Abstract
Objective
To determine whether rates of physician visits for ambulatory care sensitive (ACS) conditions are lower for people of low-socioeconomic status than of high-socioeconomic status in an urban population with universal health care coverage.
Data Sources/Study Setting
Physician claims and hospital discharge abstracts from fiscal years 1998 to 2001 for urban residents of Manitoba, Canada. The 1996 Canadian Census public use database provided neighborhood household income information. The study included all continuously enrolled urban residents in the Manitoba Health Services Insurance Plan.
Study Design
Twelve ACS conditions definable using 3-digit ICD-9-CM codes permitted cross-sectional and longitudinal comparison of ambulatory visits and hospitalizations. Neighborhood household income data provided a measure of socioeconomic status.
Data Collection/Extraction Methods
Files were extracted from administrative data housed at the Manitoba Centre for Health Policy.
Principal Findings
All conditions showed a socioeconomic gradient with residents of the lowest income neighborhoods having both more visits and more hospitalizations than their counterparts in higher income areas. Six of nine conditions with a sufficient N showed individuals living in the lowest income neighborhoods to have significantly more ambulatory visits before hospitalization for an ACS condition than did those in the most affluent neighborhoods. Many conditions showed a gradient in rate of hospitalization even after controlling for the number of ambulatory care visits.
Conclusions
In the Canadian universal health care plan, the poor have reasonable access to ambulatory care for ACS conditions. Ambulatory care may be more effective in preventing hospitalizations among relatively affluent individuals than among the less well off.
Keywords: Access to care, ambulatory care sensitive, hospitalization, physician visits, socioeconomic status
Recent cost containment efforts have increased the need to identify where resources might be most efficiently targeted and to monitor the effects of such interventions. At the same time, the economic and health benefits of appropriate primary and preventive care have received increasing attention (Starfield 1998; Shi et al. 2002). Population-based studies are central to “sentinel” approaches to evaluation, where rates of hospitalization or outcomes for selected medical conditions determine whether problems exist in the organization or quality of care (Rutstein et al. 1976; Weissman, Gatsonis, and Epstein 1992).
Combining these perspectives, Billings et al. (1993) developed the concept of ambulatory care sensitive (ACS) conditions, conditions for which “timely and effective outpatient care can help to reduce the risks of hospitalization by either preventing the onset of an illness or condition, controlling an acute episodic illness or condition, or managing a chronic disease or condition.” Such conditions include asthma, angina, pelvic inflammatory disease, gastroenteritis, and congestive heart failure. To independently identify ACS conditions, Brown et al. (2001) used three different groups of physicians and somewhat different methodologies (Delphi panel, Modified Delphi panel, and Questionnaire panel). The degree of consensus among panels provided the basis for our ordering of these conditions.
The literature has stressed the socioeconomic gradient: hospitalizations for ACS conditions had a much stronger negative association with area income than did those for other diagnoses (Billings et al. 1993). Similar associations between ACS hospitalizations and neighborhood income have been replicated in adult, elderly, and pediatric populations (Schreiber and Zielinski 1997; Blustein, Hanson, and Shea 1998; Shi et al. 1999; Parchman and Culler 1999; Parker and Schoendorf 2000). This relationship has often been interpreted as vulnerable populations having inadequate primary care or greater barriers to such care than their more well-off counterparts.
Hospitalization rates for ACS conditions have been suggested as a proxy for the presence or absence of appropriate primary and preventive care; more physician visits within a community should result in fewer ACS hospitalizations (Billings et al. 1993; Billings, Anderson, and Newman 1996; Gadomski, Jenkins, and Nichols 1998). Reports of access to medical care in a small area were inversely correlated with hospitalization rates for five ACS conditions in urban California (Bindman et al. 1995). Increased physician supply and penetration of primary care have been associated with lower ACS hospitalizations in several studies (Krakauer et al. 1996; Friedman and Basu 2001; Ricketts et al. 2001; Backus et al. 2002; Basu, Friedman, and Bursten 2002). Research examining associations between the presence of a regular or continuous source of care and ACS hospitalizations has produced contradictory results (Gill 1997; Gill and Mainous 1998; Epstein 2001; Falik et al. 2001).
On the other hand, gradients in ACS hospitalizations may simply reflect socioeconomic gradients in health status and not in health care. In Manitoba, those with the poorest health status have the highest hospital use and expenditure rates (Roos et al. 2004). In the United Kingdom, hospital admission rates reflect socioeconomic differences and patient morbidity, not quality in primary care (Giuffrida, Gravelle, and Roland 1999; Reid, Cook, and Majeed 1999).
Individual-level (rather than aggregate) data in a defined population can examine such questions as: What is the relationship between ambulatory visits and hospitalizations for ACS conditions for patients of differing socioeconomic statuses? Do patients resident in low-income neighborhoods have fewer or more ambulatory visits before hospitalizations than their counterparts in more affluent neighborhoods?
This paper explores these issues for urban residents of the province of Manitoba, Canada. Canadians are provided complete coverage for physician visits and hospital stays under Canadian national health insurance. Within Canada, Manitoba has generally ranked in the mid-range of a series of indicators of health status, socioeconomics, and health care expenditures (Shanahan and Gousseau 1999). In Winnipeg (which included 77 percent of Manitoba's 1996 urban population), physician supply figures are influenced by the city's serving as a referral center for the entire province of 1.1 million people. A cross-Canada study of 57 health regions (which did not take the rural referral base into account) ranked Winnipeg fourteenth in physicians per capita and eighth in per capita supply of specialists (Maclean's 2003).
The effective bed supply in urban Manitoba is not high by North American standards (Roos, Burchill, and Carriere 2003). Hospital use patterns across socioeconomic groups were largely similar to those found elsewhere in Canada and internationally (Manga, Broyles, and Angus 1987; Haan, Kaplan, and Camacho 1987; Carstairs and Morris 1989; Pappas et al. 1993). Urban Manitoba has experienced slightly lower overall rates of ACS hospitalizations (6.99 per 1,000 in 1990 and 5.72 in 2000 after significant health care reform) than three other Canadian centers (Hamilton, Ottawa, and Toronto) (Billings, Anderson, and Newman 1996). In 1990, Canadian rates were generally in the lower part of the range for 15 American cities.
Methods
In Manitoba, a single diagnosis is available on physician claims, while up to sixteen diagnoses are present on the hospital discharge abstracts. One diagnosis—that labeled as “most responsible” in the Canadian implementation of ICD-9-CM coding—was used to define the relevant hospital diagnosis. The “most responsible” diagnosis is essentially equivalent to that specified as the “principal” diagnosis in American data sets (Tu et al. 2001).
