Abstract
The importance of lymphotoxin α (LTα) in lymphoid organogenesis is well established. Although LTα has been implicated in the pathogenesis of T-cell-mediated immunopathologies, the requirement for LTα in T-cell activation and effector function in vivo is not well understood. To determine the role of LTα in T-cell activation in vivo, we compared the generation of antigen-specific T-cell responses between wild type (+/+) and LTα-deficient (LTα−/−) mice during an acute infection with lymphocytic choriomeningitis virus (LCMV). Our studies showed that LCMV-infected LTα−/− mice had a profound impairment in the activation and expansion of virus-specific CD8 T cells in the spleen, as determined by cytotoxicity assays, intracellular staining for gamma interferon, and staining with major histocompatibility complex class I tetramers. Further, the nonlymphoid organs of LTα−/− mice also contained substantially lower number of LCMV-specific CD8 T cells than those of +/+ mice. Greatly reduced virus-specific CD8 T-cell responses in LTα−/− mice led to a defect in LCMV clearance from the tissues. In comparison to that in +/+ mice, the activation of LCMV-specific CD4 T cells was also significantly attenuated in LTα−/− mice. Adoptive transfer experiments were conducted to determine if abnormal lymphoid architecture in LTα−/− mice caused the impairment in the activation of LCMV-specific T-cell responses. Upon adoptive transfer into +/+ mice, the activation and expansion of LCMV-specific LTα−/− T cells were restored to levels comparable to those of +/+ T cells. In a reciprocal cell transfer experiment, activation of +/+ T cells was significantly reduced upon transfer into LTα−/− mice. These results showed that impairment in the activation of LCMV-specific T cells in LTα−/− mice may be due to abnormal lymphoid architecture and not to an intrinsic defect in LTα−/− T cells.
The tumor necrosis factor (TNF) superfamily represents an expanding family of pleiotropic mediators regulating cellular processes ranging from proliferation and differentiation to apoptosis (5, 17, 22, 26). Among the TNF family members, the genes for TNF, lymphotoxin alpha (LTα), and lymphotoxin beta (LTβ) are tightly linked within the major histocompatibility complex (MHC) (26). TNF is produced by lymphocytes, NK cells, and macrophages; LTα and LTβ are made by CD4+ CD3− cells in the developing lymph node, lymphocytes, and NK cells (18, 26). LTα exists as a soluble homotrimer (LTα3) and as membrane-bound heterotrimers with LTβ (3, 4). Emulating TNF, LTα3 exerts its effects via TNF receptors I and II in inflammation and cytotoxicity (24, 26). LTα-deficient (LTα−/−) mice lack lymph nodes and Peyer's patches and also exhibit severe disruptions in splenic architecture (6). Although the importance of LTα in lymphoid organogenesis is well established, its role in orchestrating T-cell responses is not well understood (6, 12, 13, 17). LTα−/− mice fail to develop murine gammaherpesvirus-induced splenomegaly and CD8 T-cell lymphocytosis (15). Mice deficient in both TNF and LTα exhibit severely attenuated cytotoxic T-cell responses to lymphocytic choriomeningitis virus (LCMV) (7). However, these studies did not distinguish the role of abnormal lymphoid architecture from that of LTα deficiency alone in the development of T-cell responses. Therefore, the regulation of CD4 and CD8 T-cell activation and effector function by LTα during a viral infection, independent of the disrupted lymphoid environment, is still unclear. To determine the role of LTα alone in T-cell activation and function in vivo, we examined the development of antigen-specific T-cell responses to LCMV in wild type (+/+) and LTα−/− mice. Our studies showed that the development of LCMV-specific T-cell responses was severely impaired in LTα−/− mice compared to that in +/+ mice. Interestingly, LTα−/− T cells did not exhibit any defect in activation, expansion, or effector function upon adoptive transfer into irradiated +/+ mice. Further, the lymphoid environment in the LTα−/− mice precluded optimal activation of +/+ T cells. Taken together, our studies suggest that impaired T-cell activation in LTα−/− mice may be due to abnormal lymphoid architecture and not to an intrinsic defect in T cells per se.
MATERIALS AND METHODS
Mice.
C57BL/6 (H-2b; Thy-1.2) and B6.PL-Thy1a/Cy (H-2b; Thy-1.1) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). The generation of LTα−/− mice has been described previously (6). The LTα−/− mice (C57BL/6 background) provided by David Chaplin were bred under specific-pathogen-free conditions at Yale University.
Virus and virus titration.
The Armstrong CA 1371 strain of LCMV was used in this study (1). Eight- to 10-week-old mice were infected intraperitoneally with 2 × 105 PFU of LCMV. Infectious LCMV levels in the serum and tissues were quantitated by plaque assay on Vero cell monolayers as described previously (1).
Cytotoxic T-lymphocyte (CTL) assay.
MHC class I-restricted LCMV-specific cytotoxic T-cell activity in the spleens of LCMV-infected mice was measured by a 51Cr release assay as previously described (1).
Intracellular cytokine staining.
LCMV-specific gamma interferon (IFN-γ)-producing T cells were quantitated by intracellular cytokine staining as described elsewhere (19, 27). Briefly, splenocytes were stimulated with LCMV epitope peptides (0.1 μg/ml; CD8 and CD4 epitopes) in vitro for 5 h at 37°C in a medium containing brefeldin A and recombinant human interleukin-2 (50 U/ml). After incubation, cells were first surface stained with anti-CD4 (clone RM4-5) or anti-CD8 (clone 53-6.7) and then stained intracellularly with anti-IFN-γ (clone XMG1.2) by using the Cytofix/Cytoperm kit from Pharmingen (La Jolla, Calif.). In some studies, surface staining with anti-Thy1.2 antibodies (clone 53-2.1) was also performed. The number of IFN-γ-producing CD8 and CD4 T cells was determined by flow cytometry using a FacsCalibur flow cytometer (Becton Dickinson, San Jose, Calif.). All antibodies were purchased from Pharmingen. Flow cytometry data were analyzed with CellQuest software (Becton Dickinson).
