Abstract
Replication of cytomegalovirus (CMV) is largely controlled by the cellular arm of the immune response. In this study the CMV-specific CD4 T-cell response was characterized in a cohort of apparently healthy individuals. In 11% of all individuals, extremely high frequencies, between 10 and 40%, were found. High-level frequencies of CMV-specific CD4 T cells persisted over several months and were not the result of an acute infection. Specific T cells were oligoclonal and were phenotypically and functionally characterized as mature effector cells, with both cytokine-secreting and proliferative potential. These high-level frequencies do not seem to compromise the immune response towards heterologous infections, and no signs of immunopathology were observed. Whereas a large temporary expansion of virus-specific T cells is well known to occur during acute infection, we now show that extremely high frequencies of virus-specific T cells may continuously exist in chronic CMV infection without overtly compromising the remaining protective immunity.
The human cytomegalovirus (CMV) is among the leading causes for virus-associated complications after transplantation (13, 25, 27). CMV is a herpesvirus that employs several strategies which interfere with the host immune system. Consequently, the virus is only rarely, if ever, eliminated from the infected host and establishes a state of latency which is clinically inapparent in healthy individuals (1). Upon the use of immunosuppressive medication that specifically inhibits T-cell function, however, the risk of developing CMV-related disease is significantly increased, demonstrating the importance of the cellular arm of the immune response for the control of extensive viral replication (23, 41).
Murine models have been instrumental in elucidating the relative importance of the individual components of the cellular immune response. Work with B-cell-deficient mice has demonstrated that CMV-specific antibodies do not contribute to the control of primary viremia; however, they seem to play some role in limiting virus dissemination during episodes of virus recurrence (18, 19). Using the same model system, both the importance and the hierarchy of the cellular arm of the immune system were emphasized. Major histocompatibility complex-unrestricted natural killer cells and MHC-restricted CD4 and CD8 T cells were the dominant effectors for suppression of CMV replication and protection from lethal virus challenge. The effect of in vivo depletion of single effector subsets was to a large extent compensated for by the remaining subsets, indicating a functional redundancy of the cellular control mechanisms (31).
In humans, the overall importance of CMV-specific CD4 and CD8 T-cell responses in controlling the extent of virus replication was convincingly demonstrated by adoptive transfer experiments (48) or by the analysis of proliferative and cytotoxic T-cell activity (20, 33). We recently added on to this knowledge by showing that a drop in the frequency of CMV-specific CD4 T cells may even be predictive of CMV-associated disease in patients after renal transplantation. This is particularly relevant immediately after transplantation, when high doses of immunosuppressive drugs may disrupt the balance between viral replication and cellular immune responses. Later, after transplantation, a balance is apparently regained that allows the efficient control of virus recurrence, and CMV-specific T-cell responses do not differ from those of healthy controls (38).
In this study a detailed analysis of CMV-specific CD4 T-cell responses and subsets thereof was performed with clinically asymptomatic individuals with persistent CMV infection. We found that absolute T-cell frequencies show a large interindividual variation, with frequencies of up to 40%. CMV-specific T cells show characteristics of mature effector cells. These high frequencies are not the result of an acute infection, demonstrating that a chronically infected organism may continuously supply enormous numbers of T cells focused on a single persistent virus.
MATERIALS AND METHODS
Subjects.
The study was conducted with 161 CMV-seropositive individuals (50 healthy individuals and 111 long-term renal transplant recipients; 51.1 ± 14.2 years old) and 77 CMV-seronegative individuals (49 healthy individuals and 28 long-term renal transplant recipients; 38.6 ± 14.0 years old). All individuals were documented as CMV-seropositive or seronegative and had no CMV-associated symptoms. All transplant recipients received an immunosuppressive double or triple drug regimen. Among seropositive individuals, nine long-term stable renal transplant recipients (54.0 ± 9.9 years old; four males, five females; 8.9 ± 4.2 years old after transplantation; range, 2.3 to 15.6 years) with high frequencies of CMV-specific CD4 T cells were chosen for further analysis. All individuals were seropositive prior to transplantation, except for one (individual #0320), for whom the respective pretransplant serostatus is not known. Serostatus of the donor was positive in four cases and unknown in the remaining cases. Seven individuals received a triple drug regimen consisting of cyclosporine A (CyA), azathioprine (Aza), and methylprednisolone (MP), except for one (individual #1325) receiving CyA/MP and one (individual #0320) receiving Aza/MP. Blood was drawn in the morning before intake of immunosuppressive drugs. To control for trough levels of immunosuppressive drugs, serum levels of CyA were determined using a standard assay (Roche Diagnostics, Mannheim, Germany). Mean trough levels were 106.6 ± 31.58 ng/ml. All patients and control persons gave informed consent.
Stimulation of CMV-specific CD4 and CD8 T cells within whole blood.
Simultaneous stimulation of CMV-specific CD4 and CD8 T cells was performed in whole blood essentially as described previously (38, 39, 42). As a stimulus, titered amounts of CMV antigen that was derived from CMV-infected fibroblasts (complement fixation reagent; BioWhittaker, Verviers, Belgium) were used in the presence of 1 μg of αCD28 and αCD49d (clones L293 and 9F10; BD PharMingen, Heidelberg, Germany)/ml, respectively. As negative controls, blood cells were stimulated with control antigen that was derived from mock-infected fibroblasts and thus does not contain any CMV proteins (BioWhittaker). In selected individuals, additional stimulations were carried out using 2.5 μg of staphylococcus aureus enterotoxin B (SEB) (Sigma, Deisenhofen, Germany)/ml. Cells were incubated in polypropylene tubes at 37°C at 6% CO2 for a total of 6 h. During the last 4 h, 10 μg of Brefeldin A (Sigma)/ml was added to block extracellular secretion of cytokines. Thereafter, the blood was treated with 2 mM EDTA for 15 min. Subsequently, erythrocytes were lysed and leukocytes were fixed for 10 min using BD lysing solution according to the manufacturer's instruction (BD PharMingen). Cells were washed once with FACS buffer (phosphate-buffered saline, 5% filtered fetal calf serum, 0.5% bovine serum albumin, 0.07% NaN3) and either immediately processed for flow-cytometric analysis or left overnight at 4°C.
