Abstract
The flexion reflex can be elicited via stimulation of skin, muscle, and high-threshold afferents inducing a generalized flexion of the limb. In spinalized animal models this reflex is quite prominent and is strongly modulated by actions of hip proprioceptors. However, analogous actions on the flexion reflex in spinal cord injured (SCI) humans have not yet been examined. In this study, we investigated the effects of imposed static hip angle changes on the flexion reflex in ten motor incomplete SCI subjects when input from plantar cutaneous mechanoreceptors was also present. Flexion reflexes were elicited by low-intensity stimulation of the sural nerve at the lateral malleolus, and were recorded from the ipsilateral tibialis anterior (TA) muscle. Plantar skin stimulation was delivered through two surface electrodes placed on the metatarsals, and was initiated at different delays ranging from 3 to 90 ms. We found that non-noxious sural nerve stimulation induced two types of flexion reflexes in the TA muscle, an early, and a late response. The first was observed only in three subjects and even in these subjects, it appeared irregularly. In contrast, the second (late) flexion reflex was present uniformly in all ten subjects and was significantly modulated during hip angle changes. Flexion reflexes recorded with hip positioned at different angles were compared to the associated control reflexes recorded with hip flexed at 10°. Hip flexion (30°, 40°) depressed the late flexion reflex, while no significant effects were observed with the hip set in neutral angle (0°). Strong facilitatory effects on the late flexion reflex were observed with the hip extended to 10°. Moreover, the effects of plantar skin stimulation on the flexion reflex were also found to depend on the hip angle. The results suggest that hip proprioceptors and plantar cutaneous mechanoreceptors strongly modulate flexion reflex pathways in chronic human SCI, verifying that this type of sensory afferent feedback interact with spinal interneuronal circuits that have been considered as forerunners of stepping and locomotion. The sensory consequences of this afferent input should be considered in rehabilitation programs aimed to restore movement and sensorimotor function in these patients.
Keywords: Cutaneous afferents, Flexion reflex, Hip proprioceptors, Rehabilitation, Sensorimotor integration
Introduction
Flexion reflexes are polysynaptic responses securing withdrawal of skin areas from a potential offending stimulus (Sherrington 1910). However, beyond this nociceptive function, flexion reflexes appear also to be tightly coupled with posture and locomotion (Lundberg 1979, Grillner 1981). Because of the consistent actions of many different afferents involved in the generation of the flexion reflex (e.g., excitation of flexors and inhibition of extensors), these were termed as flexion reflex afferents (FRA) (see reviews of Lundberg 1979; Hultborn and Illert 1991), although diverse actions not belonging to the classical FRA model have also been reported (Schomburg 1990), verifying that these FRA responses, which ‘may’ evoke flexion reflexes, have access to other mutually exclusive alternative pathways, as originally proposed by Eccles and Lundberg (1959).
Excitation of FRA induces two different classes of flexion reflexes, for example, an early and a late reflex. The early flexion reflex in individuals with a spinal cord injury (SCI) resembles the early flexion reflex observed in neurologically intact subjects and in spinal cats (Meinck et al. 1985; Anden et al. 1966a). In the acute spinal cat, FRA excitation at a non-nociceptive level generates a widespread short latency excitation of many flexor motor pools (Anden et al. 1966a). However, activation of spinal monoaminergic terminals by intravenous injection of l-dopa depresses transmission in the short-latency flexion reflex and releases long-latency FRA pathways resulting in a long lasting excitation of flexor motor pools with concomitant extensor inhibition (Anden et al. 1966b, c). Because of the strong reciprocal expression of this response between excitatory interneuronal pathways (from ipsi- and contralateral FRA) to flexor and extensor motoneurons, Jankowska et al. (1967a, b) suggested that this reflex is also involved in spinal stepping, verified by the alternating bursts of flexor and extensor activity following administration of Nialamide (Jankowska et al. 1967a). Excitation of FRAs at a non-nociceptive level induces also a late flexion reflex in individuals with a motor complete SCI (Roby-Brami and Bussel 1987; Knikou and Conway 2005), which displays similar characteristics to that observed in the spinal cat after l-dopa (Roby-Brami and Bussel 1990, 1992).
Several studies have concentrated also on the nociceptive flexion withdrawal reflex, which combines flight and defence movements (Sherrington 1910). In healthy humans, this reflex has a short central conduction time and is thus related to early flexion reflexes (Hagbarth and Finer 1963). Following a SCI, the nociceptive early flexion reflex is still observable in the biceps femoris muscle (Dimitrijevic and Nathan 1968). It is apparent that nociception or cutaneous sensation determines the type of the flexion reflex elicited, and it should be mentioned that the nociceptive flexion reflex is not synonym to the late flexion reflex evoked by non-noxious stimulus, which presents the ‘‘flexion phase of the alternating reflex act of stepping,’’ as originally proposed by Sherrington (1910).
Previous work has shown that the flexion reflex is influenced by limb posture, site of stimulation, electrically-induced muscle contraction, and by actions of descending pathways (Hagbarth 1952; Andersen et al. 1999; Baxendale and Ferrell 1981; Schomburg 1990; Baldissera et al. 1981; Knikou and Conway 2005). In the acute spinal cat preparation, late flexion reflexes are generated by spinal interneurons that are strongly modulated by signals transmitting hip position (Grillner and Rossignol 1978). Similar findings have also been reported in spinal-injured man (Conway and Best 1995). Hip proprioceptors modulate substantially soleus H-reflex excitability, actions of spinal inhibitory interneurons exerted either from group Ia or Ib afferents, and interact with plantar cutaneous mechanoreceptors to alter soleus H-reflex magnitude in both SCI and able-bodied subjects (Knikou and Rymer 2002a, b; Knikou 2005 Knikou 2006). These actions signify that hip proprioceptors participate in complex interneuronal circuits that have been linked to human posture and locomotion (see review of Baldissera et al. 1981). Recent studies have demonstrated that hip-mediated afferent input modulates locomotion in human SCI by enhancing the swing phase following a pronounced hip extension (Dietz et al. 1998, 2002). Similar results were also reported in infants during walking, where hip proprioceptors interacted with load pathways leading to an enhanced swing driven by exaggerated hip extension (Pang and Yang 2000).
