Abstract
Objective
To examine the relationship between patient case-mix, utilization, primary care physician (PCP) payment method, and the probability that patients switch their PCPs.
Data Sources/Study Setting
Administrative enrollment and claims/encounter data for 1994–1995 from four physician organizations.
Study Design
We developed a conceptual model of patient switching behavior, which we used to guide the specification of multivariate logistic analyses focusing on interactions between patient case-mix, utilization, and PCP reimbursement methods.
Data Collection/Extraction Methods
Claims data were aggregated to the encounter level; a switch was defined as a change in PCP since the previous encounter. The PCPs were reimbursed on either a capitated or fee-for-service (FFS) basis.
Principal Findings
Patients with stable chronic conditions (Ambulatory Diagnostic Groups [ADG] 10) and capitated PCPs were 36 percent more likely to switch PCPs than similar patients with FFS PCPs, controlling for patient age and sex and physician fixed effects. When the number of previous encounters was included in the model, this relationship was no longer significant. Instead high utilizers with capitated PCPs were significantly more likely to switch PCPs than were similar patients with FFS PCPs.
Conclusions
A patient's demographics and utilization are associated with the probability that the patient will switch PCPs. Capitated PCP payment was associated with higher rates of switching among high utilizers of health care resources. These findings raise concerns about the continuity and quality of care experienced by vulnerable patients in an era of changing financial incentives.
Keywords: Physician payment, panel data, managed care, primary care physicians
Capitation creates potential conflicts of interest between physicians’ incomes and patients’ expectations for treatment, especially among the chronically ill (Blumenthal 1994; Rodwin 1993). Physicians worry that capitation will cause them to resent their sick patients, and experts are concerned that physicians will avoid costly patients for financial reasons (Blumenthal 1994). Regardless of whether or not physicians respond to these financial incentives, the stage has been set for reduced levels of trust and satisfaction with the medical profession, resulting in patients who are increasingly doubtful, critical, and demanding of their physicians (Mechanic 1998). One potential manifestation of a lack of trust or reduced satisfaction is the switching of PCPs. A more complete understanding of the consequences of capitated financial arrangements is important. While experts disagree on the future of managed care and capitation in the employer-sponsored health insurance market, capitation remains the predominant provider payment method in Medicaid and SCHIP (State Children's Health Insurance Program) (Robinson 2001; Berwick 2002; Draper and Gold 2002).
The rate that patients switch physicians is a measure of the quality of the doctor–patient relationship. Experts recommend linking patient loyalty to physician performance assessment and incentive systems (Rodwin 1993), though only a minority of HMOs does so (Gold et al. 1995).
Although a small minority of individuals switches physicians annually (4–11 percent), almost half of individuals have switched physicians in the past. While early research found physician or patient relocation and physician retirement were the predominant reasons for switching physicians, more recent research shows dissatisfaction with aspects of care is important in patients’ decisions to change doctors (Cahal 1962; Cousins 1985; Slomski 1995). Safran et al. (2001) found both interpersonal aspects of care and structural features of care are contributors to the decision to change physicians. Few studies have focused specifically on PCPs (as opposed to specialists) who are important in a managed care environment. We are aware of no work examining the impact physician payment methods have on switching behavior.
We use data from physician organizations that reimburse PCPs on either a capitated or fee-for-service basis to examine whether patients with chronic conditions are more likely to switch PCPs when physicians are capitated. We estimate reduced-form models that develop evidence about switching patterns and assess whether observed behavior is consistent with predictions from a conceptual model based on the literature.
Conceptual Framework
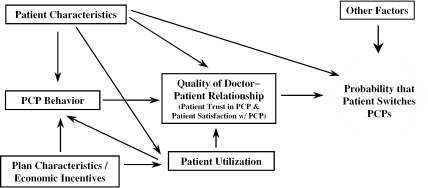
Figure 1 depicts a conceptual model of PCP switching. In the model, the PCP–patient relationship, a combination of satisfaction and trust in the PCP, is central. Satisfaction is an assessment of the care received and interactions with the PCP, and trust implies an expectation that the PCP will act in the patient's best interest (Mechanic 1998; Shortell et al. 1998). Previous research shows both satisfaction (Marquis, Davies, and Ware 1983) and trust (Kao et al. 1998; Safran et al. 2001) predict switching and thought of switching regular care physicians. Patients choose to switch PCPs when the quality of the PCP–patient relationship does not meet their minimum expectations or when other factors make continuing the relationship infeasible (e.g., changing health plans and the former PCP is not in the new plan's provider network). The PCP–patient relationship is affected by patient characteristics and utilization. It is also influenced by plan characteristics, in particular financial incentives, through their influences on PCP behavior and utilization.
Figure 1.
Model of Patient Switching Behavior
The effect of patient characteristics has been investigated in the literature. Patient age and gender, but not patient race, education, or income have been found to be related to satisfaction and switching behavior (Cleary and McNeil 1988; Hall and Dornan 1990; Kane, Maciejewski, and Finch 1997; Marquis, Davies, and Ware 1983; Slomski 1995; Safran et al. 2001).
