Abstract
Objective
To determine how the addition of generalist care managers and collaborative information technology to an ambulatory team affects the care of patients with diabetes.
Study Setting
Multiple ambulatory clinics within Intermountain Health Care (IHC), a large integrated delivery network.
Study Design
A retrospective cohort study comparing diabetic patients treated by generalist care managers with matched controls was completed. Exposure patients had one or more contacts with a care manager; controls were matched on utilization, demographics, testing, and baseline glucose control. Using role-specific information technology to support their efforts, care managers assessed patients' readiness for change, followed guidelines, and educated and motivated patients.
Data Collection
Patient data collected as part of an electronic patient record were combined with care manager-created databases to assess timely testing of glycosylated hemoglobin (HbA1c) and low-density lipoprotein (LDL) levels and changes in LDL and HbA1c levels.
Principal Findings
In a multivariable model, the odds of being overdue for testing for HbA1c decreased by 21 percent in the exposure group (n=1,185) versus the control group (n=4,740). The odds of being tested when overdue for HbA1c or LDL increased by 49 and 26 percent, respectively, and the odds of HbA1c <7.0 percent also increased by 19 percent in the exposure group. The average HbA1c levels decreased more in the exposure group than in the controls. The effect on LDL was not significant.
Conclusions
Generalist care managers using computer-supported diabetes management helped increase adherence to guidelines for testing and control of HbA1c levels, leading to improved health status of patients with diabetes.
Keywords: Patient care management, chronic illness, diabetes mellitus, medical informatics
Diabetes mellitus and its complications comprise one of the most expensive categories of chronic disease in the United States, contributing to at least 213,062 deaths in 2000 and $132 billion in costs in 2002. There is significant potential for improvement when appropriate medical care is provided (American Diabetes Association 2003). The highest potential for improvement comes from the capability to prevent the deadly complications of this disease; careful control of blood pressure, control of glycoslylated hemoglobin (HbA1c) and low density lipoprotein (LDL) level, and administration of appropriate medications (including ACE inhibitors, statins, aspirin, and β-blockers) have been shown to slow, and, in many cases, stop the progression of microvascular disease in people with diabetes (Matthews 1999; Nicollerat 2000).
However, the United States' success in achieving tight control of HbA1c levels and appropriate medication administration in these patients has been limited at best (Toth et al. 2003). Despite implementation efforts at over half of the major health systems in the United States, compliance with management guidelines remains low. In a recent study, only 10.4 percent of patients met HbA1c, blood pressure, and LDL goals and only 13 percent met medication standards after guideline implementation (Toth et al. 2003). Clearly, people with diabetes and those caring for them have difficulty adhering to these guidelines.
Guideline compliance can be increased through improved processes of care or disease management. One heavily studied approach involves an additional team member called a care manager who facilitates changes in clinic processes and patient knowledge and behaviors. Several studies have shown that interventions involving care managers can help patients and other care providers improve the quality of care and outcomes in diabetes (Pan et al. 1997; Tuomilehto et al. 2001; Knowler et al. 2002; New et al. 2003; Taylor et al. 2003; The California Medi-Cal Type 2 Diabetes Study Group 2004) and other diseases (Bond et al. 1988; Allen 1994; McGrew et al. 1995; Crystal, Lo Sasso, and Sambamoorthi 1999; Naylor et al. 1999; Bull, Hansen, and Gross 2000).
These studies focus almost entirely on specific diseases or conditions and are mostly efficacy-style trials of disease management, or “a coordinated system … for a specific chronic illness” (AHM 2001), as opposed to a more broadly defined vision of care management, e.g., “a collaborative process of assessment, planning, facilitation and advocacy” (CMSA 2003). Disease management programs frequently create specialized clinics, which represent a highly focused setting where providers have in-depth training in a single disease whether they are specialists or trained primary care providers. In these specialized clinics or disease-specific clinic sessions, processes can be more easily controlled than in a general clinic where a multitude of acute and chronic illnesses are treated. In contrast, we studied the impact when care management was used to help treat a patient population with multiple chronic and acute illnesses and needs; care management was characterized by generalist care managers and specially developed information technology to support collaboration during the general primary care workflow. As persons with multiple chronic illnesses are known to suffer higher rates of complications and mortality, the generalist approach has the theoretical advantage of treating the whole person with one or more chronic disease rather than focusing on one disease (Rothman and Wagner 2003; Norris and Olson 2004). In practice, however, this approach is challenging. One study in which a broader patient population was treated demonstrated increases in adherence to guidelines and patient satisfaction, but did not find reductions in HbA1c in the patients with diabetes (Wagner et al. 2001). In other studies, it was found that care management programs increased the use of resources (D'Ercole et al. 1997). This finding is of special concern for overworked primary care clinics that frequently only receive a fraction of the savings that result from improving the health of their patients (Casalino 2003). Thus, it is important that implementation of such programs be carried out carefully, especially in real-world settings with diverse patient populations and limited resources.
Given these concerns, we hypothesized that specialized care could be generalized into a multidisease care management model. To do so, we implemented Wagner's Chronic Care Model (CCM) (Bodenheimer, Wagner, and Grumbach 2002a, Bodenheimer, Wagner, and Grumbach 2002b) in a way different from many of the previous studies. At Intermountain Health Care (IHC) in Salt Lake City, we adopted a team approach (with the patient at the center) to encourage patient self-management and improved connection to community resources, and created core health care organization goals as part of a model to improve the care of chronic illness; these interventions are all standard parts of Wagner's CCM. Two major capabilities from the CCM were implemented to address the need to integrate the care management program into primary care workflow. Care managers were placed in the clinics and trained to facilitate team collaboration and general patient education, a more central role than advocated for in the CCM. In addition, existing information technology was leveraged and new applications were created to enable the primary care teams (including the care manager) to adopt many different guidelines at once. We hypothesized that the use of computerized alerts, summarized patient information, and electronic communication would allow an integrated approach to successfully meet the needs of patients with chronic illnesses without the need for specialized clinics for each disease or patient population. This information technology would aid the care manager, who would also work with the patient to assess their readiness to change and create a specific care plan based on any of the patient's particular chronic illness(es) (Spencer et al. 2002; Duran 2003). The generalist care manager, with support of the information system, can then act as a catalyst in each clinic, creating and then helping enact the care plan with the patient.
