Abstract
Objective
To determine factors affecting compliance with guidelines for annual eye examinations for persons diagnosed with diabetes mellitus (DM) or age-related macular degeneration (ARMD).
Data Sources/Study Setting
Nationally representative, longitudinal sample of individuals 65+ drawn from the National Long-Term Care Survey (NLTCS) with linked Medicare claims records from 1991 to 1999.
Study Design
Medicare beneficiaries were followed from 1991 to 1999, unless mortality intervened. All claims data were analyzed for presence of ICD-9 codes indicating diagnosis of DM or ARMD and the performance of eye exams. The dependent variable was a binary indicator for whether a person had an eye exam or not during a 15-month period. Independent variables for demographics, living conditions, supplemental insurance, income, and other factors affecting the marginal cost and benefit of an eye exam were assessed to determine reasons for noncompliance.
Data Collection/Extraction Methods
Panel data were created from claims files, 1991–1999, merged with data from the NLTCS.
Principal Findings
The probability of having an exam reflected perceived benefits, which vary by patient characteristics (e.g., education, no dementia), and factors associated with the ease of visit. African Americans were much less likely to be examined than were whites.
Conclusions
Having an exam reflects multiple factors. However, much of the variation in the probability of an exam remained unexplained as were reasons for the racial differences in use.
Keywords: Practice guidelines, compliance, eye care, diabetes mellitus, age-related macular degeneration
National practice guidelines specify recommended diagnostic and treatment patterns for individuals at risk for particular conditions. Optimal care assumes adherence to guidelines. Yet, for a variety of conditions, compliance tends to be far from complete (Brenes and Paskett 2000; Cheng, Kalis, and Feifer 2001; Erhardt 1999; Hilber et al. 2000; Hsia et al. 2002; Lawler and Viviani 1997; Pate et al. 2002).
Well-established guidelines exist for most common eye diseases. The American Academy of Ophthalmology (AAO) and American Diabetes Association (ADA) recommend that individuals with diabetes should be screened annually for diabetic retinopathy (DR) (American Academy of Ophthalmology 1998; American Diabetes Association 2002), although other guidelines recently allow exams every two years in specific situations. The AAO also recommends annual visits (at a minimum) for those diagnosed with age-related macular degeneration (ARMD) (2000, 2001). Since treatment options were more limited for ARMD in the 1990s, the benefit of timely eye exams was lower, providing an interesting contrast to diabetes (American Academy of Ophthalmology 2001).
In the national longitudinal data on Medicare beneficiaries used for this study, compliance was far below levels specified in guidelines. Only 25 percent of persons with DM received an exam for each 15-month consecutive period spanning 1991–1999. For ARMD, the corresponding percentage was 41–70, depending on the ARMD form. To better understand why this occurs, we analyzed patient demographic health-related and system-related factors from the NLTCS Medicare sample. Because effective therapeutic interventions were far better for treating ocular complications of diabetes than for ARMD, this provided a unique opportunity to empirically assess the potential role of effective therapy availability in compliance behavior.
Conceptual Framework
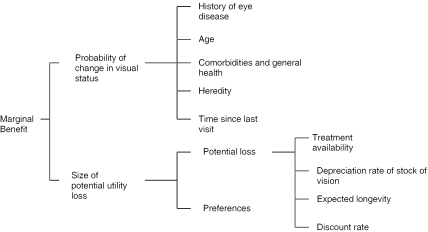
The purpose of regular visual examinations is to monitor disease onset and progression so that timely interventions can be applied to prevent or at least reduce vision loss. In an economic framework, in any period, an individual visits an eye care provider if the expected marginal benefit of the visit in terms of better maintenance of vision is greater than or equal to the visit's marginal cost. The expected marginal benefit of a visit depends on the probability of a change in visual status, and the size of utility loss due to lack of timely application of the intervention, discounted to reflect the person's rate of time preference (Figure 1).
Figure 1.
Expected Marginal Benefit of a Visit
The probability of detecting a change in visual status is a function of heredity (e.g., parents' history of eye diseases), the patient's history of eye disease, age, general health (e.g., control of blood glucose for diabetes), and time since the last eye exam. Many of these factors are known to the patient and provider, but not observed by the researcher. Size of potential utility loss depends on the magnitude of the loss and patient preferences, including preferences for good vision and risk preferences. Potential loss depends on (1) the effectiveness of the intervention, (2) the rate at which vision is expected to decline—if the disease appears likely to progress rapidly, the individual will be more likely to demand a visit because the cost of having an undetected illness or disease progression is greater, (3) the duration of the expected benefit from better maintenance of vision, and (4) the discount rate. The marginal utility of good vision reflects the marginal utility of engaging in activities requiring good vision (e.g., reading).
The full cost of the visit depends on the monetary price of the visit, the time price of the visit, and other associated costs (Phelps 2002). A higher price should lower the probability of an eye exam. Time prices should depend in part on distance from the individual's home to the ophthalmologist or optometrist. Further, cognitively or physically disabled elderly persons typically require assistance in getting to the appointment, and even some nondisabled elderly persons may want to be accompanied by others. The full cost of the visit should be higher when such assistance is used, but is reduced somewhat when informal caregivers live nearby.
Data
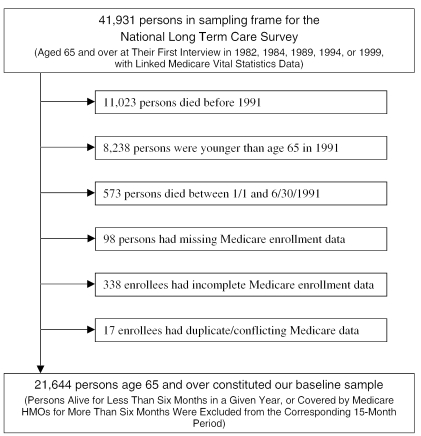
The study population consisted of individuals aged 65+ at time of entry in the study drawn for purposes of the National Long Term Care Survey (NLTCS). The NLTCS is a random longitudinal study of U.S. adults 65+, conducted in 1982, 1984, 1989, 1994, and 1999. The sample included institutionalized adults, enabling us to generalize results to the entire population aged 65+.
