Abstract
Epstein-Barr virus (EBV) transformation of B cells from fetal cord blood in vitro varies depending on the individual sample. When a single preparation of EBV was simultaneously used to transform fetal cord blood samples from six different individuals, the virus transformation titer varied from less than zero to 105.9. We show that this variation in EBV transformation is associated with a marked primary immune response in cord blood samples predominately involving CD4+ T cells and CD16+ CD56+ NK cells. After virus challenge both CD4+ T cells and NK cells in fetal cord blood cultures expressed the lymphocyte activation marker CD69. The cytotoxic response against autologous EBV-infected lymphoblastoid cell line (LCL) targets correlated with the number of CD16+ CD69+ cells and was inversely correlated with the virus transformation titer. Although NK activity was detected in fresh cord blood and increased following activation by the virus, killing of autologous LCLs was detected only following activation by exposure to the virus. Both activated CD4+ T cells and CD16+ NK cells were independently able to kill autologous LCLs. Both interleukin-2 and gamma interferon were produced by CD4+ T cells after virus challenge. The titer of EBV was lower when purified B cells were used than when whole cord blood was used. Addition of monocytes restored the virus titer, while addition of resting T cells or EBV-activated CD4+ T-cell blasts reduced the virus titer. We conclude that there are primary NK-cell and Th1-type CD4+ T-cell responses to EBV in fetal cord blood that limit the expansion of EBV-infected cells and in some cases eliminate virus infection in vitro.
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus that is carried by more than 90% of the human population as a lifelong latent infection (reviewed in reference 41). EBV transforms or immortalizes B cells in vitro (37), and this property has been used as an assay for the virus itself (31) and for virus-neutralizing antibodies (18, 35). Despite the transforming properties of the virus, EBV infection is asymptomatic in the vast majority of individuals. Nevertheless, it is associated with a number of important cancers including undifferentiated nasopharyngeal carcinoma, endemic Burkitt's lymphoma, certain Hodgkin's lymphomas, and posttransplant lymphoproliferative disease (1). A normal, healthy immune response appears to be essential in maintaining the asymptomatic carrier state.
Most work on cell-mediated immune responses to EBV has focused on secondary immune responses to the virus in seropositive individuals and has concentrated on the major histocompatibility complex (MHC) class I-restricted cytotoxic response of peripheral blood CD8+ T cells to EBV-transformed lymphoblastoid B-cell lines (LCL) (reviewed in references 40 and 43). Activation of memory T cells from peripheral blood from seropositive individuals causes the growth of EBV-transformed B cells to regress in vitro (42). More recently, there have been a number of reports of cytotoxic CD4+ T cells which are able to inhibit LCL growth (22, 32, 46, 50). Several mechanisms of killing by CD4+ T cells, including Fas/Fas ligand, granzyme, and perforin, have been reported (50). Furthermore, CD4+ T-cell effectors from seropositive individuals have been shown to inhibit the initial phase of EBV-induced B-cell proliferation (34). However, under certain circumstances CD4+ T cells can also enhance LCL growth in vitro (20), and T cells are required for the optimal development of EBV B lymphomas in SCID-Hu mice (13, 23, 24, 36), providing further evidence of potential T-helper function in B-cell growth transformation by EBV.
EBV infection is usually acquired in infancy, at which time it is not associated with any defined clinical disease and is presumed to be asymptomatic. However, if primary infection is delayed until adolescence or adulthood, a high proportion of individuals develop infectious mononucleosis (IM) (reviewed in reference 2). IM is characterized by increased numbers of EBV-infected B cells in peripheral blood and massive oligoclonal expansion of EBV-specific CD8+ T cells (11, 12). Although the reason why IM occurs only in some individuals is not known, one possible interpretation of the above observations is that IM occurs when primary EBV infection is not adequately controlled, leading to a subsequent overstimulation of CD8 T cells by EBV-infected B cells.
Primary EBV-specific cytotoxic CD8+ T-cell responses to LCL have not been demonstrated in vitro. Several studies have detected primary cytotoxic CD4+ T-cell responses either in fetal cord blood or in lymphocytes from seronegative adults following stimulation by autologous EBV-infected LCL (28, 29, 46, 50). In addition. EBV-transformed LCL can activate NK cells from both adult and fetal blood (29). However, LCL are not able to restimulate in vitro all of the responses that occur to EBV in vivo. These include responses to those lytic cycle antigens not present in LCL (38) and some EBV antigens that require presentation by bystander dendritic cells (7). The limitations of studies using LCL to stimulate a response and the potential importance of primary immune responses to EBV have prompted us to examine the immune reaction of unprimed fetal cord blood lymphocytes to EBV challenge with live virus.
Fetal cord blood lymphocytes have been used for many years to assay EBV in terms of its ability to transform cord blood B cells. It has been tacitly assumed that fetal cord blood has no immune response to EBV and is therefore ideal for this purpose. Here we present evidence to the contrary and describe primary cellular immune responses to EBV-infected cells by fetal cord blood lymphocytes. The variation in these responses relates to the variation in the observed B-cell transformation titer of a standard sample of virus. The data shown demonstrate that cellular immune responses of naive fetal cord blood lymphocytes can regulate EBV infection in vitro and that these responses vary greatly among individuals.
MATERIALS AND METHODS
Collection of fetal cord blood.
Samples of fetal cord blood were collected from placental veins, following elective caesarean section, by aspiration into 50-ml heparinized syringes (20 U/ml) within 10 min of delivery. The blood was processed immediately following collection. Each sample was diluted 1:1 with RPMI 1640 medium containing 10 mM glutamine, 100 IU of penicillin/ml, and 10 μg of streptomycin/ml. The diluted blood was layered over Ficoll Hypaque (Lymphocyte separation medium; ICN Flow) and centrifuged at 400 × g for 30 min at room temperature. Cells from the interface were collected and washed twice by suspension and centrifugation in phosphate-buffered saline (PBS). At the second wash, a 10-μl sample was mixed in a 1:1 ratio with red blood cell-lysing fluid (Haemolyse; Coulter), and nucleated cells were counted. Following centrifugation, the cells were resuspended at 2 × 107/ml in RPMI 1640 with 50% fetal calf serum (FCS) and 10% dimethyl sulfoxide, and 1-ml aliquots were frozen and stored in liquid nitrogen.
Virus preparation.
High-titer stocks of the B958 laboratory strain of EBV were prepared from B958 cells (American Type Culture Collection [ATCC]) as previously described (49). Briefly, 10 liters of cell culture was centrifuged at 700 × g for 10 min to remove cells and debris. The supernatant was then centrifuged at 100,000 × g for 90 min to pellet the virus, which was resuspended in RPMI 1640-10% FCS, filtered through a 0.22-μm-pore-size filter, and stored frozen at −70°C.
