Abstract
The regulatory proteins Nef, Rev, and Tat of human immunodeficiency virus type 1 (HIV-1) are attractive targets for vaccine development, since induction of effective immune responses targeting these early proteins may best control virus replication. Here we investigated whether vaccination with biologically active Tat or inactive Tat toxoid derived from HIV-1IIIB and simian-human immunodeficiency virus (SHIV) strain 89.6p would induce protective immunity in rhesus macaques. Vaccination induced high titers of anti-Tat immunoglobulin G in all immunized animals by week 7, but titers were somewhat lower in the 89.6p Tat group. Dominant B-cell epitopes mapped to the amino terminus, the basic domain, and the carboxy-terminal region. Tat-specific T-helper responses were detected in 50% of immunized animals. T-cell epitopes appeared to map within amino acids (aa) 1 to 24 and aa 37 to 66. In addition, Tat-specific gamma interferon responses were detected in CD4+ and/or CD8+ T lymphocytes in 11 of 16 immunized animals on the day of challenge. However, all animals became infected upon intravenous challenge with 30 50% minimal infective doses of SHIV 89.6p, and there were no significant differences in viral loads or CD4+ T-cell counts between immunized and control animals. Thus, vaccination with HIV-1IIIB or SHIV 89.6p Tat or with Tat toxoid preparations failed to confer protection against SHIV 89.6p infection despite robust Tat-specific humoral and cellular immune responses in some animals. Given its apparent immunogenicity, Tat may be more effective as a component of a cocktail vaccine in combination with other regulatory and/or structural proteins of HIV-1.
Over the past decade, numerous candidate human immunodeficiency virus (HIV) vaccines have been tested in nonhuman primates with various degrees of success (3). Of these, live-attenuated vaccines have provided the most potent protection against simian immunodeficiency virus (SIV) or simian-human immunodeficiency virus (SHIV) infection (3). However, safety issues associated with this approach preclude its direct application in humans. Given the apparent failure of envelope-based subunit vaccines, novel alternative approaches to designing an effective HIV vaccine are urgently required. In this study, we focused on the HIV type 1 (HIV-1) Tat protein as a target for vaccine design. Tat is an 86- to 102-amino-acid transcriptional activator that is encoded by two exons and is highly conserved among HIV isolates (13, 14, 30). It is produced early during the virus life cycle and is essential for efficient virus replication (4, 9). Several in vitro studies have demonstrated that Tat is secreted from virus-infected cells (11, 12) and exerts its biological effects on neighboring cells by (i) inhibiting T-cell proliferation (32, 34, 35), (ii) inducing apoptotic cell death (8, 20, 22), and (iii) increasing permissiveness for infection by both macrophage-tropic and T-cell-tropic HIV isolates as a result of enhanced expression of CXCR4 and CCR5 on susceptible cells (19, 23). Thus, Tat appears to be involved in both host immune suppression and viral dissemination.
Our rationale for using Tat as a vaccine target for HIV is supported by the following. First, Tat induces both humoral and cellular immune responses in humans (16, 17, 28). Second, anti-Tat antibodies protect against the increased permissiveness for HIV infection and the inhibitory effects on T-cell proliferation (12, 31, 36) and thus may control disease progression. Third, in HIV-1-infected individuals, anti-Tat antibodies correlate inversely with progression to AIDS (25, 36). In addition, in a recent study of 57 HIV-1-infected subjects, CD8+ T-cell responses against Tat were evident in 19% of these individuals, indicating that Tat is frequently targeted by HIV-1-specific cytotoxic T lymphocytes (CTL) (1). Furthermore, in nonhuman primates, there appears to be selective pressure on Tat CTL epitopes during the acute phase of SIV infection, suggesting that CTL against Tat may play an important role in disease control (2).
Vaccination studies performed with rhesus or cynomolgus macaques have demonstrated protection or control against SHIV infection by using Tat protein, peptides, or DNA as immunogens. In rhesus macaques, although complete protection against intrarectal challenge with the highly pathogenic SHIV strain 89.6p was not afforded by vaccination with Tat or Tat toxoid in the presence of incomplete Freund's adjuvant, a significant level of disease attenuation was observed as judged by viral load measurements (24). Importantly, 88% of vaccinated animals that had Tat-specific cellular and humoral immune responses were protected against disease progression. In addition, vaccination of rhesus macaques with peptide sequences corresponding to two known B-cell epitopes in HIV-1 Tat induced high anti-Tat antibodies to these specific epitopes, which caused a reduction in viral loads during the chronic phase of SHIV 33 infection (15). In a separate study using cynomolgus macaques, five of seven animals with humoral and cellular immune responses elicited by vaccination with Tat protein using ribi or alum as an adjuvant were protected against intravenous challenge with SHIV 89.6p (5). More recently, in a study designed to enhance the innate immune response, it was demonstrated that vaccination with DNA containing tat coding sequences and CpG motifs protects cynomolgus macaques against intravenous challenge with SHIV 89.6p (7). Thus, several approaches using Tat as a vaccine target have yielded promising results in nonhuman primates.
In a separate report, we demonstrated that biologically active Tat and inactive Tat toxoid preparations derived from HIV-1IIIB and SHIV 89.6p are immunogenic in rhesus macaques (unpublished data). Here we have extended our studies to test whether the immune responses induced by these Tat preparations would confer protection against intravenous challenge with SHIV 89.6p. Our data show that despite the induction of robust Tat-specific humoral and cellular immune responses in a subset of animals, we failed to protect animals against intravenous challenge with the highly pathogenic SHIV strain 89.6p.
MATERIALS AND METHODS
Animals.
The 20 adult rhesus macaques (Macaca mulatta) used in this study were captive-bred and were obtained from a breeding colony in the United States. Prior to their inclusion in the study, all animals were screened and confirmed to be free of antibodies to SIV, simian retrovirus (SRV), and simian T-cell leukemia virus type 1 and to be free of isolatable SIV and SRV following culture. The animals were housed at the Walter Reed Army Institute of Research in accordance with American Association for Accreditation of Laboratory Animal Care standards.
Immunization schedule.
We have previously described the production and purification of biologically active Tat and chemically inactivated Tat toxoid derived from HIV-1IIIB or SHIV 89.6p, which were used as immunogens in this study (unpublished data). Our immunization schedule consisted of five groups of four animals each (Table 1). Group 1 served as controls receiving adjuvant only. Groups 2 and 3 were immunized intramuscularly at 0, 5, 9, and 29 weeks with 100 μg of HIV-1IIIB or SHIV 89.6p Tat toxoid, respectively, in the presence of incomplete Freund's adjuvant (Seppic ISA 51). Groups 4 and 5 were similarly immunized with biologically active HIV-1IIIB or SHIV 89.6p Tat, respectively.
TABLE 1.
Immunization schedule
| Groupa | Immunogen | Dose (μg) given at 0, 5, 9 and 29 wk |
|---|---|---|
| 1 | Adjuvant | |
| 2 | HIV-1 HXBc2 Tat toxoid | 100 |
| 3 | SHIV 89.6p Tat toxoid | 100 |
| 4 | HIV-1 HXBc2 Tat | 100 |
| 5 | SHIV 89.6p Tat | 100 |
There were four animals in each group. At week 33, all animals were challenged intravenously with 30 MID50 of SHIV 89.6p.