Patient health status may influence the decision to switch physicians in two ways. First, sicker patients tend to be less satisfied with medical care than healthier patients (Hall, Milburn, and Epstein 1993; Marshall, Hays, and Mazel 1996). Social conversation acts as a mediating factor between health status and patient satisfaction, wherein sicker patients have less social conversation with their physicians, reducing their satisfaction (Hall et al. 1996). This may be because physicians spend more time providing sicker patients with biomedical information, leaving less time for social conversation (Hall et al. 1996), like their sicker patients less than healthier patients (Hall et al. 1993), experience frustration with patients who are demanding or have too many problems (Levinson et al. 1993), or have negative opinions about patients who abuse themselves (Levinson et al. 1993). Physicians' negative feelings could also be unintentionally communicated to patients, reducing patients' satisfaction with medical care (Hall, Milburn, and Epstein 1993; Hall et al. 1998), all of which would be expected to increase the probability of switching physicians. Sicker patients, however, have more regular contact with their PCPs, which is expected to increase loyalty and reduce their propensity to switch PCPs (Hirschman 1970). In addition, sicker patients, particularly those with chronic conditions who become experienced with their disease, are more likely to voice concerns they have with their medical care (Haug and Lavin 1983; Hall et al. 1996), which may reduce their propensity to switch PCPs (Hirschman 1970).
Aspects of medical care utilization are expected to be important in switching PCPs. Increased use of services results in a more established relationship and increased loyalty toward the PCP, reducing the likelihood patients switch PCPs. Shorter office visits result in less social conversation (Hall et al. 1998) and less PCP knowledge about patients (Hall et al. 1996; Gross et al. 1998), reducing patient satisfaction. Shorter office visits also reduce patient involvement in treatment decisions, which increases the probability of switching PCPs (Kaplan et al. 1996). Other aspects of utilization that may damage patient satisfaction and increase the propensity to switch PCPs include difficulty getting appointments (Jatulis, Bundek, and Legorreta 1997; Hays et al. 1998), lack of continuity of care (Cleary and McNeil 1988; Hays et al. 1998; Safran et al. 2001), and perceived difficulty obtaining referrals for specialty care (Grumbach et al. 1999; Kasteler et al. 1976).
Capitation may affect utilization in ways damaging to the doctor–patient relationship and mediate the way patients (particularly those with chronic conditions), experience the health care system. Capitation creates incentives for physicians to minimize services provided to patients (Hillman 1987) and to attract large numbers of patients to their panels (Pauly et al. 1992). Hence, capitation may result in shorter office visits (Blumenthal et al. 1999) and longer waiting times for appointments, both of which may increase switching. Research comparing capitated PCP payment to managed care FFS finds capitation is associated with reduced access to care, physicians' knowledge of patients, clinician–patient communication, and interpersonal treatment (Safran et al. 2000).
In addition to the above factors that would affect all patients, capitation generates potentially strong financial incentives for physicians to avoid high-cost patients. Those patients with identifiable characteristics that are predictive of high costs, or those who are otherwise high utilizers, may switch more quickly from capitated PCPs than from FFS PCPs. It is the interaction between financial incentives and health status or utilization that we try to isolate and quantify in the empirical work that follows.
Data
The data obtained for this research are administrative enrollment and claims or encounter data for one year from three independent practice associations and one large, multispecialty medical group in New York, Ohio, Idaho, and California for 1994 and 1995.1 The main advantage of these data is that we know how the physician organizations paid individual PCPs for their services. In two of the physician organizations, PCPs were paid a monthly fee for each patient for ambulatory care, inpatient evaluation, and management encounters provided. The PCPs’ capitation payment did not include non-PCP services, such as services provided by specialists or hospitalizations. The capitated arrangements in these two physician organizations were very similar. The two other physician organizations paid PCPs on a discounted FFS scale. We generated a dichotomous measure of PCP payment type (capitation versus FFS) and used it in the methods section.
Representativeness of Data
We compared our four markets to the U.S. managed care market at the time, using information on HMO penetration. The average HMO penetration in the four markets was somewhat higher than the U.S. average (32.7 percent versus 24.0 percent) due to one of the physician organizations being located in California, the state with the highest HMO penetration. Nationally, HMOs capitate 46.7 percent of primary care services provided in solo or single specialty practices and 54.9 percent of services provided in multispecialty practices (Interstudy 1997). Capitation was more prevalent in the market areas of the two physician organizations that capitated PCPs and was approximately at or below the national average in the market areas of the two physician organizations that paid PCPs on an FFS basis (Table 1).
Table 1.
