Abstract
Objective
Influenza-associated deaths in healthy children that were reported during the 2003–2004 influenza season heightened the public awareness of the seriousness of influenza in children. In 1996–1998, a pivotal phase III trial was conducted in children who were 15 to 71 months of age. Live attenuated influenza vaccine, trivalent (LAIV-T), was shown to be safe and efficacious. In a subsequent randomized, double-blind, placebo-controlled LAIV-T trial in children who were 1 to 17 years of age, a statistically significant increase in asthma encounters was observed for children who were younger than 59 months. LAIV-T was not licensed to children who were younger than 5 years because of the concern for asthma. We report on the largest safety study to date of the recently licensed LAIV-T in children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a 4-year (1998–2002) community-based trial that was conducted at Scott & White Memorial Hospital and Clinic (Temple, TX).
Methods
An open-label, nonrandomized, community-based trial of LAIV-T was conducted before its licensure. Medical records of all children were surveyed for serious adverse events (SAEs) 6 weeks after vaccination. Health care utilization was evaluated by determining the relative risk (RR) of medically attended acute respiratory illness (MAARI) and asthma rates at 0 to 14 and 15 to 42 days after vaccination compared with the rates before vaccination. Medical charts of all visits coded as asthma were reviewed for appropriate classification of events: acute asthma or other. We evaluated the risk for MAARI (health care utilization for acute respiratory illness) 0 to 14 and 15 to 42 days after LAIV-T by a method similar to the postlicensure safety analysis conducted on measles, mumps, and rubella and on diphtheria, tetanus, and whole-cell pertussis vaccines.
Results
All children regardless of age were administered a single intranasal dose of LAIV-T in each vaccine year. In the 4 years of the study, we administered 18 780 doses of LAIV-T to 11 096 children. A total of 4529, 7036, and 7215 doses of LAIV-T were administered to children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age, respectively. In vaccination years 1, 2, 3, and 4, we identified 10, 15, 11, and 6 SAEs, respectively. None of the SAEs was attributed to LAIV-T. In vaccination years 1, 2, 3, and 4, we identified 3, 2, 1, and 0 pregnancies, respectively, among adolescents. All delivered healthy infants. The RR for MAARI from 0 to 14 and 15 to 42 days after LAIV-T was assessed in vaccinees during the 4 vaccine years. Compared with the prevaccination period, there was no significant increase in risk in health care utilization attributed to MAARI from 0 to 14 and 15 to 42 days after vaccination in children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in the 4 vaccine years. In children who were 18 months to 4 years of age, there was no significant increase in the risk in health care utilization for MAARI, MAARI subcategories (otitis media/sinusitis, upper respiratory tract illness, and lower respiratory tract illness), and asthma during the 0 to 14 days after vaccination compared with the prevaccination period. No significant increase in the risk in health care utilization for MAARI, MAARI subcategories, and asthma was detected when the risk period was extended to 15 to 42 days after vaccination, except for asthma events in vaccine year 1. A RR of 2.85 (95% confidence interval [CI]: 1.01–8.03) for asthma events was detected in children who were 18 months to 4 years of age but was not significantly increased for the other 3 vaccine years (vaccine year 2, RR: 1.42 [95% CI: 0.59–3.42]; vaccine year 3, RR: 0.47 [95% CI: 0.12–1.83]; vaccine year 4, RR: 0.20 [95% CI: 0.03–1.54]). No significant increase in the risk in health care utilization for MAARI or asthma was observed in children who were 18 months to 18 years of age and received 1, 2, 3, or 4 annual sequential doses of LAIV-T. Children who were 18 months to 4 years of age and received 1, 2, 3, or 4 annual doses of LAIV-T did not experience a significant increase in the RR for MAARI 0 to 14 days after vaccination; this was also true for children who were 5 to 9 and 10 to 18 years of age.
Conclusions
We observed no increased risk for asthma events 0 to 14 days after vaccination in children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age, In vaccine year 1, children who were 18 months to 4 years of age did have a significantly higher RR (2.85; 95% CI: 1.01–8.03) for asthma events 15 to 42 days after vaccination. In vaccine year 2, the formulation of LAIV-T was identical to the vaccine formulation used in vaccine year 1; however, in children who were 18 months to 4 years of age, no statistically significant increased risk was detected for asthma events 15 to 42 days after vaccination. Similarly, in vaccine years 3 and 4, children who were 18 months to 4 years of age did not have a statistically significant increased risk for asthma events 15 to 42 days after vaccination. Also, LAIV-T did not increase the risk for asthma in children who received 1, 2, 3, or 4 annual doses of LAIV-T. Although the possibility for a true increased risk for asthma was observed in 1 of 4 years in children who were 18 months to 4 years at 15 to 42 days after vaccination, it is more likely that the association is a chance effect because of the 190 comparisons made without adjustment for multiple comparisons. We conclude that LAIV-T is safe in children who are 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age. The hypothesis that LAIV-T is associated with an increase in asthma events in children who are younger than 5 years is not supported by our data. Reassessment of the lower age limit for use of LAIV-T in children is indicated.
Keywords: asthma exacerbation, children and adolescents, health service utilization, influenza vaccine, outcome assessment
ABBREVIATIONS: RSV, respiratory syncytial virus; LAIV-T, live attenuated influenza vaccine, trivalent; MAARI, medically attended acute respiratory illness; FDA, Food and Drug Administration; SAE, serious adverse event; SWHP, Scott & White Health Plan; RR, relative risk; ICD-9, International Classification of Diseases, Ninth Revision; CI, confidence interval
Acute respiratory illness is the leading burden of disease worldwide.1 Influenza and respiratory syncytial virus (RSV) are the major viral respiratory pathogens of children. During any given year, influenza or RSV is the leading cause of hospitalization for lower respiratory tract disease.2–4 RSV causes hospitalizations in children, primarily in those who are younger than 1 year.5 Influenza causes high rates of hospitalization in children who are younger than 5 years, and the rate is comparable to that observed in adults who are older than 50 years.6–9 Infection rates and morbidity ascribed to influenza are greatest among individuals with minimal previous exposure to influenza.10–14 In interpandemic periods, infants and children are highly susceptible to influenza because as a group, they either have not been infected or have been infected less often with 1 of the major influenza virus types (H1N1, H3N2, or influenza B) compared with adults. School-aged children also experience high rates of influenza infection, febrile illness, and school absenteeism.15 During an influenza outbreak, an estimated 63 school days were missed for every 100 children.15 Increase in work-related absenteeism also occurred among the parents who missed ~1 day of work for every 3 days of school missed by the children. Significant hospitalization and medical visits occur in school-aged children with chronic medical conditions.16,17 In children, influenza is recognized for causing secondary bacterial pneumonia and serious disease associated with organ systems other than the respiratory tract.18–22 Hospitalization as a result of acute febrile illness and central nervous system disease adds to the spectrum of serious illness in children attributed to influenza.2,19,21,22 Influenza-associated deaths in healthy children that were reported during the 2003–2004 influenza season heightened the public awareness of the seriousness of influenza in children.23
In 1996–1998, a pivotal phase III trial was conducted in children who were 15 to 71 months of age.23–25 Live attenuated influenza vaccine, trivalent (LAIV-T), was shown to be safe and efficacious.24–26 In a subsequent randomized, double-blind, placebo-controlled LAIV-T trial in children who were 1 to 17 years of age, a statistically significant increase in asthma encounters was observed for children who were younger than 59 months.27 LAIV-T was licensed in June 2003 in the United States for use in healthy individuals 5 to 49 years of age. LAIV-T was not licensed for use in children who were younger than 5 years because of the concern for asthma. LAIV-T is highly efficacious in children who are younger than 5 years, and they would benefit from a safe influenza vaccine.