Stimulation of CMV-specific T cells from isolated PBMC.
peripheral blood mononuclear cells (PBMC) were isolated using density gradient centrifugation. PBMC (106) from a CMV-positive and a CMV-negative blood donor, respectively, were mixed and stimulated in a final volume of 450 μl in polypropylene round bottom tubes (Greiner, Frickenhausen, Germany) as described above using CMV antigen or control antigen. In order to assign the cells in the respective mixture to individual donors, cells from one donor were prestained using fluorescein isothiocyanate (FITC)-conjugated anti-CD45 monoclonal antibody (F4149; Sigma). After 2 h at 37°C, 10 μg of Brefeldin A/ml was added, and cells were vortexed, centrifuged for 5 min at 300 × g, and incubated for an additional 4 h. Subsequently, PBMC were treated with 2 mM EDTA as described above and fixed at 37°C for 5 min using prewarmed 4% paraformaldehyde solution. Thereafter, cells were washed once with FACS buffer and processed for flow-cytometric analysis.
Polyclonal stimulation of Th1 and Th2 cells in whole blood.
The protocol for polyclonal stimulation was adapted from reference 40 for the use in whole blood. One hundred fifty microliters of heparinized blood was supplemented with 300 μl of RPMI containing 5% fetal calf serum (Biochrom, Berlin, Germany), 2 mM glutamine, and antibiotics (PAA, Cölbe, Germany). T cells were stimulated in polypropylene tubes at 37°C at 6% CO2 for 4 h using 10 ng of phorbol myristate acetate (PMA)/ml and 2 μM ionomycin (both from Sigma) in the presence of 10 μg of Brefeldin A/ml. Mock-treated samples contained only Brefeldin A. Thereafter, samples were fixed as described above and processed for flow cytometry.
Determination of the frequency and characterization of CMV-specific T cells and T-helper-cell profiles by flow cytometry.
Fixed leukocytes were permeabilized with 2 ml of FACS buffer containing 0.1% saponin (Sigma) for 10 min at room temperature. Thereafter, they were immunostained for 30 min at room temperature in the dark using saturating conditions of the following antibodies: anti-CD4 or anti-CD8 (clones SK3 or SK1), anti-gamma interferon (IFN-γ) (clone 4S.B3), and anti-CD69 (clone L78) (all antibodies from BD PharMingen). In addition, the following cell surface molecules were stained on cytokine-positive cells: CD45RO, CD27, CD62L (all antibodies from BD PharMingen), T-cell receptor (TCR)-Vβ2, -Vβ3, -Vβ5.1, -Vβ5.2, -Vβ8, -Vβ13.1, -Vβ14, -Vβ16, -Vβ17, -Vβ20, -Vβ21.3, and -Vβ23 (antibodies from Beckman Coulter, Krefeld, Germany), and CCR7 (antibody clone 3D12, kindly provided by M. Lipp, MDC, Berlin, Germany). Staining of CCR7 was performed as an indirect procedure using phycoerythrin-conjugated F(ab′)2 donkey-anti-rat immunoglobulin G fragments (Dianova, Hamburg, Germany) as secondary antibody. For the determination of T-helper-cell profiles, cells treated with PMA-ionomycin were stained with antibodies against CD4, interleukin 4 (IL-4) (clone 8D3-8, BD PharMingen), and IFN-γ. Specifically stimulated PBMC were stained using antibodies against CD4 and IFN-γ. After staining, cells were washed once with 3 ml of FACS buffer and fixed with 1% paraformaldehyde. At least 10,000 CD4- or CD8-positive lymphocytes were analyzed on a FACScan (Becton Dickinson, Heidelberg, Germany) using Cellquest software. Usually, control antigens did not stimulate any IFN-γ production. Nevertheless, the percentage of specific T cells was calculated by subtraction of the frequency obtained by the respective control stimulations.
Determination of antigen-specific T-cell frequencies using enzyme-linked immunospot (ELISPOT) assay.
The ELISPOT assay was essentially done as described previously (34, 35). PBMC were isolated using density gradient centrifugation, and 2 × 105 PBMC per well were stimulated as described above using CMV antigen, control antigen, or SEB. This cell number was found to be superior to more diluted cell suspensions and ensures proper cell-cell contact during stimulation. In order to exclusively detect cytokine-secreting CD4 T cells, a subset of PBMC was depleted for CD8 T cells by negative selection (Dynal, Hamburg, Germany) and treated similarly. In general, ELISPOT assay wells containing more than 500 spot-forming cells cannot be counted accurately because the spots start to coalesce. This would correspond to a frequency of 0.5% activated cells of all PBMC.
Determination of antigen-specific proliferation.
PBMC were isolated using density gradient centrifugation. PBMC (105 per well) were stimulated in triplicate as described above using CMV antigen, control antigen, or SEB. Cells were pulsed with 0.25 μCi of [3H]thymidine/well after 1, 2, 3, 4, or 5 days and incubated for a further 20 h. Thereafter, counts per minute were determined using a standard harvesting apparatus (Wallac, Freiburg, Germany).
Determination of CMV serostatus and viral load.
The CMV serostatus was determined by a commercial CMV immunoglobulin G test (IMX, MEIA; Abbott Diagnostics, Wiesbaden, Germany), and CMV loads were measured as virus DNA from whole blood using the hybrid capture assay (Digene Hybrid Capture system, CMV-DNA, version 2.0; Abbott Diagnostics) according to the manufacturer's instructions.
Statistical analysis.
Statistical analysis was performed using the Prism V3.00 Software (Graphpad, San Diego, Calif.). Significant differences were determined using the Mann-Whitney test.
RESULTS
The frequency of CMV-specific CD4 T cells shows a wide distribution among CMV-seropositive individuals.
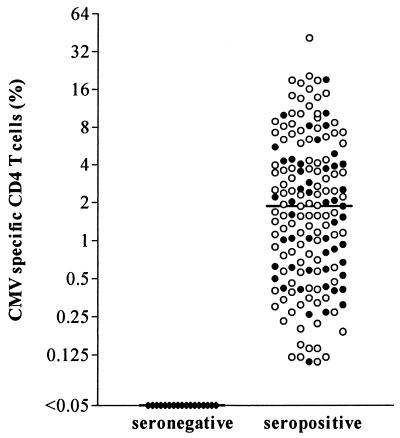
Heparinized whole blood of CMV-seropositive healthy individuals (n = 50) or long-term renal transplant recipients (n = 111) with stable graft function and without any evidence of infectious complications was incubated with CMV antigen or control antigen for 6 h in vitro, and antigen-specific T-cell cytokine induction was subsequently analyzed by flow cytometry. The frequency of CMV-specific CD4 T cells was determined as the percentage of CD69- and IFN-γ-positive CD4 T cells. CMV-specific CD4 T cells with median frequencies of 1.87% were detectable in all seropositive donors, whereas none of the 77 seronegative individuals tested had any T cells reactive against CMV (all below 0.05%) (Fig. 1). There was a large variation between seropositive individuals in the number of CMV-specific CD4 T cells, and 17 individuals had frequencies exceeding 10% (total range, 0.1 to up to 43.8%).