Cell culture.
All cells were cultured in RPMI 1640 supplemented with10 mM glutamine, 100 IU of penicillin/ml, 10 μg of streptomycin (Gibco)/ml, and 10% FCS. EBV-transformed LCL were recovered from the initial virus titration plates; for samples where no initial virus transformation occurred, LCL were initiated by culturing an aliquot of cord blood with EBV in the presence of 5 μg of phytohemagglutinin A (PHA)/ml. Established lines were cultured in 10-ml flasks and split 1:5 twice weekly. Other cell lines used as targets in cytotoxicity assays were the MHC class I-negative myeloid cell line K562 (ATCC) and the β-2 microglobulin-negative Burkitt's lymphoma line Daudi (ATCC). The MHC class I-negative lymphoblastoid cell line LCL721.221 (referred to below as 221) (45) was a gift from V. Braud, University of Oxford.
Virus titration.
Simultaneous titration of a single aliquot of EBV was carried out on six unfractionated cord blood samples as previously described (49). Briefly, serial 10-fold dilutions of 10−1 to 10−6 were made from a single vial of concentrated virus. A vial of each of the six cord blood samples was thawed, and the cells were resuspended in tissue culture medium at a concentration of 2.2 × 106/ml. For each cord blood sample, six 900-μl aliquots of cell suspension were mixed with 100-μl aliquots of each dilution of virus. Following a 1-h incubation at 37°C, each dilution of cells and virus was plated out into 10 replicate wells of a 96-well microtiter plate containing a further 100 μl of medium to give a final volume of 200 μl/well. Samples were incubated at 37°C in a humidified atmosphere of 5% CO2 and fed weekly by replacement of 100 μl of medium per well. After 6 weeks the wells were examined under an inverted microscope and scored for the presence of typical colonies of EBV-transformed cells. The virus titer was defined as the highest dilution at which 50% of the wells were positive for EBV-transformed growth, calculated as previously described (39, 49).
Virus challenge.
An aliquot of cord blood lymphocytes was resuspended in RPMI 1640-10% FCS at 2 × 106/ml. A 10-μl volume of concentrated virus was added to each milliliter of cell suspension (equivalent to a virus concentration of at least 100 times its 50% end point titer assayed in a permissive sample of cord blood). The samples were cultured for 7 days, the cells were harvested, and their phenotypes were determined by flow cytometry, or the cells were assayed for cytotoxic function in a chromium release assay.
Cytotoxic assay.
Cytotoxic function was assayed by chromium release. A total of 106 target cells were labeled by incubation with 0.1 μCi of 51Cr (Amersham) in 100 μl of Hanks' balanced salt solution at 37°C for 1 h. The target cells were washed in PBS and then resuspended at a concentration of 105/ml in tissue culture medium. A 100-μl volume of target cells was placed in wells of a 96-well V-bottom plate, and 100 μl of effector cells was added to triplicate wells. Control wells containing 100 μl of medium alone (background counts per minute) or 100 μl of 1% Triton X-100 (total counts per minute) were also prepared. The plates were centrifuged at 400 × g for 30 s to pellet the cells and were incubated for 5 h at 37°C, at which time 50 μl of supernatant was removed and mixed with 250 μl of scintillant (Optiphase Hi Safe; LKB) in 96-well polyethyltolbuamide (PET) plates, and counts per minute were then determined with a scintillation counter. The average of each triplicate was taken, and the percent specific cytotoxicity was calculated by the following formula: (specific counts per minute − background counts per minute)/(total counts per minute − background counts per minute) × 100.
Flow cytometry.
Debris and dead cells were removed from 7-day cultures by overlaying T cells on an isotonic density gradient of 18.36% (wt/vol) metrizamide made up by using 1.02 ml of 36% (wt/vol) metrizamide in distilled water (ICN Flow) plus 0.94 ml of PBS and 0.04 ml of FCS. Live cells were collected from the interface after centrifugation at 500 × g for 15 min.
Prior to staining, both fresh and cultured cord blood cells were pelleted, resuspended in 5 ml of 0.15 M ammonium chloride-10 mM potassium bicarbonate-100 mM EDTA, and incubated for 5 min at room temperature to lyse red blood cells. Cells were washed and resuspended at a concentration of 106/ml in PBS-0.01% sodium azide. Aliquots (100 μl) were stained for 1 h at 4°C with the following fluorochrome-conjugated antibodies: anti-CD4-phycoerythrin (PE) (clone MT310; Dako), anti-CD8-fluorescein isothiocyanate (FITC) (clone DK25; Dako), anti-CD3-PE-Cy5 (UCHT1; Sigma), anti-CD45RA-FITC (clone F8.11.13; Sigma), anti-CD45RO-PE (UCHL1; Sigma), anti-CD16-FITC (Serotec), or anti-CD69-PE-Cy5 (Immunotech). Cells were washed and analyzed on a FACScan (Becton Dickinson). Data were analyzed with WinMDI software (Scripps). Cells were prepared and stained for fluorescence sorting as described for flow cytometry by using azide-free buffers, and the final suspension was made in PBS-0.05 mM EDTA-1% bovine serum albumin. Samples were analyzed by using a FACSscan. Ten thousand gated events were collected for each sample. Cell sorting was carried out by using a FACS Vantage flow cytometer (Becton Dickinson).
Intracellular cytokine staining was carried out on fresh cord blood lymphocytes, cells cultured for 7 days after virus challenge, or cells cultured for 4 days with PHA. The latter were maintained until day 10 by resuspension in a medium containing 10 IU of recombinant interleukin-2 (IL-2)/ml. Cells were first restimulated by culturing for 5 h with the addition of 50 ng of phorbol myristate acetate (PMA)/ml, 250 ng of ionomycin/ml, and 10 mM monensin (Sigma) before intracellular cytokine staining was carried out. Cell debris was removed by centrifugation through metrizamide as described above, and cells were washed and resuspended in 0.1 M lysine-0.05 M phosphate buffer. Cells were fixed by addition of 8% stock paraformaldehyde to a final concentration of 0.5%. After 1 h, cells were pelleted, resuspended in fresh lysine phosphate buffer, and then kept at 4°C until analysis. Cells were made permeable for staining by washing and suspension in PBS containing 0.1% saponin (Sigma). The cells were stained with anti-CD4-FITC, anti-CD3-Cy5, and either a PE-conjugated immunoglobulin G1 (IgG1) control antibody (Sigma) or anti-gamma interferon (anti-IFN-γ) (Serotec), PE-anti-IL-2 (Sigma), or PE-anti-IL-4 (Sigma). Results were analyzed on a FACSscan flow cytometer.
Fractionation of cord blood cells.
