Abstract
Assembly of hepatitis delta virus (HDV) in infected human hepatocytes involves association of the 1,679- nucleotide single-stranded genomic RNA (δRNA) with multiple copies of both small and large forms of the delta protein (δAg) to form a ribonucleoprotein particle which in turn interacts with envelope proteins of the natural helper virus, hepatitis B virus. Subsequently, for initiation of a new round of replication, the amount of small δAg within the assembled HDV particle is both necessary and sufficient. Quantitative assays were used in order to better understand just how much δAg is needed. The molar ratio of δAg species to genomic δRNA in assembled HDV particles was approximately 200. Next, this ratio was determined for cells under several different experimental situations in which HDV genome replication was occurring. These included replication in woodchuck liver and also in mouse liver and skeletal muscle, as well as replication in stably and transiently transfected cultured human hepatoblastoma cells. Surprisingly, in almost all these situations the molar ratios were comparable to that observed for HDV particles. This was true for different times after the initiation of replication and was independent of whether or not virus assembly was occurring. Cell fractionation combined with quantitative assays was used to test whether the majority of δAg and δRNA were colocalized during HDV replication in transfected cells. The cytoplasmic fraction contained the majority of δAg and genomic δRNA. Finally, the quality of δAg and δRNA, especially at relatively late times after the initiation of replication, was examined by using reverse transcription-PCR, cloning, and sequencing through the entire δAg open reading frame. When virus assembly and spread were not possible, 20% or less of the predicted δAg would have been able to support HDV replication. In summary, an examination of the quantity, quality and intracellular distribution of δAg and δRNA in several different experimental systems has provided a better understanding of the parameters associated with the initiation, maintenance, and ultimate decline of HDV genome replication.
Human hepatitis delta virus (HDV) was discovered in 1977 (34) and was soon recognized as a satellite of hepatitis B virus (HBV) (35). The helper role of HBV is limited to providing the envelope proteins needed for HDV assembly and subsequent cycles of infection (20). In other words, all other aspects of the intracellular replication of the HDV genome can be studied independently of assembly and infection.
The genome of HDV is a small 1,679-nucleotide single-stranded circular RNA (δRNA) (8, 19, 42). Replication of this RNA genome involves RNA-directed RNA transcription by a host polymerase, most likely RNA polymerase II (26, 27). During replication, an exact complement of the genome called the antigenome is produced. Within the antigenome sequence is the open reading frame (ORF) for a small 195-amino-acid protein, delta antigen (δAg), that is essential for genome replication (20), although its role(s) remains obscure. This protein is translated from a third species, one that is linear, shorter than full-length antigenomic RNA, capped, and polyadenylated (17, 18). During replication, some of the antigenomic RNA species undergo posttranscriptional RNA editing at a site corresponding to the amber termination codon for the small δAg (24). The editing enzyme is of a class known as adenosine deaminases that act on RNA (ADAR) (4, 38). The editing change is transmitted to new genomic RNAs and ultimately leads to the translation of a 19-amino-acid-longer form of δAg. This 214-amino-acid large δAg inhibits replication as supported by small δAg (7), and in addition, it is essential for virus assembly (5).
Previous studies have reported that in the liver of an HDV-infected chimpanzee or woodchuck, the amount of genomic δRNA reaches about 300,000 copies per average cell (8). Relative to that amount, that of antigenomic δRNA is 5 to 20 times less abundant and that of the mRNA is about 500 times less abundant (8). There are no published estimates of how many molecules of δAg are present in an infected cell. However, two previous studies have attempted to determine the molar ratio of δAg to genomic δRNA in virus particles. In one study, using equilibrium density centrifugation, a ratio of 70 was deduced (37), while in the other study, using electrophoresis under nondenaturing conditions, a ratio of 220 was obtained (13).
Initiation of HDV genome replication cannot be achieved by genomic δRNA alone (14). Somehow there must be provided copies of small δAg. In a natural situation, this δAg is provided, at least initially, by the δAg within the infecting virus particle. With this in mind, the experiments described here were undertaken to test the hypothesis that the molar ratio of δAg/δRNA might be a biologically relevant parameter, not just for assembly but also for initiation and possibly maintenance of HDV genome replication. We suspected that this molar ratio could be a parameter that is independent of the fraction of total cells in which replication was occurring. Our strategy was therefore to apply a method that could determine the ratio not just for virions but also for cells in which HDV replication had been initiated. Thus, our beginning aim was to compare the ratio for virions with that for intracellular values obtained during genome replication.
As summarized in Table 1, there are now many experimental systems available for studying HDV genome replication; these include various infections and transfections, both of animal tissues and of cultured cells. Only in some of these cases is a helper virus, either HBV or woodchuck HBV (WHV), also present so as to allow assembly of the replicating HDV genomes. As reported here, we determined the molar ratio of δAg/δRNA in several of these systems and compared the values with that for virus particles. In addition, we applied cell fractionation to transfected cells in order to determine whether the majority of δAg and δRNA share similar intracellular distributions. Finally, we used RT-PCR cloning and sequencing to study the quality of the δAg and δRNA, especially under conditions where genome replication had peaked and was beginning to decline.
TABLE 1.
Partial list of experimental systems for studying HDV genome replication
| Strategy for initiation of HDV genome replication | Species and tissue or cells or cell lines | Reference |
|---|---|---|
| Infection | Human liver | 34 |
| Chimpanzee liver | 8 | |
| Woodchuck liver | 32 | |
| Mouse liver | 28 | |
| Chimpanzee-cultured hepatocytes | 39 | |
| Woodchuck-cultured hepatocytes | 41 | |
| DNA transfection | Chimpanzee liver | 40 |
| Woodchuck liver | 33 | |
| Mouse liver | 6 | |
| Mouse skeletal muscle | 30 | |
| Variety of cultured cell lines | 20 | |
| RNA transfection | Mouse liver | 6 |
| Variety of cultured cell lines | 25 |
MATERIALS AND METHODS
Initiation of HDV genome replication.
As referred to elsewhere in the text, we used several different experimental systems to initiate HDV genome replication. As cited in a footnote to Table 3, each of these systems was previously described by us or by others.
TABLE 3.