The Manitoba Centre for Health Policy maintains a comprehensive, longitudinal, population-based administrative database containing all claims routinely submitted by physicians and health care facilities for all individuals registered with the Manitoba Health Services Insurance Plan (Roos et al. 1993). Nonparticipation is minimal since there are no premium payment requirements. Reflecting the fee-for-service environment, just 7 percent of physicians submit “evaluation claims” (claims for which remuneration is not attached) for any portion of their visits (Watson et al. 2004). Surgical procedures and patient location are recorded with a high degree of accuracy (Roos and Nicol 1999). The available diagnostic information is generally satisfactory, but using hospital abstracts alone underestimates the prevalence of the conditions studied (Robinson et al. 1997; Huzel et al. 2003).
Manitoba ICD-9-CM codes have been coded on hospital discharge abstracts up to the 5-digit level for certain conditions but only at the 3-digit level from the physician claims. However, twelve relatively common ACS conditions (Billings et al. 1993) can be specified almost completely on the basis of the 3-digit ICD-9-CM code. The conditions are: asthma, angina, pelvic inflammatory disease, gastroenteritis, congestive heart failure, severe ear–nose–throat (ENT) infections, epilepsy, bacterial pneumonia, tuberculosis (pulmonary and other), iron deficiency anemia in children up to 5 years of age, cellulitis, and dental conditions (Table 1).
Table 1.
Differing Definitions of Selected ACS Conditions (Hospital Separations in Manitoba, 1998–2000)
| ACS Conditions* | N using Billings et al. Definitions† | N Using 3-Digit Adaptations | % Increase Using 3-Digit Adaptations |
|---|---|---|---|
| Asthma | 4,115 | 4,115 | 0.0 |
| Angina | 4,998 | 5,050 | 1.0 |
| Pelvic inflammatory disease | 1,409 | 1,409 | 0.0 |
| Gastroenteritis | 2,959 | 2,993 | 1.1 |
| Congestive heart failure | 9,759 | 10,582 | 8.4 |
| Severe ENT infections | 2,451 | 2,487 | 1.5 |
| Epilepsy | 825 | 825 | 0.0 |
| Bacterial pneumonia | 11,906 | 12,036 | 1.1 |
| Pulmonary/other tuberculosis | 258 | 258 | 0.0 |
| Iron deficiency anemia‡ | 113 | 114 | 0.9 |
| Dental conditions | 1,509 | 1,509 | 0.0 |
| Cellulitis | 3,547 | 3,547 | 0.0 |
| 18,158 | 18,289 |
Diagnoses are ordered according to the degree of consensus among three panels reported by Brown et al. (2001). The top five diagnoses in this table were identified by all panels as ACS.
Billings et al. (1993) suggest 4-digit definitions for several conditions. Some 5-digit codes were used to specify congestive heart failure.
Iron deficiency anemia in children up to 5 years of age.
ACS, ambulatory care sensitive; ENT, ear–nose–throat.
Data
Four fiscal years (1998–2001) of inpatient, day surgery, and ambulatory visit data from Manitoba Health were used; emergency room and hospital outpatient visits were not complete. Inpatient and day surgery stays were counted as hospitalizations. The urban population of Manitoba (n of 794,555) was studied to reduce variability in access to primary care, physician practice, and hospitalization patterns across different settings.
Income Quintiles
Following Roos and Mustard (1997), Manitoba urban residents were divided into five equal-sized groups based on average household income in each census enumeration area on the 1996 Canadian Census. The ordering of urban neighborhood income is quite stable, with correlations around 0.85 over 5-year census intervals. The category “urban” was defined as representing areas with “minimum population concentrations of 1,000 and a population density of 400 or more per square kilometer, based on the previous census population counts” (Statistics Canada 1996, p. 229). The maximum number of households in large urban areas is 375. Postal or municipal code was used to link each resident to an enumeration area; this permitted assigning the hospital discharge abstracts and physician visits to income quintiles (Q1 being the lowest).
Exclusions
Overall, 4.1 percent of hospital discharge abstracts and 1.7 percent of physician visits were eliminated because of missing or inappropriate income values for an enumeration area (for example, residence in a personal care home or other institution). Records reflecting non-Manitoba residence, discharge dates or physician visit dates outside the indicated study period, duplicate records, and errors in age values were also removed; each category removed represented less than 1 percent of all records.
Calculating Rates
In analyzing rates, the numerator comprised all indicated events for the three fiscal years, 1998–2000. The denominator consisted of all persons classified as urban and registered with Manitoba Health from April 1, 1998 through March 31, 2001 and those who were born or had died during this period. Rates are expressed on the basis of person years, calculated from the duration of registration for all persons in the denominator. Utilization rates were age-standardized by the direct method. Eleven age groups were generated, beginning with age 0–14, continuing with 10-year groupings to age 74, and using 5-year groupings thereafter. The values of key variables (age, residential postal code) at the time of the first-occurring ACS event were assigned to all subsequent records for that individual.
Results
Rates of ACS Visits and Hospitalizations
Rates of ambulatory visits for the twelve individual ACS conditions varied dramatically from 4,310.28 per 10,000 person years (PY) for severe ENT infections to 8.05 per 10,000 person years for pulmonary and other tuberculosis (Table 2). Hospitalization rates varied much less than those for ambulatory visits, from a high of 27.26 per 10,000 PY for congestive heart failure to a low of 0.59 per 10,000 PY for tuberculosis. Other frequent hospitalizations for bacterial pneumonia, angina, asthma, and pelvic inflammatory disease showed rates of 25.81, 12.77, 10.77, and 7.74 per 10,000 PY, respectively. The least frequent hospitalizations were for epilepsy (1.74 per 10,000 PY) over the entire population and for iron-deficiency anemia (1.67) among children up to 5 years of age.
Table 2.