B cells and monocytes were purified from whole cord blood by magnetic cell sorting. Cells were labeled with either the anti-CD19 antibody clone HD37 or the anti-CD14 antibody clone TUK4 (Dako), followed by a goat anti-mouse paramagnetic microbead conjugate (Miltenyi Biotec, Bisley, Surrey, United Kingdom). Cells were washed in PBS-0.1% bovine serum albumin-1 mM EDTA and passed over a “Minimacs” column (Miltenyi Biotec). Retained CD19+ B cells or CD14+ monocytes were eluted, washed in PBS, and then resuspended in RPMI medium. The flowthrough was further passed through a glass bead column coated with human IgG and anti-human immunoglobulin to remove all remaining Fc receptor- or immunoglobulin-positive cells; the remaining cells were analyzed by flow cytometry and shown to be 90% CD3+ T cells.
Generation of CD4+ T-cell lines.
Autologous LCL were produced from cord blood by transformation with B958 virus. Once established, the LCL were cultured in 10% autologous plasma and used to stimulate T-cell responses from cord blood lymphocytes as previously described (50). Briefly 2 × 106 cord blood lymphocytes were mixed with 5 × 104 irradiated (3,000 rads) autologous LCL in 2 ml of RPMI-10% autologous plasma. On day 10, the cultures were stimulated with a further 2 × 105 autologous LCL in autologous plasma. On day 14 and weekly thereafter, the cultures were adjusted to 106 cells per ml and stimulated with the addition of 106 autologous LCL and 10 U of IL-2 per ml. Once established, the cultured cells were more than 80% CD4+ T cells. They were purified to 100% CD4+ T cells by magnetic activated cell sorting.
RESULTS
Not all fetal cord blood samples support EBV transformation.
Following EBV challenge of fetal cord blood, clumps of cells were visible after 4 days (Fig. 1). Surprisingly, in as many as 20% of individual cord blood samples, the clumps were seen to disperse after 2 weeks and the cells died out, indicating an apparent regression of transformation. Figure 2 shows virus titers following transformation of five separate samples of fetal cord blood, demonstrating the variation in virus titer between different cord bloods, including one that failed to transform. By comparison, purified B cells from the same samples gave a 10-fold-lower titer of virus in four out of five cases. This indicates that B cells require the help of some accessory cells for optimal transformation. In contrast, the purified B cells from the sample which failed to transform also grew into LCL, indicating that failure to transform the unfractionated sample of cord blood was not due to a functional deficit of either the virus or the B cells. Furthermore, addition of CD14+ monocytes to the B cells partly restored their transformation, providing evidence for the role of monocytes as accessory cells enhancing virus transformation, whereas addition of resting T cells to the purified B-cell cultures reduced or eliminated the transformation. These results indicate that primary cellular immune responses in cord blood can exert a controlling influence on EBV transformation of B cells in vitro and that this may account for the variation in virus transformation titer that has been observed between different samples of cord blood lymphocytes.
FIG. 1.
Photomicrograph of fetal cord blood 4 days after infection with EBV. Large clumps of cells have formed throughout the well, indicating that many cell types become activated and adhere to one another in association with EBV-infected B cells.
FIG. 2.
Bar graph showing the transformation titers of EBV on five separate samples of unfractionated cord blood. The titer of virus was lower when measured on purified B cells. The titer was restored by addition of monocytes (Mφ) to the purified B cells. In contrast, addition of T cells to purified B cells reduced the virus titer.
The same EBV preparation gives different transformation titers against cord blood lymphocytes from different individuals.
In order to control for variation between cord blood samples, possibly due to differences in the method of collection or the length of time between collection and processing, we collected six samples of cord blood in an identical manner, separated the lymphocytes, and froze them within 1 h. We then titrated a single sample of EBV on an aliquot of each cord blood sample. The results shown in Table 1 demonstrate that, depending on the cord blood sample on which it was titrated, a single sample of virus can have an apparent transformation titer ranging from zero up to a maximum of 105.9. This could not be explained by the variation in the number of B cells between samples.
TABLE 1.
Apparent transformation titers of a single sample of EBV measured simultaneously on six samples of fetal cord blood containing various proportions of CD19+ cells
| Cord blood sample | % CD19+ cells | Log10 virus titer |
|---|---|---|
| 1 | 2.5 | 4.52 |
| 2 | 2.7 | 4.91 |
| 3 | 12.0 | 1.2 |
| 4 | 8.0 | <1.0 |
| 5 | 3.0 | 5.9 |
| 6 | 5.7 | 3.85 |
Fetal cord blood CD4+ T cells and NK cells become activated following EBV challenge.
To determine which cord blood lymphocytes might be involved in responses to EBV challenge in vitro, we determined the phenotypes of the fresh fetal cord blood lymphocytes and the cells remaining in a fetal cord blood culture 7 days after virus challenge. The proportions of T cells, monocytes, and NK cells in freshly isolated fetal cord blood are similar to those in adult blood. Analysis of the resting cells in the six samples used in the above experiment showed that CD4+ (43% ± 6.3%) and CD8+ (21% ± 4.3%) T cells together make up the majority of the cells (Fig. 3a). The remaining cells were mostly CD14+ monocytes (12% ± 3.3%), CD16+ NK cells (16.4% ± 4.4%), and CD19+ B cells (5.7% ± 3.7%) (data not shown). The CD45RA isotype indicative of naive cells was present on a proportion of the cord blood lymphocytes (Fig. 3b). A few CD45RO+ memory cells were also found in most samples (Fig. 3b). Fewer than 1% of resting cells expressed CD69, a marker of early activation on a range of cell types (data not shown).
FIG. 3.
Flow cytometry plots of fetal cord blood stained with different combinations of fluorescent antibodies. (a) CD4-PE and CD8-FITC staining of fresh fetal cord blood, showing the typical pattern of CD4high T cells and CD4low monocytes on the y axis versus CD8high T cells and CD8low NK cells on the x axis. Third-color staining with CD3-PE-Cy5 confirmed the phenotype of the T cells (data not shown). (b) CD45 expression on freshly isolated cord blood. Many of the cells express the CD45RA isoform, which is typical of naive lymphocytes; only a few cells express the CD45RO isoform, present on memory cells. A considerable number of cells do not express either isoform of CD45. (c) There is a slight increase in the proportion of fetal cord blood cells expressing CD45RO after 7 days of culture, indicating that some cells have become activated and developed a memory phenotype. (d) There is a greater increase in the proportion of cells expressing CD45RO 7 days after EBV challenge of fetal cord blood, indicating that many cells have become activated and developed a memory phenotype.