Examples of quantitation during HDV genome replicationa
| Experimental system | No. of δAg per average cell (105) | No. of δRNA per average cell (105) | δAg/δRNA ratio |
|---|---|---|---|
| Woodchuck liver, infected | |||
| Animal no. 1 | 73 | 0.66 | 112 |
| Animal no. 2 | 61 | 0.64 | 94 |
| HuH7 cells, transiently transfected with RNA | |||
| Day 3 | 19 | 0.07 | 266 |
| Day 5 | 95 | 0.27 | 359 |
| Day 9 | 240 | 0.59 | 410 |
| Day 15 | 85 | 0.29 | 292 |
| Day 20 | 9.3 | 0.04 | 210 |
| HuH7 cell clones stably transfected with DNA | |||
| Clone 8 | 8.7 | 0.052 | 167 |
| Clone 12 | 18 | 0.060 | 306 |
| Clone 14 | 5.2 | 0.026 | 200 |
| Mouse liver, transfected | |||
| Day 5 | 2.3 | 0.020 | 118 |
| Day 9 | 6.8 | 0.034 | 200 |
| Day 15 | 0.39 | 0.024 | 16 |
| Day 20 | 1.4 | 0.028 | 48 |
| Day 30 | 0.11 | 0.003 | 38 |
| Mouse skeletal muscle, transfected | |||
| Day 8 | <0.72 | 0.018 | <40 |
| Day 20 | 0.72 | 0.039 | 18 |
| Day 40 | 0.96 | 0.037 | 26 |
| Day 80 | <0.72 | 0.004 | <180 |
Five different methods for the initiation of HDV genome replication are shown here. Woodchucks already infected with woodchuck hepatitis virus were superinfected with serum from an HDV-infected animal, as previously described (29). Transient transfection of HuH7 cells with antigenomic HDV RNAs was as previously described (27). The three HuH7 cell clones obtained by stable transfection of HuH7 cells with pSVL(D3) (20) have been described previously (9). Mice were transfected with the HDV cDNA construct pDL456 (22) by a hydrodynamics-based procedure to achieve replication in the liver (6). After moving the HDV insert of pDL456 from pSVL (Pharmacia) to pcDNA3 (Invitrogen), the resulting construct, pJC126, was directly injected into mouse skeletal muscle by following a previously described procedure (30). Indicated for each sample are the deduced numbers of molecules of δAg and genomic δRNA per average cell along with the molar ratio. Quantitation of δAg refers to both small and large forms.
Extraction of protein and RNA.
Total protein and RNA were extracted from cells and tissues by using Tri Reagent (Molecular Research Center) according to the manufacturer's instructions.
Quantitation of HDV protein.
As a protein standard, we used purified small δAg, as generously provided by H. Zuccola and J. Hogle. This δAg had been expressed in Escherichia coli and subsequently purified almost to homogeneity (>85% pure by gel electrophoresis and staining) as previously described (12). Protein samples were examined by immunoblotting by using a rabbit polyclonal antibody and staphylococcal protein A labeled with 125-I (DuPont). Quantitation was performed using a Bio-Imager (Fuji).
Quantitation of HDV RNA.
As a nucleic acid standard, genomic RNA was transcribed in vitro from a cloned HDV cDNA and then gel purified and quantitated by optical density. Using this standard, RNA samples were examined by Northern analysis, all as previously described (20).
Cell fractionation.
Cell fractionation was based on a previously described procedure (1). At the indicated times after transfection, monolayer cultures of HuH7 cells were washed with PBS and scraped directly into NP-40 lysis buffer (10 mM sodium chloride, 10 mM Tris-HCl [pH 7.4], 3 mM MgCl2, and 0.5% NP-40). After 5 min on ice, cells were collected by centrifugation. The resulting first supernatant (C1) was removed, and the pellet was resuspended in lysis buffer. After centrifugation, the second supernatant (C2) was separated from the nuclear pellet (N). In all cases, total protein and RNA were then extracted with Tri Reagent.
RT-PCR cloning and sequencing.
Total RNA samples were first fractionated to obtain poly(A)-containing species and then reverse transcribed by using oligo(dT) as primer, as previously described (16). Nested PCR was used to amplify the entire ORF for both small and large δAg. The products were cloned by using a TOPO TA cloning kit (Invitrogen), and selected plasmids were subjected to automated nucleotide sequencing. Screening for single nucleotide changes relative to the sequence of Kuo et al. (21) was performed by using the program Sequencher 3.1.1 (Gene Codes), with results as presented in Fig. 2 to 5. Overall, for 66 clones sequenced we detected only three changes that could have represented insertions or deletions. Since these were infrequent and at different locations, they were not considered further.
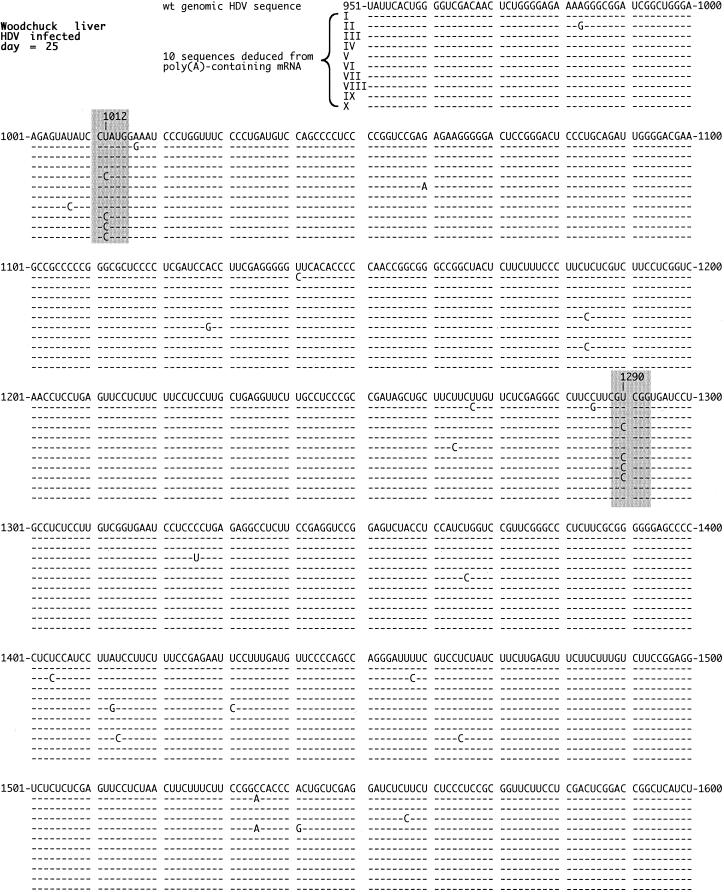
FIG. 2.
Quality of HDV RNA detected in infected woodchuck liver. At the peak of an acute HDV infection of a woodchuck, the animal was sacrificed and total RNA was extracted from the liver. The poly(A)-containing RNA was then isolated, and the region spanning the ORF for the δAg was subjected to RT-PCR, cloning, and sequencing. The figure shows 10 sequences deduced for genomic RNA in relationship to the small δAg wild-type sequence of Kuo et al., using the same numbering system (21). Highlighted in gray are the changes at nt 1012 and 1290 that occurred multiple times and are discussed in the text. The nonhighlighted changes did not occur more than twice. Note that the 3 nt changes on sequences III and VI, in addition to that at nt 1290, were silent in terms of the predicted amino acid sequence.