Detection of LCMV-specific CD8 T cells by MHC class I tetramer staining.
The two immunodominant epitopes of LCMV are amino acid residues 396 to 404 and 33 to 41 of the viral nucleoprotein (NP) and glycoprotein (GP), respectively. Construction of MHC class I Db tetramers containing the LCMV CTL epitope peptide NP396-404 or GP33-41 has been described previously (19). Single-cell suspensions of spleen and lymph nodes were prepared by standard procedures. Mononuclear cells were isolated from peripheral blood, livers, and lungs according to published procedures (16). Single-cell suspensions of mononuclear cells from spleens, lymph nodes, peripheral blood, livers, and lungs were surface stained with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD8 and allophycocyanin-labeled MHC tetramers for 1 h at 4°C. In some experiments, PE-labeled anti-Thy1.2 and FITC-labeled anti-Thy1.1 antibodies were also used. The number of tetramer-binding CD8 T cells was quantitated by flow cytometry as described above.
Adoptive transfer experiment to examine activation of LTα−/− T cells in lymphoid organs of +/+ mice.
B6.PL-Thy1a/Cy (H-2b; Thy-1.1) +/+ mice were exposed to 540 rads of radiation. Spleen cells (10 × 107 cells/mouse) from C57BL/6 +/+ or LTα−/− mice were adoptively transferred (intravenously) into irradiated B6.PL-Thy1a/Cy (H-2b; Thy-1.1) mice 1 day after irradiation. Simultaneously, mice receiving adoptive transfer were infected with LCMV (intraperitoneal injection), and virus-specific T-cell responses were analyzed 8 days later. The sex of each spleen cell recipient mouse was matched to that of its donor.
Adoptive transfer experiment to examine activation of +/+ T cells in lymphoid organs of LTα−/− mice.
Spleen cells (8 × 107 cells/mouse) from B6.PL-Thy1a/Cy (H-2b; Thy-1.1) +/+ mice were adoptively transferred into irradiated (540 rads) C57BL/6 +/+ and LTα−/− mice. These mice were infected with LCMV on the day of adoptive transfer, and LCMV-specific CD8 T-cell responses in the spleen were analyzed 8 days later.
RESULTS
Impaired CD8 T-cell responses in LTα−/− mice.
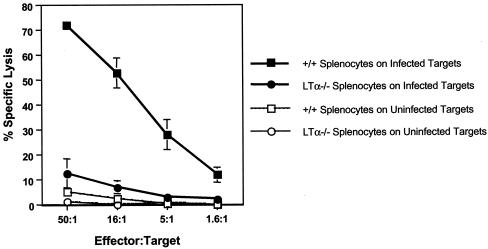
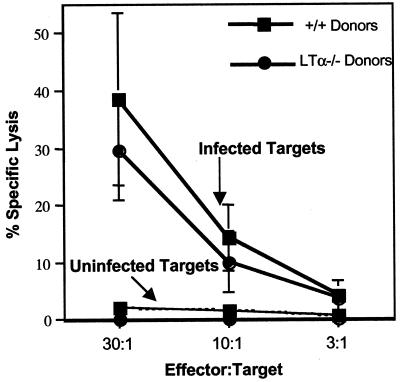
To determine the role of LTα in the generation of CD8 T-cell responses, virus-specific CD8 CTL responses in +/+ and LTα−/− mice following an acute infection with LCMV were compared. Eight days following infection with LCMV, we measured LCMV-specific MHC class I-restricted CTL activity directly ex vivo in the spleens of +/+ and LTα−/− mice. As shown in Fig. 1, spleen cells from LCMV-infected +/+ mice showed potent CTL activity. In striking contrast, splenocytes from LTα−/− mice showed weak CTL activity, which was ∼10-fold lower than that of +/+ mice. Immunocompetent mice resolve LCMV infection within 8 to 10 days; resolution is dependent on perforin-mediated cytotoxicity by CD8 T cells (10, 25). As shown in Table 1, +/+ mice resolved LCMV infection successfully, as expected. Consistent with the development of reduced CTL activity, LTα−/− mice exhibited impaired clearance of LCMV (Table 1).
FIG. 1.
CTL responses in LTα−/− mice. Eight days following infection with LCMV, MHC class I-restricted CTL activity in the spleens of +/+ and LTα−/− mice was measured by a 51Cr release assay using uninfected and LCMV-infected MC57 cells as target cells. Data are means for three mice per group.
TABLE 1.
Impaired clearance of LCMV in LTα-deficient micea
| Mouse genotype | LCMV titerb in:
|
|
|---|---|---|
| Serum | Liver | |
| +/+ | <1.6 | <2.7 |
| +/+ | <1.6 | 2.7 |
| +/+ | <1.6 | <2.7 |
| LTα−/− | 3.3 | 5.7 |
| LTα−/− | 3.3 | 5.8 |
| LTα−/− | 4.1 | 5.8 |
On day 8 postinfection, infectious LCMV levels in the sera and livers of +/+ and LTα−/− mice were measured by plaque assay.
Expressed as log10 PFU per ml of serum or per g of tissue.
