Abstract
Context
Place of residence is associated with health outcomes.
Objective
To examine neighborhood effects on mortality after the onset of serious disease and to assess whether these effects vary for different sociodemographic or diagnostic subgroups.
Design, Setting, Patients
Our sample consists of a complete cohort of 10,557 elderly Medicare beneficiaries throughout the city of Chicago newly diagnosed and hospitalized for the first time with one of five common serious diseases in 1993 (stroke, myocardial infarction, congestive heart failure, hip fracture, and lung cancer) followed until 1999. Attributes of 51 zip code neighborhoods were obtained both from census data (1990) and from a comprehensive social survey of neighborhood residents (1994–1995). Cox proportional hazards models with robust standard errors were specified.
Main Outcome Measure
Survival after hospitalization.
Results
People who lived in neighborhoods with higher socioeconomic status (SES) or with a better social environment had significantly longer survival after disease onset. We evaluated the differential impact of neighborhood attributes on survival depending on gender, race, and poverty using interaction terms. Only the interaction terms between neighborhood social-structural factors and individual poverty were significant, suggesting that neighborhood SES and social environment were especially helpful for people with higher income. Neighborhood attributes did not differ in their impact depending on the race or sex of the subjects. Analyses of cause-specific mortality showed that myocardial infarction was the primary force driving the associations between neighborhood attributes and mortality.
Conclusions
Where people live matters with respect to posthospitalization mortality, but how neighborhoods affect this outcome depends on individual demographic and diagnostic characteristics. Myocardial infarction in particular may be a “neighborhood sensitive” condition. Individuals' health may depend not just on individuals' characteristics but also on their neighborhoods'.
Keywords: Neighborhood, aging, MI, social capital, Medicare
Geographic variation in health status, at levels ranging from states to counties to neighborhoods, is well documented (LaVeist 1989; 1992; 1995; Collins and David 1990; 1992; Clarke, Farmer, and Miller 1994; Chang and Christakis 2005). One explanation is that areas differ in health status because residents differ in characteristics such as demographic attributes, socioeconomic status (SES), and health risks (Diez-Roux 2002). However, recent work on neighborhood effects using multilevel statistical methods has pointed to the possible contextual effects of residential areas on health even after controlling for individual characteristics. That is, neighborhood SES and other neighborhood social factors may independently affect individuals' self-rated health, morbidity, or mortality over and above individual sociodemographic backgrounds (Robert 1999; Yen and Syme 1999; Pickett and Pearl 2001).
However, there are important gaps in our knowledge about whether neighborhood environment has an impact on mortality among older persons and how such neighborhood effects vary for different social or clinical subgroups in a population. Theoretically, people with different sociodemographic attributes or different illnesses may have different exposure or reactivity to such neighborhood effects. For example, older people may spend more time in their home area or have longer-standing ties to it and therefore be more vulnerable to poor conditions in their local environment. Despite this theoretical expectation, however, evidence is not consistent regarding neighborhood effects on mortality in old life. While some studies have reported a prominent effect of neighborhood SES on mortality in older persons (Waitzman and Smith 1998a; Wen, Cagney, and Christakis forthcoming), others have found insignificant or weaker neighborhood effects among the elderly (Haan, Kaplan, and Camacho 1987; Anderson et al. 1997; Waitzman and Smith 1998b).
The effect of neighborhood characteristics, including neighborhood-level SES, may also depend on individual-level SES. For example, the unhealthy consequences of individual poverty could be intensified for those who live in poverty areas (termed the “double jeopardy” hypothesis); on the other hand, poor people living in poor neighborhoods may be less stressed from perceived income inequality and thus suffer less from relative deprivation (termed the “relative deprivation” hypothesis) (Robert and House 2000; Wen, Browning, and Cagney 2003). Only a handful of studies have examined how environmental effects depend on individual characteristics, with mixed findings (Anderson et al. 1997; Collins, Herman, and David 1997; O'Campo, Xue, and Wang 1997; Pearl, Braveman, and Abrams 2001; Merkin, Stevenson, and Powe 2002).
This study first documents the effects of neighborhood SES and social environment on posthospitalization mortality and then explores how the effects vary by gender, race, and poverty status. It examines the role of the neighborhood environment in differentially contributing to a higher risk of death in specific diseases. And it emphasizes the way in which neighborhoods might affect the course, as distinct from the onset, of disease. Our sample consists of a complete cohort of Medicare beneficiaries newly diagnosed and hospitalized with one of five serious and common diseases in 1993 throughout the entire city of Chicago (stroke, myocardial infarction, congestive heart failure, hip fracture, and lung cancer). One advantage of this design is that many sources of unobserved neighborhood and individual heterogeneity are implicitly controlled for by the selection of a patient population at the time of their diagnosis with one of the specific five diseases. Here, we take advantage of several data sets with both diagnostic and social information to advance understanding of how neighborhood of residence might affect mortality after the onset of disease. In so doing, we also attempt to advance the evidentiary base regarding the nature of health disparities by social status in our society.
Data and methods
Data Sources
We merged and used three distinct data sources: the 1990 decennial census; the 1994–1995 Project on Human Development in Chicago Neighborhoods—Community Survey (PHDCN-CS); and the 1993–1999 Care after the Onset of Serious Illness (COSI) data set. Zip codes were available for subjects in the COSI data and were used to define neighborhoods and to link the three data sources into one merged file. Although zip code boundaries do not perfectly circumscribe neighborhoods, they do represent local residential areas and they are frequently used in studies of neighborhoods (Finch, Kolody, and Vega 1999; Lipton and Gruenewald 2002; Merkin et al. 2002; Zwanziger, Mukamel, and Indridason 2002). We studied 10,557 patients from COSI residing in 51 zip code areas in Chicago. Geocoding to smaller levels of aggregation (e.g., census tracts) was not possible because of sample size constraints and data limitations.
