Abstract
Objective
To estimate the effects of smoking on quality of life over time, using the Years of Healthy Life (YHL) construct.
Data Sources/Study Setting
The Health and Retirement Study (HRS) survey (N = 12,652) of persons 50 to 60 years old and the Asset and Health Dynamics Among the Oldest Old (AHEAD) survey (N = 8,124) of persons≥70 years old, plus spouses regardless of age, followed from 1992/1993 to 2000.
Study Design
Years of healthy life from baseline to death were estimated. Regression models were developed with smoking as the main explanatory variable and with both YHL and years of life remaining as the outcome variables.
Principal Findings
Smoking was strongly and consistently related to YHL lost. In HRS, individuals who had quit smoking at least 15 years prior to baseline had a similar number of YHL left as never smokers.
Conclusions
Efforts to encourage smoking cessation should emphasize the impact of these factors on quality of life.
Keywords: Smoking, quality of life, years of healthy life, longitudinal study, middle aged, elderly
There is overwhelming evidence of the adverse effect of tobacco smoking on overall mortality (Centers for Disease Control and Prevention 2002) and of the ability of smoking cessation to extend life (Taylor et al. 2002). Furthermore, strong evidence links smoking to the development of and mortality from a number of specific diseases, including the three leading causes of death in the United States: heart disease, cancer, and stroke (U.S. Department of Health and Human Services 1990). Less work has been done to investigate the relationship between smoking and disability or healthy life, more generally. Better understanding the impact of smoking on quality of life is important, first, to create a more complete view of the burden of smoking on society, and second, to provide information to smokers that may be more salient in encouraging smoking cessation attempts.
Messages concerning the effect of smoking on disability and quality of life may be more likely to invoke changes in smoking behavior than are messages about loss of life years, recent experimental research suggests. In this work, smokers' perceptions about their likelihood of surviving to age 75 were reduced more by information highlighting the quality of life effects of smoking than by messages presenting mortality risks (Sloan, Smith, and Taylor 2003). This is important because other work indicates that when people's perception of the likelihood of living to age 75 is lowered, they are more likely to stop smoking (Smith, Taylor, and Sloan 2001). Furthermore, smoking must be considered together with other modifiable risk factors, namely exercise, body mass index (BMI), and alcohol consumption.
Quality of life is a commonly used concept, but it has no universally accepted definition. It can be interpreted as the degree to which persons perceive themselves able to function physically, emotionally, and socially. In a general sense, it is that which makes life worth living. In a more quantitative sense, it is an estimate of remaining life free of impairment, disability, or handicap, as used in the expression quality adjusted life years (Last 2001). Quality of life has been measured in a number of different ways, ranging from more complex, multidimensional scales such as the SF-36 (8 subscales) (McHorney et al. 1993) to very simple, one-item instruments such as the Excellent/Very Good/Good/Fair/Poor (EVGGFP) question (Diehr et al. 1998). This simple, latter measure shows surprisingly high reliability and validity (Idler and Benyamini 1997). Quality of life can be measured at a single point in time or over a period of time using, for example, QALYs (quality adjusted life years), HALYs (health adjusted life years), DALYs (disability adjusted life years), or YHLs (years of healthy life) (Gold, Stevenson, and Fryback 2002).
A few earlier studies have estimated the loss of life adjusted for quality of life, broadly defined, due to specific diseases (Melse et al. 2000) as well as due to modifiable risk factors (Mathers et al. 2001; 2000). Using DALYs as their outcome, Mathers et al. (2001) found that smoking was responsible for 12.1 percent of the lost DALYs, while physical inactivity (6.0 percent) and obesity (4.3 percent) accounted for less. Among females, the relative percentages were 6.8 percent for smoking, 7.5 percent for physical inactivity, and 4.3 percent for obesity. That study did not distinguish the effect of these modifiable risk factors by age. A global assessment of the burden of modifiable risk factors on quality of life estimated that smoking contributed 11.7 percent of the lost DALYs among developed nations (compared to 10.7 percent for alcohol and 4.3 percent for physical inactivity) (Murray and Lopez 1997).
Other studies have assessed the effect of a particular modifiable risk factor in isolation. For example, physical activity and quality of life have been found to be related, with elderly persons who are more active being better off (Ruuskanen and Ruoppila 1995; Parkatti et al. 1998). However, these studies have cross-sectional designs, making inferences about the causal direction difficult. Obesity has also been identified as a significant factor in reducing quality of life, with one study suggesting the magnitude of reduction is similar to that of poverty, smoking, and problem drinking (Sturm and Wells 2001). The effect of alcohol on quality of life is complex. A consensus is emerging that the relative balance of the positive effect of moderate alcohol consumption on cardiovascular health and the various negative effects of alcohol (including violence, traffic accidents, liver disease, etc.) varies among populations and especially by age (U.S. Department of Health and Human Services 2000).
The objective of our study is to use the Years of Healthy Life methodology (based on the EVGGFP question and mortality status) to estimate the impact of smoking on YHL, while also considering the effect of inactivity, alcohol consumption, and weight (both extremely low and high body mass index). Our analysis is both national and longitudinal, and will allow us to assess both the average individual-level effect of these modifiable risk factors on YHL as well as an estimated aggregate effect on American society among middle-aged and elderly persons.
Materials and Methods
Data
The Health and Retirement Study (HRS) is a national panel study (the same individuals were interviewed at different time points) with an initial sample of more than 12,600 persons (Juster and Suzman 1995). The survey includes information about health behaviors, ill health and disability, medical care usage, and other topics. The baseline survey was an in-home interview conducted in 1992 for the 1931–1941 birth cohort (and their spouses, regardless of age). The respondents were resurveyed in 1994 (wave 2), 1996 (wave 3), 1998 (wave 4), and 2000 (wave 5).
The Asset and Health Dynamics Among the Oldest Old (AHEAD) database is a companion national panel study designed to monitor similar topics in an older age group (Myers, Juster, and Suzman 1997). The initial sample (wave 1: 1993) consisted of 7,447 respondents aged 70 and older (and their spouses, regardless of age) who lived in the community. If the subject or “gatekeeper” (usually a spouse) indicated that the subject was unable to participate in the interview, a proxy respondent was identified and interviewed instead. Persons aged 80+ were oversampled to allow for more precise estimates in this group. The follow-up interviews were conducted by telephone, or, for those ≥80, mostly in person (wave 2: 1995; wave 3: 1998; wave 4: 2000). As in HRS, people of Hispanic origin, African Americans, and Florida residents were also oversampled (http://hrsonline.isr.umich.edu/intro/index.html).