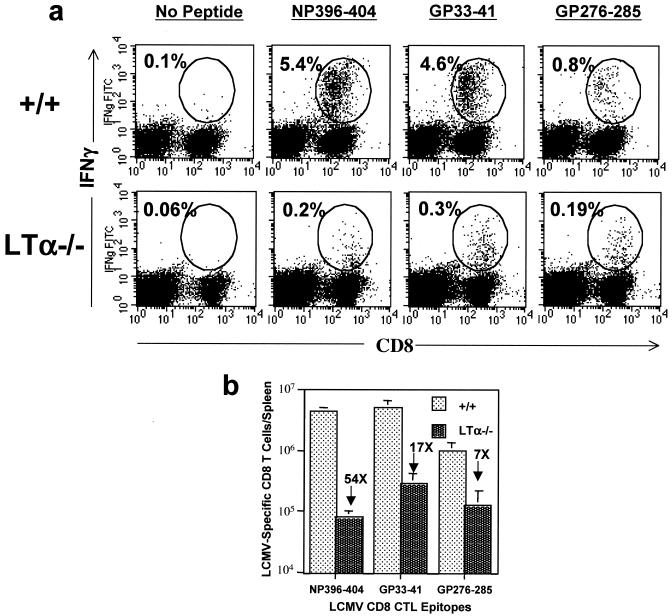
The effector function of CD8 T cells is mediated via cell-mediated cytotoxicity and by production of cytokines such as IFN-γ. In immunocompetent mice, on day 8 postinfection (p.i.), LCMV-specific CD8 T cells produce IFN-γ in response to a short stimulation with the specific peptide (19). We assessed the ability of CD8 T cells in LCMV-infected +/+ and LTα−/− mice to produce IFN-γ by intracellular staining. As depicted in Fig. 2a, CD8 T cells from +/+ mice produced readily detectable levels of IFN-γ in response to stimulation with all of the LCMV CTL epitope peptides. On the other hand, strikingly, very few CD8 T cells from LTα−/− mice produced IFN-γ. Further, as indicated by lower fluorescence intensity for IFN-γ staining, CD8 T cells from LTα−/− mice produced smaller amounts of IFN-γ than CD8 T cells from +/+ mice. On a per-spleen basis (Fig. 2b), the total numbers of NP396-404-, GP33-41-, and GP276-285-specific IFN-γ-producing CD8 T cells in LTα−/− mice were 7- to 54-fold lower than those in +/+ mice. Taken together, these data show that LTα−/− mice exhibit a profound defect in the development of virus-specific CD8 T-cell functions, i.e., cytotoxicity and IFN-γ production.
FIG. 2.
LCMV-specific IFN-γ-producing CD8 T cells in LTα−/− mice. On day 8 after infection with LCMV, splenocytes from +/+ and LTα−/− mice were stimulated in vitro with CTL epitope peptides (NP396-404, GP33-41, or GP276-285) for 5 h. The number of LCMV-specific CD8 T cells was determined by intracellular staining for IFN-γ. (a) Flow cytometry profiles of staining for IFN-γ following stimulation with specific peptides. Percentages of IFN-γ-producing CD8 T cells among splenocytes are given. (b) Total numbers of IFN-γ-producing CD8 T cells in the spleens of +/+ and LTα−/− mice. Note that the CD8 T-cell responses to NP396-404, GP33-41, and GP276-285 were 54-, 17-, and 7-fold lower, respectively, in LTα−/− mice than in +/+ mice.
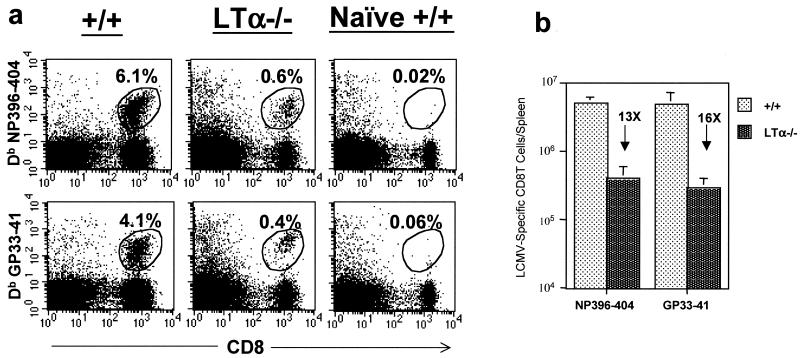
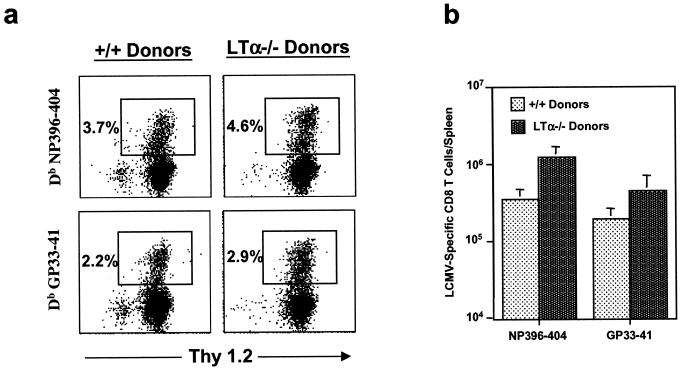
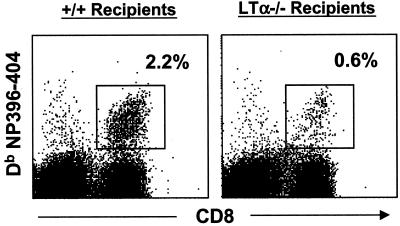
We have previously shown that effector function-negative LCMV-specific CD8 T cells (functionally unresponsive) can persist in virally infected mice (28). To examine this possibility, we used MHC class I tetramers to visualize LCMV-specific CD8 T cells in +/+ and LTα−/− mice (day 8 p.i.). As shown in Fig. 3a, CD8 T cells specific to two immunodominant epitopes were detected in the spleens of LCMV-infected +/+ and LTα−/− mice. However, the proportions of LCMV-specific CD8 T cells were ∼10-fold lower in LTα−/− mice than in +/+ mice. As determined by tetramer staining, the spleens of LTα−/− mice had ∼13- and 16-fold fewer NP396-404- and GP33-41-specific CD8 T cells, respectively, than those of +/+ mice (Fig. 3b). In the +/+ mice, the number of LCMV-specific CD8 T cells detected by tetramer staining (Fig. 3) was comparable to that detected by intracellular IFN-γ staining (Fig. 2). Interestingly, in the LTα−/− mice, more LCMV-specific CD8 T cells (especially NP396-404-specific cells) were detected by tetramer staining (Fig. 3) than by intracellular IFN-γ staining (Fig. 2). Accordingly, the fold difference between +/+ and LTα−/− mice in the total number of NP396-404-specific cells was magnified in the analysis for intracellular IFN-γ (54-fold by IFN-γ staining versus 13-fold by tetramer staining). These data imply that a proportion of NP396-404-specific CD8 T cells in LTα−/− mice are functionally unresponsive. The functional unresponsiveness of LCMV-specific CD8 T cells is likely due to high antigenic load on account of delayed viral clearance (28). In summary, defective CD8 T-cell responses to LCMV in LT−/− mice are associated with impaired expansion and some degree of functional unresponsiveness.