After culture for 7 days in medium alone, there was a considerable reduction in the total number of live cells recovered, and the proportion of cells of each phenotype also changed. Invariably, some cells present after culture in FCS alone became activated, as demonstrated by the expression of CD45RO (Fig. 3c) or CD69 (Fig. 4 a through c). However, by comparison, following virus challenge there was both a greater recovery of total viable cells after a week in culture and a more marked increase in the proportion of cells expressing CD45RO or CD69 (Fig. 3d and 4e through g). Overall, following virus challenge, there was a reduction in the average percentage of CD4+ T cells (24.6% ± 10%); however, a substantial portion (40% ± 13%) of these CD4+ T cells were now activated, as indicated by their expression of CD69 (Fig. 4d). Conversely, there was an increase in the average percentage of CD16+ cells (38% ± 17%), the majority of which (74% ± 9.8%) now coexpressed CD69 (Fig. 4f), indicating that they too had become activated. There was also an increase in the percentage of CD69+ CD8+ cells (Fig. 4e); however, these were almost entirely CD8low CD3− CD16+ cells, with only a small proportion of CD8high CD3+ T cells remaining, of which only a few expressed CD69 (Fig. 4e).
FIG. 4.
(a through c) Expression of CD4 and CD69 (a), CD8 and CD69 (b), or CD16 and CD69 (c) on fetal cord blood cells after 7 days in culture with FCS alone. (d through f) Expression of CD4 and CD69 (d), CD8 and CD69 (e), or CD16 and CD69 (f) on fetal cord blood cells 7 days after challenge with EBV.
We analyzed the data for correlation between the activated phenotype of the cells from each of the six cord blood samples and the transformation titer of EBV virus obtained for that sample. Figure 5 depicts the statistically significant negative correlation (Spearman's rank correlation coefficient [r] = 0.943; P < 0.035; n = 6) between the percentage of CD16+ NK cells on day 7 and the ultimate virus transformation titer, i.e., samples with the lowest virus transformation titers had the highest numbers of NK cells on day 7 postchallenge. Conversely, there was a trend toward higher percentages of CD4+ cells in samples with high virus transformation titers, but this was not statistically significant (P > 0.05) (data not shown).
FIG. 5.
Significant negative correlation between the transformation titer of EBV obtained with each cord blood sample and the percentage of CD16+ cells present in cultures of the same cord blood sample 7 days after virus challenge.
Cytotoxic function of EBV-activated cord blood lymphocytes.
We went on to test the cytotoxic activity, against autologous LCL and NK target cells, of EBV responder cells from fetal cord blood 1 week after stimulation with virus. First, we measured the cytotoxicities of 100-μl aliquots of the resuspended cultures without recovering or adjusting the numbers of viable cells. In this way we could obtain a measure of the total cytotoxic capacity induced by EBV exposure in any cord blood sample. The results of this experiment are shown in Fig. 6. There was some cytotoxicity toward K562 NK and Daudi target cells following virus challenge in most of the samples. This cytotoxic activity was greatest in those samples that had a high percentage of NK cells and gave a low virus transformation titer. In addition, the two samples with the highest NK activities were also able to kill autologous LCL. However, these results take no account of differences in the numbers of live cells present on day 7 or of potential differences between the NK activities of the original individual resting cord blood samples.
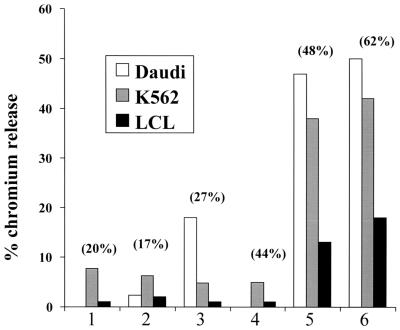
FIG. 6.
Cytotoxic activities in 100-μl aliquots of cells taken from cord blood cultures 7 days post-virus challenge. The percentage of CD16+ NK cells in each culture is given. All cultures had some cytotoxicity for the NK K562 target cells. The cultures with high percentages of CD16+ cells had the highest NK activities; they were also able to kill the Burkitt's lymphoma cell line Daudi, which is a target for lymphokine-activated killer cells, as well as autologous LCL.
To address these issues, we compared the cytotoxic function of the fresh cord blood samples with the cytotoxic activity of the live cells on day 7. In this experiment the live cultured cells were recovered on metrizamide gradients and used at the same effector/target ratios. Figure 7 shows the cytotoxic activities of fresh and cultured cord blood lymphocytes from three of the six cord blood samples. The first of these did not support virus transformation (Fig. 7a), the second showed an intermediate transformation titer (Fig. 7b), and a third showed a high transformation titer (Fig. 7c) following exposure to virus. All of the samples from fresh cord blood had NK cell activity against the MHC class I-negative 221 cells but not against autologous LCL (Fig. 7). After 7 days of culture following exposure to EBV, there was a marked increase in NK activity in the sample with a low transformation titer, and this sample showed high levels of killing of autologous LCL. A similar pattern was seen in the samples with intermediate virus titers. In contrast, 7 days after exposure to EBV, there was minimal change in the NK activities of the samples with high transformation titers. These samples also showed only a marginal increase in killing of autologous LCL at this time. Overall, the results show that the high levels of cytotoxicity seen in cord blood samples with low virus transformation titers are associated with activation of cells by exposure to EBV, leading to both quantitative and qualitative increases in cytotoxic capacity against both NK targets and autologous LCL.
FIG. 7.
Comparison of cytotoxic activities in three samples of cord blood before virus challenge and on day 7 after virus challenge. Open rectangles, NK activity of fresh cord blood against the MHC class I-negative cell line 221; filled rectangles, NK activity of the cells against 221 target cells on day 7; open triangles, cytotoxic activity of fresh cord blood against autologous LCL; filled triangles, cytotoxic activity of the same sample against autologous LCL 7 days after virus challenge. (a) Cells from a cord blood sample with a low virus transformation titer of <101; (b) cells from a sample with an intermediate titer of 103.9; (c) cells from a cord blood sample with a high titer of 105.9. All of the resting samples had NK activity but showed no significant lysis of autologous LCL. After 7 days of culture, there was an increase in NK activity in all the samples; this was greatest for samples a and b, with a low or intermediate virus titer, respectively. This increase in NK activity was accompanied by the development of cytotoxicity toward autologous LCL, which was most marked in the samples with lower virus titers.
Both NK and CD4+ T cells are cytotoxic for LCL after activation.