An open-label, nonrandomized, community-based trial in children who were 18 months through 18 years of age was conducted in Texas from 1997 to 2002. We report on the largest safety study to date of LAIV-T in children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age. LAIV-T was equally safe among all age groups. The hypothesis that LAIV-T is associated with an increase for acute asthma events was not supported by our trial.
METHODS
Study Design
An open-label, nonrandomized, community-based trial using LAIV-T was conducted from 1997 to 2002 in children who were 18 months through 18 years of age before LAIV-T licensure.28–30 The first year was to establish a baseline for medically attended acute respiratory illness (MAARI) rate in the community. In the subsequent 4 years, children were enrolled after satisfying all of the inclusion criteria and none of the exclusion criteria. Children were excluded from the study when they had significant egg allergy, were immunocompromised, lived with a household member who was immunodeficient, had a chronic condition for which the inactivated influenza vaccine was recommended, or were pregnant or planned to become pregnant. Children who were 2 years and older were excluded when they were hospitalized or seen in the emergency department in the previous 12 months for asthma, reactive airway disease, or wheezing illness or in the previous 6 months when they were younger than 2 years. LAIV-T administration was postponed for children with fever within 2 days of vaccination or history of receiving or planning to receive an inactivated or live vaccine within 14 or 28 days of LAIV-T, respectively. Children with a history of wheezing or mild, intermittent asthma were not excluded. Mild, intermittent asthma was defined as children who did not use steroids (oral or inhaled) or bronchodilator therapy daily or every other day for asthma control. LAIV-T administration was postponed in children who had mild, intermittent asthma and a history of wheezing in the past 2 weeks that required bronchodilator therapy for 2 or more consecutive days. Children were not excluded when they used daily nasal steroids or had exercise-induced asthma that required bronchodilator treatment before exercise. The institutional review boards of Baylor College of Medicine, Scott & White Memorial Hospital and Clinic, and Texas Department of Health approved this study. Informed consent was obtained from the legal guardians of enrolled participants or adult participants.
Vaccine
At enrollment all participants received one 0.5-mL dose of LAIV-T (~107 median tissue culture infectious dose of each of 3 vaccine components in egg allantoic fluid with sucrose-phosphate-glutamate) by nasal spray. LAIV-T was provided by MedImmune Vaccines, formerly Aviron (Mountain View, CA), frozen in single-dose, intranasal applicators. Each year, LAIV-T contained the 3 influenza virus strains that were antigenically comparable to those that were chosen by the Food and Drug Administration (FDA) for the licensed inactivated influenza vaccine. In vaccine year 1 (1998–1999), the formulation of LAIV-T was A/Beijing/262/95 (H1N1), A/Sydney/05/97 (H3N2), and B/Beijing/184/93-like. In vaccine year 2 (1999–2000), the vaccine formulation was unchanged. In vaccine year 3 (2000–2001), A/New Caledonia/20/99 replaced the previous H1N1 vaccine strain. In vaccine year 4 (2001–2002), A/Panama/2007/99 (H3N2) and B/Sichuan/379/99-like replaced the previous H3N2 and B vaccine strains.
Safety Assessment
All enrolled participants were surveyed for serious adverse events (SAEs) and pregnancies during the 6 weeks after vaccination. Monthly administrative database searches for SAEs and pregnancies were conducted for vaccine recipients who were patients of the Scott & White Memorial Hospital and Clinic. A 6-week follow-up postcard or a telephone contact was performed for all vaccine participants except for those who were members of the Scott & White Health Plan (SWHP). The monthly administrative database searches provided the 42-day follow-up information for SWHP vaccinees. An SAE was defined as an event that was fatal; was immediately life-threatening; or resulted in or prolonged a hospitalization, a permanent or substantial disability, an important medical event, or a congenital anomaly (an offspring of participant regardless of the time to diagnosis). SAEs that occurred within the first 42 days after vaccination regardless of causality and all vaccine-related SAEs for the duration of the study were reported to the FDA and institutional review boards. All SAEs were followed with appropriate medical management until resolved.
The likelihood (Pr) of observing an SAE is Pr = 1 − e−xy, where x is the probability of an SAE in an individual participant and y is the number of observed participants. This formula can address the number of participants that must be followed to be certain that LAIV-T is not associated with a significant risk for SAEs.
Increase in health care utilization attributed to LAIV-T was evaluated by determining the relative risk (RR) of MAARI at 0 to 14 days (risk period) and 15 to 42 days (risk period) after vaccination compared with the rates before vaccination (reference period). Demographic information and MAARI and asthma events were extracted from the administrative database. MAARI was identified by database search for specific International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes. The ICD-9 codes for MAARI included those for otitis media and sinusitis, (381–383,461) upper respiratory tract illness (79, and 460–487), and lower respiratory tract illness (464, 466, 480–487, 490–492, 494–496, 510–513, 515–516, 518, and 786.1). Asthma ICD-9 codes were 493 and 486.07. Each medical encounter had up to 2 ICD-9 codes. Medical encounters included those in clinics, emergency departments, and hospitals. Multiple entries on a single day were counted as 1 encounter. Some enrollees were referred for vaccination by clinicians on the same day of a clinic visit; therefore, medical charts were reviewed for all events on day 0 of vaccination, and appropriate assignments were made to risk or reference period. Day 0 events that were documented to occur before vaccination were assigned to the reference period; all others were assigned to the risk period. Medical charts of all asthma visits were reviewed to classify appropriately the event as acute asthma or other. The MAARI and asthma data used in the analysis were from participants of the SWHP, a subset of the vaccinated group, because it contained more complete information on health care utilization compared with that available for patients in the Scott & White Memorial Hospital and Clinic administrative database.
Statistical Methods
We evaluated the risk for MAARI (health care utilization for acute respiratory illness) 0 to 14 and 15 to 42 days after LAIV-T by a method similar to the safety evaluation of childhood vaccines.31,32 Poisson regression analysis, a multivariate analysis for log-linear modeling of incidence rates, was used to estimate the RR of an event controlling for age category (18 months–4 years, 5–9 years, and 10–18 years) and time period determined from virus surveillance data.33–35 The RR of MAARI and asthma encounters 0 to 14 and 15 to 42 days after vaccination (risk periods) were compared with that occurring in the prevaccination interval (reference period). Age of a study participant was held constant for all analyses. The specific age of a study participant was used only to assign a participant to an age category (18 months–4 years, 5–9 years, or 10–18 years). Age category was used in the analysis and treated as a nominal variable. Three time intervals were identified for each enrollment year except in the last year (2001–2002), which included 2 time intervals. These time intervals reflected the activity of respiratory viruses that would affect MAARI. For example, higher background rates of MAARI not attributed to LAIV-T would be expected during a parainfluenza or RSV outbreak. Time interval was treated as a nominal variable in the analyses.