FIG. 1.
Heterogeneous frequencies of CMV-specific CD4 T cells in healthy seropositive individuals (n = 50) (black circles) and long-term renal transplant recipients (n = 111) (open circles). Frequencies of CMV-specific CD4 T cells were analyzed using flow cytometry and may range from 0.1 to up to 43.8% (median, 1.87%). Neither median T-cell frequencies nor the frequency distribution differed between control individuals (median, 1.57%) and long-term transplant patients (median, 1.90%; P = 0.11). Renal transplant recipients tested negative for CMV DNA. No CMV-specific T cells were detectable in CMV-seronegative individuals (n = 77).
The frequency of CMV-specific CD4 T cells may exceed superantigen responses.
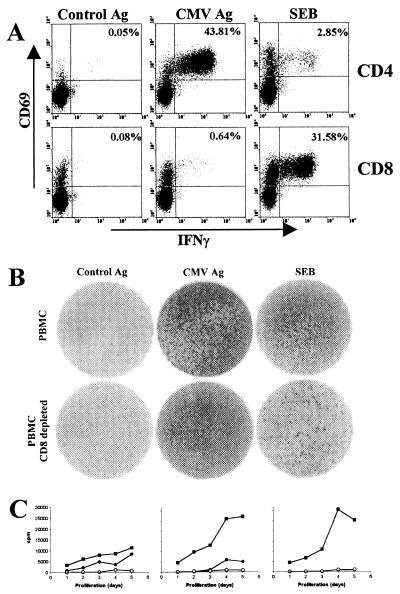
So far, antigen-specific responses of extremely high frequencies have been found during acute viral infections or for responses to strong polyclonal stimuli, such as superantigens. In individual cases, however, CMV-specific CD4 T cells may reach frequencies as high as 43.81% of all CD4 T cells (individual #0320) (Fig. 2A), well above the respective frequency found for the bacterial superantigen SEB (2.85%). In contrast, the respective CMV-specific CD8 T-cell frequency was lower, while SEB stimulation induced cytokine production in 31.58% of CD8 T cells. Within the same individual, control antigen failed to induce any relevant cytokine production.
FIG. 2.
Detection of CMV-specific CD4 T cells. (A) Whole blood of a CMV-positive individual (#0320) was stimulated with control antigen, CMV antigen, or SEB, and specifically induced cytokine induction in CD4 and CD8 T cells was analyzed using flow cytometry. Numbers indicate percentages of specifically stimulated CD4 T cells. (B) Determination of CMV-specific T cells using the ELISPOT assay. IFN-γ secretion from 2 × 105 PBMC/well stimulated with control antigen, CMV antigen, or SEB was analyzed. The lower panel shows IFN-γ secretion from CD8-depleted PBMC. The mean numbers of spot-forming units per well after stimulation with control antigen, CMV antigen, and SEB were 23, ≫500, and >>500 (upper panel) and 19, ≫500, and 404 (lower panel), respectively. Given the percentage of 52% CD4 T cells among CD8-depleted PBMC, 404 spots correspond to an SEB-reactive CD4 T-cell frequency of 0.4%. Thus, the ELISPOT assay leads to an approximately sevenfold underestimation of frequencies compared to the respective frequencies obtained by flow cytometry (A) 2.85%). (C) PBMC were stimulated with control antigen (o), CMV antigen (•), and SEB (▪), and proliferative responses were assayed after 1, 2, 3, 4, or 5 days. The left diagram corresponds to the individual analyzed in panels A and B, and the middle and right panels represent proliferative responses in individuals with CMV-specific CD4 T-cell frequencies of 2.9 and 0%, respectively.
The existence of extremely high frequencies of CMV-specific CD4 T cells was confirmed using another well-established assay system for the determination of antigen-specific T cells. PBMC were isolated, and the stimulation-induced IFN-γ secretion was determined using the ELISPOT assay (Fig. 2B). As expected, using the cellular concentration of 2 × 105 unfractionated PBMC per well, spot distribution nearly reached confluency in both CMV- and SEB-stimulated samples, indicating that the frequencies were far above the upper limit for accuracy (Fig. 2B, upper panel). The use of unfractionated PBMC does not allow assignment of a single spot-forming unit to individual CD4 or CD8 T cells. To exclusively determine the IFN-γ secretion capacity of CD4 T cells, PBMC were depleted for CD8 T cells and stimulated similarly. In line with results obtained by flow cytometry (Fig. 2A), the frequency of IFN-γ-positive spots after CMV antigen stimulation was not significantly reduced, whereas a large fraction of SEB-reactive cells was (Fig. 2B, lower panel). Together this demonstrates that the CMV-reactive cells mainly represent CD4 T cells.
In addition to the ability of cytokine production after antigenic stimulation, the proliferative capacity of specific T cells was analyzed by the incorporation of [3H]thymidine in vitro, which was added on either day 1, 2, 3, 4, or 5 (Fig. 2C). The so determined proliferation in individuals with high CMV-specific T-cell frequencies was compared with the proliferative responses in persons with T-cell frequencies in the normal range or seronegative subjects. Three representative examples with CMV-specific CD4 T-cell frequencies of 43.8, 2.9, and 0% are shown (Fig. 2C). CMV-specific proliferative responses in the individual with the highest T-cell frequencies were detectable already after 1 day (left diagram), whereas the respective response in an individual with a CMV-specific CD4 T-cell frequency of 2.9% required 3 days of proliferation to be detectable (Fig. 2C, center). The CMV-negative individual does not show any CMV-specific proliferation, whereas in the control stimulation with SEB, proliferation was readily detectable (right diagram). Taken together, the use of three assay systems confirmed the existence of exceedingly high frequencies of CMV-specific CD4 T cells with both cytokine-secreting and proliferative capacity.
No evidence of an unspecific bystander activation after CMV stimulation.