Rates (per 10,000 PY) of Ambulatory Visits and Hospitalizations (Urban Manitoba, 1998–2000)
| Income Quintile* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Overall | Q1/Q5 | 95% CI‡ | |
| Ambulatory visits | ||||||||
| Asthma | 1,080.86 | 860.00 | 824.66 | 811.79 | 706.20 | 853.16 | 1.53 | 1.51–1.55 |
| Angina | 180.31 | 174.19 | 170.04 | 169.19 | 154.68 | 169.69 | 1.17 | 1.13–1.21 |
| Pelvic inflammatory disease | 29.80 | 25.10 | 20.70 | 15.61 | 11.91 | 20.56 | 2.50 | 2.20–2.88 |
| Gastroenteritis | 467.43 | 351.76 | 319.71 | 303.13 | 255.86 | 336.52 | 1.83 | 1.79–1.87 |
| Congestive heart failure | 342.76 | 314.26 | 294.40 | 251.72 | 216.83 | 289.79 | 1.58 | 1.53–1.63 |
| Severe ENT infections | 5,162.16 | 4,293.46 | 4,155.78 | 4,178.97 | 3,834.42 | 4,310.28 | 1.35 | 1.34–1.35 |
| Epilepsy | 98.06 | 73.01 | 50.44 | 39.25 | 30.32 | 57.05 | 3.23 | 3.03–3.45 |
| Bacterial pneumonia | 283.77 | 262.15 | 239.46 | 237.88 | 212.06 | 246.78 | 1.34 | 1.30–1.37 |
| Pulmonary/other tuberculosis | 10.78 | 9.33 | 7.83 | 7.70 | 4.53 | 8.05 | 2.38 | 2.03–2.86 |
| Iron deficiency anemia† | 78.32 | 44.94 | 44.13 | 36.44 | 32.55 | 48.11 | 2.41 | 1.94–3.04 |
| Dental conditions | 276.34 | 161.96 | 143.92 | 124.38 | 98.28 | 158.86 | 2.81 | 2.71–2.91 |
| Cellulitis | 544.16 | 423.43 | 395.51 | 367.07 | 333.54 | 410.05 | 1.63 | 1.60–1.66 |
| Hospitalizations | ||||||||
| Asthma | 16.53 | 13.51 | 9.91 | 8.27 | 5.70 | 10.77 | 2.90 | 2.50–3.37 |
| Angina | 13.54 | 15.83 | 12.75 | 11.08 | 9.73 | 12.77 | 1.39 | 1.21–1.58 |
| Pelvic inflammatory disease | 10.30 | 7.88 | 7.81 | 6.60 | 6.25 | 7.73 | 1.65 | 1.34–2.02 |
| Gastroenteritis | 6.33 | 8.98 | 5.94 | 4.44 | 3.59 | 5.92 | 1.76 | 1.46–2.17 |
| Congestive heart failure | 33.19 | 31.82 | 27.06 | 21.72 | 19.20 | 27.26 | 1.73 | 1.58–1.92 |
| Severe ENT infections | 6.14 | 6.75 | 3.79 | 3.41 | 2.81 | 4.60 | 2.18 | 1.77–2.74 |
| Epilepsy | 2.68 | 2.47 | 1.46 | 1.29 | 0.90 | 1.74 | 2.98 | 2.17–4.36 |
| Bacterial pneumonia | 35.62 | 32.06 | 21.83 | 22.14 | 16.48 | 25.81 | 2.16 | 1.95–2.40 |
| Pulmonary/other tuberculosis | 1.92 | 0.42 | 0.28 | 0.33 | 0.05 | 0.59 | 41.65 | 14.68–inf§ |
| Iron deficiency anemia† | 3.98 | 1.72 | 1.14 | 0.90 | 0.31 | 1.67 | 12.72 | 3.39–inf§ |
| Dental conditions | 4.52 | 4.23 | 2.86 | 2.59 | 2.27 | 3.24 | 1.99 | 1.57–2.59 |
| Cellulitis | 10.93 | 8.22 | 6.69 | 5.48 | 4.62 | 7.23 | 2.37 | 1.99–2.86 |
Rates were age-adjusted by the direct method. Q1 was the lowest neighborhood income quintile and Q5 the highest. The Q1/Q5 ratio was generated from the rates without rounding off to two decimal places.
Crude rates because of small age range.
Confidence intervals were generated from 1,000 bootstrap replications per ACS condition. Other confidence intervals are available on request.
Because of a 0 in the denominator, there were no upper limits to the bootstrapped confidence interval.
ACS, ambulatory care sensitive; ENT, ear–nose–throat; PY, person years.
Utilization and Poverty
Visit and hospitalization rates for all twelve conditions were higher among residents of the low-income neighborhoods than among their intermediate and high-income counterparts (Table 2). Individuals in the lowest income quintile were much more likely to use ambulatory care for such conditions as epilepsy, dental conditions, pelvic inflammatory disease, iron deficiency anemia, and pulmonary/other tuberculosis. Visit rates were more than two to three times the rates of visits for such conditions compared with high-income populations (as measured by the Q1/Q5 ratio). Asthma, congestive heart failure, cellulitis, and gastroenteritis showed rates of ambulatory care visits by the residents of neighborhoods with the lowest income 50–80 percent higher than visit rates for their counterparts in the highest income neighborhoods. Only angina demonstrated few socioeconomic differences in primary care utilization (Q1/Q5 ratio of 1.17).
Hospitalization rates for tuberculosis and pediatric iron deficiency anemia exhibited the most dramatic socioeconomic gradients, with the rates among individuals in the lowest income neighborhoods (Q1) being 38.40 and 12.84 times the rates of those in the highest income areas (Q5), respectively. Such rates for epilepsy, asthma, and immunization-related diseases among residents of the poorest urban neighborhoods were nearly three times those of the most prosperous areas, and over twice as high for cellulitis, ENT infections, pneumonia, and dental conditions. Similar Q1/Q5 ratios for hospitalizations (from 1.65 to 1.76) were observed for pelvic inflammatory disease, congestive heart failure, and gastroenteritis. Even for angina, the rates of hospitalization were 40 percent higher among residents of the poorer urban areas than those among more affluent residents.
Primary Care and Hospitalizations
The ratio of rates of ACS visits to hospitalizations (V/H ratio) varied considerably across conditions, reflecting both the prevalence of the condition and the nature of the disease. Care for severe ENT infections was primarily ambulatory (937 visits per one hospitalization). The lowest ratio of visits to hospitalizations was for the relatively infrequent pelvic inflammatory disease with one hospitalization for every 2.66 primary care visits (Table 3). Congestive heart failure, pneumonia, and angina were among the most frequent ACS hospitalizations, while in the middle of the ambulatory visit rates. Asthma, the second most frequent reason for ambulatory visits, was the fourth most frequent reason for hospitalization (V/H ratio of 79.22).
Table 3.