In the cat, activation of mechanoreceptor afferents of the foot sole delay the initiation of swing, inhibit late flexion reflexes, and promote stance (Duysens and Pearson 1976; Duysens 1977; Conway et al. 1995), signifying the functional interaction of the plantar mechanoreceptors with flexion reflex pathways. Recent studies support the notion that cutaneous afferents shape the locomotor pattern and contribute to the recovery of stepping following spinalization in the cat (Bouyer and Rossignol 2003). In humans, proprioceptive information from the foot sole contributes to the maintenance of erect posture (Kavounoudias et al. 2001), probably by strengthening the ongoing motoneuronal discharge (Kernell and Hultborn 1990).
Collectively, there is considerable evidence that activation of plantar cutaneous afferents or hip proprioceptors can independently influence flexion reflex excitability. The present study was undertaken to investigate the contribution of hip proprioceptors and plantar cutaneous afferents on flexion reflex excitability in ten motor incomplete SCI subjects under precisely controlled experimental conditions. We found that the late flexion reflex was either inhibited or facilitated depending on the imposed hip angle of the ipsilateral leg. We also found that excitation of plantar cutaneous mechanoreceptor afferents during hip angle changes either facilitated or depressed the flexion reflex depending on the hip angle. These findings strongly support the notion that hip-mediated afferent input interacts with complex spinal interneuronal circuits associated with control of walking in humans. Preliminary accounts of this work have been presented in abstract form (Knikou et al. 2005).
Materials and methods
Subjects
All experiments were approved by the Institutional Review Board (IRB) of the Northwestern University (Chicago, IL, USA), and conducted according to the 1964 Declaration of Helsinki. Informed consent was obtained from all subjects or from their parents prior to testing. Ten subjects with SCI ranging from C5–8 and T3–11 participated in the study. Subjects participating in the current tests are the same who participated in a previous study (Knikou 2005), and are identified with the same numbers.
The impairment scale of the American Spinal Injury Association (ASIA) (Maynard et al. 1997) was employed to classify the completeness of the lesion. Joint and muscle proprioception was assessed by asking the subjects to identify the position and direction of movement in the joints of both lower extremities with eyes closed, performed passively by the experimenter. For cutaneous sensation assessment, subjects were asked to identify with eyes closed, the site where a piece of cotton and/or needle touched them. None of the subjects had signs of lower motoneuron disorder. Subjects’ characteristics are summarized in Table 1.
Table 1.
SCI subjects’ characteristics
| Subjects | Gender | Age (years) | Post-injury (months) | Ashworth score | ASIA scale | Lesion level | Sensation | Medication/day |
|---|---|---|---|---|---|---|---|---|
| S1 | M | 23 | 78 | 1 | C | C5 | Intact | - |
| S3 | M | 27 | 26 | 0 | C | C5 | Intact | - |
| S4 | M | 45 | 54 | 2 | C | C6 | Intact | Baclofen 60 mg |
| S5 | M | 20 | 21 | 2 | C | T11 | Intact | Baclofen 20 mg |
| S6 | M | 17 | 27 | 0 | D | C6 | Intact | Baclofen 20 mg |
| S7 | F | 59 | 26 | 0 | C | T9 | Intact | Baclofen 10 mg |
| S8 | M | 15 | 26 | 2 | C | T3 | No sensation of cold/warm | - |
| S9 | M | 42 | 38 | 0 | C | C8 | Intact | Plavix Lipotor 75 mg |
| S10 | M | 50 | 186 | 1 | C | C5 | No sensation of cold/warm | Baclofen 30 mg |
| S11 | M | 50 | 162 | 3 | C | C5 | No sensation of cold/warm | - |
Spasticity at the ankle was scaled according to the Ashworth scale (Ashworth 1964). The completeness of the lesion was classified according to the American Spinal Injury Association (ASIA) scale (Maynard et al. 1997), and represents ASIA B: motor complete but sensory incomplete; ASIA C: sensory and motor incomplete, but with more than half of the muscles below injury level having a muscle grade of less than 3 out of 5, and ASIA D: sensory and motor incomplete with at least half of the muscles below the level of the injury having a muscle grade of 3 or higher. F, Female; M, Male.
Subject position
Each subject was transferred to the adjustable chair of a Biodex Rehabilitation/Testing System 2 (Biodex Medical Systems, Shirley, NY, USA), and was positioned supine. The right lower limb was secured to a knee ankle foot orthosis (KAFO), previously used in similar studies in SCI subjects (Knikou and Rymer 2002b; Knikou 2005). For each subject, adjustments were made to fit shank and thigh lengths, with ankle and knee joints set at 20° of plantar flexion and 30° of flexion, respectively. The KAFO was connected to the motor head of the Biodex. With the KAFO secured to the lower limb, the center of the subjects’ hip joint was aligned with the center of the Biodex motor head unit. The leg was moved in the sagittal plane by the experimenter. During the experiment, the head and arms were fully supported and subjects were asked to relax.
TA flexion reflex (elicitation and recording protocol)
The flexion reflex was evoked by electrical stimulation of the sural nerve, according to the procedures described by Roby-Brami and Bussel (1987). The sural nerve was stimulated at the right lateral submalleolar region through two disposable pre-gelled Ag-AgCl electrodes (Ambu Inc., Denmark). The rationale for choosing the sural nerve was that it is a pure sensory nerve, in contrast to the posterior tibial nerve which is a mixed nerve innervating both skin and muscle. The reflex was evoked by a 30 ms pulse train of 1–ms pulses delivered at 300 Hz once every 10 s, generated by a constant current stimulator (DS7A, Digitimer, UK). At this interstimulus interval, no evidence of habituation in the flexion reflex was encountered (Fuhrer 1976).