Virus challenge.
The SIV/HIV chimeric virus SHIV 89.6, which contains the env gene of the primary isolate HIV-189.6, was constructed by Reimann et al. (26). The pathogenic isolate SHIV 89.6p (27), which arose after in vivo serial passage in rhesus macaques and which causes high viral loads and a rapid decline in CD4+ T-cell levels, was used as the challenge virus. SHIV 89.6p grown in CEMx174 cells was kindly provided by J. Sodroski and Y. Lu and was used to produce a virus challenge stock by acute infection of rhesus peripheral blood mononuclear cells (PBMC) as described previously (21). Four weeks after the final boost given at week 29, all animals were challenged intravenously with 30 50% minimal infective doses (MID50) of SHIV 89.6p.
Plasma viremia.
SIV RNA was quantitated by a procedure described previously (18, 33). Briefly, 500 μl of plasma was added to 1 ml of Dulbecco’s phosphate-buffered saline and spun for 1 h at 10,000 rpm. The viral pellet was then lysed by using RNASTAT-60 (Tel-Test “B”). Samples were then amplified as previously described (33), with the exception of the primers and probe. The gag primers used were SIV-F (5′ AGTATGGGCAGCAAATGAAT 3′) and SIV-R (5′ TTCTCTTCTGCGTGAATGC 3′), and the gag probe used was SIV-P (6 FAMA-GATTTGGATTAGCAGAAAGCCTGTTGGA-TAMRA). The assay has a threshold sensitivity of 200 RNA copies/ml of plasma, with interassay variations averaging 0.5 log10 unit.
Virus isolation.
Rhesus macaque PBMC were isolated by using a 95% Ficoll-Hypaque separation solution (Sigma, St. Louis, Mo.). Approximately 2.5 × 106 cells were placed in 2.5 ml of RPMI 1640 medium containing 10% fetal bovine serum (FBS), 0.5% gentamicin, 5.0% glutamine, and 5 mg of phytohemagglutinin (PHA) (Sigma)/ml. Following 3 days of culture, the supernatant was removed and a portion was tested for the presence of SIV p27. Cells were washed free of PHA, adjusted to 106/ml in a medium containing 10% recombinant interleukin-2 (Boehringer Mannheim, Indianapolis, Ind.), and cultured with an equal number of human 3-day-PHA-blasted PBMC at 37°C. Cultures were readjusted to 106 cells/ml every 3 to 4 days with a 100% medium change and were assayed weekly for the presence of SIV p27. Cultures were held for as long as 4 weeks. A culture was designated positive following positive tests from two successive specimens. SIV p27 was detected in culture supernatant fluids by using an SIV p27-specific enzyme-linked immunosorbent assay (ELISA) (Coulter Diagnostics, Miami, Fla.).
Detection of anti-Tat and anti-SIV antibodies.
Anti-Tat antibodies were measured by ELISA as described previously (unpublished data). Briefly, 96-well ELISA plates (Nalge/Nunc) were coated with Tat, Tat toxoid, or Tat peptide (Table 2) in 50 nM sodium bicarbonate (pH 9.0) at 0.5 μg/well and were incubated overnight at 4°C. Plates were blocked with 3% bovine serum albumin (Sigma) in 1× phosphate-buffered saline (PBS) and washed six times in PBS containing 0.05% Tween 20 by using an automated plate washer (Dynex). Serum samples were analyzed at a 1:1,000 dilution (for peptide-specific antibodies) or were serially diluted in PBS-0.05% Tween 20 and added at 50 μl/well. Specific anti-Tat antibodies were detected by using horseradish peroxidase-conjugated protein G (Bio-Rad) at a 1:1,000 dilution as the secondary antibody. Plates were developed with 50 μl of TM Blue (Intergen)/well for 5 min, stopped with 50 μl of 2 N HCl, and read at 450 nm with a plate reader (Dynex). Circulating anti-SIV antibodies after challenge were detected by an HIV-2 ELISA (Genetic Systems, Seattle, Wash.) which is cross-reactive for SIV antibody. The ELISA used a fixed plasma dilution of 1:200 and was run as directed except that the plates were read on a Vmax kinetic ELISA reader (Molecular Devices, Menlo Park, Calif.) at 650 nm. Values of milli-optical density (OD) units per minute minus background were used as ELISA units.
TABLE 2.
Tat peptides derived from SHIV 89.6p and HIV-1IIIB used in this study
| Peptide origin and no. | Amino acids | Sequencea |
|---|---|---|
| SHIV 89.6p | ||
| 1 | 1-15 | MEPVDPRLEPWKHPG |
| 2 | 9-23 | EPWKHPGSKPKTAST |
| 3 | 16-30 | SKPKTASTNSYSKKS |
| 4 | 24-38 | NSYSKKSSFHSQVSF |
| 5 | 31-45 | SFHSQVSFTTKALGI |
| 6 | 39-53 | TTKALGISYGRKKRR |
| 7 | 46-60 | SYGRKKRRQRRRAHQ |
| 8 | 54-68 | QRRRAHQNSQTHQAS |
| 9 | 61-75 | NSQTHQASLSKQPSS |
| 10 | 69-83 | LSKQPSSQPRGDPTG |
| 11 | 76-90 | QPRGDPTGPKEQKKK |
| 12 | 84-98 | PKEQKKKVERETETD |
| 13 | 91-102 | VERETETDPVHQ |
| HIV-1IIIB | ||
| 1.1 | 1-12 | MEPVDPRLEPWK |
| 2.1 | 7-18 | RLEPWKHPGSQP |
| 3.1 | 13-24 | HPGSQPKTASTN |
| 4.1 | 19-30 | KTASTNSYSKKS |
| 5.1 | 25-36 | SYSKKSSFHSQV |
| 6.1 | 31-42 | SFHSQVSFITKA |
| 7.1 | 37-48 | SFITKALGISYG |
| 8.1 | 43-54 | LGISYGRKKRRQ |
| 9.1 | 49-60 | RKKRRQRRRPPQ |
| 10.1 | 55-66 | RRRPPQGSQTHQ |
| 11.1 | 61-72 | GSQTHQVSLSKQ |
| 12.1 | 67-78 | VSLSKQPTSQSR |
| 13.1 | 73-84 | PTSQSRGDPTGP |
| 14.1 | 79-86 | GDPTGPKE |
| 86- | 86-101 | EQKKKVERETETDQGN |
A boldfaced “S” indicates that a cysteine residue has been replaced by serine.
Lymphocyte proliferation assay.
Freshly isolated macaque PBMC were added, in triplicate, at 2 × 105 cells/well to 96-well round-bottom plates in 200 μl of RPMI 1640 growth medium containing 5% FBS, 0.5% gentamicin, and 5.0% glutamine and were incubated for 4 to 5 days at 37°C in the presence of 5 or 10 μg of Tat toxoid or HIV-1 HXBc2 Tat peptides/ml (Table 2). Unstimulated PBMC or PBMC stimulated with 10 μg of PHA (Sigma)/ml served as negative and positive controls, respectively. PBMC were then pulsed with 1 μCi of [3H]thymidine (NEN)/well and incubated for a further 18 h at 37°C. PBMC were then harvested onto filter mats, and thymidine incorporated into cellular DNA was measured as counts per minute in a β-counter (Wallac). Results were expressed as stimulation index (SI) values, calculated as the mean counts per minute of stimulated PBMC divided by the mean counts per minute of unstimulated PBMC. An SI value of >5 was considered positive.