Description of Data by Physician Organization
| Physician Organization 1 | Physician Organization 2 | Physician Organization 3 | Physician Organization 4 | |
|---|---|---|---|---|
| Location | California | New York | Ohio | Idaho |
| HMO penetration in market area | 50.1% | 23.3% | 21.0% | 13.1% |
| Method of PCP reimbursement | Partial Capitation | Fee-For-Service | Fee-For-Service | Partial Capitation |
| PCP services capitated by HMOs in market area: | ||||
| Solo/Single Spec. Group | 66.4% | 35.0% | 46.4% | 85.7% |
| Multi-Spec. Group | 85.4% | 47.8% | 60.9% | 95.2% |
| Dates covered by data | Jan.–Dec. 1994 | Jan.–Dec. 1995 | Apr. 1994 Mar. –1995 | Jan.–Dec. 1995 |
| Number of PCPs | 662 | 341 | 180 | 208 |
| Number of enrollees age 21–64 | 51,811 | 39,166 | 50,845 | 12,907 |
| Number of enrollees continuously enrolled for six months (%) | 35,235(68.0%) | 20,076(51.3%) | 33,031(65.0%) | 7,065(54.7%) |
| Percent of continuously enrolled enrollees with at least 1 service in claims data | 91.3% | 83.8% | 79.8% | 96.4% |
| Number of continuously enrolled enrollees with at least 2 encounters (%) | 27,217(77.2%) | 14,124(70.4%) | 21,213(64.2%) | 4,706(66.6%) |
| Number of continuously enrolled enrollees excluded because only 1 patient seen by PCP | 13 | 9 | 14 | 29 |
| Number of continuously enrolled enrollees excluded because claims data shows more than 1 PCP on given date | 38 | 25 | 1 | 0 |
| Number of continuously enrolled enrollees (patients) included in analyses | 27,166 | 14,090 | 21,198 | 4,677 |
| Number of patients randomized to estimation dataset | 13,477 | 6,779 | 9,582 | 2,109 |
| % patients switching PCPs | 6.8% | 4.9% | 0.4% | 7.1% |
| % encounters that are switches from previous encounter | 1.6% | 1.3% | 0.2% | 2.1% |
Sample
The four physician organizations provided care for a total of 154,729 enrollees from the ages of 21 to 64 years. Patients were excluded from analyses if they were continuously enrolled for less than six months or if they had fewer than two encounters with physicians or physician substitutes, thus eliminating the opportunity to observe switching behavior (Table 1). The final sample included 67,131 patients and 682 PCPs.
Outcome Variable: Switching PCP Since Previous Encounter
Patients were defined as having switched their PCP if the PCP of record with the physician organization changed from one encounter with a physician or physician substitute to the next.2 As such, encounters with specialists and cross-coverage by other PCP type physicians (i.e., when the PCP is out of town) are not considered switches.
Explanatory Variables
Case-Mix Adjustment and Categories of Diagnoses
We used the Ambulatory Diagnostic Groups (ADGs) component of the Johns Hopkins University ACG Case-Mix Adjustment System (ACGs), version 4.1, to adjust for differences in patient case-mix and to identify subpopulations of interest.3 While ADGs are usually assigned at the patient level, we wanted to examine the effect of individual ADGs on the probability that patients switch their PCPs. Therefore, we determined ADGs based on diagnoses coded prior to a given encounter, including only the information available at the time the patient would have decided to switch PCPs. Thus, the ADGs were cumulative over encounters.4
In addition to the ADGs, three categories of diagnoses were created; two of the categories were created to identify patients about whom physicians may have negative opinions because of their self-destructive behavior: drug and alcohol abusers and patients with sexually transmitted diseases. A third category captured diagnoses that are vague, since not being able to specifically diagnose a condition may frustrate the physician and may cause the patient to question the physician's abilities.
Utilization Measures
Four categories of variables were created to control for utilization intensity, including the number of encounters the patient had prior to the current encounter and the time between encounters and this value squared. Longer times between encounters, controlling for patient case-mix and number of previous encounters, may represent difficulty in obtaining an appointment. The third category of variables assessed the time spent with physicians in office visits. The CPT-4 procedure codes representing office visits were separated into short, medium, and long office visits.5 We calculated the percentage of previous PCP encounters that were short for each encounter. Patients with a high percentage of short encounters, controlling for case-mix, may be less satisfied with their PCP. The fourth category represents utilization with different types of physicians. Each encounter was identified as being with the patient's PCP, another primary care type physician (OPCTP—physician other than the patient's PCP with a specialty of internal medicine, family practice, or general practice), or a specialist. For each encounter, the percentage of previous encounters with the PCP, OPCTP, and specialists were calculated. Patients with higher percentages of visits with OPCTP experience less continuity of care. In addition, controlling for case-mix, patients with lower percentages of visits with specialists may perceive limited access to referrals.
Methods
Bivariate analyses were performed at the patient level to examine differences between switchers and nonswitchers using t-tests for continuous variables and chi-squared tests for categorical variables. Multivariate analyses were performed at the encounter level because switchers tended to have more encounters than nonswitchers, thus having a greater opportunity for a switch to be observed. The encounter-level analyses eliminate this endogeneity. In addition, encounter-level analyses allow the identification of the sequence of events, such as whether the diagnosis of a chronic condition occurred before or after switching PCPs. Prior to conducting analyses, the data were split into two random samples based on unique patient identifiers. One sample was used in model development, while the other sample was used for model validation, thus avoiding overfitting the model.
Logistic regression analyses with PCP fixed effects6 were conducted to determine the influence of patient characteristics, case-mix, utilization, and capitated PCP payment on the probability that patients switch their PCP. Huber-White robust standard errors were used to account for the correlation between the multiple observations contributed by the same patient. The dependent variable was whether the patient switched PCPs since the previous encounter.
Two models were run; the first included patient demographics, ADGs, and ADG interacted with capitated PCP payment. The second also included utilization measures and interactions with capitated PCP payment. This sequential modeling was done to determine if coefficients changed, particularly the coefficient on the ADG representing “chronic medical stable” conditions interacted with capitated PCP payment, when utilization measures were added to the model. The Wald statistic was used to test whether the coefficients on sets of variables were jointly equal to zero. The c-statistic was used to assess model discrimination, and the Hosmer-Lemeshow statistic was used to evaluate model calibration (Hosmer and Lemeshow 1989).
