Abstract
The etiology of the lymphadenopathy and follicular hyperplasia associated with human immunodeficiency virus type 1 (HIV-1) infection has remained unclear. To determine whether the B-lymphocyte expansions characteristic of this syndrome represent polyclonal and virus-specific processes, the antigen specificity of B cells in lymphoid tissues of monkeys infected with simian-human immunodeficiency virus (SHIV) chimeras was assessed using an inverse immunohistochemical assay with biotinylated HIV-1 envelope gp120 (Env) as an antigen probe. Env-binding B cells were found aggregated in lymph node and splenic germinal centers (GCs). Most Env-binding GCs also contained an unstained population of B cells, suggesting the GCs were formed by a polyclonal (oligoclonal) process. By day 42 following infection, Env-binding B cells were present in 19% of all lymph node GCs. Env-binding cells were present in 25% of GCs even during chronic infection. This extraordinarily high frequency of Env-specific B lymphocytes suggests that the expansion of virus-specific B cells may largely account for the follicular hyperplasia in AIDS virus-infected individuals.
Striking alterations in B-lymphocyte populations occur in secondary lymphoid tissues of human immunodeficiency virus type 1 (HIV-1)-infected individuals. B cells in lymph nodes and spleens become activated early in the course of infection, resulting in increases in the size and number of the secondary lymphoid follicles. This follicular hyperplasia persists throughout an AIDS virus infection until its end stage and is manifested clinically as lymphadenopathy. The lymphadenopathy and follicular hyperplasia are so extreme that a state of nonspecific B-lymphocyte activation has been hypothesized to account for them (26). The present study examines the alternative hypothesis that the B-cell expansion results from a vigorous virus-specific immune response.
The process of secondary lymphoid follicle formation has been characterized in a number of model systems. The central region of the secondary follicles is termed the germinal center (GC). GCs develop by the oligoclonal expansion of B cells that have been activated following exposure to antigen and interaction with helper CD4+ T lymphocytes. During this cellular expansion, each B lymphocyte clone diversifies by somatic mutation of its immunoglobulin (Ig) variable region. Progeny cells with high affinity for antigen are selected for further expansion, while low-affinity cells are deleted by apoptosis. This iterative process culminates in the generation of high-affinity memory B lymphocytes and plasma cells.
GCs play diverse roles in the pathogenesis of AIDS. They are a major reservoir for infectious virions (16, 45) and an important site where permissive cells become infected (18). Moreover, GC abnormalities are commonly seen throughout the course of HIV-1 or simian immunodeficiency virus (SIV) infections (9, 15, 46), suggesting that B-cell development and antibody formation may be dysfunctional in HIV-infected individuals. Perhaps most importantly, GCs are likely an important site where HIV-1 envelope (Env)-specific virus-neutralizing antibodies develop, since neutralizing antibodies generally contain somatic mutations (2).
In view of the importance of GCs in AIDS pathogenesis, it is important to elucidate the ontogeny, frequency, and epitope specificity of antigen-specific B cells in GCs elicited in an AIDS virus infection. Because GCs develop for the most part in secondary lymphoid organs and can only be studied longitudinally in sequential biopsy specimens, it would be difficult to obtain relevant tissue specimens for evaluation from humans. Moreover, as GCs first develop during the primary immune response to an antigen, subjects would have to be identified for evaluation very early following virus exposure. The evolution of GCs is therefore most readily studied in animal models.
The infection of rhesus monkeys with chimeric immunodeficiency viruses that express HIV-1 envelope glycoproteins on an SIV backbone (SHIVs) has proven a valuable animal model for studying AIDS immunopathogenesis. Previous studies of SHIV-infected monkeys have documented the occurrence of follicular hyperplasia (31), characterized the time course for seroconversion to Env reactivity (23), and examined the evolution of HIV-1 neutralizing antibodies (10). In the present study, lymphoid tissues of SHIV-infected monkeys were studied to determine the time course for the recruitment of Env-specific B cells into GCs during primary infection and to define the frequency of these B cells in GCs. An inverse immunohistochemical technique was employed to identify cells producing antienvelope antibodies by their ability to bind a labeled recombinant HIV-1 envelope glycoprotein (rgp120). These studies demonstrate an extraordinarily high frequency of Env-specific B lymphocytes in GCs of SHIV-infected monkeys.
MATERIALS AND METHODS
Antibodies and recombinant proteins.
HIV-1 89.6 recombinant gp120 (rgp120-89.6) and gp140 (rgp-89.6) were prepared by Robert Doms (University of Pennsylvania, Philadelphia, Pa.) as described previously (5, 8) from supernatants of cells infected with recombinant vaccinia viruses expressing HIV envelope glycoproteins and purified by lectin affinity chromatography. The rgp120-89.6 obtained from Doms was biotinylated in 100-μg batches using the FluoReporter Mini-biotin-XX protein labeling kit (Molecular Probes, Eugene, Oreg.) according to the manufacturer's instructions. Unreacted biotin was removed by filtration over a Chroma Spin LC-30 column (Clontech Laboratories, Palo Alto, Calif.). Biotinylated rgp120-IIIB was obtained from Intracel (Rockville, Md.). Unlabeled and biotinylated keyhole limpet hemocyanin (KLH) were obtained from Sigma (St. Louis, Mo.). Recombinant soluble human CD4 (rsCD4) was provided by Biogen, Inc. (Cambridge, Mass.).
The antigenic structure of biotinylated rgp120 was probed by immunoprecipitation using human anti-HIV-1 envelope monoclonal antibodies (MAbs) F105 (49), 17b (48), and CG10 (13), which recognize conformational epitopes of gp120, and murine anti-human CD4 MAb 5A8 (34).
The phenotype of Env-binding lymph node cells was determined by confocal fluorescence microscopy after indirect immunostaining using antibodies recognizing rhesus monkey T cells, B cells, follicular dendritic cells, and the proliferation-associated antigen Ki-67. T cells were stained with rabbit polyclonal anti-human CD3 (Dako Corp., Carpinteria, Calif.) with goat anti-rabbit IgG conjugated to Alexa 568 (Molecular Probes Inc., Eugene, Oreg.). B cells were stained using mouse IgG2a MAb anti-human CD20 (Dako) with goat anti-mouse IgG conjugated to Alexa 568 (Molecular Probes). Follicular dendritic cells were stained using mouse IgM MAb anti-human DRC-1 (Dako) with goat anti-mouse IgM conjugated to Alexa 568 (Molecular Probes). Ki-67 was stained using mouse IgG1 MAb anti-human Ki-67 (Pharmingen, San Diego, Calif.) with goat anti-mouse IgG conjugated to Alexa 568. The optimal dilution for each reagent was determined in advance.
Immunoprecipitation of biotinylated rgp120.
Biotinylated rgp120-89.6 at a final concentration of 3.3 μg/ml (27.5 nM) was incubated overnight at 4°C with an equal concentration of an MAb, either F105, 17b, CG10, or 5A8, together with protein A-Sepharose. In studies using the MAbs CG10 and 5A8, rsCD4 was added to the incubation at a final concentration of 3.3 μg/ml. After removal of the supernatant, the protein A-Sepharose was washed twice in phosphate-buffered saline (PBS) containing 2% heat-inactivated fetal bovine serum (FBS) and once in PBS and then boiled for five minutes in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The immunoprecipitated proteins were separated by electrophoresis in a 4% stacking gel and 7.5% separating gel and transferred to nitrocellulose using the Protean II apparatus (Bio-Rad, Hercules, Calif.). Biotinylated molecular size standards (Sigma) were run in an adjacent lane. After blocking with 2% FBS and 0.05% Tween 20 in Tris-buffered saline (TBS), the nitrocellulose was incubated with alkaline phosphatase-conjugated streptavidin-biotin complex (streptABComplex-AP; Dako Corporation) diluted 1:50 in TBS for 1 h at room temperature. The nitrocellulose was washed three times in TBS and developed with Vector Blue (Vector Labs) chromogenic alkaline phosphatase substrate.
