Abstract
Objective
To estimate the effect of reference pricing (RP) of nonsteroidal anti-inflammatory drugs (NSAIDs) on drug subsidy program and beneficiary expenditures on analgesic drugs.
Data Sources/Study Setting
Monthly claims data from Pharmacare, the public drug subsidy program for seniors in British Columbia, Canada, over the period of February 1993 to June 2001.
Study Design
RP limits drug plan reimbursement of interchangeable medicines to a reference price, which is typically equal to the price of the lowest cost interchangeable drug; any cost above that is borne by the patient. Pharmacare introduced two different forms of RP to the NSAIDs, Type 1 in April 1994 and Type 2 in November 1995. Under Type 1 RP, generic and brand versions of the same NSAID are considered interchangeable, whereas under Type 2 RP different NSAIDs are considered interchangeable. We extrapolated average reimbursement per day of NSAID therapy over the months before RP to estimate what expenditures would have been without the policies. These counterfactual predictions were compared with actual values to estimate the impact of the policies; the estimated impacts on reimbursement rates were multiplied by the postpolicy volume of NSAIDS dispensed, which appeared unaffected by the policies, to estimate expenditure changes.
Principal Findings
After Type 2 RP, program expenditures declined by $22.7 million (CAN), or $4 million (CAN), annually cutting expenditure by about half. Most savings accrued from the substitution of low-cost NSAIDs for more costly alternatives. About 20 percent of savings represented expenditures by seniors who elected to pay for partially reimbursed drugs. Type 1 RP produced one-quarter the savings of type 2 RP.
Conclusions
Type 2 RP of NSAIDs achieved its goal of reducing drug expenditures and was more effective than Type 1 RP. The effects of RP on patient health and associated health care costs remain to be investigated.
Keywords: Reference pricing, generic substitution, prescription drugs, drug cost containment, NSAIDs
Faced with growing drug expenditures, drug insurance plan executives have adopted various cost containment measures. One such policy, reference pricing (RP), limits drug plan reimbursement of interchangeable medicines to a reference price, which is typically equal to the price of the lowest cost interchangeable drug; any cost above that is borne by the patient. RP policies vary in the extent to which drugs are considered interchangeable by a particular plan (Lopez-Casasnovas and Puig-Junoy 2000). Under its most restrictive form—Type 1 RP—only chemically equivalent drugs (i.e., branded and “generic” versions of the same drug) are considered interchangeable. Under Type 2 RP, all drugs from the same therapeutic class are considered interchangeable. For instance, under such a system all nonsteroidal antiinflammatory drugs (NSAIDs), used for analgesia, would be reimbursed at the same rate. Under Type 3 RP, by contrast, all the different analgesic drugs, including opiates and NSAIDs, would be considered interchangeable.
There have been calls to integrate RP into the Medicare prescription drug benefit (Huskamp et al. 2000; Morgan, Barer, and Agnew 2003). Unlike traditional patient cost sharing policies, RP fully subsidizes lower cost medicines, and, for those who meet exemption criteria, higher cost medicines as well. RP might therefore save money while avoiding the adverse impacts on patient health associated with patient cost sharing, which typically applies to all drugs (Tamblyn et al. 2001). While the debate on whether RP should be widely used in the U.S. has been heated, evidence on its outcomes is limited (Kanavos and Reinhardt 2003). In this paper, we use retrospective population-based claims data to compare the net drug program savings realized by the application of Type 1 and then Type 2 RP with the NSAIDs by Pharmacare, the publicly funded drug subsidy program for seniors and various other residents of British Columbia (BC), Canada. NSAIDs are among the most commonly used medications worldwide, with over 70 million prescriptions and more than 30 billion over-the-counter tablets sold each year in the U.S. (Wolfe, Lichtenstein, and Singh 1999). Over 15 percent of North Americans suffer from arthritis and/or musculoskeletal disease (Lawrence et al. 1998), and current guidelines endorse both NSAIDs and acetaminophen as first-line therapies for symptomatic osteoarthritis (ACR 2000).
Potential program savings from RP depend on the vector of reimbursement prices of the drugs considered interchangeable as well as on the quantities dispensed. Potential savings are greatest when use is skewed toward higher priced drugs and the price spread—the difference between the highest and lowest drug prices—is large. The price spread can only increase (or at least not decrease) the greater the number of drugs that are deemed interchangeable; potential savings are therefore largest for Type 3 RP and lowest for Type 1 RP. There were considerable differences in the prices of the different NSAIDs in BC prior to the introduction of Type 2 RP. The cost of generic ibuprofen, for instance, varied between $0.11 and $0.16 per day (depending on the amount used), whereas the daily cost of a newer NSAID, etodolac, varied from $1.79 to $3.58 (Therapeutics Initiative 1995).
Several factors can mitigate drug plan savings from RP. The first of these is the generosity of the criteria, if any, by which patients are exempted from RP. Pharmacare exempts patients who have failed or are likely to fail on a lower cost, fully reimbursed drug. Although the physician must submit a written petition for review by Pharmacare's pharmacist personnel, exemption requests are usually granted within 48 hours. Pharmacare also exempts all NSAID prescriptions written by rheumatologists from Type 2 RP. Second, physicians might “prescribe around” the RP restrictions. In other words, they might substitute relatively costly analgesic drugs, including various opiates, that are not subject to RP for those that are. Third, economic theory suggests that setting reimbursement rates according to the prices of a set of reference standard drugs might encourage the manufacturers of those drugs to raise retail prices (Zweifel and Crivelli 1996; Morton 1997; Anis and Wen 1998). On the other hand, experience from Type 2 RP introduced in European countries suggests that such price increases are offset by decreases in the retail prices of drugs that are only partially reimbursed by drug plans. Fourth, although the drug plan saves money on those beneficiaries who elect to pay extra for the higher cost drugs, these expenditures are merely shifted—overall drug costs do not decline.