The analysis sample included all subjects who had responded to the self-reported health question (Excellent, Very Good, Good, Fair, or Poor) in all five waves of HRS (n = 6,719) or all four waves of AHEAD (n = 3,766). In addition, for subjects who had one missing value (HRS n = 1,049; AHEAD n = 942), this value was interpolated as the average of the preceding and subsequent values, or, if the missing value was in the first wave, the value at wave 1 was set to be identical to wave 2. Those who died during the study period (HRS n = 555; AHEAD n = 1,301) were also included. Subjects who were lost to follow-up or who had more than one missing value for the self-reported health variable were excluded from the analyses. Consistent with earlier studies using HRS and AHEAD (Østbye et al. 2002; Østbye, Taylor, and Jung 2002), we found that the excluded subjects were somewhat more likely to be younger, male, and to report a slightly better health status at baseline than those included in the analyses.
Deaths
If a respondent had died between two waves, this would usually be reported when contact attempts were made. Furthermore, both the HRS and the AHEAD datasets have been matched, using Social Security numbers, to the National Death Index to confirm date of death.
Outcome Variables: Years of Life and Years of Healthy Life Remaining
Our primary outcome, Years of Healthy Life (YHL) is a longitudinal measure of quality of life. It has been developed and validated by Diehr (Diehr et al. 1998; Diehr et al. 2001; Diehr and Patrick 2001) and combines mortality status with a longitudinal series of measures of self-reported health status.
The health status measure (EVGGFP) was asked consistently and phrased in identical manner in all waves of both HRS and AHEAD: “Next I have some questions about your health. Would you say your health is excellent, very good, good, fair, or poor?” This standard self-reported health question is a simple but well-known measure that has been studied in detail (Gold, Franks, and Erickson 1996), and been found to be strongly predictive of future health events, including death (Idler and Benyamini 1997). Plotting the proportion of people who are “healthy,” that is, in excellent, very good, or good health over time (this proportion represents the mean health status of the population at each point in time), and then estimating the area under the curve, provides a useful longitudinal summary measure of quality of life for the population, that is, the number of healthy years during the period in question.
In addition, using a more sophisticated and innovative coding scheme, we also used a distinct value for each of the six health states as suggested by Diehr, based on her empirical validation of this measure with the Cardiovascular Health Survey data (Diehr et al. 2001; Diehr and Patrick 2001) (by relating the answer to the EVGGPF question at baseline to the probability of being “healthy” [excellent, very good, or good health] two years later). The values assigned to the different health states are 0.95 (excellent), 0.90 (very good), 0.80 (good), 0.30 (fair), 0.15 (poor), 0 (dead), respectively, following Diehr (Diehr et al. 2001). These weights reflect the probability of being healthy (health excellent, very good, or good) next year given a certain health status level (including death) in the current year, and allow for comparison across studies.
We refer to this quantity as YHL rather than as quality-adjusted life years (QALYs), because QALYs are usually based on preference-ratings, and maximizing QALYs can be thought of as maximizing utility-weighted population health. Maximizing YHL will maximize the amount of time the population is “healthy” but this may not agree perfectly with a preference-rated utility (Diehr et al. 2001), since the weighting of years of life is based on responses to the EVGGFP self-reported health question instead of a question specifically designed to more explicitly value the state of life.
For each individual in HRS or AHEAD, the number of healthy life years from baseline until death was estimated by combining two periods. First, for each individual, the years of healthy life lost during the study were calculated by summarizing the area under the curve (generated by plotting the health status over time). Second, YHL lost during the period from the end of the study until death were projected (extracted from tables in Diehr et al. 2001) based on the subject's self-reported health, age, and gender in the last wave of the of the study. The sum of these two time estimates represents the projected number of years of healthy life from baseline to death for an individual and was used as the outcome variable in the models of years of healthy life (YHL). The number of years of healthy life during the study was calculated using both the cruder (healthy = 1 versus not healthy = 0) and the more refined (specific numerical value for each health state) method outlined above.
The number of years alive during the study were calculated. Also, the expected remaining years of life after the end of the study were projected based on the subject's self-reported health, age, and gender in the last wave (also using estimates from Diehr et al. 2001). The sum of these two time periods represents survival from baseline to death and was used as the outcome variable in the models of years of life.
Explanatory Variables
In HRS, current smokers were divided into heavy (a pack or more per day) and light smokers (less than one pack per day). Former smokers were divided into three groups: quit less than 3 years prior, between 3 and 15 years prior, and more than 15 years prior to the baseline interview. In AHEAD, only three categories could be distinguished: current, former, and never smokers.
In HRS, all persons who reported heavy physical activity three or more times per week were classified as doing heavy exercise All persons who reported heavy physical activity one to two times per week, as well as those who reported light physical activity three or more times per week, were classified as doing moderate exercise. The remaining persons were classified as doing light exercise. In the first wave of AHEAD, it was not possible to identify a reasonable measure of exercise. Alcohol consumption was divided into none, light to moderate drinking (up to two drinks/day), and heavy drinking (more than two drinks/day). Self-reported history of drinking problems was coded as a binary variable that indicated if the respondent answered affirmatively to at least one of the following four questions: (1) Ever been told to cut down on their drinking? (2) Ever taken a drink first thing in the morning as an “eye opener”? (3) Ever been told by anyone that their drinking annoyed them? and (4) Ever felt a need to “cut down” on their drinking? Very low Body Mass Index (BMI) (<18.5 kg/m2) and obesity (BMI≥30.0) were contrasted with the ideal and slightly overweight category according to recent guidelines (Strawbridge, Wallhagen, and Shema 2000).
Demographic variables including gender, age group (HRS: 50–54, 55–59, 60–64 at baseline; AHEAD: 70–74, 75–79, 80–84, 85–89 at baseline), race (white versus other [predominantly black]), marital status (married versus not married), and, as a measure of socioeconomic status, education (not completed high school, completed high school, completed college) were controlled for in the analyses.