We also hypothesized that the care of patients with diabetes would especially improve in our multidisease, collaborative care management model as patients with diabetes have a very high rate of co-occurring conditions that can worsen disease outcomes (Rothman and Wagner 2003). Improvement was measured by assessing changes in processes (such as timely testing for disease markers), and outcomes (changes in the levels of these markers indicating control) as defined by current diabetes guidelines (AACE 2000; ADA 2003; Goldstein et al. 2004; Haffner 2004). The demands and benefits of successful multidisease care management programs that can be implemented in the workflow of primary care clinics need to be defined, especially in diseases where they have the most impact. When one attempts to integrate multidisease care management systems into primary care, one may dilute the benefit, that might accrue to patients who are treated in a specialized setting. Integrated care management systems offer the promise to improve quality in a cost-effective manner. By examining the changes in adherence and outcomes in a generalist implementation within diabetes, we hoped to determine whether positive effects can be substantial when examining the impact on a single disease.
Methods
Health Care Organization
IHC is an integrated delivery network consisting of 20 hospitals and more than 1,200 employed and affiliated physicians in Utah and Idaho. The 450 physicians employed by IHC work in one of 92 clinics, and provide for more than three million outpatient visits each year. For this study, IHC augmented the services of selected primary care providers in seven IHC-owned ambulatory clinics by installing care managers on-site and adding specific information technology. On average, each care manager serves as a resource to 6 to 10 primary care physicians and has a panel of 350 to 500 active patients. Care managers are trained professionals; all seven in this study were either registered nurses or social workers. Four similar reference clinics without care managers, but serving a similar patient population, were used to generate a control population. The control clinics were matched on provider type and experience, staffing, and variety of patient conditions. This study was approved by the local Institutional Review Board as meeting the criteria for ethical human subjects research.
Care Delivery Design and Information Technology
Exposure to the intervention was defined as referral to, and at least one visit with, a generalist care manager who adhered to the care management delivery model, and used the advanced information technology applications. Patients were referred to care managers by primary care physicians at the physicians' discretion; the providers were encouraged to refer when the patient or their family needed education, cognitive, and community/social support to deal with illness. Referral was not based on specific criteria as perceived need was felt to be the most inclusive indicator for the effectiveness study. For instance, only a subset of patients with diabetes are sent; reasons for referral range from out-of-control glucose levels to those with complicating conditions (e.g., nonsupportive home environment).
Once a patient is referred, the care managers offer all pertinent services to the patients and their families, regardless of diagnosis. The general care management program of which they are a part has several components. With referral, a care management team is activated with the care manager acting to provide continuity, regular follow-up, and collaboration. The care managers meet initially with patients, providing education for disease-specific and general problem-solving skills, motivation to encourage self-management, and development of care specific plans, which frequently include several diseases. The self-management component is facilitated by a care manager assessing the patients' readiness to change to self-managing behaviors, providing ongoing motivation/feedback, and encouraging patient independence, usually through a series of phone calls to patients. The care managers put the patients and their caregivers in touch with community resources and advocate for the patient within and beyond the immediate care team both in person at case conferences and via the phone.
Substantial informational technology access was given to all team members, whether care managers were involved or not. The information technology provided Access to patient information, provided reminders and structures for Best practices, and enabled virtual Communication. For Access, team members have access to a longitudinal electronic health record (EHR). The EHR includes the option to use a summarized patient worksheet for chronic diseases. The patient-specific electronic summary gives an overview based upon the chronic conditions of the patient. Team members have access to computer alerts (such as drug–drug interactions) and chronic disease reminders on the summarized form to help support Best practices. The logic in guidelines is extracted in order to generate reminders automatically either via active alerts or on the patient summary as passive prompts.
For the exposure group only, care managers have an additional alerting system that reminds them of specific process-based tasks to perform, such as calling a set of people with diabetes when their tests are overdue. The care management system also has a specific interface that allows care managers to store and retrieve information specific to their workflow. For example, a phone contact for depression has coded elements that easily link to standardized mental health forms.
Finally, all team members have access to an electronic Communication system that allows providers to exchange electronic messages that are ultimately attached to a specific patient's chart. As both control and intervention patients cared for in this study have providers who have the option to use the clinical information system, the information technology portion of the intervention is restricted to the specific care management components and the activities of the care managers themselves.
The diabetes-specific component of this intervention is two-fold. First, all team members are trained in several chronic disease guidelines further developed by IHC from national sources, including ones for diabetes and hyperlipidemia (AACE 2000; ADA 2003; Goldstein et al. 2004; Haffner 2004). From these guidelines, specific diabetic reminders are built into the general information system in the summarized, structured form. In addition, tickler lists in the care manager application display lists of patients who need follow-up calls for missed tests and patients with high test values. Another aspect of the care model is the specific diabetes education provided by care managers; although two of the seven are Certified Diabetes Educators, all are trained in basic diabetes education.
Sample Size and Eligibility
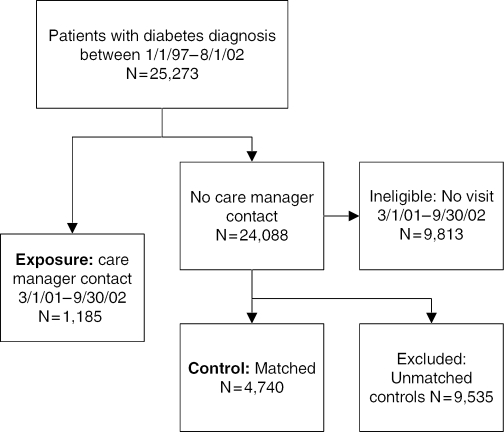
For the purposes of this study, a diabetes registry containing 25,273 patients (as shown in Figure 1) was created by analyzing data from patients seen in the seven care manager clinics and four control clinics. The diabetes registry was created by identifying patients with two or more separate ambulatory visits within the 5-year period between January 1, 1997 and August 1, 2002 with an ICD-9 code of 250.xx (where xx indicates a subdiagnosis of diabetes). Patients were assigned to the exposure group if they had had any encounter with a care manager from March 1, 2001 to September 30, 2002 (the study period). This criterion produced a total of 1,185 exposure patients, who were seen in seven clinics and were co-managed by any of 65 physicians and 7 care managers. Of the 24,088 patients remaining who did not see a care manager, 9,813 had no outpatient encounters during the study period and were excluded. The remaining patients (n=14,275) were used to match control patients in a 4:1 ratio (n=4,740 matches); clinics with and without care managers contributed similar numbers of controls.