Finally, the health of patients who switch to lower quality drugs might suffer, resulting in an off-setting increase in drug and other treatment costs. While we do not have data on patient health outcomes and individual patients commonly report better efficacy and/or tolerability with particular NSAIDs (Walker, Chan, and Yood 1992; Langman et al. 2001), we note that there is no consistent evidence of clinically significant differences in the anti-inflammatory and analgesic effect of the numerous different NSAIDs (Brooks and Day 1991; Holbrook 2001). Retrospective analyses of observational data have suggested a hierarchy among conventional NSAIDs in their potential for gastrointestinal injury, but these differences can be attributed to variations in effective dose and channeling bias (Henry et al. 1996; Rodriguez 1998). Others have studied the effects of prior authorization programs targeting higher cost NSAIDs on the health-related quality of life (Momani, Madhavan, and Nau 2002) and medical services use (Kotzan et al. 1993; Smalley et al. 1995) of chronic NSAID users enrolled in various U.S. state Medicaid programs. None of these studies detected any deleterious effects among those who were denied Medicaid subsidies for the higher cost NSAIDs.
To address the net effect of Type 1 and 2 RP on Pharmacare and patient analgesic expenditures, we used monthly Pharmacare claims data aggregated across its senior (age 65+ years) beneficiaries, over the period February 1993 to June 2001, to examine prescribing patterns, NSAID prices, Pharmacare expenditure, and patient out-of-pocket expenditure on individual NSAIDs and other analgesic drugs. A previous report indicated high accuracy and completeness of provincial government drug claims data (Williams and Young 1996a Williams and Young 1996b). We focused on seniors given that they are the highest per capita users of analgesics (Health Canada 2003), and the size and composition of the beneficiary population is relatively stable over time.
Methods
Pharmacare NSAID Reimbursement Policies
Type 1 RP, introduced in April 1994, limited Pharmacare reimbursement of all multisourced drugs (i.e., brand and generic drugs with the same active ingredient, dosage form, and strength) to the average of the lowest cost (typically generic) drugs. Type 2 RP was applied to the NSAIDs in November 1995. Under the policy, the less costly “unrestricted” NSAIDs, enteric-coated acetylsalicylic acid (ASA) (650 mg), ibuprofen, and naproxen remained fully reimbursed (at an average rate of about $0.23 daily). Pharmacare also began to reimburse acetaminophen (500 mg). The decision to provide full reimbursement for acetaminophen, ASA, ibuprofen, and naproxen was consistent with earlier recommendations by an independent academic research group, the BC Therapeutics Initiative, that these drugs be used as first line therapy for osteoarthritis (Therapeutics Initiative 1995).
Reimbursement of the “First Line Restricted” NSAIDs (diclofenac, diclofenac/misoprostol [Arthrotec], diflunisal, fenoprofen, flurbiprofen, indomethacin, ketoprofen, naproxen SR and enteric coated tablets, and salsalate) was initially limited to $0.45/day (which was less than half their existing reimbursement), then reduced to $0.43/day on March 1, 2001. Patients intolerant of unrestricted NSAIDS or with specific diagnoses (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, collagen vascular disease, or gout) were eligible for exemption from the policy. Exemption for a “second line restricted” NSAID (nabumetone, piroxicam, tenoxicam, tiaprofenic acid, tolmetin, sulindac, ketorolac, or diclofenac potassium) required failure on a first line restricted NSAID. These second line drugs were delisted a year later (November 1996), but concerns expressed by physicians and pharmacists led to the reinstatement of all but ketorolac and diclofenac potassium under the “Special Authority” program in February 1997. This prior-authorization program is similar to RP in that Pharmacare will fully reimburse a drug only if it approves the drug's use. Special authority differs from RP, however, in that Pharmacare will not reimburse any of the cost of targeted drugs for patients who fail to receive prior approval.
Various other NSAIDs1 were either delisted or required prior authorization at various times over the sample period, but these drugs collectively accounted for less than 7 percent of all NSAID prescribing in the 19 months before Type 2 RP. Notably, the cyclooxygenase-2 selective inhibitors (COX-2s) were also placed under special authority at the time of their introduction in September 2000. We assessed these drugs separately because they were reimbursed by Pharmacare only late into our sample period.
Estimation of Effects of Types 1 and 2 RP on Pharmacare NSAID Expenditure
We proceeded in three steps. First, expenditure is, by definition, price × quantity. Quantity was measured as the number of days of NSAID therapy dispensed and price as Pharmacare expenditure per day of NSAID therapy. Days therapy was constructed as the total number of milligrams dispensed divided by estimates (WHO Collaborating Centre for Drug Statistics Methodology 2000) of the typical daily maintenance dose (in milligrams). Expenditures data exclude dispensing fees, as RP affected only drug ingredient cost, and are expressed in Canadian dollars. In March 2004, $1CAN=$0.75U.S.
The introduction of RP likely affected the mix of high- and low-cost NSAIDs dispensed and therefore the price, but possibly did not affect the total volume of NSAIDs dispensed. If this is indeed the case, then our task reduces to estimating the impact of RP on price. Estimating the impact of RP on expenditure (price × quantity) is more difficult because there are more potential confounders in this case than if we were estimating the impact of RP on price alone. Moreover, even if we could control for all confounders, because quantity is more variable than price, we would likely get more precise parameter estimates from models of price than expenditure. The hypothesis that quantity was independent of RP was tested by means of the following regression:
| (1) |
where DaysNSDPopt is the number of days NSAID therapy dispensed per eligible senior Pharmacare beneficiary in month t (February 1993 ≤t≤ June 2001); data on the size of the BC senior population were obtained from Statistics Canada (Statistics Canada 2004). The RP1t indicator equals 1 in April 1994 and thereafter (the period during which the Type 1 RP policy was in effect), and equals 0 otherwise; RP2t equals 1 in November 1995 and thereafter (the Type 2 RP policy period), and equals 0 otherwise. The γj, j=0, …, 5 are unknown parameters estimated using ordinary least squares (OLS) and ɛt is the random error term.
There is reason to believe that the ɛt are autocorrelated. If dispensing volumes are atypically large in month t, as reflected by a large positive value of ɛt, beneficiaries will take longer to consume their inventory of medicines and may delay filling subsequent prescriptions. Given that Pharmacare will reimburse a maximum of 100 days supply, a positive shock to ɛt can reduce dispensing volumes by up to 4-months hence. Autoregressive errors of this sort render the standard OLS covariance matrix estimator inconsistent. We therefore used the Newey–West autocorrelation consistent covariance matrix estimator (Newey and West 1987) throughout to ensure that hypothesis testing was valid in the presence of up to a 4 month autocorrelation.