We were able to match 41,931 Medicare beneficiaries to Medicare Vital Statistics records to obtain the exact dates of birth and death. After deletions, we obtained a baseline analysis sample of 21,644 (Figure 2). From this sample, using Medicare enrollment files, we removed individuals in Medicare HMOs for more than six months in the year. Individuals who subsequently reenrolled in Medicare fee-for-service were added back into each year's sample (51 individuals/year on average). Six percent of the base sample enrolled in an HMO for six-plus months in 1991; by 1999, more than 17 percent of the surviving individuals enrolled in an HMO for greater than half the year.
Figure 2.
Sample Selection Process
We then identified disease cohorts for diabetes mellitus (DM) and ARMD by searching for specific ICD-9-CM diagnosis codes (primary or secondary) on seven different types of Medicare claims files for the period 1984–1999: Carrier (Physician Supplier/Part B, 1991–1999 only), Outpatient, Inpatient, Skilled Nursing, Home Health Agency, and Hospice. The file of Durable Medical Equipment (DME) claims was only searched for diabetes diagnoses. ICD-9-CM diagnosis codes used for DM and ARMD are shown in Table 1. We identified the DM sample as all individuals with diabetes diagnosis codes on two-plus claims of nonhospital services, or one-plus claims of hospital, hospice, and skilled nursing services (Hebert et al. 1999). For individuals who met this criterion, we began tracking them in the DM sample from their earliest claim.
Table 1.
Diagnosis and Procedures Codes for Diabetes, ARMD, Comorbidities, and Eye Exams
| Panel A: Diabetes, ARMD, and Comorbidities | |
|---|---|
| Diseases | ICD-9 Diagnosis Code |
| Diabetes mellitus | 250.x |
| ARMD, dry | 362.51, 362.57 |
| ARMD, wet | 362.52, 362.53 |
| ARMD, unspecified | 362.5, 362.50 |
| Diabetic retinopathy (BDR), background | 362.01 |
| Diabetic retinopathy (PDR), proliferative | 362.02 |
| Diabetic retinopathy (UDR), unspecified | 362.0 |
| Glaucoma, suspected | 365.0, 365.00, 365.01, 365.04 |
| Glaucoma, primary open-angle | 365.1, 365.10, 365.11, 365.12, 365.15 |
| Glaucoma, narrow-angle | 364.73, 365.02, 365.2x, 365.61 |
| Glaucoma, other | 365.03, 365.13, 365.14, 365.3x, 365.4x, 365.5x, 365.6, 365.60, 365.62, 365.63, 365.64, 365.65, 365.8x, 365.9 |
| Cataract | 365.51, 366.x, 379.31, 743.30–743.34, V43.1 |
| Panel B: Eye Examination Codes for All Persons | |
| Restrictions | CPT-4 or ICD-9 Procedure Codes |
| None | 92002, 92004, 92012, 92014, 92018, 92019, 92225, 92226, 92230, 92235, 92250, 92260 |
| With provider code 18 or 41 only (optometrist or ophthalmologist) on claims where available. If unavailable, with ICD-9-CM diagnosis codes 360.x-379.x only. | 99024, 99025, 99201–99205, 99211–99215, 99241–99245, 99251–99255, 99261–99263, 99271–99275, 99281–99285 |
| None | 16.21, 95.02, 95.03, 95.11, 95.12 |
| Panel C: Eye Examination Codes for Persons with Eye Diseases | |
| Diseases | CPT-4 or ICD-9 Procedure Codes |
| Cataract | 366xx, 36551, 37931, 74330–74334, V431 |
| Diabetes with any ocular complication | 76511–76513, 76516, 67208, 67210, 67227, 67228 |
| Diabetes with any form of diabetic retinopathy | 92287 |
| Diabetes, claims only with 362.02 as primary diagnosis | 67036, 67038–67040, 67101, 67105, 67107–67110, 67112 |
| Glaucoma, any form | 92020, 92081–92083, 92100, 92120, 92130, 92140, 92275, 65850, 65855, 66150, 66155, 66160, 66165, 66170, 66172, 66180, 66184, 66185, 66700, 66710, 66720, 66740 |
| Glaucoma, narrow-angle only | 65865, 65870, 65880, 66500, 66505, 66600, 66625, 66630, 66761, 66762 |
| Glaucoma, other | 92287, 65900, 65930 |
| ARMD, any form | 92240, 92283, 92284, 67208, 67210, 67218, 67228 |
| ARMD, wet only (362.52, 362.53) | 67036, 67038, 67039, 67040, 67108, 67110, 67220, 67221 |
We searched claims data from 1984–1990 to obtain a more accurate measure of persons with each disease. Because Carrier (Part B) files lacked information on diagnoses prior to 1991, we were limited to the other types of claims for 1984–1990. (DME was included in the Part B files during this period.) Nonetheless, first diagnoses for nearly a third of the patients with diabetes were recorded before 1991. Among persons with diagnosed ARMD, only 1 percent was selected for the ARMD sample based on diagnoses recorded before 1991.
Each beneficiary was included for each 15-month period before and following the date of diagnosis through date of death or year-end 1999, whichever occurred first. Only those persons classified as having diabetes before the end of 1999 were included in the DM sample. Likewise, persons who were diagnosed with ARMD were included in the ARMD sample. Since persons could be diagnosed with more than one disease, some persons were included in both samples. After the above screens, there were 23,944 and 22,611 observed claims in the two analytic samples, respectively, 4,906 distinct individuals for DM and 4,280 for ARMD. The samples are not entirely independent: 16.5 percent of the claims for persons with diabetes also had ARMD, and 20.5 percent of the claims for persons with ARMD also had DM.