Figure 1.
Sample Size and Eligibility
The exposure group start date was defined as the first outpatient encounter with a care manager during the study period, and the start date of the control group as the first outpatient encounter during the study period in which diabetes was included on the diagnosis list. Follow-up time, which began accruing after the individually defined start date, ranged from 4 to 18 months.
Design
The study design was a retrospective cohort design with matched controls in a 4:1 ratio. Each case was matched to four controls by sex, age, a comorbidity index, the testing regularity pattern (regular, irregular, no testing, or unknown), and previous pattern for glycemic control (controlled, uncontrolled, or unknown) of LDL or HbA1c. Ages were grouped in 10-year intervals based on clinically significant formulations from previous studies (Turner et al. 1999; Mokdad et al. 2003; Engelgau et al. 2004). Regularity and control definitions were based on patient data during the 2-year period prior to the start date, and are described in Table 1. The target goal for desirable HbA1c levels changed from the eligibility period (2001–2002) to the study period (2002–2003) from 7.2 to 7.0 percent; this change is reflected in the differences between baseline control and study control definitions in Table 1. The comorbidity index was based on the work by Deyo, Cherkin, and Ciol (1992). In their approach, the co-existing diseases in a single patient during the baseline period (represented by ICD-9-CM codes from outpatient visit billing codes) are weighted and summed, with a maximum score of 14 comorbidities. This scale was collapsed into three categories (1, 2, or 3 or more comorbidities) for matching and data analysis purposes.
Table 1.
Definitions of Regularity of Testing and Control Categories for Matching, Process Measures, and Outcomes
| Category | Definition | Reference |
|---|---|---|
| Matching | ||
| Previous regularity of HbA1c or LDL testing* | AACE (2000), Goldstein et al. (2004) | |
| Regular | Patient was tested yearly for previous 2 years | |
| Irregular | Patient was tested once in previous 2 years | |
| No testing | Not tested in previous 2 years | |
| Previous HbA1c or LDL control† | AACE (2000), Haffner (2004) | |
| Controlled | One or more test and | |
| Mean HbA1c≤7.2 | ||
| Mean LDL≤100 | ||
| Uncontrolled | One or more test and | |
| Mean HbA1c>7.2 | ||
| Mean LDL>100 | ||
| Unknown | Not testing or unknown regularity | |
| Process measures | ||
| Timeliness of testing | Test ordered and … | Goldstein et al. (2004), Haffner (2004) |
| HbA1c | ≤6 months since last test | |
| LDL | <1 year since last test | |
| Tested if overdue | Test ordered and … | Goldstein et al. (2004), Haffner (2004) |
| HbA1c | ≥7 months since last test | |
| LDL | ≥13 months since last test | |
| In control | Most recent test result | Goldstein et al. (2004), Haffner (2004) |
| HbA1c | <7.0 | |
| LDL | <100 | |
| Outcome measures | ||
| HbA1c and LDL | Last level and adjusted change in level | |
Definitions are for those diagnosed 2 or more years prior to trigger date. If diagnosis was between 1 and 2 years from trigger date, testing was regular if conducted last year. If the patient was diagnosed within last year, testing regularity was unknown.
Control was unknown if no testing was conducted or diagnosis was made within 1 year.
HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein.
Outcome Measures
Outcomes were process and health status indicators as shown in Table 1. Process variables were adherence to established diabetic and hyperlipidemia guidelines, including the conformity to testing frequency. Use of information systems by the care managers was assessed by audit trails and self-report. Beginning from the treatment initiation date, automated retrospective analysis was carried out for each individual to determine whether laboratory tests were current or overdue based on agreed-upon standards of care and whether observed laboratory test values fell above or below a desired threshold. Patients were overdue for testing if 7 months (for HbA1c) or 13 months (for LDL) had elapsed since the last abnormal test. The desired guideline thresholds were set at HbA1c≤7.0 and LDL≤100 during the period demarcated by this study. Health status outcome indicators were the levels of both HbA1c and LDL.
Statistical Analysis
The effects of generalist care managers on outcomes were assessed using logistic and linear regression. Estimates for the main effect of care management were adjusted for patient age (in 10-year age categories), sex, comorbidities, history of testing regularity, race, and history of HbA1c and LDL control. The monthly snapshots of the data presented the potential for each patient to have multiple observations, so variance estimation techniques clustered on patient identifier were used to correct for the effects of multiple observations (Huber 1967; White 1982). Although patients were matched on previous control of diabetes (see Table 1) as measured by HbA1c level at baseline, they were not matched on exact HbA1c levels as it was thought that this would lead to overmatching. Differences in baseline levels and subsequent changes were adjusted for possible regression to the mean using the method of Trochim (2003). In this conservative adjustment, intraindividual correlations (r) of change are used to estimate the proportion of change in HbA1c levels that may be because of statistical artifacts.
Results
During the study period, 4,421 patients were referred to seven care managers by 65 physicians; of these referrals, 1,185 (26.8 percent) had diabetes and were assigned to the exposure group. From patients with diabetes seen by physicians and not care managers, 4,470 controls were matched to the study subsets via the criteria described in the methods. The demographic information for the exposure, control, and eligible registry patients is displayed in Table 2. For the unmatched categorical variables (including race), the exposure group distribution was not significantly different from the control one. As an entire group, the registry patients were different from exposure and control groups in that they had a slightly higher disease burden (29.7 percent with two or more diseases versus 26 percent for the other two groups), and had significantly less follow-up and thus more missing information.