Surface electromyograms (EMG) were recorded by a single differential electrode (DE-2.1; DelSys, Boston, MA) placed over the ipsilateral TA muscle following light mechanical abrasion of the skin. The recorded EMG signals were amplified and band-pass filtered (10 Hz–1 kHz) before being sampled at 5 kHz (LabView PCI-MIO-16E-4, Texas National Instruments Inc., USA).
At the beginning of each test, the stimulus delivered to the sural nerve was set at 0 mA and was increased slowly so to observe the evoked response on the digital oscilloscope screen. The stimulus intensity during which the initial EMG TA activity was induced was identified as the reflex threshold (RT). This reflex response was categorized as early if its latency was less than 100 s, and as late when its latency was beyond 120 ms (Roby-Brami and Bussel 1987). Further increments in stimulus strengths were delivered so to observe if the expression of the flexion reflex changed with increments in the stimulation intensity. During testing, the sural nerve was stimulated at 1.5×RT. At this intensity, no limb movement was evoked and subjects reported no pain following sural nerve stimulation, indicating that the test afferent volley included large muscle and cutaneous afferents eliciting the non-nociceptive flexion reflex (Sherrington 1910).
Conditioning stimulation (electrical): excitation of plantar cutaneous afferents
The experimental protocol employed to excite plantar cutaneous afferents was identical to that previously employed to condition the soleus H-reflex with hip set at different angles in the same SCI subjects (Knikou 2005). Two Ag-AgCl surface electrodes (Ambu Inc., Ølstykke, Denmark) were placed transversely across the first and third metatarsals. Using a constant current stimulator (DS7, Digitimer, UK), the perceptual threshold (PT) that corresponded to the stimulus intensity first perceived by the subject was established. All conditioning stimuli were equivalent to 3×PT. At this stimulation intensity no movement of the intrinsic muscles of the foot was elicited and no pain was reported, signifying that the conditioning afferent volley excited mainly low-threshold plantar cutaneous afferents. Tactile units remain largely intact after chronic SCI (Thomas and Westling 1995), thus transmission of plantar skin sensation was not regarded as problematic in the current study.
The conditioning stimulus train consisted of five pulses with an inter-stimulus interval of 4.8 ms and pulse train duration of 24 ms. The conditioning stimulation train was repeated once every 10 s and preceded the flexion reflex at different conditioning test (C-T) intervals, measured as the time between the end of the pulse train delivered to the foot sole and the beginning of the pulse train delivered to the sural nerve. The C-T intervals tested ranged from 3 to 90 ms (3, 6, 9, 15, 30, 60, 90 ms), so to observe short-and long-lasting effects of plantar skin stimulation on the flexion reflex with hip positioned at different hip angles (10°, 30° flexion; 10° extension). These intervals were selected so to compare the effects on the flexion reflex with the ones previously reported on the soleus H-reflex (Knikou 2005). For each control and conditioned flexion reflex recorded in all subjects, a minimum of 15 reflex responses were acquired every 10 s and saved for further analysis.
Experimental procedures
For the tests examining the modulation pattern of the flexion reflex, the hip was passively flexed at 10°. Flexion reflexes recorded at this angle were considered to be the control reflexes. Reflexes were subsequently recorded with the hip positioned randomly at 30° and at 40° of flexion, at a neutral position (0°), and at 10° of extension. These flexion reflexes were considered as the conditioned ones and were expressed as a percentage of the mean size of the control reflex.
In the second test, flexion reflexes were conditioned with plantar cutaneous afferent stimulation at varying time delays with the hip positioned randomly at 10°, 30° of flexion, and at 10° of extension. The stimulus strength evoking the control flexion reflex recorded at different hip angles (30° of flexion, 10° of extension) was carefully adjusted for these reflexes to have similar amplitude with the control flexion reflex recorded with hip flexed at 10°. This allowed to counteract the modulatory effects that hip angle changes have on the flexion reflex and thus to compare the amount of facilitation/inhibition due to the conditioning stimulation across hip angles and subjects tested. The conditioned flexion reflexes were expressed as a percentage of the control flexion reflex recorded at each hip angle. Tests were completed in two different experimental sessions and each session lasted about 2 h.
Data analysis
The digitized EMG signals were full-wave rectified and the size of the flexion reflexes (early and/or late) were quantified by calculating the area under the EMG records corresponding to the period between the onset latency and the point in time at which the response fell below 1.5 standard deviation of the baseline EMG activity. In all tests, the conditioned reflexes were expressed as a percentage of the associated control flexion reflex.
In the tests examining the effects of static hip angle changes on flexion reflex excitability, the conditioned flexion reflexes recorded with hip flexed (30°, 40°), extended (10°), and at a neutral angle (0°) were expressed as a percentage of the mean size of the control flexion reflex recorded with hip flexed at 10°. A one-way analysis of variance (ANOVA) and post hoc Bonferroni tests were applied to the data to establish significant differences between control and conditioned reflexes.
The conditioned flexion reflexes that utilized stimulation of plantar cutaneous afferents were expressed as a percentage of the mean size of the control reflex recorded at each hip angle tested. For each subject, a one-way ANOVA along with post hoc Bonferroni tests were applied to the data to establish significant differences between control and conditioned reflexes across different C-T intervals at each hip angle. The mean size of the conditioned flexion reflex from each subject was then grouped according to the hip angle and the C-T interval investigated. A two-way ANOVA with repeated measures was applied to the data to establish if changes in the magnitude of the conditioned flexion reflex across hip angles and C-T intervals investigated were statistically significant different. For all statistical tests, alpha was set at 95% of confidence interval. The results are presented as mean values and standard error of the mean (SEM).
