceptibility indicates that integrins alone, as seems to be the case for hsc70, are not the only molecules needed to transform a poorly permissive cell line into one fully susceptible (like MA104) to the virus. Altogether, these data suggest that hsc70 is involved in the entry of rotaviruses, by working in combination with other proteins, most likely integrins, although other, hitherto unidentified cell receptors cannot be discarded.
It has recently been shown that sphingolipids and cholesterol are important for rotavirus infection and has been proposed that a complex of proteins immersed in the lipid microdomains known as rafts might serve as the functional receptor for rotaviruses (1, 20). Integrins and hsc70 might be components of this complex. In this regard, there has been an interesting, recent observation that bacterial lipopolysaccharide (LPS) from gram-negative bacteria and lipoteichoic acid (LTA) from the outer cell wall of gram-positive bacteria bind to CD14, a glycosylphosphatidylinositol-linked protein which functions as their cell surface receptor on human monocytic and endothelial cell lines. After their initial binding to CD14, LPS and LTA are rapidly transferred to the heat shock proteins hsp70 and hsp90 on the cell membrane. Antibodies to these heat shock proteins were found to block the transfer of LPS and to inhibit interleukin 6 production upon LPS stimulation. These data indicate that LPS transfers from CD14 to hsp70 and hsp90, which may be part of an LPS-LTA multimeric receptor-transducing complex that might be present in lipid microdomains (53).
It remains to be determined if, during the infection of cells by rotaviruses, hsc70 serves only as an anchor on the membrane for the viruses during their transit to the cell's interior or if the chaperone activity of the protein is important for this event.
Acknowledgments
We thank R. I. Morimoto, Northwestern University, for kindly providing the human hsc70 gene and H. B. Greenberg, Stanford University, for making available the antirotavirus MAbs. We are also thankful to Xochitl Alvarado for her help with confocal microscopy and to Elizabeth Mata and Graciela Cabeza for assistance with the animal handling.
This work was partially supported by grants 75197-527106 and 55000613 from the Howard Hughes Medical Institute, G0012-N9607 from the National Council for Science and Technology-Mexico, and IN201399 from DGAPA-UNAM.
REFERENCES
- 1.Arias, C. F., C. A. Guerrero, E. Méndez, S. Zárate, P. Isa, R. Espinosa, P. Romero, and S. López. 2001. Early events of rotavirus infection: the search for the receptor(s), p. 47-63. In D. Chadwick and J. A. Goode (ed.), Gastroenteritis viruses. John Wiley & Sons, Ltd., Chichester, United Kingdom. [DOI] [PubMed]
- 2.Arispe, N., and A. De Maio. 2000. ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J. Biol. Chem. 275:30839-30843. [DOI] [PubMed] [Google Scholar]
- 3.Arnold-Schild, D., D. Hanau, D. Spehner, C. Schmid, H. Rammensee, H. de la Salle, and H. Schild. 1999. Cutting edge: receptor-mediated endocytosis of heat shock protein by professional antigen-presenting cells. J. Immunol. 162:3757-3760. [PubMed] [Google Scholar]
- 4.Binder, R. J., M. L. Harris, A. Ménoret, and P. K. Srivastava. 2000. Saturation, competition, and specificity in interaction of heat shock proteins (hsp) gp96, hsp90, and hsp70 with CD11b+ cells. J. Immunol. 165:2582-2587. [DOI] [PubMed] [Google Scholar]
- 5.Botzler, C., G. Li, R. D. Issels, and G. Multhoff. 1995. Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones 3:6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulanger, J., D. Faulds, E. M. Eddy, and C. A. Lingwood. 1995. Members of the 70 kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and mycoplasma-related infertility. J. Cell Physiol. 165:7-17. [DOI] [PubMed] [Google Scholar]
- 7.Brenner, B. G., and M. A. Wainberg. 1999. Heat shock protein-based therapeutic strategies against human immunodeficiency virus type 1 infection. Infect. Dis. Obstet. Gynecol. 7:80-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chouchane, L., F. S. Bowers, S. Sawasdikosol, R. M. Simpson, and T. J. Kindt. 1994. Heat-shock proteins expressed on the surface of human T cell leukemia virus type I-infected cell lines induce autoantibodies in rabbits. J. Infect. Dis. 169:253-259. [DOI] [PubMed] [Google Scholar]
- 9.Ciarlet, M., and M. K. Estes. 1999. Human and most animal rotavirus strains do not require the presence of sialic acid on the cell surface for efficient infectivity. J. Gen. Virol. 80:943-948. [DOI] [PubMed] [Google Scholar]