FIG.3.
Detection of LCMV-specific CD8 T cells in the spleens of +/+ and LTα−/− mice by using MHC class I tetramers. Eight days following infection with LCMV, splenocytes from +/+ and LTα−/− mice were stained with anti-CD8 antibodies and fluorochrome-labeled Db MHC class I tetramers (specific to NP396-404 and GP33-41) and were analyzed by flow cytometry. (a) Flow cytometry profiles of CD8 T cells staining for the tetramers. The flow cytometry profiles are gated on total splenocytes based on forward and side scatter. Percentages of tetramer-binding CD8 T cells among splenocytes are given. (b) Total number of tetramer-binding LCMV-specific CD8 T cells per spleen in +/+ and LTα−/− mice. Note that the total numbers of CD8 T cells specific to NP396-404 and GP33-41 epitopes were 13- and 16-fold lower, respectively, in LTα−/− mice than in +/+ mice.
Reduced numbers of LCMV-specific CD8 T cells in nonlymphoid organs of LTα−/− mice.
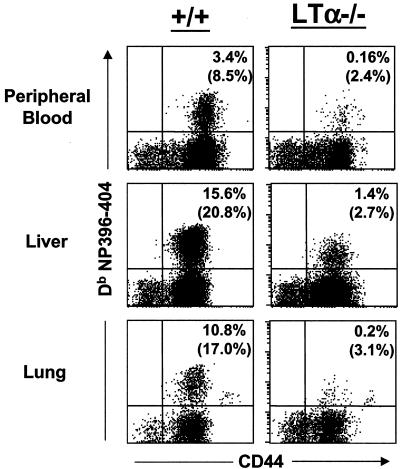
The data presented in Fig. 1 to 3 clearly show that activation and expansion of LCMV-specific CD8 T-cell responses are severely impaired in the spleens of LTα−/− mice. Since LTα−/− mice lack lymph nodes and exhibit aberrations in splenic architecture, it was of interest to examine activation of LCMV-specific CD8 T cells in the nonlymphoid organs. To this end, on day 8 p.i., we quantitated LCMV-specific CD8 T cells in the peripheral blood, livers, and lungs of LCMV-infected +/+ and LTα−/− mice. Mononuclear cells were isolated from peripheral blood, livers, and lungs, and LCMV-specific CD8 T cells were quantitated by staining with MHC class I tetramers. As shown in Fig. 4, the peripheral blood, livers, and lungs of LCMV-infected +/+ mice contained high numbers (8 to 20% of CD8 T cells) of LCMV-specific CD8 T cells. In striking contrast, remarkably low numbers of LCMV-specific CD8 T cells were detected in the livers and lungs of LTα−/− mice. These data demonstrate a substantial reduction in the activation and expansion of LCMV-specific CD8 T cells in nonlymphoid organs of LTα−/− mice.
FIG. 4.
Reduced numbers of LCMV-specific CD8 T cells in nonlymphoid organs of LTα−/− mice. On the 8th day p.i., mononuclear cells isolated from peripheral blood, livers, and lungs of +/+ and LTα−/− mice were stained with anti-CD8 and anti-CD44 antibodies and with fluorochrome-labeled Db MHC class I tetramers (specific to NP396-404) and were analyzed by flow cytometry. The flow cytometry profiles are gated on total CD8 T cells. Percentages in upper right-hand corners are proportions of mononuclear cells that are tetramer-binding CD8 T cells. Note that all tetramer-binding CD8 T cells are CD44hi. Numbers in parentheses are percentages of tetramer-binding CD8 T cells to total CD8 T cells.
Reduced CD4 T-cell responses in LTα−/− mice.
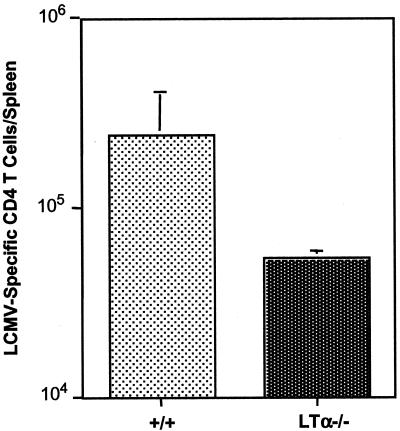
We compared the activation of LCMV-specific CD4 T cells between +/+ and LTα−/− mice. Eight days after infection with LCMV, LCMV-specific CD4 T-cell responses were measured by intracellular staining for IFN-γ. As shown in Fig. 5, the numbers of LCMV-specific CD4 T cells in LTα−/− mice were ∼6-fold lower than those in +/+ mice. These data show that both CD8 and CD4 T-cell responses are affected in LTα−/− mice.
FIG. 5.