We can manipulate ((1)) to identify the effect of RP on the expected DaysNSDPopt as follows:
| (2) |
| (3) |
Under the null hypothesis that Type 1 RP did not affect quantity, γ2+γ3t=0. We evaluated this expression at the average value of t in the post–Type 1 RP introduction period and tested it with Student's t-test. The same approach was used to test the null hypothesis that the introduction of Type 2 RP did not affect post–Type 2 RP quantities dispensed.
In the second step, assuming that RP did not affect quantity and using the same regression approach we estimated the impact of the policies on price. The regression model is
| (4) |
where CostDayNSDt refers to Pharmacare expenditure per day of NSAID therapy was dispensed in month t. The effect of Type 1 and Type 2 RP on the expected price paid is as follows:
| (5) |
| (6) |
Third, we multiplied the estimated price changes (5) and ((6)) by total quantity to arrive at an estimate of the impact of each policy on expected monthly Pharmacare NSAID expenditures:
where DaysNSDt refers to the total number of days NSAID therapy dispensed in month t. Our identifying assumption in this and all other regressions is that the pre-RP trends in the outcome variable would have continued into the post-RP period had RP not been implemented.
Estimation of Secondary Effects of Type 1 and Type 2 RP
As discussed, Pharmacare savings are attenuated if physicians substitute relatively costly opiate analgesics, which were not targeted by Type 2 RP, for the NSAIDs that were. We ran a regression of the form ((1)) to test whether DaysOpiatePopt, the number of days opiate therapy dispensed per eligible senior Pharmacare beneficiary per month, increased after the introduction of RP. We then estimated the impact of RP on post-RP opiate dispensing volumes using the same methods to test for the impact of RP on DaysNSDPopt, as described by ((2)) and ((3)) above.
Next, we tested the hypothesis that RP causes convergence in the retail prices of the NSAID drugs. To do so, we estimated the impact of Type 1 and Type 2 RP on PriceURNSDt, the average monthly retail unit prices of the unrestricted NSAIDs, and on PriceRNSDt, the average monthly retail unit prices of the restricted NSAIDs, using the same methods that were used to test the impact of Type 1 and Type 2 RP on DaysNSDPopt, outlined above. The average retail unit price of NSAID group k∈{Unrestricted, Restricted} was measured as the weighted average of the total (Pharmacare plus patient) expenditure per unit dispensed of each of the lk NSAIDs in group k with weights equal to the drug lk's share of total days therapy dispensed in NSAID group k in the 3 months preceding the introduction of Type 1 RP (i.e., January–March 1994).
Finally, we assessed the extent to which the introduction of RP shifted drug expenditures to beneficiaries who elected to pay extra to use their NSAID of choice. Our data record total beneficiary-paid expenditure but do not record the number of beneficiaries who elected to pay. We thus cannot estimate the financial burden on those who elected to pay. We elected to estimate the impact of RP on patient expenditures using the same approach as was used to estimate the impact of RP on Pharmacare expenditures, that is by modeling the impact of RP on BenCostDayNSDt the average beneficiary expenditures per day of NSAID therapy dispensed, and multiplying the estimated price changes by the total days of NSAID therapy dispensed.
Results
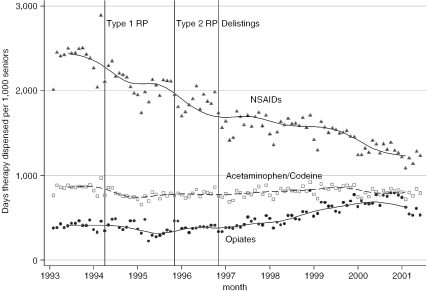
In order to assess whether we could feasibly model the impact of RP on prices alone, we assessed whether or not RP affected NSAID quantities. Based on the data displayed in Figure 1, the introduction of RP was not associated with any marked drops in the use of NSAIDs. We formally tested this hypothesis by estimating the parameters of (1) (which are displayed along with other parameter estimates in an online-only appendix) and, using these estimates, testing the hypotheses that (2)(3) were both equal to 0. We could not reject these hypotheses at the 5 percent level (Table 1). Moreover, the estimated values of ((2)) and ((3)), also displayed in Table 1, were modest: the introductions of Type 1 RP and Type 2 RP were associated with reductions of only 1.3 and 0.04 days of NSAID therapy per senior per month, respectively.
Figure 1.
Days of Analgesic Therapy Dispensed per 1,000 Seniors, by Analgesic Type and Month
Table 1.
Effect of RP on Days of Analgesic Therapy Dispensed per 1,000 Seniors over the Post-RP Period, by Analgesic Type
| Estimate | p-Value | 95% CI | ||
|---|---|---|---|---|
| Effect of Type 1 RP | ||||
| NSAIDs | ||||
| Average monthly effect | −1,293 | .055 | −2,617 | 30 |
| Average annual effect | −15,517 | .055 | −31,399 | 366 |
| Cumulative effect post-RP | −112,497 | .055 | −227,646 | 2,653 |
| Acetaminophen/Codeine | ||||
| Average monthly effect | −302 | .068 | −626 | 23 |
| Average annual effect | −3,621 | .068 | −7,516 | 274 |
| Cumulative effect post-RP | −26,251 | .068 | −54,490 | 1,987 |
| Opiates | ||||
| Average monthly effect | −343 | .023 | −636 | −49 |
| Average annual effect | −4,112 | .023 | −7,634 | −590 |
| Cumulative effect post-RP | −29,812 | .023 | −55,344 | −4,279 |
| Effect of Type 2 RP | ||||
| NSAIDs | ||||
| Average monthly effect | −38 | .896 | −618 | 542 |
| Average annual effect | −457 | .896 | −7,414 | 6,499 |
| Cumulative effect post-RP | −2,591 | .896 | −42,010 | 36,828 |
| Acetaminophen/Codeine | ||||
| Average monthly effect | 150 | .126 | −43 | 342 |
| Average annual effect | 1,794 | .126 | −511 | 4,099 |
| Cumulative effect post- RP | 10,167 | .126 | −2,895 | 23,228 |
| Opiates | ||||
| Average monthly effect | 492 | <.001 | 310 | 674 |
| Average annual effect | 5,904 | <.001 | 3,722 | 8,085 |
| Cumulative effect post-RP | 33,454 | <.001 | 21,094 | 45,814 |
Notes: We fit a moving average trend line through the data points. The vertical lines indicate the introduction of the three major NSAID cost control policies in the 1990s; see text for details of these policies.