Ratio of Ambulatory Visit Rates to Hospitalization Rates for ACS Conditions (Urban Manitoba, 1998–2000)
| Income Quintile* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ratio of Visit Rates to Hospitalization Rates | ||||||||
| Q1 V/H | Q2 V/H | Q3 V/H | Q4 V/H | Q5 V/H | Overall V/H | Q5 V/H versus Q1 V/H | 95% CI‡ | |
| ACS conditions | ||||||||
| Asthma | 65.39 | 63.66 | 83.21 | 98.16 | 123.89 | 79.22 | 1.89 | 1.63–2.20 |
| Angina | 13.32 | 11.00 | 13.34 | 15.27 | 15.9 | 13.29 | 1.19 | 1.04–1.36 |
| Pelvic inflammatory disease | 2.89 | 3.19 | 2.65 | 2.37 | 1.91 | 2.66 | 0.66 | 0.52–0.85 |
| Gastroenteritis | 73.84 | 39.17 | 53.82 | 68.27 | 71.27 | 56.84 | 0.96 | 0.80–1.18 |
| Congestive heart failure | 10.33 | 9.88 | 10.88 | 11.59 | 11.29 | 10.63 | 1.03 | 0.99–1.23 |
| Severe ENT infections | 840.74 | 636.07 | 1,096.5 | 1,225.5 | 1,364.6 | 937.02 | 1.62 | 1.32–2.04 |
| Epilepsy | 36.59 | 29.56 | 34.55 | 30.43 | 33.69 | 32.79 | 0.92 | 0.66–1.35 |
| Bacterial pneumonia | 7.97 | 8.18 | 10.87 | 10.74 | 12.87 | 9.56 | 1.61 | 1.46–1.79 |
| Pulmonary/other tuberculosis | 5.61 | 22.21 | 27.96 | 23.33 | 90.6 | 13.64 | 16.13 | 6.16–inf§ |
| Iron deficiency anemia | 19.68 | 26.13 | 38.71 | 40.49 | 105.00 | 29.34 | 5.35 | 1.45–inf§ |
| Dental conditions | 61.14 | 38.29 | 50.32 | 48.02 | 43.3 | 49.03 | 0.71 | 0.56–0.92 |
| Cellulitis | 49.79 | 51.51 | 59.12 | 66.98 | 72.19 | 56.72 | 1.45 | 1.22–1.75 |
Rates were age-adjusted by the direct method.
Q1 was the lowest neighborhood income quintile and Q5 the highest.
Confidence intervals for the ratio (Q5 V/H versus Q1 V/H) were generated from 1,000 bootstrap replications per ACS condition. Other confidence intervals are available on request.
Because of a 0 in the denominator, there were no upper limits to the bootstrapped confidence interval.
ACS, ambulatory care sensitive; ENT, ear–nose–throat.
Over half of the ACS conditions showed individuals of higher socioeconomic status to have considerably more visits per hospitalization. Most strikingly, one tuberculosis hospitalization was found for each 5.61 visits among patients in the lowest income neighborhoods compared with one admission for 90.60 visits among those in the highest income areas. Similar, although less dramatic, patterns were found for iron deficiency anemia, asthma, bacterial pneumonia, severe ENT infections, pelvic inflammatory disease, and cellulitis (Table 3). Other ACS conditions (angina, congestive heart failure, epilepsy, and gastroenteritis) have similar V/H ratios across socioeconomic groups.
Visits Prior to Hospitalization
The poorest individuals were found to see physicians more frequently than the most affluent. Does ambulatory care prevent subsequent hospitalizations? Table 4 presents the frequency both of all visits prior to hospitalization and of those with the same diagnosis as the index hospitalization. Without considering diagnosis on the physician claim, significantly more visits were recorded for individuals in Q1 than in Q5 for six of the nine ACS conditions studied. Eight of the nine conditions averaged more visits having the same diagnosis as the index hospitalization for individuals in Q1; given the small numbers, only two of these differences proved statistically significant.
Table 4.
Ambulatory Visits in Year before Index Hospitalization for Highest and Lowest Income Quintiles (1999–2000 Hospitalizations)
| Visits in Year before Index Hospitalization | ||||||
|---|---|---|---|---|---|---|
| Mean | Mean | |||||
| ACS Diagnosis at Hospitalization (Number of Hospitalizations) | Any Diagnosis | Q1/Q5 Ratio | p-Value for Q1/Q5 Ratio* | Same Diagnosis as Hospitalization | Q1/Q5 Ratio | p-Value for Q1/Q5 Ratio* |
| Asthma | ||||||
| Q1 (308) | 12.89 | 1.20 | .020 | 2.70 | 1.04 | .721 |
| Q5 (112) | 10.77 | 2.59 | ||||
| Angina | ||||||
| Q1 (424) | 13.52 | 1.05 | .404 | 1.15 | 1.19 | .151 |
| Q5 (178) | 12.89 | 0.97 | ||||
| Pelvic inflammatory disease | ||||||
| Q1 (149) | 13.16 | 1.36 | .001 | 0.28 | 2.00 | .068 |
| Q5 (91) | 9.65 | 0.14 | ||||
| Gastroenteritis | ||||||
| Q1 (156) | 13.39 | 1.29 | .015 | 0.69 | 1.13 | .554 |
| Q5 (80) | 10.39 | 0.61 | ||||
| Congestive heart failure | ||||||
| Q1 (930) | 13.84 | 1.01 | .879 | 1.73 | 1.47 | .001 |
| Q5 (237) | 13.74 | 1.18 | ||||
| Severe ENT infections | ||||||
| Q1 (173) | 13.47 | 1.33 | .003 | 2.56 | 1.04 | .822 |
| Q5 (79) | 10.14 | 2.47 | ||||
| Bacterial pneumonia | ||||||
| Q1 (897) | 12.48 | 1.08 | .142 | 0.73 | 1.30 | .007 |
| Q5 (277) | 11.56 | 0.56 | ||||
| Dental conditions | ||||||
| Q1 (133) | 10.12 | 1.30 | .042 | 0.54 | 1.23 | .429 |
| Q5 (61) | 7.79 | 0.44 | ||||
| Cellulitis | ||||||
| Q1 (302) | 12.36 | 1.20 | .032 | 1.22 | 0.98 | .917 |
| Q5 (109) | 10.30 | 1.24 | ||||
Individuals resident in urban Manitoba and hospitalized in fiscal years 1999 or 2000, but having no hospitalizations in the previous year, were used in this analysis. The nine conditions included in Table 4 had 60 or more index hospitalizations in Q5, the quintiles with the fewest hospitalizations. The other three conditions had 18 (epilepsy) or fewer Q5 hospitalizations.