We specified a 15-month rather than the 12-month interval in the guidelines to allow for noncompliance with the 12-month recommendation for such reasons as difficulties in scheduling, patient illness, holidays, and bad weather (Lee et al. 2003). Our results thus reflect a conservative assessment of nonconformance with regular follow-up care.
We identified eye exams using CPT-4 and ICD-9-CM procedure codes. Exams included visits during which only an eye exam was performed, or for therapeutic procedures, diagnostic services before such treatments were performed. For individuals without any eye diseases, we used codes shown in Table 1. Subsequent to being diagnosed with one-plus study diseases, we used additional disease-specific codes (Table 1) to identify other forms of contact with an eye care provider. The date of a person's initial diagnosis counted as an eye exam whether or not an eye exam was coded by one of the procedure codes.
A person was considered fully compliant with recommended annual eye exam guidelines if at least one visit was recorded during each 15-month period. This method of accounting biases in favor of conformance with regular visits. Someone may be in the first month of one time period and not be seen until the end of the next time period, a gap of 29 months, and still be considered to have had regular eye exams.
Empirical Specification
The observational unit was the person for a 15-month period. The binary dependent variable was one if the person had an eye examination within a period. Explanatory variables reflected marginal benefit and cost of an examination.
Factors determining benefit fell into two categories: (1) probability of change in visual status and (2) size of potential utility loss. For the first, we included variables for age, time before initial diagnosis, number of periods since initial diagnosis of DM (for diabetic retinopathy) or ARMD, and ocular comorbidities. Each variable was defined as of day one of the observational period. Thus, age was defined as of January 1, 1991, April 1, 1993, and so forth.
For duration of diagnoses, we defined intervals for before and after diagnosis. For example, for a person diagnosed with diabetes on April 3, 1994, the variable for before diagnosis would apply to the first (January 1991–March 1992), second (April 1992–June 1993), and third observational periods (July 1993–September 1994). The variable for “first period after diagnosis” would apply to the fourth period (October 1994–December 1995). For the fifth period, the variable would be for “second period after diagnosis.” Having been diagnosed four-plus periods previously was the omitted reference group.
For ocular comorbidities, we defined binary variables using the ICD-9-CM diagnosis codes in Table 1 for DM, background diabetic retinopathy (BDR), proliferative DR (PDR), and unspecified DR (UDR); suspected glaucoma, primary open-angle glaucoma (POAG), narrow-angle glaucoma (NAG), and other glaucoma; dry ARMD, wet ARMD, and unspecified ARMD; and cataract. Binary variables were equal to one in the period in which diagnosis of the comorbidity occurred and in all future periods. These variables are listed in approximate increasing order of disease progression, except for unspecified categories. Other factors equal, especially for DR, the probability that a therapeutic intervention will be productive increases with severity of the disease. Thus, for example, it is less likely that a patient diagnosed with DM but without DR would receive a recommendation for treatment at the next visit than would a patient with BDR. For ARMD, the relationship between severity and treatment during the observational period was much less clear, given the paucity of effective treatment options during the 1990s.
Diabetes complications may indicate that blood glucose levels and blood pressure have not been well controlled. We did not have measures of blood glucose levels or blood pressure, but we included a variable for the number of complications from diabetes (based on ICD-9 codes of 250.x). We did not include a variable for time since last eye exam since this would be endogenous to the current decision to obtain an eye exam.
For the magnitude of the potential utility loss, we included binary variables for death in the current and in the next period, which measured the time horizon over which any potential benefit an intervention to improve the future course of vision would accrue. We included explanatory variables for household income, education, and dementia. The education and income measures came from the NLTCS. For dementia, we searched the data for claims with a diagnosis of Alzheimer's or related dementias back to 1984.
We included a vision variable from the NLTCS, indicating whether or not the respondent said she could read newsprint, with or without glasses. Since the measures were only available once every five years, we merged the survey information corresponding most closely to the start of the 15-month observational period. Thus, for example, for January 1, 1991, we used information from the 1989 NLTCS interviews. For a date 30 months hence, July 1, 1993, we used information from the 1994 NLTCS. Until 1994, the NLTCS was only administered to persons with at least one limitation in activities of daily living (ADL) or instrumental activities of daily living (IADL) and, even after this date, persons without ADL and IADL limitations were underrepresented. When a response was not available from the NLTCS, we set the value of the variable equal to zero and set a binary variable for the variable equal to one, indicating that the variable was missing. This specification permitted us to use the full sample, including individuals without ADL or IADL limitations that may not have completed a NLTCS interview. We had no direct measure of the effectiveness of treatment. However, since treatments for DR were much more effective on average for DM than for ARMD during the observational period, this difference should be reflected in differences between the two conditions in the propensity to be examined.
The remaining variables reflected differences in the marginal cost of obtaining an exam: number of ADL limitations reported at the NLTCS interview nearest the period; a binary variable for residence in a nursing home at that interview; number of months during the period that the person was enrolled in an HMO (ranging from zero to six, since individuals with more than six months were removed from the sample); number of months the person was covered by Medicaid (from Medicare enrollment data); and other supplemental coverage to Medicare (from NLTCS interviews). We included a variable for the ratio of optometrists and ophthalmologists to 10,000 population in the Primary Sampling Unit (PSU) in which the person resided and a binary variable for rural PSUs. A PSU was a Standard Metropolitan Statistical Area for persons in metropolitan areas and a county for persons outside of metropolitan areas at the NLTCS interview.