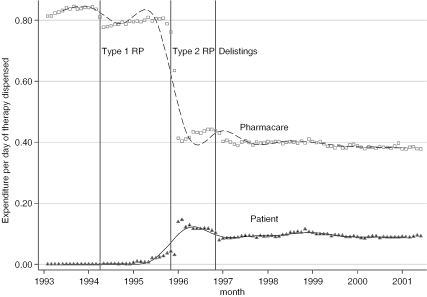
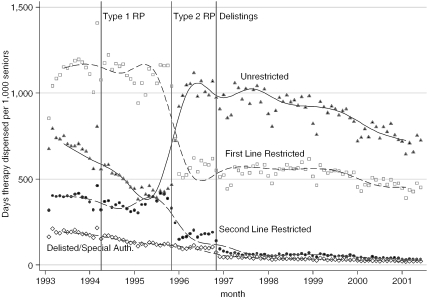
While Type 2 RP appeared to have little effect on the total volume of NSAIDs dispensed, it did have a substantial effect on reimbursement prices (see Figure 2 and Table 2). Pharmacare expenditure per day of NSAID therapy dispensed dropped by almost half, from $0.80 to $0.44, after Type 2 RP, and dropped to $0.40 after the “second line restricted” NSAIDs were delisted in November 1996 (Figure 2). Type 1 RP, by contrast had a much smaller effect on prices. About one-quarter of the savings that Pharmacare realized from the introduction of RP represented additional costs to nonexempt patients who elected to use higher priced NSAIDs. Patients paid nothing pre-RP but paid about $0.10 per day of NSAID therapy after RP (Figure 2). Most of the savings from the introduction of RP were therefore attributable to substitutions of low cost unrestricted for higher cost restricted NSAIDs. The price differences between these NSAIDs were considerable. Prior to the introduction of RP, the unrestricted NSAIDs cost Pharmacare $0.23 per day whereas the other NSAIDs cost up to six times as much (Table 2). As Figure 3 indicates, rates of prescribing unrestricted NSAIDs doubled after the introduction of Type 2 RP. This increase was entirely because of the increased use of naproxen; indeed, Table 3 indicates that rates of use of the other two unrestricted NSAIDs, ibuprofen and ASA, actually declined after the introduction of RP. Before the introduction of RP, the restricted NSAID diclofenac was the most widely used NSAID; a year after Type 2 RP was introduced, however, rates of use dropped to about one-quarter of its pre-RP rate of use.
Figure 2.
Average Expenditure per Day of NSAID Therapy Dispensed, by Month and Payer (Pharmacare and Patients)
Table 2.
Average Pharmacare Expenditure per Day of Therapy Dispensed, by Analgesic and Time Period
| Time Period | ||||
|---|---|---|---|---|
| Analgesic Category | Pre-RP | Type 1 RP | Type 2 RP | Delistings |
| Feb 93–Mar 94 | Apr 94–Oct 95 | Nov 95–Oct 96 | Nov 96–Jun 01 | |
| NSAIDS | ||||
| Unrestricted | ||||
| ASA ect 650 mg tab | 0.22 100 | 0.13 59 | 0.12 55 | 0.12 55 |
| Ibuprofen tab | 0.20 100 | 0.11 55 | 0.11 55 | 0.11 55 |
| Naproxen tab | 0.27 100 | 0.22 81 | 0.22 81 | 0.21 78 |
| Subtotal | 0.23 100 | 0.16 71 | 0.19 82 | 0.19 82 |
| First line restricted | ||||
| Diclofenac | 1.03 100 | 0.85 83 | 0.63 61 | 0.62 60 |
| Diclofenac/misoprostol | 1.24 100 | 1.23 99 | 1.00 81 | 0.90 72 |
| Diflunisal | 1.12 100 | 0.85 76 | 0.63 56 | 0.64 57 |
| Fenoprofen | 1.20 100 | 1.18 99 | 0.90 75 | 0.95 79 |
| Flurbiprofen | 1.23 100 | 0.84 69 | 0.61 50 | 0.61 49 |
| Indometacin | 0.49 100 | 0.43 88 | 0.41 84 | 0.35 71 |
| Ketoprofen | 0.89 100 | 0.55 62 | 0.46 52 | 0.41 46 |
| Naproxen ect & sr | 0.82 100 | 0.85 103 | 0.52 63 | 0.49 59 |
| Salsalate | — | 1.44 — | 1.23 — | 1.14 — |
| Subtotal | 0.96 100 | 0.87 91 | 0.66 69 | 0.62 65 |
| Second line restricted | ||||
| Sulindac | 1.27 100 | 1.04 82 | 0.81 64 | 1.00 79 |
| Nabumetone | — | 1.49 — | 1.22 — | 1.26 — |
| Piroxicam cap | 1.01 100 | 0.81 80 | 0.63 62 | 0.73 72 |
| Tenoxicam | 1.36 100 | 1.34 98 | 1.03 76 | 1.06 78 |
| Tiaprofenic acid | 1.46 100 | 1.23 84 | 0.78 53 | 0.93 64 |
| Tolmetin | 1.10 100 | 0.98 89 | 0.78 71 | 0.91 83 |
| Subtotal | 1.29 100 | 1.19 92 | 0.93 72 | 1.06 82 |
| Delisted/special auth. | 1.38 100 | 1.32 95 | 0.97 70 | 1.08 78 |
| All NSAIDs | 0.83 100 | 0.79 95 | 0.47 57 | 0.39 47 |
| COX-2 NSAIDS | — | — | — | 0.98 — |
| Acetaminophen & Codeine | ||||
| Acetaminophen | 0.41 100 | — | 0.14 35 | 0.11 27 |
| Codeine | 0.48 100 | 0.43 90 | 0.38 79 | 0.36 75 |
| Codeine/acetaminophen | 0.18 100 | 0.12 67 | 0.12 65 | 0.13 74 |
| Codeine/ASA | 0.90 100 | 0.80 89 | 0.83 92 | 0.81 89 |
| All Acetam./Codeine | 0.23 100 | 0.16 70 | 0.16 70 | 0.16 70 |
| OPIATES | ||||
| Oxycodone/acetaminophen | 3.10 100 | 1.04 34 | 0.87 28 | 0.85 27 |
| Fentanyl | 0.20 100 | 0.21 101 | 0.20 99 | 0.19 94 |
| Hydromorphone | 0.39 100 | 0.32 82 | 0.33 86 | 0.39 101 |
| Meperidine | 1.56 100 | 1.53 98 | 1.48 95 | 1.44 92 |
| Morphine | 3.05 100 | 3.03 99 | 2.89 95 | 2.56 84 |
| Propoxyphene | 0.87 100 | 0.86 98 | 0.84 96 | 0.83 95 |
| Anileridine | 4.60 100 | 4.59 100 | 4.46 97 | 4.29 93 |
| Pentazocine | 1.53 100 | 1.53 100 | 1.49 97 | 1.44 94 |
| All Opiates | 1.01 100 | 1.02 101 | 1.00 99 | 0.75 74 |
Notes: The bold values indicate period-specific average monthly rates relative to rates in the pre-RP period.