Q1 was the lowest neighborhood income quintile and Q5 the highest. Q1/Q5 ratios were tested using a negative binomial regression in the SAS GENMOD procedure (Pedan 2001).
ACS, ambulatory care sensitive; ENT, ear–nose–throat.
Although not shown in a table, ambulatory visits in the year after the index hospitalization showed few regular patterns; for six out of the nine conditions the most affluent had slightly more visits than the least affluent. Individuals with complicated histories (those with an index hospitalization and one or more hospitalizations in the previous year) were too few for detailed analyses. Finally, analysis of 1999 ambulatory visits for the large number of Manitobans having no hospitalizations in 1998–2000 showed individuals in the lowest income quintile (n=100,471) to be somewhat more likely to visit the doctor (means of 4.38 ambulatory visits overall and 0.66 visits for ACS conditions) than those in the other quintiles.
Hospitalizations Controlling for Ambulatory Visits
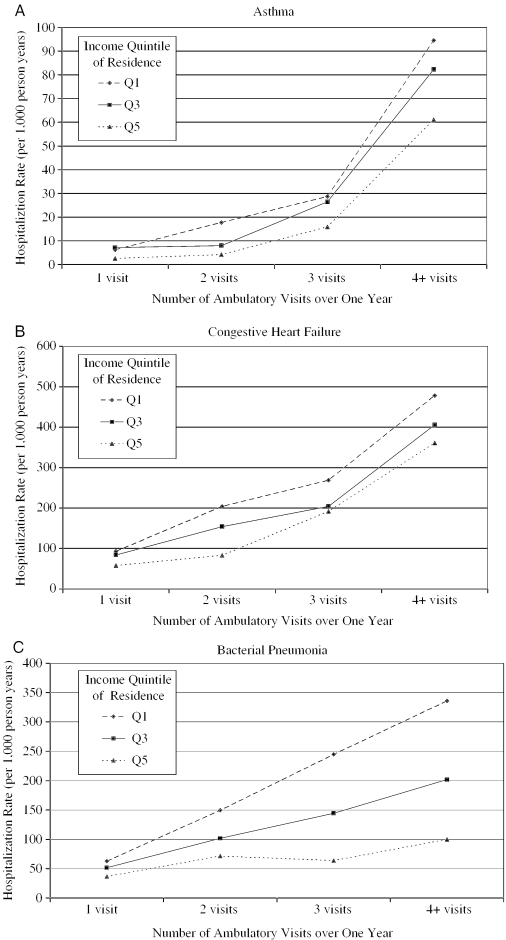
A regression predicting hospitalization rate and controlling for the number of ambulatory visits for each condition in Table 4 tallied hospital stays and visits in each year (1998–2000) separately; thus, an individual with hospitalizations in each year would be counted three times. The six ACS conditions with the most hospitalizations (asthma, angina, congestive heart failure, severe ENT infections, bacterial pneumonia, and cellulitis) showed hospitalization rates for residents of the lowest income neighborhoods to be significantly geater than those of their counterparts from the highest income neighborhoods with the same number of visits. Lower income individuals with the most primary care visits tended to have the highest hospitalization rates for these conditions (see the three examples in Figure 1). The other conditions (pelvic inflammatory disease, gastroenteritis, and dental conditions) had relatively few hospitalizations for comparison.
Figure 1.
Hospitalization Rate by Frequency of Ambulatory Visits and Income Quintile of Residence for Three ACS Conditions (Urban Manitoba, 2000)
Sensitivity Testing
Additional hypotheses were explored. Residents of low-income neighborhoods might have been more likely to have several visits within a single episode of illness. Rather than counting each visit separately, physician visits 14 or fewer days apart were considered as part of the same episode. Multiple visits per episode were greatest for bacterial pneumonia (21 percent reduction in overall rate using episodes) and for cellulitis (17 percent reduction). Q1/Q5 ratios were altered only minimally by this episode-based approach.
A given individual might have appeared more than once in counting ambulatory visits and hospitalizations, affecting the Q1/Q5 ratio. This possibility was tested in two different ways:
For any given ACS diagnosis, a particular individual was allowed to appear only once in the 3-year period.
A particular individual was allowed to appear only once in the 3-year period. The first ACS diagnosis found was noted, then the next individual considered.
Counting in terms of individuals rather than visits markedly lowered some of the rates, but sensitivity testing changed the Q1/Q5 ratios relatively little.
Discussion
ACS conditions are of considerable research interest; typing “ACS conditions” into the PubMed search facility generated a listing of 17 papers in 2003. No single study has been able to deal with all possible types of care which might affect hospitalization rates. Thus, this paper has not included emergency department and outpatient visits; these visits are not part of the standard Manitoba hospital data sets. A relatively small number of such visits (based in the Winnipeg teaching hospitals) are captured as ambulatory visits. An earlier, more labor-intensive analysis using 1 year of Winnipeg data found 4.9 percent of ambulatory care to be provided in emergency departments. Residents of lower income neighborhoods were disproportionately likely to receive such care (Mustard et al. 1998).
Characterizing socioeconomic status using mean neighborhood income seems generally appropriate in studying ACS conditions; physician supply and hospital bed supply are typically measured to assess residents' access to care in a given geographic area. Income data from the 1996 National Population Health Survey were available at the household level for a relatively small urban Manitoba sample (weighted n=4725). First Nations (aboriginal) individuals were not included; they tend to be among the poorest members of the population (and thus heavily represented in Q1). The three conditions having an adequate number of visits in the 1994–1998 period (severe ENT infections, asthmas, and cellulitis) showed Q1/Q5 ratios between 1.19 and 1.27.
Canadian Medicare's “natural experiment” was designed to provide equality in access to all medically necessary hospital, diagnostic, and physician services. The American literature implies that low-income individuals will have inadequate, infrequent primary care and therefore higher hospitalization rates. Relatively high visit rates should be associated with lower rates of hospitalizations. This was not the case in urban Manitoba.
With no formal barriers to primary care, both physician visits and hospital admissions varied substantially across areas of differing socioeconomic status. Residents of the poorest urban neighborhoods not only utilized primary care significantly more than residents of comparatively affluent areas, but were also more often hospitalized for each ACS condition. Ambulatory care's capability to prevent or reduce hospitalization appears to vary across income groups (Figure 1).