Results
Descriptive statistics for the four physician organizations are shown in Table 1. Almost 5 percent of patients with two or more encounters switched PCPs in our data, ranging from 0.4 percent to 7.1 percent for the four physician organizations. The average number of encounters per patient was comparable across the four physician organizations.
Table 2 compares switchers and nonswitchers, overall and within PCP payment method. Unless otherwise specified, the results reported are for the two payment methods combined. Switchers were younger and more likely to be female than nonswitchers. Switchers had more ADGs and more major ADGs than nonswitchers. Although not significant overall, capitated and FFS switchers were more likely to have the “chronic medical stable” ADG than nonswitchers. Consistent with the finding that patients who switched PCPs are sicker than patients who did not switch PCPs, switchers had more encounters than nonswitchers. In addition, switchers had a higher percentage of short encounters than nonswitchers, experienced less continuity of care (a higher percentage of encounters with OPCTP), and a higher percentage of encounters with specialists.
Table 2.
Switchers Compared to Nonswitchers
| ALL | Capitated | FFS | ||||
|---|---|---|---|---|---|---|
| Switchers n=1,447 | Nonswitchers n=30,500 | Switchers n=1,073 | Nonswitchers n=14,513 | Switchers n=374 | Nonswitchers n=15,987 | |
| Demographics | ||||||
| Female | 73.7% | 64.7%*** | 74.6% | 66.2%*** | 71.1% | 63.3%** |
| Age | 39.8 | 41.4*** | 39.4 | 40.6*** | 41.1 | 42.1 |
| Case-Mix /Diagnoses | ||||||
| ADG10: chronic medical stable | 38.7% | 37.2% | 33.6% | 27.0%*** | 53.2% | 46.3%** |
| Total # ADGs | 4.6 | 3.9*** | 4.2 | 3.3*** | 5.5 | 4.4*** |
| ♯ Major ADGs | 0.49 | 0.41*** | 0.44 | 0.35*** | 0.63 | 0.47*** |
| General Diagnoses | 69.0% | 60.1%*** | 66.5% | 55.2%*** | 75.9% | 64.5%*** |
| STD Diagnoses | 2.6% | 1.3%*** | 2.2% | 1.5% | 3.7% | 1.1%*** |
| Drug/Alcohol Use/Abuse Diagnoses | 1.5% | 0.7%** | 1.0% | 0.4%*** | 2.7% | 1.1%** |
| Utilization | ||||||
| Number of encounters | 8.0 | 6.0*** | 7.4 | 5.5*** | 9.8 | 6.4*** |
| Average ♯ of days between encounters | 56.7 | 61.0*** | 60.9 | 57.2** | 44.4 | 64.5*** |
| % Encounters that were short visits | 16.9% | 12.4%*** | 17.6% | 13.8%*** | 14.9% | 11.1%** |
| % Encounters with PCP | 32.5% | 36.7%*** | 43.1% | 50.5%*** | 2.4% | 24.1%*** |
| % Encounters with PCP type physicians other than PCP | 31.9% | 30.4% | 20.5% | 14.1%*** | 64.5% | 45.2%*** |
| % Encounters with specialists | 35.6% | 32.9%** | 36.4% | 35.4% | 33.1% | 30.6% |
Differences between switchers and nonswitchers: significant at p<.01;
significant at p<.001
Table 3 contains selected odds ratios resulting from logistic regressions with PCP fixed effects. Model 1 included patient age, gender, ADGs, ADG-capitation interactions, and indicators for drug use, sexually transmitted diseases, and general diagnoses. Model fit was good as assessed by the Hosmer-Lemeshow test (χ28df=6.61, p=0.58) and the c-statistic (0.82). Female gender was associated with an increased likelihood of switching PCPs, while increasing patient age was associated with decreasing likelihood of switching PCPs. The main effect of “chronic medical stable” conditions (ADG 10) was insignificant. However, its interaction with capitated PCP payment was positively associated with switching PCPs (OR=1.36, p<0.05). Drug/alcohol abuse diagnoses were associated with increased likelihood of switching PCPs, while diagnoses of sexually transmitted diseases and general, unspecified diagnoses were not significantly related to switching PCPs.
Table 3.