Figure 3.
Days of NSAID Therapy Dispensed per 1,000 Seniors, by NSAID Reimbursement Category and Month
Table 3.
Average Monthly Number of Days of Therapy Dispensed per 1,000 Seniors, by Analgesic and Time Period
| Time Period | ||||
|---|---|---|---|---|
| Analgesic Category | Pre-RP | Type 1 RP | Type 2 RP | Delistings |
| Feb 93–Mar 94 | Apr 94–Oct 95 | Nov 95–Oct 96 | Nov 96–Jun 01 | |
| NSAIDS | ||||
| Unrestricted | ||||
| ASA ect 650 mg tab | 313 100 | 196 63 | 141 45 | 78 25 |
| Ibuprofen tab | 117 100 | 87 74 | 109 93 | 112 96 |
| Naproxen tab | 271 100 | 191 70 | 702 259 | 682 252 |
| Subtotal | 701 100 | 474 68 | 952 136 | 872 124 |
| First line restricted | ||||
| Diclofenac | 533 100 | 374 70 | 176 33 | 138 26 |
| Diclofenac/misoprostol | 0 — | 6 — | 27 — | 32 — |
| Diflunisal | 137 100 | 262191 | 156 114 | 160 117 |
| Fenoprofen | 21 100 | 15 71 | 7 33 | 4 19 |
| Flurbiprofen | 5 100 | 4 80 | 2 40 | 1 20 |
| Indometacin | 38 100 | 25 66 | 12 32 | 8 21 |
| Ketoprofen | 135 100 | 116 86 | 105 78 | 96 71 |
| Naproxen ect & sr | 150 100 | 98 65 | 56 37 | 32 21 |
| Salsalate | 118 100 | 210 178 | 78 66 | 43 36 |
| Subtotal | 1,137 100 | 1,110 98 | 619 54 | 514 45 |
| Second line restricted | ||||
| Sulindac | 57 100 | 42 74 | 20 35 | 6 11 |
| Nabumetone | — | 57 — | 57 — | 21 — |
| Piroxicam cap | 89 100 | 62 70 | 30 34 | 7 8 |
| Tenoxicam | 97 100 | 97 100 | 33 34 | 7 7 |
| Tiaprofenic acid | 126 100 | 96 76 | 44 35 | 10 8 |
| Tolmetin | 22 100 | 15 68 | 9 41 | 4 18 |
| Subtotal | 391 100 | 369 94 | 193 49 | 55 14 |
| Delisted/special auth. | 189 100 | 137 72 | 103 54 | 47 25 |
| All NSAIDs | 2,407 100 | 2,081 86 | 1,870 78 | 1,478 61 |
| COX-2 NSAIDS | — | — | — | 72 — |
| Acetaminophen & Codeine | ||||
| Acetaminophen | 0 — | 0 — | 30 — | 45 — |
| Codeine | 10 100 | 10 100 | 11 110 | 19 190 |
| Codeine/acetaminophen | 788 100 | 707 90 | 703 89 | 709 90 |
| Codeine/ASA | 59 100 | 47 80 | 42 71 | 35 59 |
| All Acetam./Codeine | 857 100 | 764 89 | 786 92 | 808 94 |
| OPIATES | ||||
| Oxycodone/acetaminophen | 15 100 | 14 93 | 16 107 | 23 153 |
| Fentanyl | 208 100 | 176 85 | 179 86 | 302 145 |
| Hydromorphone | 55 100 | 54 98 | 64 116 | 131 238 |
| Meperidine | 4 100 | 4 100 | 4 100 | 4 100 |
| Morphine | 73 100 | 75 103 | 89 122 | 98 134 |
| Propoxyphene | 42 100 | 37 88 | 34 81 | 3 7 |
| Anileridine | 5 100 | 5 100 | 5 100 | 6 120 |
| Pentazocine | 7 100 | 5 71 | 5 71 | 2 29 |
| All Opiates | 409 100 | 370 90 | 396 97 | 569 139 |
Notes: The bold values indicate period-specific average monthly rates relative to rates in the pre-RP period.