Medical record reviews have shown essentially no differences among socioeconomic groups in the acuity levels of hospitalized patients in Winnipeg (Strumwasser, Paranjpe, and Ronis 1990; DeCoster et al. 1999; Bruce et al. 2002). Lower income individuals do not appear to have been differentially hospitalized because of social circumstances (e.g., homelessness, alcoholism), person-centered factors (e.g., inability to follow a prescribed outpatient treatment regimen), or behavioral problems (e.g., lack of compliance) while being in better physical health than their higher income counterparts.
The Winnipeg poor have more frequent contact with general practitioners; socioeconomic groups differ little in contact with specialists (Roos et al. 1999). Winnipeg residents of lower income neighborhoods have been shown to have a higher need for health care (estimated from an index combining age, gender, socioeconomic status, and health status) (Roos et al. 1999; 2004). The rate of use among lower income groups appears to be “needs-driven and hence not easily managed away” (Roos, Burchill, and Carriere 2003, p. 9).
While socioeconomic status and rates of ACS hospitalizations have been associated in many North American studies, factors other than access doubtlessly contribute to the differences (Billings et al. 1993; Parchman and Culler 1994; Blustein, Hanson, and Shea 1998). As noted outside Manitoba, higher rates of visits and hospitalizations may be because of the poor's higher disease prevalence, increased disease severity, and multiple comorbidities (Weissman, Gatsonis, and Epstein 1992; Billings et al. 1993; Anderson et al. 1996; Blustein, Hanson, and Shea 1998). Interestingly, in Spain (with universal financial access to health care), no association between socioeconomic status, primary care, and ACS hospitalizations existed either within adult or pediatric populations (Casanova and Starfield 1995; Casanova, Colomer, and Starfield 1996).
Would different kinds of care—perhaps more effectively integrating visits with other services— reduce overall rates of hospitalization among the poor? A recent Manitoba study of the elderly found continuity of care to reduce both hospitalizations for all conditions and hospitalizations for ambulatory sensitive conditions (Menec et al. 2004). Manitoba's population-based programs directed toward childhood immunizations, screening mammography, and Papanicolaou testing are also worth noting (Gupta et al. 2003). Are particular barriers to care (time constraints, costs of transportation, lack of information, and so on) significantly affecting primary care and eventual hospitalization rates?
Work might focus on the ACS conditions for which costs can be contained without damaging outcomes. Boston and New Haven, two cities with leading teaching hospitals, differ dramatically in the rates of hospital utilization and associated expenditures for heart failure and pneumonia (Wennberg, Freeman, and Culp 1987). Such variation vis-à-vis congestive heart failure has been found among Medicare patients of 77 major American hospitals (Wennberg et al. 2004). Attention to the prevention, treatment, and out-of-hospital care of these two conditions might prove particularly fruitful, given the high hospitalization rates and observed socioeconomic gradients in utilization.
Doing “more of the same,” which in Canada means increasing physician supply to deal with apparent shortages, is unlikely to change the socioeconomic gradient accompanying visits and hospitalizations. American studies suggesting that having more physicians will decrease ACS hospitalizations among the poor may be predicated on visit rates considerably lower than those found in urban Manitoba. Indeed, problems with primary care and health system performance have recently been reported across five English-speaking countries (Schoen et al. 2004). Regardless of the health care system, markedly reducing ACS hospitalizations is likely to prove difficult.
Acknowledgments
This research has been funded by the Canadian Population Health Initiative (CPHI), a program of the Canadian Institute for Health Information (CIHI), by the Canadian Institutes for Health Research, and the Manitoba Centre for Health Policy (Manitoba Health Project Number 2000/2001-23). The results and conclusions are those of the authors and no official endorsement by Manitoba Health was intended or should be inferred. The authors thank Jo-Anne Baribeau, Carole Ouelette, and Phyllis Jivan for manuscript preparation.
References
- Anderson RT, Sorlie PD, Backlund E, Johnson NJ, Kaplan GA. “Mortality Effects of Community Socioeconomic Status.”. Epidemiology. 1996;8(1):42–7. doi: 10.1097/00001648-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Backus L, Moron M, Baccetti P, Baker LC, Bindman AB. “Effect of Managed Care on Preventable Hospitalization Rates in California.”. Medical Care. 2002;40(4):315–24. doi: 10.1097/00005650-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Basu J, Friedman B, Bursten H. “Primary Care, HMO Enrolment and Hospitalization for Ambulatory Care Sensitive Conditions: A New Approach.”. Medical Care. 2002;40(12):1260–9. doi: 10.1097/00005650-200212000-00013. [DOI] [PubMed] [Google Scholar]
- Billings J, Anderson GM, Newman LS. “Recent Findings on Preventable Hospitalizations.”. Health Affairs (Millwood) 1996;15(3):239–49. doi: 10.1377/hlthaff.15.3.239. [DOI] [PubMed] [Google Scholar]
- Billings J, Zeitel L, Lukomnik J, Carey TS, Blank AE, Newman L. “Datawatch: Impact of Socioeconomic Status on Hospital Use in New York City.”. Health Affairs (Millwood) 1993;12(1):162–73. doi: 10.1377/hlthaff.12.1.162. [DOI] [PubMed] [Google Scholar]
- Bindman AB, Grumbach K, Osmond D, Komaromy M, Vranizan K, Lurie N, Billings J, Stewart A. “Preventable Hospitalizations and Access to Health Care.”. Journal of the American Medical Association. 1995;274(4):305–11. [PubMed] [Google Scholar]