In resting cord blood, two populations of CD16+ cells can be seen by flow cytometry: a CD16high CD56neg/low and a CD16low CD56low population (Fig. 8a). Gating on forward and side scatter showed that the latter population was large and granular, properties typical of NK cells. Following culture with EBV, the CD16high cells were no longer found in the culture and only a CD16low CD56low population of cells remained (Fig. 8b). Further analysis showed that the CD16low cells were CD8low, CD3−, and CD4− (Fig. 8c through e). This phenotype is consistent with their being NK cells. NK cells are generally associated with killing of MHC class I-negative targets; however, LCL express MHC class I. Therefore, we used fluorescence-activated cell sorting (FACS) to investigate if the killing of LCL was mediated by an NK CD16+ CD3− cell population or by CD4+ CD3+ T cells. Figure 8e shows the two distinct populations of either CD4+ or CD16+ cells seen in cord blood samples on day 7 after virus challenge. Following FACS separation, these cells were placed in a cytotoxic assay against 221 cells and autologous LCL. The results of this assay on sorted cells from three samples of cord blood (Fig. 9) demonstrate that the autologous LCL were killed by both CD16+ and CD4+ cells. The CD16+ cells also killed the MHC class I-negative LCL 221 NK target, confirming the identity of the cultured CD16+ cells as NK cells.
FIG. 8.
Phenotypes of NK cells in resting fetal cord blood and cultured fetal cord blood 7 days post-virus challenge. (a) Dual staining of fresh fetal cord blood showing two populations of CD16+ and CD56+ cells. The CD56+ CD16low population was shown to be large and more granular, consistent with their being NK cells. CD16 was also found on CD14+ monocytes (data not shown). (b) Seven days after virus challenge, there was a mixed population of CD16+ and CD56+ cells. CD14+ monocytes were absent from these cultures (data not shown). (c through e) The CD16+cells also expressed low levels of CD8 (c) but were CD3− (d) and CD4− (e). (f) The CD8low cells did not express CD3, but the CD8high cells were CD3+ T cells. The data indicate that the CD16+ cells present in day-7 cultures have an NK cell phenotype.
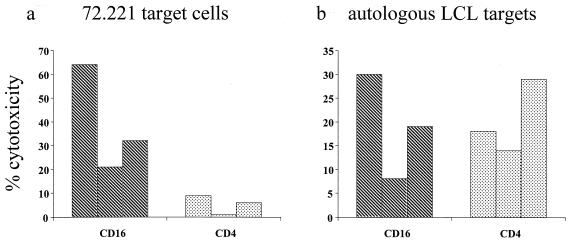
FIG. 9.
Cytotoxic activities of CD16+ and CD4+ populations purified by FACS. (a) Cytotoxic activity of CD16+ and CD4+ cell populations derived from cultures of fetal cord blood 7 days after virus challenge and FACS purification. The MHC class I-negative 221 target cells were killed most effectively by the CD16+ population. They were only slightly affected by purified CD4+ T cells. (b) In contrast, autologous LCL were killed both by CD16+ and by CD4+ populations of effector cells.
EBV-activated CD4+ T cells from cord blood produce IFN-γ.
In addition to an alteration in the CD45 isoform, CD69 expression, and enhanced cytotoxic function, the CD4+ T cells also showed a change in their pattern of cytokine production 7 days after virus challenge. Fresh cord blood lymphocytes, when stimulated with PMA and ionomycin, produced only IL-2; this is consistent with their naive phenotype (data not shown). Furthermore, T-cell blasts, generated by PHA stimulation and maintained for 10 days in IL-2-containing medium, also produced only IL-2 (as detected by intracellular staining) upon restimulation with PMA and ionomycin (Fig. 10a). In contrast, following 7 days of culture with EBV, CD4+ T cells developed a Th1 phenotype, as demonstrated by their ability to produce both IL-2 and IFN-γ, but not IL-4, when restimulated with PMA and ionomycin (Fig. 10b).
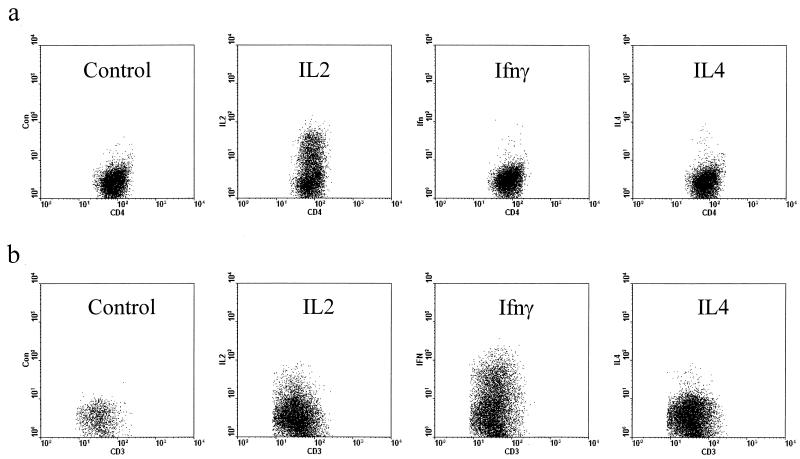
FIG. 10.
Flow cytometry of intracellular cytokines in T-cell blasts derived from fetal cord blood. Cells were stained with anti-CD4-FITC and anti-CD3-Cy5 plus control IgG-PE or anti-cytokine-PE. Plots show the gated CD4+ CD3+ population. (a) Cord blood cells were stimulated with PHA for 3 days and then washed and cultured in fresh medium with IL-2 until day 7, at which time they were restimulated with PMA and ionomycin for 6 h in the presence of monensin prior to intracellular staining. (b) Cord blood cells cultured for 7 days after infection with EBV. Prior to staining, cells were restimulated with PMA and ionomycin in the presence of monensin for 6 h. The results show that PHA blasts retained a naive phenotype and produced only IL-2 upon restimulation. In contrast, EBV-stimulated blasts produced both IL-2 and IFN-γ, but not IL-4.
Inhibition of transformation by CD4+ T cells activated by coculture with autologous LCL.
The addition of resting CD4+ T cells to purified B cells resulted in a reduction in the virus transformation titer in four out of five samples, indicating that they may directly inhibit virus transformation (Fig. 2). However, this experiment also demonstrates that monocytes act as accessory cells for optimal B-cell transformation. Monocytes coexpress CD4 and CD16, and depletion experiments to further assess the role of CD4+ T cells or CD16 NK cells are likely to be confounded by codepletion of monocytes. We therefore measured the effect of adding autologous EBV-reactive CD4+ T-cell blasts on the transformation of cord blood. CD4+ T-cell lines were generated from cord blood by stimulation with autologous LCL. The cells were cultured in autologous serum to avoid generating CD4+ T cells reactive to components of FCS. Further duplicate titrations of virus were carried out on another aliquot of cord blood. Autologous CD4+ T-cell blasts were added to one of the titration plates at a ratio of 1 CD4+ T cell to 100 cord blood lymphocytes. Table 2 shows the results from four separate samples. In three samples the addition of autologous CD4+ T-cell blasts completely eliminated virus transformation, while in the fourth sample the T cells had no effect. Taken together, these results indicate that CD4+ T cells mostly inhibit virus transformation.