Analysis
Parallel data elements from the two surveys were used to relate smoking and other explanatory variables, all measured at baseline, to the two outcome variables: years of life remaining and Years of Healthy Life (YHL) remaining. Multivariate linear regression models with years of life remaining and YHL remaining as the dependent variables were developed.
Finally, the number of years of healthy life and years lost to smoking per year for the entire U.S. population were calculated. For each age and gender group, we multiplied the number of individuals in that group in the United States (U.S. Census Bureau 2000) with the proportion of individuals in each smoking category (from HRS and AHEAD), and multiplied this again with the ratio of the number of YHL lost (from Table 2a and 2b) for members of that smoking category over the number of YHL remaining if they had not been smoking (the intercept in Table 2a and 2b). Since we did not have good data for those “falling between” HRS and AHEAD, that is, Americans aged 65–69 at baseline, the values for this age group were interpolated as the average between the 60–64 and the 70–74 age groups. Finally, YHL lost per year in each age/gender/smoking category were then summed to give the number of YHL lost per year to smoking in the U.S. population 50–84. Parallel calculations were performed for total number of years of life lost per year in the total U.S. population.
Table 2a.
Years of Life and Years of Healthy Life Remaining, by Gender and Age Group: HRS, Multivariate Regression Models
| Years of Life Remaining | Years of Healthy Life Remaining | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||||
| Variables | 50–54 | 55–59 | 60–64 | 50–54 | 55–59 | 60–64 | 50–54 | 55–59 | 60–64 | 50–54 | 55–59 | 60–64 |
| Intercept | 24.27 | 19.20 | 15.57 | 28.60 | 23.99 | 18.65 | 17.05 | 11.93 | 10.01 | 19.79 | 15.71 | 11.77 |
| Smokinga | ||||||||||||
| Heavy (1 pack or more) | −2.04 | −3.12 | −3.01 | −1.44 | −2.55 | −1.45 | −2.04 | −2.77 | −3.12 | −1.66 | −2.18 | −0.98 |
| Light (less than 1 pack) | −2.12 | −1.79 | −2.41 | −1.05 | −0.32 | −1.12 | −1.80 | −1.53 | −2.36 | −0.98 | −0.42 | −1.33 |
| Former (quit less than 3 years) | −1.41 | −2.14 | −3.32 | −2.44 | −1.43 | −5.93 | −1.00 | −2.28 | −3.55 | −2.25 | −1.44 | −4.96 |
| Former (quit 3–15 years) | −0.38 | −0.84 | −0.78 | −0.83 | −0.57 | −0.62 | −0.03 | −0.76 | −0.86 | −1.04 | −0.64 | −0.62 |
| Former (quit more than 15 years) | 0.06 | −0.75 | −0.21 | −0.31 | −0.33 | 0.64 | 0.11 | −0.40 | −0.18 | 0.21 | −0.34 | 0.50 |
| Exerciseb | ||||||||||||
| Light | 0.46 | 2.26 | 2.64 | 2.33 | 2.73 | 3.61 | 0.69 | 2.76 | 2.52 | 2.37 | 3.10 | 2.98 |
| Moderate | 0.45 | 3.27 | 3.26 | 2.64 | 2.49 | 3.95 | 0.89 | 3.72 | 3.23 | 2.74 | 3.10 | 3.86 |
| Heavy | 1.05 | 4.24 | 4.20 | 2.75 | 3.12 | 4.64 | 1.97 | 4.78 | 4.29 | 3.37 | 3.87 | 4.93 |
| Body Mass Index (BMI)c | ||||||||||||
| BMI less than 18.5 | −6.66 | −10.41 | −11.57 | −3.14 | −4.71 | −0.72 | −6.19 | −6.53 | −7.88 | −1.66 | −3.09 | −0.83 |
| BMI 30 or greater | −0.51 | −0.42 | 0.37 | −0.43 | −1.10 | 0.31 | −1.08 | −0.90 | −0.26 | −0.98 | −1.76 | −0.32 |
| Alcohol Consumptiond | ||||||||||||
| Light (up to 2 drinks/day) | 0.54 | 0.34 | 0.63 | 0.41 | 0.60 | 0.73 | 0.85 | 0.52 | 0.78 | 0.90 | 0.68 | 0.82 |
| Heavy (more than 2 drinks/day) | 0.22 | 0.58 | −0.64 | −1.25 | 0.58 | 2.51 | 0.37 | 0.43 | 0.12 | −0.45 | 0.59 | 1.83 |
| Past drinking problem | −0.17 | −1.03 | −0.79 | −0.98 | −1.54 | −1.00 | −0.54 | −1.00 | −0.60 | −1.13 | −1.42 | −1.56 |
| Racee | ||||||||||||
| White | 0.83 | 1.35 | 0.64 | 0.21 | 1.11 | 1.82 | 1.31 | 1.53 | 1.11 | 0.78 | 1.13 | 1.82 |
| Marital Statusf | ||||||||||||
| Married | 1.55 | 0.77 | 0.54 | 0.52 | 1.13 | 0.14 | 1.54 | 0.97 | 0.32 | 0.88 | 1.32 | 0.40 |
| Educationg | ||||||||||||
| Less than high school | −0.85 | −0.28 | −0.32 | −0.57 | −0.71 | −1.18 | −1.88 | −1.06 | −0.92 | −2.13 | −2.23 | −2.34 |
| College | 0.24 | 0.54 | 0.10 | 0.72 | 0.65 | −0.14 | 0.72 | 1.14 | 0.93 | 1.01 | 1.21 | 0.34 |
| Adjusted R2 | 0.075 | 0.123 | 0.117 | 0.069 | 0.103 | 0.115 | 0.175 | 0.192 | 0.168 | 0.196 | 0.234 | 0.225 |
| n | 1,440 | 1,675 | 1,078 | 1,753 | 1,931 | 833 | 1,394 | 1,612 | 1,047 | 1,815 | 1,893 | 814 |
Reference category:
Never;
Sedentary;
BMI 18.5–30;
Never;
Others;
Others;
Completed high school.
Numbers are coefficients representing years; bold indicates statistical significance at p<.05.
Table 2b.