FIG. 5.
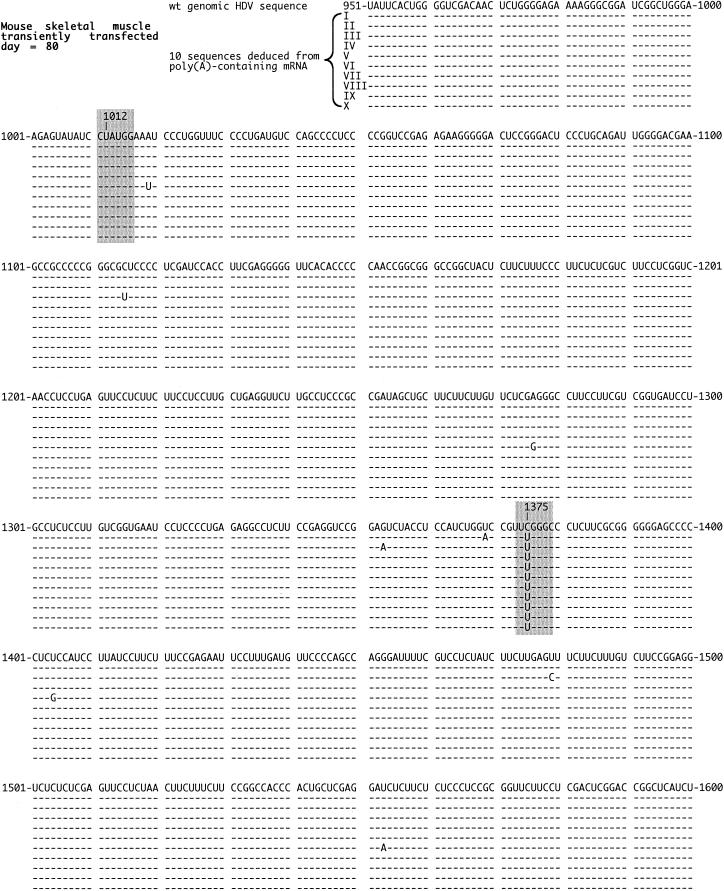
Quality of HDV RNA detected in DNA-transfected mouse skeletal muscle. Total RNA was extracted from mouse skeletal muscle at day 80 after DNA transfection. Otherwise, details are as described in the legend to Fig. 2. As highlighted in gray, no changes were detected at nt 1012, while 10 of 10 sequences were changed at nt 1375.
RESULTS
Quantitation of δAg and δRNA in virions.
We began with quantitation for HDV particles from the sera of three infected woodchucks. As summarized in Table 2, we deduced the average number of molecules of δAg and δRNA per milliliter of serum. And, of more general significance, we deduced the molar ratio of δAg to δRNA. The molar ratio value was approximately the same for each of the three sera studied, with an average of 204 ± 25. As mentioned in the introduction, previous studies from this lab have used quite different experimental procedures to deduce this ratio and obtained values of 70 (37) and 220 (12). It is considered that the value obtained by the present standardized procedures is more direct and thus more reliable.
TABLE 2.
Quantitation of HDV serum particlesa
| Serum no. | No. of δAg/ml (1010) | No. of δRNA/ml (1010) | δAg/δRNA ratio |
|---|---|---|---|
| 1 | 200 | 0.89 | 225 |
| 2 | 67 | 0.38 | 176 |
| 3 | 93 | 0.44 | 211 |
Each serum was assayed at two dilutions to determine the values shown. The amounts were deduced relative to the protein and RNA standards and expressed as molecules per milliliter. Also deduced was the molar ratio of δAg to genomic δRNA. Quantitation of δAg refers to both small and large forms.
We were concerned that the deduced molar ratio might be affected if there was significant assembly of δAg into particles in the absence of δRNA. This can happen for virus assembly using cultured cells (36), but it was unclear whether it was occurring during an experimental infection of the woodchuck. Therefore, to test whether such an assembly was functioning as a significant source of error, serum particles were disrupted with nonionic detergent and then the released ribonucleoprotein (RNP) was centrifuged away from what might have been free delta protein (37). It was found that the molar ratio of δAg/δRNA in the purified RNP was not significantly different from that obtained for the intact virus particles (data not shown). Thus, the ratios summarized in Table 2 can be considered to reflect typical requirements for assembly of HDV RNP into particles. Of course, this ratio is also the ratio that is associated with the natural process of infection and successful initiation of new rounds of HDV genome replication.
Quantitation during genome replication.
Several different experimental systems involving HDV genome replication were subjected to a similar quantitation. In some cases, measurements were made at various times after the initiation of genome replication. Five sets of data are given in Table 3.
Consider first samples of woodchuck liver collected near the peak of an HDV infection. Data are shown for two infected animals. We deduced that there were about 70,000 molecules of genomic δRNA per average liver cell. (In all calculations, a cell was assumed to have a total RNA content of 25 pg. In terms of the liver, we have not allowed either for the fact that maybe only 70% of liver cells are hepatocytes or for the possibility that <100% of hepatocytes were infected.) This value is several fold lower than that deduced in our earlier report (8). However, this difference is understandable, since in that study, the HDV standard used was not RNA but DNA. We have recently observed that under the hybridization conditions used, DNA standards can give signals two- to sixfold lower than those of RNA standards of the same sequence (data not shown). Also shown in the table is that the amount of δAg per average cell was at least 6 million molecules. It is worth noting that this large number is actually close to the number of ribosomal particles per cell (11).
We also calculated the molar ratio of δAg to δRNA. For the two infected animals, that value was essentially the same (about 100). This value was only twofold different than the ratio obtained earlier for the three serum samples. Since this difference could be within what we consider to be the observed experimental error range, we cannot tell whether it is significant. (Note that we did not consider inclusion of antigenomic δRNA data in these ratio calculations. The main reason was that we know that during HDV replication, the amount of antigenomic δRNA is only 5 to 20% of that of genomic δRNA [8].)
Following these studies with HDV replication in woodchuck tissues, we considered replication in transfected cultured cells. Table 3 shows results for two models used with a human hepatoblastoma cell line (HuH7), namely, transient transfection with antigenomic RNAs and stable transfection with DNA. Monitoring the transient transfection from day 3 to day 20, we detected an initial increase followed by an ultimate decrease for both δAg and δRNA. However, the molar ratio of δAg/δRNA remained what we interpret to have been relatively constant throughout this time period.