The rates of MAARI events by age category were calculated by standard methods. The number of children vaccinated per time interval by age category was determined using the variables date of vaccination and age at vaccination. For each age category, the numbers of child-days during the 0 to 14 and 15 to 42 days after vaccination (risk periods) and prevaccination (reference period) periods were counted, dependent on the date of vaccination and time interval, and summed. Total child-days produced the denominators for the rates calculated for 0 to 14 and 15 to 42 days after vaccination and prevaccination periods. For each age category, MAARI and asthma events were counted, dependent on date of vaccination and time interval, and summed. The totals of MAARI and asthma events provided the numerators for the rates calculated for 0 to 14 and 15 to 42 days after vaccination and prevaccination periods.
RR Estimates
The risk ratios of MAARI events comparing the rate of MAARI and asthma during 0 to 14 and 15 to 42 days after vaccination (risk periods) with the rate of MAARI and asthma during the prevaccination period (reference period) were calculated using Poisson regression analysis while controlling for age category and time interval.33–35 The Poisson models were generated using the maximum likelihood technique. The estimated coefficients were transformed to incidence rate ratios. The 95% confidence intervals (CIs) were similarly transformed. These analyses were similar to the postlicensure safety analysis conducted on measles, mumps, and rubella and on diphtheria, tetanus, and whole-cell pertussis vaccines.31,32
RESULTS
SAE Safety Assessment
All children regardless of age were administered a single intranasal dose of LAIV-T in each vaccine year. In the 4 years of the study, we administered 18 780 doses of LAIV-T to 11 096 children (Table 1). A total of 4529, 7036, and 7215 doses of LAIV-T were administered to children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age, respectively. More than 95% of LAIV-T recipients in all 4 years had a successful 6-week follow-up for SAEs. In vaccination years 1, 2, 3, and 4, we identified 10, 15, 11, and 6 SAEs, respectively (Table 2). Ten, 13, and 19 of the SAEs occurred 0 to 14, 15 to 28, and 29 to 42 days after vaccination. None of the SAEs was judged by the principal investigators to be related to LAIV-T. In vaccination years 1, 2, 3, and 4, we identified 3, 2, 1, and 0 pregnant teenagers, respectively, during the safety period. All delivered full-term, healthy infants except for 1 teenager, who delivered a healthy preterm infant (gestational age: 33 weeks; birth weight: 1915 g). No LAIV-T–related SAE was observed with administration of 18 780 doses of LAIV-T in children; the true incidence of LAIV-T–related SAE is <1/5900 doses.
TABLE 1.
LAIV-T Recipients by Age Group and Vaccine Year
| Vaccine Year
|
|||||
|---|---|---|---|---|---|
| Age Group, Y | 1 (1998–1999) | 2 (1999–2000) | 3 (2000–2001) | 4 (2001–2002) | Total No. of Doses |
| 1.5–4 | 1034 | 1318 | 1216 | 961 | 4529 (24.1%) |
| 5–9 | 1696 | 1959 | 1891 | 1490 | 7036 (37.5%) |
| 10–18 | 1568 | 1974 | 2043 | 1630 | 7215 (38.4%) |
| Total no. of doses | 4298 | 5251 | 5150 | 4081 | 18 780 |
Children were given 1 dose of LAIV-T annually. A total of 18 780 doses of LAIV-T were administered to 11 096 children during the 4 vaccine years.
TABLE 2.
Compilation of SAEs* Detected in Children Who Received LAIV-T from 1998 to 2002
| Days After Vaccination
|
|||
|---|---|---|---|
| SAE | 0–14 | 15–29 | 29–42 |
| Psychiatric disorder | 3 | 1 | 2 |
| Trauma/skeletal pain | 0 | 3 | 1 |
| Migraine headache | 0 | 1 | 0 |
| Malignancy/tumor | 0 | 1 | 1 |
| Surgery or complications from surgery | 1 | 1 | 2 |
| Threatened abortion | 0 | 1 | 0 |
| Ruptured ovarian cyst | 0 | 0 | 1 |
| Soft tissue and bone infection | 1 | 1 | 0 |
| Urinary tract infection | 1 | 0 | 0 |
| Fever with absence of bacteremia | 0 | 1 | 1 |
| Mononucleosis | 0 | 1 | 0 |
| Aseptic meningitis | 0 | 1 | 0 |
| Lower respiratory tract illness | 1† | 0 | 4 |
| Gastroenteritis | 0 | 1 | 3 |
| Gastroesophageal reflux disease | 0 | 0 | 1 |
| Constipation | 1 | 0 | 0 |
| Appendicitis | 2 | 0 | 3 |
| Total | 10 | 13 | 19 |
None were vaccine related.
RSV culture–positive lower respiratory tract illness.
SWHP Administrative Database
Two large administrative databases were available to conduct the MAARI and asthma safety analyses: the Scott & White Memorial Hospital and Clinic and the subset SWHP. The advantage of the Scott & White Memorial Hospital and Clinic administrative database was that it included all children who were seen at the Scott & White Memorial Hospital and Clinic. Some of these children, however, did not receive all of their primary care at the Scott & White Memorial Hospital and Clinic; therefore, not all health care utilization events would be captured in that administrative database. The advantage of the SWHP administrative database was that it captured essentially all health care utilization events for SWHP members; ~50% of the vaccinees were members of the SWHP (Table 3). A total of 8671 doses were given to SWHP members; 3669 had 2 or more annual doses, and 2115 children were younger than 5 years. Incidence rates for MAARI during the safety analysis period were compared for both databases for all 4 years. In 1999–2000 and 2000–2001, the incidence rates for MAARI were significantly higher using the SWHP administrative database, and in the other 2 years, the rates were comparable (data not shown). SWHP captured encounters at non–Scott & White Memorial Hospital and Clinic facilities. Therefore, taking a conservative approach, all MAARI safety analyses were conducted using the SWHP administrative database.
TABLE 3.
LAIV-T Vaccine Recipients Who Are Members of the SWHP
| Annual Doses | Age Group | 1998–1999 | 1999–2000 | 2000–2001 | 2001–2002 | Total |
|---|---|---|---|---|---|---|
| 1 | 18 mo–4 y | 504 | 409 | 332 | 233 | 1478 |
| 5–9 y | 888 | 445 | 331 | 125 | 1789 | |
| 10–18 y | 833 | 471 | 334 | 97 | 1735 | |
| Total | 2225 | 1325 | 997 | 455 | 5002 | |
| 2 | 18 mo–4 y | 197 | 167 | 119 | 483 | |
| 5–9 y | 482 | 183 | 101 | 766 | ||
| 10–18 y | 520 | 228 | 97 | 845 | ||
| Total | 1199 | 578 | 317 | 2094 | ||
| 3 | 18 mo–4 y | 71 | 69 | 140 | ||
| 5–9 y | 317 | 108 | 425 | |||
| 10–18 y | 388 | 134 | 522 | |||
| Total | 776 | 311 | 1087 | |||
| 4 | 18 mo–4 y | 14 | 14 | |||
| 5–9 y | 191 | 191 | ||||
| 10–18 y | 283 | 283 | ||||
| Total | 488 | 488 | ||||
| Total | 2225 | 2524 | 2351 | 1571 | 8671 |
MAARI Safety Assessments
The RR of MAARI from 0 to 14 and 15 to 42 days after LAIV-T was assessed in vaccinees during the 4 vaccine years (Table 4). Compared with the prevaccination period, there was no significant increase in risk in health care utilization attributed to MAARI from 0 to 14 and 15 to 42 days after vaccination in children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in the 4 vaccine years (Table 4).