Years of Life and Years of Healthy Life Remaining, by Gender and Age Group: AHEAD, Multivariate Regression Models
| Years of Life Remaining | Years of Healthy Life Remaining | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||||
| Variables | 70–74 | 75–79 | 80–84 | 70–74 | 75–79 | 80–84 | 70–74 | 75–79 | 80–84 | 70–74 | 75–79 | 80–84 |
| Intercept | 11.06 | 8.75 | 6.33 | 14.59 | 11.49 | 8.99 | 7.15 | 5.47 | 3.56 | 9.14 | 7.45 | 5.45 |
| Smokinga | ||||||||||||
| Current | −2.12 | −1.35 | −1.01 | −1.63 | −0.70 | −1.11 | −2.11 | −1.52 | −0.53 | −1.63 | −1.18 | −0.61 |
| Former | −1.18 | −0.68 | −0.79 | −0.57 | −0.61 | −0.66 | −1.26 | −0.64 | −0.15 | −0.39 | −0.81 | −0.57 |
| Body Mass Index (BMI)b | ||||||||||||
| BMI less than 18.5 | −1.20 | −3.82 | −3.45 | −2.46 | −2.83 | −2.08 | −1.27 | −2.33 | −2.73 | −1.53 | −2.20 | −1.39 |
| BMI 30 or greater | 0.72 | 0.75 | 1.33 | −1.17 | 0.79 | 0.73 | 0.36 | 0.44 | 0.25 | −1.24 | 0.09 | 0.16 |
| Alcohol Consumptionc | ||||||||||||
| Light (up to 2 drinks/day) | 1.84 | 1.58 | 1.81 | 0.30 | 0.50 | 0.54 | 1.77 | 1.44 | 1.36 | 0.87 | 0.84 | 0.81 |
| Heavy (more than 2 drinks/day) | 2.28 | 0.96 | 1.44 | −0.05 | 1.57 | 1.95 | 2.68 | 0.67 | 1.13 | 0.59 | 1.99 | 1.94 |
| Past drinking problem | 0.14 | −0.11 | 0.40 | −0.45 | −1.83 | 0.08 | 0.05 | −0.40 | −0.18 | −0.46 | −1.15 | 0.36 |
| Raced | ||||||||||||
| White | 0.04 | −0.33 | 0.39 | 0.99 | 0.56 | 0.34 | 0.48 | 0.06 | 0.45 | 1.09 | 0.52 | 0.34 |
| Marital Statuse | ||||||||||||
| Married | 0.81 | 0.97 | 0.71 | 0.40 | 0.15 | 0.67 | 0.50 | 0.44 | 0.23 | 0.28 | −0.14 | 0.10 |
| Educationf | ||||||||||||
| Less than high school | −0.14 | −1.03 | −0.20 | −1.52 | −0.87 | −0.05 | −0.71 | −0.83 | −0.40 | −1.69 | −1.26 | −0.41 |
| College | 1.43 | −0.30 | 0.37 | 0.87 | 0.75 | 0.17 | 1.59 | 0.24 | 1.04 | 1.02 | 0.80 | −0.04 |
| Adjusted R2 | 0.062 | 0.056 | 0.070 | 0.063 | 0.045 | 0.012 | 0.123 | 0.076 | 0.094 | 0.113 | 0.084 | 0.015 |
| n | 941 | 643 | 451 | 1389 | 1022 | 715 | 922 | 632 | 437 | 1363 | 1013 | 699 |
Reference category:
Never;
Sedentary;
BMI 18.5–30;
Never;
Others;
Completed high school
Bold numbers: p<.05.
SAS version 8.0 was used for data management and statistical analysis.
Results
More than a quarter of HRS respondents were smokers, while current smoking was less common among the AHEAD respondents (age 70+), with only 11 percent reporting smoking as of wave 1. Forty-five percent of respondents reported never smoking, while a nearly identical percentage reported being a former smoker (Table 1).
Table 1.
Characteristics of the Health and Retirement Study (HRS) and Asset and Health Dynamics among the Oldest Old (AHEAD) Samples Used in Multivariate Models
| HRS | AHEAD | |||
|---|---|---|---|---|
| Characteristic | n | Percentage | n | Percentage |
| Smoking | ||||
| Heavy (≥1 pack/day) | 1,646 | 16.8 | ||
| Light (<1 pack/day) | 975 | 10.0 | ||
| Former (quit <3 years) | 468 | 4.8 | ||
| Former (quit 3–15 years) | 1,548 | 15.8 | ||
| Former (quit>15 years) | 1,481 | 15.1 | ||
| Current | 702 | 11.0 | ||
| Former | 2,821 | 44.1 | ||
| Never | 3,681 | 37.6 | 2,881 | 45.0 |
| Exercise | ||||
| Sedentary | 931 | 9.5 | ||
| Light | 1,556 | 15.9 | ||
| Moderate | 5,468 | 55.8 | ||
| Heavy | 1,844 | 18.8 | ||
| Body Mass Index (BMI) | ||||
| <18.5 | 178 | 1.8 | 278 | 4.3 |
| 18.5–29.9 | 7,375 | 75.0 | 5,225 | 81.6 |
| ≥30 | 2,277 | 23.2 | 901 | 14.1 |
| Alcohol Consumption | ||||
| None | 3,238 | 33.0 | 3,017 | 47.1 |
| Light (≤2 drinks/day) | 5,364 | 54.7 | 2,787 | 43.5 |
| Heavy (>2 drinks/day) | 495 | 5.1 | 152 | 2.4 |
| Past drinking problem | 702 | 7.2 | 448 | 7.0 |
| Gender/Age Group | ||||
| Male | ||||
| 50–54 | 1,596 | 38.0 | ||
| 55–59 | 1,497 | 35.7 | ||
| 60–64 | 1,103 | 26.3 | ||
| 70–74 | 1,164 | 45.5 | ||
| 75–79 | 820 | 32.1 | ||
| 80–84 | 573 | 22.4 | ||
| Female | ||||
| 50–54 | 2,272 | 40.6 | ||
| 55–59 | 2,311 | 41.3 | ||
| 60–64 | 1,020 | 18.2 | ||
| 70–74 | 1,629 | 42.3 | ||
| 75–79 | 1,264 | 32.9 | ||
| 80–84 | 954 | 24.8 | ||
| Race | ||||
| White | 7,794 | 79.3 | 5,390 | 84.2 |
| Other | 2,036 | 20.7 | 1,014 | 15.8 |
| Marital Status | ||||
| Married | 7,491 | 76.2 | 3,441 | 53.7 |
| Not married | 2,339 | 23.8 | 2,963 | 46.3 |
| Education | ||||
| Less than high school | 2,891 | 29.4 | 2,753 | 43.0 |
| Completed high school | 5,475 | 55.7 | 2,906 | 38.4 |
| College | 1,464 | 14.9 | 745 | 11.6 |
Nearly half of the elderly (AHEAD) respondents did not consume any alcohol and 43.5 percent were moderate drinkers, while only 2.4 percent reported current heavy drinking. Seven percent reported past drinking problems. Among the HRS sample, only one-third did not drink alcohol, over half of the respondents drank moderately, and nearly twice as many (compared to AHEAD) reported being a heavy drinker at baseline (5.1 percent); a similar percentage (7.2 percent) reported a history of drinking problems. The younger sample had a higher percentage of respondents (23.2 percent) who were obese (BMI of 30+) compared to the older sample (14.1 percent), and very low BMI (<18.5) was more common among the older respondents (4.3 percent AHEAD versus 1.8 percent HRS). Among the HRS sample, 1 in 10 respondents were sedentary, while 18.8 percent reported heavy exercise at baseline. The percentage of racial minorities was slightly lower in AHEAD (15.8 percent) compared to HRS (20.7 percent), but the proportion of respondents with less than a high school education was much higher in AHEAD (43.0 percent) than in HRS (29.4 percent).