Logistic Regression Results†
| Model 1 ADG Model | Model 2 Add Patient Utilization and Utilization–Capitation Interactions | |
|---|---|---|
| Patient Demographics | ||
| 26–35 years | 0.83(0.65, 1.07) | 0.84(0.65, 1.09) |
| 36–55 years | 0.73(0.56, 0.93)* | 0.76(0.59, 0.99) |
| 56–65 years | 0.44(0.32, 0.61)*** | 0.51(0.37, 0.70)*** |
| Female | 1.29(1.12, 1.48)*** | 1.20(1.04, 1.39)* |
| Patient Case-Mix | ||
| ADG10: chronic medical stable | 0.94(0.18, 4.83) | 0.65(0.02, 18.33) |
| ADG10-capitation interaction | 1.36(1.02, 1.80)* | 1.28(0.95, 1.73) |
| Other Diagnoses Categories | ||
| Sexually transmitted diseases | 1.08(0.68, 1.74) | 1.15(0.73, 1.83) |
| Drug use | 2.07(1.11, 3.86)* | 2.01(1.08, 3.73)* |
| General diagnoses | 1.04(0.92, 1.18) | 1.10(0.96, 1.25) |
| Patient Utilization | ||
| Time since previous encounter | 1.01(1.01, 1.02)*** | |
| Time since previous encounter squared | 0.99(0.99, 0.99)*** | |
| 2 previous encounters | 0.70(0.57, 0.87)** | |
| 3 previous encounters | 0.29(0.20, 0.41)*** | |
| 4 or 5 previous encounters | 0.16(0.10, 0.26)*** | |
| 6–19 previous encounters | 0.08(0.04, 0.14)*** | |
| 20+previous encounters | 0.16(0.05, 0.48)** | |
| % of previous encounters—short | 1.19(0.97, 1.46) | |
| % of previous encounters with OPCTP | 3.10(2.46, 3.92)*** | |
| % of previous encounters with specialists | 1.69(1.35, 2.10)*** | |
| Patient Utilization–Capitation Interactions | ||
| Time since previous encounter | 1.02(1.01, 1.03)*** | |
| Time since previous encounter squared | 0.99(0.99, 1.00) | |
| 2 previous encounters | 1.06(0.82, 1.38) | |
| 3 previous encounters | 2.11(1.44, 3.11)*** | |
| 4 or 5 previous encounters | 3.06(2.05, 4.56)*** | |
| 6–19 previous encounters | 5.14(3.21, 8.23)*** | |
| 20 + previous encounters | 2.49(0.89, 6.97) | |
| Cstatistic | .817 | .853 |
| Hosmer-Lemeshow Test | χ2 8df=6.61 p=0.58 | χ2 8df=12.31 p=0.14 |
Results are reported as odds ratios with confidence intervals in parentheses.
Significant at p<.05;
significant at p<.01;
significant at p<.001
Model 2 included the utilization measures and utilization–capitation interactions. The model was well calibrated (χ28df=12.31, p=0.14), and discriminated well (c-statistic=0.85).7 The main effect of the number of previous encounters was negative. The capitation–previous encounters interactions, however, were positive, indicating capitated patients were significantly more likely than their FFS counterparts with the same case-mix and number of previous encounters to switch PCPs. The combination of the main effect of previous encounters and the interactions with capitation reveal the overall effect of the number of prior encounters is negative for capitated patients.
Both the percentage of previous encounters that were short and its interaction with capitated PCP reimbursement were not significantly associated with patients' propensity to switch their PCP. The percentage of previous encounters with OPCTPs and the percentage of previous encounters with specialists both had strong positive associations with switching PCPs.
The time between patient encounters and the likelihood that patients switch PCPs had a U-shaped relationship. Initially, longer times between encounters were associated with greater likelihood that patients switch PCPs during the interval, with the probability of switching peaking at 100 days between encounters for FFS patients and 180 days between encounters for capitated patients. With the inclusion of the utilization measures, the chronic medical stable ADG-capitation interaction was no longer significant (OR=1.28, p <0.34).
Discussion
We found PCP payment method was an important predictor of patient switching of PCPs when interacted with the presence of stable chronic conditions (ADG 10) or patients’ prior utilization. For those patients with ADG 10 (stable, chronic medical conditions), capitation relative to FFS was associated with a 36 percent increase in the likelihood of switching PCPs, not controlling for utilization. Although inclusion of utilization controls eliminated this result, the number of previous encounters interacted with capitation were strongly associated with switching. Capitated patients with 6–19 encounters were more than five times as likely to switch PCPs as FFS patients with the same number of previous encounters.
There are two main plausible interpretations of these results. One interpretation is that capitated PCPs are dumping their high-cost patients, either intentionally or unintentionally. If the dumping is intentional, it appears they identify patients based on utilization (actual burden) rather than case-mix (expected burden). This assumes PCPs are aware of their patients’ insurance coverage and can distinguish between patients based on this. Alternatively, the dumping could be unintentional and the pressures of capitated arrangements cause PCPs stress that is then inadvertently “leaked” to high-utilizing patients during encounters. While studies have shown capitated contracts are associated with reduced physician and patient satisfaction with various aspects of care (Kerr et al. 1997; Safran et al. 2000), Flood and Bott (1998) found that few individual physicians practice different styles of medicine based on patients' insurance. However, their research focused on acute conditions, which may have less room for discretionary treatment and may be less likely to invoke capitated physicians' reactions to high-utilization patients.
A second possible interpretation is that high utilization patients may be more sophisticated than other patients and more easily dissatisfied in a heavily managed care environment. Many studies have found health status to be positively related to patient satisfaction. Safran et al. (2000) found capitated PCP payment was negatively related to physicians’ knowledge of patients, doctor–patient communication, and physicians’ interpersonal treatment of patients, all of which patients with chronic diseases may be more sensitive to than other patients since they have more frequent contact with physicians. Schlesinger et al. (1999) found sicker patients had to be much more dissatisfied than healthier patients to disenroll from their health plan. It is plausible that a similar relationship would exist for switching from a PCP. Therefore, the increased propensity of high utilization patients to switch PCPs under capitation may be indicative of severe deterioration of the relationship between these patients and their PCPs.