TABLE 2.
Influence of autologous EBV-reactive CD4+ T-cell lines on EBV transformation of cord blood
| Log10 virus titer on cord blooda | Log10 virus titer on cord blood plus CD4 blastsb |
|---|---|
| 3.3 | <1 |
| 3.0 | <1 |
| 2.76 | 2.87 |
| 3.7 | <1 |
Virus was titrated on whole cord blood as described in Materials and Methods.
Virus was titrated on whole cord blood with the addition of 104 autologous T-cell blasts to each individual well.
DISCUSSION
For many years fetal cord blood has been used to assay the concentration of virus in EBV preparations. The assay calculates the virus dilution at which 50% of wells in a 96-well plate, each containing 2 × 105 lymphocytes, are transformed by virus, resulting in outgrowth of an EBV-infected LCL (49). It has been tacitly assumed in the use of this assay that cord blood contains no significant immune response to inhibit the growth of EBV-infected B cells. However, a growing number of reports demonstrate the ability of EBV-transformed LCL to induce primary responses in fetal cord blood (28, 29, 46, 50). Furthermore, it has been routine practice in this laboratory to remove adherent cells from cord blood lymphocytes, because this was found to reduce variation between EBV transformation assays. The mechanism by which the removal of adherent cells caused this effect was never investigated, but one possible explanation would be that depletion of antigen-presenting cells limits the activation of an immune response. Here we report a large variation in the apparent virus transformation titer when samples of fetal cord blood from six individual donors were transformed with the same virus sample without depletion of adherent cells.
This variation in apparent transformation titer could not be explained by variation in the virus preparations themselves, since the virus dilutions were prepared simultaneously from the same stock. Furthermore, flow cytometry did not reveal any large differences in the numbers of B cells in the six fetal cord blood samples prior to addition of virus that could have accounted for the variation. Likewise, the cord blood samples all had similar numbers of T cells, monocytes, and NK cells as well as similar levels of NK activity at the start of the trial. In contrast, at 1 week following virus challenge there was a clear association between the number of activated NK cells in the culture and failure of virus transformation.
The importance of NK cells in controlling virus infections has been highlighted by numerous studies, and their role is of particular importance in herpesvirus infections (6, 21, 25). NK cells are regulated by a number of activating and inhibitory receptors that control their cytotoxic function. These include the inhibitory receptors regulated by MHC class I, which allow the killing of a number of MHC class I-negative cells (6, 30, 47). It has been suggested that some inhibitory signals can be mediated by MHC class I-independent mechanisms (4). In humans at least eight activating receptors belonging to immunoglobulin or lectin families of proteins have been identified on NK cells, and the ultimate decision as to whether a cell is lysed or not is thought to depend on the relative balance of inhibitory and activation signals (30, 47). Several studies of NK cells from freshly isolated seropositive and seronegative adult blood have shown that NK cells can inhibit the outgrowth of EBV-infected cells (8, 9, 21, 27). Importantly, it has been shown that NK cells freshly isolated from fetal cord blood were unable to inhibit LCL outgrowth (27) but that NK cells from cord blood can be activated by culturing in the presence of EBV-infected LCL (29). It has also been shown that superinfection of B-cell targets with EBV or induction of lytic replication enhances the susceptibility of cells to NK lysis (8, 9). These earlier observations are in agreement with our findings that freshly isolated fetal cord blood lymphocytes do not kill LCL in cytotoxicity assays and that cord blood NK cells are able to do so only subsequent to activation in cultures challenged with virus. The interaction between CD48 on EBV-infected B cells and 2B4 (CD244) on activated NK cells has recently been shown to be critical for regulation of EBV infection by NK cells. Defective NK cell signaling through this pathway has been shown to underlie X-linked lymphoproliferative disease, in which fatal fulminant infectious mononucleosis follows primary EBV infection (25, 30). Further quantitative studies on the expression of NK-activating and -inhibitory ligands associated with EBV infection will be required to clarify the mechanism of NK target recognition in primary EBV infection.
Numerous cytokines, including interferons, IL-2, IL-12, and IL-15, have been shown to activate NK cells, inducing them to proliferate and enhancing their cytotoxic activity in vitro (5, 6, 9, 16, 21, 44). In vivo, following culture of peripheral blood lymphocytes in medium containing IL-2, the resultant lymphokine-activated killer cells are able to regulate the outgrowth of EBV-associated posttransplant lymphomas in humans (33). In murine models, NK cells activated in the presence of IL-2 up-regulated CD95 ligand and acquired the ability to kill CD95-positive tumor cells (10, 26). CD95 ligand is also up-regulated on human NK cells in response to cytokines (6). Cytokine secretion by T cells or monocytes may therefore represent a mechanism by which NK cells become activated and kill EBV-infected cells during a primary immune response. We have previously shown that CD4+ T cells can kill LCL by CD95/CD95 ligand-induced apoptosis (50). If CD95 ligand on NK cells is up-regulated by cytokines, then the killing of LCL would be an intrinsic property of these NK cells and could be independent of further activation or inhibitory signals. Our results suggest that, in primary EBV infection, CD4+ T cells may play a central role in regulating EBV transformation, both by directly killing EBV-infected B cells and by secreting IL-2, which would induce NK cells to kill these infected targets. Others have recently shown that CD4+ T-cell effectors can inhibit the early phase of EBV-induced B-cell proliferation (34).
Our earlier observation that monocyte depletion enhanced EBV transformation was confirmed and may in part be explained by the influence of monocytes on the activation of NK cells through cytokine production. IL-12 and IL-15 are both produced by monocytes and can activate NK cells and enhance their production of IFN-γ (14, 15, 48). IL-12 was first described as a product of EBV-transformed B-cell lines that activated NK cells (48). IL-15 also has an important role in the early activation of NK cells in response to virus infections, including EBV and human herpesvirus-7 infections (3, 21, 48). Clearly, further investigation of the role of monocytes in the control of EBV transformation is needed.