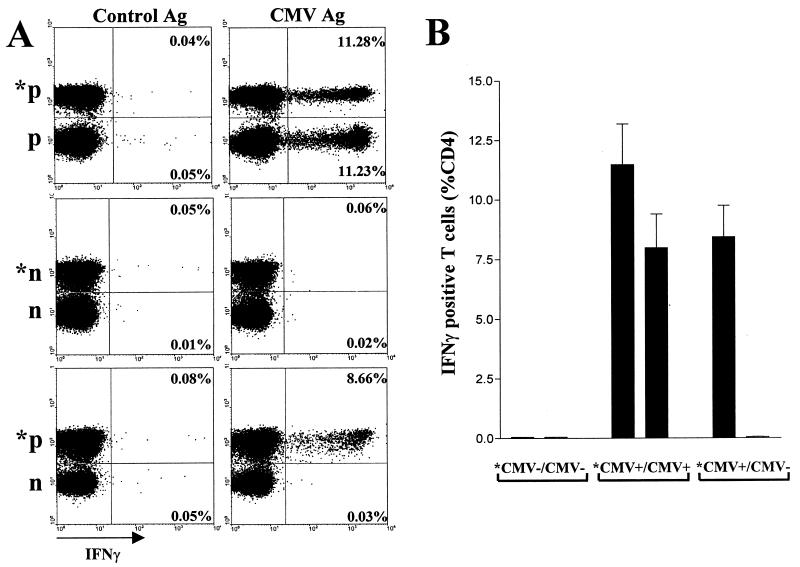
Cytokines secreted by activated T cells may lead to a potential bystander activation of antigen-nonspecific T cells. To test whether this process may confound the determination of CMV-specific T cells, a simultaneous stimulation of a mixture of PBMC from a CMV-positive donor and a CMV-negative donor was performed using CMV antigen and control antigen (Fig. 3). In order to flow-cytometrically distinguish the T cells from the two different donors within the mixture, cells from one donor were prestained using FITC-conjugated anti-CD45 antibody. This did not affect the determination of specific T-cell frequencies (Fig. 3A, upper panel and data not shown). Remarkably, only CD4 T cells from the CMV-positive donor were activated to produce cytokines, whereas T cells from the CMV-negative donor remained cytokine negative (Fig. 3A). This result was confirmed using all combinations of three CMV-positive donors and three negative donors (Fig. 3B). Consequently, bystander activation does not seem to confound the determination of CMV-specific-T-cell frequencies.
FIG. 3.
Lack of antigen-nonspecific CD4 T-cell activation. PBMC from pairs of three CMV-positive and three CMV-negative blood donors in all combinations were mixed and stimulated for 6 h using CMV antigen and control antigen. To distinguish cells of the respective donor within the mixture, PBMC of one donor were prestained using FITC-conjugated anti-CD45 antibody (prestained cells are denoted with an asterisk). After stimulation, IFN-γ production was flow-cytometrically analyzed in T cells from each donor in the mixture. (A) PBMC from a CMV-negative (n) and a CMV positive (p) donor were mixed (*p/p, *n/n, *p/n; 106 PBMC from each donor) and stimulated. (B) PBMC from three CMV-positive (prestained) and three CMV-negative donors were mixed in all possible combinations (n = 9; *CMV+/CMV−). Six different combinations of PBMC from CMV-negative (*CMV−/CMV−) and CMV positive donors (*CMV+/CMV+) were stimulated as controls.
A highly focused CD4 T-cell response against CMV may be stable without clinical symptoms.
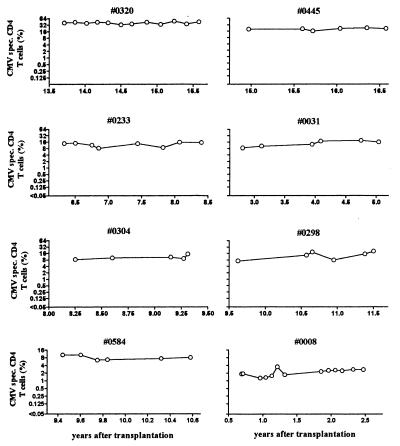
The high frequency of virus-specific T cells is reminiscent of the large temporary clonal T-cell expansion after primary infection or reactivation with Epstein-Barr virus or lymphocytic choriomeningitis virus (4-6, 15, 46). However, all the individuals with high CMV-specific CD4-T-cell frequencies described here were remarkably stable in their CMV responses during the study period of more than 2 years (Fig. 4) and showed no evidence of intercurrent infection or CMV replication. The respective CMV-specific CD8-T-cell frequencies were consistently lower and sometimes not detectable (Fig. 2A) (38; also data not shown). Interestingly, despite this highly focused virus-specific CD4 T-cell response, all individuals were without any clinical symptoms and showed no signs of increased susceptibility to heterologous infections. While the reasons for the extent of the CMV response remain unclear, it may reflect the requirement to maintain an individually constant percentage of CD4 T cells to efficiently control CMV replication.
FIG. 4.
Extremely high frequencies of CMV-reactive T cells are stable over time. Respective T cells in eight individuals were followed during at least 1.2 to up to 2.3 years using flow cytometry. CMV-DNA was not detectable in any of the patients (hybrid capture assay; data not shown).
The CMV-specific CD4 T-cell response is oligoclonal.
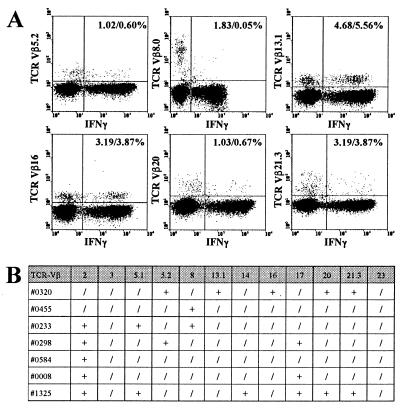
Exceedingly high frequencies of T cells directed against a single pathogen might be the result of a large monoclonal expansion, i.e., after infection with a superantigen-carrying microbe (17). Therefore, the clonal composition of antigen-specific T cells in individual subjects was determined by staining various TCR-Vβ chains in stimulated cytokine-positive CD4 T cells. A representative example of TCR Vβ distributions among CMV-specific CD4 T cells is shown in Fig. 5A (subject #0320). Recognition of CMV antigen is mediated by a variety of different TCR specificities, and prominent fractions of CMV-specific cells were found among T cells bearing TCR-Vβ13.1 or -Vβ16. Several TCR, such as TCR-Vβ8.0, did not recognize any CMV antigen (for other examples see Fig. 5B). Interestingly, the relative distribution of TCR-Vβ chains among CMV-specific T cells varies in different individuals (Fig. 5B). Within one individual, however, not only the absolute frequency (Fig. 4) but also the clonal distribution of CMV-specific T-cell responses are highly stable over time (data not shown). Thus, CMV-specific T cells are not derived from a monoclonal expansion but rather represent a repertoire of individually differing oligo- or polyclonal responses. This also implies that the postulated CMV superantigen (9) does not exert any biasing effect on the CMV-specific T-cell repertoire.