Measures of neighborhood SES were derived from the 1990 census, including measures of affluence, poverty, and education at the zip code level. Substantially all residents of Chicago appear in this data set.
Measures of neighborhood social environment were constructed from the PHDCN-CS (Sampson, Raudenbush, and Earls 1997). The PHDCN-CS is a probability sample of 8,782 residents of Chicago focusing on respondent assessments of the neighborhoods in which they live. Each record in the PHDCN-CS data was identified by a census block group in Chicago. Using the geographic centroids of census block groups, we linked each census block with its corresponding zip code, thus aggregating individuals' responses in the PHDCN-CS up to the zip code level. That is, in order to characterize the 51 Chicago neighborhoods, we used reports by thousands of Chicago residents regarding a great variety of attributes of their local neighborhoods clustered into zip codes. On average, each zip code was described by 243 participants in the PHDCN-CS.
The core data of COSI are rooted in the 1993 inpatient hospitalization records from the Health Care Financing Administration's Medicare program (Christakis, Iwashyna, and Zhang 2002; Iwashyna, Zhang, and Christakis 2002). The COSI data set consists of an inception cohort of patients newly diagnosed in 1993 with one of several serious illnesses. These diseases were selected mainly for their epidemiological significance; acute MI, CHF, and stroke are leading causes of death; lung cancer is the most common cancer-related cause of death (Sarna 1993); and hip fracture commonly causes mortality and disability in the elderly (Hayes et al. 1996; Sloan, Taylor, and Picone 1999). Given the coverage of Medicare in the elderly (over 96 percent of the elderly are represented; Hatten 1980), and given the reliable methods employed specifically to detect the onset of illness in COSI (thus excluding prevalent cases), substantially all elderly patients in Chicago in 1993 with incident hospitalizations for these conditions are captured in this data set. The patients were hospitalized in 316 different hospitals for this initial hospitalization.
The collection and analysis of these data sets were approved by our institutional review board.
Dependent Variable
Our health outcome was the duration of survival for COSI cohort members. The survival time was the number of days from the date of the index hospitalization in 1993 for the onset of disease until either death or cessation of follow-up; people still alive on June 30, 1999 (n=2,746) were right censored. Death dates were obtained from the highly complete and accurate Social Security Administration records, as provided by HCFA.
Independent Variables
Individual demographic and health measures include age, gender, race (white versus nonwhite), diagnosis, comorbidity score, and a dichotomous indicator of Medicaid recipient in 1993 as a measure of individual poverty status. Most of these variables have been previously validated or extensively exploited. Investigators have assessed, for example, the optimal use of Medicare data for measuring age (Kestenbaum 1992) and race (Lauderdale and Goldberg 1996). The poverty indicator was developed using previously described methods (Escarce et al. 1993; Carpenter 1998; Clark and Hulbert 1998; Pope et al. 1998). Comorbidity is measured by the Charlson method based on 3 years of prior data for each patient, as described elsewhere (Charlson et al. 1987; Zhang, Iwashyna, and Christakis 1999).
Measures of neighborhood SES were obtained or constructed from the 1990 U.S. Census Summary Tape File STF 3B (data at the zip code level). These measures include the percentage of residents with household annual income $50,000 or over (concentrated affluence), the percentage of households in a neighborhood that were below the Federal poverty threshold (concentrated poverty), and the percentage of college graduates (aggregate education). Table 1 illustrates SES statistics for the 51 zip code areas included in this study.
Table 1.
Zip Code Area SES Characteristics
| N=51 | ||||
|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | |
| Concentrated poverty* | 0.19 | 0.13 | 0.03 | 0.57 |
| Concentrated affluence† | 0.22 | 0.12 | 0.04 | 0.58 |
| Aggregate education‡ | 0.15 | 0.14 | 0.02 | 0.56 |
| Median family income ($) | 28,089 | 10,195 | 6,917 | 59,117 |
Concentrated poverty is measured by the percentage of households living in poverty in 1990.
Concentrated affluence is measured by the percentage of residents with household annual income $50,000 or over in 1990.
Aggregate education is measured by the percentage of college graduates in 1990.
SES, socioeconomic status.
Measures of neighborhood social environment were constructed with self-reported data from the PHDCN-CS. Following the procedures of Sampson et al. (1997), a “collective efficacy” scale was constructed through combining items of social cohesion and informal social control. The social cohesion items from the PHDCN-CS assessed the respondent's level of agreement with the following statements: (1) “People around here are willing to help their neighbors,” (2) “This is a close-knit neighborhood,” (3) “People in this neighborhood can be trusted,” (4) “People in this neighborhood generally do not get along with each other,” and (5) “People in this neighborhood do not share the same values.” The last two items were reverse coded. Informal social control was tapped through respondents' level of agreement with the following statement: “You can count on adults in this neighborhood to watch out that children are safe and do not get in trouble.” An additional informal control item asked respondents how likely it was that people in their neighborhood would intervene if a fight broke out in front of their house. Social cohesion and informal social control were closely correlated across the zip code areas (r=0.93, p<.0001).
The social support scale contained four items corresponding to the following questions: (1) “How often do you and other people in the neighborhood ask each other advice about personal things such as child rearing or job openings?” (2) “How often do you and people in your neighborhood do favors for each other?” (3) “When a neighbor is not at home, how often do you and other neighbors watch over their property?” (4) To what extent “if I were sick I could count on my neighbors to shop for groceries for me?”