Results
Observations on the expression of the early and late flexion reflex
Examples of the two different classes of flexion reflexes are illustrated in Fig. 1. In subject 3, sural nerve stimulation at 7.4 (=RT), and at 9.2 mA induced both the early and the late flexion reflex, with the latter showing an increase in magnitude and duration with increases in the stimulus intensity. Similarly, in subject 5, at the stimulation intensity of 10.4 (=RT) and 17.4 mA both early and late flexion reflexes were present. However, this was not the case for subject 10 (Fig. 1). In this subject, sural nerve stimulation at 16.2 mA (=RT) and at 17.5 mA induced no early flexion reflex. The early flexion reflex was observed in three out of the ten subjects. Based on the expression of the early flexion reflex, it was apparent that this response was not regularly observed, whatever the stimulus intensity employed. The late flexion reflexes (>120 ms) were consistently observed under all conditions tested in all ten subjects. The occurrence of a late response was independent of whether or not an early response was present.
Fig. 1.
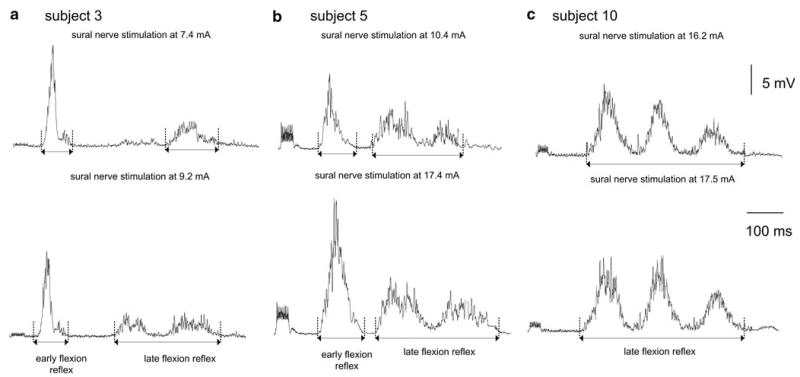
The full-wave rectified EMG averages of the two classes of the flexion reflex are identified by vertical cursors placed at the start and at the end of the corresponding EMG burst. For all three subjects, the top EMG corresponds to the stimulation intensity during which a response in the tibialis anterior muscle was first observed. In subject 10 (c), the early flexion reflex was absent when the sural nerve was stimulated at non-nociceptive stimulus intensities
Effects of imposed hip angle changes on the flexion reflex
An early flexion reflex (<100 ms) was present sporadically in three subjects (s3, s5, and s8) during hip angle changes. In subjects 3 and 5, the early flexion reflex was depressed with hip flexed at 30° and in neutral angle (0°), while with hip extended to 10° it was absent (or depressed). Interestingly, the absence of the early component with hip extended at 10° in these two subjects coincided with facilitation of the late flexion reflex. In contrast, in subject 8 the early flexion reflex was equally depressed with hip either flexed (30°, 40°) or extended at 10°.
In Fig. 2a, the effects of controlled static hip angle changes on the late flexion reflex are indicated for one subject (s7) with the hip flexed at 10°, 30°, and 40° and with the hip extended at 10°. As illustrated in the EMG recordings, positioning the hip in flexion angles resulted in depression of the flexion reflex, while a significant facilitation of the reflex was induced when the hip was extended to 10°.
Fig. 2.
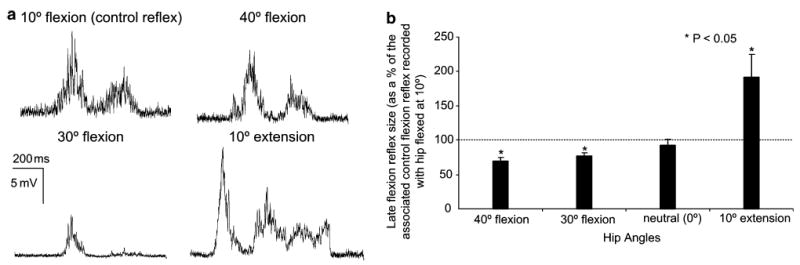
Effects of imposed static hip angle changes on the late flexion reflex.(a) The average late flexion reflex recorded with the ipsilateral hip set at 10° of flexion (control hip angle), 30° of flexion, 40° of flexion and at 10° of extension for one subject (s7) is presented. (b) Pool data showing the effects of hip angle variations on the late flexion reflex. For each hip angle tested, the average size of the conditioned late flexion reflexes (as a percentage of the control late flexion reflex recorded with hip flexed at 10°) was calculated for all subjects tested. Asterisks indicate cases of statistically significant differences between the control and the conditioned reflex sizes (P<0.05). Error bars indicate the SEM
Positioning the hip at different flexion and extension angles resulted in significant changes on the size of the late flexion reflex compared to control reflex values obtained with hip flexed at 10°. No significant changes were observed in the magnitude of the reflex with the hip positioned in neutral (0°) (Fig. 2). The late flexion reflex was reduced when the hip was positioned at 30° and at 40° of flexion, reaching overall amplitudes of only 77 ± 5% and 70 ± 6% of the control reflex values. In contrast, with the hip extended at 10°, the flexion reflex was significantly increased compared to control reflex values, reaching a mean level of 190 ± 34% of control reflex values (P<0.05). The amplitude of the conditioned flexion reflex across hip angles was statistically significant different (P<0.05).
Effects of plantar cutaneous stimulation on the flexion reflex during hip angle changes
In these tests, the early flexion reflex was sporadically observed across the three subjects (s3, s5, s8) resulting in no possible establishment of the conditioning effects of plantar cutaneous afferents excitation on the early flexion reflex during hip angle changes.