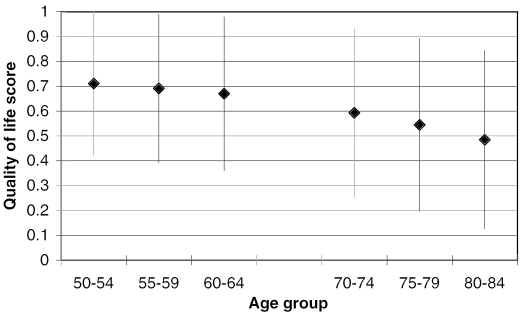
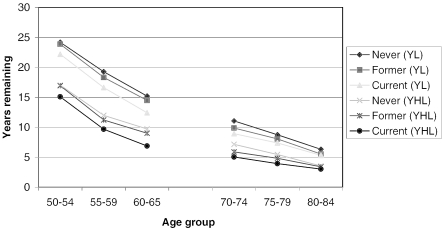
The average quality of life score declined by age, somewhat more rapidly among men (Figure 1a) than among women (Figure 1b), reflecting the higher mortality levels among men. The number of years remaining, as well as the number of healthy years remaining, was lower for men than for women across all age groups, and individuals in their fifties (HRS respondents) had a predictably larger number of years of life and years of healthy life remaining (Figures 2a and 2b). For bother genders, never smokers had the most years and years of healthy life remaining, and current smokers had the lowest values, with former smokers falling between the other two.
Figure 1a.
Means and SD's of Quality of Life (Based on EVGGFP Question and Mortality Status) by Age Group (Stacked Values), HRS and AHEAD; Men.
Figure 1b.
Means and SD's of Quality of Life (Based on EVGGFP Question and Mortality Status) by Age Group (Stacked Values), HRS and AHEAD; Women
Figure 2a.
Years of Life and Years of Healthy Life Remaining by Smoking Status, HRS and AHEAD; Men
Figure 2b.
Years of Life and Years of Healthy Life Remaining by Smoking Status, HRS and AHEAD; Women
Smoking had a clear dose–response relationship with both years of life remaining and years of healthy life remaining among middle-aged respondents (Table 2a). Among heavy smokers in the age 50–54 group, males had just over two years fewer expected years of life remaining, while females had 1.44 fewer years, compared to never smokers, net of the other modifiable risk factors and other control variables. In the same age group, male smokers also had just over two years less healthy life remaining compared to never smokers, and female smokers had 1.66 fewer such years. Across the age groups in the HRS, one to three years of healthy life was lost for heavy smokers relative to never smokers. Light smokers lose one to two years of healthy life in the younger age groups. These trends also held true for older individuals (Table 2b), but the total number of years of life lost and years of healthy life lost was smaller. However, even among the oldest men and women (age 80–84), the point estimate for years of healthy life lost to smoking was more than one-half a year, though this was not statistically significant.
Smoking cessation yielded improvements in both expected years and healthy years remaining only after an individual survived 15+ years past cessation. In HRS, those who quit less than 3 years before baseline in some cases experienced a greater loss of years and healthy years relative to never smokers than did continuing light smokers, likely due to recent quitters who had quit because of health problems. Those in the youngest age groups who quit between 3 and 15 years before baseline still lost up to a year of healthy life, but the number of years of healthy life lost among those who quit more than 15 years ago was similar to that of the never smokers. In AHEAD, former smokers lost up to a year of healthy life.
The effect of other modifiable risk factors on YHL were plausible given known linkages with mortality. Light alcohol consumption was associated with an increase in years of healthy life left for the older sample individuals, a consistent finding for both males and females and across age groups (70–74, 75–79, and 80+). Above age 80, there was a similar increase in years of life left for men, but not for women. The relationship was not consistent for heavy alcohol consumption; past drinking problems were not generally associated with years of life left or years of healthy life left.
Individuals with very low BMI had significantly fewer years of healthy life remaining, particularly among males in HRS, where 6–8 years of healthy life were lost (these estimates were based on a relatively small number of individuals). In AHEAD, 1.4–2.7 years of healthy life were lost by persons with a very low BMI. Obesity had a fairly weak effect on both years of life and years of healthy life, but those coefficients that are statistically significant show an expected loss of years and healthy years of a magnitude less than 2 years among middle-aged persons. Among the elderly, however, obesity did not typically have an effect. The effect of exercise in the younger age groups was very strong in both genders, an increase of more than 4 healthy years for heavy exercise in several groups. Furthermore, there was a clear dose–response relationship between level of exercise and years of life and years of healthy life remaining.