- 10.Cripe, T. P., S. E. Delos, P. A. Estes, and R. L. Garcea. 1995. In vivo and in vitro association of hsc70 with polyomavirus capsid proteins. J. Virol. 69:7807-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delorme, C., H. Brussow, J. Sidoti, N. Roche, K. Karlsson, J. Neeser, and S. Teneberg. 2001. Glycosphingolipid binding specificities of rotavirus: identification of a sialic acid-binding epitope. J. Virol. 75:2276-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estes, M. K. 1996. Rotaviruses and their replication, p. 1625-1655. In B. N. Fields, D. N. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Virology, vol. 2. Raven Press, New York, N.Y. [Google Scholar]
- 13.Fang, D., Y. Haraguchi, A. Jinno, Y. Soda, N. Shimizu, and H. Hoshino. 1999. Heat shock cognate protein 70 is a cell fusion-enhancing factor but not an entry factor for human T-cell lymphotropic virus type I. Biochem. Biophys. Res. Commun. 261:357-363. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes, J., D. Tang, G. Leone, and P. W. Lee. 1994. Binding of reovirus to receptor leads to conformational changes in viral capsid proteins that are reversible upon virus detachment. J. Biol. Chem. 269:17043-17047. [PubMed] [Google Scholar]
- 15.Ferrarini, M., S. Heltai, M. R. Zocchi, and C. Rugarli. 1992. Unusual expression and localization of heat-shock proteins in human tumor cells. Int. J. Cancer 51:613-619. [DOI] [PubMed] [Google Scholar]
- 16.Fukudome, K., O. Yoshie, and T. Konno. 1989. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology 172:196-205. [DOI] [PubMed] [Google Scholar]
- 17.Gaudin, Y., R. W. H. Ruigrok, and J. Brunner. 1995. Low-pH induced conformational changes in viral fusion proteins: implications for the fusion mechanism. J. Gen. Virol. 76:1541-1556. [DOI] [PubMed] [Google Scholar]
- 18.Glotzer, J. B., M. Saltik, S. Chiocca, A. Michou, P. Moseley, and M. Cotten. 2000. Activation of heat-shock response by an adenovirus is essential for virus replication. Nature 407:207-211. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero, C. A., E. Méndez, S. Zárate, P. Isa, S. López, and C. F. Arias. 2000. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc. Natl. Acad. Sci. USA 97:14644-14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrero, C. A., S. Zárate, G. Corkidi, S. López, and C. F. Arias. 2000. Biochemical characterization of rotavirus receptors in MA104 cells. J. Virol. 74:9362-9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo, C., O. Nakagomi, M. Mochizuki, H. Ishida, M. Kiso, Y. Ohta, T. Suzuki, D. Miyamoto, K. I. J. Hidari, and Y. Suzuki. 1999. Ganglioside GM(1a) on the cell surface is involved in the infection by human rotavirus KUN and MO strains. J. Biochem. 126:683-688. [DOI] [PubMed] [Google Scholar]
- 22.Hammond, C., and A. Helenius. 1994. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science 266:456-458. [DOI] [PubMed] [Google Scholar]
- 23.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-580. [DOI] [PubMed] [Google Scholar]
- 24.Haywood, A. M. 1994. Virus receptors: binding, adhesion, strengthening, and changes in viral structure. J. Virol. 68:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewish, M. J., Y. Takada, and B. S. Coulson. 2000. Integrins a2b1 and a4b1 can mediate SA11 rotavirus attachment and entry into cells. J. Virol. 74:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jindal, S., and M. Malkovsky. 1994. Stress responses to viral infection. Trends Microbiol. 2:89-90. [DOI] [PubMed] [Google Scholar]
- 27.Jindal, S., and R. A. Young. 1992. Vaccinia virus infection induces a stress response that leads to association of hsp70 with viral proteins. J. Virol. 66:5357-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keljo, D. J., and A. K. Smith. 1988. Characterization of binding of simian rotavirus SA-11 to cultured epithelial cells. J. Pediatr. Gastroenterol. Nutr. 7:249-256. [DOI] [PubMed] [Google Scholar]
- 29.López, S., R. Espinosa, P. Isa, M. T. Merchant, S. Zárate, E. Méndez, and C. F. Arias. 2000. Characterization of a monoclonal antibody directed to the surface of MA104 cells that blocks the infectivity of rotaviruses. Virology 273:160-168. [DOI] [PubMed] [Google Scholar]
- 30.Macejak, D. G., and P. Sarnow. 1992. Association of heat shock protein 70 with enterovirus capsid precursor P1 in infected human cells. J. Virol. 66:1520-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manara, G. C., P. Sansoni, L. Badiali-De Giorgi, G. Gallinella, C. Ferrari, V. Brianti, F. F. Fagnoni, C. L. Ruegg, G. De Panfilis, and G. Pasquinelli. 1993. New insights suggesting a possible role of a heat shock protein 70-kD family-related protein in antigen processing/presentation phenomenon in humans. Blood 82:2865-2871. [PubMed] [Google Scholar]
- 32.Mayer, M. P., and B. Bukau. 1998. Hsp70 chaperone systems: diversity of cellular functions and mechanism of action. Biol. Chem. 379:261-268. [PubMed] [Google Scholar]