Multiplying the price reductions attributable to the introduction of RP by the quantity of NSAIDs dispensed post-RP produced (undiscounted) total savings estimates of $7.5 million for Type 1 RP and $22.7 million for Type 2 RP (Table 4). The annualized savings are $1 million (95 percent CI: $0.6 to $1.5 million) and $4 million (95 percent CI: $3.6 to $4.4 million), respectively, or about 11 percent and 44 percent, respectively, of the $9.1 million Pharmacare spent on NSAIDs for seniors in the 12 months prior to Type 2 RP. Some of the savings attributed to Type 2 RP are actually because of the delistings of the second line restricted NSAIDs. This latter policy was responsible for about $0.04 or 10 percent of the $0.40 reduction in Pharmacare reimbursement prices that eventually accrued after the introduction of Type 2 RP (Figure 2). Hence, the delistings policy produced savings of about $400,000 annually (10 percent ×$4,000,000).
Table 4.
Effect of RP on Pharmacare NSAID Expenditures
| NSAIDs | ||||
|---|---|---|---|---|
| Estimate | p-Value | 95% CI | ||
| Effect of Type 1 RP | ||||
| Cumulative effect over all months | −$7,506,214 | <.001 | −$10,900,000 | −$4,098,872 |
| Cumulative effect per year | −$1,035,340 | <.001 | −$1,505,318 | −$565,362 |
| Effect of Type 2 RP | ||||
| Cumulative effect over all months | −$22,700,000 | <.001 | −$24,800,000 | −$20,600,000 |
| Cumulative effect per year | −$4,007,322 | <.001 | −$4,378,332 | −$3,636,312 |
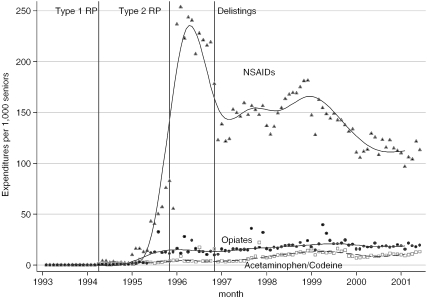
Pharmacare savings were partially offset by increased total patient spending of $92,000 and $820,000 annually after the introduction of Type 1 and Type 2 RP, respectively (Table 5). Patient spending increased rapidly immediately after the introduction of Type 2 RP but dropped off thereafter, stabilizing at about $150 per 1,000 seniors a year after the introduction of the policy (Figure 4). The rapid increase and then decrease in patient spending likely reflects payments by seniors who elected to pay out of their pocket when filling their first refill prescription for a restricted NSAID after the introduction of the policy, but who subsequently received exemption or switched to a lower cost NSAID. These figures exclude out-of-pocket costs for those seniors who did not receive a special authority exemption for second line restricted drugs used after November 1996. (As Pharmacare did not pay for any portion of these drugs, data on their use and expenditures are not available.) Moreover, our data exclude patient spending on nonreimbursed over-the-counter analgesics, such as low strength ASA.
Table 5.
Effect of RP on Patient NSAID Expenditures
| NSAIDs | ||||
|---|---|---|---|---|
| Estimate | p-Value | 95% CI | ||
| Effect of Type 1 RP | ||||
| Cumulative effect over all months | $666,340 | .041 | $27,884 | $1,304,795 |
| Cumulative effect per year | $91,909 | .041 | $3,846 | $179,972 |
| Effect of Type 2 RP | ||||
| Cumulative effect over all months | $4,637,901 | <.001 | $4,024,745 | $5,251,057 |
| Cumulative effect per year | $818,453 | <.001 | $710,249 | $926,657 |
Figure 4.
Patient Out-of-Pocket Drug Expenditures per 1,000 Seniors, by Analgesic Category and Month
Pharmacare savings from RP might also be mitigated by increased spending on more costly analgesics, such as opiates, which cost Pharmacare about $1 per day of therapy prior to the introduction of Type 1 RP (Table 2). According to the estimates presented in Table 1, and as is evident from Figure 1, Type 1 RP had only negligible effects on the prescribing of other analgesics, but there was an increase in rates of opiate use after the introduction of Type 2 RP in the order of 492 days of therapy per month per 1,000 patients. It is unclear from our aggregate data if opiates were being used as a substitute for NSAIDs, or if they were increasing for other reasons. Whatever the cause, the increased opiate use did not translate into increased Pharmacare expenditures because of offsetting reductions in Pharmacare expenditure per day of opiate therapy over the same time period (Table 2).
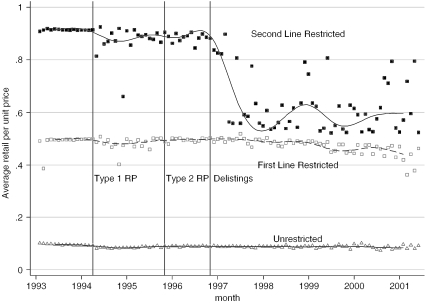
We next assessed the effects of Type 2 RP on the pricing decisions of the firms whose drugs were used to set the reference price (the unrestricted NSAIDs) or whose drugs were no longer fully reimbursed under the policy (the first and second line restricted NSAIDs). On the basis of the data displayed in Figure 5, there is no evidence that RP increased retail prices of either the unrestricted or restricted drugs. Indeed, Type 1 RP was associated with a small but sustained drop in the prices of unrestricted NSAIDs, and a larger but transitory drop in prices of second line restricted NSAIDs. Type 2 RP had virtually no effect on drug prices although the delisting of the various NSAIDs in November 1996 did coincide with a marked drop in the second line restricted drug prices. Based on the results of the models of the impact of RP on drug prices, displayed in Table 6, the introduction of Type 2 RP was associated with an average price drop of $0.23 per unit per month over the post–Type 2 RP period. As we did not control for the effect of the delistings policy in the regressions, this estimate can be interpreted as the combined influence of Type 2 RP and the delistings policy. Presumably, if this price reduction is a consequence of the delistings policy alone, then the price drop associated with this policy alone would be larger.
Figure 5.
Average Retail Unit Prices of NSAIDs, by Their Type 2 RP Reimbursement Status and Month
Table 6.