Tat-specific intracellular IFN-γ production.
One million PBMC were incubated for 2 h at 37°C with 10 μg of the homologous Tat toxoid/ml in RPMI 1640 growth medium containing 5% FBS, 0.5% gentamicin, and 5.0% glutamine. Unstimulated or staphylococcal enterotoxin B-stimulated PBMC served as negative and positive controls, respectively. To allow for intracellular accumulation of gamma interferon (IFN-γ), 2 μl of Golgiplug (brefeldin A; Pharmingen) was added and incubation was continued overnight at 37°C. PBMC were then stained for CD4+ and CD8+ T-cell subsets by using anti-human CD4 and CD8 monoclonal antibodies conjugated to fluorescein isothiocyanate (CD4-FITC; catalog no. 71024L; Becton Dickinson [BD] Pharmingen, Mountain View, Calif.) and peridinin chlorophyll protein (CD8-PerCP; catalog no. 347314; BD Pharmingen), respectively. After 30 min of incubation at 4°C, PBMC were washed in PBS, fixed, and permeabilized with 100 μl of Cytofix/Perm solution (BD Pharmingen), followed by staining for intracellular IFN-γ by using 0.4 μg of phycoerythrin-conjugated anti-human IFN-γ (BD Pharmingen). Finally, PBMC were washed, resuspended in PBS containing 2% formaldehyde, and analyzed by using a FACScalibur flow cytometer and CellQuest software. By generating dot plots of forward scatter versus side scatter, 30,000 events were acquired in the lymphocyte population, from which levels of IFN-γ-positive CD4+ and CD8+ T lymphocytes were calculated. Results were expressed as percent IFN-γ-positive cells for each lymphocyte subset.
CD4+ T-cell counts.
CD4 T-lymphocyte counts in peripheral blood were performed on a FACScalibur flow cytometer (Becton Dickinson) by using phycoerythrin-conjugated anti-human CD4 (Becton Dickinson). Analysis was performed by a whole-blood lysis procedure as directed by the manufacturer.
RESULTS
Circulating anti-Tat antibodies.
We have shown that after three immunizations at 0, 5, and 9 weeks, biologically active Tat and chemically inactivated Tat toxoid preparations derived from HIV-1IIIB and SHIV 89.6p elicited high titers of anti-Tat antibodies, ranging from approximately 1 × 105 to 4.8 × 106, 2 weeks after the third immunization. After a 20-week rest period, all immunized animals were given a final boost at week 29 and challenged 4 weeks later with pathogenic SHIV 89.6p. Anti-Tat antibody levels at the time of the final boost and on the day of challenge are shown in Table 3. At week 29, the HIV-1IIIB Tat toxoid- and Tat-immunized animals had anti-Tat antibodies titers ranging from <1 × 105 to 2 × 105. These titers were boosted in all animals on the day of challenge at week 33, when levels ranged from 1.25 × 105 to 8 × 105 (mean titer, 5.8 × 106) in the HIV-1IIIB Tat toxoid group and from 3 × 105 to 2.4 × 106 (mean titer, 1 × 106) in the HIV-1IIIB Tat group. Anti-Tat antibody titers at week 29 in the SHIV 89.6p Tat toxoid- and Tat-immunized animals ranged from <1 × 105 to 2 × 105. Like those for the HIV-1IIIB Tat- and Tat toxoid-immunized animals, these titers were also boosted to day-of-challenge levels ranging from 1.25 × 105 to 6 × 105 (mean titer, 3.1 × 105) in the SHIV 89.6p Tat toxoid group and from 0.75 × 105 to 1.2 × 106 (mean titer, 5.4 × 105) in the SHIV 89.6p Tat group. However, comparison of antibody titers between each group revealed no significant differences (P > 0.05). As expected, nonimmunized control animals were negative for anti-Tat antibodies on every occasion tested. Thus, the final boost given at week 29 enhanced anti-Tat antibodies in all immunized animals.
TABLE 3.
Anti-Tat antibodies at time of final boost and on day of challenge
| Animal no. | Vaccine | Reciprocal end point dilution
|
|
|---|---|---|---|
| Wk 29 | Day of challenge | ||
| 3M1 | None | Negative | Negative |
| 016D | None | Negative | Negative |
| P729 | None | Negative | Negative |
| 90B069 | None | Negative | Negative |
| 400D | HIV-1IIIB Tat toxoid | <100,000 | 125,000 |
| JZ | HIV-1IIIB Tat toxoid | <100,000 | 800,000 |
| A135 | HIV-1IIIB Tat toxoid | 100,000 | 800,000 |
| N627 | HIV-1IIIB Tat toxoid | 200,000 | 600,000 |
| A105 | SHIV 89.6p Tat toxoid | 200,000 | 600,000 |
| N616 | SHIV 89.6p Tat toxoid | <100,000 | 125,000 |
| 101X | SHIV 89.6p Tat toxoid | <100,000 | 300,000 |
| 110D | SHIV 89.6p Tat toxoid | <100,000 | 200,000 |
| IKO | HIV-1IIIB Tat | 200,000 | 2,400,000 |
| G408 | HIV-1IIIB Tat | <100,000 | 800,000 |
| 571Z | HIV-1IIIB Tat | 100,000 | 600,000 |
| 89-162 | HIV-1IIIB Tat | <100,000 | 300,000 |
| G898 | SHIV 89.6p Tat | <100,000 | 75,000 |
| 44I | SHIV 89.6p Tat | <100,000 | 800,000 |
| N839 | SHIV 89.6p Tat | <100,000 | 1,200,000 |
| A144 | SHIV 89.6p Tat | <100,000 | 100,000 |
Mapping linear immunogenic B-cell epitopes.