Understanding the effects of capitation is and will remain important because capitation will remain in the marketplace for years to come. Managed care in the employer-sponsored market is currently undergoing a transformation with enrollment in point-of-service plans and preferred provider organizations increasing and enrollment in HMOs stagnating or even decreasing (Dudley and Luft 2001). While some experts are stating the end of managed care is at hand (Robinson 2001), others predict the use of capitation will increase as a result of currently increasing health care costs and a weakened economy (Berwick 2002). Regardless of what occurs in the private health insurance environment, capitation is firmly entrenched in Medicaid and SCHIP, with 76 percent of plans making some use of either global capitation or professional services capitation arrangements with their Medicaid providers (Draper and Gold 2002).
Limitations
There are several features of the data that complicate interpretation of the results. First, because claims/encounter data were used to identify switches, the results are only relevant to individuals who utilize health care. Those who switch PCPs without utilization are missed. Second, the study sample was limited to patients obtaining their health care through only four physician organizations, using two different PCP payment methods. The results, consequently, may not be generalizable to all physician organizations, or other PCP payment methods. In addition, the physician organizations are in nonoverlapping markets, and while we know some of the methods the physician organizations use to manage care, we cannot assume that we are aware of all of the methods used. If the characteristics of the market and the methods used to manage care are unrelated to the PCP payment method, then the results of the analyses are unaffected. However, to the extent that they are correlated, the results may be biased by omitted variables, with the direction of the bias unknown. It is unlikely, however, that the interactions between PCP payment method and other variables are impacted.
In our data, we are able to observe switching within a physician organization, but not across physician organizations. This problem is minimized because individuals are most likely to switch physician organizations when they switch plans, and plan switching is only possible when individuals change employers or during open enrollment periods. This would not affect the main result regarding the capitation interactions unless the interactions were different for physician organization switching than for PCP switching.
Last, we lack complete information of how each PCP in the study was reimbursed for all of their patients. PCPs may distinguish between patients with different types of insurance (Kerr et al. 1997) or they may have a single practice style for all of their patients, regardless of payment type (Flood and Bott 1998). If the former is the case, our results are not impacted by our lack of knowledge of the payment methods for PCPs' unobserved patients. However, if physicians do adopt a single practice style, then our results may be a function of the percentage of the PCP's patients that are covered by capitation rather than whether the individual patient was capitated. Not only would this affect the PCP fixed effects, but it could also affect the interactions between capitation and utilization and health status. We have substantial variation in the number of patients observed per PCP and in the HMO penetration in the market areas of the physician organizations that capitate PCPs. Thus, it is likely that the error is random rather than systematic, biasing the coefficients on the capitation interactions toward zero.
Conclusions
This study adds to the evidence that capitated PCP payment systematically affects the doctor–patient relationship. Patients who are expected to be the most loyal, those with regular physician contact, are relatively more likely to switch PCPs if their PCP is paid capitation rather than FFS. The findings suggest that the quality of care received by the most vulnerable patients may be compromised under capitation, which raises concerns about capitation as a PCP payment method.
Notes
We obtained the data from HCIA, Inc.
Contact authors for the list of diagnosis and procedure codes used in classifications.
All records with laboratory or x-ray procedure codes were excluded from ADG assignments to prevent the inclusion of “rule out” diagnoses, as recommended in the 1998 Johns Hopkins University ACG Case-Mix Adjustment System: Implementation Guide.
Because our method results in varying time periods over which ADGs could be observed, systematic measurement error is induced into the ADGs. The measurement error is systematic in the length of the observed time period. We account for it in our multivariate models by including a second-order Taylor series expansion in the length of the time period. We have shown this method eliminates the bias in Monte Carlo experiments (Dick and Sorbero 2002).
See note 2.
In our data, PCPs are paid by either capitation or FFS, but not both. As a result, we cannot disentangle the PCP effects from the capitation effects (i.e., PCP payment method is a linear combination of PCP fixed effects). As a result, the PCP capitation payment main effect is subsumed by the PCP fixed effects. The PCPs who did not have any patients switching out of their practices were dropped from the analyses due to lack of variation in the dependent variable for the fixed effect. In effect, this selection based on the value of the dependent variables could induce bias. We investigated this by estimating random-effects models with and without the affected observations, and we compared the results using Hausman tests. We also reestimated the fixed effects model on a subset of data that included only PCPs with relatively large observed panels. The large panel sizes guaranteed switching for these PCPs, eliminating the need to selectively drop observations. There were no substantive differences in the results from the various estimation methods, which indicates the selection bias was minimal.
Model performance was also assessed using the validation dataset, limited to those PCPs also included in the estimation data set to allow the use of the PCP fixed effects. The discrimination of the model remained very good; the c-statistic was 0.83 compared to 0.86 in the estimation dataset. The model, however, was not well calibrated (χ2 10df=29.14, p=0.001). We reestimated the model in the validation dataset to determine the consistency of the parameter estimates with those from the estimation dataset. With the exceptions of age and alcohol/drug abuse, the substantive and statistical significance of the findings were unchanged.