Despite the evidence in favor of NK cells being the major effector cell in regulating EBV infection of fetal cord blood, this does not explain why cord blood samples were not all able to eliminate EBV transformation. Both cytotoxic function assays and flow cytometry showed only small differences in NK activity and numbers of CD16+ cells at the start of the culture, so a lack of NK precursors is unlikely to be the explanation for the failure of some cord blood samples to eliminate EBV. Likewise, the proportion of CD14+ monocytes showed little variation between samples. The situation with CD4+ T cells is, however, different. Although the total numbers are similar at the start, the response of each individual will vary depending on the MHC alleles and T-cell-receptor repertoire. Such variation in the numbers of responding CD4+ T cells could, therefore, explain the differences between samples. Evidence for the central role of CD4+ T cells in the primary immune response comes from the observation that T-cell depletion led to a larger increase in virus titer than either CD16 or CD14 depletion. In the cottontop tamarin model of EBV lymphomagenesis (17, 19), immunity is mediated by a combination of CD4+ T cells and CD8+ NK or CD8+ T cells. Vaccination studies with this model showed that priming of an antigen-specific CD4+ T-cell cytotoxic response was required for optimum activation of the NK cell component (51, 52). Several studies with humans have highlighted the cytotoxic activity of CD4+ T cells following stimulation by EBV-transformed LCL in seropositive adults (22, 31, 33, 50) and in fetal cord blood (29, 46, 50). In contrast, other studies have shown that under certain circumstances CD4+ T cells can enhance EBV reactivation by CD40/CD40 ligand-mediated signals (20). We have observed that some primary cytotoxic CD4+ T cells are even able to enhance LCL growth in a long-term assay (data not shown). Further studies are required to clarify the role of CD4+ T cells in primary EBV infection; these would include studies on the precursor frequency of CD4 T cells and investigations of whether CD4+ T cells in cord blood samples with high transformation titers can provide helper functions to enhance EBV infection.
An important conclusion from the data presented above is that different primary immune responses in different individuals might be reflected in different susceptibilities to primary EBV infection, infectious mononucleosis, or posttransplant lymphoma. Furthermore, the NK and CD4+ T-cell activities described above might have a long-term role in the control of, or susceptibility to, other EBV-associated diseases in adults.
Acknowledgments
We are grateful to the Wellcome Trust for funding this work (grant 055388). The Wellcome Trust and the Higher Education Funding Council (HEFC) provided funds for the purchase of a FACS Vantage flow cytometer.
We thank Sasha Srekovich for assistance in flow cytometry. We are also grateful to Margaret Callan and Ann Pullen for helpful criticism of the manuscript.
REFERENCES
- 1.Ablashi, D., G. W. Bornkamm, C. Boshoff, S. H. Chan, I. Ernberg, I. T. Magrath, M. G. Masucci, M. Melbye, P. S. Moore, A. J. Morgan, N. Muller, G. Niedobitek, P. P. Pastoret, N. Raab-Traub, C. Rabkin, T. F. Schulz, G. de The, A. O. Williams, and M. C. Yu. 1997. Epstein-Barr virus and Kaposi's sarcoma herpesvirus/human herpesvirus. IARC Monogr. Eval. Carcinog. Risks Hum. 70:497. [Google Scholar]
- 2.Andersson, J. 1998. Infectious mononucleosis: clinical characteristics, complications and management. Herpes 5:15-19. [Google Scholar]
- 3.Atedzoe, B. N., A. Ahmad, and J. Menezes. 1997. Enhancement of natural killer cell cytotoxicity by the human herpesvirus-7 via IL-15 induction. J. Immunol. 159:4966-4972. [PubMed] [Google Scholar]
- 4.Avril, T., A. C. Jarousseau, H. Watier, J. Boucraut, P. Le Bouteiller, P. Bardos, and G. Thibault. 1999. Trophoblast cell line resistance to NK lysis mainly involves an HLA class I-independent mechanism. J. Immunol. 162:5902-5909. [PubMed] [Google Scholar]
- 5.Bamford, R. N., A. J. Grant, J. D. Burton, C. Peters, G. Kurys, C. K. Goldman, J. Brennan, E. Roessler, and T. A. Waldmann. 1994. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA 91:4940-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 7.Blake, N., S. Lee, I. Redchenko, W. Thomas, N. Steven, A. Leese, P. Steigerwald-Mullen, M. G. Kurilla, L. Frappier, and A. Rickinson. 1997. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity 7:791-802. [DOI] [PubMed] [Google Scholar]
- 8.Blazar, B., M. Patarroyo, E. Klein, and G. Klein. 1980. Increased sensitivity of human lymphoid lines to natural killer cells after induction of the Epstein-Barr viral cycle by superinfection or sodium butyrate. J. Exp. Med. 151:614-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blazar, B. A., M. Strome, and R. Schooley. 1984. Interferon and natural killing of human lymphoma cell lines after induction of the Epstein-Barr viral cycle by superinfection. J. Immunol. 132:816-820. [PubMed] [Google Scholar]
- 10.Bradley, M., A. Zeytun, A. Rafi-Janajreh, P. S. Nagarkatti, and M. Nagarkatti. 1998. Role of spontaneous and interleukin-2-induced natural killer cell activity in the cytotoxicity and rejection of Fas+ and Fas− tumor cells. Blood 92:4248-4255. [PubMed] [Google Scholar]
- 11.Callan, M. F. C., L. Tan, N. Annels, G. S. Ogg, J. D. K. Wilson, C. A. O'Callaghan, N. Steven, A. J. McMichael, and A. B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callan, M. F. C., N. Steven, P. Krausa, J. D. K. Wilson, P. A. H. Moss, G. M. Gillespie, J. I. Bell, A. B. Rickinson, and A. J. McMichael. 1996. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 2:906-911. [DOI] [PubMed] [Google Scholar]
- 13.Cannon, M. J., P. Pisa, R. I. Fox, and N. R. Cooper. 1990. Epstein-Barr virus induces aggressive lymphoproliferative disorders of human B cell origin in SCID/hu chimeric mice. J. Clin. Investig. 85:1333-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carson, W. E., J. G. Giri, M. J. Lindemann, M. L. Linett, M. Ahdieh, R. Paxton, D. Anderson, J. Eisenmann, K. Grabstein, and M. A. Caligiuri. 1994. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 180:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan, S. H., B. Perussia, J. W. Gupta, M. Kobayashi, M. Pospisil, H. A. Young, S. F. Wolf, D. Young, S. C. Clark, and G. Trinchieri. 1991. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J. Exp. Med. 173:869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chehimi, J., N. M. Valiante, A. D'Andrea, M. Rengaraju, Z. Rosado, M. Kobayashi, B. Perussia, S. F. Wolf, S. E. Starr, and G. Trinchieri. 1993. Enhancing effect of natural killer cell stimulatory factor (NKSF/interleukin-12) on cell-mediated cytotoxicity against tumor-derived and virus-infected cells. Eur. J. Immunol. 23:1826-1830. [DOI] [PubMed] [Google Scholar]