White respondents had a larger number of healthy years remaining than African American respondents, over one year in HRS, less than one year in AHEAD. Marital status provided a similar advantage: among the oldest age groups, men gained more healthy years than women from being married. There was also a strong dose response relationship between level of education and healthy years remaining.
The regression models with the YHL outcome measure based on the more crude calculation of quality of life over time, as outlined above, gave very similar results to those in Tables 2a and 2b, and are not shown here.
Overall (Table 3), an estimated 3.1 million years of healthy life were lost per year among U.S. men aged 50–84, while 1.6 million YHL were lost to U.S. women in the same age group.
Table 3.
Total Number of Years of Life and Healthy Life Lost to Smoking Annually, Total U.S. Population Ages 50–84
| Men (Millions) | Women (Millions) | Total | |
|---|---|---|---|
| Years of Life Lost | 2.4 | 1.2 | 3.6 |
| Years of Healthy Life Lost | 3.1 | 1.6 | 4.7 |
| N (Total U.S. Pop. Ages 50–84) | 33.4 | 39.2 | 72.6 |
Discussion
Quitting smoking and increasing exercise are the lifestyle interventions most likely to improve the overall health of the many middle-aged and older Americans who continue to smoke. The benefits of smoking cessation are substantial, with the number of healthy years remaining among former smokers returning to that of never smokers 15 years after quitting. Simulation studies (Tengs, Osgood, and Lin 2001) suggest smoking cessation yields the biggest increase in QALYs than any other intervention among middle-aged persons. In efforts to encourage smoking cessation, the clear message that cessation will provide additional years of healthy life should complement information regarding mortality benefits. This agrees with the general direction of the literature that as the population ages, healthy lifespan is also increasing, and it is not the case that extra years are increasingly being lived in a poor quality of life.
Overall, the number of healthy years remaining is 7–8 years less than the number of years remaining among the middle aged, and 3–5 years less among the old. These differences represent the average number of years lived in a state of less than good health. In our analyses, smoking has a consistent and strong relationship with both years of life lost and years of healthy life lost in middle-aged and old men and women, demonstrating both the morbidity and mortality effects of smoking. Earlier studies have found lifetime life years lost to smoking to be as much as 14 years for smokers (Centers for Disease Control and Prevention 2002). Our estimates are shorter since they reflect the reduced life (and healthy life) expectancy of someone who already has lived until the baseline of our study. Our results are more similar to those found in Finland (Kiiskinen et al. 2002): the number of work years lost to smoking in that study and the number of healthy years lost to smoking in our study correspond well to each other.
It takes a long time before the risk of former smokers returns to that of never smokers, but from a health promotion perspective it is very encouraging that, among those who quit smoking more than 15 years ago, the risk is about the same as that of never smokers. This finding is consistent with a review by LaCroix (LaCroix and Omenn 1992), which concluded that, although the time from quitting until the risk of mortality approached those of never smokers varied substantially by type of illness, the overall risk of death approached that of never smokers after 15 to 20 years of abstinence, and that, at the population level, the prospects are excellent that smoking cessation even after the age of 65 will extend both the number of years of life and the quality of life.
In spite of the fewer number of healthy years available, male smokers still lose up to a year more of healthy life to smoking than women, so both in absolute and, especially, in relative terms, smoking appears to have a more detrimental effect on men than in women (Tengs, Osgood, and Lin 2001). Alcohol consumption is generally beneficial to extending life, consistent with other studies (Mukamal et al. 2003; Reynolds et al. 2003), though it is not as powerful a predictor of increased years of healthy life as not smoking. However, persons with a history of problem drinking lose years of healthy life, illustrating why there is often an ambivalence among public health experts toward recommending alcohol consumption on the basis of extension of life, or as we have shown, healthy life. This ambivalence stems from the fact that a certain proportion of the population who use alcohol abuse it, resulting in a reduction of life and healthy life.
Having very low BMI is worse than having a larger BMI after controlling for other modifiable risk factors. These findings may seem odd at first glance. However, it is likely that the observed strong negative effect of being underweight is a marker of early and incipient disease rather than an effect of underweight per se. The changing effect of obesity, from having an adverse effect on years and healthy years among the middle aged to having a slightly positive effect among the old, is consistent with our own work (Østbye et al. 2002; Østbye, Taylor, and Jung 2002) as well as that of others (Stevens et al. 1998; Campbell et al. 1990). It is likely that these age differences represent a healthy survivor effect.
The overall estimated life expectancies in our analyses correspond with recent U.S. life tables (National Center for Health Statistics 2002), which strengthens the validity of our findings. Our estimates of life expectancy for nonsmokers (Tables 2a, 2b and Figures 2a, 2b) are somewhat lower than the average life expectancy for the nonsmoking U.S. population at a given age. This is likely due to the fact that our estimate is based on the intercept of the regression model, which in our model represents a person less healthy (i.e., sedentary, never drinker, African American, unmarried, completed high school) than the average life expectancy at that specific age, which is what is found in a standard life table. This discrepancy was strongly influenced by physical activity, and, therefore, in AHEAD, where a good exercise measure was not available, the life expectancy of our nonsmokers is very similar to that of the U.S. population in the same age groups. We observed a strong dose–response relationship between the amount of exercise at baseline and the subsequent number of both years and healthy life years. It should be noted that since it is not possible to control for early or incipient illness at baseline, the causal pathway between quality of life and health on the one hand and physical activity on the other cannot fully be determined in observational studies, even in a longitudinal study like ours. The apparent increase in the effect of exercise with age is likely due to a combination of the fact that exercise is more likely in individuals without incipient illness or disability and the beneficial effect of exercise per se. However, the strong effect of physical activity on healthy years of life observed in our study points toward the importance not only of not smoking, but also of encouraging physical activity and exercise among elderly Americans. A number of smaller clinical intervention studies show that it is never too late to start an exercise program (Jette et al. 1999; Stewart et al. 1998).
Strengths and Limitations
Our study is based on a large and nationally representative sample of elderly and near elderly Americans. In contrast to other studies, which have considered the effect of smoking and other modifiable risk factors on quality of life, in this dataset, individual-level information on both smoking and health status, as well as on important confounding variables, are available. Furthermore, our estimates are grounded in long periods of follow-up with a series of quality of life observations for each individual, supplemented with an extrapolation of healthy life remaining beyond the study (based on the status of the individual at the last observation). This longitudinal approach contrasts with other studies, which have been mostly based on synthetic cohorts generated from cross sectional data.
