Abstract
In polarized epithelial cells, the vesicular stomatitis virus glycoprotein is segregated to the basolateral plasma membrane, where budding of the virus takes place. We have generated recombinant viruses expressing mutant glycoproteins without the basolateral-membrane-targeting signal in the cytoplasmic domain. Though about 50% of the mutant glycoproteins were found at the apical plasma membranes of infected MDCK cells, the virus was still predominantly released at the basolateral membranes, indicating that factors other than the glycoprotein determine the site of virus budding.
For most viruses, epithelial cells of the respiratory or the gastrointestinal tract are the primary targets for replication. The plasma membranes of these cells are divided by tight junctions into apical and basolateral domains differing in composition and function (11). The polarized organization of epithelial cells has important consequences for virus pathogenicity (3). Following replication in these cells, some viruses are released preferentially from the apical surface, thus favoring the establishment of a localized infection. Most respiratory viruses, such as influenza virus, parainfluenza virus, rhinovirus, or respiratory syncytial virus, behave in this way. Conversely, viruses that are released from the basolateral membrane find access to the underlying tissue and the blood system, facilitating the development of a systemic infection. Viruses belonging to this category include vesicular stomatitis virus (VSV), Semliki Forest virus, vaccinia virus, and certain retroviruses, including human immunodeficiency virus type 1. Though this rule is not applicable to all viruses, the importance of a directional virus release from polarized epithelial cells is illustrated by Sendai virus. This paramyxovirus normally buds from the apical plasma membrane and causes a localized infection of the bronchial epithelium. In contrast to the wild-type virus, the F1-R mutant is released in a bipolar manner and shows an extended tissue tropism and increased virulence (16). For many enveloped viruses that are released from epithelial cells in a polarized manner, it has been shown that the envelope glycoproteins per se are targeted to the same plasma membrane domain from which the virus buds (6, 10). These observations have led to the hypothesis that the site where the envelope glycoproteins accumulate determines the site of virus budding.
In the case of VSV, a member of the family Rhabdoviridae, the basolateral release of the virus coincides with the basolateral localization of single-surface glycoprotein G (5). A basolateral-membrane-targeting signal in the cytoplasmic domain of the G protein has been identified as YTDI504, which fits the general motif Y-X-X-aliphatic amino acid responsible for basolateral targeting and/or the endocytosis of many cellular and viral membrane proteins (17). When either the tyrosine or the isoleucine of this motif was replaced by alanine, about 70% of vector-expressed G protein was targeted to the apical plasma membranes of polarized Madin-Darby canine kidney (MDCK) cells. In this study, we made use of reverse genetics with VSV to investigate the importance of the basolateral-membrane-targeting signal in the cytoplasmic tail of the G protein for polarized virus release. A Y501A, I504A, or Y501A/I504A mutation was introduced into the G protein gene, which was then cloned into the full-length antigenomic vector pVSV-XN2 by making use of the single restriction sites MluI and XhoI (14). For the recovery of infectious recombinant VSVs, BSR-T7/5 cells (2) were grown in 50-mm-diameter dishes and each cell was infected with 5 focus-forming units of MVA-T7, a recombinant vaccinia virus encoding T7 RNA polymerase (15). Subsequently, the cells were transfected with 5 μg of pVSV-XN2, 1.5 μg of pBS-N, 2.5 μg of pBS-P, and 1 μg of pBS-L by using Lipofectamin 2000 transfection reagent (Life Technologies). The last three plasmids encode the components N, P, and L, respectively, of the VSV polymerase complex, which are expressed from the T7 promotor (14). Two days after transfection, virus was recovered from the cell culture supernatant, propagated in BHK-21 cells, and plaque purified. When the three VSV mutants rVSV-G(Y502A), rVSV-G(I505A), and rVSV-G(Y502/I505A) in nonpolarized BHK-21 cells were characterized with regard to plaque morphology, one-step growth kinetics, and incorporation of G protein into virions, they did not significantly differ from rVSV, a recombinant VSV containing parental G protein (data not shown). We studied the vectorial transport of the G protein in polarized type I MDCK (MDCK-I) cells that were grown on 0.4-μm-pore-size Transwell polycarbonate filters (Costar). The cells were infected with either rVSV or one of the three mutant