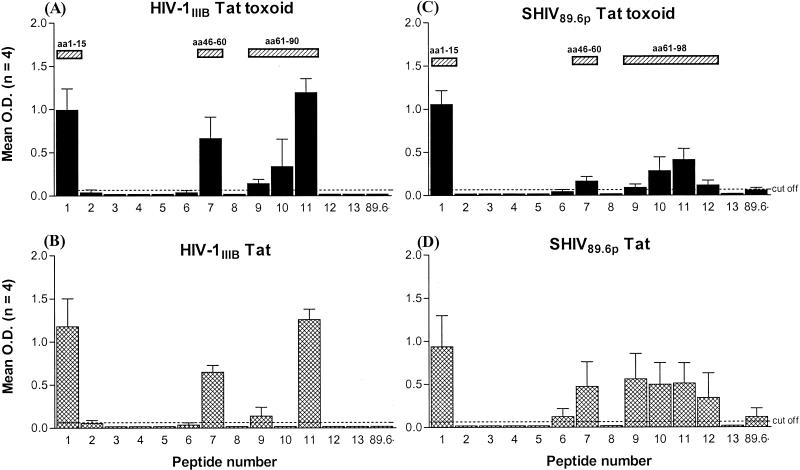
In an attempt to further characterize the antibody response induced by Tat vaccination, we utilized overlapping 15-mer peptides spanning the entire sequence of SHIV 89.6p Tat in an ELISA to identify linear immunogenic B-cell epitopes. Figure 1 shows the mean reactivity of serum samples obtained from HIV-1IIIB and SHIV 89.6p Tat- and Tat toxoid-immunized animals on the day of challenge for each Tat peptide. Antibodies from HIV-1IIIB Tat toxoid-immunized (Fig. 1A) and Tat-immunized (Fig. 1B) animals recognized peptides 1, 7, 9, and 11, corresponding to SHIV 89.6p Tat amino acid (aa) sequences 1 to 15, 46 to 60, 61 to 75, and 76 to 90, respectively; the strongest reactivity was observed with peptides 1, 7, and 11. Antibodies from the HIV-1IIIB Tat toxoid-immunized group, but not those from the HIV-1IIIB Tat-immunized group, recognized peptide 10, which corresponds to aa 69 to 83. In addition to those peptides recognized by the HIV-1IIIB Tat and Tat toxoid groups, antibodies from animals immunized with SHIV 89.6p Tat toxoid (Fig. 1C) and Tat (Fig. 1D) demonstrated reactivity with peptides 6 (aa 39 to 53) and 12 (aa 84 to 98) and the tail peptide “86-” (aa 86 to 101). Thus, vaccination with HIV-1IIIB and SHIV 89.6p Tat vaccines induced antibodies to linear B-cell epitopes within the amino terminus, the basic domain, and the carboxy-terminal region of SHIV 89.6p Tat.
FIG. 1.
Mean reactivities of antibodies obtained on the day of challenge from animals immunized with HIV-1IIIB (A) or SHIV 89.6p (C) Tat toxoid (solid bars) or with HIV-1IIIB (B) or SHIV 89.6p (D) Tat (cross-hatched bars) against SHIV 89.6p Tat overlapping peptides 1 to 13 and the carboxy-terminal peptide 86-. Serum samples were analyzed at a 1:1,000 dilution. Hatched rectangles above bar graphs highlight reactive regions and indicate the corresponding amino acid sequences. Error bars each represent the standard error of the mean of OD readings for the four animals. All control animals were nonreactive against all the peptides, with OD values below cutoff (data not shown).
T-cell responses and epitope mapping.
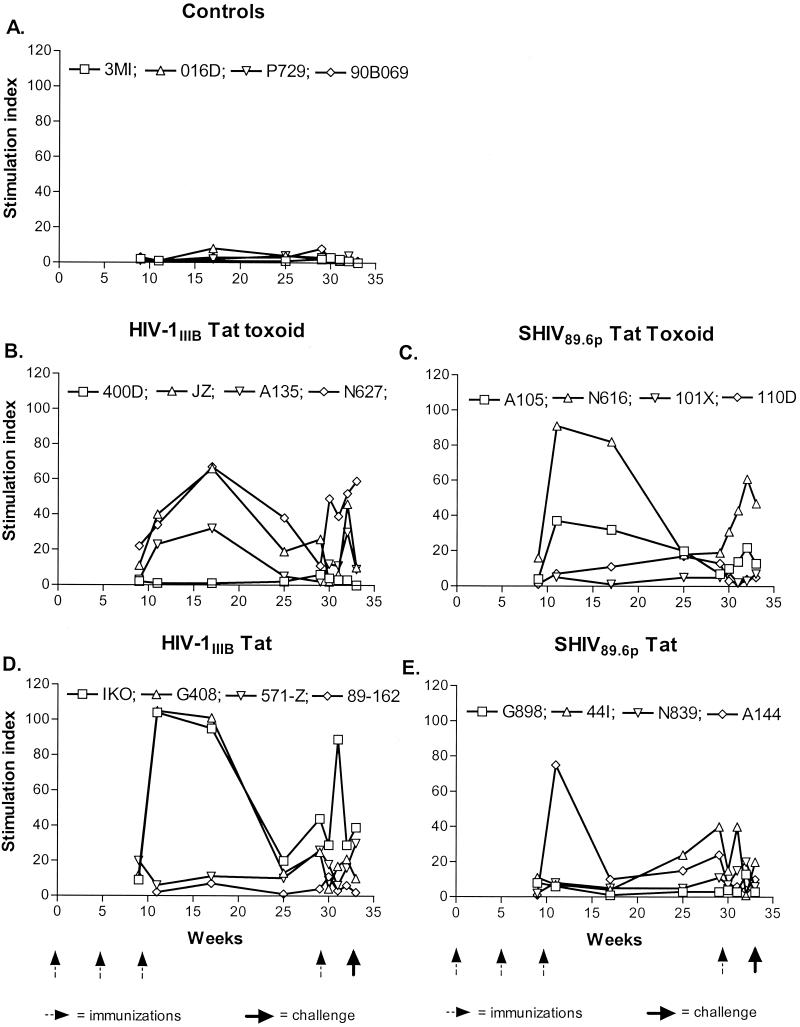
In order to assess the cell-mediated immune response induced by Tat vaccination, we monitored T-lymphocyte proliferative responses throughout the course of the study. Shown in Fig. 2 are the proliferative responses for each immunized group as they developed over time, leading up to the day of challenge. As expected, no Tat-specific proliferative responses were detected in any of the nonimmunized control animals (Fig. 2A). Three of four (JZ, N627, and A135; Fig. 2B) HIV-1IIIB Tat toxoid-immunized animals and two of four (IKO and G408; Fig. 2D) HIV-1IIIB Tat-immunized animals developed substantial proliferative responses after the third immunization at week 9, peaking between weeks 11 and 17. These responses were strongest in IKO and G408, with SI values of >100 at week 11. After week 17, proliferative responses declined but were boosted in the majority of animals by the final immunization at week 29. No response was detected in 400D (Fig. 2B) or 89-162 (Fig. 2D) throughout the course of the study. Borderline responses were evident in animal 571-Z (Fig. 2D). In the SHIV 89.6p Tat toxoid- and Tat-immunized groups, peak proliferative responses were detected at week 11 for N616 and A105 (Fig. 2C) and for A144 (Fig. 2E). Responses waned subsequently, and only N616 and A105 (Fig. 2C) experienced a boosting effect after the final immunization. Delayed proliferative responses, peaking at week 29, were observed for 44I (Fig. 2E). No response or very low responses were observed in the remaining animals.
FIG. 2.
Longitudinal assessment of Tat-specific T-helper responses in controls (A) and in animals immunized with HIV-1IIIB Tat toxoid (B), SHIV 89.6p Tat toxoid (C), HIV-1IIIB Tat (D), or SHIV 89.6p Tat (E) in response to stimulation with the homologous Tat toxoid. Arrows indicate times of immunization and virus challenge.
In an attempt to map proliferative responses to linear epitopes, we utilized a series of 12-mer overlapping peptides encompassing the entire sequence of HIV-1IIIB Tat (Table 2). Four peptide pools, each consisting of three or four peptides, were prepared and used to stimulate T cells obtained from three nonimmunized controls and four HIV-1IIIB Tat toxoid- or Tat-immunized animals at week 19. These four immunized animals demonstrated robust proliferative responses, as shown in Fig. 2B and D. SI values in response to each of those peptide pools are shown in Table 4. SI values in response to PHA stimulation ranged from 19 to 247. None of the Tat peptide pools induced T-cell proliferation in cells from control animals. In contrast, peptide pool 3, which includes aa 37 to 66, induced proliferative responses (SI values, 12 and 28) in two immunized animals, and the remaining two animals responded weakly to peptide pool 1 (aa 1 to 24). Thus, immunization with Tat or Tat toxoid preparations derived from HIV-1IIIB or SHIV 89.6p induced robust T-helper responses, which appear to map to linear epitopes within aa 1 to 24 and aa 37 to 66, in a subset of animals.