REFERENCES
- Berwick DM. “Implementing the 21st Century Health Care Chassis.”. Washington, DC: 2002. Keynote address at the annual research meeting of Academy for Health Services Research and Health Policy, June 24. [Google Scholar]
- Blumenthal D. “The Vital Role of Professionalism in Health Care Reform”. Health Affairs. 1994;13(1):252–6. doi: 10.1377/hlthaff.13.1.252. [DOI] [PubMed] [Google Scholar]
- Blumenthal D, Causino N, Chang Y, Culpepper L, Marder W, Saglam D, Stafford R, Starfield B. “The Duration of Ambulatory Visits to Physicians.”. Journal of Family Practice. 1999;48(4):264–71. [PubMed] [Google Scholar]
- Cahal MF. “What the Public Thinks of the Family Doctor—Folklore and Facts.”. General Practice. 1962;225(2):146–57. [PubMed] [Google Scholar]
- Cleary PD, McNeil BJ. “Patient Satisfaction as an Indicator of Quality Care.”. Inquiry. 1988;25(1):25–36. [PubMed] [Google Scholar]
- Cousins N. “How Patients Appraise Physicians.”. New England Journal of Medicine. 1985;313(22):1422–4. doi: 10.1056/NEJM198511283132227. [DOI] [PubMed] [Google Scholar]
- Dick AW, Sorbero MES. “A Second-Order Taylor Series Method to Eliminate Bias in Case-Mix Adjustment Due to Measurement Error”. University of Rochester; 2002. Manuscript. [Google Scholar]
- Draper D, Gold MR. “Risk Contracting in Medicaid-Participating Plans”. Washington, DC: 2002. Poster session at the annual research meeting of Academy for Health Services Research and Health Policy, June. [Google Scholar]
- Dudley RA, Luft HS. “Managed Care in Transition.”. New England Journal of Medicine. 2001;344(14):1087–92. doi: 10.1056/NEJM200104053441410. [DOI] [PubMed] [Google Scholar]
- Flood AB, Bott DM. “Managed Care and Physicians”. Washington, DC: 1998. Paper presented at annual meeting of the Academy for Health Services Research. [Google Scholar]
- Gold MR, Hurley R, Lake T, Ensor T, Berenson R. “A National Survey of the Arrangements Managed-Care Plans Make with Physicians.”. New England Journal of Medicine. 1995;333(25):1678–83. doi: 10.1056/NEJM199512213332505. [DOI] [PubMed] [Google Scholar]
- Gross DA, Zyzanski SJ, Borawski EA, Cebul RD, Stange KC. “Patient Satisfaction with Time Spent with Their Physician.”. Journal of Family Practice. 1998;47(2):133–7. [PubMed] [Google Scholar]
- Grumbach K, Selby JV, Damberg C, Bindman AB, Quesenberry CP, Truman A, Uratsu C. “Resolving the Gatekeeper Conundrum: What Patients Value in Primary Care and Referrals to Specialists.”. Journal of the American Medical Association. 1999;282(3):261–6. doi: 10.1001/jama.282.3.261. [DOI] [PubMed] [Google Scholar]
- Hall JA, Dornan MC. “Patient Sociodemographic Characteristics as Predictors of Satisfaction with Medical Care: A Meta-Analysis.”. Social Science and Medicine. 1990;30(7):811–8. doi: 10.1016/0277-9536(90)90205-7. [DOI] [PubMed] [Google Scholar]
- Hall JA, Epstein AM, DeCiantis ML, McNeil BJ. “Physicians’ Liking for Their Patients: More Evidence for the Role of Affect in Medical Care.”. Health Psychology. 1993;12(2):140–6. doi: 10.1037//0278-6133.12.2.140. [DOI] [PubMed] [Google Scholar]
- Hall JA, Milburn MA, Epstein AM. “A Causal Model of Health Status and Satisfaction with Medical Care.”. Medical Care. 1993;31(1):84–94. doi: 10.1097/00005650-199301000-00007. [DOI] [PubMed] [Google Scholar]
- Hall JA, Milburn MA, Roter DL, Daltroy LH. “Why Are Sicker Patients Less Satisfied with Their Medical Care? Test of Two Explanatory Models.”. Health Psychology. 1998;17(1):70–5. doi: 10.1037//0278-6133.17.1.70. [DOI] [PubMed] [Google Scholar]
- Hall JA, Roter DL, Milburn MA, Daltroy LH. “Patients’ Health as a Predictor of Physician and Patient Behavior in Medical Visits.”. Medical Care. 1996;34(12):1205–18. doi: 10.1097/00005650-199612000-00006. [DOI] [PubMed] [Google Scholar]
- Haug M, Lavin B. Consumerism in Medicine: Challenging Physician Authority. Beverly Hills, CA: Sage; 1983. [Google Scholar]
- Hays R, Brown JA, Spritzer KL, Dixon WJ, Brook RH. “Member Ratings of Health Care Provided by 48 Physician Groups.”. Archives of Internal Medicine. 1998;158(7):785–90. doi: 10.1001/archinte.158.7.785. [DOI] [PubMed] [Google Scholar]
- Hillman AL. “Financial Incentives for Physicians in HMOs: Is There a Conflict of Interest?”. New England Journal of Medicine. 1987;317(27):1743–8. doi: 10.1056/NEJM198712313172725. [DOI] [PubMed] [Google Scholar]