The late flexion reflex was present in all subjects following plantar cutaneous afferent excitation at all hip joint angles tested. A summary of the induced changes on the late flexion reflex is illustrated in Fig. 3. In this figure, each data point presents the average size of the conditioned flexion reflex of all subjects. Plantar cutaneous afferent stimulation delivered with hip extended to 10° resulted in no significant effects on the flexion reflex for C-T intervals that ranged from 3 to 9 ms (Fig. 3a). In contrast, for the longer C-T intervals tested (15–90 ms) the conditioned flexion reflex was facilitated. The reflex magnitude for these intervals ranged from 118 ± 6% to 140 ± 9% of the control reflex (P<0.05). Similarly, with hip flexed at 30° (Fig. 3c) no significant effects on the flexion reflex were encountered for C-T intervals that ranged from 3 to 30 ms, while at the C-T intervals of 60 and 90 ms the flexion reflex was facilitated. Moreover, excitation of plantar cutaneous afferents with hip flexed at 10° induced no significant effects on the flexion reflexes for C-T intervals of 3, 6, and 9 ms (P>0.05) (Fig. 3b). During the longer C-T intervals the flexion reflex was depressed compared to control reflex values (Fig. 3b).
Fig. 3.
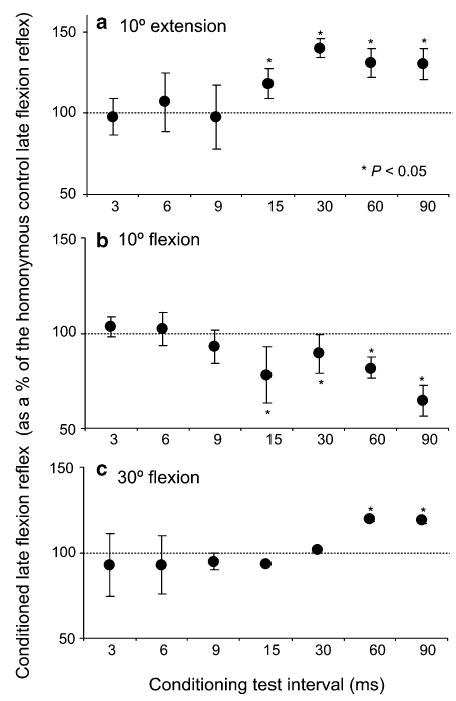
Time course of the effects of plantar cutaneous afferent excitation on the late flexion reflex with hip positioned at 10° of extension (a), 10° of flexion (b), and at 30° of flexion (c). For each conditioning test interval, the overall average size of the conditioned late flexion reflex is presented as a percentage of the associated control flexion reflex recorded at each hip angle tested. Asterisks indicate statistically significant differences between the conditioned and the control flexion reflex (P<0.05). Error bars represent the SEM
Discussion
The present work extends our previous observations that hip proprioceptors modulate significantly both the soleus H-reflex and the actions of spinal inhibitory interneurons in neurologically intact and in spinal-injured subjects (Knikou and Rymer 2002a, b; Knikou 2005 Knikou 2006). Current results suggest that hip proprioceptors are also able to strongly modulate flexion reflex pathways. During concurrent tactile input from the plantar aspect of the foot, hip proprioceptors interacted with the interneurons of the FRA pathway modulating the magnitude of the flexion reflex. Finally, the two classes of the flexion reflex did not depend on the intensity employed to excite FRA, with the early response shown to be absent (or depressed) in most subjects, suggesting that different interneuronal pathways might be involved during their expression.
Observations on the two types of the flexion reflex
The incidence of the early flexion reflex was low (30%). This is in agreement with previous observations in SCI subjects (Shahani and Young 1971; Roby-Brami and Bussel 1987; Knikou and Conway 2005), and in spinal l-dopa-treated cats (Conway et al. 1987; Anden et al. 1966a). In the present study, the test reflex stimulus intensity was normalized to the threshold of the first evoked TA EMG activity, whether this corresponded to the early or to the late flexion reflex. Thus, the presence of the early flexion reflex was not related to the stimulus intensity employed to excite the FRA. In addition, the late flexion reflex appeared independently of the early reflex (see Fig. 1c).
Our findings support the notion that the early and the late flexion reflexes are transmitted via different interneuronal pathways (Schomburg 1990). The irregularity of the appearance of the early flexion reflex suggests that it might be suppressed following a SCI in humans, in a similar manner to that reported for the acute and chronic spinal cats following l-dopa administration (Anden et al. 1966a, b).
Possible receptors contributing to the flexion reflex modulation during hip angle changes
Based on the current experimental protocol, it is difficult to identify the afferents or the receptors that contributed to the flexion reflex modulation during imposed hip angle changes. Nonetheless, evidence from studies in both humans and lower vertebrates suggest that the contribution of hip muscle spindle afferents responding to stretch cannot be ignored. Muscle spindle afferents are considered as receptors for detecting limb position (Prochazka et al. 1989), with their static discharges known to depend on the amount of the muscle shortening or lengthening (Ribot-Ciscar et al. 2003). These findings are in agreement with the reports of patients, stating that they can still detect the position of their hip after a total hip joint replacement (Grigg et al. 1973).
Stretch-sensitive group II afferents, considered as part of the FRA system, have diverse actions on both flexors and extensors in acute-spinal preparations at rest (reviewed in McCrea 2001). During fictive locomotion, FRA stimulation including the group II afferents, enhances the ongoing flexion or resets the step cycle to flexion (Schomburg et al. 1998), while changes in hip position, presumably via activity in flexor muscle stretch receptors and mainly group II afferents entrain the step cycle during fictive locomotion (Kriellaars et al. 1994; Perreault et al. 1995; Hiebert et al. 1996). Although the above studies were conducted under dynamic conditions, they signify the impact of hip flexor muscle spindle afferents in FRA spinal pathways. To conclude, it is likely that muscle spindle afferents responding to stretch contributed to the observed effects.
Interaction of hip proprioceptors with FRA pathways
Sustained changes in hip extension angle induced a significant facilitation of the late flexion reflex, while hip flexion resulted in reflex depression. No significant effects on the magnitude of the reflex were observed when the hip was positioned at a neutral angle (0°), when compared to the control reflex recorded with hip flexed at 10°. Our results show that during an imposed hip extension, the late flexion reflex magnitude increases, which is in line with the modulation pattern of the flexion reflex reported previously in SCI subjects, in human infants during walking, and in the spinal cat model (Conway and Best 1995; Pang and Yang 2000, 2001; Grillner and Rossignol 1978).