Mouse immunizations, sample collection, and handling.
The inverse immunohistochemical techniques used in the monkey studies were validated in studies using BALB/c mice immunized with unlabeled KLH or HIV-1 Env. Each mouse was immunized intraperitoneally with 100 μg of a purified protein emulsified in complete Freund's adjuvant. Each mouse received secondary immunizations 8 weeks later by intraperitoneal injection of 15 μg of HIV-1 Env or 25 μg of KLH emulsified in incomplete Freund's adjuvant. Blood samples collected from retroorbital veins were allowed to clot, and serum was recovered and stored frozen until it was evaluated for antibodies specific for the immunizing antigens.
Mice were euthanatized 8 days after the secondary immunization by inhalation of carbon dioxide. Their spleens were frozen in OCT cryoembedding medium (Fisher Scientific) immediately upon removal and stored at −80°C until use. Frozen sections were cut at 6-μm thickness, thaw-mounted onto Superfrost Plus slides (Fisher), air dried, and stored at −20°C.
Assay of mouse serum antibodies to Env and KLH.
Serum was obtained from mice after primary and secondary immunizations with purified unlabeled KLH or HIV-1 Env. The serum was assayed by enzyme-linked immunosorbent assay (ELISA) to confirm the development of humoral immunity to the immunizing antigens. Wells of MaxiSorp 96-well ELISA plates (Nunc) were incubated overnight at 4°C with 100-μl volumes of PBS containing 0.1 μg of HIV-1-89.6 Env rgp120 or 0.2 μg of KLH per well. After aspiration, the wells were blocked with 2% bovine serum albumin (BSA) in PBS for 2 h at room temperature. Serum samples were diluted in the blocking buffer and added in volumes of 100 μl/well. After 45-min incubations, the plates were washed four times with 0.05% Tween 20 in PBS and incubated for 45 min with a 1:2,000 dilution of peroxidase-conjugated goat anti-mouse Igs (Dako) at room temperature. After another four washes, the chromogen TMB (KPL, Gaithersburg, Md.) was added, and the reaction was halted by addition of TMB stop solution (KPL). Absorption at 450 nm was measured using a Dynatech MR5000 ELISA plate reader.
SHIV clones, animal infections, and sample handling.
The SHIVs employed for this study are described elsewhere (23). In brief, the env, tat, and rev genes of HIV-1 HXBc2 were introduced into an infectious molecular clone of SIVmac239. The env gene of the resulting virus was replaced with the env gene of HIV-1 clone 89.6 to generate SHIV-89.6 (40). Serial passage of SHIV-89.6 in vivo generated the highly pathogenic SHIV-89.6P quasispecies. Molecular clones recovered from the quasispecies include SHIV-KB1 and SHIV-KB9 (22). Monkeys infected with these viruses were used in the experiments depicted in Fig. 2 and 4. In order to optimize the detection of Env-specific lymph node B cells for the prospective and quantitative studies of primary infection, two SHIV recombinants (SHIV-89.6* and SHIV-KB9ct) that expressed a gp120 identical to that of the rgp120-89.6 employed as an inverse immunohistochemical probe were selected. SHIV-89.6*, SHIV-KB9ct, and SHIV-KB1 all infect rhesus monkeys and cause a modest depletion of CD4+ T lymphocytes (22, 23).
FIG. 2.
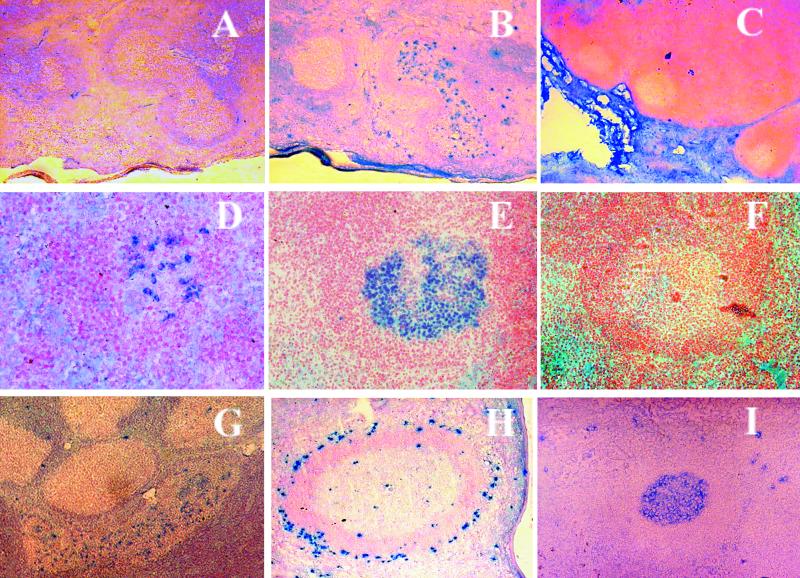
Inverse immunohistochemical staining of lymphoid tissue. (A and B) Serial splenic sections from monkey 18284, infected with SHIV-KB9ct 9 months earlier, stained with hematoxylin and immunostained with biotinylated rgp120-89.6 (Env) without (panel A) or with (panel B) catalyzed signal amplification. (C) Uninfected monkey lymph nodes, stained using Env with catalyzed signal amplification. Note absence of staining in germinal center cells. (D to F) Inverse immunostaining of murine splenic sections. (D) Mouse immunized with KLH and probed with biotinylated KLH with catalyzed signal amplification. (E and F) Mouse immunized with Env and probed with biotinylated Env (E) or KLH (F) with catalyzed signal amplification. (G to I) Lymphoid tissues from SHIV-infected monkeys stained with Env with catalyzed signal amplification. (G) Lymph nodes from monkey 15243, obtained 4 months postinfection with SHIV-KB1, counterstained with hematoxylin. (H) Spleen from monkey 19941, obtained 3 months postinfection with SHIV-KB9. Note staining of cells in marginal zone. (I) Lymph nodes from monkey 15655, obtained 16 months postinfection with SHIV-89.6ct. Original magnifications vary from ×10 to ×40.
FIG. 4.
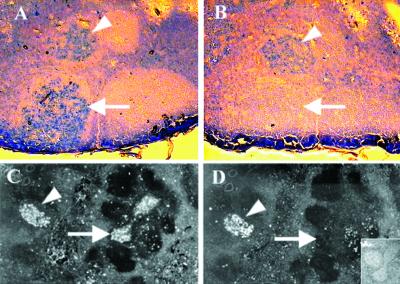
Differential staining of SHIV-infected lymph node GCs by different recombinant HIV-1 envelope glycoproteins. Panels A and B depict serial sections, stained with biotinylated rgp120 of two different HIV-1 strains, 89.6 (A) and IIIB (B) with catalyzed signal amplification. (C and D) A different pair of serial sections, viewed with fluorescent imaging to demonstrate the results of a competition inverse immunohistochemistry assay employing biotinylated rgp120-89.6 with catalyzed signal amplification and Vector Red chromogen alone (C) and in the presence of a 100-fold excess of an unlabeled rgp140-89.6 oligomer (D). An inset shows the slide in D now counterstained with hematoxylin and viewed under visible light to demonstrate that the germinal center indicated by an arrow, though unstained, is present. Arrowheads indicate GCs that are stained in both panels; arrows indicate GCs that are differentially stained in the two panels.