We tested three different clones of HuH7 cells that had been stably transfected with HDV DNA sequences. As previously reported, these lines carry 20 to 200 DNA copies per cell (9). For these lines, the ratio of δAg/δRNA was approximately the same, with an average value of 224. At the same time, it should be noted that in these lines, although every cell was transfected, the average amounts of δAg and δRNA were significantly lower, especially with respect to the liver of an infected woodchuck.
The last two examples in Table 3 represent HDV replication in mouse liver and skeletal muscle transiently transfected with HDV cDNA. Transfection of hepatocytes was achieved by using a hydrodynamics-based procedure (6), while that for skeletal muscle was by direct injection (30). Consider first the results for liver. At 5 and 9 days after transfection, the levels of δAg and δRNA were at their highest. The ratio of δAg/δRNA was approximately the same as that for the liver of an infected woodchuck. However, at later times, days 15, 20, and 30, the amounts of δAg decreased faster than those of δRNA, with the consequence that the ratio dropped at least threefold. Consider next the results for skeletal muscle. At days 20 and 40, where we were able to determine the molar ratio of δAg/δRNA, the values were comparable to those for liver at days 15, 20, and 30. In both situations, we interpret these lower values as evidence that not only the quantity but also the quality of HDV genome replication was now suboptimal. It might be that most of the replication occurred at earlier times and so we were largely observing the slowly progressing degradation of previously accumulated RNA and protein species. Independent evidence supports this interpretation. Researchers had previously observed by immunostaining that δAg in the mouse hepatocytes was redistributed from predominantly nuclear at day 5 to predominantly cytoplasmic at day 15 (6). Additional evidence is presented later in this article, when we consider the quality of the δAg species.
In summary, Tables 2 and 3 represent quantitation of δAg and δRNA in several different experimental systems. The most striking feature of these data was that the molar ratio of δAg/δRNA, with two exceptions, was relatively constant, with a value similar to that for HDV particles released into the serum of an infected animal.
Intracellular distribution of δAg and δRNA.
The above studies addressed only the quantitation of δAg and δRNA per cell. An equally relevant question concerned the intracellular distribution of these molecules. In previous reports of δAg localization by immunostaining, accumulation was typically detected predominantly in the nucleus, with variations only as to whether this protein was in the nucleolus, the nucleoplasm, nucleoplasmic speckles, etc. (2, 3, 10). Similarly, in situ hybridization typically detected most of the δRNA, both genomic and antigenomic, as nuclear (10, 15).
We decided to obtain quantitation based upon cell fractionation studies. For this, we used HuH7 cells at different times after RNA transfection. The results for duplicate cultures fractionated at 12 days after transfection are shown in Fig. 1. Panels A to D show the results of assays for δAg, genomic δRNA, antigenomic δRNA, and 18S rRNA, respectively. Quantitation of these panels is summarized in Table 4, along with duplicate fractionations of cells at day 4 after transfection.
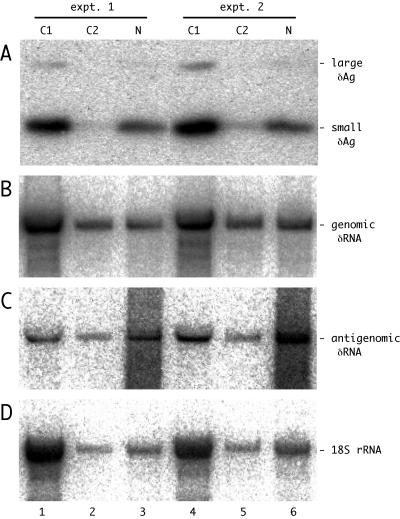
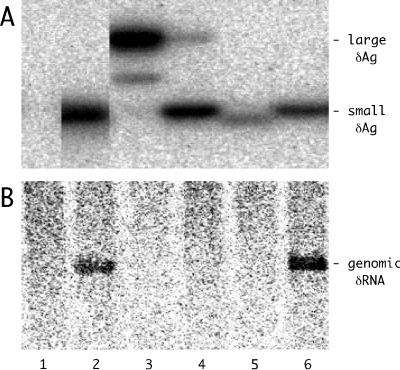
FIG. 1.
Detection of δAg and genomic and antigenomic δRNA in fractions of HuH7 cells transfected with HDV RNAs. Duplicate cultures were transfected with antigenomic HDV RNAs. After 12 days the cells were fractionated to provide the cytoplasmic fractions C1 and C2 and the final nuclear fraction N. The total protein and RNA were extracted from each of these fractions. Panels A to D show the results of assays for δAg, genomic δRNA, antigenomic δRNA, and 18S rRNA, respectively. Note that in panel A we detected both small and large forms of δAg.
TABLE 4.
Nuclear localization during HDV genome replicationa
| Experiment no. (day)b | % δAg | % Genomic δRNA | % Antigenomic δRNA | % 18S rRNA |
|---|---|---|---|---|
| 1 (12) | 27 | 12 | 47 | 8 |
| 2 (12) | 20 | 22 | 53 | 22 |
| 3 (4) | 13 | 28 | 46 | 11 |
| 4 (4) | 20 | 9 | 61 | 13 |
The data quantitated are as described for panels A to D in the legend to Fig. 1. Shown are the percentages in the nuclear fraction of total detected δAg, genomic and antigenomic δRNA, and 18S rRNA.
These four experiments represent individual fractionations of HuH7 cells transfected, in duplicate, 12 or 4 days previously. Figure 1 shows the data for experiments 1 and 2 only. Quantitation of δAg refers to both small and large forms.
The protein assays detected both forms of δAg (Fig. 1A). For all the assays there were no significant differences in the intracellular distributions deduced for cultures at day 12 versus day 4 (Table 4). The distribution was such that about 20% of δAg was in the nuclear fraction. Quantitation of δRNA showed that the nuclear fraction contained 18% of the unit-length genomic RNA and 52% of the antigenomic RNA. These results raise the question of why the nuclear fraction contains less of the δAg and the genomic δRNA. Three lines of evidence support the interpretation that it is not an artifact of fractionation. First, for the 18S rRNA only about 12% was in the nuclear fraction (Fig. 1D). This is consistent with a not unreasonable level of adherent cytoplasmic contamination. Second, as part of the immunoblot assays we used India ink to stain the proteins electrotransferred from the gel to the nylon membrane. This showed that the majority of the abundant fast-migrating histone proteins were in the nuclear fraction (data not shown). Thirdly, in separate fractionations of cultures at day 5 after transfection with a plasmid that expresses only the small δAg, we found 88% of this protein in the nuclear fraction (data not shown). In summary, if an artifact of cell fractionation is really not involved, then the enhanced distribution of δAg and genomic δRNA in the cytoplasm required some other explanation.