The number of voluntary associations measured local civil engagement. In the PHDCN-CS, respondents were asked about whether they belonged to (1) a religious organization, (2) watch programs, (3) block group, tenant associations, or neighborhood council, (4) business or civic groups, (5) ethnic or nationality clubs, (6) neighborhood ward group or local political organizations.
The perceived violence scale was created to reflect the perceived prevalence of armed fights, violent arguments between neighbors, gang fights, sexual assault or rape, and robbery or mugging.
Analytic Strategy
To assess neighborhood collective efficacy, social support, voluntary associations, and perceived violence, we replicated a novel “ecometric” approach based on a three-level item response model. Detailed description and illustration of this approach has appeared elsewhere (Sampson et al. 1997; Raudenbush and Sampson 1999; Wen, Cagney, and Christakis forthcoming). Briefly, this method allows an overall characterization of neighborhoods that corrects for the clustering of individuals within neighborhoods and for various features of surveys used to collect such data.
However, once the above-listed attributes of each neighborhood were determined, we then proceeded to combine them, at the neighborhood level, using factor analysis—a data reduction technique concerned with finding a small number of common factors that linearly reconstruct the original variables (Harman 1976). Based on factor analyses, we found that collective efficacy, social support, voluntary association, and perceived violence were tightly clustered around a single latent concept. A factor score was thus generated and labeled as the neighborhood social environment index. This is one of the two key independent variables in our study. Collective efficacy, social support, and participation in local voluntary associations have positive correlations with this latent variable, with factor loadings of 0.91, 0.70, and 0.48, respectively. Perceived violence is also strongly loaded on this factor but in opposite direction, with a factor loading of −0.72. The internal consistency of the contextual social index scale is reasonably good with a reliability coefficient (Cronbach's α) of 0.72.
To capture the overall neighborhood SES context, we integrated concentrated poverty, concentrated affluence, and aggregate education into a single summary measure labeled neighborhood SES using the same method. This is the second key independent variable in our study. The factor loadings of the three variables are −0.78, 0.94, and 0.71, respectively. The neighborhood SES scale has a high level of internal consistency (Cronbach's α=0.83) and is significantly and positively correlated with the contextual social index (r=0.48; p=.0003).
We then used Cox proportional hazards models to test the independent effects of these two neighborhood measures on individual hazard of death among the members of the Chicago COSI cohort. Cox models yield results in the form of “hazard ratios” similar to odds ratios. Insofar as we are primarily interested in testing the overall effects of neighborhood social environment on individual outcomes, only the composite measures of neighborhood SES and social environment are included in the analytical models, both of which are standardized. This strategy also serves to ensure the parsimony of models that include community–individual interaction terms. The Huber–White robust method of calculating the variance–covariance matrix was used to account for the possible correlation in survival experiences among patients living within the same zip code area (Lin and Wei 1989). The Breslow method was adopted to handle tied values (Breslow 1974). Log-likelihood ratio tests were performed to statistically evaluate the interactions of neighborhood characteristics by poverty or diagnosis. We tested for violations of the proportionality assumption and no meaningful violations were found.
We used this overall modeling framework in preference to a hierarchical linear models approach because of the existence of censoring in our individual-level survival data (Lin and Wei 1989).
Results
Table 2 illustrates characteristics of COSI patients in Chicago. The average age of this cohort is about 79 with 14 percent of them labeled as living in poverty. About 40 percent of COSI members are men and the majority of them are white. Interestingly, the baseline comorbidity scores present an ascending gradient toward the time of diagnosis.
Table 2.
Characteristics of 10,557 Chicago COSI Patients Diagnosed with Stroke, MI, CHF, Hip Fracture, and Lung Cancer
| Variables | Mean (%) | Standard Deviation |
|---|---|---|
| Demographic | ||
| Age | 78.88 | 7.29 |
| Male | 0.39 | 0.49 |
| Poverty (Medicaid recipient) | 0.14 | 0.34 |
| Race (white) | 0.70 | 0.46 |
| Baseline comorbidity* | ||
| Charlson score for year 1 | 1.38 | 1.22 |
| Charlson score for year 2 | 1.25 | 0.99 |
| Charlson score for year 3 | 1.19 | 0.89 |
| Diagnosis in 1993 | ||
| Acute MI | 0.19 | 0.39 |
| CHF | 0.29 | 0.45 |
| Hip fracture | 0.16 | 0.37 |
| Lung cancer | 0.08 | 0.28 |
| Stroke | 0.27 | 0.45 |
N=10,557.
Baseline comorbidity was measured by the Charlson scores for the first, the second, and the third year of lookback.
MI, myocardial infarction; CHF, congestive heart failure; COSI, Care after the Onset of Serious Illness.
Table 3 provides the results from Cox proportional hazards models that assess the effects of neighborhood attributes on the mortality of elderly Medicare recipients. Model 1 is the baseline model that only includes individual-level variables. As expected, age, male gender, poverty, baseline comorbidity, and diagnosis are all significant predictors for mortality. Men and those in poverty have, respectively, 34.4 and 9.4 percent increased hazards of death after onset of serious illness. The main effects of the diagnoses in these models show that lung cancer patients have the highest hazard of death and that hip fracture is the least life-threatening condition among the five diseases; compared with MI, the hazard of death from lung cancer is 222 percent higher and the hazard of death from hip fracture is 23 percent lower. The individual-level pattern remains largely consistent after controlling for neighborhood variables.
Table 3.