The rhesus monkeys used in this study were maintained at the Oregon Regional Primate Research Center in accordance with the guidelines of the committee on Animals for the Harvard Medical School and the Guide for the Care and Use of Laboratory Animals (3). Healthy rhesus monkeys were inoculated intravenously with 400 times the dose of cell-free SHIV that causes infection in 50% of susceptible tissue cultures. Infection of all animals was confirmed by serologic and virologic assays, with peak viremia occurring from 10 to 14 days after inoculation (22, 23, 40). Viral loads in plasma in the cohort during this primary viremia were similar to viral loads seen in monkeys infected with pathogenic SIVmac, except for monkey 18331, in which plasma p27 assays remained negative despite a transient CD4+ T-lymphocyte decline and seroconversion. Immunologic and virologic data from some of these monkeys have been reported previously (23, 31, 40).
Monkeys were anesthetized with ketamine-HCl (5 mg/kg intramuscularly) for all blood sampling and with Telazol (Fort Dodge Animal Health, Overland Park, Kans.) (8 mg/kg intramuscularly) for biopsies. Animals were euthanatized by an overdose of sodium pentobarbital administered intravenously. Lymph nodes and pieces of spleen were frozen in OCT cryoembedding medium (Fisher Scientific) immediately upon removal and stored at −80°C until use. Frozen sections were cut at 10-μm thickness, thaw-mounted onto Superfrost Plus slides (Fisher), and immediately refrozen. Tissue sections were stored at −20°C.
Inverse immunohistochemistry and immunofluorescence.
Slide-mounted tissue sections of lymph nodes or spleen from SHIV-infected and uninfected control monkeys and from KLH- or Env-immunized mice were fixed in cold acetone for 10 min, air dried, and rehydrated in Tris-buffered saline (TBS, pH 8). Slides were then processed using the Sequenza immunostaining workstation (Shandon Lipshaw, Pittsburgh, Pa.). Tissue sections were pretreated to block endogenous peroxidase activity and endogenous biotin (Dako). Inverse immunostaining was enhanced by using catalyzed signal amplification with the Renaissance TSA-indirect kit (NEN-Life Science Products, Boston, Mass.). Each slide was first treated with blocking reagent buffer (Renaissance kit) to reduce nonspecific staining and then incubated for 1 h at room temperature with biotinylated recombinant HIV-1 envelope gp120-89.6 or -IIIB at a maximum concentration of 0.2 μg/ml (1.67 nM) or with biotinylated KLH (Sigma) at a concentration of 2.5 μg/ml.
For competition binding experiments, where noted, unlabeled rgp140-89.6 was added at a 100-fold-greater concentration. Slides were rinsed three times with TBS containing 0.05% Tween 20 (TBST) after each subsequent staining step. The slides were incubated with streptavidin-horseradish peroxidase (1:100 dilution; Renaissance kit) for 30 min at room temperature and subsequently incubated with biotinyl tyramide at a 1:50 dilution for 10 min to catalyze amplification of the biotin signal. The amplified biotin signal, indicating bound biotinylated rgp120, was detected histochemically by using alkaline phosphatase-conjugated streptavidin-biotin complex (streptABComplex-AP; Dako) and either Vector Blue or Vector Red chromogens (Vector Laboratories) together with levamisole (Dako) to suppress endogenous alkaline phosphatase activity. After counterstaining with either Nuclear Fast Red (Vector) or Gill's hematoxylin (Fisher), slides were covered with Crystal/Mount permanent mounting medium (Fisher) and air dried. Digitized images of tissue sections were formatted using Adobe Photoshop v4 software.
For phenotyping of Env-binding cells, selected tissue sections were stained first using a modification of the procedure described above, wherein a streptavidin conjugated to Alexa 488 (Molecular Probes) was substituted for the streptABComplex-AP. Next, after washing in TBST, the tissue sections were stained with one of a panel of fluorescently labeled antibodies (described in the section on antibodies and recombinant proteins). The fluorescent labels were imaged by confocal microscopy, performed with a Leica TCS SP laser scanning microscope equipped with three lasers. The fluorescence of individual fluorochromes was captured separately in sequential mode after optimization using Leica software to reduce bleed-through between channels (photomultiplier tubes). Adobe Photoshop v4 software was used to assign correct colors of up to three channels collected, including the fluorochromes Alexa 488 (green) and Alexa 568 (red), and the differential interference contrast image (gray scale).
Statistical analysis.
The number of GCs in each lymph node and the number of GCs containing Env-binding cells were determined independently by two observers by manual counting with a light microscope. These tallies provided the denominator and numerator, respectively, for calculation of the percentage of GCs containing B cells that bound rgp120 in each node. Statistical significance was evaluated by pairwise comparisons among all time points using a paired two-sample t test for means, as implemented in Microsoft Excel 2000 software. The P values were computed for two-tailed analyses.
Manual counts of individual Env-stained and unstained cells in cross-sectional views of GCs were performed by a single observer with a light microscope. These tallies provided the numerator and denominator, respectively, for calculation of the percentage of Env-binding cells for each GC analyzed. A relationship between the size of a GC (i.e., the number of cells in cross-section) and the percentage of Env-binding cells in the GC was sought using the Pearson correlation coefficient and Microsoft Excel 2000 software.
RESULTS
Recombinant gp120 retains its conformational and functional properties after biotinylation.
The aim of the present study was to characterize the evolution of the HIV-1 Env-specific B-lymphocyte populations in secondary lymphoid tissues of SHIV-infected rhesus monkeys. To this end, an inverse immunohistochemistry assay was employed to localize cells producing anti-gp120 antibodies in situ. A soluble, biotin-labeled, recombinant envelope protein, rgp120-89.6, was selected as the antigen probe in the inverse immunohistochemistry assay. Because preparation of this biotin-labeled probe required covalent modification of exposed amino acid residues of the envelope glycoprotein, it was important to determine whether this reaction disrupted important conformational or functional properties of the envelope glycoprotein. To examine this possibility, the recognition of biotin-labeled rgp120-89.6 by MAbs that recognize conformation-dependent HIV-1 Env epitopes was tested by immunoprecipitation (Fig. 1).
FIG. 1.
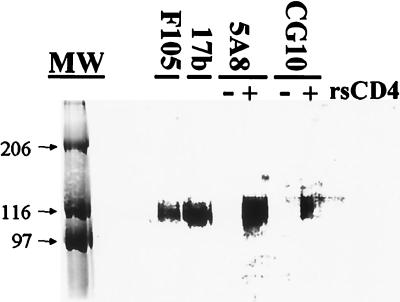
Immunoprecipitation of biotinylated rgp120-89.6 by monoclonal antibodies. Recombinant soluble CD4 (rsCD4) was added with MAb to some samples, shown in lanes marked with a plus sign (+). Lane MW, molecular size standards, labeled by size (in kilodaltons).