Table 2.
Group Baseline Characteristics
| Exposure | Control | Registry | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 1,185 | 100.0 | 4,740 | 100.0 | 14,275 | 100.0 |
| Female | 603 | 50.9 | 2,412 | 50.9 | 7,170 | 50.2 |
| Age | ||||||
| 18–29 | 52 | 4.4 | 208 | 4.4 | 787 | 5.5 |
| 30–39 | 74 | 6.2 | 296 | 6.2 | 929 | 6.5 |
| 40–49 | 199 | 16.8 | 796 | 16.8 | 2,049 | 14.4 |
| 50–59 | 264 | 22.3 | 1,056 | 22.3 | 3,053 | 21.4 |
| 60–69 | 296 | 25.0 | 1,184 | 25.0 | 3,249 | 22.8 |
| 70–79 | 219 | 18.5 | 876 | 18.5 | 2,995 | 21.0 |
| 80+ | 81 | 6.8 | 324 | 6.8 | 1,213 | 8.5 |
| Mean (SD), years | 59.9 (15) | 59.8 (15) | 60.1 (16) | |||
| Race* | ||||||
| American Indian | 2 | 0.2 | 11 | 0.2 | 28 | 0.2 |
| Asian/Pacific Islander | 22 | 1.9 | 111 | 2.4 | 301 | 2.1 |
| Black | 9 | 0.8 | 30 | 0.7 | 123 | 0.9 |
| Hispanic | 67 | 5.7 | 15 | 6.4 | 918 | 6.4 |
| Unknown | 26 | 2.2 | 106 | 2.2 | 1,268 | 8.9 |
| Caucasian | 1,059 | 89.4 | 3,718 | 88.5 | 11,637 | 81.5 |
| Risk score | ||||||
| 1 | 867 | 73.2 | 3,468 | 73.2 | 10,031 | 70.3 |
| 2 | 264 | 22.3 | 1,056 | 22.3 | 3,717 | 26.0 |
| 3+ | 53 | 4.5 | 212 | 4.5 | 527 | 3.7 |
| Exposure | Control | Registry | ||||
| HbA1c (%) | LDL (%) | HbA1c (%) | LDL (%) | HbA1c (%) | LDL (%) | |
| Testing history | ||||||
| Unknown | 0.9 | 6.9 | 0.9 | 6.9 | 8.5 | 15.2 |
| Not tested | 11.5 | 11.2 | 11.5 | 11.2 | 11.9 | 19.8 |
| Irregular | 34.3 | 32.1 | 34.3 | 32.1 | 28.2 | 31.8 |
| Regular | 53.2 | 49.8 | 53.2 | 49.8 | 51.4 | 33.1 |
| Control history | ||||||
| Unknown | 11.8 | 17.6 | 11.8 | 17.6 | 20.2 | 34.4 |
| Uncontrolled | 44.6 | 40.3 | 44.6 | 40.3 | 45.3 | 34.7 |
| Controlled | 43.6 | 42.2 | 43.6 | 42.2 | 34.6 | 30.9 |
Race was not a matching variable; the race distribution between control and exposure groups was not significantly different.
HbAlc, glycosylated hemoglobin; LDL, low-density lipoprotein
Care managers had encounters with patients an average of 4.5±1.8 times per 1 year of follow-up. Diabetes was the most frequent reason for referral (26.8 percent), followed by mental health (24 percent), and resource assistance (12 percent) needs. Comparing patients referred for diabetes with others in the care management group, the patients with diabetes had more visits than care-managed patients with other diagnoses, or 5.8±2.0 visits per year. In all, there were 6,876 visits completed by care managers for patients with diabetes; 39.4 percent were via phone, 36 percent were visits with the patient, 11.9 percent were care conferences or other advocating activities, and 5.5 percent were in a group education session. Seventy percent of all encounters in patients with diabetes involved diabetic education or protocol adherence checks; 14 percent of encounters were for financial assistance with medications, and the remaining 16 percent of encounters in patients with diabetes were solely for other diseases, including depression, hypertension, and drug dependency. During the study period, care managers addressed at least one other major issue besides diabetes in 35 percent of patients with diabetes. Care managers accessed the electronic records of every patient at least once during the study period, using the computer to see laboratory and radiology test results, to read physician progress notes, or to review measures of chronic disease adherence on the patient worksheet. Best practice support provided by the patient worksheet or tickler lists to remind care managers of follow-up appointments were used daily by three (of seven) of the care managers, used weekly by three additional care managements, and used at least monthly by all seven. In addition to phone calls, communication among team members via the electronic messaging system was used by care managers at twice the rate of physicians per patient seen; as their receipt of messages was higher, physicians saw 29 percent more electronic messages in the care of exposure patients (1.0±3.7 messages per patient) versus controls (0.7±2.1 messages per patient). Physicians of control patients used the information system on 93 percent of all patients, including alerts, the summarized worksheet, and communication between providers about patient status. Beyond the care manager-specific applications and message log use, no significant difference in information system use by physicians was seen in the care of exposure versus control patients.
Table 3 shows the unadjusted and adjusted odds ratio (OR) for the exposure group versus the control group in adherence to the diabetes guidelines. Before adjustment for other variables, the patients in the exposure group had 20 percent lower odds of being overdue for HbA1c testing, were 42 and 20 percent more likely to be tested for HbA1c and LDL if overdue, and were 24 percent more likely to have an HbA1c under the goal threshold of 7.0. All of these values were significant at the p<.01 level in both the single and multivariable model except LDL testing when overdue (p=.10 for single and p=.04 for multivariable models).
Table 3.