A case-mix index, the DxCG score, was included to measure severity of illness other than for eye conditions that were excluded by our DxCG measure (Ellis, Pope, and Iezzoni 1996). The effect of other conditions may be bimodal. On the one hand, persons with other ongoing health problems may experience greater ease in obtaining referrals or might have other providers arrange visits for eye care; on the other, they may also have so many medical visits that they have to schedule other services, including eye exams. Marginal cost of an eye exam may be lower for married persons, for elderly persons living with children, and for elderly persons with children within an hour's drive. We defined binary variables for children living with parents and for children living within an hour's driving distance, with no children or children more than one hour's distance away as the omitted reference group. Marriage might not only reduce marginal cost, but also increase marginal benefit. Education may also affect marginal cost to the extent that more educated persons are better at processing health information (Kenkel 2000). We included demographic variables for gender and race/ethnicity (African American, Hispanic)—variables that do not fit into framework but plausibly affect variation in the probability of obtaining an eye exam and binaries variables for year.
We used logit analysis (SAS Version 8.1), given that the dependent variable was a binary for eye care visits during a 15-month period.
Results
Descriptive Statistics
On average, half of the persons in the diabetes and two-thirds in the ARMD sample were examined in a given 15-month period (Table 2). Ocular comorbidities were very common. Thus, although the guidelines for a particular condition specify regular eye examinations, many persons had more than one reason to be examined. African Americans were more highly represented in the diabetes (10 percent) than in the ARMD sample (3 percent).
Table 2.
Sample Means
| Diabetes | ARMD | |||
|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | |
| Eye Exam | 0.50 | 0.50 | 0.67 | 0.47 |
| Probability of change in visual status | ||||
| Read newsprint | 0.86 | 0.35 | 0.86 | 0.35 |
| Age | 78.72 | 5.99 | 80.41 | 6.40 |
| DR (background) | 0.07 | 0.26 | 0.03 | 0.17 |
| DR (proliferative) | 0.02 | 0.14 | 0.01 | 0.10 |
| DR (unspecified) | 0.01 | 0.09 | 0.00 | 0.06 |
| Glaucoma (narrow-angle) | 0.03 | 0.16 | 0.03 | 0.17 |
| Glaucoma (primary open-angle) | 0.10 | 0.30 | 0.12 | 0.32 |
| Glaucoma (other) | 0.02 | 0.14 | 0.03 | 0.16 |
| Glaucoma (suspect) | 0.05 | 0.22 | 0.06 | 0.24 |
| ARMD (dry) | 0.06 | 0.24 | 0.24 | 0.43 |
| ARMD (wet) | 0.02 | 0.15 | 0.08 | 0.28 |
| ARMD (unspecified) | 0.08 | 0.28 | 0.32 | 0.47 |
| Cataract | 0.58 | 0.49 | 0.70 | 0.46 |
| Dx not yet | 0.27 | 0.44 | 0.45 | 0.50 |
| Dx last period | 0.19 | 0.39 | 0.16 | 0.37 |
| Dx two periods ago | 0.16 | 0.37 | 0.13 | 0.34 |
| Dx three periods ago | 0.13 | 0.34 | 0.10 | 0.30 |
| Diabetes | 0.73 | 0.44 | 0.20 | 0.40 |
| Diabetes complications (#) | 0.54 | 0.75 | 0.57 | 0.79 |
| Time left to benefit | ||||
| Death this period | 0.10 | 0.29 | 0.07 | 0.25 |
| Death next period | 0.10 | 0.30 | 0.07 | 0.26 |
| Marginal valuation of loss | ||||
| Dementia | 0.15 | 0.35 | 0.12 | 0.33 |
| Education (years) | 10.08 | 3.82 | 10.85 | 3.81 |
| Income | 17802 | 13451 | 19397 | 14322 |
| Marginal cost | ||||
| ADLs | 2.32 | 2.24 | 2.03 | 2.16 |
| Married | 0.46 | 0.50 | 0.43 | 0.50 |
| Children at home | 0.14 | 0.34 | 0.13 | 0.34 |
| Children within 1-hr drive | 0.59 | 0.49 | 0.57 | 0.49 |
| In nursing home | 0.10 | 0.30 | 0.10 | 0.30 |
| Months on Medicaid | 1.88 | 4.35 | 1.42 | 3.86 |
| Supplemental insurance | 0.67 | 0.47 | 0.67 | 0.47 |
| Months in HMO | 0.74 | 2.55 | 0.54 | 2.06 |
| Eye doctors per 10,000 pop. | 12.15 | 3.99 | 12.32 | 4.20 |
| Rural resident | 0.18 | 0.39 | 0.22 | 0.43 |
| DxCG score | 1.33 | 1.39 | 1.10 | 1.09 |
| Death this period * DxCG | 0.13 | 0.78 | 0.08 | 0.60 |
| Death next period * DxCG | 0.21 | 0.87 | 0.14 | 0.69 |
| Other demographic characteristics | ||||
| Male | 0.38 | 0.49 | 0.33 | 0.47 |
| Black | 0.10 | 0.29 | 0.03 | 0.18 |
| Hispanic | 0.01 | 0.12 | 0.01 | 0.08 |
| Other races | 0.02 | 0.13 | 0.01 | 0.10 |
Logit Results for the Diabetes Sample
Although many of the explanatory variables had statistically significant impacts on the dependent variable (in bold), the analysis overall explained only 20 percent of the variation in the probability of compliance with guidelines (Table 3). Factors associated with a greater probability a visit included: (1) being unable to read a newspaper; (2) having an ocular comorbidity or having more advanced disease; (3) nonocular DM complications; (4) more education; (5) no dementia; (6) fewer ADL limitations; (7) being married or having children in the home; (8) higher DxCG score, indicating poorer general health; (9) being female; (10) being white; and (11) being a nursing home resident.
Table 3.