CD4 T-cell activation in LTα−/− mice. At day 8 after infection with LCMV, splenocytes from +/+ and LTα−/− mice were stimulated in vitro with the MHC class II-restricted peptide GP61-80. Following stimulation with the peptide, the number of LCMV-specific CD4 T cells was determined by intracellular staining for IFN-γ. Unstimulated controls showed no staining for IFN-γ. Data are means for three mice.
Activation of LTα−/− T cells is restored after adoptive transfer into wild-type mice.
LTα−/− mice have severe defects in lymphoid organogenesis (6). LTα−/− mice lack all lymph nodes and Peyer's patches; spleen architecture is remarkably disorganized, with improper segregation of B- and T-cell zones and lack of germinal centers (6). It has been previously shown that secondary lymphoid organ architecture may be important for the optimal elicitation of T-cell responses to LCMV infection (11). Defective activation of CD8 T cells in LTβ-deficient mice has also been reported to be due to disrupted splenic architecture (2). Therefore, it is possible that defective activation of CD8 and CD4 T cells in LTα−/− mice following an acute LCMV infection may be due to abnormal lymphoid architecture. Alternatively, LTα−/− T cells may have an intrinsic defect in activation and expansion in vivo. We used an adoptive transfer system to ask the question whether LTα−/− T cells could be activated in the splenic microenvironment of a wild-type mouse.
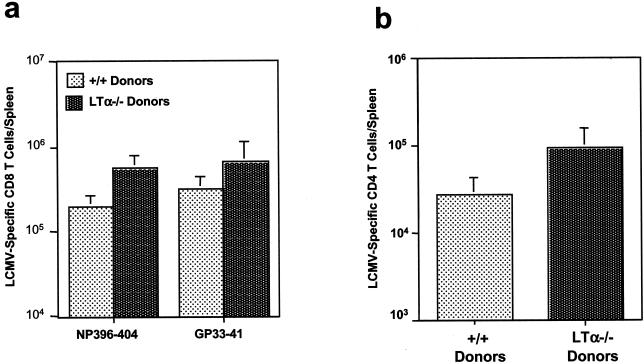
We transferred 10 × 107 spleen cells from C57BL/6 +/+ or LTα−/− mice into sublethally irradiated congenic B6.PL-Thy1a/Cy (Thy-1.1) mice. C57BL/6 +/+ mice and LTα−/− mice whose spleen cells were transferred into Thy-1.1 mice are designated +/+ donors and LTα−/− donors, respectively. Irradiated mice receiving adoptive transfer were infected with LCMV, and virus-specific T-cell responses were analyzed 8 days later. As shown in Fig. 6, LCMV-specific MHC class I-restricted CTL activities in the spleens of Thy-1.1 mice that received spleen cells from +/+ mice (+/+ donors) and LTα−/− mice (LTα−/− donors) were comparable. By use of this assay, it was not possible to exclude the contribution of endogenous host T cells to CTL activity. In the host receiving adoptive transfer, the donor and recipient (endogenous) T cells can be distinguished based on surface expression of congenic Thy1.1 and Thy1.2 molecules. While the donor T cells express only Thy1.2, recipient (host) T cells are Thy1.1+. We performed three-color flow cytometry analysis using anti-CD8, anti-Thy1.2, and MHC class I tetramers to type and enumerate LCMV-specific CD8 T cells. As shown in Fig. 7a, spleens of mice receiving T cells from +/+ donors and LTα−/− donors had comparable numbers of LCMV-specific CD8 T cells. Further, all of the LCMV-specific CD8 T cells (tetramer-binding CD8 T cells) were Thy1.2+, showing that they were of donor origin; very few to no host-derived CD8 T cells (Thy1.2−) were detected. The total number of LCMV-specific CD8 T cells from LTα−/− donors was ∼2-fold higher than that from +/+ donors (Fig. 7b). These data clearly demonstrate that LTα−/− CD8 T cells underwent normal activation and expansion upon transfer into normal mice. Although LTα−/− mice lack lymph nodes (6), LTα−/− LCMV-specific CD8 T cells homed normally into lymph nodes in mice receiving adoptive transfer (data not shown). We also determined the numbers of LCMV-specific CD8 T cells in adoptive transfer recipients by intracellular staining for IFN-γ. In agreement with the tetramer data (Fig. 7), this analysis showed that the total numbers of LCMV-specific IFN-γ-producing CD8 T cells in the spleens of mice receiving T cells from LTα−/− donors were similar to those for mice receiving T cells from +/+ donors (Fig. 8a). Costaining for Thy1.2 during intracellular cytokine staining confirmed that all IFN-γ-producing CD8 T cells were of donor origin (data not shown). Activation of LCMV-specific CD4 T cells (as determined by intracellular staining for IFN-γ) in the spleens of mice receiving T cells from LTα−/− donors was similar to that for mice receiving T cells from +/+ donors (Fig. 8b). Taken together, these data convincingly demonstrate that LTα−/− T cells do not have an intrinsic defect in activation in vivo.
FIG. 6.
CTL activity in the spleens of mice receiving adoptive transfer. A total of 10 × 107 spleen cells from naive C57BL/6 +/+ or LTα−/− mice (designated +/+ donors and LTα−/− donors, respectively) were transferred into irradiated congenic B6.PL-Thy1a/Cy (Thy-1.1) mice, which were infected with LCMV. At day 8 p.i., LCMV-specific class I-restricted CTL activities in the spleens of mice receiving adoptive transfer were measured by a 51Cr release assay using LCMV-infected and uninfected MC57 cells as target cells.
FIG. 7.