Effect of RP on the Average Monthly Unit Prices of Restricted and Unrestricted NSAIDs
| NSAIDs | ||||
|---|---|---|---|---|
| Estimate | p-Value | 95%CI | ||
| Effect of Type 1 RP | ||||
| Unrestricted | 0.027 | <.001 | 0.013 | 0.042 |
| First line restricted | −0.147 | .028 | −0.279 | −0.016 |
| Second line restricted | −0.014 | .625 | −0.070 | 0.042 |
| Effect of Type 2 RP | ||||
| Unrestricted | −0.003 | .655 | −0.015 | 0.010 |
| First line restricted | −0.016 | .680 | −0.094 | 0.061 |
| Second line restricted | −0.229 | <.001 | −0.313 | −0.145 |
Discussion
We found that Type 2 RP applied to the NSAIDs, coupled with the delisting of some higher-cost NSAIDs, reduced BC Pharmacare's expenditure on its senior beneficiaries by about $4 million annually during the 5 years following its introduction. Most of these savings accrued because of the increased use of lower cost NSAIDs (especially naproxen) and decreased use of higher cost NSAIDs (especially diclofenac). About 10 percent of these savings are because of delisting of some higher cost NSAIDs. A further 20 percent of savings represented expenditures by seniors who elected to pay for partially reimbursed drugs. A smaller proportion of these savings are because of reductions in the prices of second line restricted NSAIDs; although the price drop was substantial, these drugs were rarely used post-RP, so that expenditure reductions were modest. It is unclear from our analyses whether these price drops, which occurred about 1 year after the introduction of Type 2 RP, are causally related to RP or to the delistings of these NSAIDs. The net effect of the changes in prescribing, patient expenditures, and drug prices was to reduce average Pharmacare expenditure per day of NSAID therapy by about half; the total volume of NSAIDs dispensed was unaffected. Similar relative expenditure reductions were realized after the Medicaid programs of Georgia and Tennessee implemented prior authorization programs for various higher cost NSAIDs (Kotzan et al. 1993; Smalley et al. 1995). However, in both programs some of the expenditure reduction was because of decreased NSAID use.
Application of the Type 1 RP (generic substitution) produced annualized savings of $1 million, or roughly one-quarter of the savings realized by Type 2 RP. Type 2 RP was able to achieve larger savings by exploiting substantial price differences between different NSAIDs; Type 1 RP could only exploit price differences that existed between generic and brand versions of multisourced NSAIDs. RP was also applied to Pharmacare's other beneficiary groups—residents of long-term care facilities, social assistance recipients, and households whose drug costs exceed an income-contingent deductible. In 1999, Pharmacare drug expenditures on these groups were sizeable—about 86 percent of Pharmacare expenditures are on seniors (British Columbia Ministry of Health Services 2001). Given that per capita analgesic use is likely lower among these groups, RP probably generated less than proportionate budgetary savings. The application of RP could have also promoted the prescribing of lower cost NSAIDs to those not covered by Pharmacare, such as those under 65 covered by private insurance.
Pharmacare program savings accrued despite liberal exemption criteria and the opportunity for physicians to prescribe higher potency and more costly analgesics that were not subject to reimbursement restriction. While there was a modest increase in opiate use after the application of Type 2 RP, reductions in opiate prices more than offset increases in use. It is unclear if the increased opiate use is causally related to RP. It is possible that the increased opiate use was because of treatment failure on the lower cost NSAIDs or because of other factors unrelated to RP. This area, like the estimation of the effect of RP on drug prices, remains an area for future research.
Acknowledgments
Support for this project from the Health Transitions Fund, Health Canada; the Canadian Health Services Research Foundation; Brogan Inc.; the BC Ministry of Health; and the Drug Information Association is acknowledged. Doreen Au provided invaluable research assistance. We thank Sean Burnett, Wendy Eyres, and Suzanne Solven of the BC Ministry of Health for assistance with the collection and interpretation of the data. Paul Grootendorst is supported by a career award from the Rx&D Health Research Foundation and the Canadian Institutes of Health Research (CIHR). Anne Holbrook is the recipient of a CIHR Career Investigator Award; Adrian Levy is supported by a Scholar award from the BC Michael Smith Foundation for Health Research. This paper is dedicated to the memory of our colleague Bernie O'Brien (1959–2004).
Note
These include etodolac, phenylbutazone, floctafenine, mefenamic, the SR, and enteric coated (other than 650 mg) forms of ASA, 1 g SR form of naproxen, as well as the injectable and/or suppository forms of indometacin, naproxen, ketoprofen, diclofenac, and piroxicam.
Supplementary Material
The following supplementary material is available for this article online:
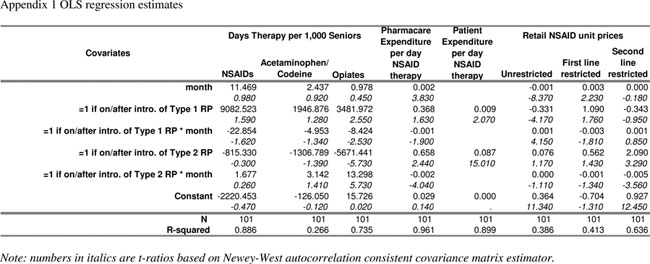
TABLE S1.
OLS regression estimates
References
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines “Recommendations for the Medical Management of Osteoarthritis of the Hip and Knee.”. Arthritis and Rheumatism. 2000;43(9):1905–15. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Anis A, Wen Q. “Price Regulation of Pharmaceuticals in Canada.”. Journal of Health Economics. 1998;17:21–38. doi: 10.1016/s0167-6296(97)00016-7. [DOI] [PubMed] [Google Scholar]
- British Columbia Ministry of Health Services “Pharmacare Trends 2000”. 2001. [accessed on September 14, 2004]. Available at http://www.healthservices.gov.bc.ca/pharme/outgoing/PcareTrends2000.pdf.