It is important to note that our studies rely on sustained changes in hip joint angle, and that the effects of dynamic hip movement may be different. Further, reorganization of the flexion reflex during assisted walking in human SCI might result in different response patterns. However, it has recently been reported that imposed extension of the hip produces a hip flexion moment in SCI subjects lying supine (Steldt and Schmit 2004), and augments the swing phase during body weight-assisted treadmill walking in human SCI (Dietz et al. 2002), suggesting that similar afferent systems and neuronal mechanisms might be involved under static and dynamic conditions.
At this point, we should consider the possibility that if motoneuronal excitability is increased by hip extension then any afferent input, regardless of origin, will be magnified. In this case, the question that arises is whether the effects of hip angle changes were mediated by changes in motoneuronal excitability alone or whether hip proprioceptors influenced directly the excitability of interneurons of the FRA pathway. In this respect, we observed that the two classes of the flexion reflex (early and late) were modulated differently during hip angle changes. A depression of the early reflex was observed with hip angle changes, but was independent of the hip angle (flexion vs. extension). In two of three subjects this reflex component was absent (or depressed) with hip extended at 10°, when the associated late flexion reflex was facilitated. Given that the early and late flexion reflex effects were different during hip angle changes, this argues against a simple increase in motoneuronal excitability. This conclusion is further supported by the interaction of hip proprioceptors with spinal inhibitory interneurons such as those of reciprocal Ia, presynaptic, and Ib inhibitory interneurons in spinal-injured man (Knikou 2005). Nonetheless, future studies using more detailed methods are necessary to address the relative contribution of interneurons in the expression of the spinal reflexes during static or dynamic imposed hip angle changes.
Based on our results and on the spinal origin of the late flexion reflex (Baldissera et al. 1981; Roby-Brami and Bussel 1987), it is apparent that the effects of hip angle changes were transmitted through spinal interneurons. The similar modulation pattern observed between the late flexion reflex and the soleus H-reflex during hip angle changes in spinal-injured man (Knikou and Rymer 2002b) also indicate that key spinal interneuronal circuits interact systematically depending on the direction of the movement.
Interaction of plantar cutaneous afferents and FRA during hip angle changes
The present study supports the significant actions of low-threshold plantar cutaneous afferents on spinal reflex pathways in individuals with an established chronic SCI. Under static conditions, excitation of plantar cutaneous afferents with hip extended and flexed at 10° resulted in a significant long lasting facilitation and inhibition of the late flexion reflex, respectively. With the hip flexed at 30°, the late flexion reflex was facilitated during the longest C-T intervals investigated (60 and 90 ms) (see Fig. 3).
We have previously shown that activation of low-threshold plantar cutaneous afferents with identical stimulation parameters in the same SCI subjects induce only facilitatory effects on the soleus H-reflex with the amount of reflex facilitation to depend on the hip angle (see Fig. 2 in Knikou 2005). Our findings agree well with the notion that cutaneous mechanoreceptors access ‘alternative reflex pathways’ to flexors and extensors so to promote stance (Hagbarth 1952; Schomburg 1990; Burke 1990). Further, sural nerve stimulation and excitation of plantar cutaneous afferents were both low-threshold, while the distances of the stimulation sites to spinal segmental levels associated with the flexion reflex were equivalent. Thus, we can assume that the conduction time of the testing and conditioning stimuli were similar. Therefore, given the long latency of the flexion reflex and the long duration of the C-T intervals tested, it is likely that the reflex modulation was mediated through long latency spinal networks, which might also be involved in the spinal locomotor centers.
Low-threshold stimulation of the deep or plantar nerves during late flexion reflexes in decerebrate spinal cats injected with L-dopa is effective in terminating the flexor phase (Conway et al. 1995), verifying that the effects of cutaneous afferents on the FRA pathways are of spinal origin. The demonstration that activation of plantar cutaneous afferents can influence differently the flexion reflex during controlled hip angle variations suggests the presence of an interneuronal switch triggered by concurrent input from hip proprioceptors and cutaneous mechanoreceptors.
Earlier studies have reported that stimulation of low-threshold sensory afferents induces a bimodal excitability pattern in extensor motoneurons, for example, an early inhibition that is followed by a substantial late facilitation of the H-reflex (Delwaide et al. 1981). It was later proposed that cutaneous input modulates transmission in presynaptic inhibitory pathways of both flexor and extensor group Ia afferents in man (Nakashima et al. 1990; Iles 1996). Studies addressing similar effects in human SCI have shown that sural nerve stimulation exciting non-nociceptive afferents facilitates the ipsilateral TA H-reflex and decreases the heteronymous Ia facilitation exerted from the quadriceps onto soleus α motoneurons (Roby-Brami and Bussel 1990). These findings suggest that FRA stimulation induces presynaptic inhibition of transmission of Ia afferent terminals, and that cutaneous afferents from the foot have spinal oligosynaptic or polysynaptic connections with the interneurons of the FRA pathway. Nonetheless, a specific neuronal mechanism involved in the flexion reflex modulation under the current experimental protocol cannot be established.
Further, plantar cutaneous afferents include mechanoreceptors mediating sensation of the applied load (Kavounoudias et al. 2000) contributing to body posture awareness (Roll et al. 2002). However, given the lack of load input in the present experiments, modulation of these pathways might be different under loading conditions. Thus, there is a need for future studies to address spinal integration of these afferent signals to both flexors and extensors during assisted walking in human SCI.
Possible effects of intersubject variability
The spinal lesions were classified as ASIA C in nine out of ten subjects with their injury level to range from C5 to T9. Similarly, six out of the ten subjects were taking varying amounts of antispastic medications at the time of the study. We found however no significant differences in the magnitude of the conditioned flexion reflex across conditioning protocols when comparing data from subjects on spasticity medications to those without medications (P>0.05). Nonetheless, the sample size for this comparison was small. The antispastic effect of baclofen, for example, on the expression of long-latency flexion reflex pathways has not been identified. Thus, the study of such effects is an important topic for future studies since hip proprioceptive input influences walking in individuals with SCI (Dietz et al. 2002).