Odds Ratios for Logit Analysis
| Diabetes | ARMD | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |||
| Probability of a change in visual status | ||||||
| Read newsprint | 0.85 | 0.72 | 1.00 | 0.83 | 0.69 | 1.00 |
| Age | 0.99 | 0.98 | 0.99 | 1.00 | 0.99 | 1.01 |
| DR (background) | 1.53 | 1.34 | 1.75 | 1.64 | 1.30 | 2.06 |
| DR (proliferative) | 1.54 | 1.21 | 1.96 | 1.17 | 0.80 | 1.72 |
| DR (unspecified) | 1.67 | 1.17 | 2.39 | 1.37 | 0.74 | 2.52 |
| Glaucoma (narrow angle) | 2.77 | 2.25 | 3.42 | 2.01 | 1.63 | 2.49 |
| Glaucoma (primary open angle) | 3.86 | 3.45 | 4.32 | 2.89 | 2.58 | 3.24 |
| Glaucoma (other) | 1.24 | 1.00 | 1.52 | 1.21 | 1.00 | 1.47 |
| Glaucoma (supect) | 1.70 | 1.47 | 1.95 | 1.44 | 1.26 | 1.64 |
| ARMD (dry) | 1.83 | 1.60 | 2.10 | 1.54 | 1.36 | 1.75 |
| ARMD (wet) | 1.18 | 0.96 | 1.45 | 1.70 | 1.48 | 1.96 |
| ARMD (unspecified) | 1.76 | 1.58 | 1.97 | 1.40 | 1.23 | 1.60 |
| Cataract | 2.90 | 2.72 | 3.09 | 2.19 | 2.04 | 2.34 |
| Dx not yet | 0.97 | 0.87 | 1.08 | 2.92 | 2.43 | 3.50 |
| Dx last period | 1.08 | 0.96 | 1.21 | 1.89 | 1.67 | 2.13 |
| Dx two periods ago | 0.99 | 0.88 | 1.11 | 1.60 | 1.42 | 1.81 |
| Dx three periods ago | 1.05 | 0.93 | 1.18 | 1.30 | 1.15 | 1.48 |
| Diabetes | 1.08 | 0.98 | 1.19 | |||
| Diabetes complications (#) | 1.08 | 1.02 | 1.15 | 1.04 | 0.93 | 1.17 |
| Time left to benefit | ||||||
| Death this period | 0.52 | 0.43 | 0.64 | 0.63 | 0.51 | 0.77 |
| Death next period | 0.73 | 0.62 | 0.85 | 0.83 | 0.69 | 0.99 |
| Marginal valuation of vision loss | ||||||
| Dementia | 0.71 | 0.65 | 0.78 | 0.76 | 0.69 | 0.84 |
| Education (years) | 1.37 | 1.23 | 1.54 | 1.22 | 1.08 | 1.37 |
| Income | 1.03 | 0.95 | 1.11 | 1.08 | 1.00 | 1.17 |
| Marginal cost | ||||||
| ADLs | 0.91 | 0.89 | 0.93 | 0.90 | 0.88 | 0.93 |
| Married | 1.10 | 1.02 | 1.18 | 1.13 | 1.06 | 1.22 |
| Children at home | 1.20 | 1.00 | 1.43 | 1.03 | 0.85 | 1.24 |
| Children within 1-hr drive | 0.85 | 0.75 | 0.96 | 0.90 | 0.79 | 1.02 |
| In nursing home | 1.96 | 1.34 | 2.85 | 0.90 | 0.64 | 1.27 |
| Months on Medicaid | 0.99 | 0.99 | 1.00 | 0.99 | 0.98 | 1.00 |
| Supplemental insurance | 1.03 | 0.91 | 1.18 | 0.98 | 0.86 | 1.12 |
| Months in HMO | 0.95 | 0.93 | 0.97 | 0.96 | 0.94 | 0.98 |
| Eye doctors per 10,000 | 1.22 | 0.87 | 1.70 | 1.45 | 1.02 | 2.06 |
| Rural resident | 0.99 | 0.91 | 1.07 | 1.01 | 0.93 | 1.09 |
| DxCG score | 1.06 | 1.02 | 1.09 | 1.09 | 1.05 | 1.14 |
| Death this period * DxCG | 0.97 | 0.92 | 1.03 | 0.93 | 0.87 | 1.00 |
| Death next period * DxCG | 0.96 | 0.91 | 1.02 | 0.99 | 0.91 | 1.07 |
| Other demographic characteristics | ||||||
| Male | 0.89 | 0.83 | 0.95 | 0.96 | 0.90 | 1.03 |
| Black | 0.68 | 0.62 | 0.76 | 0.82 | 0.70 | 0.97 |
| Hispanic | 0.84 | 0.66 | 1.07 | 0.95 | 0.66 | 1.35 |
| Other races | 0.81 | 0.65 | 1.01 | 0.95 | 0.72 | 1.25 |
| Pseudo R-square | 0.20 | 0.13 | ||||
Note: Odds ratios in bold indicate statistical significance at the 5% level or better. Parameter estimates for variables indicating missing and binary variables identifying each of the 15-month periods are not shown.
Among factors that were not significantly associated with differences in compliance with others were: (1) time since diagnosis; (2) household income; (3) having other supplemental insurance; (4) having fewer months on Medicaid; and (5) greater density of eye care providers. Patients enrolled in an HMO were less likely to have an exam in the fee-for-service claim files; eye exams paid by an HMO were not captured in the Medicare claims data.
Logit Results for ARMD
For ARMD, the analysis explained only 14 percent of the variation in the probability of having an exam during a given 15-month period. Results for ARMD overall were quite similar to those for diabetes, with a few important differences.