Odds of Case-Managed Patients Being Adherent to Diabetes Guideline for HbA1c and LDL
| HbA1c Overdue | HbA1c Completed if Overdue | HbA1c<7.0 | LDL Completed if Overdue | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value |
| Unadjusted odds: single variable model with exposure to case managers versus usual care | ||||||||
| Care manager | 0.80 (0.74, 0.86) | <.01 | 1.42 (1.31, 1.64) | <.01 | 1.24 (1.08, 1.41) | <.01 | 1.20 (0.98, 1.54) | .10 |
| Adjusted odds: multivariable model included age, sex, race, risk score (number of comorbidities), testing history, and control history | ||||||||
| Care manager | 0.79 (0.72, 0.85) | <.01 | 1.49 (1.3, 1.71) | <.01 | 1.31 (1.14, 1.51) | <.01 | 1.26 (1.02, 1.57) | .04 |
| Significant variables* | Age, risk score, testing history | Age, risk score, testing history | Age, race, risk score, testing history, control history | Age, control history | ||||
| Selected odds from model | ||||||||
| Age (reference=60–69) | <.01 | <.01 | <.01 | <.01 | ||||
| 20–29 | 1.8 (1.5, 2.15) | 0.64 (0.48, 0.85) | 0.45 (0.36, 0.58) | 0.61 (0.41, 0.9) | ||||
| 80+ | 1.26 (1.08, 1.46) | 0.84 (0.68, 1.05) | 0.92 (0.81, 1.03) | 0.5 (0.32, 0.77) | ||||
| Deyo risk score (reference=1) | <.01 | <.01 | <.01 | |||||
| 2 | 1.25 (1.15, 1.36) | 0.77 (0.67, 0.88) | 0.87 (0.8, 0.94) | Nonsignificant | ||||
| 3 or more | 1.42 (1.11, 1.83) | 0.9 (0.63, 1.29) | 0.76 (0.59, 0.97) | |||||
| Testing history (reference=regular) | <.01 | <.01 | <.01 | |||||
| No tests | 10.85 (8.47, 13.87) | 0.27 (0.19, 0.39) | 0.1 (0.07, 0.15) | |||||
| Irregular | 2.48 (2.28, 2.71) | 0.69 (0.61, 0.79) | 0.57 (0.52, 0.63) | |||||
Sex was not significant in any component, but was included in the final multivariable model.
HbAlc, glycosylated hemoglobin; LDL, low-density lipoprotein.
In the multivariable model, the exposure group was 21 percent less likely to be overdue for HbA1c testing (OR 0.79, 95 percent confidence interval [CI] 0.72–0.85), and 31 percent more likely to have an HbA1c under 7.0 percent, as shown in Table 3. Also significant in the model were testing regularity, age (with the very young and the very old at higher risk of being overdue), and increasing comorbidity index score. The group of patients whose past testing was irregular or nonexistent had 2.5 and 10.9 times the odds of being overdue, respectively, versus patients in whom testing had been regular (no previous tests: OR 10.85, 95 percent CI 8.47–13.87; irregular testing: OR 2.48, 95 percent CI 2.28–2.71; p=<.01).
Exposure to care managers significantly increased the odds of completing the testing once the patient was overdue for HbA1c (OR 1.49; 95 percent CI 1.3–1.71) and LDL (OR 1.26; 95 percent CI 1.02–1.57) testing, as seen in Table 3. Patients in the exposure and control groups with age between 20 and 29 (younger) and older than 80 years (very old), higher risk patients, and those with an irregular testing history had worse odds of being tested when overdue for HbA1c. Being of younger and very old age also decreased the odds of being tested for LDL by 40–50 percent.
Table 4 compares the absolute and relative differences in HbA1c and LDL levels between the care-managed (exposure) and control groups. The average changes between the initial and final levels for HbA1c and final levels of LDL were significantly lower for the exposure group as compared with the control group (as shown in Table 4). Despite matching on history of glycemic control, the initial level of HbA1c in the exposure group was 0.25 percentage units higher than that of the matched controls. The correlation (r) between pretest and posttest was 0.64, and the maximum amount of decline in HbA1c levels because of regression from the mean is expected to be 0.09 percent HbA1c greater in the exposure than the control group; the 0.09 percent is subtracted from the exposure groups' difference. The odds of the HbA1c being in the controlled range were also significantly higher for the exposure group (Table 3; OR 1.19, 95 percent CI 1.10–1.28). Again, younger age, higher risk, and irregular testing history all lowered the odds of being in control; a history of being uncontrolled or being of nonwhite or unknown race also lowered the odds that the current test result demonstrated control. No significant difference was seen between the two groups for odds of LDL below 100 mg/dl.
Table 4.
Reduction in HbA1c and LDL Levels in Case-Managed versus Reference Patients
| HbA1c | LDL | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||||
| Baseline | Post | Reduction | Adj. Reduction | Baseline | Post | Reduction | |
| Care management | 7.96 (1.74) | 7.41 (1.38) | 0.55% | −0.46% | 102.8 (32.7) | 96.7 (28.3) | −6.1 mg/dl |
| Reference | 7.71 (1.53) | 7.53 (1.36) | 0.18% | −0.18%† | 104.3 (33.2) | 100.6 (30.4) | −3.7 mg/dl |
| Difference | 0.25 | (−0.12) | (−0.37) | (−0.28) | (−1.5) | (−3.9) | (−2.4 mg/dl) |
| p-value | <.01 | .02 | <.001 | <.01 | NS | <.01 | .09 |
Adjusted for regression to the mean; for details, see Davis (1976) or Trochim (2003).
The baseline value for the lower mean does not change in the method.
HbAlc, glycosylated hemoglobin; LDL, low-density lipoprotein.
Discussion
This study demonstrates a statistically significant improvement in adherence to diabetic guidelines when generalist care managers with enhanced computer support are involved in the care of people with diabetes as compared with usual care—including computer support—for matched controls. In addition, the average values for LDL and HbA1c were ultimately lower for the care-managed group versus the controls, and the odds of having glycemic control were higher in the care-managed group. These improvements, if sustained, are predicted by previous studies to lead to a 15–20 percent reduction in complications (Viberti 2003; Vinik and Vinik 2003). A notable exception to these positive results were the very old (age 80 and older), who were less likely to achieve adherence to the guidelines at each step. These results are tempered by the nature of our effectiveness study, which makes it difficult to measure individual components of the intervention.