FIG. 5.
CMV-specific CD4 T cells are oligoclonal. CMV-specific T cells were found among T cells expressing different T-cell receptors. (A) Shown are the respective specific T cells among cells expressing TCR-Vβ5.2, -Vβ8.0, -Vβ13.1, -Vβ16, -Vβ20, and -Vβ21.3 in a representative example (individual #0320). A particularly high percentage of CMV-specific T cells express TCR-Vβ13.1 or -Vβ16, whereas no specific T cell expresses TCR-Vβ8.0. The two numbers in each diagram indicate the percentages of TCR-Vβ-positive cells among all CD4 T cells and among CMV-specific CD4 T cells, respectively. (B) TCR-Vβ distribution among CMV-specific CD4 T cells in different individuals. + and / indicate the presence or absence, respectively, of IFN-γ-positive CD4 T cells within the respective T-cell population bearing a particular TCR-Vβ subunit.
The CMV-specific CD4 T-cell response is mediated by mature effector cells.
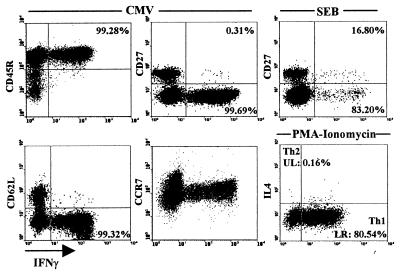
In order to characterize the phenotype and maturation status of CMV-specific T cells, CMV- and SEB-reactive T cells were further analyzed (Fig. 6 and Table 1). The majority of CMV-specific T cells had an activated/memory phenotype with expression of CD45RO, downregulation of CD62L, and low CCR7 expression. As shown in a representative example, 99.7% of CMV-specific T cells were negative for CD27, which is indicative of a mature differentiation state. This does not apply to the SEB-reactive T cells, since 16.8% of the respective fraction were CD27 positive (Fig. 6; see also Table 1). These observations may explain the discordance of CMV/SEB-specific CD4 T-cell frequencies and their proliferative responses (Fig. 2C), which are determined by both the frequency and the differentiation status of specific T cells in the population (21, 26).
FIG. 6.
Characterization of CMV-specific CD4 T cells. CMV-specific CD4 T cells are mainly positive for CD45RO and are largely negative for CD27, CD62L, and CCR7. In contrast, a considerable percentage of SEB-responsive T cells are CD27 positive (16.8%). Numbers indicate percentages of T cells positive (CD45R) or negative (CD27, CD62L, and CCR7) for the respective cell surface molecule among IFN-γ-positive CD4 T cells. The percentage of Th1 cells was determined after polyclonal stimulation with PMA-ionomycin (lower right diagram).
TABLE 1.
CMV-specific CD4 T cells are mature effector cellsa
| Marker | CD4 T-cell response (%) specific to:
|
|||||
|---|---|---|---|---|---|---|
| CMV
|
SEB | CMV/PMA-ionomycin (% CMV-specific T cells among Th1 cells) | ||||
| CD45RO (pos) | CD62L (neg) | CCR7 (neg) | CD27 (neg) | CD27 (neg) | ||
| #0320 | 99.28 | 98.32 | 99.88 | 99.69 | 83.20 | 56.07 |
| #0445 | 99.43 | 95.52 | 99.54 | 98.32 | 77.74 | 44.49 |
| #0031 | 77.65 | 98.02 | 99.51 | 90.45 | 86.10 | 25.23 |
| #0233 | 70.00 | 92.67 | 98.55 | 93.15 | ND | 25.28 |
| #0298 | 92.66 | 99.14 | 99.36 | 98.71 | 87.54 | 29.20 |
| #0584 | 10.11 | 95.92 | 98.95 | 96.27 | 63.98 | 14.95 |
| #0008 | 94.78 | 89.29 | ND | 96.82 | ND | 10.90 |
| #1325 | 71.66 | 97.46 | 99.82 | 97.24 | 75.56 | 22.58 |
Numbers indicate the percentages of cells positive (pos) (CD45RO) or negative (neg) (CD62L, CCR7, CD27) for the respective cell surface antigen among IFN-γ-positive CD4 T cells stimulated with CMV antigen or SEB. The last column gives the percentages of CMV-specific CD4 T cells among all Th1 cells. The latter were calculated from the percentage of Th1 that was determined by a separately performed polyclonal stimulation with PMA-ionomycin. ND, not determined.
Interestingly, although the majority of CMV-specific T cells are positive for CD45RO, certain individuals show a distinct fraction of CMV-reactive CD45RO-negative T cells (Table 1). Given the fact that within the short incubation time of 6 h naive cells are not able to respond with relevant cytokine production (49), these observations support the concern about the general use of CD45RO to characterize antigen-experienced memory T cells (3, 7, 24, 28, 29).
CMV-specific CD4 T cells were generally expressing Th1 cytokines, such as IFN-γ and TNF-α, but not the Th2 cytokines IL-4 or IL-10 (data not shown). In connection with their high frequency, the T-helper profile may be significantly biased towards Th1. For example, among the 80% Th1 cells of donor #0320 that produced IFN-γ after polyclonal stimulation with PMA-ionomycin (Fig. 6), 56% were CMV specific (Table 1). This illustrates how antigenic challenge from a single virus may affect the composition of the total CD4 T-cell pool.
DISCUSSION
In this study an analysis of the frequencies and phenotypic characteristics of CMV-specific CD4 T cells in seropositive individuals was performed. The most striking observation was the existence of extremely high frequencies of CMV-specific CD4 T cells in individuals with stable control of viral replication and no signs of clinical symptoms. In about 11% of all cases (17 blood donors out of 161), frequencies ranging from 10 to 43% were detected. These T cells were oligoclonal and showed a considerably homogenous expression of markers characteristic of antigen-experienced mature effector cells. They were functionally characterized by their capacity to secrete IFN-γ and to proliferate upon antigenic stimulation.