First, the relationship between time since diagnosis and the probability of having an exam was very different from diabetes. For ARMD, with and without the ocular comorbidity variables included in the analysis, there was a negative relationship between the probability of receiving an exam and time since diagnosis of the condition. Before diagnosis with ARMD, persons were almost three times more likely to have had an exam than those in the omitted reference group, being diagnosed four or more periods earlier (OR=2.92, 95 percent CI=2.43–3.50). By contrast, those diagnosed three periods earlier were only 30 percent more likely to have had an exam than those in the omitted reference group (OR=1.30, 95 percent CI=1.15–1.50). The pattern between time since diagnosis and the probability of an exam was similar when the ocular comorbidity variables were excluded (not shown). Persons diagnosed with wet ARMD were 70 percent more likely to have been examined than those without a diagnosis (OR=1.70, 95 percent CI=1.48–1.96) and more likely to be examined than those with other types of ARMD.
Second, higher income increased the probability of an exam, implying that the probability of an exam rose by 8 percent for each $1,000 increase in household income, a result that was almost statistically significant at conventional levels (OR=1.08, 95 percent CI=1.00–1.17).
Unlike the diabetes analysis, presence of adult children in the household, residence in a nursing home, and being female had no statistically significant effects on the probability of having an eye exam for persons in the ARMD sample. Finally, other factors significantly related to a greater probability of a visit for ARMD, but not for diabetes, included fewer months on Medicaid, and greater density of eye care providers.
Discussion and Conclusions
The results provide insight into specific factors associated with having the eye exams, including many social support factors. Our results are consistent with our framework. For example, based on a comparison of anticipated benefits and costs, persons who have more reason to believe that a visit may lead to a therapeutic intervention and who are likely to place a higher valuation on vision loss are more likely to have obtained an examination. Factors leading to higher marginal costs of obtaining an exam were associated with a lower probability of being examined. For example, fewer eye care providers would imply greater transportation costs, and for people with ARMD, fewer providers were significantly associated with fewer visits.
In general, compliance patterns were similar for persons in both samples, ARMD and DM, but yet with substantial differences that may reflect differences in disease course and thus patient perceptions of the benefit of care or seriousness of disease. For diabetes, there was no change in the probability of having an exam with increase in time since diagnosis, but there was an increase with diagnosed severity of retinopathy. Patients appear to have reasoned that, once diagnosed with ocular findings, they needed to have their eyes examined. For ARMD, however, the pattern was one of monotonic decline in probabilities of an exam from before the date of diagnosis through four or more periods after diagnosis. Whatever the guidelines state, it appears that providers have given patients the bad news. (Treatment options for ARMD were extremely limited until after the observational period.) As such, patients were less likely to have subsequent visits. Similarly, those with problems reading were more likely to have exams than those that did not.
That patients' decisions to obtain care are consistent with a weighing of private costs and benefits is reassuring at one level. Socially optimal rates of compliance fall short of the 100 percent mark. Results of this study are instructive in indicating why rates fall short of the ideals specified in the guidelines. But without having more clinical detail, more information on patient preferences and the costs patients and families face, it is not possible to address the larger question of the gap between socially optimal and actual rates of use. Also, with such information, it is likely that more variation in the probability of an exam could be explained.
At a finer level of detail, our study offers several other important findings. These results may be used to develop interventions to improve compliance with guidelines to the extent that current compliance rates are considered to be inadequate.
The most important factors associated with demand for examinations were specific characteristics of the individual and living situation (for DM). For this cohort of elderly individuals, insurance coverage was not a major factor. Of course, all persons in the sample had some form of coverage, at least if the providers were willing to record an ocular diagnosis on the claim. The results serve to emphasize that even universal coverage does not guarantee full patient compliance with recommended care. This point is further emphasized by the lack of effect of supplemental health insurance. The net out-of-pocket cost of the visit should have been lower for Medicare beneficiaries with some form of supplementary health insurance. Indeed, those with Medicaid were slightly less likely, not more likely, to have had an exam. We attribute this finding to some unmeasured characteristic of Medicaid since those recipients had more favorable cost-sharing arrangements.
Among the individual characteristics, a few stand out. Many beneficiaries had more than one of the major eye diseases. Persons with most severe eye disease were generally the ones most likely to be examined at least once during the 15-month period.
Persons near death were less likely to have an eye exam, likely in part because of the need to attend to major illnesses related to their general health and also because the expected duration over which the benefit of mitigating the trajectory of vision loss was lower on average for persons near death. Although demand for some types of medical care increases near the end of life, demand for nonemergent eye care decreases.
Variables related to willingness (and ability) to pay for improved vision also explained variations in the probability of having an exam. Persons with a dementia diagnosis should be less likely to require near vision, such as for reading. More highly educated persons may be more likely to read, although the association between education and use may reflect other factors as well, including better recognition of the potential benefit of timely receipt of preventive care (Kenkel 2000).
The availability of eye care professionals was a factor for ARMD, suggesting that difficulty of access to a provider is an impediment to compliance with guidelines for some eye diseases. An alternative explanation, which seems unlikely because persons with ARMD represent a distinct minority of patients seen, is that the relationship is not causal, but rather that eye care providers locate in areas where patients have preferences for eye care on dimensions we did not measure. Persons with ADL limitations were less likely to be examined as were persons who were not married, both factors related to convenience of obtaining care.
We found substantial racial disparities in the probability of receipt of an eye exam, after accounting for factors that are correlated with race including educational attainment, income, and location. Such disparities may reflect access barriers, attitudes of providers toward patients based on race, differences in trust of the health care system, or distance from providers. Although we could account for variation among metropolitan areas and rural counties, an appreciable amount of variation within these jurisdictions exists. Differences between blacks and whites are particularly noteworthy given the large number of covariates. Our finding is in contrast to another study (Schaumberg et al. 2000), which found that African American women were more likely than white women to have had an eye exam in the past two years, but other research, like ours, has shown that African Americans use eye care services at much lower rates than whites (Devgan et al. 2000; Wang, Javitt, and Tielsch 1997).