Quality of δAg and δRNA.
We reasoned that the best way to determine the quality of the HDV RNA and protein translated from it would be to sequence the entire ORF under different conditions of HDV replication. Furthermore, rather than sequence this region on full-length genomic or antigenomic δRNA, we considered it better to analyze the polyadenylated mRNA species, since these are more likely to reflect the proteins that can be translated. We used RT-PCR, cloning, and sequencing. Figures 2 to 5 present a summary of such results for four different examples. In each case we indicate only the sequence changes relative to those of the known wild-type genomic RNA sequence (21).
Figure 2 summarizes the sequences of 10 clones obtained from the HDV mRNA of a woodchuck at the peak of an acute infection. In total, there were 31 single-nucleotide changes on these 10 sequences. Of these changes, 23 occurred only once or twice in 10 sequences; while some of these might have been relevant to the quality of the δAg, the observed low frequency and almost random location inhibits further study. In contrast, there were two sites at which changes occurred in more than 2 of 10 clones. The first such site was nucleotide (nt) 1012, at which the genome was changed from U to C. From previous studies we recognize this site as the amber/W mutation in the termination codon of the small delta protein (24). Further, we know the change arises posttranscriptionally as an A-to-I editing of antigenomic RNA sequences by ADAR activity (31). The second site has not previously been reported; it is equivalent to a U-to-C change at nt 1290 on the genomic RNA. We note that in terms of the predicted delta protein, this would be a silent mutation, simply changing one arginine codon to another arginine codon. We speculate that it arose, like the change at nt 1012, via A-to-I editing on antigenomic RNA sequences.
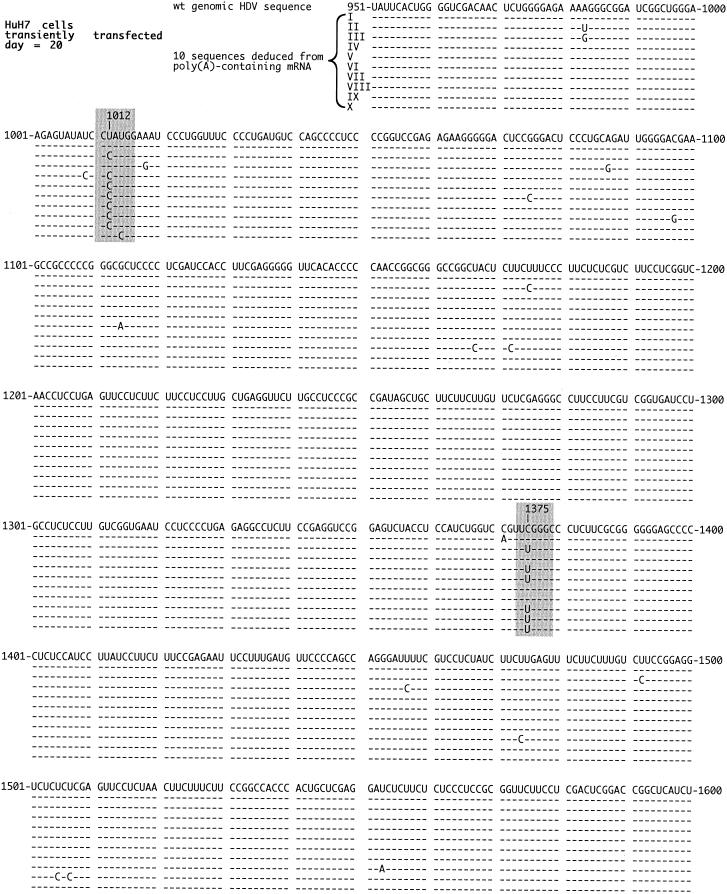
For the second example, presented in Fig. 3, we tested RNA from cultured HuH7 cells at 20 days after transfection with HDV RNA species. We detected only two predominant sites of change, nt 1012 and 1375. Similar results, in terms of these two sites of change, were also obtained with sequences derived from RNAs isolated at day 12 after transfection (data not shown). There were other single nucleotide changes, but these demonstrated no specificity, at least as based on 10 complete sequences. As before, the nt 1012 change we consider to have been a consequence of posttranscriptional ADAR editing. However, the second site of change, at nt 1375, was abundant (6 of 10). It corresponded to a change of C to U on the genomic RNA, although in the absence of further data it could also be a change of G to A on the antigenomic RNA. Either way, in terms of the predicted δAg, it would correspond to a change of arginine to glutamine at amino acid 75. We also examined the RNA from one of the clones of HuH7 cells that had been stably transfected with HDV cDNA sequences (9). Again, we detected only those two specific and frequently altered sites, nt 1012 and 1375 (data not shown).
FIG. 3.
Quality of HDV RNA detected in RNA-transfected HuH7 cells. Total RNA was extracted from HuH7 cells at 20 days after RNA transfection. Otherwise, details are as described in the legend to Fig. 2. Highlighted in gray are the changes at nt 1012 and 1375. An 11th sequence, not shown, was identical to that of the wild type.
The third example, presented in Fig. 4, is RNA from the liver of a transfected mouse at day 30. Ten sequences are shown. Again there were only single nucleotide changes, and of these, as in Fig. 3, only two sites exhibited changes that were repeated, namely, nt 1012 and 1375. In this case, the change at nt 1375 was even more abundant (8 of 10).
FIG. 4.
Quality of HDV RNA detected in DNA-transfected mouse liver. Total RNA was extracted from mouse liver at day 30 after DNA transfection. Otherwise, details are as described in the legend to Fig. 2. Highlighted in gray are the changes at nt 1012 and 1375. An 11th sequence, not shown, was identical to that of the wild type.
In Fig. 5 we show the last example, that of transfected mouse skeletal muscle at day 80. In these sequences only one change was repeated. For all 10, the same change was observed at nt 1375. Note that no change was detected at nt 1012.
Stability and functionality of mutant δAg.
The above studies revealed many sequences with a specific change at nt 1375. As can be seen in Fig. 3 to 5, there were sequences for which this was the only change. This mutation at nt 1375 on the mRNA, as mentioned earlier, would change the arginine of the δAg at amino acid 75 to glutamine. This change is located within what is considered the core nuclear localization signal (45) and following what is considered to be the dimerization domain (44, 46). We therefore transferred this changed cDNA sequence to an expression vector in order to determine whether the predicted δAg could be stably expressed and if so, whether it was somehow functional in HDV genome replication.