TABLE 4.
RR for MAARI 0 to 14 and 15 to 42 Days After LAIV-T
| RR Postvaccination/Prevaccination Period (95% CI)
|
||||
|---|---|---|---|---|
| Vaccine Year | Age Groups | Prevaccination Rate (Reference) Per 10 000 Child-Days | 0–14 D Postvaccination | 15–42 D Postvaccination |
| 1 (1998–1999) | 18 mo–4 y | 68.5 | 0.85 (0.61–1.19) | 0.86 (0.62–1.16) |
| 5–9 y | 35.5 | 0.74 (0.50–1.09) | 0.97 (0.71–1.33) | |
| 10–18 y | 21.4 | 1.04 (0.64–1.67) | 1.10 (0.72–1.68) | |
| 2 (1999–2000) | 18 mo–4 y | 94.2 | 0.72 (0.54–0.96) | 1.03 (0.80–1.33) |
| 5–9 y | 45.9 | 1.18 (0.89–1.58) | 1.09 (0.81–1.45) | |
| 10–18 y | 25.5 | 0.88 (0.59–1.33) | 1.20 (0.84–1.70) | |
| 3 (2000–2001) | 18 mo–4 y | 104.6 | 0.72 (0.53–0.99) | 1.02 (0.72–1.44) |
| 5–9 y | 54.2 | 0.73 (0.52–1.02) | 0.71 (0.49–1.05) | |
| 10–18 y | 29.3 | 0.73 (0.47–1.13) | 0.97 (0.59–1.59) | |
| 4 (2001–2002) | 18 mo–4 y | 69.4 | 0.82 (0.55–1.24) | 0.70 (0.45–1.09) |
| 5–9 y | 50.1 | 0.62 (0.40–0.98) | 0.82 (0.53–1.27) | |
| 10–18 y | 15.6 | 1.22 (0.67–2.24) | 1.26 (0.67–2.37) | |
Boldface indicates a significant decrease in health care utilization.
The relative incidence of MAARI, MAARI subcategories (otitis media/sinusitis, upper respiratory tract illness, and lower respiratory tract illness), and asthma 0 to 14 days after vaccination was lower compared with the prevaccination period in children who were 18 months to 18 years of age (Table 5). No significant increase in the risk in health care utilization for MAARI, MAARI subcategories, and asthma was detected in vaccinees 0 to 14 days after vaccination. There was a significant decrease in the RR for MAARI, otitis media/sinusitis, upper respiratory tract illness, and lower respiratory tract illness 0 to 14 days after vaccination in some of the vaccine years (Table 5, boldface).
TABLE 5.
RR for MAARI and Asthma Visits 0 to 14 Days After LAIV-T in Children Who Were 18 Months to 18 Years of Age
| RR 0–14 D/Prevaccination Period (95% CI)
|
||||
|---|---|---|---|---|
| Outcome | Year 1 (1998–1999) | Year 2 (1999–2000) | Year 3 (2000–2001) | Year 4 (2001–2002) |
| MAARI | 0.85 (0.68–1.07) | 0.91 (0.76–1.10) | 0.73 (0.59–0.90) | 0.80 (0.61–1.05) |
| Otitis media/sinusitis | 0.76 (0.51–1.14) | 0.62 (0.43–0.90) | 0.57 (0.39–0.84) | 0.78 (0.50–1.23) |
| URTI | 0.92 (0.70–1.21) | 1.04 (0.84–1.30) | 0.77 (0.6–0.98) | 0.83 (0.58–1.19) |
| LRTI | 0.50 (0.19–1.35) | 0.42 (0.19–0.96) | 0.73 (0.39–1.35) | 0.25 (0.08–0.76) |
| Asthma | 0.49 (0.19–1.30) | 1.25 (0.69–2.27) | 0.48 (0.22–1.07) | 1.31 (0.64–2.67) |
Boldface indicates a significant decrease in health care utilization; URTI, upper respiratory tract illness; LRTI, lower respiratory tract illness.