- Blustein J, Hanson K, Shea S. “Preventable Hospitalizations and Socioeconomic Status.”. Health Affairs (Millwood) 1998;17(2):177–89. doi: 10.1377/hlthaff.17.2.177. [DOI] [PubMed] [Google Scholar]
- Brown AD, Goldacre MJ, Hicks N, Rourke JT, McMurtry RY, Brown JD, Anderson GM. “Hospitalization for Ambulatory Care-Sensitive Conditions: A Method for Comparative Access and Quality Studies Using Routinely Collected Statistics.”. Canadian Journal of Public Health. 2001;92(2):155–9. doi: 10.1007/BF03404951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce S, DeCoster C, Trumble Waddell J, Burchill C. “Patients Hospitalized for Medical Conditions in Winnipeg, Canada: Appropriateness and Level of Care.”. Healthcare Management Forum. 2002;15(4, suppl):53–7. doi: 10.1016/s0840-4704(10)60183-4. [DOI] [PubMed] [Google Scholar]
- Carstairs V, Morris R. “Deprivation: Explaining Differences in Mortality between Scotland and England and Wales.”. British Medical Journal. 1989;299(6704):886–9. doi: 10.1136/bmj.299.6704.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova C, Colomer C, Starfield BH. “Pediatric Hospitalization Due to Ambulatory Care-Sensitive Conditions in Valencia (Spain).”. International Journal for Quality in Health Care. 1996;8(1):51–9. doi: 10.1093/intqhc/8.1.51. [DOI] [PubMed] [Google Scholar]
- Casanova C, Starfield BH. “Hospitalizations of Children and Access to Primary Care: A Cross-National Comparison.”. International Journal of Health Services. 1995;25(2):283–94. doi: 10.2190/PCF7-ALX9-6CN3-7X9G. [DOI] [PubMed] [Google Scholar]
- DeCoster C, Peterson S, Carriere KC, Kasian P. “Assessing the Extent to Which Hospitals Are Used for Acute Care Purposes.”. Medical Care. 1999;37(6, suppl):JS151–66. doi: 10.1097/00005650-199906001-00014. [DOI] [PubMed] [Google Scholar]
- Epstein AJ. “The Role of Public Clinics in Preventable Hospitalizations among Vulnerable Populations.”. Health Services Research. 2001;36(2):405–20. [PMC free article] [PubMed] [Google Scholar]
- Falik M, Needleman J, Wells BL, Korb J. “Ambulatory Care Sensitive Hospitalizations and Emergency Visits: Experiences of Medicaid Patients Using Federally Qualified Health Centers.”. Medical Care. 2001;39(6):551–61. doi: 10.1097/00005650-200106000-00004. [DOI] [PubMed] [Google Scholar]
- Friedman B, Basu J. “Health Insurance, Primary Care and Preventable Hospitalization of Children in a Large State.”. American Journal of Managed Care. 2001;7(5):473–81. [PubMed] [Google Scholar]
- Gadomski A, Jenkins P, Nichols M. “Impact of a Medicaid Primary Care Provider and Preventive Care on Pediatric Hospitalization.”. Pediatrics. 1998;101(3):E1. doi: 10.1542/peds.101.3.e1. [DOI] [PubMed] [Google Scholar]
- Gill JM. “Can Hospitalizations Be Avoided by Having a Regular Source of Care?”. Family Medicine. 1997;29(3):166–71. [PubMed] [Google Scholar]
- Gill JM, Mainous AG. “The Role of Provider Continuity in Preventing Hospitalizations.”. Archives of Family Medicine. 1998;7(4):352–7. doi: 10.1001/archfami.7.4.352. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Gravelle H, Roland M. “Measuring Quality of Care with Routine Data: Avoiding Confusion between Performance Indicators and Health Outcomes.”. British Medical Journal. 1999;319(7202):94–8. doi: 10.1136/bmj.319.7202.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Roos LL, Walld R, Traverse D, Dahl M. “Delivering Equitable Care: Comparing Preventive Services in Manitoba, Canada.”. American Journal of Public Health. 2003;93(12):2086–92. doi: 10.2105/ajph.93.12.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan M, Kaplan GA, Camacho TC. “Poverty and Health: Prospective Evidence from the Alameda County Study.”. American Journal of Epidemiology. 1987;152(6):989–98. doi: 10.1093/oxfordjournals.aje.a114637. [DOI] [PubMed] [Google Scholar]
- Huzel L, Roos LL, Anthonisen NR, Manfreda J. “Diagnosing Asthma: The Fit between Survey and Administrative Database.”. Canadian Respiratory Journal. 2003;9(6):407–12. doi: 10.1155/2002/921497. [DOI] [PubMed] [Google Scholar]
- Krakauer H, Jacoby I, Millman M, Lukomnik JE. “Physician Impact on Hospital Admission and on Mortality Rates in the Medicare Population.”. Health Services Research. 1996;31(2):191–211. [PMC free article] [PubMed] [Google Scholar]
- Maclean's “The Top 20 Health Regions, and How They Ranked in Each Category.”. 2003;116(24):24–6. [Google Scholar]
- Manga P, Broyles RW, Angus DE. “The Determinants of Hospital Utilization under a Universal Public Insurance Program in Canada.”. Medical Care. 1987;25(7):658–70. doi: 10.1097/00005650-198707000-00009. [DOI] [PubMed] [Google Scholar]
- Menec V, Sirski M, Attawar D, Katz A. “What Is the Potential of Patient Registration in Canada? Looking at the Right Measure of “Regular Doctor” Is Important.”. 2004 Unpublished. [Google Scholar]
- Mustard CA, Kozyrskyj A, Barer ML, Sheps S. “Emergency Department Use as a Component of Total Ambulatory Care: A Population Perspective.”. Canadian Medical Association Journal. 1998;158(1):49–55. [PMC free article] [PubMed] [Google Scholar]
- Pappas G, Queen S, Hadden WC, Fisher GF. “The Increasing Disparity in Mortality between Socioeconomic Groups in the United States, 1960 and 1986.”. New England Journal of Medicine. 1993;329(2):103–9. doi: 10.1056/NEJM199307083290207. [DOI] [PubMed] [Google Scholar]
- Parchman ML, Culler SD. “Primary Care Physicians and Avoidable Hospitalizations.”. Journal of Family Practice. 1994;39(2):123–8. [PubMed] [Google Scholar]
- Parchman ML, Culler SD. “Preventable Hospitalization in Primary Care Shortage Areas. An Analysis of Vulnerable Medicare Beneficiaries.”. Archives of Family Medicine. 1999;8(6):487–97. doi: 10.1001/archfami.8.6.487. [DOI] [PubMed] [Google Scholar]
- Parker JD, Schoendorf KC. “Variation in Hospital Discharges for Ambulatory Care-Sensitive Conditions among Children.”. Pediatrics. 2000;106(4, suppl):942–8. [PubMed] [Google Scholar]