HuH7 cells were cotransfected with vectors encoding mRNAs for the mutant small δAg and/or wild-type forms of large and small δAg. In each case, transfection included a construct that expressed an HDV antigenomic RNA that was greater than unit length but, because of a 2-nt deletion, was unable to express δAg (20, 43). After 4 days, total protein and RNA were extracted and analyzed for HDV protein (Fig. 6A) and RNA species (Fig. 6B).
FIG. 6.
Expression and functional tests of the R(75)Q small δAg produced following the change at nt 1375. In lanes 1 to 6, HuH7 cells were each transfected with pDL481, a plasmid expressing greater-than-unit-length antigenomic HDV RNA with a frameshift mutation in the δAg ORF (20, 43), along with the indicated combinations of plasmids expressing the small, large, or mutated small forms of δAg. The transfection strategy was as previously described (7). The expression of the mutated small form of δAg was from pSG206, constructed by modification of pDL444, the plasmid expressing wild-type small δAg. In lanes 2 to 6, the cotransfection included the following: lane 2, plasmids expressing wild-type small δAg; lane 3, wild-type large δAg; lane 4, both small and large δAg in the ratio 0.8:0.2; lane 5, mutant δAg; lane 6, mutant δAg and small δAg in the ratio 0.5:0.5. Panels A and B show the immunoblot and Northern analyses, respectively.
As shown in Fig. 6A, the mutant δAg was stably expressed (lane 5). The accumulation was about 30% relative to that of wild-type small δAg (lane 2). As expected from the nature of the mutation, the mobility was very close to that of the small form of δAg.
In terms of HDV RNA accumulation (Fig. 6B), wild-type small δAg supported HDV genome replication (lane 2) but the mutant did not (lane 5). Furthermore, while large δAg was able to act as a dominant negative inhibitor of genome replication as supported by small δAg (lane 4), the mutant protein did not (lane 6).
In summary, the mutant protein was expressed but it demonstrated neither support nor inhibition of HDV genome replication. Further experiments are needed to determine how the change at nt 1375 arose and why in some situations, especially for the transfected mouse liver and skeletal muscle at late times after transfection, 80% or more of the HDV mRNA was changed at this site.
DISCUSSION
The objective of this study was to compare HDV genome replication in several different experimental situations in the hope that it would provide insights into the requirements of replication. We began with virus particles present in the sera of infected animals and deduced that there are, on average, about 200 molecules of δAg per δRNA (Table 2). We showed that this number was not overestimated due to the presence of particles containing δAg but not δRNA.
What does this ratio of 200 mean in terms of the structure of a viral RNP? Previous studies have shown that the function of the δAg depends upon dimerization (23, 44). For comparison, a ratio of 140 corresponds to an average of one δAg dimer per 12 bp of HDV rod-like RNA. One model is that dimers of δAg bind to δRNA and confer protection against nuclease. A second and quite different model is based on the observation that the δAg, per se, forms higher order multimers (46). Such protein complexes would have significant positive charges, and so it could be to these complexes that δRNA, with its negative charge, binds. In fact, 140 molecules of δAg, each with a predicted charge of +12 (21), would neutralize the charge of a unit-length δRNA molecule. This second model is supported by previous studies in which HDV RNP structures were treated with vanadyl ribonucleosides; this totally released δRNA and left behind multimers of δAg (37).
As mentioned earlier, we suspected that the molar ratio of δAg/δRNA in virus particles might also be a parameter relevant for HDV genome replication. After all, it is also the ratio, following infection, which is sufficient to initiate HDV RNA-directed RNA synthesis, RNA processing, and accumulation of processed RNA species. In addition, this ratio is a parameter that should be independent of the multiplicity of infection. Therefore, with the ratio for virions as a reference point, we determined the ratio during various conditions of HDV RNA transcription and accumulation. In almost every case we observed values that were comparable and were maintained during genome replication. These findings support the biological relevance of the ratio and maybe imply that there must be some mutual regulation as the mechanism that maintains the ratio during HDV replication. The two exceptions were at late times in transfected mouse liver and skeletal muscle, when δAg levels had already decreased significantly. The observed ratios were several fold lower than for virus particles (Table 2). Our interpretation of such findings is that they are consistent with replication having largely come to a halt. In other words, the detected RNA and protein species were largely the residue of those synthesized at an earlier time. Incidentally, such lowered ratios support the idea that, at least in these experimental situations, a higher amount of δAg was not needed to protect the δRNA.
As part of these studies, we also determined the absolute numbers of δAg and δRNA per average cell. Such quantitation revealed major differences in the extent of HDV protein and RNA accumulation in different experimental situations. It also showed for the first time that the amounts of δAg per average cell were often greater than six million copies (Table 3). Surely at such levels many and diverse interactions are possible with host cell components.
Next, we considered the intracellular distribution of δAg and δRNA using a combination of cell fractionation and quantitation. Such studies have to be considered in the context of previous immunomicroscopy to detect δAg and in situ hybridization to detect δRNA (10, 15). Such qualitative studies found that most of the viral components were in the nucleus. In our present study, after cell fractionation, about half of the antigenomic δRNA was nuclear (Fig. 1 and Table 4). However, much lower amounts of δAg and genomic δRNA were found in the nucleus. The same results were observed for cells at both day 4 and day 12 after transfection; thus, the distributions seem independent of the extent of HDV genome replication. In addition, we obtained some evidence against the interpretation that the distributions were a consequence of artifactual elution from the nucleus during cell fractionation. Therefore, since the molar ratio was maintained in both the cytoplasmic and nuclear fractions, it could be that the δAg and genomic δRNA were behaving as part of a RNP complex, but in truth, we still do not have a detailed explanation. However, we note that one possible interpretation is that our results confirm the original report, from Macnaughton and Lai in 2001, that the HDV genomic RNA is transported to the cytoplasm immediately after synthesis, while antigenomic RNA remains in the nucleus (T. B. Macnaughton and M. M. C. Lai [University of Southern California], personal communication).
Finally, we considered the quality of δRNA and δAg under various replication conditions. This was achieved by sequencing of the complete δAg ORF on poly(A)-containing antigenomic RNA species. Other than editing at nt 1012, which leads to the appearance of the large δAg, we only found two specific changes that occurred with a frequency of greater than 20%. One change, at nt 1290, would produce a protein with the same amino acid sequence. The other, at nt 1375, would produce a protein with an altered sequence. This protein could be stably expressed, but it neither supported nor inhibited HDV replication (Fig. 6). From the sequence analysis, we found that this mutation was present in transfected human liver cells and on 80% or more of δAg mRNA in transfected mouse liver (Fig. 4) and skeletal muscle (Fig. 5) but it was not detected (<10%) in infected woodchuck liver (Fig. 2). We have also searched the GenBank, EMBL, DDBJ, and PDB databases for HDV RNA and protein sequences obtained for infected primates and woodchucks. The specific change was not found on 81 RNA sequences (<1%) or on a total of 109 protein sequences (<1%). This may mean the mutation is not without consequences and can be selected against in an infection involving virus spread and initiation of new rounds of infection. Certainly, this change at nt 1375 is unlike the one at nt 1012 in that it cannot be explained by ADAR editing. Studies are under way to determine whether it reflects another form of posttranscriptional RNA editing.