In children who were 18 months to 4 years of age, there was no significant increase in the risk in health care utilization for MAARI, MAARI subcategories, and asthma during the 0 to 14 days after vaccination compared with the prevaccination period (Fig 1). No significant increase in the risk in health care utilization for MAARI, MAARI subcategories, and asthma was detected when the risk period was extended to 15 to 42 days after vaccination, except for asthma events in vaccine year 1 (Fig 1E). A RR of 2.85 (95% CI: 1.01–8.03) for asthma events was detected in children who were 18 months to 4 years of age but was not significantly increased for the other 3 vaccine years (vaccine year 2, RR: 1.42 [95% CI: 0.59–3.42]; vaccine year 3, RR: 0.47 [95% CI: 0.12–1.83]; vaccine year 4, RR: 0.20 [95% CI: 0.03–1.54]).
Fig 1.
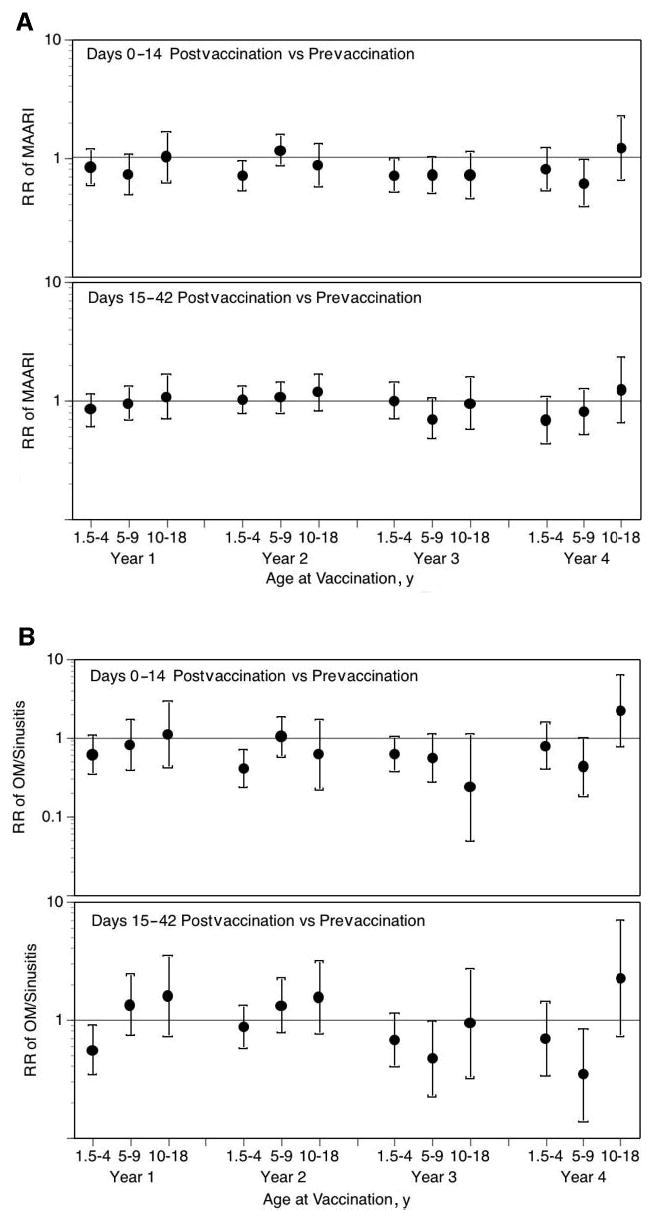
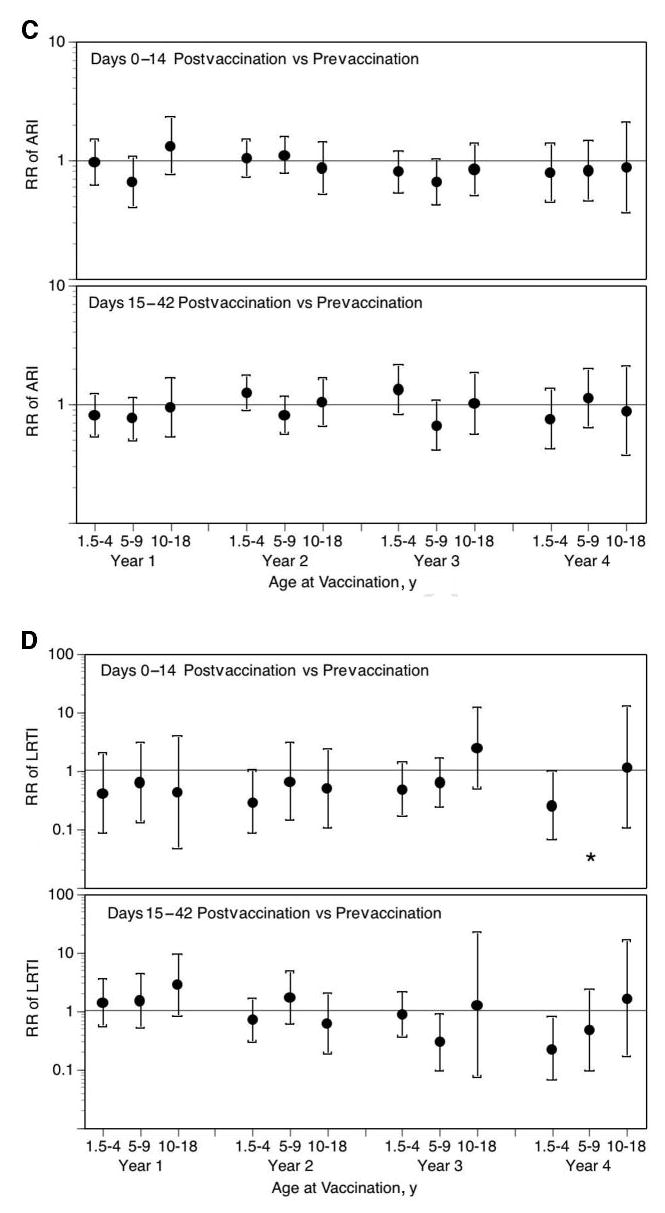
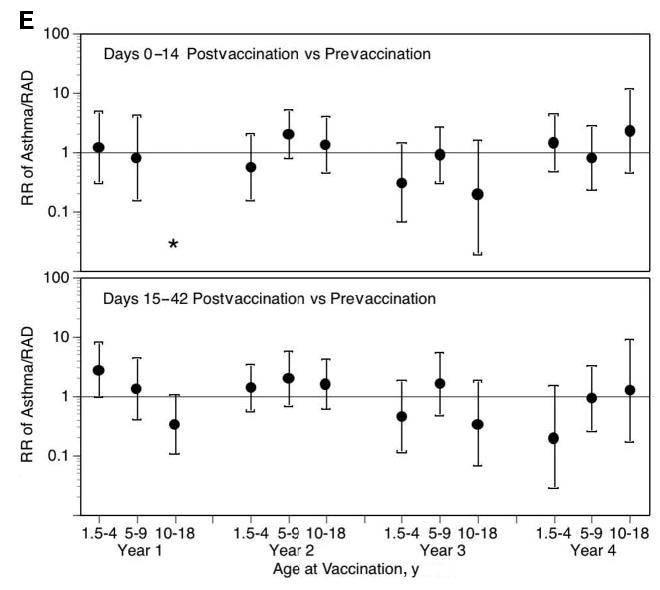
LAIV-T is safe in children who are 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age. The relative incidence of MAARI (A), MAARI subcategories (otitis media/sinusitis [B], upper respiratory tract illness [ARI; C], and lower respiratory tract illness [LRTI; D], and asthma/reactive airway disease [asthma/ RAD; E]) 0 to 14 and 15 to 42 days after vaccination was compared with the prevaccination period in children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age. No significant increase in the risk in health care utilization for MAARI, MAARI subcategories, and asthma was detected in LAIV-T vaccinees 0 to 14 and 15 to 42 days after vaccination except in vaccine year 1. In vaccine year 1, children who were 18 months to 4 years of age had a significantly higher RR (2.85; 95% CI: 1.01–8.03) for asthma events 15 to 42 days after vaccination but not in the subsequent 3 vaccine years.
No significant increase in the risk in health care utilization for MAARI or asthma was observed in children who were 18 months to 18 years of age and received 1, 2, 3, or 4 annual sequential doses of LAIV-T (Table 6). Children who were 18 months to 4 years of age and received 1, 2, 3, or 4 annual doses of LAIV-T did not experience a significant increase in the RR for MAARI 0 to 14 days after vaccination; this was also true for children who were 5 to 9 and 10 to 18 years of age (data not shown). Analysis of MAARI subcategories and asthma by age categories was not performed because of small cell size.
TABLE 6.
RR for MAARI and Asthma After Multiple Annual Doses of LAIV-T in Children Who Were 18 Months to 18 Years of Age
| RR 0–14 D/Prevaccination Period (95% CI)
|
|||||
|---|---|---|---|---|---|
| Category | No. of Annual Doses | Year 1 (1998–1999) | Year 2 (1999–2000) | Year 3 (2000–2001) | Year 4 (2001–2002) |
| MAARI | 1 | 0.85 (0.68–1.07) | 0.97 (0.76–1.23) | 0.77 (0.57–1.03) | 0.94 (0.62–1.42) |
| 2 | 0.86 (0.65–1.14) | 0.75 (0.49–1.15) | 0.86 (0.43–1.69) | ||
| 3 | 0.61 (0.39–0.96) | 0.27 (0.10–0.71) | |||
| 4 | 0.94 (0.51–1.74) | ||||
| Asthma | 1 | 0.49 (0.19–1.30) | 1.84 (0.83–4.08) | 0.65 (0.22–1.85) | 1.42 (0.47–4.29) |
| 2 | 0.80 (0.32–2.02) | 0.64 (0.17–2.34) | 0.42 (0.05–3.58) | ||
| 3 | 0.0 | 1.80 (0.11–28.80) | |||
| 4 | 0.80 (0.14–4.70) | ||||
Boldface indicates a significant decrease in health care utilization.