TABLE 4.
Proliferative responses to HIV-1IIIB Tat peptides, measured at week 19
| Animal no. | Vaccine | SI in response to:
|
||||
|---|---|---|---|---|---|---|
| PHA (10 μg/ml)
|
Tat peptide poola (10 μg of peptide/ml)
|
|||||
| 1 | 2 | 3 | 4 | |||
| 016D | None | 247 | 2 | 2 | 4 | 2 |
| P729 | None | 72 | 2 | 4 | 1 | 1 |
| 90B069 | None | 112 | 1 | 1 | 1 | 1 |
| JZ | HIV-1IIIB Tat toxoid | 19 | 9 | 2 | 3 | 4 |
| N627 | HIV-1IIIB Tat toxoid | 91 | 6 | 6 | 12 | 1 |
| IKO | HIV-1IIIB Tat | 71 | 1 | 5 | 28 | 3 |
| G408 | HIV-1IIIB Tat | 36 | 9 | 2 | 31 | 2 |
Composition of Tat peptide pools: pool 1, peptides 1 to 3 (aa 1 to 24); pool 2, peptides 4 to 6 (aa 19 to 42); pool 3, peptides 7 to 10 (aa 37 to 66); pool 4, peptides 11 to 14 (aa 61 to 86).
Tat-specific IFN-γ-producing CD4+ and CD8+ T cells.
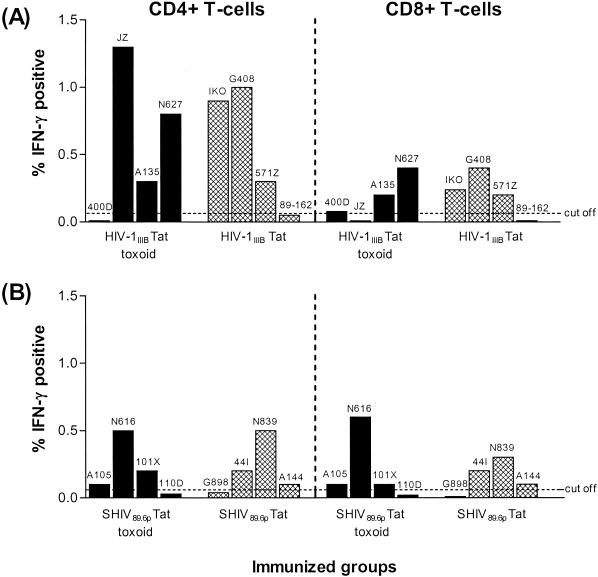
To further characterize the T-cell response induced by Tat vaccination, we used intracellular flow cytometry to measure the ability of the homologous Tat toxoid to induce production of the antiviral cytokine IFN-γ in CD4+ and CD8+ T cells on the day of challenge. These results, after subtraction of background levels observed in unstimulated PBMC, are shown in Fig. 3. A cutoff value of 0.05% was set based on the mean IFN-γ response observed in control animals. As a positive control, T cells from all animals responded to staphylococcal enterotoxin B stimulation to produce IFN-γ levels ranging from 1.5 to 9.7% on CD4+ T cells and from 1 to 11.2% on CD8+ T cells (data not shown). Tat-specific IFN-γ-producing CD4+ and/or CD8+ T cells were clearly evident in six of eight HIV-1IIIB Tat toxoid- and Tat-immunized animals, with levels of IFN-γ-positive CD4+ and CD8+ T cells ranging from 0.3 to 1.3% and 0.2 to 0.4%, respectively (Fig. 3A). Although these IFN-γ responses were noticeably higher in the CD4+ T-cell population, they were not significantly (P > 0.05) higher than those observed in the CD8+ T-cell population. A similar number of animals in the SHIV 89.6p Tat toxoid and Tat groups also produced a Tat-specific IFN-γ response, with levels of IFN-γ-producing CD4+ and CD8+ T cells ranging from 0.1 to 0.5% and 0.1 to 0.6%, respectively (Fig. 3B). Statistical analysis revealed that the IFN-γ responses on CD4+ T cells, but not on CD8+ T cells, observed in the SHIV 89.6p Tat toxoid and Tat groups were significantly lower (P = 0.047) than those observed in the HIV-1IIIB Tat toxoid and Tat groups. These results clearly demonstrate that these vaccine preparations are capable of eliciting Tat-specific CD4+ and CD8+ T-cell responses and that these memory responses can be recalled in vitro in response to the homologous Tat toxoid to produce the antiviral cytokine IFN-γ.
FIG. 3.
Tat-specific IFN-γ production in CD4+ and CD8+ T lymphocytes obtained from HIV-1IIIB Tat toxoid- and Tat-immunized (A) and SHIV 89.6p Tat toxoid- and Tat-immunized (B) groups on the day of challenge. Shown are the specific responses after subtraction of background levels obtained with unstimulated PBMC. Tat-specific IFN-γ responses were not detected in T cells from control animals (data not shown).
Outcome of challenge.
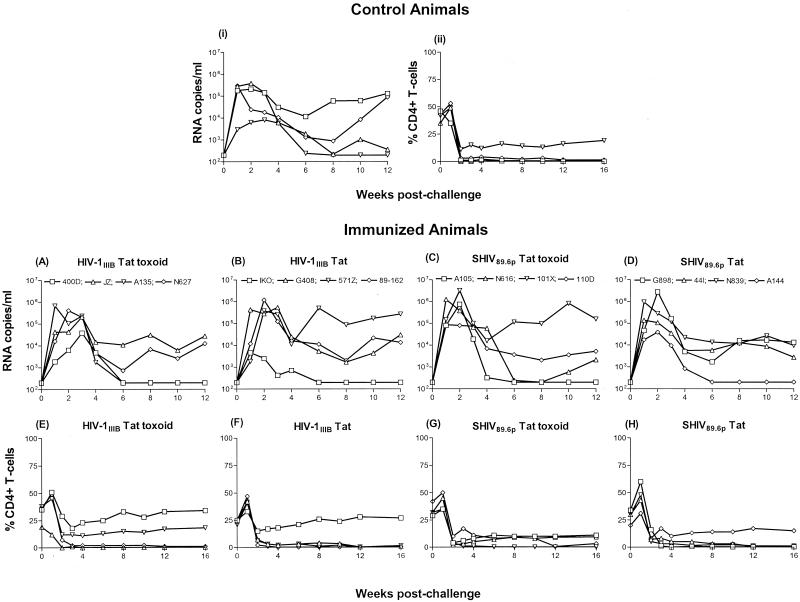
Having demonstrated that both Tat and Tat toxoid preparations induce humoral and cellular immune responses in some animals, we tested whether these immune responses would protect against intravenous challenge with SHIV 89.6p by monitoring postchallenge viral loads and CD4+ T-cell levels in control and immunized animals. Shown in Fig. 4 are the viral loads (Fig. 4i) and CD4+ T-cell levels (Fig. 4ii) monitored longitudinally in the four control animals after challenge. Three of four control animals (3MI, 016D, and 90B069) showed peak viral loads of approximately 105.5 RNA copies/ml between weeks 1 and 2 postchallenge (Fig. 4i). The remaining animal (P729) had a lower peak of approximately 104 RNA copies/ml at 3 weeks postchallenge. This animal appeared to control the infection, since we failed to detect the virus in plasma at week 6 and thereafter up to week 12. In addition, we isolated virus from PBMC of P729 on only one occasion between weeks 6 and 16 (data not shown). Animal 016D, with a peak viral load of 105.5 RNA copies/ml, also appeared to control the infection somewhat, with a viral load just above the limit of detection at week 12. By contrast, after an initial decline, animals 3MI and 90B069 had viral loads of approximately 105 RNA copies/ml by week 12. During the acute phase of the infection and coinciding with peak viremia, all control animals experienced dramatic declines in their CD4+ T-cell levels (Fig. 4ii). By week 2 postchallenge, three of four animals (3MI, 016D, and 90B069) had <1% CD4+ T cells circulating in the periphery and remained so up to week 16. Animal P729, which controlled the infection, maintained a CD4+ T-cell level of approximately 15% from week 3 up to week 16. Moreover, this was the only control animal that mounted a measurable antibody response against the challenge (data not shown).