As described herein, we can use the mRNA sequence information presented in Fig. 2 to 5 to make deductions about HDV replication competence in each of these systems. First, we know that the single nucleotide changes at nt 1012 and 1375 are ones that lead to δAgs that cannot support virus replication. Second, we can look for silent mutations, such as that at nt 1290. Third, while we can detect other predicted amino acid changes, we cannot without further study conclude whether or not the altered proteins will support replication. Using just this information, we can deduce important differences in HDV replication competence in the infected woodchuck, where virus assembly and spread was possible, relative to the other experimental systems. Specifically, 40 to 80% of the δAg in infected woodchucks could not support replication. In contrast, at relatively late times after transfection of HuH7 cells and mouse liver and skeletal muscle, the value was higher (≥80%). These data support the interpretation that in transfected cells, in the absence of virus assembly and spread, there is an accumulation of mutated δAg species unable to support replication. This molecular study thus confirms a similar hypothesis first made on the basis of qualitative immunomicroscopy observations of variations in the intracellular redistribution of δAgs in transfected cultured cells (3) and a more recent study with transfected mouse liver (6).
We detected many other single-nucleotide changes for which the frequency per site was ≤20%. There were 92 such changes in a total of 42,000 sequenced nucleotides, that is, a frequency of 2.2 × 10−3 per nucleotide. However, most of these may be technical errors, because in a reconstruction experiment, using in vitro-transcribed HDV mRNA mixed with total RNA from untransfected HuH7 cells, we determined that the combined error rate for the RT, PCR amplification, and cloning processes was 2.0 × 10−3 per nucleotide (data not shown).
Overall, our studies of HDV genome replication both in cultured cells and in infected livers showed that there was a significant conservation of the molar ratio of δAg to δRNA relative to that for assembled virus particles. In addition, examination of the quality of intracellular δAg showed that in the absence of virus assembly and new rounds of infection, HDV replication led to the accumulation of mutated mRNA species, for the majority of which the δAg quality was inconsistent with continued support of genome replication. Thus, under these situations, one is observing largely the progressive decline of previously accumulated δAg and δRNA species.
Acknowledgments
Harmon Zuccola and James Hogle (Harvard University) provided the purified delta protein. John Monjardino (Imperial College) provided the three cell lines stably transfected with HDV cDNA. Anthony Lerro of the Laboratory Animal Facility carried out all the mouse injections. We thank Anita Cywinski of the DNA Sequencing Facility for automated sequencing. Constructive comments on the manuscript were given by Richard Katz, Glenn Rall, and William Mason.
This work was supported by grants AI-26522 and CA-06927 from the NIH and by an appropriation from the Commonwealth of Pennsylvania.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. A. Smith, J. G. Seidman, and K. Struhl. 1987. Current protocols in molecular biology. Wiley, New York, N.Y.
- 2.Bell, P., R. Brazas, D. Ganem, and G. G. Maul. 2000. Hepatitis delta virus replication generates complexes of large hepatitis delta antigen and antigenomic RNA that affiliate with and alter nuclear domain 10. J. Virol. 74:5329-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bichko, V. V., and J. M. Taylor. 1996. Redistribution of the delta antigens in cells replicating the genome of hepatitis delta virus. J. Virol. 70:8064-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey, J. L., and J. L. Gerin. 1995. Hepatitis D virus RNA editing: specific modification of adenosine in the antigenomic RNA. J. Virol. 69:7593-7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, F. L., P. J. Chen, S. J. Tu, M. N. Chiu, C. J. Wang, and D. S. Chen. 1991. The large form of hepatitis δ antigen is crucial for the assembly of hepatitis δ virus. Proc. Natl. Acad. Sci. USA 88:8490-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, J., L. J. Sigal, A. Lerro, and J. Taylor. 2001. Replication of the human hepatitis delta virus genome is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. J. Virol. 75:3469-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao, M., S.-Y. Hsieh, and J. Taylor. 1990. Role of two forms of the hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J. Virol. 64:5066-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, P.-J., G. Kalpana, J. Goldberg, W. Mason, B. Werner, J. Gerin, and J. Taylor. 1986. Structure and replication of the genome of hepatitis δ virus. Proc. Natl. Acad. Sci. USA 83:8774-8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, D., A. Yang, H. Thomas, and J. Monjardino. 1993. Characterization of stable hepatitis delta expressing hepatoma cell lines: effect of HDAg on cell growth. Prog. Clin. Biol. Res. 382:149-153. [PubMed] [Google Scholar]
- 10.Cunha, C., J. Monjardino, D. Chang, S. Krause, and M. Carmo-Fonseca. 1998. Localization of hepatitis delta virus RNA in the nucleus of human cells. RNA 4:680-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darnell, J., H. Lodish, and D. Baltimore. 1986. Molecular cell biology. Scientific American Books Ltd., New York, N.Y.