- Hirschman AO. Exit, Voice, and Loyalty: Responses to Decline in Firms, Organizations, and States. Cambridge, MA: Harvard University Press; 1970. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley and Sons, Inc; 1989. [Google Scholar]
- Inter Study . Competitive Edge. Minneapolis, MN: InterStudy Publications; 1997. (release 7.1) [Google Scholar]
- Jatulis DE, Bundek NI, Legorreta AP. “Identifying Predictors of Satisfaction with Access to Medical Care and Quality of Care.”. American Journal of Medical Quality. 1997;12(1):11–8. doi: 10.1177/0885713X9701200103. [DOI] [PubMed] [Google Scholar]
- Kane RL, Maciejewski M, Finch M. “The Relationship of Patient Satisfaction with Care and Clinical Outcomes.”. Medical Care. 1997;35(7):714–30. doi: 10.1097/00005650-199707000-00005. [DOI] [PubMed] [Google Scholar]
- Kao AC, Green DC, Zaslavsky AM, Koplan JP, Cleary PD. “The Relationship between Method of Physician Payment and Patient Trust.”. Journal of the American Medical Association. 1998;280(19):1708–14. doi: 10.1001/jama.280.19.1708. [DOI] [PubMed] [Google Scholar]
- Kaplan SH, Greenfield S, Gandek B, Rogers WH, Ware JE. “Characteristics of Physicians with Participatory Decision-Making Styles.”. Annals of Internal Medicine. 1996;124(5):497–504. doi: 10.7326/0003-4819-124-5-199603010-00007. [DOI] [PubMed] [Google Scholar]
- Kasteler J, Kane RL, Olsen DM, Thetford C. “Issues Underlying Prevalence of ‘Doctor-Shopping’ Behavior.”. Journal of Health and Social Behavior. 1976;17:328–39. [PubMed] [Google Scholar]
- Kerr EA, Hays RD, Mittman BS, Siu AL, Leake B, Brook RH. “Primary Care Physicians’ Satisfaction with Quality of Care in California Capitated Medical Groups.”. Journal of the American Medical Association. 1997;278(4):308–12. [PubMed] [Google Scholar]
- Levinson W, Stiles WB, Inui TS, Engle R. “Physician Frustration in Communicating with Patients.”. Medical Care. 1993;31(4):285–95. doi: 10.1097/00005650-199304000-00001. [DOI] [PubMed] [Google Scholar]
- Marquis MS, Davies AR, Ware JE. “Patient Satisfaction and Change in Medical Care Provider: A Longitudinal Study.”. Medical Care. 1983;21(8):821–9. doi: 10.1097/00005650-198308000-00006. [DOI] [PubMed] [Google Scholar]
- Marshall GN, Hays RD, Mazel R. “Health Status and Satisfaction with Health Care: Results from the Medical Outcomes Study.”. Journal of Consulting and Clinical Psychology. 1996;64(2):380–90. doi: 10.1037//0022-006x.64.2.380. [DOI] [PubMed] [Google Scholar]
- Mechanic D. “The Functions and Limitations of Trust in the Provision of Medical Care.”. Journal of Health Politics, Policy and Law. 1998;23(4):661–86. doi: 10.1215/03616878-23-4-661. [DOI] [PubMed] [Google Scholar]
- Pauly MV, Feldman R, Eisenberg JM, Erder MH, Schwartz JS. Ann Arbor, MI: Health Administration Press; 1992. Paying Physicians: Options for Controlling Cost, Volume, and Intensity of Services. [Google Scholar]
- Robinson JC. “The End of Managed Care.”. Journal of the American Medical Association. 2001;285(20):2622–8. doi: 10.1001/jama.285.20.2622. [DOI] [PubMed] [Google Scholar]
- Rodwin MA. Medicine, Money, and Morals: Physicians’ Conflicts of Interest. New York: Oxford University Press; 1993. [Google Scholar]
- Safran DG, Rogers WH, Tarlov AR, Inui TS, Tairá DA, Montgomery JE, Ware JE, Slavin CP. “Organizational and Financial Characteristics of Health Plans: Are They Related to Primary Care Performance?”. Archives of Internal Medicine. 2000;160(1):69–76. doi: 10.1001/archinte.160.1.69. [DOI] [PubMed] [Google Scholar]
- Safran DG, Montgomery JE, Chang H, Murphy J, Rogers WH. “Switching Doctors: Predictors of Voluntary Disenrollment from a Primary Physician's Practice.”. Journal of Family Practice. 2001;50(2):130–6. [PubMed] [Google Scholar]
- Schlesinger M, Druss B, Thomas T. “No Exit? The Effect of Health Status on Dissatisfaction and Disenrollment from Health Plans.”. Health Services Research. 1999;34(2):547–76. [PMC free article] [PubMed] [Google Scholar]
- Shortell SM, Waters TM, Clarke KWB, Budetti PP. “Physicians as Double Agents: Maintaining Trust in an Era of Multiple Accountabilities.”. Journal of the American Medical Association. 1998;280(12):1102–8. doi: 10.1001/jama.280.12.1102. [DOI] [PubMed] [Google Scholar]
- Slomski AJ. “Will Patients Leave You for Cheaper Care?”. Medical Economics. 1995;8(21):47–57. [PubMed] [Google Scholar]