It should be noted that the wording of the health status question is not asked “relative to others your age.” We believe that the current wording represents a more absolute measure of health status and should be more sensitive to the long-term effects of different risk factors.
In general, there is a difference between years of healthy life lost among those with a risk factor and years of healthy life lost due to the same risk factor, the latter implying direct causality. When looking for causal relationships in our analyses, we believe this distinction is most important to make for BMI, less so for exercise and for alcohol use, while for tobacco smoking the distinction is likely least important.
Comparing the coefficients for smoking and the other risk factors to each other, as well as comparing those relating to remaining years of life to those relating to remaining YHL directly, must be done with caution since, for example, it is possible to lose two YHL by being dead for two years or by having a health status of 0.8 for 10 years.
It is possible that, given age, gender, and self-reported health status, smokers have a poorer prognosis than nonsmokers. If so, the portion of our outcome variables projected from the last observation are underestimates of the actual life expectancy and healthy life expectancy from our last observation for those with these risk factors. That would imply that our results are underestimates of the impact of smoking on healthy years of life lost and on years of life lost. Since the participants in the surveys were living in the community at baseline (elderly who were institutionalized during the study are included), the calculations do not fully capture the extent to which institutionalized elderly are more or less likely to smoke than those in the community.
It has been shown that burden of disease and burden of smoking and other risk factors on quality of life estimates are to some extent measure dependent. The QALYs, HALYs, DALYs, and YHL measures are likely to provide somewhat different values (Gold, Franks, and Erickson 1996). The quality of life question (EVGGFP), on which the longitudinal YHL measure is based, is easy for the respondent to understand, simple to administer and collect, and has a low respondent burden. Also, although it strictly speaking is not a utility, it is much easier to administer for the interviewer and much easier to understand for the respondent than more theoretical and complicated time-tradeoff or standard gamble measures (Drummond et al. 1999). Since the EVGGFP measure is widely used in longitudinal health surveys, it is simple to calculate the longitudinal YHL measure used in the analyses above. We believe that more comparative studies using YHL as well as further investigations into its methodological properties will be useful. For comparative studies, it is preferable to use the standardized values for the different quality of life states as well as the projected life and healthy life expectancies suggested by Diehr (Diehr et al. 2001), but it is of interest to evaluate these values based on other, large representative longitudinal surveys.
Conclusion
Clinical and public health messages emphasizing improvements in quality of life and health may be more salient to many smokers than mortality-based messages. Interventions aiming to reduce smoking have the potential to greatly increase the number of healthy years among middle-aged and old Americans.
Acknowledgments
Thanks to Lynn van Scoyoc for her assistance with data management and analysis and to Katrina Krause, M.A., for her editing of this manuscript.
Footnotes
This research was funded by grants 1RO1-AG-15868-03 from the National Institute on Aging, and 1RO1-AA-12162-03, from the National Institute of Alcoholism and Alcohol Abuse.
References
- Campbell A J, Spears G F, Brown J S, Busby W J, Borrie M J. “Anthropometric Measurements as Predictors of Mortality in a Community Population Aged 70 Years and Over.”. Age and Ageing. 1990;19(2):131–5. doi: 10.1093/ageing/19.2.131. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. “Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Economic Costs—United States, 1995–1999.”. Morbidity and Mortality Weekly Report. 2002;51(14):300–3. [PubMed] [Google Scholar]
- Diehr P, Patrick D L. “Probabilities of Transition among Health States for Older Adults.”. Quality of Life Research. 2001;10(5):431–42. doi: 10.1023/a:1012566130639. [DOI] [PubMed] [Google Scholar]
- Diehr P, Patrick D L, Bild D E, Burke G L, Williamson J D. “Predicting Future Years of Healthy Life for Older Adults.”. Journal of Clinical Epidemiology. 1998;51(4):343–53. doi: 10.1016/s0895-4356(97)00298-9. [DOI] [PubMed] [Google Scholar]
- Diehr P, Patrick D L, Spertus J, Kiefe C I, McDonell M, Fihn S D. “Transforming Self-Rated Health and the SF-36 Scales to Include Death and Improve Interpretability.”. Medical Care. 2001;39(7):670–80. doi: 10.1097/00005650-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Drummond M F, O'Brian B, Stoddart G L, Torrance G W. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1999. [Google Scholar]
- Gold M, Franks P, Erickson P. “Assessing the Health of The Nation: The Predictive Validity of a Preference Based Measure and Self-rated Health.”. Medical Care. 1996;34(2):163–77. doi: 10.1097/00005650-199602000-00008. [DOI] [PubMed] [Google Scholar]
- Gold M, Stevenson D, Fryback G. “HALYs and QALYs and DALYs, Oh My: Similarities and Differences in Summary Measures of Population Health.”. Annual Review of Public Health. 2002;23:115–34. doi: 10.1146/annurev.publhealth.23.100901.140513. [DOI] [PubMed] [Google Scholar]
- Idler E L, Benyamini Y. “Self-rated Health and Mortality: A Review of Twenty-Seven Community Studies.”. Journal of Health and Social Behavior. 1997;38(1):21–37. [PubMed] [Google Scholar]
- Jette A M, Lachman M, Giorgetti M M, Assmann S F, Harris B A, Levenson C, Wernick M, Krebs D. “Exercise: It Is Never Too Late: The Strong-for-Life Program.”. American Journal of Public Health. 1999;89(1):66–72. doi: 10.2105/ajph.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster F, Suzman R. “An Overview of the Health and Retirement Study.”. Journal of Human Resources. 1995;30(5):S7–56. [Google Scholar]