Conclusions and considerations for SCI rehabilitation
The late flexion reflex in human SCI has been compared with the late flexion reflex observed in l-dopa-treated acute and chronic spinal cats (Roby-Brami and Bussel 1990, 1992; Anden et al. 1966a, b). This reflex in the cat and in man is considered to utilize interneurons of spinal locomotor centers (Lundberg 1979; Baldissera et al. 1981; Bussel et al. 1989). Our findings demonstrate that the late flexion reflex in spinal-injured man is influenced by afferent feedback from both cutaneous mechanoreceptors and hip proprioceptors.
Flexion reflexes are also utilized to promote the swing phase of gait during functional electrical stimulation (FES)-assisted walking in individuals with a SCI (Postans et al. 2004). In this set up, the flexion reflex provides a synchronized flexion movement of hip, knee, and ankle promoting the swing phase of gait. It is thus advisable for optimizing this intervention FRA excitation to occur before the hip has reached a neutral angle.
In the light of the emerging new findings during body weight-assisted treadmill walking (Harkema 2001), employed alone or in combination with FES utilizing the flexion reflex to assist stepping after SCI, and that the influence of plantar sensitivity on postural control is likely to increase in a neurological disorder (Meyer et al. 2004), the activity of these interneuronal pathways should be further investigated during assisted walking in motor incomplete SCI patients, thus afferent input of proper amplitude and time be present during their locomotor training.
Acknowledgments
Authors are in debt to the subjects for their willingness to participate on many hours of experimentation throughout the project. This study was supported by the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Grant No. 5R03HD43951 (MK).
References
- Anden NE, Jukes MG, Lundberg A, Vyklicky L. The effect of Dopa on the spinal cord 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966a;67:373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Anden NE, Jukes MG, Lundberg A. The effect of Dopa on the spinal cord. 2. A pharmacological analysis. Acta Physiol Scand. 1966b;67:387–397. doi: 10.1111/j.1748-1716.1966.tb03325.x. [DOI] [PubMed] [Google Scholar]
- Anden NE, Jukes MG, Lundberg A, Vyklicky L. The effect of Dopa on the spinal cord. 3. Depolarization evoked in the central terminals of ipsilateral Ia afferents by volleys in the FRA. Acta Physiol Scand. 1966c;68:322–336. [Google Scholar]
- Andersen OK, Sonnenborg FA, Arendt-Nielsen L. Modular organization of human leg withdrawal reflexes elicited by electrical stimulation of the foot sole. Muscle Nerve. 1999;22:1520–1530. doi: 10.1002/(sici)1097-4598(199911)22:11<1520::aid-mus6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practioner. 1964;192:540–542. [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M (1981) Integration in spinal neuronal systems. In: Handbook of physiology, the nervous system II, pp 509–595
- Baxendale RH, Ferrell WR. The effect of knee joint discharge on transmission in flexion reflex pathways in decerebrate cats. J Physiol (Lond) 1981;315:231–242. doi: 10.1113/jphysiol.1981.sp013744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer LJG, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol. 2003;90:3640–3653. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- Burke D. Exteroceptive reflexes and flexor spasms. Adv Neural Regenerat Res. 1990:379–389. [Google Scholar]
- Bussel B, Roby-Brami A, Yakovleff A, Bernis N. Late flexion reflex in paraplegic patients: evidence for a spinal stepping generator. Brain Res Bull. 1989;22:53–56. doi: 10.1016/0361-9230(89)90127-5. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Conway BA, Best N. The effect of changes in static hip position on the flexion reflex in motor complete spinal cord injured humans. J Physiol Suppl. 1995;487:75–76. [Google Scholar]
- Conway BA, Scott DT, Riddell JS (1995) The effects of plantar nerve stimulation on long latency flexion reflexes in the acute spinal cat. In: A. Taylor, M.N. Gladden, R. Durbada (eds) Alpha and gamma motor systems. Plenum Press, pp. 593–595
- Delwaide PJ, Crenna P, Fleron MH. Cutaneous nerve stimulation and motoneuronal excitability. I: Soleus and tibialis anterior excitability after ipsilateral and contralateral sural nerve stimulation. J Neurol Neurosurg Psychiatry. 1981;44:699–707. doi: 10.1136/jnnp.44.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Wirtz M, Curt A, Colombo G. Locomotor pattern in paraplegic patients: training effects recovery of spinal cord function. Spinal Cord. 1998;36:380–390. doi: 10.1038/sj.sc.3100590. [DOI] [PubMed] [Google Scholar]
- Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Nathan PW. Studies of spasticity in man. 3. Analysis of reflex activity evoked by noxious cutaneous stimulation. Brain. 1968;91:349–368. doi: 10.1093/brain/91.2.349. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976;24:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- Duysens J. Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J Neurophysiol. 1977;40:737–751. doi: 10.1152/jn.1977.40.4.737. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Lundberg A. Synaptic actions in motoneurones by afferents which may evoke the flexion reflex. Arch Ital Biol. 1959;97:199–221. [Google Scholar]
- Fuhrer MJ. Interstimulus interval effects on habituation of flexor withdrawal activity mediated by the functionally transected human spinal cord. Arch Phys Med Rehabil. 1976;57:577–582. [PubMed] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Grillner S (1981) Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB (ed) Handbook of physiology: the nervous system: motor control, Vol. 2. Am. Physiol. Soc. Bethesda, pp. 1179–1236
- Grigg P, Finerman GA, Riley LH. Joint-position sense after total hip replacement. J Bone J Surg. 1973;55:1016–1025. [PubMed] [Google Scholar]
- Hagbarth KE. Excitatory and inhibitory skin areas for flexor and extensor motoneurones. Acta Physiol Scand Suppl. 1952;94:1–57. [PubMed] [Google Scholar]
- Hagbarth KE, Finer BL. The plasticity of human withdrawal reflexes to noxious skin stimuli in lower limbs. Prog Brain Res. 1963;1:65–81. [Google Scholar]