The Effects of Neighborhood Social Factors on Mortality after Hospitalization in 10,557 Elderly Chicago Patients†
| Models‡ | ||||||
|---|---|---|---|---|---|---|
| Independent Variables | 1 | 2 | 3 | 4 | 5 | 6 |
| Individual-level variables | ||||||
| Age | 1.053*** | 1.053*** | 1.052*** | 1.053*** | 1.053*** | 1.052*** |
| Male | 1.344*** | 1.344*** | 1.347*** | 1.346*** | 1.341*** | 1.348*** |
| Poverty (Medicaid recipient) | 1.094*** | 1.082** | 1.079** | 1.077** | 1.136*** | 1.101*** |
| Race (nonwhite) | 1.046 | 1.013 | 1.033 | 1.016 | 1.015 | 1.032 |
| CHF | 1.113*** | 1.110*** | 1.110*** | 1.109*** | 1.110*** | 1.110*** |
| Hip fracture | 0.810*** | 0.811*** | 0.808*** | 0.809*** | 0.809*** | 0.808*** |
| Lung cancer | 3.220*** | 3.218*** | 3.218*** | 3.217*** | 3.227*** | 3.221*** |
| Stroke | 1.009 | 1.007 | 1.007 | 1.007 | 1.006 | 1.007 |
| Charlson score (lookup year 1) | 1.099*** | 1.099*** | 1.098*** | 1.099*** | 1.099*** | 1.098*** |
| Charlson score (lookup year 2) | 1.064*** | 1.064*** | 1.064*** | 1.064*** | 1.065*** | 1.065*** |
| Charlson score (lookup year 3) | 1.035*** | 1.035*** | 1.035*** | 1.035*** | 1.035*** | 1.035*** |
| Neighborhood-level variables | ||||||
| Neighborhood SES | 0.959** | 0.973 | 0.944*** | |||
| Neighborhood social index | 0.967** | 0.979 | 0.958*** | |||
| Neighborhood SES × poverty | 1.137*** | |||||
| Neighborhood social index × poverty | 1.065** | |||||
| Log likelihood | −67,253 | −67,249 | −67,249 | −67,248 | −67,244 | −67,248 |
p<=.10.
p<=.05.
p<=.01 (two-tailed tests).
Zip code level, N=51; individual level, N=10,557.
The estimates presented in this table are hazard ratios.
CHF, congestive heart failure; SES, socioeconomic status.
Models 2 and 3 in Table 3 show that people who live in neighborhoods with higher SES and better social environment have significantly better chances of survival. Specifically, a one standard deviation (SD) increase in neighborhood SES and neighborhood social index is associated with 4.3 and 3.4 percent lower risk of death, respectively. The magnitude of these neighborhood effects can be understood more intuitively by comparison with the mortality effect of individual attributes. For example, according to model 2 in Table 3, the difference in the relative hazard of death between two subpopulations which differ by one SD in neighborhood SES (or roughly by $10,483 in the median income of the residents in the two neighborhoods) is approximately equivalent to the difference that would be generated by a 10-month age difference at the individual level. The corresponding figure for neighborhood social index is approximately an 8-month age difference. Model 4 examines neighborhood SES and social index simultaneously; there appears to be some collinearity between the two variables.
We then evaluate the differential impact of neighborhood attributes on survival depending on individuals' gender, race, and poverty using interaction terms, which allow us to assess whether neighborhood attributes are particularly powerful in particular types of individuals. Only the interaction terms between neighborhood factors and individual poverty are statistically significant (models 5 and 6 in Table 3). Log-likelihood ratio tests further confirmed the significance of the interaction between neighborhood SES and individual poverty (p=.002). That the effect of neighborhood SES is more beneficial for people not in poverty is shown in Figure 1. Conversely, for those in poverty, the effect of neighborhood SES is detrimental with borderline significance. Neighborhood attributes did not differ in their overall impact depending on the race or sex of the subjects.
Figure 1.
Effect Sizes of Zip Code Area SES with 95 Percent Confidence Interval (Stratified by Individual Poverty Status)
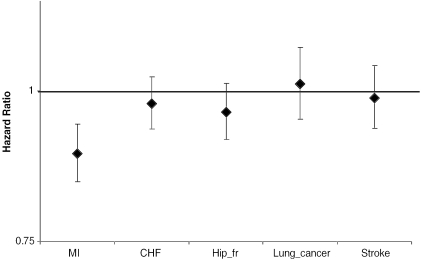
Finally, Table 4 presents findings from two models that assess interaction effects between neighborhood attributes and individual diagnosis. In this table, models 1 and 2 illustrate a consistent pattern that neighborhood SES and social index are significantly more relevant for MI patients than for patients with the other diagnoses. Log-likelihood ratio tests show a p-value of .03 for the interaction effect by diagnosis of the social index and a p-value of .09 for that of the neighborhood SES. The differential effect sizes of neighborhood social index by individual diagnosis are illustrated in Figure 2, which indicates that neighborhood social environment is particularly salient to posthospitalization mortality from MI.
Table 4.
The Effects of Neighborhood Social Factors on Mortality after Hospitalization in 10,557 Elderly Chicago Patients†
| Modelsठ| ||
|---|---|---|
| Independent Variables | 1 | 2 |
| Individual-level variables | ||
| Stroke | 1.000 | 0.993 |
| MI | – | – |
| CHF | 1.103*** | 1.096*** |
| Hip fracture | 0.803*** | 0.798*** |
| Lung cancer | 3.195*** | 3.155*** |
| Neighborhood-level variables | ||
| Neighborhood SES | 0.884*** | |
| Neighborhood social index | 0.896*** | |
| Neighborhood SES × stroke | 1.114** | |
| Neighborhood SES × MI | – | |
| Neighborhood SES × CHF | 1.104*** | |
| Neighborhood SES × hip fracture | 1.098** | |
| Neighborhood SES × lung cancer | 1.086 | |
| Neighborhood social index × stroke | 1.103*** | |
| Neighborhood social index × MI | – | |
| Neighborhood social index × CHF | 1.093** | |
| Neighborhood social index × hip fracture | 1.077* | |
| Neighborhood social index × lung cancer | 1.129** | |
| Log likelihood | −67,245 | −67,244 |
p<=.10.
p<=.05.
p<=.01 (two-tailed tests).