FIG. 4.
Plasma viremia as measured by RNA copies per milliliter and percent CD4+ T cells in control and immunized animals following intravenous challenge with 30 MID50 of SHIV 89.6p at week 33. The upper section shows RNA copies per milliliter (i) and percent CD4+ T cells (ii) in nonimmunized control animals. Plasma viremia (A through D) and percent CD4+ T cells (E through H) in immunized animals are shown in the lower panels.
Peak viral loads ranging from 104.5 to 106 RNA copies/ml in the HIV-1IIIB Tat toxoid group and from 103.5 to 106 RNA copies/ml in the HIV-1IIIB Tat group were detected between weeks 1 and 3 postchallenge (Fig. 4A and B). Two of four HIV-1IIIB Tat toxoid-immunized animals (400D and A135) controlled the infection, and plasma viremia was not evident in these animals past week 6 (Fig. 4A). Furthermore, although these two animals experienced initial declines in their CD4+ T-cell levels, they maintained levels between 15 and 30% during the chronic phase of the infection (Fig. 4E) and seroconverted to the challenge virus (data not shown). By contrast, animals JZ and N627 had <1% CD4+ T cells circulating in the periphery at week 3 postchallenge and thereafter (Fig. 4E), failed to seroconvert, and had set-point viral loads of approximately 104 RNA copies/ml (Fig. 4A). In the HIV-1IIIB Tat group (Fig. 4B), one animal (IKO) had a relatively low peak viral load, controlled the infection, was virus isolation negative between weeks 8 and 16 (data not shown), maintained CD4+ T cells at the 25% level (Fig. 4F), and seroconverted to the challenge virus. It is worth noting that this animal exhibited the highest cellular response to the final vaccine boost at week 29 (Fig. 2D). By contrast, the remaining three animals in this group (G408, 571Z, and 89-162) had 3% or fewer CD4+ T cells at 3 weeks postchallenge and thereafter (Fig. 4F), made no antibodies to the challenge virus (data not shown), and had set-point viral loads between 104 and 105 RNA copies/ml (Fig. 4B).
Similar observations were made for the SHIV 89.6p Tat toxoid and Tat groups. Peak viral loads ranging from 105 to 106.5 RNA copies/ml in the SHIV 89.6p Tat toxoid group (Fig. 4C) and from 104.5 to 106.5 RNA copies/ml in the SHIV 89.6p Tat group (Fig. 4D) were detected between weeks 1 and 2 postchallenge. The two SHIV 89.6p Tat toxoid-immunized animals (A105 and N616) with the lowest viral loads (and yielding no or intermittent virus-positive PBMC between weeks 6 and 16) also had higher percentages of CD4+ T cells in the circulation (Fig. 4G) and seroconverted to the challenge virus. These two animals also exhibited a boost in their cellular responses after the final immunization (Fig. 2C). The opposite was true for the other two animals with higher set-point viral loads (Fig. 4C). Likewise, in the SHIV 89.6p Tat group, animal A144 (Fig. 4D) controlled the infection, was virus isolation positive only once between weeks 6 and 16, maintained CD4+ T cells at approximately 12% during the chronic phase (Fig. 4H), and made antibodies to the challenge virus. This animal was the only member of this group that had a strong cellular response at week 11 (Fig. 2E), but interestingly, it failed to respond to the final immunization at week 29.
Statistical analysis revealed a trend toward a significant inverse correlation between CD4+ T-cell levels and viral load measurements at week 8 postchallenge (P = 0.056), highlighting the prognostic value of CD4+ T-cell counts in predicting disease outcome. These data clearly show that all animals became infected after SHIV 89.6p challenge and that one or two animals from each group controlled the infection. However, comparison of viral loads at week 12 postchallenge between control animals and each of the four immunized groups revealed no significant differences (P > 0.05). Although there does not appear to be a statistically significant difference between the vaccinated and control animals, there appears to be some correlation between the ability to mount a cellular response to Tat and the outcome of challenge. Of six vaccinated animals (A105, N616, A144, A135, 400D, and IKO) that controlled the virus after challenge, four (IKO, A105, N616, and A135) showed a cellular response after the final boost. One of these six controllers (A144) showed a strong early response at week 11 but failed to respond to the final boost, and animal 400D did not mount a cellular response to the vaccine. The fact that the proportion of controllers mounting cellular immune responses (5 of 6) is higher than that for the group overall (P = 0.09 by Pearson's chi-square test) might suggest that the cellular proliferative response is protective.
DISCUSSION
Vaccination studies using biologically active Tat or inactive Tat toxoid derived from HIV-1IIIB as immunogens have been described previously (5, 6, 10, 24). Our study is the first to test whether the 102-aa Tat protein of SHIV 89.6p could induce protective immunity against homologous virus challenge in rhesus macaques. We reported elsewhere that Tat or Tat toxoid preparations derived from HIV-1IIIB and SHIV 89.6p could induce robust humoral and cellular immune responses in nonhuman primates. Here we show that these responses failed to confer protection against intravenous challenge with SHIV 89.6p.