Frequencies in a high range are well known during acute infections of mice, monkeys, and humans, although the analysis has mainly been restricted to virus-specific CD8 T cells (4-6, 15, 22, 36, 46). Acute infection with lymphocytic choriomeningitis virus leads to a profound temporary expansion of virus-specific T cells (4). Likewise, primary human Epstein-Barr virus infection is accompanied by the induction of specific CD8 T cells, which may reach frequencies of up to 40% (6). These frequencies, however, decline upon control of viremia and are far less abundant in chronic infection.
During chronic infection with CMV, high levels of virus-specific T cells do exist, but CMV-specific CD4 T cells seem to dominate respective CD8 T-cell responses. Recently, high frequencies of CMV-specific CD8 T cells have been found in chronically infected individuals by using peptide-specific tetramers (12). Although this peptide specificity may lead to an underestimation of absolute CD8 T-cell frequencies, levels did not exceed 5% of total CD8 T cells and thus lay far beyond frequencies of CMV-specific CD4 T cells reported in this study. In support of the dominance of CMV-specific CD4 T cells, measurable CMV-specific CD8-T-cell frequencies in our cohort were almost always lower than respective CD4 T-cell responses (38) (Fig. 2A). Thus, taken together, these high CD4 T-cell frequencies are among the highest reported for chronically infected individuals, and these levels are highly stable over time.
The continuous presence of such high frequencies of CMV-reactive CD4 T cells in the chronic phase of CMV infection was unexpected and calls into question whether the stimulation conditions were really CMV specific. Thus, it might be possible that a significant proportion of the activated CD4 T cells were cross-reactive or were triggered by bystander activation. However, the following observations made this unlikely: (i) CD4 T cells of CMV-seronegative individuals were not activated under the activation conditions (Fig. 1); (ii) mixing labeled PBMC of CMV-seronegative and CMV-seropositive blood donors revealed a specific stimulation of the CD4 T cells of the seropositive donors only (Fig. 3); (iii) CMV-induced cytokine-producing T cells were found among distinct sets of TCR-Vβ-positive T cells, whereas cytokine production in T cells with different Vβ specificities was not concomitantly induced, although the latter population contained memory T cells (Fig. 5 and data not shown); and (iv) the CMV antigen itself does not contain any broadly activating cofactor, as its addition to the stimulation of PBMC from CMV-seronegative donors with adenovirus antigen or SEB did not lead to an enhancement of the respective CD4 T-cell frequency (data not shown). One might still argue that the high CMV response might in part be due to an auto- or allo-antigen that is present in the CMV preparation or to cross-reactivity of CMV-specific T cells with auto-antigens (14). However, control antigen did not stimulate any cytokine production, and a highly frequent CMV-specific CD4 T-cell response is rather common among CMV-seropositive individuals (see Fig. 1). Moreover, no signs of autoimmunity have ever been detected in the persons studied. Together this strongly supports our view of a highly frequent CD4 T-cell response focused on CMV.
Several causes may contribute to the generation of these high frequencies. First, the individual frequency might directly be linked to primary infection. As has been shown in the mouse model, the course of primary CMV infection defines the overall load of viral genomes in tissues (32). Induction of protective immunity requires entry of viral antigens into secondary lymphoid tissues. The dose of antigen and the duration of antigenic stimulation critically determine the outcome of the immune response (50), and the clonal burst size of T cells during the acute response is directly related to the frequencies of T cells in the memory pool (16). Second, homeostatic proliferation in lymphopenic hosts has been shown to drive the expansion of T cells to maintain a certain size of the memory T-cell pool (43). Although conditions of homeostatic proliferation are difficult to assess in systems other than animals, strong immunosuppressive therapy immediately after transplantation may similarly lead to lymphopenic conditions where the presence of persistent viruses, such as CMV, may be prone to bias the clonal size of an immune response towards CMV. Third, the quantity might be linked to the CMV strain or HLA type, which determine the overall repertoire of displayed antigens. Consequently, different subsets of immunogenic peptides are presented which may influence the absolute frequency of an antigen-specific response (2, 11).
The particular stability of virus-specific CD4 T cells has already been demonstrated in elegant murine models, where frequencies of virus-specific CD4 T cells are stable even upon infection with heterologous viruses (45, 47). In contrast, in the same setting, virus-specific CD8 T cells experience a considerable attrition and are displaced by memory T cells of other specificities (37). Nevertheless, it is currently unclear how these high levels of virus-specific CD4 T-cell frequencies are maintained. For persistent viruses, such as CMV or human immunodeficiency virus (HIV), it is generally appreciated that the presence of viral antigen in the lymphatic tissue represents a continuous stimulus for the maintenance of virus-specific T cells. In support of this view, members of our group and others recently reported that a therapy-induced decline in HIV load leads to a progressive loss of both virus-specific CD4 and CD8 T cells in HIV-infected individuals (30, 39). In the case of CMV, it was shown in murine models that productive replication cycles are frequently initiated at the sites of CMV latency (31). The same may hold true for human CMV, although the CMV load in healthy seropositive subjects is generally not measurable. Upon the use of highly sensitive assays, however, viral DNA has also been detected (44). Thus, it is reasonable to assume that boosting of T-cell responses by periodic subclinical CMV replication accounts for the stability of CMV-specific T-cell frequencies over time.
CMV-specific CD4 T cells correlate with control of CMV replication in vivo (38) and have a median frequency of approximately 1.9%. Neither the frequency nor its interindividual distribution differs between healthy individuals and long-term transplant recipients (Fig. 1). While a decline of CMV-specific CD4 T cells to below 0.25% is associated with an increase in viral replication and the development of CMV-associated symptoms (38), it is unclear what consequences the continued presence of extremely high specific T-cell frequencies might have. Substantial evidence has been provided that T cells can mediate not only protective immunity but also immunopathology (8, 10, 51). Thus, it is conceivable that the localized effector function of highly numerous T cells with distinct oligoclonal specificity may induce immunopathogenesis at sites of viral replication. Moreover, the existence of highly frequent T cells directed against a single virus raises concerns about whether the affected individual is able to mount an adequate immune response against viruses other than CMV. Interestingly, however, our group of individuals does not show any clinical signs of immunopathology or any evidence of a compromised control of heterologous infections. Nevertheless, it still seems possible that an existing low-level immunity, i.e., towards heterologous viruses, is further reduced to below protective levels by conditions of severe immune deterioration, such as extensive surgical procedures or serious acute infections.