The biotin-labeled rgp120-89.6 was immunoprecipitated by the MAbs F105 and 17b (Fig. 1), indicating that at least a portion of the rgp120 in the preparation retained the corresponding epitopes after biotinylation. The ability of biotinylated rgp120-89.6 to bind to human CD4 was also confirmed, as shown by the immunoprecipitation of the biotinylated envelope glycoprotein by the CD4-specific MAb 5A8 only in the presence of recombinant soluble human CD4 (Fig. 1). There was no binding of the MAb 5A8 to the biotinylated envelope in the absence of recombinant soluble human CD4. Similarly, the biotinylated rgp120-89.6 was recognized by the MAb CG10, an antibody that binds to a CD4-induced gp120 epitope, only when recombinant soluble human CD4 was present. Therefore, the biotinylated rgp120-89.6 retained its functional capacity to bind to CD4 and then to undergo a CD4-induced conformational change.
Augmentation of inverse immunostaining with catalyzed signal amplification.
Earlier studies suggested that inverse immunohistochemistry might prove useful in localizing HIV-1-specific B cells in histologic specimens (25, 26). However, initial attempts to apply this technique to the study of lymph nodes of chronically SHIV-infected rhesus monkeys met with little success. Neither formalin-fixed, paraffin-embedded tissue sections nor acetone-fixed frozen sections from lymph nodes or spleens showed convincing staining with the biotin-labeled rgp120-89.6 probes. Allowing for the possibility that the signal-to-noise ratio might be insufficient to detect rgp120-89.6 binding to cells in tissue sections, a catalyzed signal amplification step was introduced into the staining protocol.
The improvement in staining after this modification was striking (Fig. 2A and 2B). Using biotinylated rgp120-89.6 and catalyzed signal amplification with immunohistochemical or immunofluorescent detection, consistent, saturable binding of HIV-1 Env to cells in B-cell zones of lymphoid tissues from the SHIV-infected monkeys was demonstrated. The specificity of the staining was demonstrated by comparison with two catalyzed signal amplification-enhanced inverse immunohistochemistry assay controls. In the first control, biotinylated rgp120-89.6 was applied to lymphoid tissue sections from an uninfected monkey (Fig. 2C). GCs in these uninfected tissues did not bind to rgp120-89.6. In the second control, a biotinylated nonviral protein, KLH, was applied to tissue sections from SHIV-infected animals. There was negligible binding of this nonviral antigen probe to Env-binding GCs (data not shown).
As an additional antigen-specific control for staining in the inverse immunohistochemistry assay, we immunized mice with either recombinant HIV-1 Env gp120 or the nonviral protein KLH and tested the serum of these mice by ELISA for antibodies to Env and to KLH. The splenic tissues from these mice were then probed using biotinylated versions of these two proteins. The mice developed antibodies reactive only with the immunizing antigen, and antibody titers increased after booster immunizations (data not shown). Inverse immunohistochemistry staining of splenic sections from these animals identified KLH-binding GCs only in KLH-immunized mice (Fig. 2D) and Env-binding GCs only in Env-immunized mice (Fig. 2E). GCs from mice immunized with one protein did not stain with the other protein (Fig. 2F). These results therefore confirm that binding of an antigen probe to GC lymphocytes in a catalyzed signal amplification-augmented inverse immunohistochemistry assay is only seen in animals that develop humoral immunity specific for that antigen.
Localization of Env-binding cells in lymphoid tissues from monkeys chronically infected with SHIV.
The tissue distribution of Env-binding cells was examined in lymph nodes and spleens of chronically SHIV-infected monkeys using biotinylated HIV-1-89.6 rgp120 in an inverse immunohistochemistry assay augmented with catalyzed signal amplification. In lymph nodes of chronically infected monkeys, Env-binding cells were present in the B-cell zones, predominantly in germinal centers. Notably, intensely stained and entirely unstained germinal centers were frequently apparent on a single tissue section, in close proximity (e.g., Fig. 2G). Scattered staining was also observed among cells in the mantle zones. Also, intensely stained cells were frequently present in medullary cords and sinuses, which are known to be rich in plasma cells.
In the spleens of chronically infected monkeys, the distribution of Env-binding cells was similar to that seen in lymph nodes, with clusters of cells in germinal centers and occasional cells scattered in the mantle zones. Additionally, Env-binding cells were found in the marginal zones, occasionally forming a ring surrounding a germinal center and mantle zone (Fig. 2H). Curiously, some rings of Env-binding marginal zone cells surrounded GCs containing few Env-binding cells. This observation is in accord with reports that splenic marginal zone B cells are often clonal but are not related to those in adjacent GCs (50).
Phenotypic characterization of Env-binding germinal center cells.
Env-binding cells were readily found in the germinal centers of spleens and lymph nodes from the chronically SHIV-infected monkeys. Occasionally a germinal center was found to stain homogeneously (Fig. 2I). More often, though, the Env-binding lymphocytes were admixed with nonstaining cells. Since GCs typically contain a mixture of B cells, T cells, and follicular dendritic cells, it was necessary to confirm that the Env-binding cells present in the lymphoid tissues of SHIV-89.6-infected monkeys belonged to the B-cell lineage. The phenotype of the Env-binding cells was determined by multilabel fluorescence microscopy of tissue sections stained in a catalyzed signal amplification-enhanced inverse immunofluorescent assay using rgp120-89.6 as an antigen probe and a panel of fluorescently labeled antibodies (Fig. 3).
FIG. 3.
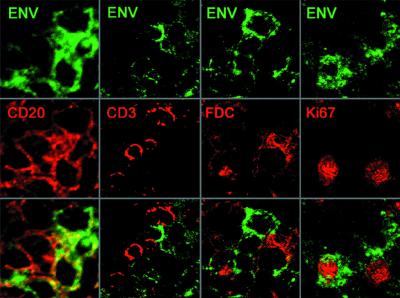
Confocal microscopy showing the phenotype of Env-binding GC cells in lymph nodes from SHIV-infected rhesus monkeys. Cells binding rgp120-89.6-biotin are shown in green. Phenotypic antibodies detected by indirect immunofluorescence are shown in red. The green and red channels are presented separately, with the indicated fluorescent markers, and superimposed. Original magnifications, ×80.
Multicolor confocal microscopy confirmed that the Env-binding GC cells were CD20+ B cells. The leftmost column in Fig. 3 shows a high-power view of a cluster of Env-binding cells (green), all of which also bound anti-CD20 (red), a B-cell marker. The second column shows that Env (green) and anti-CD3 (red) bound to nonoverlapping cell populations, although weak staining of a subset of CD3+ cells could be detected by confocal microscopy at higher concentrations of Env (data not shown). This faint binding to T cells could reflect the binding of the rgp120 probe to CD4, a finding observed in immunoprecipitation assays (Fig. 1).
Although the Env (green) and antibodies to the follicular dendritic cell network did not colocalize (Fig. 3, third column), three-dimensional confocal microscopic views revealed an intimate apposition of follicular dendritic cell processes to Env-binding GC B cells. The proximity of B cells to follicular dendritic cell processes should allow interactions between membrane IgG on B cells and follicular dendritic cell-bound immune complexes, as required for the clonal selection processes to occur. Moreover, these GCs had an extensive and intact follicular dendritic cell network, which is consistent with the presence of follicular hyperplasia in the lymph node (54). There was no evidence of the follicular dendritic cell loss and concomitant follicular involution that typify the later stages of immunodeficiency virus infection (38, 39).
The B-cell population of a GC is conventionally divided into two groups, centrocytes and the larger, mitotically active centroblasts. In order to determine whether the Env-binding GC cells were centrocytes, centroblasts, or both, we performed two-color confocal microscopy using rgp120-89.6 and the MAb Ki-67, a marker for proliferating cells. As shown in the rightmost column of Fig. 3, some Ki-67+ GC B cells bound the envelope antigen probe, as did some Ki-67− B cells. The binding of Env to centroblasts was somewhat surprising, as centroblasts typically downregulate their expression of immunoglobulins (reviewed in reference 28). It appears that the catalyzed signal amplification-enhanced inverse immunostaining is sufficiently sensitive to detect Env-binding antibodies even on GC centroblasts. Alternatively, the gp120-binding Ki-67+ cells may belong to a putative subset of mitotically active centrocytes (24).