Zip code level, N=51; individual level, N=10,557.
The estimates presented in this table are hazard ratios.
To save space, the coefficients of age, gender, poverty, race, and comorbidity are not presented.
MI, myocardial infarction; CHF, congestive heart failure; SES, socioeconomic status.
Figure 2.
Effect Sizes of Zip Code Area Social Index with 95 Percent Confidence Interval (Stratified by Diseases)
To help distinguish possible hospital-based effects on mortality from neighborhood-based effects, we repeated all the above analyses in subsets of the data restricted to include only those who survived 7 or 30 days posthospitalizations, and our results were not different.
Discussion
Numerous studies have suggested that local areas may exert an independent impact on health over and above individual characteristics, at scales ranging from census tract (Anderson et al. 1997; LeClere, Rogers, and Kimberley 1997) to larger neighborhood areas (Browning, Cagney, and Wen 2003; Wen et al. 2003; Cagney, Browning, and Wen forthcoming). Our work advances this literature in several important ways. First, we examined a relatively novel outcome in this area, namely, mortality after onset of serious disease. Second, we focused on two broad and important, but distinct, dimensions of neighborhood ecology: local SES and local social environment. Third, we examined whether neighborhood attributes can explain or contribute to health disparities in old life. Fourth, we investigated whether neighborhood effects vary by individual sociodemographic factors and diagnosis, and, if so, how. And fifth, we studied a large and complete population-based sample of patients experiencing the onset of various serious diseases in an entire city.
We find that neighborhood SES and social context are associated with the relative hazard of death net of individual sociodemographic factors and baseline diagnostic factors. Moderate variation in neighborhood context had approximately the same impact on individuals' outcomes as nearly a year of life at the individual level.
In addition, neighborhood factors had varying effects depending on the poverty status but not the gender or race of individuals. Neighborhood of residence was especially relevant when individual income was adequate. This evidence that patients in poverty do not gain survival time (after the onset of disease) from living in better neighborhoods lends some support to the “relative deprivation hypothesis.” In other words, low-income older patients might suffer particular health decrements when living in neighborhoods more advantaged than their own status. Interestingly, this was not the case with race. There was no evidence that neighborhood effects change by race (that is, there was no interaction with race). On the one hand, this suggests that neighborhood environment is important to health regardless of racial background. On the other hand, it has been well documented that disadvantaged racial groups are much more likely than whites to live in distressed neighborhoods (Massey, Condran, and Denton 1987; Wilson 1987; Massey 1996); as a result, they may suffer from poor neighborhood environments in addition to any individual disadvantageous conditions.
Moreover, we examined possible neighborhood effects on disease-specific mortality. Studying disparate diseases may help advance knowledge about the specific processes underlying neighborhood effects. It is plausible that individuals with different diagnoses are differentially affected by neighborhood environment. For example, if local social support is a major mechanism explaining the effects of neighborhood social-structural features on health, then the magnitude of neighborhood effects on mortality might depend on the degree of amenability of various diseases to such a factor. Previous work has argued for the need to differentiate causes of death in studying the way informal social support might influence mortality (Litwak et al. 1989). We found that neighborhood SES and social environment are especially potent predictors for mortality following MI. Indeed, MI appears to be the primary force driving the associations between neighborhood SES and total mortality in our sample. Several studies have directly tested the effects of social support on mortality or prognosis in post-MI patients, and they have generally found that social support had strong and beneficial effects on recovering from MI (Ruberman et al. 1984; Berkman, Leo-Summers, and Horwitz 1992; Case et al. 1992; Iwashyna 2001). Previous work has also found that the quality of health care significantly affected post-MI mortality rate (Ayanian et al. 2002). Our work suggests that these phenomena may also operate at the neighborhood level and contribute to health disparities.
Several limitations and methodologically controversial aspects of this study are noteworthy. First, we used zip code boundaries to define residential communities. Zip code areas are drawn for the purpose of delivering mail and may not be the most appropriate unit for analyzing the impact of the social ecological environment on health. Arguably, census tracts are preferred to zip codes in that tracts are originally drawn to encompass socially homogeneous populations and better conform to local neighborhoods. They are also smaller areas than zip codes, which may or may not be desirable conceptually or methodologically. Empirically, do zip code level data perform substantially worse in health outcome equations than data collected at the census-tract level? This is one of the research questions investigated by Geronimus and Bound (1998) in a study using data from the PSID linked to census 1970 and 1980. They specifically examined whether aggregate SES variables measured at the zip code level compared with those measured for census tracts generate different estimates of effects on self-rated health and whether estimates differ when using 1970 compared with 1980 census data. Results show little difference in either coefficient estimates or goodness of fit between the zip code or tract levels of aggregation or between 1970 and 1980 census data. These findings suggest that regression coefficients may not be sensitive to the timing of census data collection or to the level of aggregation of the census data. To date, the majority of neighborhood effects research has relied on census data to characterize neighborhood environment and has used administrative definitions of neighborhoods such as census tract and zip code areas. In general, these definitions do not perfectly circumscribe the boundaries of relevant neighborhood dimensions (Kawachi and Berkman 2003). The appropriate definitions may differ across places, individuals, and outcomes being studied. Places have different ecological characteristics of natural and built environment, which potentially affects the geography and human interactions across the space. People have different opinions on the boundary and amount of residential space that can be viewed as relevant territory of their neighborhoods. So, if objective or independently assessed survey-based neighborhood measures are used, geocoded, and linked to individual-level health data, a certain amount of neighborhood exposure misclassification is inevitable in neighborhood effects research.