Evaluating our generalist care management system on a set of patients with diabetes—an expensive, complicated chronic disease with effective therapies—is an important component of the overall success of the system, especially given that patients with diabetes were only approximately 26 percent of the patients treated. Several recent studies demonstrate the differences between this study design and the current literature (Pan et al. 1997; Tuomilehto et al. 2001; Knowler et al. 2002; New et al. 2003; Taylor et al. 2003; The California Medi-Cal Type 2 Diabetes Study Group 2004). Most used an idealized trial format, with separate diabetes clinics, endocrinologists, and/or nurse specialists to improve out-of-control blood pressure and lipid levels in people with diabetes. These investigators have been able to show improvement in control, adherence, and even mortality of diabetics randomized to treatment clinics versus control. The effectiveness format in the present study uses a different implementation method. No specialized clinics were created; rather, the usual day-to-day activities of PCPs were augmented by the presence of the generalist care managers in a team-based approach. Wagner et al. (2001) conducted trials with both frail geriatric and diabetic patients using chronic care clinics that were closer to our approach (although still disease specific). Unlike the current study, the diabetic arm of Wagner's study showed improvement in adherence but no change in HbA1c in the intervention (N=278) versus control patients (Wagner et al. 2001).
Our approach has a strong basis in theory; the care managers receive training in and apply the stages of change model (Prochaska 2003), the coaching model (Koenigsberg, Bartlett, and Cramer 2004), and Wagner's CCM (Wagner et al. 2001), as they care for patients with a variety of illnesses. Our implementation of the CCM is different from most, however, in that it adds several aspects of information system components. The care managers and other team members have access to and use multiple features of a shared electronic medical record, specifically a summarized, structured form with patient-specific results; the success of the team approach with generalist care managers may indeed depend on this distributed, longitudinal technology, which enables the team to apply multiple guidelines with ease. Components that facilitate this process included the ability to access the entire patient record easily, specific decision support mechanisms that help them to efficiently address the needs of a population by providing lists of alerts for patients who require attention, and patient-specific electronic communication. Importantly, this intervention allows for smoother integration into the primary care workflow, as information technology helps facilitate communication and the application of multiple disease guidelines and other resources in a single visit rather than the creation of specialized clinics or additional visits for other comorbidities. Studies indicate that this is an important factor in the inefficiency of primary care clinics (Flocke, Frank, and Wenger 2001; Rothman and Wagner 2003). In addition, the generalist approach may allow the care managers to focus more on the needs of the patient than the needs of one particular disease, improving patient-centric care delivery and prioritizing care delivery (Allen 1994; Crystal et al. 1999; Naylor et al. 1999; Bull et al. 2000). Finally, this generalist implementation had all of the elements of the CCM, including health care organization, self-management support, clinical information systems, decision support, connection to the community, and delivery system redesign. We focused on delivery system redesign with team care and information system support; a previous study found the care delivery design to be the most influential component (Sperl-Hillen et al. 2004) but the information technology element included in our implementation is very broad and may contribute significantly to the success of our program, as described by Casalino et al. (2003).
Several potential biases exist in this study. First, referral bias may create differences between this population and other pertinent populations. Although attempts were made to match control and exposure variables based on available pertinent variables and risk factors, there are possibly other factors that would contribute to the effect seen in this paper. While it is possible that the patients who did not receive care management were in some way different from those who did, the matching variables were chosen to ensure similar previous outcomes and baseline probability of adherence and control of diabetes. Also, utilization was matched by determining the eligibility of controls based on previous visit history. Most biases in referral for this system (patients who are more ill or more difficult to control, for example) would favor a result of no differences between the groups. The higher baseline HbA1c confirms the direction of these biases; the correction for regression to the mean provides an appropriate perspective given this bias. The inclusion of both Type I (estimated at <5 percent of the study population) and Type II diabetes as well as a broad range of ages indicates a number of potential different subpopulations who might have very different treatment recommendations. To account for these different subpopulations, the chosen process and control measures are the same in the various guidelines that cover these groups, while the differing treatment recommendations were largely excluded from analysis. Thus, recommendations exist to measure LDL in even the youngest groups with diabetes, but treatment recommendations differ. Adjusting for age and estimated Type I diabetics did not affect the significance of the results. Another source of bias was the initial selection of HbA1c 7.2 percent as the cutoff for control; at the start of the study, and this was the internal guideline at the health system under study; it was selected during a period when external guidelines were shifting from 8.0 to 7.0 percent as the goal. The results do not differ with control criteria set at 7.0 or 7.2 percent; for generalizability, 7.0 percent is given in the results.
Biases based on environmental variables, such as clinic milieu or other provider care, were minimized by including a large proportion of control patients who were seen in the same or similar clinics or by the same physicians but not referred. Biases as a result of information system effects were also minimized by ensuring that all clinicians included had access to and generally used the clinical information system. The effect of individual components of the care management system described in this study is difficult to disambiguate because of the study design. For instance, five of the care managers were not certified diabetic educators; although a separate analysis indicates that outcomes did not differ in these five care managers, the relative role of diabetic education is difficult to discern. All patients had access to diabetes education through groups and individual educators, but the kind, amount, and quality of diabetic education may differ; part of this difference is as a result of the design of the system, however. Finally, some of the improvements may be because of the increased scrutiny of these patients (Hawthorne effect) and could attenuate over time.
The averaged difference in HbA1c and LDL between the groups is consistent with previous studies, despite the fact that both the initial and final HbA1c levels of both our groups were lower than most studies. Many patients with out-of-control HbA1c and LDL levels were likely excluded from the control group in this study because they were never tested (substantiated by the data on irregular and unknown testing)—the effect of care manager is likely underestimated because of this difficulty in study design.