In order to characterize the diversity of HIV-1 strains recognized by the Env-binding cells, the staining of tissues with biotinylated envelope glycoproteins derived from two different HIV-1 isolates was compared. Some germinal centers contained cells whose antibodies bound only to envelope of the infecting strain (Fig. 4A and 4B, linear arrows), while cells in other GCs produced cross-reactive antibodies, capable of binding to envelopes from both 89.6 and IIIB isolates (arrowheads). Consistently, an antigen probe based on the envelope of the infecting viral strain identified more Env-binding GCs than did a heterologous probe, most likely because only a subset of antibodies elicited by infection with a cloned virus can recognize heterologous HIV-1 serotypes (10, 33).
Another important aspect of diversity among anti-Env antibodies is the ability of these antibodies to recognize various conformational forms of the envelope glycoproteins. One important subset of anti-Env antibodies can recognize the native, trimeric HIV-1 envelope glycoprotein complex; inability to bind to the native envelope usually implies a lack of virus-neutralizing potential (11, 37). We therefore compared the ability of GC B cells to recognize two conformationally different preparations of HIV-1 envelope glycoprotein in a competitive inverse immunohistochemistry assay. In this experiment, a biotinylated monomeric rgp120-89.6 was observed to bind to numerous GCs in lymph nodes of SHIV-89.6-infected monkeys (Fig. 4C). Staining of some of these GCs was completely abrogated by competition with a 100-fold excess of unlabeled oligomeric rgp140-89.6 envelope (linear arrow in Fig. 4C and 4D), suggesting that the antibodies present on these cells were able to bind to oligomeric as well as to monomeric envelope.
By contrast, other GC B cells (arrowheads, Fig. 4C and 4D) were stained by monomeric rgp120-89.6 even in the presence of a 100-fold excess of the unlabeled oligomeric rgp140-89.6, implying that the antibodies in these GCs do not bind to this oligomeric envelope glycoprotein. Presumably, antibodies present on those cells recognize epitopes of gp120 that are not exposed on the oligomer, probably occluded by intermolecular contacts (35, 53). In any case, the differential staining pattern of these GCs demonstrates that the antibodies recognized distinct epitopes. This result is important because it rules out the possibility that B cells were binding to the envelope probe nonspecifically or were only recognizing a putative HIV-1 envelope superantigen (6).
Increase in lymph node GCs during primary SHIV infection.
The etiology of the follicular hyperplasia seen during AIDS virus infections has remained unexplained. In order to examine the potential role of viremia as a stimulus for the expansion of secondary follicles, it was important to seek a correlation between the two. Since viremia and follicular hyperplasia develop very early after an AIDS virus infection, a complete characterization of the relationship must include analysis of specimens obtained during the period of primary infection. Accordingly, a prospective, quantitative study of viral load and lymphoid follicle development during primary SHIV infection was performed.
Five rhesus monkeys were infected intravenously with a SHIV that expressed an envelope gp120 identical in sequence to the gp120-89.6 immunohistochemical antigen probe that was selected for use in the study. Peripheral blood samples were collected twice weekly through 5 weeks postinfection and quantitatively tested for viral p27 antigen concentration to determine the plasma viral load (data previously reported in reference 23). Axillary and inguinal lymph nodes were obtained by biopsy on postinfection days 10, 21, and 42 and at necropsy on postinfection day 270. Frozen lymph nodes were sectioned in their entirety, and approximately every 20th section was studied. A single node per animal from each time point was analyzed. After staining with Nuclear Fast Red to reveal the tissue architecture, the number and size of any GCs present were recorded for each lymph node section.
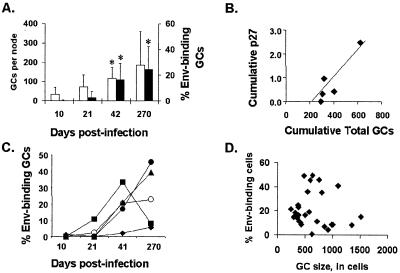
The abundance of GCs increased during the course of infection (Fig. 5A, hollow bars). Tissues from postinfection day 10 contained few GCs. In screening 64 cutting levels from postinfection day 10 lymph nodes from five different monkeys, a total of 174 GCs were identified. GCs were more plentiful in lymph nodes obtained on postinfection day 21 from four of the five monkeys; one monkey had fewer GCs on postinfection day 21 than on postinfection day 10. In screening 79 cutting levels from lymph nodes of the five monkeys obtained on postinfection day 21, 365 GCs were identified. In postinfection day 42 lymph nodes, 472 GCs were identified in 59 cutting levels, a significant increase from postinfection day 10 (P < 0.05). The number of lymph node GCs was even larger at a later time point for two of the five monkeys. Overall, at postinfection day 270, 932 GCs were found in 68 cutting levels.
FIG. 5.
Quantitative analysis of GCs following infection of rhesus monkeys (n = 5) with SHIV-89.6. Biopsied lymph nodes were sectioned in entirety, every 20th section was probed with Env gp120-89.6 using inverse immunohistochemistry with catalyzed signal amplification, and GCs were manually counted microscopically over the entire section. (A) Cohort averages for total and Env-binding GCs at each time point. Open bars indicate a tally of all GCs; solid bars indicate the percentage of Env-binding GCs. (B) Correlation of GC formation with the magnitude of viral antigenemia in each animal. The x axis shows the sum of GCs present in all four lymph nodes obtained from a subject after infection. The y axis shows the sum of p27gag values for eight plasma samples obtained from each subject between postinfection days 3 and 36. (C) Percentage of Env-binding GCs for each subject over time. Each data point represents the average for one lymph node. (D) Plot showing the maximum cross-sectional size of each Env-binding GC and the percentage of Env-binding cells in those GCs. No correlation was found between these values.
The abundance of GCs in the lymph nodes examined varied among the animals. One factor accounting for the variation in GC abundance may have been viral load. For each monkey, the combined number of GCs present in lymph nodes from all four time points was correlated with the cumulative value of serial p27 assays (p27 data were previously reported in reference 23) performed on plasma specimens obtained from that animal during primary infection (R2 = 0.86; Fig. 5B). Thus, animal 18331 had a cumulative p27 level in plasma of less than 0.16 ng/ml and only 318 GCs, while animal 18284 had a cumulative p27 level in plasma of 3.0 ng/ml and 620 GCs.
rgp120-binding B cells in lymph node GCs during primary SHIV infection.
Viremia could lead to follicular hyperplasia by antigen-specific or nonspecific mechanisms. It was therefore important to determine whether substantial proportions of the GCs in these monkeys comprised SHIV-specific B lymphocytes or rather represented nonspecifically activated B-lymphocyte populations. To identify the Env-specific B cells for enumeration, rgp120 binding to GC B cells was assessed by inverse immunohistochemistry in frozen sections of the lymph nodes obtained during primary SHIV infection from the five rhesus monkeys described above. The tally of all GCs present in each lymph node, as described above, provided the denominators for calculation of the percentages of GCs containing B cells that bind rgp120 in each node.
rgp120-binding B cells were rare in tissues obtained on postinfection day 10, constituting less than 1% of GCs in lymph nodes from the five monkeys (Fig. 5C). This was not surprising, as viral load was very low until postinfection day 10. The number of GCs containing rgp120-binding cells had increased by postinfection day 21, constituting almost 3% of GCs overall. This increase did not achieve statistical significance. However, the rgp120-binding B cells were significantly more frequent in GCs on postinfection day 42 than on either postinfection day 10 or 21 (P = 0.048 and P = 0.029, respectively, using a paired two-sample t test for means). On postinfection day 42, they were present on average in 19% of all GCs in a node (range, 2 to 33%; Fig. 5A, solid bars). By 9 months after infection, Env-binding cells were present on average in 24% (range, 6 to 46%) of GCs, still significantly increased compared with postinfection day 10 (P = 0.042).