Second, although this study has prospective survival data, no time-varying neighborhood and other individual-level information are available. As a result, this study does not address the issue of residential mobility and advance our knowledge about the relative importance of residential selection versus social causation in explaining neighborhood effects on health. While all of the respondents were older and sick, it seems likely that some moved between their initial diagnosis and either death or censorship. The issue of residential mobility has not been well addressed in the literature of neighborhood effects on health. This has been noted as a major limitation in the field (Kawachi and Berkman 2003; Macintyre and Ellaway 2003). Indeed, this gap in the literature warrants future investigation equipped with data longitudinal both at the individual level and neighborhood level to explore and clarify this issue.
Third, our models did not control for some individual characteristics, thus raising the possibility of confounding at the individual level. For example, because of the well-known limitations of the Medicare data, race is dichotomized into white versus nonwhite group and individual poverty status was measured by a dummy indicator of Medicaid recipients. Nonetheless, we controlled for baseline illness burden in individuals in our empirical analyses in addition to the initial diagnosis and still found significant neighborhood-level effects on health—the latter level being the main focus of our work. Moreover, our analyses are conditioned on the onset of disease, a fact which helps to account for the impact of any prior unmeasured social or medical risks for disease. These efforts in controlling for individual background notwithstanding, it is conceptually debatable whether some individual factors can be labeled as “confounders” versus “mediators.” As Diex-Roux argued, “because disease is expressed at the level of the individual, neighborhood factors necessarily exert their effect through individual-level processes … . Whether an individual-level variable is conceptualized as a confounder or a mediator depends on the question being asked” (Diez-Roux 2001, p. 1787). Macintyre and Ellaway (2003, p. 26) have similarly commented that “people creates places, and places create people.” In the current research, we control for several individual variables in an attempt to detect a residual effect of neighborhood environment on mortality, whereby implicitly treating them as confounders. But, in theory, it is arguably the case that neighborhood environment may affect posthospitalization mortality via its impact on residents' SES profile and the risk of falling ill. Without controlling for individual factors such as poverty and comorbidity in the prior 3 years, neighborhood effects would have been even stronger. In this sense, the findings reported in this study may have underestimated the true neighborhood effects on mortality following serious diseases in old life.
Elderly individuals' attributes and their environments may both affect posthospitalization outcomes from serious disease. Moreover, the association between individual attributes and health outcomes may partly represent the impact of individuals' neighborhoods. Interventions directed at decreasing health disparities in our society might therefore focus not only on the interactions of people of certain races or incomes with the health care system, but also on the more population-level determinants of neighborhood inequality and on the social-structural factors that make individuals' place of residence so relevant to their lives.
Acknowledgments
We wish to thank Robert Sampson, Felton Earls, and members of the Project on Human Development in Chicago neighborhoods for generously providing access to the Community Survey data. This research is supported in part by a grant from NIA (R01-AG15326-01) (NAC). An earlier version of this paper was presented at the Midwest Sociological Association (MSA) Annual Meeting in Chicago, 2003, and the ASA annual meeting in San Francisco, 2004.
References
- Anderson RT, Sorlie P, Backlund E, Johnson N, Kaplan GA. “Mortality Effects of Community Socioeconomic Status.”. Epidemiology. 1997;8:42–7. doi: 10.1097/00001648-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Ayanian JZ, Landrum MB, Guadagnoli E, Gaccione P. “Specialty of Ambulatory Care Physicians and Mortality among Elderly Patients after Myocardial Infarction.”. New England Journal of Medicine. 2002;347(21):1678–86. doi: 10.1056/NEJMsa020080. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Leo-Summers L, Horwitz RI. “Emotional Support and Survival after Myocardial Infarction. A Prospective, Population-Based Study of the Elderly.”. Annals of Internal Medicine. 1992;117(12):1003–9. doi: 10.7326/0003-4819-117-12-1003. [DOI] [PubMed] [Google Scholar]
- Breslow NE. “Covariance Analysis of Censored Survival Data.”. Biometrics. 1974;30:89–99. [PubMed] [Google Scholar]
- Browning CR, Cagney KA, Wen M. “Explaining Variation in Health Status across Space and Time: Implications for Race and Ethnic Disparities in Self-rated Health.”. Social Science and Medicine. 2003;57:1221–35. doi: 10.1016/s0277-9536(02)00502-6. [DOI] [PubMed] [Google Scholar]
- Cagney KA, Browning CR, Wen M. “Race and Self-rated Health at Older Ages: What Difference Does the Neighborhood Make?”. Journal of Gerontology: Social Sciences. doi: 10.1093/geronb/60.4.s181. forthcoming. [DOI] [PubMed] [Google Scholar]
- Carpenter L. “Evolution of Medicaid Coverage of Medicare Cost Sharing.”. Health Care Financing Review. 1998;1998(20):11–18. [PMC free article] [PubMed] [Google Scholar]