DISCUSSION
We report our safety experience with LAIV-T in children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age. We delivered 18 780 doses of LAIV-T to 11 096 children in this community-based, nonrandomized, open-label trial. This is the largest safety experience with LAIV-T in children reported to date. LAIV-T was safe and well tolerated by children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age. No increased risk for MAARI, MAARI subcategories (otitis media/sinusitis, upper respiratory tract illness, and lower respiratory tract illness), or asthma were observed among children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in the first 14 days after vaccination. LAIV-T in children who were 18 months to 4 years of age was as safe as that observed among children in the older age groups. The safety data generated in this study in children who were 18 months to 18 years of age are in strong agreement with the safety of LAIV-T reported in the pivotal phase III efficacy trial that was conducted in children who were 15 to 71 months of age.24–26
In this community-based, nonrandomized, open-label trial, we were successful in contacting >95% of the LAIV-T vaccinees to assess for SAEs 0 to 42 days after vaccination. None of the 42 SAEs detected in the 4 vaccine years was related to LAIV-T. Safety was also assessed by determining whether there was an increase in health care utilization attributed to respiratory illnesses (MAARI and asthma) that may be associated with LAIV-T. The risk period was 0 to 14 and 15 to 42 days after vaccination, and the reference period was the prevaccination period (the start date of the vaccination program to the date of vaccination). The Data Safety Monitoring Board of our study recommended the Poisson regression analysis to determine the RR for MAARI and asthma. This approach has been used in the safety evaluation of licensed childhood vaccines.31,32 The design of our study—nonrandomized, open-labeled, and without placebo—resembled those of phase IV safety evaluation. Having access to the SWHP administrative database facilitated the safety assessment of LAIV-T by an established method, the Poisson regression analysis, used in postlicensure vaccine safety assessment.31,32
Children were often seen by their primary physician for a MAARI event on the day of vaccination and subsequently referred to the study to receive LAIV-T. Therefore, before MAARI safety analyses, the medical charts of all participants were reviewed for all MAARI events that occurred on day 0 of vaccination, and appropriate assignments were made to risk or reference period. In addition, many children with a diagnosis of asthma were seen for a nonasthmatic event. For preventing misclassification of asthma events, the medical charts of all asthma visits were reviewed and assigned an appropriate classification of events: acute asthma or other. For reducing biases introduced by respiratory virus activity in the community, the MAARI rates were adjusted by time intervals that encompassed the presence or absence of respiratory virus activities. In each of the 4 vaccine years, we documented respiratory virus outbreaks with 1 or more viral pathogens that included RSV, parainfluenza virus, picornaviruses, or influenza virus. The community viral outbreaks caused an increase in MAARI and asthma events. Thus, the time-interval adjustment controlled for the impact of community viral outbreaks on MAARI and asthma events in the reference and risk periods that occurred after school opening each year.
The RR for MAARI was in general lower 0 to 14 days compared with 15 to 42 days after vaccination (Table 4), although the RR for MAARI at 15 to 42 days after vaccination was not significantly increased above the prevaccination (reference) period. This observation suggests that the lower RR for MAARI observed during 0 to 14 days after vaccination might be an enrollment artifact (healthy child effect) or a true biological phenomenon. We used inclusion and exclusion criteria that selected for children who were healthy at the time of enrollment. These selection criteria might contribute to a lower baseline illness rate. Another possibility is that LAIV-T induced an innate antiviral state (eg, interferon production) for 1 to 2 weeks after vaccination. The innate antiviral state could protect children from illnesses associated with circulating respiratory viruses. Protection against disease with wild-type influenza has been observed in the ferret model with co-administration of LAIV and wild-type influenza virus.36 Results from an influenza challenge study performed in human volunteers suggested that LAIV induced an antiviral effect that protected against illness from an experimental challenge with wild-type influenza virus.37 In support of LAIV-T’s inducing an innate antiviral state, we observed a significant reduction in the RR of MAARI, otitis media/sinusitis, upper respiratory tract illness, and lower respiratory tract illness 0 to 14 days after vaccination in some of the vaccine years (Table 5).
Limited information is available on the safety of administering LAIV-T during an influenza outbreak. In vaccine years 1999–2000, 2000–2001, and 2001–2002, influenza virus activity started toward the end of enrollment, midway through enrollment, and at the end of enrollment, respectively (Table 7). No increase risk in MAARI, MAARI subcategories, or asthma was observed in those vaccine years. LAIV-T contains the hemagglutinin and neuraminidase genes of the contemporary influenza strains and the 6 internal genes of the attenuated master virus strains.38,39 The 6 internal genes of the LAIV-T parent attenuated strains provide the phenotypic characteristic of attenuation, cold adaptation, and temperature restriction. The polygenic nature and multiple gene mutations contained in the 6 internal genes contribute to the stability of the attenuated phenotype of LAIV-T.40,41 The formation of new reassortants between LAIV-T and a contemporary interpandemic influenza virus would most likely yield attenuated to wild-type–like variants. Genetic reassortants during interpandemic periods do not seem to have a selective advantage.42–44 Our data support the safety of LAIV-T when administered to children in a community that experiences natural influenza virus activity during an interpandemic period.
TABLE 7.
Delivery of LAIV-T in Relation to Influenza Outbreak
| Vaccine Delivery Period
|
Influenza Outbreak Period
|
|||
|---|---|---|---|---|
| Vaccine Year | Start Date | End Date | Start Date | End Date |
| 1998–1999 | August 17 | December 19 | January 17 | April 3 |
| 1999–2000 | September 15 | December 30 | December 5 | January 29 |
| 2000–2001 | November 6 | January 20 | December 3 | February 24 |
| 2001–2002 | October 30 | December 28 | January 6 | March 2 |
LAIV-T is not currently recommended for children with mild, moderate, or severe asthma. Furthermore, LAIV-T is not recommended for children who are younger than 5 years because of the increased risk for asthma events observed in 1 clinical trial.27 In that trial, an increased risk for asthma-coded encounters occurred primarily in LAIV-T recipients who were 18 to 35 months of age. No clustering of asthma-coded encounters was observed within the first 42 days of vaccination. No increased risk was observed in children who were 18 to 35 months of age when the asthma outcome was expanded to include wheezing illness and shortness of breath.27 Also, no increased risk for asthma was observed in LAIV-T recipients with a previous diagnosis of asthma.27 Young children frequently have virus-induced wheezing illnesses that are treated as asthma. Misclassification of asthma in young children may explain the discrepant data.45 More than 1500 comparisons were made without adjustments for multiple comparisons. For independent outcomes at an α level of .10, a mean of 150 positive tests are expected for when there is no difference in rates between LAIV-T and placebo.27,45 For dependent outcomes, the number of statistically significant results expected as a result of chance alone may be more or less depending on the degree of dependence and the nature of the true association of the vaccine with the outcome. In no other clinical trials has an increased risk for asthma in healthy LAIV-T vaccinees been observed.24–26,46–48 In a small study, LAIV-T was safe and well tolerated in children and adolescents with moderate to severe asthma.49
In our community-based, nonrandomized, open-label trial ~10% of LAIV-T recipients had a history of mild intermittent asthma, reactive airway disease, or wheezing illness.50 We did not exclude children with mild, intermittent asthma or exercise-induced asthma. We observed no increased risk for asthma events 0 to 14 days after vaccination in children who were 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age. In vaccine year 1, children who were 18 months to 4 years of age did have a significantly higher RR (2.85; 95% CI: 1.01–8.03) for asthma events 15 to 42 days after vaccination. In vaccine year 2, the formulation of LAIV-T was identical to the vaccine formulation used in vaccine year 1; however, in children who were 18 months to 4 years of age, no statistically significant increased risk was detected for asthma events 15 to 42 days after vaccination (RR: 1.42; 95% CI: 0.59–3.42). Similarly, in vaccine years 3 and 4, children who were 18 months to 4 years of age did not have a statistically significant increased risk for asthma events 15 to 42 days after vaccination (vaccine year 3, RR: 0.47 [95% CI: 0.12–1.83]; vaccine year 4, RR: 0.20 [95% CI: 0.03–1.54]). Also, LAIV-T did not increase the risk for asthma in children who received 1, 2, 3, or 4 annual doses of LAIV-T (Table 6). Although the possibility for a true increased risk for asthma was observed in 1 of 4 years in children who were 18 months to 4 years at 15 to 42 days after vaccination, it is more likely that the association is a chance effect because of the 190 comparisons made without adjustment for multiple comparisons. In 3 of those 4 vaccine years, the composition of LAIV-T was revised according to the recommendation of the FDA, thus adding to the robustness of safety with multiple LAIV-T compositions. Finally, we controlled for misclassification of asthma events by reviewing the medical records of all events with an ICD-9 asthma diagnostic code and making a priori assignments to acute asthma or other. We believe that this process was crucial in reducing misclassification and in obtaining reliable safety data for administrative database analyses.