In conclusion, a highly frequent CMV-specific cellular immune response is detectable in a number of persistently infected individuals without any obvious signs of immunopathology or enhanced susceptibility towards heterologous infections. These findings emphasize that the remaining repertoire is sufficiently large to compensate for the expansion of T cells with single viral specificities. Furthermore, it indicates that CMV may have a particular pathogenic potential against which the immune system has to concentrate its resources.
Acknowledgments
We thank M. Lipp (Max-Delbrück Center; Berlin, Germany) for the kind gift of monoclonal antibody against CCR7. We thank Candida Guckelmus and Claudia Schormann for excellent technical assistance and Hans-Gerhard Burgert for critical review of the manuscript.
A.M. was supported by grants from the Deutsche Forschungsgemeinschaft/SFB 399.
REFERENCES
- 1.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Immunol. Today 21:447-455. [DOI] [PMC free article] [PubMed]
- 2.Alexander Miller, M. A., G. R. Leggatt, and J. A. Berzofsky. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA 93:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butz, E. A., and M. J. Bevan. 1998. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callan, M. F., N. Steven, P. Krausa, J. D. Wilson, P. A. Moss, G. M. Gillespie, J. I. Bell, A. B. Rickinson, and A. J. McMichael. 1996. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 2:906-911. [DOI] [PubMed] [Google Scholar]
- 6.Callan, M. F., L. Tan, N. Annels, G. S. Ogg, J. D. Wilson, C. A. O'Callaghan, N. Steven, A. J. McMichael, and A. B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106-111. [DOI] [PubMed] [Google Scholar]
- 8.Chang, K. M., B. Rehermann, and F. V. Chisari. 1997. Immunopathology of hepatitis C. Springer Semin. Immunopathol. 19:57-68. [DOI] [PubMed] [Google Scholar]
- 9.Dobrescu, D., B. Ursea, M. Pope, A. S. Asch, and D. N. Posnett. 1995. Enhanced HIV-1 replication in V beta 12 T cells due to human cytomegalovirus in monocytes: evidence for a putative herpesvirus superantigen. Cell 82:753-763. [DOI] [PubMed] [Google Scholar]
- 10.Doherty, P. C., and R. M. Zinkernagel. 1974. T-cell-mediated immunopathology in viral infections. Transplant. Rev. 19:89-120. [DOI] [PubMed] [Google Scholar]
- 11.Gallimore, A., H. Hengartner, and R. Zinkernagel. 1998. Hierarchies of antigen-specific cytotoxic T-cell responses. Immunol. Rev. 164:29-36. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie, G. M., M. R. Wills, V. Appay, C. O'Callaghan, M. Murphy, N. Smith, P. Sissons, S. Rowland-Jones, J. I. Bell, and P. A. Moss. 2000. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J. Virol. 74:8140-8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebart, H., L. Kanz, G. Jahn, and H. Einsele. 1998. Management of cytomegalovirus infection after solid-organ or stem-cell transplantation. Current guidelines and future prospects. Drugs 55:59-72. [DOI] [PubMed] [Google Scholar]
- 14.Hiemstra, H. S., N. C. Schloot, P. A. van Veelen, S. J. Willemen, K. L. Franken, J. J. van Rood, R. R. de Vries, A. Chaudhuri, P. O. Behan, J. W. Drijfhout, and B. O. Roep. 2001. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA 98:3988-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshino, Y., T. Morishima, H. Kimura, K. Nishikawa, T. Tsurumi, and K. Kuzushima. 1999. Antigen-driven expansion and contraction of CD8+-activated T cells in primary EBV infection. J. Immunol. 163:5735-5740. [PubMed] [Google Scholar]
- 16.Hou, S., L. Hyland, K. W. Ryan, A. Portner, and P. C. Doherty. 1994. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature 369:652-654. [DOI] [PubMed] [Google Scholar]
- 17.Huber, B. T., P. N. Hsu, and N. Sutkowski. 1996. Virus-encoded superantigens. Microbiol. Rev. 60:473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonjic, S., W. Mutter, F. Weiland, M. J. Reddehase, and U. H. Koszinowski. 1989. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 169:1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonjic, S., I. Pavic, B. Polic, I. Crnkovic, P. Lucin, and U. H. Koszinowski. 1994. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J. Exp. Med. 179:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause, H., H. Hebart, G. Jahn, C. A. Muller, and H. Einsele. 1997. Screening for CMV-specific T cell proliferation to identify patients at risk of developing late onset CMV disease. Bone Marrow Transplant. 19:1111-1116. [DOI] [PubMed] [Google Scholar]
- 21.Laouar, Y., and I. N. Crispe. 2000. Functional flexibility in T cells: independent regulation of CD4+ T cell proliferation and effector function in vivo. Immunity 13:291-301. [DOI] [PubMed] [Google Scholar]
- 22.Letvin, N. L., Y. Yasutomi, L. Shen, K. A. Reimann, Z. W. Chen, J. E. Schmitz, and M. J. Kuroda. 1998. The CD8+ T lymphocyte response during primary SIVmac infection. Adv. Exp. Med. Biol. 452:177-179. [DOI] [PubMed] [Google Scholar]
- 23.Lowance, D., H. H. Neumayer, C. M. Legendre, J. P. Squifflet, J. Kovarik, P. J. Brennan, D. Norman, R. Mendez, M. R. Keating, G. L. Coggon, A. Crisp, I. C. Lee, et al. 1999. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N. Engl. J. Med. 340:1462-1470. [DOI] [PubMed] [Google Scholar]
- 24.Maini, M. K., N. Gudgeon, L. R. Wedderburn, A. B. Rickinson, and P. C. Beverley. 2000. Clonal expansions in acute EBV infection are detectable in the CD8 and not the CD4 subset and persist with a variable CD45 phenotype. J. Immunol. 165:5729-5737. [DOI] [PubMed] [Google Scholar]