Activation of +/+ and LTα−/− CD8 T cells following adoptive transfer into +/+ mice. A total of 10 × 107 spleen cells from naive C57BL/6 +/+ or LTα−/− mice (designated +/+ donors and LTα−/− donors, respectively) were transferred into irradiated congenic B6.PL-Thy1a/Cy (Thy-1.1) mice, which were infected with LCMV. Eight days after infection with LCMV, splenic CD8 T cells specific to CTL epitopes NP396-404 and GP33-41 were quantitated by staining with anti-CD8, anti-Thy1.2 antibodies, and fluorochrome-labeled MHC class I tetramers. Donor-derived T cells are Thy1.2+, and recipient-derived (endogenous) T cells are Thy1.2−. (a) Flow cytometry profiles gated on total CD8 T cells. The percentage of total splenocytes represented by tetramer-binding CD8 T cells is given in each dot plot. Note that all tetramer-positive cells are also Thy1.2+ (of donor origin) and that very few Thy1.2− cells (endogenous [of recipient origin]) are present. (b) Total number of LCMV-specific Thy1.2+ CD8 T cells in the spleens of +/+ Thy-1.1 mice receiving spleen cells from +/+ or LTα−/− mice. Data are means for four mice per group.
FIG. 8.
LCMV-specific IFN-γ-producing CD8 and CD4 T cells in mice receiving adoptive transfer. Irradiated congenic B6.PL-Thy1a/Cy (Thy-1.1) mice received 10 × 107 spleen cells from +/+ or LTα−/− mice (designated +/+ donors and LTα−/− donors, respectively). Eight days after infection with LCMV, virus-specific CD8 and CD4 T cells in the spleens of mice receiving spleen cells from +/+ donors and LTα−/− donors were quantitated by intracellular cytokine staining as described in the legends to Fig. 2 and 4. Costaining with anti-Thy1.2 antibodies showed that all of the IFN-γ-producing T cells were of donor origin. Panels a and b show the total numbers of LCMV-specific CD8 and CD4 T cells, respectively, in the spleens of mice receiving spleen cells from +/+ donors and LTα−/− donors. Data are means for four mice per group.
Activation of +/+ T cells is impaired after adoptive transfer into LTα−/− mice.
To examine if disrupted splenic architecture in LTα−/− mice precludes activation and expansion of +/+ T cells, we performed an adoptive transfer experiment. In this experiment, we adoptively transferred 8 × 107 spleen cells from congenic B6.PL-Thy1a/Cy +/+ (Thy-1.1) mice into irradiated C57BL/6 +/+ and LTα−/− mice. Irradiated C57BL/6 +/+ and LTα−/− mice that received spleen cells from Thy-1.1 mice are designated +/+ recipients and LTα−/− recipients, respectively. Following adoptive transfer of spleen cells, these mice were infected with LCMV, and CD8 T-cell responses were analyzed 8 days later. LCMV-specific CD8 T cells were quantitated in the spleens by staining with anti-Thy1.1, anti-CD8, and MHC class I tetramers. It is worth emphasizing that adoptively transferred T cells (of donor origin) are Thy1.1+, while endogenous T cells (of recipient origin) are Thy1.1−. As shown in Fig. 9, spleens of control +/+ C57BL/6 mice (+/+ recipients) contained readily detectable numbers of LCMV-specific CD8 T cells. On the other hand, spleens of LTα−/− mice (LTα−/−recipients) contained substantially fewer numbers of LCMV-specific CD8 T cells than those of +/+ recipients (Fig. 9). All of the tetramer-binding CD8 T cells detected in the spleens of +/+ recipients and LTα−/− recipients were of donor origin (Thy1.1+) (data not shown). The total numbers of LCMV-specific CD8 T cells in the spleens of LTα−/− recipients were ∼70% lower than those in the spleens of control +/+ recipients (data not shown). Consistent with reduced LCMV-specific CD8 T-cell responses, LTα−/− recipients showed impaired viral clearance from liver and lungs; no infectious LCMV was detected in the livers and lungs of +/+ recipients (data not shown). Taken together, the data presented in Fig. 7 to 9 strongly suggest that defective T-cell responses in LTα−/− mice may be primarily due to abnormal lymphoid architecture.
FIG. 9.
Activation of +/+ CD8 T cells following adoptive transfer into LTα−/− mice. A total of 8 × 107 spleen cells from naive B6.PL-Thy1a/Cy +/+ (Thy-1.1) mice were transferred into irradiated C57BL/6 +/+ and LTα−/− mice (referred to as +/+ recipients and LTα−/− recipients, respectively), which were infected with LCMV. Eight days after infection with LCMV, splenic CD8 T cells specific to CTL epitope NP396-404 were quantitated by staining with anti-CD8, anti-Thy1.1 antibodies, and fluorochrome-labeled-MHC class I tetramers. All the tetramer-binding CD8 T cells were Thy1.1+ (donor derived). The flow cytometry profiles are gated on total splenocytes based on forward and side scatter. The percentage of total splenocytes represented by tetramer-binding CD8 T cells is given in each dot plot.
DISCUSSION
In the present study, we determined the requirement for LTα in the generation of T-cell responses during acute infection with LCMV in mice. Our studies show that the development of LCMV-specific T-cell responses was profoundly impaired in LTα−/− mice. This impairment in the generation of virus-specific T-cell responses was associated with a lack of lymph nodes and disruption of splenic architecture in LTα−/− mice and was not due to an intrinsic defect in LTα−/− T cells.
LTα is considered a proinflammatory cytokine, thought to play an important role in lymphocyte-dependent immunopathologies such as multiple sclerosis and experimental autoimmune encephalitis (21, 23). However, the requirement for LTα in the activation, expansion, and effector function of T cells in vivo is not well understood. We addressed this issue by examining the development of antigen-specific T-cell responses in LTα−/− mice in response to an acute LCMV infection. Our studies showed that the development of LCMV-specific MHC class I-restricted CTL activity was severely impaired in LTα−/− mice (Fig. 1). Resolution of an LCMV infection is dependent on perforin-mediated killing of infected cells by CD8 T cells (10, 25). As a consequence of the suboptimal CTL response in LTα−/− mice, clearance of LCMV from the tissues was impaired (Table 1). Like LCMV infection, herpes simplex virus (HSV) infection in LTα−/− mice induced poor CD8 CTL activity, resulting in impaired control of the virus in the central nervous system (14). Mice doubly deficient in TNF and LTα, and mice deficient in LTβ, also show diminished CTL responses to LCMV (7). Bone marrow chimera experiments have indicated that the impaired CTL response in LTβ-deficient mice is due to disrupted lymphoid architecture rather than to a lack of LTβ function (2).