In summary, in this large community-based trial in children who were 18 months to 18 years of age, the LAIV-T vaccine was safe and not associated with an increased risk in health care utilization attributed to MAARI and asthma. Children who were 18 months to 4 years of age tolerated LAIV-T as well as children who were 5 to 18 years of age. Approximately 10% of children who were enrolled in this study had a history of mild, intermittent asthma; reactive airway disease; or wheezing. Lowering the age limit for LAIV-T to 15 or 18 months of age and expanding the current LAIV-T recommendations to include children with mild, intermittent asthma should be considered.
Acknowledgments
This trial was supported by National Institutes of Health Grant UO1AI41050 and Aviron (MedImmune Vaccines Inc; Mountain View, CA), which also provided the investigational intranasal influenza vaccine.
We appreciate the support provided by Linda Lambert, PhD, Influenza Program Officer at National Institute of Allergy and Infectious Diseases. This study would not be possible without the staff and physician support from Baylor College of Medicine, Scott & White Memorial Hospital and Clinic, and Scott & White Health Plan (Nadine Zimmerman for data extraction). We are extremely grateful to the Temple and Belton communities for support and participation.
Footnotes
Conflict of interest: This trial was supported by the National Institutes of Health and Aviron (now MedImmune). Dr Piedra is an ad hoc consultant to Sanofi Pasteur; Drs Piedra, Gaglani, and Glezen are ad hoc consultants to MedImmune and in the speaker bureau supported by MedImmune; Dr Herschler belongs to the speaker bureau of Wyeth; Mr Hessel is an employee of MedImmune.
References
- 1.Michaud CM, Murray CJL, Bloom BR. Burden of disease—implications for future research. JAMA. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Paredes A, Taber LH. Influenza in children. Relationship to other respiratory agents. JAMA. 1980;243:1345–1349. doi: 10.1001/jama.243.13.1345. [DOI] [PubMed] [Google Scholar]
- 3.Sugaya N, Mitamura K, Nirasawa M, Takahashi K. The impact of winter epidemics of influenza and respiratory syncytial virus on paediatric admissions to an urban general hospital. J Med Virol. 2000;60:102–106. [PubMed] [Google Scholar]
- 4.Chiu SS, Lau YL, Chan KH, Wong WHS, Peiris JSM. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med. 2002;347:2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 5.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 6.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 7.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effects of influenza on hospitalization, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 8.Glezen WP, Decker M, Joseph SW, Mercready RG., Jr Acute respiratory disease associated with influenza epidemic in Houston, 1981–1983. J Infect Dis. 1987;155:1119–1126. doi: 10.1093/infdis/155.6.1119. [DOI] [PubMed] [Google Scholar]
- 9.Couch RB, Kasel JA, Glezen WP, et al. Influenza: its control in persons and populations. J Infect Dis. 1986;153:431–440. doi: 10.1093/infdis/153.3.431. [DOI] [PubMed] [Google Scholar]
- 10.Jennings LC, Miles JAR. A study of acute respiratory disease in the community of Port Chalmers. II. Influenza A/Port Chalmers/1/73: intrafamilial spread and the effect of antibodies to the surface antigens. J Hyg (Lond) 1978;81:67–75. doi: 10.1017/s0022172400053778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox JP, Hall CE, Cooney MK, Foy HM. Influenzavirus infections in Seattle families, 1975–1979. I. Study design, methods, and the occurrence of infections by time and age. Am J Epidemiol. 1982;116:212–227. doi: 10.1093/oxfordjournals.aje.a113407. [DOI] [PubMed] [Google Scholar]
- 12.Fox JP, Cooney MK, Hall CE, Foy HM. Influenzavirus infections in Seattle families, 1975–1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am J Epidemiol. 1982;116:228–242. doi: 10.1093/oxfordjournals.aje.a113408. [DOI] [PubMed] [Google Scholar]
- 13.Frank AL, Taber LH, Wells JM. Comparison of infection rates and severity of illness for influenza A subtypes H1N1 and H3N2. J Infect Dis. 1985;151:73–80. doi: 10.1093/infdis/151.1.73. [DOI] [PubMed] [Google Scholar]
- 14.Glezen WP, Taber LH, Frank AL, Gruber WC, Piedra PA. Influenza virus infection in infants. Pediatr Infect Dis J. 1997;16:1065–1068. doi: 10.1097/00006454-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season. Arch Pediatr Adolesc Med. 2002;156:986–991. doi: 10.1001/archpedi.156.10.986. [DOI] [PubMed] [Google Scholar]
- 16.Neuzil KM, Wright PF, Mitchel EF, Griffin MR. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr. 2000;137:856–864. doi: 10.1067/mpd.2000.110445. [DOI] [PubMed] [Google Scholar]
- 17.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 18.Sperber SJ, Gross PA. Influenza: manifestations, treatment and prevention. Infect Med. 1994;11:675–683. [Google Scholar]
- 19.Glezen WP. Consideration of the risk of influenza in children and indications for prophylaxis. Rev Infect Dis. 1980;2:408–420. doi: 10.1093/clinids/2.3.408. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien KL, Walters MI, Sellman J, et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis. 2000;30:784–789. doi: 10.1086/313772. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto S, Kobayashi M, Uemura O, et al. PCR on cerebrospinal fluid to show influenza-associated acute encephalopathy or encephalitis. Lancet. 1998;352:873–875. doi: 10.1016/S0140-6736(98)12449-2. [DOI] [PubMed] [Google Scholar]
- 22.McCullers JA, Facchini S, Chesney PJ, Webster RG. Influenza B virus encephalitis. Clin Infect Dis. 1999;28:898–900. doi: 10.1086/515214. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Update: influenza-associated deaths reported among children aged <18 years—United States, 2003–04 influenza season. MMWR Morb Mortal Wkly Rep. 2004;52:1286–1288. [PubMed] [Google Scholar]
- 24.Belshe RB, Mendelman P, Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 25.Belshe RB, Gruber WC, Mendelman P, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000;136:168–175. doi: 10.1016/s0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 26.Piedra PA, Yan L, Kotloff K, et al. Safety of the trivalent, cold-adapted influenza vaccine (CAIV-T) in preschool aged children. Pediatrics. 2002;110:662–672. doi: 10.1542/peds.110.4.662. [DOI] [PubMed] [Google Scholar]
- 27.Bergen R, Black S, Shinefield H, et al. Safety of cold-adapted live attenuated influenza vaccine in a large cohort of children and adolescents. Pediatr Infect Dis J. 2004;23:138–144. doi: 10.1097/01.inf.0000109392.96411.4f. [DOI] [PubMed] [Google Scholar]
- 28.Piedra PA, Gaglani M, Herschler G, et al. Safety and effectiveness of the trivalent, cold-adapted influenza vaccine (CAIV-T) in children. In: Osterhaus ADME, ed. The World Congress on Options for the Control of Influenza IV Amsterdam, Netherlands: Elsevier Science 2001;1219:939–943.