The final boost given at week 29 enhanced anti-Tat antibodies in all immunized animals, and on the day of challenge these levels were substantially higher than those reported for humans (16, 17) or monkeys (10, 24) vaccinated with Tat or Tat toxoid. In attempts to map the binding of anti-Tat antibodies to linear B-cell epitopes, we used overlapping peptides spanning the entire sequence of the 102-aa SHIV 89.6p Tat protein. We found that the major B-cell epitopes were located at aa 1 to 15 (MEPVDPRLEPWKHPG) at the amino-terminal end, aa 46 to 60 (SYGRKKRRQRRRAHQ) within the basic domain, and aa 61 to 90 (NSQTHQASLSKQPSSQPRGDPTGPKEQKKK) at the carboxy-terminal region. Not surprisingly, given the 86-aa length of HIV-1IIIB Tat, on the day of challenge antibodies from HIV-1IIIB Tat- and Tat toxoid-immunized animals failed to react with peptides beyond peptide 11 (aa 76 to 90). We showed that the additional 16-aa tail region present in the Tat protein of SHIV 89.6p is immunogenic, since antibodies obtained on the day of challenge from animals immunized with SHIV 89.6p Tat or Tat toxoid reacted with peptide 12, which corresponds to aa 84 to 98. Our findings are in agreement with a recent report showing that aa 4 to 12 (VDPRLEPWK, or epitope 1) and aa 42 to 50 (LGISYGRK, or epitope 2) are major B-cell epitopes within the first exon of Tat that have limited or no antigenic polymorphism within geographically diverse strains (14), highlighting their potential as vaccine components. Interestingly, immunization of rhesus macaques with peptide sequences corresponding to major B-cell epitopes in Tat reduced viral load during the chronic phase of SHIV 33 infection (15).
Robust CD4+ T-helper responses were detected after the third immunization in a subset of animals; these declined during the course of the study but were again boosted in the majority after the final immunization at week 29. In addition, Tat-specific IFN-γ-producing CD4+ and CD8+ T lymphocytes were detected in 50% of immunized animals. These IFN-γ responses were more robust in the HIV-1IIIB group, particularly in the CD4+ T-cell compartment. Furthermore, T-helper responses induced by HIV-1IIIB Tat and Tat toxoid cross-reacted with SHIV 89.6p and SIVmac251 Tat, thus highlighting the potential for these immunogens to elicit broad-based cell-mediated immunity--a requirement of an effective AIDS vaccine. A recent report has described the first CTL epitope in HIV-1 Tat as residing within the amino terminus at aa 2 to 11, with the corresponding sequence EPVDPRLEPW, and noted that this epitope is frequently targeted by CTL in HIV-1-infected individuals (1). Earlier, it was demonstrated for SIVmac239-infected rhesus macaques that virus isolates with mutations in the Mamu-A*01-restricted CTL epitope SL8 (positioned at Tat28-35) escaped immune recognition, resulting in increased disease progression (2). Our preliminary data concur with those findings for HIV, indicating that T-cell epitopes reside within aa 1 to 24 and aa 37 to 66. Taken together, these data justify the inclusion of at least the amino termini of HIV and SIV Tat as important components in vaccine design.
Having demonstrated that Tat and Tat toxoid preparations derived from HIV-1IIIB and SHIV 89.6p elicited strong humoral and cellular immune responses in some animals, we challenged all animals 4 weeks after the final immunization by intravenous inoculation with the highly pathogenic SHIV 89.6p. As in previous vaccination studies using this challenge virus, the pattern of the ensuing infection was typical in three of four control animals. Peak plasma viremia was detected between weeks 1 and 2 postchallenge, coincident with CD4+ T-cell counts declining to almost undetectable levels. One control animal (P729) appeared to control the infection by clearing the virus by week 6 and maintaining CD4+ T cells at the 15% level throughout the follow-up period. The opposite was true for the remaining three controls. The outcome of challenge in each immunized group was similar to that seen in unimmunized control animals, and there was no significant difference in viral load or CD4+ T-cell counts between controls and any group of immunized animals. Therefore, our results conflict with the findings of previous studies that have shown protection or control of SHIV 89.6p infection, although these studies failed to achieve sterilizing immunity (5, 10, 24). However, differences in the study design could explain these conflicting results. Specifically, Ensoli and Cafaro (10) and Cafaro et al. (5) showed control of SHIV 89.6p infection after a 10-MID50 intravenous challenge in Tat-immunized cynomolgus macaques, a species that exhibits lower peak viremia after challenge with this chimeric virus (29). Furthermore, these authors used more-extensive immunization regimens via the intradermal or subcutaneous routes in the presence of RIBI or alum as an adjuvant. In addition, they incorporated immune-stimulating complexes in the final boost to enhance cellular responses. Although Pauza et al. (24) used rhesus macaques in their study, they also used more immunizations via the intradermal or intramuscular route, but the challenge with SHIV 89.6p was performed intrarectally to model sexual transmission. Therefore, these differences in monkey species, adjuvant, and dose and route of both immunization and challenge viruses used could explain these conflicting results. However, given the highly pathogenic nature of SHIV 89.6p in the rhesus macaque model, it is conceivable that the protective effects of Tat immunization in macaques may be overwhelmed by challenging with such a highly pathogenic virus and therefore may underestimate the potential for Tat to confer protection.
In conclusion, we have clearly demonstrated that biologically active Tat and Tat toxoid preparations derived from HIV-1IIIB and SHIV 89.6p are immunogenic in rhesus macaques, eliciting robust humoral and cellular immune responses in a subset of animals. However, these Tat immunogens failed to confer protection against the highly pathogenic SHIV 89.6p. Our findings suggest that Tat may be more effective in a vaccine strategy when combined with other regulatory and structural proteins of HIV.
Acknowledgments
This work was supported by NIH grant R21AI47471 to J.R. and M.G.L.
We thank Norman Wiltshire of the Walter Reed Army Institute of Research for veterinary assistance.