- 25.Mayer, A. D., J. Dmitrewski, J. P. Squifflet, T. Besse, B. Grabensee, B. Klein, F. W. Eigler, U. Heemann, R. Pichlmayr, M. Behrend, Y. Vanrenterghem, J. Donck, J. van Hooff, M. Christiaans, J. M. Morales, A. Andres, R. W. Johnson, C. Short, B. Buchholz, N. Rehmert, W. Land, S. Schleibner, J. L. Forsythe, D. Talbot, E. Pohanka, et al. 1997. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 64:436-443. [DOI] [PubMed] [Google Scholar]
- 26.Merica, R., A. Khoruts, K. A. Pape, R. L. Reinhardt, and M. K. Jenkins. 2000. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J. Immunol. 164:4551-4557. [DOI] [PubMed] [Google Scholar]
- 27.Nashan, B., S. Light, I. R. Hardie, A. Lin, J. R. Johnson, et al. 1999. Reduction of acute renal allograft rejection by daclizumab. Transplantation 67:110-115. [DOI] [PubMed] [Google Scholar]
- 28.Pape, G. R., T. J. Gerlach, H. M. Diepolder, N. Gruner, M. Jung, and T. Santantonio. 1999. Role of the specific T-cell response for clearance and control of hepatitis C virus. J. Viral Hepat. 6(Suppl. 1):36-40. [DOI] [PubMed] [Google Scholar]
- 29.Pilling, D., A. N. Akbar, P. A. Bacon, and M. Salmon. 1996. CD4+ CD45RA+ T cells from adults respond to recall antigens after CD28 ligation. Int. Immunol. 8:1737-1742. [DOI] [PubMed] [Google Scholar]
- 30.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518-525. [DOI] [PubMed] [Google Scholar]
- 31.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Luccaronin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddehase, M. J., M. Balthesen, M. Rapp, S. Jonjic, I. Pavic, and U. H. Koszinowski. 1994. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J. Exp. Med. 179:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reusser, P., G. Cathomas, R. Attenhofer, M. Tamm, and G. Thiel. 1999. Cytomegalovirus (CMV)-specific T cell immunity after renal transplantation mediates protection from CMV disease by limiting the systemic virus load. J. Infect. Dis. 180:247-253. [DOI] [PubMed] [Google Scholar]
- 34.Scheibenbogen, C., P. Romero, L. Rivoltini, W. Herr, A. Schmittel, J. C. Cerottini, T. Woelfel, A. M. Eggermont, and U. Keilholz. 2000. Quantitation of antigen-reactive T cells in peripheral blood by IFNγ-ELISPOT assay and chromium-release assay: a four-centre comparative trial. J. Immunol. Methods 244:81-89. [DOI] [PubMed] [Google Scholar]
- 35.Schmittel, A., U. Keilholz, S. Bauer, U. Kuhne, S. Stevanovic, E. Thiel, and C. Scheibenbogen. 2001. Application of the IFN-gamma ELISPOT assay to quantify T cell responses against proteins. J. Immunol. Methods 247:17-24. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 37.Selin, L. K., M. Y. Lin, K. A. Kraemer, D. M. Pardoll, J. P. Schneck, S. M. Varga, P. A. Santolucito, A. K. Pinto, and R. M. Welsh. 1999. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11:733-742. [DOI] [PubMed] [Google Scholar]
- 38.Sester, M., U. Sester, B. Gärtner, G. Heine, M. Girndt, N. Mueller-Lantzsch, A. Meyerhans, and H. Köhler. 2001. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation 71:1287-1294. [DOI] [PubMed] [Google Scholar]
- 39.Sester, M., U. Sester, H. Köhler, T. Schneider, L. Deml, R. Wagner, N. Mueller-Lantzsch, H. W. Pees, and A. Meyerhans. 2000. Rapid whole blood analysis of virus-specific CD4 and CD8 T cell responses in persistent HIV infection. AIDS 14:2653-2660. [DOI] [PubMed] [Google Scholar]
- 40.Sester, U., M. Sester, M. Hauk, H. Kaul, H. Kohler, and M. Girndt. 2000. T-cell activation follows Th1 rather than Th2 pattern in haemodialysis patients. Nephrol. Dial. Transplant. 15:1217-1223. [DOI] [PubMed] [Google Scholar]
- 41.Smith, S. R., D. W. Butterly, B. D. Alexander, and A. Greenberg. 2001. Viral infections after renal transplantation. Am. J. Kidney Dis. 37:659-676. [DOI] [PubMed] [Google Scholar]
- 42.Suni, M. A., L. J. Picker, and V. C. Maino. 1998. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J. Immunol. Methods 212:89-98. [DOI] [PubMed] [Google Scholar]
- 43.Tanchot, C., M. M. Rosado, F. Agenes, A. A. Freitas, and B. Rocha. 1997. Lymphocyte homeostasis. Semin. Immunol. 9:331-337. [DOI] [PubMed] [Google Scholar]
- 44.Toro, A. I., and J. Ossa. 1996. PCR activity of CMV in healthy CMV-seropositive individuals: does latency need redefinition? Res. Virol. 147:233-238. [DOI] [PubMed] [Google Scholar]
- 45.Varga, S. M., L. K. Selin, and R. M. Welsh. 2001. Independent regulation of lymphocytic choriomeningitis virus-specific T cell memory pools: relative stability of CD4 memory under conditions of CD8 memory T cell loss. J. Immunol. 166:1554-1561. [DOI] [PubMed] [Google Scholar]
- 46.Varga, S. M., and R. M. Welsh. 1998. Detection of a high frequency of virus-specific CD4+ T cells during acute infection with lymphocytic choriomeningitis virus. J. Immunol. 161:3215-3218. [PubMed] [Google Scholar]
- 47.Varga, S. M., and R. M. Welsh. 1998. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J. Immunol. 161:367-374. [PubMed] [Google Scholar]
- 48.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann, C., A. Prevost-Blondel, C. Blaser, and H. Pircher. 1999. Kinetics of the response of naive and memory CD8 T cells to antigen: similarities and differences. Eur. J. Immunol. 29:284-290. [DOI] [PubMed] [Google Scholar]
- 50.Zinkernagel, R. M., S. Ehl, P. Aichele, S. Oehen, T. Kundig, and H. Hengartner. 1997. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol. Rev. 156:199-209. [DOI] [PubMed] [Google Scholar]
- 51.Zinkernagel, R. M., O. Planz, S. Ehl, M. Battegay, B. Odermatt, P. Klenerman, and H. Hengartner. 1999. General and specific immunosuppression caused by antiviral T-cell responses. Immunol. Rev. 168:305-315. [DOI] [PubMed] [Google Scholar]