VSVs 4 days after being seeded, when the cells had formed a tight monolayer that displayed an electrical resistance of at least 4,000 Ω · cm2. Infection was successful only when the virus was applied to the basolateral surfaces of the filter-grown cells, reflecting the polarity of cellular receptors (5). Since the filters presented a strong diffusion barrier, a very high multiplicity of infection was used to make infection more efficient (100 PFU/cell or 5 × 108 PFU/filter). To determine the location of VSV G protein, the surface proteins of either the apical or the basolateral plasma membrane were selectively labeled at 6.5 h postinfection by adding a water-soluble sulfated N-hydroxysuccinimide ester of biotin (Pierce) to either the upper or the lower compartment of the filter chamber (18). VSV G protein was immunoprecipitated from the cell lysates, and the biotin label was detected in Western blots by using a streptavidin-horseradish peroxidase complex (Fig. 1). The G protein of rVSV was found to reside predominantly at the basolateral membranes of filter-grown MDCK cells. Only a small fraction of the protein (<5%) was recovered from the apical membranes. In contrast, all three of the mutants rVSV-G(Y501A), rVSV-G(I504A), and rVSV-G(Y501/I504A) expressed G proteins that were almost equally distributed between the apical and basolateral plasma membranes. This finding confirms the previous observation that Tyr501 and Ile504 are the most critical components of the basolateral-membrane-targeting signal in the cytoplasmic domain (17). In the viral context, the distribution of the mutant G proteins is not as polarized as it is in the plasmid-driven expression system described above. This discrepancy might be due to other viral proteins, e.g., the matrix protein, that may have a modulating effect on the localization of the G protein. In both systems, a significant portion of the G protein mutants was still targeted to the basolateral membrane, which may be due to another basolateral-membrane targeting signal in the ectodomain of the protein (4). To evaluate whether the change in the distribution of VSV G protein has any consequences on polarized virus release, 9 h postinfection the apical and basolateral supernatants of filter-grown MDCK-I cells were plaque titrated (Table 1). The parental rVSV was found to be released predominantly into the basolateral medium; only 1% of the virus was recovered from the apical medium. Very similar virus distributions between apical and basolateral media were observed with the three virus mutants. This finding was confirmed by electron microscopy (8), since budding forms and virions of parental and mutant rVSVs were observed almost exclusively at the lateral plasma membranes of filter-grown MDCK-I cells (Fig. 2). We therefore conclude that the G glycoprotein does not determine the site of virus release. Compared to the level of parental-virus release, the levels of the mutants released from the infected cells were somewhat reduced (Table 1). This is most probably due to the fact that the G proteins in the apical plasma membrane are not available for virus budding at the basolateral membrane. Interestingly, a discrepancy between the localization of the viral glycoprotein and the site of virus release has also been observed with Marburg virus (13). The glycoprotein of this filovirus was found to be transported predominantly to the apical plasma membrane, whereas the virus was released exclusively from the basolateral surface, indicating that factors other than the glycoprotein are responsible for vectorial budding. Another negative-strand RNA virus, measles virus, is released from the apical plasma membrane, though the viral glycoproteins F and H reside predominantly at the basolateral domains of polarized epithelial cells (7). Recent findings indicate that the matrix protein, not the glycoproteins, of measles virus determines apical virus release (9). The matrix protein of VSV is also a prime candidate for the performance of this function; it has been shown to have budding activity in the absence of other VSV proteins (12) and was demonstrated to bind to the basolateral plasma membranes of polarized epithelial cells (1).
FIG. 1.
Distribution of G protein in infected polarized MDCK-I cells. Filter-grown MDCK-I cells were infected with the rVSVs indicated above the gel. At 6.5 h postinfection, cell surface proteins of the apical (lanes a) or basolateral (lanes b) plasma membrane were selectively labeled by biotinylation. Following immunoprecipitation, sodium dodecyl sulfate-gel electrophoresis, and transfer to nitrocellulose, the biotinylated G protein was detected with a streptavidin-peroxidase complex by chemiluminescence. The results of a representative example of three independent experiments are shown.