We acknowledge several limitations of our analysis not already discussed. Most importantly, except for designation of provider's field (ophthalmologist or optometrist), we had no information on provider characteristics. Although eye examinations are mostly patient initiated, providers can potentially influence compliance by communicating benefits of regular vision monitoring, scheduling future examinations, and reminding patients of appointments (Roter and Hall 1997). A precondition for communicating benefit is a belief on the part of providers that regular monitoring is effective. Providers may sometimes recognize a lack of benefit in individual cases (e.g., in patients with severe dementia) or in cost (e.g., difficulty in getting the patient to the provider's office).
However, some providers may not be sufficiently effective in communicating the benefit of regular monitoring in cases in which a well-informed patient would choose to demand such monitoring. In another context, compliance with mammography guidelines is well-studied, and many of those studies have found physician recommendation for mammography to be an important factor in boosting compliance (Eilat-Tsanani et al. 2001); physician recommendations have also been found to influence patients in other areas (Champion 1992; Fox, Murata, and Stein 1991; Lieberman, Meana, and Stewert 1998; MacDowell, Nitz-Wiess, and Short 2000; Ore et al. 1997; Reno et al. 1997). Several studies found physicians to be less than fully compliant in recommending mammography (Chambers et al. 1989; Costanza et al. 1992; Roetzheim et al. 1991), and a similar finding has been found for physicians treating patients with diabetes (Kraft et al. 1997).
In this study, we used a 15-month rather than the 12-month interval specified by the diabetes and ARMD guidelines. One study found that altering the interval definition of compliance by as little as one month could produce a 27 percent difference in compliance estimates (Partin et al. 1998). However, we also estimated compliance using 12-month intervals and found similar patterns to those reported here.
There was some overlap between the ARMD and DM samples of about 20 percent. In a sense, the results are not completely independent.
Our study also has a limitation in how we defined duration of disease. The bias present is in underreporting duration, since individuals may have had the disease prior to 1991, which would have been evident if Medicare Part B claims had recorded diagnostic information before this year, or before age 65. In each case, it would bias toward the null hypothesis, which we found with DM. Thus, we cannot conclusively state that duration had an effect on DM.
Finally, although this study was limited to eye examinations for elderly persons with two diagnoses, the same data could be used to study compliance with guidelines in many other areas as well, ranging from receipt of recommended vaccines to various types of screening programs. Even in a population with universal coverage, actual use rates fall short of recommended levels. Also, there is much to be learned about patient perceptions of probabilities of health change and valuations of loss associated with such changes. Learning about the reasons for the substantial differences in the probability of use between blacks and whites reported in this study is an important area for future research.
Footnotes
This research was supported in part by a grant from the National Institute on Aging, “Visual Impairment, Treatment, and Effects on the Elderly,” grant no. 1RO1-AG-17473. We thank Lynn Van Scoyoc for the statistical analysis.
References
- American Academy of Ophthalmology. Preferred Practice Pattern: Diabetic Retinopathy. San Francisco: American Academy of Ophthalmology; 1998. [Google Scholar]
- American Academy of Ophthalmology. Preferred Practice Pattern: Glaucoma. San Francisco: American Academy of Ophthalmology; 2000. [Google Scholar]
- American Academy of Ophthalmology. Preferred Practice Pattern: Age-Related Macular Degeneration. San Francisco: American Academy of Ophthalmology; 2001. [Google Scholar]
- American Diabetes Association. “Diabetic Retinopathy.”. Diabetes Care. 2002;25(S1):S90–3. [Google Scholar]
- Brenes G A, Paskett E D. “Predictors of State of Adoption for Colorectal Cancer Screening.”. Preventive Medicine. 2000;31(4):410–6. doi: 10.1006/pmed.2000.0729. [DOI] [PubMed] [Google Scholar]
- Chambers C V, Balaban D J, Carlson B L, Ungemack J A, Grasberger D M. “Microcomputer-Generated Reminders. Improving the Compliance of Primary Care Physicians with Mammography Screening Guidlines.”. Journal of Family Practice. 1989;29(3):273–80. [PubMed] [Google Scholar]
- Champion V L. “Compliance with Guidelines for Mammography Screening.”. Cancer Detection and Prevention. 1992;16(4):253–8. [PubMed] [Google Scholar]
- Costanza M E, Stoddard A M, Zapka J G, Gaw V P, Barth R. “Physician Compliance with Mammography Guidelines: Barriers and Enhancers.”. Journal of the American Board of Family Practice. 1992;5(2):143–52. [PubMed] [Google Scholar]
- Cheng J W, Kalis M M, Feifer S. “Patient Adherence to Guidelines of the Sixth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.”. Pharmacotherapy. 2001;21(7):828–41. doi: 10.1592/phco.21.9.828.34557. [DOI] [PubMed] [Google Scholar]