In addition to reduced CD8 CTL function, CD8 T cells in LCMV-infected LTα−/− mice exhibited a profound impairment in IFN-γ production (Fig. 2). Compared to those in +/+ mice, substantially lower numbers of LCMV-specific IFN-γ-producing CD8 T cells were detected in the spleens of LTα−/− mice. Interestingly, this reduction in the number of virus-specific IFN-γ-producing CD8 T cells in LTα−/− mice seems to be epitope dependent. In LTα−/− mice, the number of IFN-γ-producing CD8 T cells specific to the immunodominant epitope NP396-404 was 54-fold lower than that in +/+ mice (Fig. 2b). On the other hand, in LTα−/− mice, the reductions in the numbers of IFN-γ-producing CD8 T cells specific to GP33-41 and GP276-285 were 17- and 7-fold, respectively (Fig. 2b). Epitope-specific regulation of CD8 T cells during a chronic LCMV infection has been described previously: NP396-404-specific CD8 T cells are selectively deleted in chronically infected mice (28). Taken together, the data in Fig. 1 to 3 convincingly demonstrate the “functionally impaired” LCMV-specific CD8 T-cell responses in LTα−/− mice. Impaired LCMV-specific T-cell responses in LTα−/− mice may be due to suboptimal expansion of antigen-specific CD8 T cells and/or to induction of a functionally unresponsive state. To explore these possibilities, we compared the expansion of LCMV-specific CD8 T cells in +/+ and LTα−/− mice by using MHC class I tetramers, which do not rely on functional assays (Fig. 3). Although readily detectable numbers of LCMV-specific CD8 T cells were generated in LTα−/− mice (Fig. 3), the magnitude of expansion was 13- to 16-fold lower than that in +/+ mice. These data indicated that functional impairment of the CD8 T-cell response in LTα−/− mice was caused by diminished expansion of LCMV-specific CD8 T cells (Fig. 3). In agreement with our findings, it has been reported that CD8 T-cell expansion associated with murine gammaherpesvirus infection is significantly reduced in LTα−/− mice (15). In contrast, in HSV-infected LTα−/− mice, CD8 T-cell expansion is normal, but all virus-specific CD8 T cells have been reported to be functionally unresponsive (unable to produce IFN-γ) (14). Unlike HSV infection, LCMV infection in LTα−/− mice led to impaired expansion of LCMV-specific CD8 T cells and did not induce complete functional unresponsiveness in virus-specific CD8 T cells (Fig. 2). Differences in pathogenesis (cell tropism, viral load, and duration of infection) between LCMV and HSV presumably lead to contrasting outcomes.
As mentioned above, LTα−/− mice lack lymph nodes and show disrupted splenic architecture characterized by complete loss of germinal centers, follicular dendritic cells, and lack of segregation of B- and T-cell areas (6). Studies from R. M. Zinkernagel's group have shown the importance of secondary lymphoid organ architecture for the generation of antiviral T-cell responses (11). Therefore, in LTα−/− mice, impaired T-cell responses to LCMV might be sequelae to serious alterations in lymphoid organogenesis. We addressed this issue by testing the ability of T cells from LTα−/− mice to respond in the lymphoid environment of a wild-type mouse. Spleen cells from LTα−/− or +/+ mice were adoptively transferred into irradiated congenic +/+ mice, which were infected with LCMV. Eight days following LCMV infection, virus-specific T-cell responses in the spleen cell recipient mice were quantitated. As expected, T cells derived from the donor spleens primarily mediated the anti-LCMV immune response in the adoptive transfer recipients. In LCMV-infected mice, LTα−/− T cells were activated and expanded to levels comparable to those for T cells from +/+ mice (Fig. 7 and 8). Taken together, these findings strongly suggest that LTα−/− T cells may not have an intrinsic defect in activation. To confirm that the lack of lymph nodes and the altered architecture in the spleens of LTα−/− mice precluded the genesis of a potent antiviral T-cell response to LCMV infection, we performed another adoptive transfer experiment, where we transferred +/+ T cells into LTα−/− mice and studied their activation and expansion during an acute LCMV infection. These studies showed that activation of +/+ T cells was greatly reduced when they were transferred into LTα−/− mice (Fig. 9); +/+ T cells transferred into +/+ mice showed normal activation. Taken together, these data provide strong evidence that LTα is not essential for normal activation and expansion of T cells in vivo, and they underscore the importance of lymph nodes and lymphoid architecture in the generation of T-cell responses during an acute viral infection. These conclusions are consistent with a recent report that secreted LTα is not required for activation of T cells during a mycobacterial infection in mice (20). Furthermore, spleen transfer experiments have shown that production of low levels of immunoglobulin G in sheep erythrocyte-immunized LTα−/− mice is not due to an intrinsic defect in LTα−/− B cells (8, 9). In summary, lack of LTα production by T lymphocytes does not lead to an intrinsic defect in activation or effector function in the LCMV model of acute viral infection.
Acknowledgments
We thank Kaja Madhavi Krishna and Shuning Zhan for excellent technical assistance.
This work was supported by National Institutes of Health grants AI-30048 and NS-21496 (to R.A.) and CA16885 (to N.H.R.). M.S. was supported by a postdoctoral fellowship from the National Multiple Sclerosis Society.