TABLE 1.
G protein distribution and virus release in polarized MDCK-I cellsa
| Virus | Amino acid exchange(s) | Total virus (PFU [106]/ml) | Level of virus in the baso- lateral medium (%) | Level of basolateral G protein (%)b |
|---|---|---|---|---|
| rVSV-G | ..KKRQIYTDIEMNRLGK | 6.0 | 99 | 95 |
| rVSV-G(Y501A) | .......A.......... | 2.0 | 96 | 48 |
| rVSV-G(I504A) | ..........A....... | 2.0 | 98 | 54 |
| rVSV-G(Y501A/I504A) | .......A..A....... | 1.5 | 93 | 51 |
Filter-grown MDCK-I cells were infected with the rVSVs indicated, and mutations were introduced into the cytoplasmic tails of the respective G proteins. Nine hours postinfection, the apical and basolateral supernatants were titrated by plaque assay. The level of basolateral virus is expressed as a percentage of total virus released. Data are the mean values of results of three independent infection experiments.
The protein levels shown in Fig. 1 were densitometrically quantified. The amount of basolateral G protein is expressed as a percentage of the total amount of G protein.
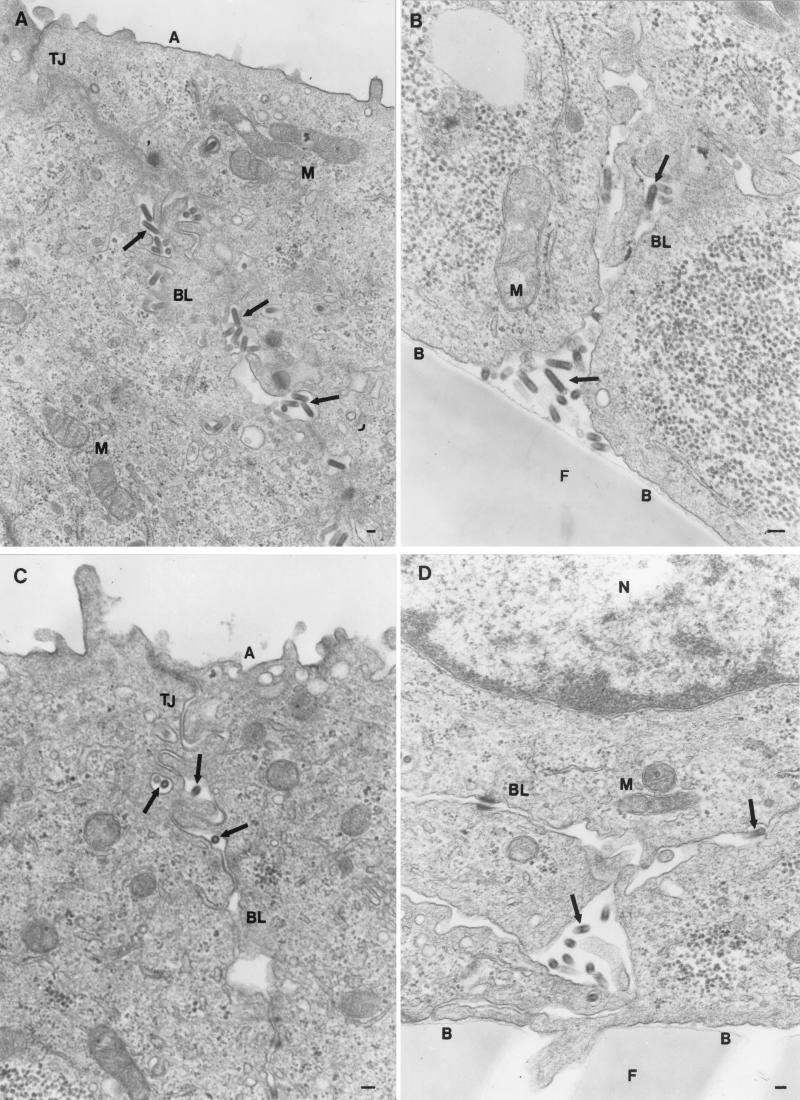
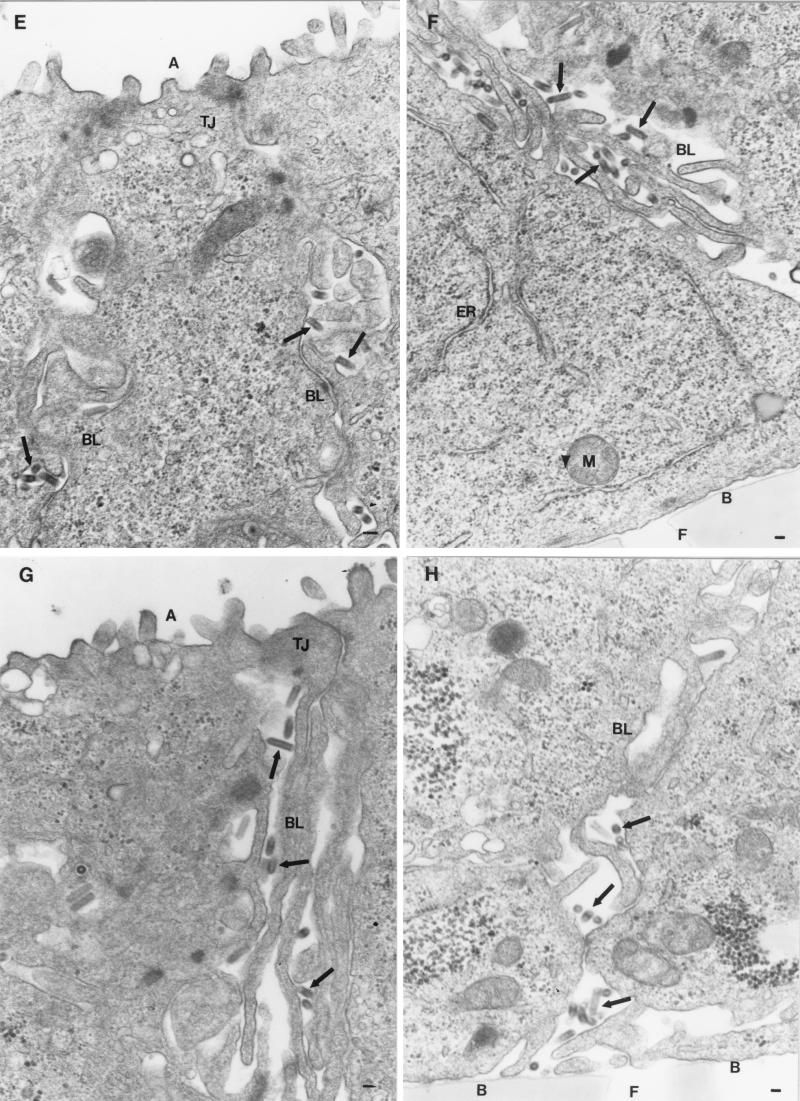
FIG. 2.
Polarity of virus maturation. Filter-grown MDCK-I cells were infected with rVSV (A and B), rVSV-G(Y501A) (C and D), rVSV-G(Y501A/I504A) (E and F), or rVSV-G(I504A) (G and H), fixed with glutaraldehyde 8 h postinfection, and prepared for electron microscopy. Abbreviations: A, apical membrane; BL, basolateral membrane; B, basal membrane; M, mitochondrium; TJ, tight junction; F, filter; ER, endoplasmic reticulum; N, nucleus. Arrows indicate VSV particles. Bars, 0.1 μm.
Acknowledgments
We thank John K. Rose and Matthias Schnell for providing the plasmids pVSV-XN2, pBS-N, pBS-P, and pBS-L.
This work was supported by Deutsche Forschungsgemeinschaft grant Zi 558/1-1 to G.Z.