- Brooks PM, Day RO. “Nonsteroidal Antiinflammatory Drugs—Differences and Similarities.”. New England Journal of Medicine. 1991;324:1716–25. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- Health Canada . Arthritis in Canada. An Ongoing Challenge (Cat. # H39-4/14-2003E) Ottawa: Health Canada; 2003. [Google Scholar]
- Henry D, Lim LLY, Rodriguez LAG, Gutthann SP, Carson JL, Griffin M, Savage R, Logan R, Moride Y, Hawkey C, Hill S, Fries JT. “Variability in Risk of Gastrointestinal Complications with Individual Non-Steroidal Anti-Inflammatory Drugs: Results of a Collaborative Meta-Analysis.”. British Medical Journal. 1996;312:1563–6. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook AM, The Ontario Musculoskeletal Therapy Review Panel . Medical Treatment Guidelines for the Treatment of Osteoarthritis, Rheumatoid Arthritis and Acute Musculoskeletal Injury. Toronto: Ontario Ministry of Health; 2001. [Google Scholar]
- Huskamp HA, Rosenthal MB, Frank RG, Newhouse JP. “The Medicare Prescription Drug Benefit: How Will the Game Be Played?”. Health Affairs. 2000;19:8–23. doi: 10.1377/hlthaff.19.2.8. [DOI] [PubMed] [Google Scholar]
- Kanavos P, Reinhardt U. “Reference Pricing for Drugs: Is It Compatible with U.S. Health Care?”. Health Affairs. 2003;22(3):16–30. doi: 10.1377/hlthaff.22.3.16. [DOI] [PubMed] [Google Scholar]
- Kotzan JA, McMillan J, Jankel C, Foster A. “Initial Impact of a Medicaid Prior Authorization Program for NSAID Prescriptions.”. Journal of Research in Pharmaceutical Economics. 1993;5:25–41. [Google Scholar]
- Langman M, Kahler KH, Kong SX, Zhang Q, Finch E, Bentkover JD, Stewart EJ. “Drug Switching Patterns among Patients Taking Non-Steroidal Anti-Inflammatory Drugs: A Retrospective Cohort Study of a General Practitioners Database in the United Kingdom.”. Pharmacoepidemiology and Drug Safety. 2001;10:517–24. doi: 10.1002/pds.653. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. “Estimates of the Prevalence of Arthritis and Selected Musculoskeletal Disorders in the United States.”. Arthritis and Rheumatism. 1998;41:778–99. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Lopez-Casasnovas G, Puig-Junoy J. “Review of the Literature on Reference Pricing.”. Health Policy. 2000;54:87–123. doi: 10.1016/s0168-8510(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Momani AA, Madhavan SS, Nau DP. “Impact of NSAIDs Prior Authorization Policy on Patients' QoL.”. Annals of Pharmacotherapy. 2002;36:1686–91. doi: 10.1345/aph.1C008. [DOI] [PubMed] [Google Scholar]
- Morgan SG, Barer ML, Agnew JD. “Whither Senior's Pharmacare: Lessons from (and for) Canada.”. Health Affairs. 2003;22:49–59. doi: 10.1377/hlthaff.22.3.49. [DOI] [PubMed] [Google Scholar]
- Morton FS. “The Strategic Response by Pharmaceutical Firms to the Medicaid Most-Favored-Customer Rules.”. Rand Journal of Economics. 1997;28:269–90. [PubMed] [Google Scholar]
- Newey W, West K. “A Simple, Positive Semi-Definite Heteroscedasticity and Autocorrelation Consistent Covariance Matrix.”. Econometrica. 1987;55:703–8. [Google Scholar]
- Rodriguez LAG. “Variability in Risk of Gastrointestinal Complications with Different Nonsteroidal Anti-Inflammatory Drugs.”. American Journal of Medicine. 1998;104:41–2. doi: 10.1016/s0002-9343(97)00208-8. [DOI] [PubMed] [Google Scholar]
- Smalley WE, Griffin MR, Fought RL, Sullivan L, Ray WA. “Effect of A Prior-Authorization Requirement on the Use of Nonsteroidal Antiinflammatory Drugs by Medicaid Patients.”. New England Journal of Medicine. 1995;332:1612–7. doi: 10.1056/NEJM199506153322406. [DOI] [PubMed] [Google Scholar]
- Statistics Canada “Estimates of Population, by Age Group and Sex, Canada, Provinces and Territories, CANSIM table 510001”. 2004. [accessed on September 14, 2004]Available at http://datacenter2.chass.utoronto.ca/cgi-bin/cansim2/getSeries.pl?s=V469836.
- Tamblyn R, Laprise R, Hanley JA, Abrahamowicz M, Scott S, Mayo N, Hurley J, Grad R, Latimer E, Perreault R, McLeod P, Huang A, Larochelle P, Mallet L. “Adverse Events Associated with Prescription Drug Cost-Sharing Among Poor and Elderly Persons.”. Journal of the American Medical Association. 2001;285:421–9. doi: 10.1001/jama.285.4.421. [DOI] [PubMed] [Google Scholar]
- Therapeutics Initiative “Should We Be Using NSAIDs for the Treatment of Osteoarthritis and “Rheumatism?”. 1995. [accessed on September 14, 2004]. Available at http://www.ti.ubc.ca/pages/letter4.html#table2. [PubMed]
- Walker AM, Chan KW, Yood RA. “Patterns of Interchange in the Dispensing of Non-Steroidal Anti-Inflammatory Drugs.”. Journal of Clinical Epidemiology. 1992;45:187–95. doi: 10.1016/0895-4356(92)90015-f. [DOI] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC Classification and DDD Assignment. Oslo: World Health Organization; 2000. [Google Scholar]
- Williams JI, Young W. Technical Report. Toronto: Institute for Clinical Evaluative Sciences; 1996a. “Inventory of Studies on the Accuracy of Canadian Health Administrative Databases.”. [Google Scholar]
- Williams JI, Young W. “A Summary of Studies on the Quality of Health Care Administrative Databases in Canada.”. In: Goel V, Williams JI, Anderson G, Blackstein-Hirsch P, Fooks C, Naylor CD, editors. Patterns of Health Care in Ontario. 2d ed. Ottawa: Canadian Medical; 1996b. pp. 339–45. [Google Scholar]
- Wolfe MM, Lichtenstein DR, Singh G. “Gastrointestinal Toxicity of Nonsteroidal Antiinflammatory Drugs.”. New England Journal of Medicine. 1999;340:1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- Zweifel P, Crivelli L. “Price Regulation of Drugs: Lessons from Germany.”. Journal of Regulatory Economics. 1996;10:257–73. [Google Scholar]