In addition to the tally of total and Env-binding GCs present on each stained section, microscopic manual counts of individual Env-stained and unstained cells were performed by a single observer for 39 representative Env-binding GCs from 14 tissue sections of three different monkeys at three time points. These GCs were of various apparent sizes. Viewed in cross-section at a single cutting level, each contained between 158 and 1,516 cells. No correlation (r2 = 0.006) was seen between the size of a GC and the proportion of Env-binding cells in that GC (Fig. 5D), indicating that virus-specific B cells were proportionately represented in both normal-sized and hyperplastic GCs.
DISCUSSION
Inverse immunostaining assays are an effective way to localize and quantify in situ cells producing specific antibodies and thereby offer a unique view of the B-cell immune response in its histological context. The present study shows that catalyzed signal amplification can greatly improve the sensitivity of inverse immunostaining assays (immunohistochemistry and immunofluorescence) without loss of binding specificity. These methods were applied to the characterization of HIV-1 envelope-specific B cells in tissues of SHIV-infected rhesus monkeys, using recombinant HIV-1 envelope glycoproteins (Env) as the antigen probes. The specificity of the inverse immunohistochemistry staining was demonstrated by (i) differential binding of Env to tissues of SHIV-infected and uninfected monkeys, (ii) selective binding of Env or KLH antigen probes to tissues of mice immunized with Env or KLH, (iii) differential binding to individual, neighboring GCs in tissues of SHIV-infected monkeys, and (iv) differential binding of GC cells to recombinant envelopes of different serotype or conformation.
Cells binding to Env were clustered in GCs and had the phenotype of B cells. Most of these GCs contained an admixture of Env-binding B cells and nonstaining cells. These nonstaining cells included both B cells and other GC-resident cell types. The presence of nonstaining B cells in Env-binding GCs probably reflects an oligoclonal composition of these GCs, as has been shown in other model systems (20). At high Env concentrations, a sensitive confocal microscope could also detect faint binding of the rgp120 to a subset of T cells, presumably CD4+ cells. The relative paucity of CD4+ cell staining in the inverse immunohistochemistry assays probably resulted from the use of a 15-fold-lower concentration of rgp120 in the inverse immunohistochemistry assay than in immunoprecipitation studies. This reduced concentration is below the dissociation constant for binding to CD4 but is evidently adequate for binding to antibodies, which presumably have higher affinity as well as the advantage of bivalent (or multivalent) avidity.
The most important observation in this study was the high frequency of virus-specific GCs present in lymphoid tissues during primary and chronic AIDS virus infection. GCs containing envelope-specific B cells were first detected in substantial numbers on approximately day 21 postinfection. Since viremia peaks between days 10 and 14 postinfection (23), these findings suggest that widespread GC formation begins approximately 1 week following exposure to viral antigens. This observation is in accord with studies of primary immune responses in mice, where GC formation begins approximately 1 week after immunization (19). The frequency of Env-specific GCs increased during subsequent weeks in the SHIV-infected monkeys, probably reflecting ongoing GC formation elicited by persistent viral antigenemia (45, 47) as well as the persistence of established GCs (4).
A number of methodological choices that were made in pursuing this study probably account for the dramatically higher frequency of virus-specific B cells seen in these AIDS virus-infected monkeys than have previously been reported by others (26). First, the evaluated lymph nodes were obtained from a cohort of animals infected with molecularly cloned SHIVs, viruses with defined envelope sequences. Consequently, recombinant proteins homologous to the infecting virus could be employed as antigen probes. Heterologous probes, as were employed in studies of HIV-infected human lymph nodes (26), detected a substantially smaller fraction of responding B cells. Second, the use of catalyzed signal amplification significantly increased the sensitivity of inverse immunohistochemical staining, allowing detection of Env-binding B cells that would otherwise be overlooked. In particular, antigen binding to germinal center B cells may be more difficult to detect because of their downregulation of immunoglobulin expression. The methods employed in the present study were shown to be sufficiently sensitive to detect cytoplasmic antibody present in GC B cells, including those that were proliferating (i.e., centroblasts).
Our finding of a high frequency of GCs containing Env-specific B cells has implications for our understanding of the lymphadenopathy associated with HIV infection. While HIV, SIV, and SHIV infections induce extensive follicular hyperplasia in humans and nonhuman primates, the antigen specificity of the follicular B cells has not previously been defined. Studies assessing spontaneous and mitogen-induced Ig secretion by peripheral blood mononuclear cells (PBMC) of HIV-1-infected donors have suggested that substantial nonspecific B-cell activation is ongoing in these individuals (1, 43, 55). Mechanisms that have been proposed to account for this nonspecific B-cell activation include bystander activation driven by T-cell costimulatory signals (21, 29, 36, 52) and the direct effects of viral gene products (6, 17, 42). It has therefore been supposed that a substantial component of the follicular hyperplasia observed in these individuals is attributable to nonspecific B-cell activation.
The results of the present study, however, suggest that sizeable proportions of the expanded GC B-lymphocyte populations are virus specific. In the weeks following seroconversion, as many as 40% of all GCs in the lymph nodes bound to rgp120-89.6. Moreover, this value likely underestimates the virus-specific B-cell response because the methods employed in the present study can detect only a fraction of the virus-specific responder B cells. The monomeric rgp120-89.6 probe would not be expected to bind to antibodies that were elicited by other viral gene products or to antibodies that selectively recognize other three-dimensional conformations of the envelope glycoprotein (41). It is conceivable that the vast majority of GC B lymphocytes are producing antibodies specific for SHIV. This overwhelming B-lymphocyte commitment to the replicating virus is reminiscent of the virus-specific CD8+ T-lymphocyte commitment in AIDS virus-infected humans and nonhuman primates recently demonstrated with the peptide/major histocompatibility complex (MHC) tetramer and single-cell gamma interferon analysis techniques (12, 32).
While GCs arising after SHIV infection appear to be composed largely of virus-specific B cells, the present results do not necessarily explain the unusual size of these GCs. An increase in the size of GCs could result from either the massive viral antigen stimulus implied by the recruitment of so many B cells or from mechanisms associated with AIDS virus infections, such as alterations in costimulation (27) or in the secretion of cytokines (7, 14, 30) or chemokines (44, 51). Whether AIDS virus-induced GCs of unusual size harbor the same number of clones as their normal-sized counterparts and whether the mechanisms of somatic mutation and affinity selection operate normally within them are unknown. Future studies addressing these issues are needed to clarify the role of virus-specific GCs in disease pathogenesis and in the evolution of virus-neutralizing antibodies.