- 33.Méndez, E., C. F. Arias, and S. López. 1993. Binding to sialic acids is not an essential step for the entry of animal rotaviruses to epithelial cells in culture. J. Virol. 67:5253-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Méndez, E., S. Lopez, M. A. Cuadras, P. Romero, and C. F. Arias. 1999. Entry of rotaviruses is a multistep process. Virology 263:450-459. [DOI] [PubMed] [Google Scholar]
- 35.Miller, D., S. Brough, and O. al-Harbi. 1992. Characterization and cellular distribution of human spermatozoal heat shock proteins. Human Reprod. 7:637-645. [DOI] [PubMed] [Google Scholar]
- 36.Morimoto, R. I., M. P. Kline, D. N. Bimston, and J. J. Cotto. 1997. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 32:17-29. [PubMed] [Google Scholar]
- 37.Multhoff, G., C. Botzler, M. Wiesnet, E. Muller, T. Meier, and W. Wilmanns. 1995. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 61:272-279. [DOI] [PubMed] [Google Scholar]
- 38.Multhoff, G., and L. E. Hightower. 1996. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones 1:167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulvey, M., and D. T. Brown. 1995. Involvement of the molecular chaperone BiP in maturation of Sindbis virus envelope glycoproteins. J. Virol. 69:1621-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newmyer, S. L., and S. L. Schmid. 2001. Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J. Cell Biol. 152:607-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oglesbee, M. J., H. Kenney, T. Kenney, and S. Krakowka. 1993. Enhanced production of morbillivirus gene-specific RNAs following induction of the cellular stress response in stable persistent infection. Virology 192:556-567. [DOI] [PubMed] [Google Scholar]
- 42.Park, S. G., and G. Jung. 2001. Human hepatitis B virus polymerase interacts with the molecular chaperonin Hsp60. J. Virol. 75:6962-6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parks, C. L., R. A. Lerch, P. Walpita, M. S. Sidhu, and S. A. Udem. 1999. Enhanced measles virus cDNA rescue and gene expression after heat shock. J. Virol. 73:3560-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prange, R., M. Werr, and M. Loffler-Mary. 1999. Chaperones involved in hepatitis B virus morphogenesis. Biol. Chem. 380:305-314. [DOI] [PubMed] [Google Scholar]
- 45.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 46.Rocchi, G., A. Pavesi, C. Ferrari, A. Bolchi, and G. C. Manara. 1993. A new insight into the suggestion of a possible antigenic role of a member of the 70 kD heat shock proteins. Cell Biol. Int. 17:83-92. [DOI] [PubMed] [Google Scholar]
- 47.Rolsma, M. D., T. B. Kuhlenschmidt, H. B. Gelberg, and M. S. Kuhlenschmidt. 1998. Structure and function of a ganglioside receptor for porcine rotavirus. J. Virol. 72:9079-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sagara, J., and A. Kawai. 1992. Identification of heat shock protein 70 in the rabies virion. Virology 190:845-848. [DOI] [PubMed] [Google Scholar]
- 49.Sagara, Y., C. Ishida, Y. Inque, H. Shiraki, and Y. Maeda. 1998. 71-Kilodalton heat shock cognate protein acts as a cellular receptor for syncytium formation induced by human T-cell lymphotropic virus type 1. J. Virol. 72:535-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sainis, L., C. Angelidis, G. N. Pagoulatos, and L. Lazaridis. 2000. HSC70 interactions with SV40 viral proteins differ between permissive and nonpermissive mammalian cells. Cell Stress Chaperones 5:132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saphire, A. C., T. Guan, E. C. Schirmer, G. R. Nemerow, and L. Gerace. 2000. Nuclear import of adenovirus DNA in vitro involves the nuclear protein import pathway and hsc70. J. Biol. Chem. 275:4298-4304. [DOI] [PubMed] [Google Scholar]
- 52.Speth, C., Z. Prohászka, M. Mair, G. Stockl, X. Zhu, B. Jobstl, G. Fust, and M. P. Dierich. 1999. A 60 kD heat-shock protein-like molecule interacts with the HIV transmembrane glycoprotein gp41. Mol. Immunol. 36:619-628. [DOI] [PubMed] [Google Scholar]
- 53.Triantafilou, K., M. Triantafilou, S. Ladha, A. Mackie, N. Fernandez, R. L. Dedrick, and R. Cherry. 2001. Fluorescence recovery after photobleaching reveals that LPS rapidly transfers from CD14 to hsp70 and hsp90 on the cell membrane. J. Cell Sci. 114:2535-2545. [DOI] [PubMed] [Google Scholar]
- 54.Zárate, S., R. Espinosa, P. Romero, C. A. Guerrero, C. F. Arias, and S. López. 2000. Integrin alpha2beta1 mediates the cell attachment of the rotavirus neuraminidase-resistant variant nar3. Virology 278:50-54. [DOI] [PubMed] [Google Scholar]
- 55.Zárate, S., R. Espinosa, P. Romero, E. Méndez, C. F. Arias, and S. López. 2000. The VP5 domain of VP4 can mediate attachment of rotaviruses to cells. J. Virol. 74:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao, Y. G., R. Gilmore, G. Leone, M. C. Coffey, B. Weber, and P. W. K. Lee. 2001. Hsp90 phosphorylation is linked to its chaperoning function. Assembly of the reovirus cell attachment protein. J. Biol. Chem. 276:32822-32827. [DOI] [PubMed] [Google Scholar]