It would be important to study the persistence of this effect through a long-term prospective study to determine assessment of the reasons for referral and overall satisfaction with the system. The independent effect of computer assistance and other intervention components is also of interest; technological assistance likely contributes to the ability of the care managers to positively impact patient outcomes by facilitating access to patient data relevant to multiple guideline compliance, by meeting specific information needs of care managers, and through the messaging abilities of the system. In effectiveness studies such as this one, the generalizability of the intervention arm and the comparability of the control arm are important. In this study, the information system components are more advanced than many other systems; however, the improvement beyond the information systems indicates that such systems are not enough: a broader care management system can further improve care. As a significant problem in health care delivery is the inadequate application of known treatments for chronic diseases and most patients with chronic diseases received their health care from primary care providers, models that can improve adherence to guidelines of care in this setting are important (Glasgow, Vogt, and Boles 1999; Rothman and Wagner 2003). Overall, this study represents an important first step in evaluating a generalist multidisease care management program in a real-world setting.
References
- AACE AACE Medical Guidelines for Clinical Practice for the Diagnosis and Treatment of Dyslipidemia and Prevention of Atherogenesis.”. Endocrine Practice. 2000;6(2):162–213. [PubMed] [Google Scholar]
- ADA Standards of Medical Care for Patients with Diabetes Mellitus.”. Diabetes Care. 2003;26(suppl 1):S33–50. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- AHM “Glossary”. 2001. [accessed on 23 October, 2001]. Available at http://www.academyforhealthcare.com/glossary/
- Allen SA. “Medicare Case Management.”. Home Healthcare Nurse. 1994;12(3):21–7. doi: 10.1097/00004045-199405000-00002. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association “Diabetes Fact Sheet”. 2003. [accessed on 7/31, 2003]. Available at http://www.diabetes.org/main/info/facts/facts_natl.jsp.
- Bodenheimer T, Wagner EH, Grumbach K. “Improving Primary Care for Patients with Chronic Illness.”. Journal of the American Medical Association. 2002a;288(14):1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Wagner EH, Grumbach K. “Improving Primary Care for Patients with Chronic Illness: The Chronic Care Model, Part 2.”. Journal of the American Medical Association. 2002b;288(15):1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- Bond GR, Miller LD, Krumwied RD, Ward RS. “Assertive Case Management in Three CMHCs: A Controlled Study.”. Hospital and Community Psychiatry. 1988;39(4):411–8. doi: 10.1176/ps.39.4.411. [DOI] [PubMed] [Google Scholar]
- Bull MJ, Hansen HE, Gross CR. “A Professional–Patient Partnership Model of Discharge Planning with Elders Hospitalized with Heart Failure.”. Applied Nursing Research. 2000;13(1):19–28. doi: 10.1016/s0897-1897(00)80015-4. [DOI] [PubMed] [Google Scholar]
- Casalino L, Gillies RR, Shortell SM, Schmittdiel JA, Bodenheimer T, Robinson JC, Rundall T, Oswald N, Schauffler H, Wang MC. “External Incentives, Information Technology, and Organized Processes to Improve Health Care Quality for Patients with Chronic Diseases.”. Journal of the American Medical Association. 2003;289(4):434–41. doi: 10.1001/jama.289.4.434. [DOI] [PubMed] [Google Scholar]
- Casalino LP. “Markets and Medicine: Barriers to Creating a ‘Business Case for Quality’.”. Perspectives in Biology and Medicine. 2003;46(1):38–51. doi: 10.1353/pbm.2003.0001. (discussion 52–4) [DOI] [PubMed] [Google Scholar]
- CMSA “Best Practices in Physician and Case Management Collaboration to Improve Patient Care.”. 2003. Consensus Paper of the 2003 Physician and Case Management Summit. [DOI] [PubMed]
- Crystal S, Lo Sasso AT, Sambamoorthi U. “Incidence and Duration of Hospitalizations among Persons with AIDS: An Event History Approach.”. Health Services Research. 1999;33(6):1611–38. [PMC free article] [PubMed] [Google Scholar]
- Davis CE. “The Effect of Regression to the Mean in Epidemiologic and Clinical Studies.”. American Journal of Epidemiology. 1976;104(5):493–8. doi: 10.1093/oxfordjournals.aje.a112321. [DOI] [PubMed] [Google Scholar]
- D'Ercole A, Struening E, Curtis JL, Millman EJ, Morris A. “Effects of Diagnosis, Demographic Characteristics, and Case Management on Rehospitalization.”. Psychiatric Services. 1997;48(5):682–8. doi: 10.1176/ps.48.5.682. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. “Adapting a Clinical Comorbidity Index for Use with ICD-9-CM Administrative Databases.”. Journal of Clinical Epidemiology. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Duran LS. “Motivating Health: Strategies for the Nurse Practitioner.”. Journal of the American Academy of Nurse Practitioners. 2003;15(5):200–5. doi: 10.1111/j.1745-7599.2003.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM. “The Evolving Diabetes Burden in the United States.”. Annals of Internal Medicine. 2004;140(11):945–50. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- Flocke SA, Frank SH, Wenger DA. “Addressing Multiple Problems in the Family Practice Office Visit.”. Journal of Family Practice. 2001;50(3):211–6. [PubMed] [Google Scholar]
- Glasgow RE, Vogt TM, Boles SM. “Evaluating the Public Health Impact of Health Promotion Interventions: The RE-AIM Framework.”. American Journal of Public Health. 1999;89(9):1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM. “Tests of Glycemia in Diabetes.”. Diabetes Care. 2004;27(suppl 1):S91–3. doi: 10.2337/diacare.27.2007.s91. [DOI] [PubMed] [Google Scholar]