- Case RB, Moss AJ, Case N, McDermott M, Eberly S. “Living Alone after Myocardial Infarction. Impact on Prognosis.”. Journal of the American Medical Association. 1992;267(4):515–9. [PubMed] [Google Scholar]
- Chang VW, Christakis NA. “Income Inequality and Weight Status in U.S. Metropolitan Areas.”. Social Science and Medicine. 2005;60(11) doi: 10.1016/j.socscimed.2004.11.036. in press. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. “A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation.”. Journal of Clinical Epidemiology. 1987;45:613–9. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Christakis NA, Iwashyna TJ, Zhang JX. “Care after the Onset of Serious Illness (COSI): A Novel Claims-based Data Set Exploiting Substantial Cross-Set Linkages to Study End-of-Life Care.”. Journal of Palliative Medicine. 2002;5(4):515–29. doi: 10.1089/109662102760269751. [DOI] [PubMed] [Google Scholar]
- Clark WD, Hulbert MM. “Research Issues: Dually Eligible Medicare and Medicaid Beneficiaries, Challenges and Opportunities.”. Health Care Financing Review. 1998;20:1–101. [PMC free article] [PubMed] [Google Scholar]
- Clarke L, Farmer F, Miller M. “Structural Determinants of Infant Mortality in Metropolitan and Nonmetropolitan America.”. Rural Sociology. 1994;59(1):84–99. [Google Scholar]
- Collins JJ, David R. “The Differential Effect of Traditional Risk Factors on Infant Birthweight among Blacks and Whites in Chicago.”. American Journal of Public Health. 1990;80:679–81. doi: 10.2105/ajph.80.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, David R. “Differences in Neonatal Mortality by Race, Income, and Prenatal Care.”. Ethnicity and Disease. 1992;2:18–26. [PubMed] [Google Scholar]
- Collins JW, Herman AA, David RJ. “Very-Low-Birthweight Infants and Income Incongruity among African American and White Parents in Chicago.”. American Journal of Public Health. 1997;87(3):414–7. doi: 10.2105/ajph.87.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Roux AV. “Investigating Neighborhood and Area Effects on Health [letter; comment].”. American Journal of Public Health. 2001;91(9):1487–93. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Roux AV. “Invited Commentary: Places, People, and Health.”. American Journal of Epidemiology. 2002;155(6):516–9. doi: 10.1093/aje/155.6.516. [DOI] [PubMed] [Google Scholar]
- Escarce JJ, Epstein KR, Colby DC, Schwartz JS. “Racial Differences in the Elderly Use of Medical Procedures and Diagnostic Tests.”. American Journal of Public Health. 1993;83:948–54. doi: 10.2105/ajph.83.7.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch BK, Kolody B, Vega WA. “Contextual Effects of Perinatal Substance Exposure among Black and White Women in California.”. Sociological Perspectives. 1999;42(2):141–56. [Google Scholar]
- Geronimus AT, Bound J. “Use of Census-based Aggregate Variables to Proxy for Socioeconomic Group: Evidence from National Samples.”. American Journal of Epidemiology. 1998;148(5):475–86. doi: 10.1093/oxfordjournals.aje.a009673. [DOI] [PubMed] [Google Scholar]
- Haan M, Kaplan GA, Camacho T. “Poverty and Health: Prospective Evidence from the Alameda County Study.”. American Journal of Epidemiology. 1987;125:989–98. doi: 10.1093/oxfordjournals.aje.a114637. [DOI] [PubMed] [Google Scholar]
- Harman HH. Modern Factor Analysis. Chicago: University of Chicago Press; 1976. [Google Scholar]
- Hatten J. “Medicare's Common Denominator: The Covered Population.”. Health Care Financing Review. 1980;21(2):53–64. [PMC free article] [PubMed] [Google Scholar]
- Hayes WC, Myers ER, Robinovitch SN, Van Den Kroonenberg A, Courtney AC, McMahon TA. “Etiology and Prevention of Age-related Hip Fractures.”. Bone. 1996;18(suppl 1):77S–86S. doi: 10.1016/8756-3282(95)00383-5. [DOI] [PubMed] [Google Scholar]
- Iwashyna TJ. In Sickness and in Health: Understanding the Effects of Marriage on Health. Chicago: University of Chicago; 2001. [Google Scholar]
- Iwashyna TJ, Zhang JX, Christakis NA. “Disease-Specific Patterns of Hospice and Related Healthcare Use among the Seriously Ill.”. Journal of Palliative Medicine. 2002;5(4):531–8. doi: 10.1089/109662102760269760. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Berkman LF. “Introduction.”. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. New York: University of Oxford Press; 2003. pp. 1–19. [Google Scholar]
- Kestenbaum B. “A Description of the Extreme Aged Population Based on Improved Medicare Enrollment Data.”. Demography. 1992;29:565–80. [PubMed] [Google Scholar]
- Lauderdale DS, Goldberg J. “The Expanded Racial and Ethnic Codes in the Medicare Data Files: Their Completeness of Coverage and Accuracy.”. American Journal of Public Health. 1996;86:712–6. doi: 10.2105/ajph.86.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVeist G. “Linking Residential Segregation to the Infant-Mortality Race Disparity in U.S. Cities.”. Sociology and Social Research. 1989;73(2):90–4. [Google Scholar]
- LaVeist G. “The Political Empowerment and Health Status of African Americans: Mapping a New Territory.”. American Journal of Sociology. 1992;97:1080–95. [Google Scholar]
- LaVeist G. “Segregation, Poverty, and Empowerment: Health Consequences for African–Americans.”. The Milbank Quarterly. 1995;71(1):41–64. [PubMed] [Google Scholar]
- LeClere FB, Rogers RG, Kimberley P. “Ethnicity and Mortality in the United States: Individual and Community Correlates.”. Social Forces. 1997;76:169–367. [Google Scholar]
- Lin D, Wei L. “The Robust Inference for the Cox Proportional Hazards Model.”. Journal of the American Statistical Association. 1989;84:1074–8. [Google Scholar]