- 29.Halloran ME, Longini IM, Gaglani MJ, et al. Protection against influenza A (H1N1) and B by trivalent, cold-adapted, influenza virus vaccine (CAIV-T) Am J Epidemiol. 2003;158:305–311. doi: 10.1093/aje/kwg163. [DOI] [PubMed] [Google Scholar]
- 30.Gaglani MJ, Piedra PA, Herschler GB, et al. Direct effectiveness of the trivalent, cold-adapted, influenza virus vaccine (CAIV-T) against the 2000–2001 influenza A (H1N1) and B epidemic in healthy children. Arch Pediatr Adolesc Med. 2004;158:65–73. doi: 10.1001/archpedi.158.1.65. [DOI] [PubMed] [Google Scholar]
- 31.Griffin MR, Taylor JA, Daugherty JR, Ray WA. No increased risk for invasive bacterial infection found following diphtheria-tetanus-pertussis immunization. Pediatrics. 1992;89:640–642. [PubMed] [Google Scholar]
- 32.Griffin MR, Ray WA, Mortimer EA, Fenichel GM, Schaffner W. Risk of seizure after measles-mumps-rubella immunization. Pediatrics. 1991;88:881–885. [PubMed] [Google Scholar]
- 33.Selvin S. Poisson regression analysis. In: Selvin S, ed. Practical Biostatistical Methods Belmont, CA: Duxbury Press; 1995
- 34.Ledley FD, Brody B, Kozinetz CA, Mize S. The challenge of follow-up for clinical trials of somatic gene therapy. Hum Gene Ther. 1992;3:657–663. doi: 10.1089/hum.1992.3.6-657. [DOI] [PubMed] [Google Scholar]
- 35.Breslow N. Multivariate cohort analysis. Natl Cancer Inst Monogr. 1985;67:149–156. [PubMed] [Google Scholar]
- 36.Whitaker-Dowling P, Maassab HF, Younger JS. Dominant-negative mutants as antiviral agents: simultaneous infection with the cold-adapted live-attenuated vaccine for influenza A protects ferrets from disease produced by wild-type influenza A. J Infect Dis. 1991;164:1200–1202. doi: 10.1093/infdis/164.6.1200. [DOI] [PubMed] [Google Scholar]
- 37.Younger JS, Treanor JJ, Betts RF, Whitaker-Dowling P. Effect of simultaneous administration of cold-adapted and wild-type influenza A viruses on experimental wild-type influenza infection in humans. J Clin Microbiol. 1994;32:750–754. doi: 10.1128/jcm.32.3.750-754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maassab HF. Adaptation and growth characteristics of influenza virus at 25°C. Nature. 1967;213:612–614. doi: 10.1038/213612a0. [DOI] [PubMed] [Google Scholar]
- 39.Maassab HF, DeBorde DC. Development and characterization of cold-adapted viruses for use as live virus vaccines. Vaccine. 1985;3:355–369. doi: 10.1016/0264-410x(85)90124-0. [DOI] [PubMed] [Google Scholar]
- 40.Snyder MH, Betts RF, DeBorde DC, et al. Four viral genes independently contribute to attenuation of live A/Ann Arbor/6/60 (H2N2) cold-adapted reassortant virus vaccine. J Virol. 1988;62:488–495. doi: 10.1128/jvi.62.2.488-495.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donabedian AM, DeBorde DC, Cook S, Smitka CW, Maassab HF. A mutation in the PA protein gene of cold-adapted B/Ann Arbor/1/66 influenza virus associated with reversion of temperature sensitivity and attenuated virulence. Virology. 1988;163:444–451. doi: 10.1016/0042-6822(88)90285-1. [DOI] [PubMed] [Google Scholar]
- 42.Shu LP, Sharp GB, Lin YP, et al. Genetic reassortment in pandemic and interpandemic influenza viruses. A study of 122 viruses infecting humans. Eur J Epidemiol. 1996;12:63–70. doi: 10.1007/BF00144430. [DOI] [PubMed] [Google Scholar]
- 43.Xu X, Smith CB, Mungall BA, et al. Intercontinental circulation of human influenza A (H1N2) reassortant viruses during the 2001–2002 influenza season. J Infect Dis. 2002;186:1490–1493. doi: 10.1086/344738. [DOI] [PubMed] [Google Scholar]
- 44.Ellis JS, Alvarez-Aguero A, Gregory V, Lin YP, Hay A, Zambon MC. Influenza AH1N2 viruses, United Kingdom, 2001–02 influenza season. Emerg Infect Dis. 2003;9:304–310. doi: 10.3201/eid0903.020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glezen WP, Piedra PA, Longini I, Halloran ME. Safety of cold-adapted live influenza vaccine [letter to editor] Pediatr Infect Dis J. 2004;23:593–594. doi: 10.1097/00006454-200406000-00030. [DOI] [PubMed] [Google Scholar]
- 46.Zangwill KM, Droge J, Mendelman P, et al. Prospective, randomized, placebo-controlled evaluation of the safety and immunogenicity of three lots of intranasal trivalent influenza vaccine among children. Pediatr Infect Dis J. 2001;20:740–746. doi: 10.1097/00006454-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Nolan T, Lee M-S, Cordova JM, et al. Safety and immunogenicity of a live-attenuated influenza vaccine blended and filled at two manufacturing facilities. Vaccine. 2003;21:1224–1231. doi: 10.1016/s0264-410x(02)00484-x. [DOI] [PubMed] [Google Scholar]
- 48.Piedra PA. Safety of the trivalent, cold-adapted influenza vaccine (CAIV-T) in children. Semin Pediatr Infect Dis. 2002;13:90–96. doi: 10.1053/spid.2002.122995. [DOI] [PubMed] [Google Scholar]
- 49.Redding G, Walker RE, Hessel C, et al. Safety and tolerability of cold-adapted influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2002;21:44–48. doi: 10.1097/00006454-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Gaglani MJ, Piedra PA, Riggs MW, Herschler GB, Griffith ME, Glezen WP. Safety of the trivalent, cold-adapted, influenza virus vaccine (CAIV-T) in a subgroup of children with history of mild intermittent wheezing/asthma/reactive airway disease (RAD) in a community-based, non-randomized, open-label trial [Abstract 2437] Pediatr Res. 2001;49:242A. [Google Scholar]