- Pedan A. “Analysis of Count Data Using the SAS® System.”. Proceedings of the Twenty-Sixth Annual SAS® Users Group International Conference, Longbeach, CA, April 22–25, 2001. 2001 [Google Scholar]
- Reid FDA, Cook DG, Majeed A. “Explaining Variation in Hospital Admission Rates between General Practices: Cross Sectional Study.”. British Medical Journal. 1999;319(7202):98–103. doi: 10.1136/bmj.319.7202.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts TC, Randolph R, Howard HA, Pathman D, Carey TS. “Hospitalization Rates as Indicators of Access to Primary Care.”. Health and Place. 2001;7(1):27–38. doi: 10.1016/s1353-8292(00)00035-6. [DOI] [PubMed] [Google Scholar]
- Robinson JR, Young TK, Roos LL, Gelskey DE. “Estimating the Burden of Disease: Comparing Administrative Data and Self-Reports.”. Medical Care. 1997;35(9):932–47. doi: 10.1097/00005650-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Roos NP, Burchill C, Carriere KC. “Who Are the High Hospital Users? A Canadian Case Study.”. Journal of Health Services Research and Policy. 2003;8(1):5–10. doi: 10.1177/135581960300800104. [DOI] [PubMed] [Google Scholar]
- Roos NP, Forget E, Walld R, MacWilliam L. “Does Universal Comprehensive Insurance Encourage Unnecessary Use? Evidence from Manitoba Says ‘No’.”. Canadian Medical Association Journal. 2004;170(2):209–14. [PMC free article] [PubMed] [Google Scholar]
- Roos NP, Fransoo R, Bogdanovic B, Carriere KC, Frohlich N, Friesen D, Patton D, Wall R. “Needs-Based Planning for Generalist Physicians.”. Medical Care. 1999;37(6, suppl):JS229–53. doi: 10.1097/00005650-199906001-00017. [DOI] [PubMed] [Google Scholar]
- Roos NP, Fransoo R, Bogdanovic B, Friesen D, MacWilliam L. “Issues in Planning for Specialist Physicians.”. Medical Care. 1999;37(6, suppl):JS229–53. doi: 10.1097/00005650-199906001-00018. [DOI] [PubMed] [Google Scholar]
- Roos NP, Mustard CA. “Variation in Health and Health Care Use by Socioeconomic Status in Winnipeg, Canada: Does the System Work Well? Yes and No.”. Milbank Quarterly. 1997;75(1):89–111. doi: 10.1111/1468-0009.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos LL, Mustard CA, Nicol JP, McLerran DF, Malenka DJ, Young TK, Cohen MM. “Registries and Administrative Data: Organization and Accuracy.”. Medical Care. 1993;31(3):201–12. doi: 10.1097/00005650-199303000-00002. [DOI] [PubMed] [Google Scholar]
- Roos LL, Nicol JP. “A Research Registry: Uses, Development, and Accuracy.”. Journal of Clinical Epidemiology. 1999;52(1):39–47. doi: 10.1016/s0895-4356(98)00126-7. [DOI] [PubMed] [Google Scholar]
- Rutstein DD, Berenberg W, Chalmers TC, Child CC, Fishman AP, Perrin E. “Measuring the Quality of Medical Care: A Clinical Method.”. New England Journal of Medicine. 1976;294(11):582–8. doi: 10.1056/NEJM197603112941104. [DOI] [PubMed] [Google Scholar]
- Schoen C, Osborn R, Huynh PT, Doly M, Davis K, Zapert K, Peugh J. “Primary Care and Health System Performance: Adults' Experiences in Five Countries.”. Health Affairs (Millwood) 2004;23(Web exclusive supplement):W96–114. doi: 10.1377/hlthaff.w4.487. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Zielinski T. “The Meaning of Ambulatory Care Sensitive Admissions: Urban and Rural Perspectives.”. Journal of Rural Health. 1997;13(4):276–84. doi: 10.1111/j.1748-0361.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Shanahan M, Gousseau C. “Using the POPULIS Framework for Interprovincial Comparisons of Expenditures on Health Care.”. Medical Care. 1999;37(6, suppl):JS83–100. doi: 10.1097/00005650-199906001-00010. [DOI] [PubMed] [Google Scholar]
- Shi L, Samuels ME, Pease M, Bailey WP, Corley EH. “Patient Characteristics Associated with Hospitalizations for Ambulatory Care Sensitive Conditions in South Carolina.”. Southern Medical Journal. 1999;92(10):989–98. doi: 10.1097/00007611-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Shi L, Starfield BH, Politzer R, Regan J. “Primary Care, Self-Rated Health, and Reductions in Social Disparities in Health.”. Health Services Research. 2002;37(3):529–50. doi: 10.1111/1475-6773.t01-1-00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starfield BH. Primary Care: Balancing Health Needs, Services, and Technology. New York: Oxford University Press; 1998. [Google Scholar]
- Statistics Canada . 1996 Census Dictionary. Final Edition Reference. Ottawa, ON: Statistics Canada; 1996. [Google Scholar]
- Strumwasser I, Paranjpe NV, Ronis DL. “Reliability and Validity of Utilization Review Criteria: Appropriateness Evaluation Protocol, Standardized Medreview Instrument, and Intensity-Severity-Discharge Criteria.”. Medical Care. 1990;28(2):95–111. doi: 10.1097/00005650-199002000-00001. [DOI] [PubMed] [Google Scholar]
- Tu JV, Austin PC, Walld R, Roos LL, Agras J, McDonald KM. “Development and Validation of the Ontario Acute Myocardial Infarction Mortality Prediction Rules.”. Journal of the American College of Cardiology. 2001;37(4):992–7. doi: 10.1016/s0735-1097(01)01109-3. [DOI] [PubMed] [Google Scholar]
- Watson D, Katz A, Reid RJ, Bogdanovic B, Roos NP, Heppner P. “Family Physician Workloads and Access to Care, 1991 to 2001.”. Canadian Medical Association Journal. 2004;171(4):339–42. doi: 10.1503/cmaj.1031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman JS, Gatsonis CA, Epstein AM. “Rates of Avoidable Hospitalization by Insurance Status in Massachusetts and Maryland.”. Journal of the American Medical Association. 1992;268(17):2388–94. [PubMed] [Google Scholar]
- Wennberg JE, Fisher ES, Stukel TA, Sharp SM. “Use of Medicare Claims Data to Monitor Provider-Specific Performance among Patients with Severe Chronic Illness.”. Health Affairs (Millwood) 2004;23(Variation supplement):VAR5–18. doi: 10.1377/hlthaff.var.5. [DOI] [PubMed] [Google Scholar]
- Wennberg JE, Freeman JL, Culp WJ. “Are Hospital Services Rationed in New Haven or Over-Utilised in Boston?”. Lancet. 1987;1(8543):1185–9. doi: 10.1016/s0140-6736(87)92152-0. [DOI] [PubMed] [Google Scholar]