- 12.Dingle, K., V. Bichko, H. Zuccola, J. Hogle, and J. Taylor. 1998. Initiation of hepatitis delta virus genome replication. J. Virol. 72:4783-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingle, K., G. Moraleda, V. Bichko, and J. Taylor. 1998. Electrophoretic analysis of the ribonucleoproteins of hepatitis delta virus. J. Virol. Methods 75:199-204. [DOI] [PubMed] [Google Scholar]
- 14.Glenn, J. S., J. M. Taylor, and J. M. White. 1990. In vitro-synthesized hepatitis delta virus RNA initiates genome replication in cultured cells. J. Virol. 64:3104-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gowans, E. J., B. M. Baroudy, F. Negro, A. Ponzetto, R. H. Purcell, and J. L. Gerin. 1988. Evidence for replication of hepatitis delta virus RNA in hepatocyte nuclei after in vivo infection. Virology 167:274-278. [DOI] [PubMed] [Google Scholar]
- 16.Gudima, S., K. Dingle, T.-T. Wu, G. Moraleda, and J. Taylor. 1999. Characterization of the 5′-ends for polyadenylated RNAs synthesized during the replication of hepatitis delta virus. J. Virol. 73:6533-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudima, S., S.-Y. Wu, C.-M. Chiang, G. Moraleda, and J. Taylor. 2000. Origin of the hepatitis delta virus mRNA. J. Virol. 74:7204-7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh, S.-Y., M. Chao, L. Coates, and J. Taylor. 1990. Hepatitis delta virus genome replication: a polyadenylated mRNA for delta antigen. J. Virol. 64:3192-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kos, A., R. Dijkema, A. C. Arnberg, P. H. van der Meide, and H. Schellekens. 1986. The hepatitis delta (δ) virus possesses a circular RNA. Nature 323:558-560. [DOI] [PubMed] [Google Scholar]
- 20.Kuo, M. Y.-P., M. Chao, and J. Taylor. 1989. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J. Virol. 63:1945-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo, M. Y.-P., J. Goldberg, L. Coates, W. Mason, J. Gerin, and J. Taylor. 1988. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: sequence, structure, and applications. J. Virol. 62:1855-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazinski, D. W., and J. M. Taylor. 1994. Expression of hepatitis delta virus RNA deletions: cis and trans requirements for self-cleavage, ligation, and RNA packaging. J. Virol. 68:2879-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazinski, D. W., and J. M. Taylor. 1993. Relating structure to function in the hepatitis delta virus antigen. J. Virol. 67:2672-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo, G., M. Chao, S.-Y. Hsieh, C. Sureau, K. Nishikura, and J. Taylor. 1990. A specific base transition occurs on replicating hepatitis delta virus RNA. J. Virol. 64:1021-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modahl, L. E., and M. M. C. Lai. 1998. Transcription of hepatitis delta antigen mRNA continues throughout hepatitis delta virus (HDV) replication: a new model of HDV RNA transcription and regulation. J. Virol. 72:5449-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modahl, L. E., T. B. Macnaughton, N. Zhu, D. L. Johnson, and M. M. C. Lai. 2000. RNA-dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol. Cell. Biol. 20:6030-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moraleda, G., and J. Taylor. 2001. Host RNA polymerase requirements for transcription of the human hepatitis delta virus genome. J. Virol. 75:10161-10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Netter, H. J., K. Kajino, and J. Taylor. 1993. Experimental transmission of human hepatitis delta virus to the laboratory mouse. J. Virol. 67:3357-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Netter, H. J., T.-T. Wu, M. Bockol, A. Cywinski, W.-S. Ryu, B. C. Tennant, and J. M. Taylor. 1995. Nucleotide sequence stability of the genome of hepatitis delta virus. J. Virol. 69:1687-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polo, J. M., B. Lim, S. Govindarajan, and M. M. C. Lai. 1995. Replication of hepatitis delta virus RNA in mice after intramuscular injection of plasmid DNA. J. Virol. 69:5203-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polson, A. G., B. L. Bass, and J. L. Casey. 1996. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature 380:454-456. [DOI] [PubMed] [Google Scholar]
- 32.Ponzetto, A., P. J. Cote, H. Popper, B. H. Hoyer, W. T. London, E. C. Ford, F. Bonino, R. H. Purcell, and J. L. Gerin. 1984. Transmission of the hepatitis B virus-associated δ agent to the Eastern woodchuck. Proc. Natl. Acad. Sci. USA 81:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapicetta, M., A. R. Ciccaglione, N. D'Urso, R. Giuseppetti, F. Negro, A. Cesolini, F. Varano, B. Forzani, G. Verme, and A. Ponzetto. 1993. Chronic infection in woodchucks infected by a cloned hepatitis delta virus. Prog. Clin. Biol. Res. 382:451. [Google Scholar]
- 34.Rizzetto, M., M. G. Canese, J. Arico, O. Crivelli, F. Bonino, C. G. Trepo, and G. Verme. 1977. Immunofluorescence detection of a new antigen-antibody system associated to the hepatitis B virus in the liver and in the serum of HBsAg carriers. Gut 18:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzetto, M., M. G. Canese, J. L. Gerin, W. T. London, D. L. Sly, and R. H. Purcell. 1980. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J. Infect. Dis. 141:590-602. [DOI] [PubMed] [Google Scholar]
- 36.Ryu, W.-S., M. Bayer, and J. Taylor. 1992. Assembly of hepatitis delta virus particles. J. Virol. 66:2310-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryu, W. S., H. J. Netter, M. Bayer, and J. Taylor. 1993. Ribonucleoprotein complexes of hepatitis delta virus. J. Virol. 67:3281-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato, S., S. K. Wong, and D. W. Lazinski. 2001. Hepatitis delta virus minimal substrates competent for editing by ADAR1 and ADAR2. J. Virol. 75:8547-8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sureau, C., J. R. Jacob, J. W. Eichberg, and R. E. Lanford. 1991. Tissue culture system for infection with human hepatitis delta virus. J. Virol. 65:3443-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sureau, C., J. Taylor, M. Chao, J. Eichberg, and R. Lanford. 1989. A cloned DNA copy of hepatitis delta virus is infectious in the chimpanzee. J. Virol. 63:4292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor, J., W. Mason, J. Summers, J. Goldberg, C. Aldrich, L. Coates, J. Gerin, and E. Gowans. 1987. Replication of human hepatitis delta virus in primary cultures of woodchuck hepatocytes. J. Virol. 61:2891-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, K.-S., Q.-L. Choo, A. J. Weiner, J.-H. Ou, C. Najarian, R. M. Thayer, G. T. Mullenbach, K. J. Denniston, J. L. Gerin, and M. Houghton. 1986. Structure, sequence and expression of the hepatitis delta viral genome. Nature 323:508-513. [DOI] [PubMed] [Google Scholar]
- 43.Wu, T.-T., H. J. Netter, D. W. Lazinski, and J. M. Taylor. 1997. Effects of nucleotide changes on the ability of hepatitis delta virus to transcribe, process, and accumulate unit-length, circular RNA. J. Virol. 71:5408-5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia, Y.-P., and M. M. C. Lai. 1992. Oligomerization of hepatitis delta antigen is required for both the trans-activating and trans-dominant inhibitory activities of the delta antigen. J. Virol. 66:6641-6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia, Y.-P., C.-T. Yeh, J.-S. Ou, and M. M. C. Lai. 1992. Characterization of nuclear targeting signal of hepatitis delta antigen: nuclear transport as a protein complex. J. Virol. 66:914-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuccola, H. J., J. E. Rozzelle, S. M. Lemon, B. W. Erickson, and J. M. Hogle. 1998. Structural basis of the oligomerization of hepatitis delta antigen. Structure 6:821-830. [DOI] [PubMed] [Google Scholar]