REFERENCES
- 1.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, D. P., D. Naniche, M. T. Crowley, P. A. Koni, R. A. Flavell, and M. B. Oldstone. 1999. Lymphotoxin-beta-deficient mice show defective antiviral immunity. Virology 260:136-147. [DOI] [PubMed] [Google Scholar]
- 3.Browning, J. L., I. D. Sizing, P. Lawton, P. R. Bourdon, P. D. Rennert, G. R. Majeau, C. M. Ambrose, C. Hession, K. Miatkowski, D. A. Griffiths, A. Ngam-ek, W. Meier, C. D. Benjamin, and P. S. Hochman. 1997. Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J. Immunol. 159:3288-3298. [PubMed] [Google Scholar]
- 4.Browning, J. L., I. Dougas, A. Ngam-ek, P. R. Bourdon, B. N. Ehrenfels, K. Miatkowski, M. Zafari, A. M. Yampaglia, P. Lawton, and W. Meier. 1995. Characterization of surface lymphotoxin forms. Use of specific monoclonal antibodies and soluble receptors. J. Immunol. 154:33-46. [PubMed] [Google Scholar]
- 5.Cosman, D. 1994. A family of ligands for the TNF receptor superfamily. Stem Cells 12:440-455. [DOI] [PubMed] [Google Scholar]
- 6.De Togni, P., J. Goellner, H. H. Ruddle, P. R. Streeter, A. Fick, S. Mariathasan, S. C. Smith, R. Carlson, L. P. Shornick, J. Strauss-Schoenberger, J. H. Russel, R. Karr, and D. D. Chaplin. 1994. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264:703-707. [DOI] [PubMed] [Google Scholar]
- 7.Eugster, H. P., M. Muller, U. Karrer, B. D. Car, B. Schnyder, V. M. Eng, G. Woerly, M. Le Hir, F. di Padova, M. Aguet, R. Zinkernagel, H. Bluethmann, and B. Ryffel. 1996. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-alpha double-deficient mice. Int. Immunol. 8:23-36. [DOI] [PubMed] [Google Scholar]
- 8.Fu, Y. X., H. Molina, M. Matsumoto, G. Huang, J. Min, and D. D. Chaplin. 1997. Lymphotoxin-alpha (LTα) supports development of splenic follicular structure that is required for IgG responses. J. Exp. Med. 185:2111-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu, Y. X., and D. D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17:399-433. [DOI] [PubMed] [Google Scholar]
- 10.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 11.Karrer, U., A. Althage, B. Odermatt, C. W. Roberts, S. J. Korsmeyer, S. Miyawaki, H. Hengartner, and R. M. Zinkernagel. 1997. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11−/−) mutant mice. J. Exp. Med. 185:2157-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koni, P. A., R. Sacca, P. Lawton, J. L. Browning, N. H. Ruddle, and R. A. Flavell. 1997. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity 6:491-500. [DOI] [PubMed] [Google Scholar]
- 13.Korner, H., M. Cook, D. S. Riminton, F. A. Lemckert, R. M. Hoek, B. Ledermann, F. Kontgen, B. Fazekas de St Groth, and J. D. Sedgwick. 1997. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol. 27:2600-2609. [DOI] [PubMed] [Google Scholar]
- 14.Kumaraguru, U., I. A. Davis, S. Deshpande, S. S. Tevethia, and B. T. Rouse. 2001. Lymphotoxin alpha−/− mice develop functionally impaired CD8+ T cell responses and fail to contain virus infection of the central nervous system. J. Immunol. 166:1066-1074. [DOI] [PubMed] [Google Scholar]
- 15.Lee, B. J., S. Santee, S. Von Gesjen, C. F. Ware, and S. R. Sarawar. 2000. Lymphotoxin-alpha-deficient mice can clear a productive infection with murine gammaherpesvirus 68 but fail to develop splenomegaly or lymphocytosis. J. Virol. 74:2786-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto, M. 1999. Role of TNF ligand and receptor family in the lymphoid organogenesis defined by gene targeting. J. Med. Investig. 46:141-150. [PubMed] [Google Scholar]
- 18.Mebius, R. E., P. Rennert, and I. L. Weissman. 1997. Developing lymph nodes collect CD4+ CD3− LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7:493-504. [DOI] [PubMed] [Google Scholar]
- 19.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 20.Roach, D. R., H. Briscoe, B. Saunders, M. P. France, S. Riminton, and W. J. Britton. 2001. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J. Exp. Med. 193:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruddle, N. H., C. N. Bergman, K. M. McGrath, E. G. Lingenheld, M. L. Grunnet, S. J. Padula, and R. B. Clark. 1990. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J. Exp. Med. 172:1193-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, C. A., T. Farrah, and R. G. Goodwin. 1994. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 76:959-962. [DOI] [PubMed] [Google Scholar]
- 23.Suen, W. E., C. M. Bergman, P. Hjelmstrom, and N. H. Ruddle. 1997. A critical role for lymphotoxin in experimental allergic encephalomyelitis. J. Exp. Med. 186:1233-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Boehmer, H. 1997. Lymphotoxins: from cytotoxicity to lymphoid organogenesis. Proc. Natl. Acad. Sci. USA 94:8926-8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh, C. M., M. Matloubian, C. C. Liu, R. Ueda, C. G. Kurahara, J. L. Christensen, M. T. Huang, J. D. Young, R. Ahmed, and W. R. Clark. 1994. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. USA 91:10854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware, C. F., T. L. VanArsdale, P. D. Crowe, and J. L. Browning. 1995. The ligands and receptors of the lymphotoxin system. Curr. Top. Microbiol. Immunol. 198:175-218. [DOI] [PubMed] [Google Scholar]
- 27.Whitmire, J. K., M. S. Asano, K. Murali-Krishna, M. Suresh, and R. Ahmed. 1998. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J. Virol. 72:8281-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]