- Haffner SM. “Dyslipidemia Management in Adults with Diabetes.”. Diabetes Care. 2004;27(suppl 1):S68–71. doi: 10.2337/diacare.27.2007.s68. [DOI] [PubMed] [Google Scholar]
- Huber P. Proceedings of the Fifth Berkeley Symposium in Mathematical Statistics and Probability. Berkeley, CA: University of California Press; 1967. “The Behavior of Maximum Likelihood Estimates under Non-Standard Conditions.”; pp. 221–33. [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. “Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin.”. New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg MR, Bartlett D, Cramer JS. “Facilitating Treatment Adherence with Lifestyle Changes in Diabetes.”. American Family Physician. 2004;69(2):309–16. [PubMed] [Google Scholar]
- Matthews DR. “The Natural History of Diabetes-Related Complications: The UKPDS ExperienceUnited Kingdom Prospective Diabetes Study.”. Diabetes Obesity & Metabolism. 1999;1(suppl 2):S7–13. doi: 10.1046/j.1463-1326.1999.0010s2007.x. [DOI] [PubMed] [Google Scholar]
- McGrew JH, Bond GR, Dietzen L, McKasson M, Miller LD. “A Multisite Study of Client Outcomes in Assertive Community Treatment.”. Psychiatric Services. 1995;46(7):696–701. doi: 10.1176/ps.46.7.696. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. “Prevalence of Obesity, Diabetes, and Obesity-Related Health Risk Factors.”. Journal of the American Medical Association. 2003;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, Schwartz JS. “Comprehensive Discharge Planning and Home Follow-Up of Hospitalized Elders: A Randomized Clinical Trial.”. Journal of the American Medical Association. 1999;281(7):613–20. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- New JP, Mason JM, Freemantle N, Teasdale S, Wong LM, Bruce NJ, Burns JA, Gibson JM. “Specialist Nurse-Led Intervention to Treat and Control Hypertension and Hyperlipidemia in Diabetes (SPLINT): A Randomized Controlled Trial.”. Diabetes Care. 2003;26(8):2250–5. doi: 10.2337/diacare.26.8.2250. [DOI] [PubMed] [Google Scholar]
- Nicollerat JA. “Implications of the United Kingdom Prospective Diabetes Study (UKPDS) Results on Patient Management.”. Diabetes Education. 2000;26(suppl):8–10. [PubMed] [Google Scholar]
- Norris SL, Olson DE. “Implementing Evidence-Based Diabetes Care in Geriatric PopulationsThe Chronic Care Model.”. Geriatrics. 2004;59(6):35–9. (quiz 40) [PubMed] [Google Scholar]
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. “Effects of Diet and Exercise in Preventing NIDDM in People with Impaired Glucose Tolerance.”. Diabetes Care. 1997;20(4):537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- Prochaska JO. “Staging: A Revolution in Helping People Change.”. Managed Care. 2003;12(9 suppl):6–9. [PubMed] [Google Scholar]
- Rothman AA, Wagner EH. “Chronic Illness Management: What Is the Role of Primary Care?”. Annals of Internal Medicine. 2003;138(3):256–61. doi: 10.7326/0003-4819-138-3-200302040-00034. [DOI] [PubMed] [Google Scholar]
- Spencer L, Pagell F, Hallion ME, Adams TB. “Applying the Transtheoretical Model to Tobacco Cessation and Prevention: A Review of Literature.”. American Journal of Health Promotion. 2002;17(1):7–71. doi: 10.4278/0890-1171-17.1.7. [DOI] [PubMed] [Google Scholar]
- Sperl-Hillen JM, Solberg LI, Hroscikoski MC, Crain AL, Engebretson KI, O'Connor PJ. “Do All Components of the Chronic Care Model Contribute Equally to Quality Improvement?”. Joint Commission Journal on Quality and Safety. 2004;30(6):303–9. doi: 10.1016/s1549-3741(04)30034-1. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Miller NH, Reilly KR, Greenwald G, Cunning D, Deeter A, Abascal L. “Evaluation of a Nurse-Care Management System to Improve Outcomes in Patients with Complicated Diabetes.”. Diabetes Care. 2003;26(4):1058–63. doi: 10.2337/diacare.26.4.1058. [DOI] [PubMed] [Google Scholar]
- The California Medi-Cal Type 2 Diabetes Study Group “Closing the Gap: Effect of Diabetes Case Management on Glycemic Control among Low-Income Ethnic Minority Populations: The California Medi-Cal Type 2 Diabetes Study.”. Diabetes Care. 2004;27(1):95–103. doi: 10.2337/diacare.27.1.95. [DOI] [PubMed] [Google Scholar]
- Toth EL, Majumdar SR, Guirguis LM, Lewanczuk RZ, Lee TK, Johnson JA. “Compliance with Clinical Practice Guidelines for Type 2 Diabetes in Rural Patients: Treatment Gaps and Opportunities for Improvement.”. Pharmacotherapy. 2003;23(5):659–65. doi: 10.1592/phco.23.5.659.32203. [DOI] [PubMed] [Google Scholar]
- Trochim WM. “The Research Methods Knowledge Base: The Effect of Regression to the Mean”. 2003. [accessed on Sept. 11, 2003]. Available at http://trochim.human.cornell.edu/kb/index.htm.
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. “Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance.”. New England Journal of Medicine. 2001;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Turner RC, Cull CA, Frighi V, Holman RR. “Glycemic Control with Diet, Sulfonylurea, Metformin, or Insulin in Patients with Type 2 Diabetes Mellitus: Progressive Requirement for Multiple Therapies (UKPDS 49).”. Journal of the American Medical Association. 1999;281(21):2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- Viberti G. “The Need for Tighter Control of Cardiovascular Risk Factors in Diabetic Patients.”. Journal of Hypertension. 2003;21(suppl 1):S3–6. [PubMed] [Google Scholar]
- Vinik AI, Vinik E. “Prevention of the Complications of Diabetes.”. American Journal of Managing Care. 2003;9(3 suppl):S63–80. (quiz S81–4) [PubMed] [Google Scholar]
- Wagner EH, Grothaus LC, Sandhu N, Galvin MS, McGregor M, Artz K, Coleman EA. “Chronic Care Clinics for Diabetes in Primary Care: A System-Wide Randomized Trial.”. Diabetes Care. 2001;24(4):695–700. doi: 10.2337/diacare.24.4.695. [DOI] [PubMed] [Google Scholar]
- White H. “Maximum Likelihood Estimation of Misspecified Models.”. Econometrica. 1982;(50):1–25. [Google Scholar]