- Lipton R, Gruenewald P. “The Spatial Dynamics of Violence and Alcohol Outlets.”. Journal of Studies on Alcohol. 2002;63(2):187–95. doi: 10.15288/jsa.2002.63.187. [DOI] [PubMed] [Google Scholar]
- Litwak E, Messeri P, Wolfe S, Gorman S, Silverstein M, Guilarte M. “Organizational Theory, Social Supports, and a Theoretical Convergence.”. American Sociological Review. 1989;54(February):49–66. [Google Scholar]
- Macintyre S, Ellaway A. “Neighborhoods and Health: An Overview.”. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. New York: University of Oxford Press; 2003. pp. 20–42. [Google Scholar]
- Massey DS. “The Age of Extremes: Concentrated Affluence and Poverty in the Twenty-first Century.”. Demography. 1996;33(4):395–412. [PubMed] [Google Scholar]
- Massey DS, Condran G, Denton N. “The Effect of Residential Segregation on Black Social and Economic Well-Being.”. Social Forces. 1987;66(1):29–56. [Google Scholar]
- Merkin SS, Stevenson L, Powe N. “Geographic Socioeconomic Status, Race, and Advanced-Stage Breast Cancer in New York City.”. American Journal of Public Health. 2002;92(1):64–70. doi: 10.2105/ajph.92.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Campo P, Xue X, Wang M-C. “Neighborhood Risk Factors for Low Birthweight in Baltimore: A Multilevel Analysis.”. American Journal of Public Health. 1997;87(7):1113–8. doi: 10.2105/ajph.87.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl M, Braveman P, Abrams B. “The Relationship of Neighborhood Socioeconomic Characteristics to Birthweight among 5 Ethnic Groups in California.”. American Journal of Public Health. 2001;91(11):1808–14. doi: 10.2105/ajph.91.11.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Pearl M. “Multi-level Analyses of Neighborhood Socioeconomic Context and Health Outcomes: A Critical Review.”. Journal of Epidemiology Community Health. 2001;55:111–22. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope GC, Adamache KW, Walsh EG, Khandker RK. “Evaluating Alternative Risk Adjusters for Medicare.”. Health Care Financing Review. 1998;20:109–29. [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S, Sampson R. “‘Ecometrics’: Toward a Science of Assessing Ecological Settings, with Application to the Systematic Social Observation of Neighborhoods.”. Sociological Methodology. 1999;29:1–41. [Google Scholar]
- Robert SA. “Socioeconomic Position and Health: The Independent Contribution of Community Socioeconomic Context.”. Annual Review of Sociology. 1999;25:489–516. [Google Scholar]
- Robert SA, House JS. “Socioeconomic Inequalities in Health: Integrating Individual-, Community- and Societal-Level Theory and Research.”. In: Albrecht GL, Fitzpatrick R, Scrimshaw S, editors. The Handbook of Social Studies in Health and Medicine. London: Sage; 2000. [Google Scholar]
- Ruberman W, Weinblatt E, Goldberg JD, Chaudhary BS. “Psychosocial Influences on Mortality after Myocardial Infarction.”. The New England Journal of Medicine. 1984;311(9):552–9. doi: 10.1056/NEJM198408303110902. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. “Neighborhoods and Violent Crime: A Multilevel Study of Collective Efficacy.”. Science. 1997;227:918–23. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Sarna L. “Women with Lung Cancer: Impact on Quality of Life.”. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 1993;2(1):13–22. doi: 10.1007/BF00642885. [DOI] [PubMed] [Google Scholar]
- Sloan FA, Taylor DHJ, Picone G. “Costs and Outcomes of Hip Fracture and Stroke, 1984 to 1994.”. American Journal of Public Health. 1999;89(6):935–7. doi: 10.2105/ajph.89.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitzman NJ, Smith KR. “Separate but Lethal: The Effects of Economic Segregation on Mortality in Metropolitan America.”. Milbank Memorial Fund Quarterly. 1998a;76(3):341–73. doi: 10.1111/1468-0009.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitzman NJ, Smith KR. “Phantom of the Area: Poverty-Area Residence and Mortality in the United States.”. American Journal of Public Health. 1998b;88:973–6. doi: 10.2105/ajph.88.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M, Browning CR, Cagney KA. “Poverty, Affluence and Income Inequality: Neighborhood Economic Structure and Its Implications for Self-rated Health.”. Social Science and Medicine. 2003;57:843–60. doi: 10.1016/s0277-9536(02)00457-4. [DOI] [PubMed] [Google Scholar]
- Wen M, Cagney KA, Christakis NA. “Community Effects on Mortality following Serious Diseases in Later Life: A Prospective Contextual Study of Medicare Beneficiaries in Chicago.”. Social Science and Medicine. forthcoming. [Google Scholar]
- Wilson WJ. The Truly Disadvantaged: The Inner City, the Underclass, and Public Policy. Chicago: The University of Chicago Press; 1987. [Google Scholar]
- Yen IH, Syme SL. “The Social Environment and Health: A Discussion of the Epidemiologic Literature.”. Annual Review of Public Health. 1999;20:287–308. doi: 10.1146/annurev.publhealth.20.1.287. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Iwashyna TJ, Christakis NA. “The Performance of Different Lookback Periods and Sources of Information for Charlson Comorbidity Adjustment in Medicare Claims.”. Medical Care. 1999;37(11):1128–39. doi: 10.1097/00005650-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Zwanziger J, Mukamel DB, Indridason I. “Use of Resident-Origin Data to Define Nursing Home Market Boundaries.”. Inquiry: A Journal of Medical Care Organization, Provision and Financing. 2002;39(1):56–66. doi: 10.5034/inquiryjrnl_39.1.56. [DOI] [PubMed] [Google Scholar]