REFERENCES
- 1.Bergmann, J. E., and P. J. Fusco. 1988. The M protein of vesicular stomatitis virus associates specifically with the basolateral membranes of polarized epithelial cells independently of the G protein. J. Cell Biol. 107:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchholz, U. J., S. Finke, and K.-K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compans, R. W., and G. Herrler. 1998. Virus infection of epithelial cells, p. 671-683. In P. L. Ogra, J. Mestecky, M. E. Lamm, and W. Strober (ed.), Mucosal immunology. Academic Press, New York, N.Y.
- 4.Compton, T., I. E. Ivanov, T. Gottlieb, M. Rindler, and D. D. Sabatini. 1989. A sorting signal for the basolateral delivery of the vesicular stomatitis virus (VSV) G protein lies in its luminal domain: analysis of the targeting of VSV G-influenza hemagglutinin chimeras. Proc. Natl. Acad. Sci. USA 86:4112-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller, S., C.-H. von Bonsdorff, and K. Simons. 1984. Vesicular stomatitis virus infects and matures only through the basolateral surface of the epithelial cell line, MDCK. Cell 38:65-77. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb, T. A., A. Gonzalez, L. Rizzolo, M. J. Rindler, M. Adesnik, and D. D. Sabatini. 1986. Sorting and endocytosis of viral glycoproteins in transfected polarized epithelial cells. J. Cell Biol. 102:1242-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maisner, A., H.-D. Klenk, and G. Herrler. 1998. Polarized budding of measles virus is not determined by viral surface glycoproteins. J. Virol. 72:5276-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marquardt, T., K. Ullrich, K. P. Zimmer, A. Hasilik, T. Deufel, and E. Harms. 1995. Carbohydrate-deficient glycoprotein syndrome (CDGS)—glycosylation, folding and intracellular transport of newly synthesized glycoproteins. Eur. J. Cell Biol. 66:268-273. [PubMed] [Google Scholar]
- 9.Naim, H. Y., E. Ehler, and M. A. Billeter. 2000. Measles virus matrix protein specifies apical virus release and glycoprotein sorting in epithelial cells. EMBO J. 19:3576-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owens, R. J., and R. W. Compans. 1989. Expression of the human immunodeficiency virus envelope glycoprotein is restricted to basolateral surfaces of polarized epithelial cells. J. Virol. 63:978-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Boulan, E., and S. K. Powell. 1992. Polarity of epithelial and neuronal cells. Annu. Rev. Cell Biol. 8:395-427. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi, T., T. Uchiyama, Y. Fujii, K. Kiyotani, A. Kato, Y. Nagai, A. Kawai, and T. Yoshida. 1999. Double-layered membrane vesicles released from mammalian cells infected with Sendai virus expressing the matrix protein of vesicular stomatitis virus. Virology 263:230-243. [DOI] [PubMed] [Google Scholar]
- 13.Sänger, C., E. Mühlberger, E. Ryabchikova, L. Kolesnikova, H.-D. Klenk, and S. Becker. 2001. Sorting of Marburg virus surface protein and virus release take place at opposite surfaces of infected polarized epithelial cells. J. Virol. 75:1274-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnell, M. J., L. Buonocore, M. A. Whitt, and J. K. Rose. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutter, G., M. Ohlmann, and V. Erfle. 1995. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 371:9-12. [DOI] [PubMed] [Google Scholar]
- 16.Tashiro, M., M. Yamakawa, K. Tobita, J. T. Seto, H.-D. Klenk, and R. Rott. 1990. Altered budding site of a pantropic mutant of Sendai virus, F1-R, in polarized epithelial cells. J. Virol. 64:4672-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas, D. C., and M. G. Roth. 1994. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J. Biol. Chem. 269:15732-15739. [PubMed] [Google Scholar]
- 18.Zimmer, G., H.-D. Klenk, and G. Herrler. 1995. Identification of a 40-kDa cell surface sialoglycoprotein with the characteristics of a major influenza C virus receptor in a Madin-Darby canine kidney cell line. J. Biol. Chem. 270:17815-17822. [DOI] [PubMed] [Google Scholar]