Acknowledgments
We thank Robert Doms (Philadelphia, Pa.) for providing rgp120-89.6 produced in his laboratory and Jonathan Gershoni (Tel Aviv, Israel) for providing MAb CG10. MAbs F105 and 17b were obtained from the AIDS Research and Reference Reagent Program of the National Institutes of Health. Biogen (Cambridge, Mass.) generously provided MAb 5A8. In addition, we are grateful to Daniele Rohne for collating viral load and CD4+ T-cell count data for the experimental animals, to Freddie Peyerl for assistance with mouse experiments, and to Paul Racz and Klara Tenner-Racz (Hamburg, Germany) for consultation on SHIV histopathology.
D.H.M. was supported by Public Health Service (PHS) grants AI-01587 and AI-45370 from the National Institute of Allergy and Infectious Diseases (NIAID). E.F.H. participated in these studies as a Medical Student Fellow of the Howard Hughes Medical Institute. M.K.A. was supported by PHS grants AI-42508 and AI-43044 from the NIAID and CA-75922 from the National Cancer Institute and by a PHS institutional grant from the National Institutes of Health to the Oregon Regional Primate Research Center, grant RR-00163. X.A. was supported by a PHS institutional grant to the New England Regional Primate Research Center, grant RR-00168. N.L.L. was supported by PHS grant AI-20729 from the NIAID.
REFERENCES
- 1.Amadori, A., R. Zamarchi, V. Ciminale, A. del Mistro, S. Siervo, A. Alberti, M. Colombatti, and L. Chieco-Bianchi. 1989. HIV-1-specific B cell activation: a major constituent of spontaneous B cell activation during HIV-1 infection. J. Immunol. 143:2146-2152. [PubMed] [Google Scholar]
- 2.Andris, J. S., and J. D. Capra. 1995. The molecular structure of human antibodies specific for the human immunodeficiency virus. J. Clin. Immunol. 15:17-24. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1996. Guide for the care and use of laboratory animals. National Research Council, National Academy Press, Washington, D.C.
- 4.Bachmann, M. F., B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 1996. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J. Exp. Med. 183:2259-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baik, S. S., R. W. Doms, and B. J. Doranz. 1999. HIV and SIV gp120 binding does not predict coreceptor function. Virology 259:267-273. [DOI] [PubMed] [Google Scholar]
- 6.Berberian, L., L. Goodglick, T. J. Kipps, and J. Braun. 1993. Immunoglobulin VH3 gene products: Natural ligands for HIV gp120. Science 261:1588-1591. [DOI] [PubMed] [Google Scholar]
- 7.Boyle, M. J., M. F. Berger, M. Tschuchnigg, J. E. Valentine, B. G. Kennedy, M. Divjak, D. A. Cooper, J. J. Turner, R. Penny, and W. A. Sewell. 1993. Increased expression of interferon-gamma in hyperplastic lymph nodes from HIV-infected patients. Clin. Exp. Immunol. 92:100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broder, C. C., P. L. Earl, D. Long, S. T. Abedon, B. Moss, and R. W. Doms. 1994. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure: oligomer-specific and -sensitive monoclonal antibodies. Proc. Natl. Acad. Sci. USA 91:11699-11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalifoux, L. V., N. W. King, and N. L. Letvin. 1984. Morphologic changes in lymph nodes of macaques with an immunodeficiency syndrome. Lab. Investig. 51:22-26. [PubMed] [Google Scholar]
- 10.Etemad-Moghadam, B., G. B. Karlsson, M. Halloran, Y. Sun, D. Schenten, M. Fernandes, N. L. Letvin, and J. Sodroski. 1998. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys. J. Virol. 72:8437-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouts, T. R., J. M. Binley, A. Trkola, J. E. Robinson, and J. E. Moore. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J. Virol. 71:2779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. C. de Quiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 13.Gershoni, J. M., G. Denisova, D. Raviv, N. I. Smorodinsky, and D. Buyaner. 1993. HIV binding to its receptor creates specific epitopes for the CD4/gp120 complex. FASEB J. 7:1185-1187. [DOI] [PubMed] [Google Scholar]
- 14.Gray, C. M., L. Morris, J. Murray, J. Keeton, S. Shalekoff, S. F. Lyons, P. Sonnenberg, and D. J. Martin. 1996. Identification of cell subsets expressing intracytoplasmic cytokines within HIV-1-infected lymph nodes. AIDS 10:1467-1475. [DOI] [PubMed] [Google Scholar]
- 15.Guarda, L. A., J. J. Butler, P. Mansell, E. M. Hersh, J. Reuben, and G. R. Newell. 1983. Lymphadenopathy in homosexual men: morbid anatomy with clinical and immunologic correlations. Am. J. Clin. Pathol. 79:559-568. [DOI] [PubMed] [Google Scholar]
- 16.Heath, S. L., J. G. Tew, J. G. Tew, A. K. Szakal, and G. F. Burton. 1995. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377:740-744. [DOI] [PubMed] [Google Scholar]
- 17.Huang, L., C. J. Li, and A. B. Pardee. 1997. Human immunodeficiency virus type 1 TAT protein activates B lymphocytes. Biochem. Biophys. Res. Commun. 237:461-464. [DOI] [PubMed] [Google Scholar]
- 18.Hufert, F. T., J. van Lunzen, G. Janossy, S. Bertram, J. Schmitz, O. Haller, P. Racz, and D. von Laer. 1997. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS 11:849-857. [DOI] [PubMed] [Google Scholar]
- 19.Jacob, J., R. Kassir, and G. Kelsoe. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob, J., and G. Kelsoe. 1992. In situ studies of the primary immune response to NP. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J. Exp. Med. 176:679-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, J. G., and R. Jemmerson. 1991. Relative frequencies of secondary B cells activated by cognate vs. other mechanisms. Eur. J. Immunol. 21:951-958. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson, G. B., M. Halloran, J. Li, I.-W. Park, R. Gomila, K. Reimann, M. Axthelm, N. L. Letvin, and J. Sodroski. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 71:4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsson, G. B., M. Halloran, D. Schenten, J. Lee, P. Racz, K. Tenner-Racz, J. Manola, R. Gelman, B. Etemad-Moghadam, E. Desjardins, R. Wyatt, N. P. Gerard, L. Marcon, D. Margolin, J. Fanton, M. K. Axthelm, N. L. Letvin, and J. Sodroski. 1998. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J. Exp. Med. 188:1159-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimoto, H., H. Nagaoka, Y. Adachi, T. Mizuochi, T. Azuma, T. Yagi, T. Sata, S. Yonehara, Y. Tsunetsugu-Yokota, M. Taniguchi, and T. Takemori. 1997. Accumulation of somatic hypermutation and antigen-driven selection in rapidly cycling surface Ig+ germinal center (GC) B cells which occupy GC at a high frequency during the primary anti-hapten response in mice. Eur. J. Immunol. 27:268-279. [DOI] [PubMed] [Google Scholar]
- 25.Laman, J. D., K. Gerritse, M. Fasbender, W. J. Boersma, N. van Rooijen, and E. Claassen. 1990. Double immunocytochemical staining for in vivo detection of epitope specificity and isotype of antibody-forming cells against synthetic peptides homologous to human immunodeficiency virus-1. J. Histochem. Cytochem. 38:457-462. [DOI] [PubMed] [Google Scholar]
- 26.Laman, J. D., P. Racz, K. Tenner-Racz, M. Klasmeier, M. J. Fasbender, C. Neelen, N. D. Zegers, M. Dietrich, W. J. Boersma, and E. Claassen. 1991. Immunocytochemical determination of antigen and epitope specificity of HIV-1-specific B cells in lymph-node biopsies from HIV-1-infected individuals. AIDS 5:255-262. [DOI] [PubMed] [Google Scholar]