- Devgan U, Yu F, Kim E, Coleman A L. “Surgical Undertreatment of Glaucoma in African-American Medicare Beneficiaries.”. Archives of Ophthalmology. 2000;118(2):253–6. doi: 10.1001/archopht.118.2.253. [DOI] [PubMed] [Google Scholar]
- Eilat-Tsanani S, Sorek M, Gay N, Chaimovitch O, Kulton L, Tabenkin H. “Family Physicians' Initiative to Increase Compliance with Screening Mammography—An Innovative Community Project.”. Israel Medical Association Journal: IMAJ. 2001;3(12):920–4. [PubMed] [Google Scholar]
- Ellis R P, Pope G C, Iezzoni L I. “Diagnosis-based Risk Adjustment for Medicare Capitation Payments.”. Health Care Financing Review. 1996;17:101–28. [PMC free article] [PubMed] [Google Scholar]
- Erhardt L R. “The Essence of Effective Treatment and Compliance Is Simplicity.”. American Journal of Hypertension. 1999;12(10, part 2):105–10S. doi: 10.1016/s0895-7061(99)00160-0. [DOI] [PubMed] [Google Scholar]
- Fox S A, Murata P J, Stein J A. “The Impact of Physician Compliance on Screening Mammography for Older Women.”. Archives of Internal Medicine. 1991;151(1):50–6. [PubMed] [Google Scholar]
- Hebert P L, Geiss L S, Tierney E F, Engelgau M M, Yawn B P, McBean A M. “Identifying Person with Diabetes Using Medicare Claims Data.”. American Journal of Medical Quality. 1999;14(6):270–7. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- Hilber D J, Whitwell K J, Krumholz D M, Pace C A. “The Effect of Continuous Quality Improvement on Compliance with Clinical Practice Guidelines in an Optometric Clinic: A Retrospective Review.”. Optometry. 2000;71(2):83–90. [PubMed] [Google Scholar]
- Hsia J, Rodabough R, Rosal M C, Cochrane B, Howard B V, Snetselaar L, Frishman W H, Stefanick M L. “Dietary and Lifestyle Factors among Older Women with Self-Reported Hypercholesterolemia: The Women's Health Initiative.”. American Journal of Medicine. 2002;113(5):384–92. doi: 10.1016/s0002-9343(02)01218-4. [DOI] [PubMed] [Google Scholar]
- Kenkel D S. “Prevention.”. In: Culyer A J, Newhouse J P, editors. Handbook of Health Economics. vol. 1. Amsterdam: Elsevier Science; 2000. pp. 1675–720. [Google Scholar]
- Kraft S K, Marrero D G, Lazaridis E N, Fineberg N, Qui C, Clark C M., Jr “Primary Care Physicians' Practice Patterns and Diabetic Retinopathy. Current Levels of Care.”. Archives of Family Medicine. 1997;6(1):29–37. doi: 10.1001/archfami.6.1.29. [DOI] [PubMed] [Google Scholar]
- Lawler F H, Viviani N. “Patient and Physician Perspectives Regarding Treatment of Diabetes: Compliance with Practice Guidelines.”. Journal of Family Practice. 1997;44(4):369–73. [PubMed] [Google Scholar]
- Lee P P, Feldman Z W, Ostermann J, Brown D S, Sloan F A. “Longitudinal Rates of Annual Eye Examinations of Persons with Diabetes and Chronic Eye Diseases.”. Opthalmology. 2003;110:1952–9. doi: 10.1016/S0161-6420(03)00817-0. [DOI] [PubMed] [Google Scholar]
- Lieberman L, Meana M, Stewert D. “Cardiac Rehabilitation: Gender Differences in Factors Influencing Participation.”. Journal of Women's Health. 1998;7(6):717–23. doi: 10.1089/jwh.1998.7.717. [DOI] [PubMed] [Google Scholar]
- MacDowell N M, Nitz-Wiess M, Short A. “The Role of Physician Communication in Improving Compliance with Mammography Screening among Women Ages 50–79 in a Commercial HMO.”. Managed Care Quarterly. 2000;8(4):11–9. [PubMed] [Google Scholar]
- Ore L, Hagoel L, Shifroni G, Rennert G. “Compliance with Mammography Screening in Israeli Women: The Impact of a Pre-scheduled Appointment and of the Letter-style.”. Israel Journal of Medical Sciences. 1997;33(2):103–11. [PubMed] [Google Scholar]
- Partin M R, Casey-Paal A L, Slater J S, Korn J E. “Measuring Mammography Compliance: Lessons Learned from a Survival Analysis of Screening Behavior.”. Cancer Epidemiology, Biomarkers and Prevention. 1998;7(8):681–7. [PubMed] [Google Scholar]
- Pate R R, Freedson P S, Sallis J F, Taylor W C, Sirard J, Trost S G, Dowda M. “Compliance with Physical Activity Guidelines: Prevalence in a Population of Children and Youth.”. Annuals of Epidemiology. 2002;12(5):3003–8. doi: 10.1016/s1047-2797(01)00263-0. [DOI] [PubMed] [Google Scholar]
- Phelps C E. Health Economics. New York: Wiley; 2002. [Google Scholar]
- Reno P L, Arfken C L, Heins J M, Fisher E B., Jr “Factors That Influence the Decision to Receive Treatment for Proliferative Diabetic Retinopathy.”. Diabetes Educator. 1997;23(6):653–5. doi: 10.1177/014572179702300604. [DOI] [PubMed] [Google Scholar]
- Roetzheim R G, Van Durme D J, Brownlee H J, Jr., Herold A H, Pamies R J, Woodard L. “Compliance with Screening Mammography: Survey of Primary Care Physicians.”. Journal of the Florida Medical Association. 1991;78(7):426–9. [PubMed] [Google Scholar]
- Roter D L, Hall J A. “Patient–Provider Communication.”. In: Glantz K, Lewis F M, Rimer B K, editors. Health Behavior and Education: Theory, Research, and Practice. 2d ed. San Francisco: Jossey-Bass; 1997. pp. 206–26. [Google Scholar]
- SAS Institute. SAS Version 8.1. Cary, NC: SAS Institute; [Google Scholar]
- Schaumberg D A, Christen W G, Glynn R J, Buring J E. “Demographic Predictors of Eye Care Utilization among Women.”. Medical Care. 2000;38(6):638–46. doi: 10.1097/00005650-200006000-00005. [DOI] [PubMed] [Google Scholar]
- Wang F, Javitt J C, Tielsch J M. “Racial Variations in Treatment for Glaucoma and Cataract among Medicare Recipients.”. Ophthalmic Epidemiology. 1997;4(2):89–100. doi: 10.3109/09286589709057101. [DOI] [PubMed] [Google Scholar]