- Kiiskinen U, Vartainen E, Puska P, Pekurinen M. “Smoking Related Costs among 25–59 Year-Old Males in a 19-Years Individual Follow-up.”. European Journal of Public Health. 2002;12(2):145–51. doi: 10.1093/eurpub/12.2.145. [DOI] [PubMed] [Google Scholar]
- LaCroix A Z, Omenn G S. “Older Adults and Smoking.”. Clinical Geriatric Medicine. 1992;8(1):69–87. [PubMed] [Google Scholar]
- Last J M. A Dictionary of Epidemiology. Oxford: Oxford University Press; 2001. [Google Scholar]
- Mathers C D, Vos E T, Stevenson C E, Begg S J. “The Australian Burden of Disease Study: Measuring the Loss of Health from Diseases, Injuries and Risk Factors.”. Medical Journal of Australia. 2000;172(12):592–6. doi: 10.5694/j.1326-5377.2000.tb124125.x. [DOI] [PubMed] [Google Scholar]
- Mathers C D, Vos E T, Stevenson C E, Begg S J. “The Burden of Disease and Injury in Australia.”. Bulletin of the World Health Organization. 2001;79(11):1076–84. [PMC free article] [PubMed] [Google Scholar]
- McHorney C, Ware J, Lu J, Sherbourne C. “The MOS 36-Item Short Form Health Survey (SF-36): II. Psychometric and Clinical Tests of Validity in Measuring Physical and Mental Health Constructs.”. Medical Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Melse J M, Essink-Bot M, Kramers P G N, Hoeymans N. “A National Burden of Disease Calculation: Dutch Disability-Adjusted Life-Years.”. American Journal of Public Health. 2000;90(8):1241–7. doi: 10.2105/ajph.90.8.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal K J, Conigrave K M, Mittleman M A, Camargo C A, Jr., Stampfer M J, Willett W C, Rimm E B. “Roles of Drinking Pattern and Type of Alcohol Consumed in Coronary Heart Disease in Men.”. New England Journal of Medicine. 2003;348(2):109–18. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- Murray C J L, Lopez A D. “Global Mortality, Disability, and the Contribution of Risk Factors: Global Burden of Disease Study.”. Lancet. 1997;349(9063):1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Myers G C, Juster F T, Suzman R M. “Asset and Health Dynamics among the Oldest Old (AHEAD): Initial Results from the Longitudinal Study. Introduction.”. Journal of Gerontology Series B: Psychology and Social Sciences. 1997;52(5):v–viii. [PubMed] [Google Scholar]
- National Center for Health Statistics. “Life Table for the Total Population: United States, 1999.”. National Vital Statistics Report. 2002;50(6):7–8. [Google Scholar]
- Østbye T, Taylor D H, Jr., Krause K M, Van Scoyoc L. “The Role of Smoking and Other Modifiable Lifestyle Risk Factors in Maintaining and Restoring Lower Body Mobility in Middle-aged and Older Americans: Results from the HRS and AHEAD.”. Journal of the American Geriatrics Society. 2002;50(4):691–9. doi: 10.1046/j.1532-5415.2002.50164.x. [DOI] [PubMed] [Google Scholar]
- Østbye T, Taylor D H, Jr., Jung S H. “A Longitudinal Study of the Effects of Tobacco Smoking and Other Modifiable Risk Factors on Ill Health in Middle-aged and Old Americans: Results from the Health and Retirement Study and Asset and Health Dynamics among the Oldest Old Survey.”. Preventive Medicine. 2002;34(3):334–45. doi: 10.1006/pmed.2001.0991. [DOI] [PubMed] [Google Scholar]
- Parkatti T, Deeg D J, Bosscher R J, Launer L L. “Physical Activity and Self-rated Health among 55 to 89 Year Old Dutch People.”. Journal of Aging and Health. 1998;10(3):311–26. doi: 10.1177/089826439801000303. [DOI] [PubMed] [Google Scholar]
- Reynolds K, Lewis L B, Nolen J D L, Kinney G L, Sathya B, He J. “Alcohol Consumption and Risk of Stroke: A Meta-analysis.”. Journal of the American Medical Association. 2003;289(5):579–88. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- Ruuskanen J M, Ruoppila I. “Physical Activity and Psychological Well-being among People Aged 65 to 84 Years.”. Age and Ageing. 1995;24(4):292–6. doi: 10.1093/ageing/24.4.292. [DOI] [PubMed] [Google Scholar]
- Sloan F, Smith V K, Taylor D H., Jr . The Smoking Puzzle: Information, Risk Perception, and Choice. Cambridge, MA: Harvard University Press; 2003. [Google Scholar]
- Stevens J, Cai J, Pamuk E R, Williamson D F, Thun M J, Wood J L. “The Effect of Age on the Association between Body-Mass Index and Mortality.”. New England Journal of Medicine. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- Stewart A L, Mills K M, Sepsis P G, King A C, McLellan B Y, Roitz K, Ritter P L. “Evaluation of CHAMPS, a Physical Activity Promotion Program for Older Adults.”. Annals of Behavioral Medicine. 1998;19(4):353–61. doi: 10.1007/BF02895154. [DOI] [PubMed] [Google Scholar]
- Strawbridge W J, Wallhagen M I, Shema S J. “New NHLBI Clinical Guidelines for Obesity and Overweight: Will They Promote Health?”. American Journal of Public Health. 2000;90(3):340–3. doi: 10.2105/ajph.90.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, Wells K B. “Does Obesity Contribute as Much to Morbidity as Poverty or Smoking?”. Public Health. 2001;115(3):229–35. doi: 10.1038/sj/ph/1900764. [DOI] [PubMed] [Google Scholar]
- Taylor D H, Jr., Hasselblad V, Henley S J, Thun M J, Sloan F A. “Benefits of Smoking Cessation for Longevity.”. American Journal of Public Health. 2002;92(6):990–6. doi: 10.2105/ajph.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengs T O, Osgood N D, Lin T H. “Public Health Impact of Changes in Smoking Behavior: Results from the Tobacco Policy Model.”. Medical Care. 2001;39(10):1131–41. doi: 10.1097/00005650-200110000-00010. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. “Census 2000 Summary File 1 (SF 1) 100-Percent Data: All States, Age Groups and Sex.”. 2000. Available at http://factfinder.census.gov/servlet/BasicFactsServlet?
- U.S. Department of Health and Human Services. “The Surgeon General's 1990 Report on the Health Benefits of Smoking Cessation. Executive Summary.”. MMWR Recommended Reports. 1990;39(RR-12):i–xv. 1–12. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Washington, DC: U.S. Department of Health and Human Services; 2000. “Tenth Special Report to the U.S. Congress on Alcohol and Health.”. [Google Scholar]