- 27.Legendre, C., M. Raphael, G. Gras, E. A. Lefevre, J. Feuillard, D. Dormont, and Y. Richard. 1998. CD80 expression is decreased in hyperplastic lymph nodes of HIV+ patients. Int. Immunol. 10:1847-1851. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Y. J., C. Arpin, O. de Bouteiller, C. Guret, J. Banchereau, H. Martinez-Valdez, and S. Lebecque. 1996. Sequential triggering of apoptosis, somatic mutation and isotype switch during germinal center development. Semin. Immunol. 8:169-177. [DOI] [PubMed] [Google Scholar]
- 29.Macchia, D., F. Almerigogna, P. Parronchi, A. Ravina, E. Maggi, and S. Romagnani. 1993. Membrane tumour necrosis factor-alpha is involved in the polyclonal B-cell activation induced by HIV-infected human T cells. Nature 363:464-466. [DOI] [PubMed] [Google Scholar]
- 30.Marfaing-Koka, A., J. T. Aubin, L. Grangeot-Keros, A. Portier, C. Benattar, D. Merrien, H. Agut, P. Aucouturier, B. Autran, J. Wijdenes, et al. 1996. In vivo role of IL-6 on the viral load and on immunological abnormalities of HIV-infected patients. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 11:59-68. [DOI] [PubMed] [Google Scholar]
- 31.Margolin, D. H., K. A. Reimann, J. B. Karlsson, J. Sodroski, K. Tenner-Racz, P. Racz, and N. L. Letvin. 1997. Immunoglobulin VH usage during primary infection of rhesus monkeys with simian-human immunodeficiency viruses. J. Virol. 71:8582-8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollet, L., T. S. Li, A. Samri, C. Tournay, R. Tubiana, V. Calvez, P. Debre, C. Katlama, and B. Autran. 2000. Dynamics of HIV-specific CD8+ T lymphocytes with changes in viral load. The RESTIM and COMET Study Groups. J. Immunol. 165:1692-1704. [DOI] [PubMed] [Google Scholar]
- 33.Montefiori, D. C., I. Y. Zhou, B. Barnes, D. Lake, E. M. Hersh, Y. Masuho, and L. B. Lefkowitz, Jr. 1991. Homotypic antibody responses to fresh clinical isolates of human immunodeficiency virus. Virology 182:635-643. [DOI] [PubMed] [Google Scholar]
- 34.Moore, J. P., Q. J. Sattentau, P. J. Klasse, and L. C. Burkly. 1992. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J. Virol. 66:4784-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima, K., O. Martinez-Maza, T. Hirano, E. C. Breen, P. G. Nishanian, J. F. Salazar-Gonzalez, J. L. Fahey, and T. Kishimoto. 1989. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J. Immunol. 142:531-536. [PubMed] [Google Scholar]
- 37.Parren, P. W., I. Mondor, D. Naniche, H. J. Ditzel, P. J. Klasse, D. R. Burton, and Q. J. Sattentau. 1998. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J. Virol. 72:3512-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Racz, P., K. Tenner-Racz, F. van Vloten, H. Schmidt, M. Dietrich, J. C. Gluckman, N. L. Letvin, and G. Janossy. 1990. Lymphatic tissue changes in AIDS and other retrovirus infections: tools and insights. Lymphology 23:85-91. [PubMed] [Google Scholar]
- 39.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I.-W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient HIV-1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reimann, K. A., J. T. Li, G. Voss, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Parritz, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary HIV-1 isolate confers high in vivo replicative capacity to a chimeric simian-human immunodeficiency virus in rhesus monkeys. J. Virol. 70:3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson, T. M., Jr., B. L. Stryjewski, C. C. Broder, J. A. Hoxie, J. R. Mascola, P. L. Earl, and R. W. Doms. 1996. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J. Virol. 70:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott-Algara, D., M. Lafon, F. Vuillier, G. Pialoux, C. Dauguet, and G. Dighiero. 1994. Viral superantigen-induced hyporesponsiveness of T cells and polyclonal B cell activation in HIV-1 infection. Eur. J. Immunol. 24:2595-2601. [DOI] [PubMed] [Google Scholar]
- 43.Shirai, A., M. Cosentino, S. F. Leitman-Klinman, and D. M. Klinman. 1992. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J. Clin. Investig. 89:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tedla, N., P. Palladinetti, M. Kelly, R. K. Kumar, N. DiGirolamo, U. Chattophadhay, B. Cooke, P. Truskett, J. Dwyer, D. Wakefield, and A. Lloyd. 1996. Chemokines and T lymphocyte recruitment to lymph nodes in HIV infection. Am. J. Pathol. 148:1367-1373. [PMC free article] [PubMed] [Google Scholar]
- 45.Tenner-Racz, K., P. Racz, M. Bofill, A. Schulz-Meyer, M. Dietrich, P. Kern, J. Weber, A. J. Pinching, F. Veronese-Dimarzo, M. Popovic, et al. 1986. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am. J. Pathol. 123:9-15. [PMC free article] [PubMed] [Google Scholar]
- 46.Tenner-Racz, K., P. Racz, C. Thome, C. G. Meyer, P. J. Anderson, S. F. Schlossman, and N. L. Letvin. 1993. Cytotoxic effector cell granules recognized by the monoclonal antibody TIA-1 are present in CD8+ lymphocytes in lymph nodes of human immunodeficiency virus-1-infected patients. Am. J. Pathol. 142:1750-1758. [PMC free article] [PubMed] [Google Scholar]
- 47.Tenner-Racz, K., H. J. Stellbrink, J. van Lunzen, C. Schneider, J. P. Jacobs, B. Raschdorff, G. Grosschupff, R. M. Steinman, and P. Racz. 1998. The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J. Exp. Med. 187:949-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thali, M., U. Olshevsky, C. Furman, D. Gabuzda, M. Posner, and J. Sodroski. 1991. Characterization of a discontinuous human immunodeficiency virus type 1 gp120 epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J. Virol. 65:6188-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tierens, A., J. Delabie, L. Michiels, P. Vandenberghe, and C. De Wolf-Peeters. 1999. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood 93:226-234. [PubMed] [Google Scholar]
- 51.Trumpfheller, C., K. Tenner-Racz, P. Racz, B. Fleischer, and S. Frosch. 1998. Expression of macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and RANTES genes in lymph nodes from HIV+ individuals: correlation with a Th1-type cytokine response. Clin. Exp. Immunol. 112:92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whalen, B. J., H. P. Tony, and D. C. Parker. 1988. Characterization of the effector mechanism of help for antigen-presenting and bystander resting B cell growth mediated by Ia-restricted Th2 helper T cell lines. J. Immunol. 141:2230-2239. [PubMed] [Google Scholar]
- 53.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, Z. Q., T. Schuler, W. Cavert, D. W. Notermans, K. Gebhard, K. Henry, D. V. Havlir, H. F. Gunthard, J. K. Wong, S. Little, M. B. Feinberg, M. A. Polis, L. K. Schrager, T. W. Schacker, D. D. Richman, L. Corey, S. A. Danner, and A. T. Haase. 1999. Reversibility of the pathological changes in the follicular dendritic cell network with treatment of HIV-1 infection. Proc. Natl. Acad. Sci. USA 96:5169-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zubler, R. H., L. H. Perrin, A. Doucet, X. Zhang, Y. P. Huang, and P. A. Miescher. 1992. Frequencies of HIV-reactive B cells in seropositive and seronegative individuals. Clin. Exp. Immunol. 87:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]