- Harkema SJ. Neural plasticity after human spinal cord injury: applications of locomotor training to the rehabilitation of walking. Prog Clin Neurosci. 2001;7:455–468. doi: 10.1177/107385840100700514. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M (1991) How is motor behavior reflected in the organization of spinal systems. In: Humphrey DR, Freud HJ (eds) Motor control: concept and issues, Wiley, New York, pp.49–73
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol (Lond) 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand. 1967a;70:369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand. 1967b;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll J-P. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol (Lond) 2001;532:869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools. Brain Res. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Effects of changes in hip joint angle on H-reflex excitability in humans. [Erratum in Exp Brain Res (2002) 144:558] Exp Brain Res. 2002a;143:149–159. doi: 10.1007/s00221-001-0978-4. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Hip angle induced modulation of H reflex amplitude, latency and duration in spinal cord injured humans. Clin Neurophysiol. 2002b;113:1698–1708. doi: 10.1016/s1388-2457(02)00285-7. [DOI] [PubMed] [Google Scholar]
- Knikou M (2005) Effects of hip joint angle changes on intersegmental spinal coupling in human spinal cord injury. Exp Brain Res DOI: 10.1007/s00221-005-0046-6, published online on July 30, 2005 [DOI] [PMC free article] [PubMed]
- Knikou M, Conway BA (2005) Effects of electrically induced muscle contraction on flexion reflex in human spinal cord injury. Spinal Cord DOI: 10.1038/sj.sc.3101772 [DOI] [PubMed]
- Knikou M, Rymer WZ, Kay E (2005) Hip proprioceptors interact with plantar cutaneous afferents modulating the flexion reflex in human spinal cord injury. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience
- Knikou M. Effects of changes in hip position on actions of spinal inhibitory interneurons in humans. Int J Neurosci. 2006;116(6) doi: 10.1080/00207450600675167. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Lundberg A (1979) Multisensory control of spinal reflex pathways. In: R. Granit O. Pomeiano (ed), Reflex control of posture and movement. Elsevier, Amsterdam, pp 11–28 [DOI] [PubMed]
- Maynard FM, Bracken MB, Creasey G, Ditunno JF, Donovan WH, Ducker TB, Garber SL, Marini RJ, Stover SL, Tator CH, Waters RL, Wilberger JP, Young W. International standards for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;5:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol (Lond) 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinck H-M, Kuster S, Benecke R, Conrad B. The flexor reflex-influence of stimulus parameters on the reflex response. Electroencephalogr Clin Neurophysiol. 1985;61:287–298. doi: 10.1016/0013-4694(85)91095-8. [DOI] [PubMed] [Google Scholar]
- Meyer PF, Oddsson LI, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res. 2004;156:502–512. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Rothwell JC, Day BL, Thompson PD, Marsden CD. Cutaneous effects on presynaptic inhibition of flexor Ia afferents in the human forearm. J Physiol (Lond) 1990;426:369–380. doi: 10.1113/jphysiol.1990.sp018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiol (Lond) 2000;528:389–404. doi: 10.1111/j.1469-7793.2000.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Yang JF. Interlimb co-coordination in human infant stepping. J Physiol (Lond) 2001;533:617–625. doi: 10.1111/j.1469-7793.2001.0617a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M-C, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol (Lond) 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postans NJ, Hasler JP, Granat MH, Maxwell DJ. Functional electric stimulation to augment partial weight-bearing supported treadmill training for patients with acute incomplete spinal cord injury: a pilot study. Arch Phys Med Rehabil. 2004;85:604–610. doi: 10.1016/j.apmr.2003.08.083. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Trend PSJ, Hulliger M, Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res. 1989;80:61–74. doi: 10.1016/s0079-6123(08)62200-1. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Bergenheim M, Albert F, Roll J-P. Proprioceptive population coding of limb position in humans. Exp Brain Res. 2003;149:512–519. doi: 10.1007/s00221-003-1384-x. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Long latency spinal reflex in man after flexor reflex afferent stimulation. Brain. 1987;110:707–725. doi: 10.1093/brain/110.3.707. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Effects of FRA stimulation on the soleus H-reflex in patients with a complete spinal cord lesion: evidence for presynaptic inhibition of Ia transmission. Exp Brain Res. 1990;81:593–601. doi: 10.1007/BF02423509. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Inhibitory effects on flexor reflexes in patients with a complete spinal cord lesion. Exp Brain Res. 1992;90:201–208. doi: 10.1007/BF00229272. [DOI] [PubMed] [Google Scholar]
- Roll R, Kavounoudias A, Roll J-P. Cutaneous afferents from human plantar sole contribute to body posture awareness. NeuroReport. 2002;13:1957–1961. doi: 10.1097/00001756-200210280-00025. [DOI] [PubMed] [Google Scholar]
- Schomburg ED. Spinal sensorimotor systems and their supraspinal control. Neurosci Res. 1990;7:265–340. doi: 10.1016/0168-0102(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp Brain Res. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Shahani BT, Young RR. Human flexor reflexes. J Neurol Neurosurg Psychiatry. 1971;34:616–627. doi: 10.1136/jnnp.34.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex and reflex stepping and standing. J Physiol (Lond) 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steldt RE, Schmit BD. Modulation of coordinated muscle activity during imposed sinusoidal hip movements in human spinal cord injury. J Neurophysiol. 2004;92:673–685. doi: 10.1152/jn.00677.2003. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Westling G. Tactile unit properties after human cervical spinal cord injury. Brain. 1995;118:1547–1556. doi: 10.1093/brain/118.6.1547. [DOI] [PubMed] [Google Scholar]