REFERENCES
- 1.Addo, M. M., M. Altfeld, E. S. Rosenberg, R. L. Eldridge, M. N. Philips, K. Habeeb, A. Khatri, C. Brander, G. K. Robbins, G. P. Mazzara, P. J. Goulder, and B. D. Walker. 2001. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals. Proc. Natl. Acad. Sci. USA 98:1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 3.Almond, N. M., and J. L. Heeney. 1998. AIDS vaccine development in primate models. AIDS 12(Suppl. A):S133-S140. [PubMed]
- 4.Arya, S. K., C. Guo, S. F. Josephs, and F. Wong-Staal. 1985. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science 229:69-73. [DOI] [PubMed] [Google Scholar]
- 5.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Akerblom, F. Corrias, S. Butto, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643-650. [DOI] [PubMed] [Google Scholar]
- 6.Cafaro, A., A. Caputo, M. T. Maggiorella, S. Baroncelli, C. Fracasso, M. Pace, A. Borsetti, L. Sernicola, D. R. Negri, P. ten Haaft, M. Betti, Z. Michelini, I. Macchia, E. Fanales-Belasio, R. Belli, F. Corrias, S. Butto, P. Verani, F. Titti, and B. Ensoli. 2000. SHIV89.6P pathogenicity in cynomolgus monkeys and control of viral replication and disease onset by human immunodeficiency virus type 1 Tat vaccine. J. Med. Primatol. 29:193-208. [DOI] [PubMed] [Google Scholar]
- 7.Cafaro, A., F. Titti, C. Fracasso, M. T. Maggiorella, S. Baroncelli, A. Caputo, D. Goletti, A. Borsetti, M. Pace, E. Fanales-Belasio, B. Ridolfi, D. R. Negri, L. Sernicola, R. Belli, F. Corrias, I. Macchia, P. Leone, Z. Michelini, P. ten Haaft, S. Butto, P. Verani, and B. Ensoli. 2001. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 19:2862-2877. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, S. S., C. Li, L. Ding, Y. Cao, A. B. Pardee, E. M. Shevach, and D. I. Cohen. 1999. Pronounced acute immunosuppression in vivo mediated by HIV Tat challenge. Proc. Natl. Acad. Sci. USA 96:10842-10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dayton, A. I., J. G. Sodroski, C. A. Rosen, W. C. Goh, and W. A. Haseltine. 1986. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell 44:941-947. [DOI] [PubMed] [Google Scholar]
- 10.Ensoli, B., and A. Cafaro. 2000. Control of viral replication and disease onset in cynomolgus monkeys by HIV-1 TAT vaccine. J. Biol. Regul. Homeost. Agents 14:22-26. [PubMed] [Google Scholar]
- 11.Ensoli, B., G. Barillari, S. Z. Salahuddin, R. C. Gallo, and F. Wong-Staal. 1990. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345:84-86. [DOI] [PubMed] [Google Scholar]
- 12.Ensoli, B., L. Buonaguro, G. Barillari, V. Fiorelli, R. Gendelman, R. A. Morgan, P. Wingfield, and R. C. Gallo. 1993. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein, G. 1996. HIV-1 Tat protein as a potential AIDS vaccine. Nat. Med. 2:960-964. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein, G., G. Tribbick, and K. Manson. 2001. Two B cell epitopes of HIV-1 Tat protein have limited antigenic polymorphism in geographically diverse HIV-1 strains. Vaccine 19:1738-1746. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein, G., K. Manson, G. Tribbick, and R. Smith. 2000. Minimization of chronic plasma viremia in rhesus macaques immunized with synthetic HIV-1 Tat peptides and infected with a chimeric simian/human immunodeficiency virus (SHIV33). Vaccine 18:2789-2795. [DOI] [PubMed] [Google Scholar]
- 16.Gringeri, A., E. Santagostino, M. Muca-Perja, H. Le Buanec, B. Bizzini, A. Lachgar, J. F. Zagury, J. Rappaport, A. Burny, R. C. Gallo, and D. Zagury. 1999. Tat toxoid as a component of a preventive vaccine in seronegative subjects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:371-375. [DOI] [PubMed] [Google Scholar]
- 17.Gringeri, A., E. Santagostino, M. Muca-Perja, P. M. Mannucci, J. F. Zagury, B. Bizzini, A. Lachgar, M. Carcagno, J. Rappaport, M. Criscuolo, W. Blattner, A. Burny, R. C. Gallo, and D. Zagury. 1998. Safety and immunogenicity of HIV-1 Tat toxoid in immunocompromised HIV-1-infected patients. J. Hum. Virol. 1:293-298. [PubMed] [Google Scholar]
- 18.Hirsch, V. M., G. Dapolito, C. McGann, R. A. Olmsted, R. H. Purcell, and P. R. Johnson. 1989. Molecular cloning of SIV from sooty mangabey monkeys. J. Med. Primatol. 18:279-285. [PubMed] [Google Scholar]
- 19.Huang, L., I. Bosch, W. Hofmann, J. Sodroski, and A. B. Pardee. 1998. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J. Virol. 72:8952-8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsikis, P. D., M. E. Garcia-Ojeda, J. F. Torres-Roca, D. R. Greenwald, L. A. Herzenberg, and L. A. Herzenberg. 1997. HIV type 1 Tat protein enhances activation but not Fas (CD95)-induced peripheral blood T cell apoptosis in healthy individuals. Int. Immunol. 9:835-841. [DOI] [PubMed] [Google Scholar]
- 21.Lewis, M. G., J. Yalley-Ogunro, J. J. Greenhouse, T. P. Brennan, J. B. Jiang, T. C. VanCott, Y. Lu, G. A. Eddy, and D. L. Birx. 1999. Limited protection from a pathogenic chimeric simian-human immunodeficiency virus challenge following immunization with attenuated simian immunodeficiency virus. J. Virol. 73:1262-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 23.Li, C. J., Y. Ueda, B. Shi, L. Borodyansky, L. Huang, Y. Z. Li, and A. B. Pardee. 1997. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc. Natl. Acad. Sci. USA 94:8116-8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauza, C. D., P. Trivedi, M. Wallace, T. J. Ruckwardt, H. Le Buanec, W. Lu, B. Bizzini, A. Burny, D. Zagury, and R. C. Gallo. 2000. Vaccination with tat toxoid attenuates disease in simian/HIV-challenged macaques. Proc. Natl. Acad. Sci. USA 97:3515-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Re, M. C., G. Furlini, M. Vignoli, E. Ramazzotti, G. Roderigo, V. De Rosa, G. Zauli, S. Lolli, S. Capitani, and M. La Placa. 1995. Effect of antibody to HIV-1 Tat protein on viral replication in vitro and progression of HIV-1 disease in vivo. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:408-416. [DOI] [PubMed] [Google Scholar]
- 26.Reimann, K. A., J. T. Li, G. Voss, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Parritz, Y. Lu, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 70:3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiss, P., F. de Wolf, C. L. Kuiken, A. de Ronde, J. Dekker, C. A. Boucher, C. Debouck, J. M. Lange, and J. Goudsmit. 1991. Contribution of antibody response to recombinant HIV-1 gene-encoded products Nef, Rev, Tat, and protease in predicting development of AIDS in HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 4:165-172. [PubMed] [Google Scholar]
- 29.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S. L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 30.Sodroski, J., C. Rosen, F. Wong-Staal, S. Z. Salahuddin, M. Popovic, S. Arya, R. C. Gallo, and W. A. Haseltine. 1985. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science 227:171-173. [DOI] [PubMed] [Google Scholar]
- 31.Steinaa, L., A. M. Sorensen, J. O. Nielsen, and J. E. Hansen. 1994. Antibody to HIV-1 Tat protein inhibits the replication of virus in culture. Arch. Virol. 139:263-271. [DOI] [PubMed] [Google Scholar]
- 32.Subramanyam, M., W. G. Gutheil, W. W. Bachovchin, and B. T. Huber. 1993. Mechanism of HIV-1 Tat induced inhibition of antigen-specific T cell responsiveness. J. Immunol. 150:2544-2553. [PubMed] [Google Scholar]
- 33.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load determination by real-time quantitation of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retrovir. 14:183-189. [DOI] [PubMed] [Google Scholar]
- 34.Viscidi, R. P., K. Mayur, H. M. Lederman, and A. D. Frankel. 1989. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science 246:1606-1608. [DOI] [PubMed] [Google Scholar]
- 35.Zagury, D., A. Lachgar, V. Chams, L. S. Fall, J. Bernard, J. F. Zagury, B. Bizzini, A. Gringeri, E. Santagostino, J. Rappaport, M. Feldman, A. Burny, and R. C. Gallo. 1998. Interferon alpha and Tat involvement in the immunosuppression of uninfected T cells and C-C chemokine decline in AIDS. Proc. Natl. Acad. Sci. USA 95:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zagury, J. F., A. Sill, W. Blattner, A. Lachgar, H. Le Buanec, M. Richardson, J. Rappaport, H. Hendel, B. Bizzini, A. Gringeri, M. Carcagno, M. Criscuolo, A. Burny, R. C. Gallo, and D. Zagury. 1998. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: a rationale for the use of Tat toxoid as an HIV-1 vaccine. J. Hum. Virol. 1:282-292. [PubMed] [Google Scholar]