- 17.Cleary, M. L., M. A. Epstein, S. Finerty, R. F. Dorfman, G. W. Bornkamm, J. K. Kirkwood, A. J. Morgan, and J. Sklar. 1985. Individual tumors of multifocal EB virus-induced malignant lymphomas in tamarins arise from different B-cell clones. Science 228:722-724. [DOI] [PubMed] [Google Scholar]
- 18.de Schryver, A., G. Klein, J. Hewetson, G. Rocchi, W. Henlé, G. Henlé, D. J. Moss, and J. H. Pope. 1974. Comparison of EBV neutralization tests based on abortive infection or transformation of lymphoid cells and their relation to membrane reactive antibodies (anti-MA). Int. J. Cancer 13:353-362. [DOI] [PubMed] [Google Scholar]
- 19.Epstein, M. A., A. J. Morgan, S. Finerty, B. J. Randle, and J. K. Kirkwood. 1985. Protection of cottontop tamarins against Epstein-Barr virus-induced malignant lymphoma by a prototype subunit vaccine. Nature 318:287-289. [DOI] [PubMed] [Google Scholar]
- 20.Fu, Z., and M. J. Cannon. 2000. Functional analysis of the CD4+ T-cell response to Epstein-Barr virus: T-cell-mediated activation of resting B cells and induction of viral BZLF1 expression. J. Virol. 74:6675-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosselin, J., A. TomoIu, R. C. Gallo, and L. Flamand. 1999. Interleukin-15 as an activator of natural killer cell-mediated antiviral response. Blood 94:4210-4219. [PubMed] [Google Scholar]
- 22.Honda, S., T. Takasaki, K. Okuno, M. Yasutomi, and I. Kurane. 1998. Establishment and characterization of Epstein-Barr virus-specific human CD4+ T lymphocyte clones. Acta Virol. 42:307-313. [PubMed] [Google Scholar]
- 23.Johannessen, I., and D. H. Crawford. 1999. In vivo models for Epstein-Barr virus (EBV)-associated B cell lymphoproliferative disease (BLPD). Rev. Med. Virol. 9:263-277. [DOI] [PubMed] [Google Scholar]
- 24.Johannessen, I., M. Asghar, and D. H. Crawford. 2000. Essential role for T cells in human B-cell lymphoproliferative disease development in severe combined immunodeficient mice. Br. J. Haematol. 109:600-610. [DOI] [PubMed] [Google Scholar]
- 25.Joncas, J., Y. Monczak, F. Ghibu, C. Alfieri, A. Bonin, G. Ahronheim, and G. Rivard. 1989. Killer cell defect and persistent immunological abnormalities in two patients with chronic active Epstein-Barr virus infection. J. Med. Virol. 28:110-117. [DOI] [PubMed] [Google Scholar]
- 26.Khar, A., B. V. Pardhasaradhi, C. Varalakshmi, A. M. Ali, and A. L. Kumari. 1997. Natural killer cell as the effector which mediates in vivo apoptosis in AK-5 tumor cells. Cell. Immunol. 177:86-92. [DOI] [PubMed] [Google Scholar]
- 27.Masucci, M. G., M. T. Bejarano, G. Masucci, and E. Klein. 1983. Large granular lymphocytes inhibit the in vitro growth of autologous Epstein-Barr virus-infected B cells. Cell. Immunol. 76:311-321. [DOI] [PubMed] [Google Scholar]
- 28.Misko, I. S., T. B. Sculley, C. Schmidt, D. J. Moss, T. Soszynski, and K. Burman. 1991. Composite response of naive T cells to stimulation with the autologous lymphoblastoid cell line is mediated by CD4 cytotoxic T cell clones and includes an Epstein-Barr virus-specific component. Cell. Immunol. 132:295-307. [DOI] [PubMed] [Google Scholar]
- 29.Moretta, A., P. Comoli, D. Montagna, A. Gasparoni, E. Percivalle, I. Carena, M. G. Revello, G. Gerna, G. Mingrat, F. Locatelli, G. Rondini, and R. Maccario. 1997. High frequency of Epstein-Barr virus (EBV) lymphoblastoid cell line-reactive lymphocytes in cord blood: evaluation of cytolytic activity and IL-2 production. Clin. Exp. Immunol. 107:312-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretta, A., C. Bottino, M. Vitale, D. Pende, C. Cantoni, M. Mingari, R. Biassoni, and L. Moretta. 2001. Activating receptors and co-receptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19:197-223. [DOI] [PubMed] [Google Scholar]
- 31.Moss, D. J., and J. H. Pope. 1972. Assay of the infectivity of Epstein-Barr virus by transformation of human leucocytes in vitro. J. Gen. Virol. 17:233-236. [DOI] [PubMed] [Google Scholar]
- 32.Munz, C., K. L. Bickham, M. Subklewe, M. L. Tsang, A. Chahroudi, M. G. Kurilla, D. Zhang, M. O'Donnell, and R. M. Steinman. 2000. Human CD4+ T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J. Exp. Med. 191:1649-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nalesnik, M. A., A. S. Rao, H. Furukawa, S. Pham, A. Zeevi, J. J. Fung, G. Klein, H. A. Gritsch, E. Elder, T. L. Whiteside, and T. E. Starzl. 1997. Autologous lymphokine-activated killer cell therapy of Epstein-Barr virus-positive and -negative lymphoproliferative disorders arising in organ transplant recipients. Transplantation 63:1200-1205. [DOI] [PubMed] [Google Scholar]
- 34.Nikiforow, S., K. Bottomly, and G. Miller. 2001. CD4+ T-cell effectors inhibit Epstein-Barr virus-induced B-cell proliferation. J. Virol. 75:3740-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson, G., F. Dewey, G. Klein, G. Henlé, and W. Henlé. 1970. Relation between neutralization of Epstein-Barr virus and antibodies to cell-membrane antigens induced by the virus. J. Natl. Cancer Inst. 45:989-995. [PubMed] [Google Scholar]
- 36.Perera, S. M., J. A. Thomas, M. Burke, and D. H. Crawford. 1998. Analysis of the T-cell micro-environment in Epstein-Barr virus-related post-transplantation B lymphoproliferative disease. J. Pathol. 184:177-184. [DOI] [PubMed] [Google Scholar]
- 37.Pope, J. H., W. Scott, and D. J. Moss. 1973. Human lymphoid cell transformation by Epstein-Barr virus. Nat. New Biol. 246:140-141. [DOI] [PubMed] [Google Scholar]
- 38.Redchenko, I. V., and A. B. Rickinson. 1999. Accessing Epstein-Barr virus-specific T-cell memory with peptide-loaded dendritic cells. J. Virol. 73:334-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent end-points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 40.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405-431. [DOI] [PubMed] [Google Scholar]
- 41.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p 2575-2627. In D. M. Knipe, and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 42.Rickinson, A. B., D. J. Moss, D. J. Allen, L. E. Wallace, M. Rowe, and M. A. Epstein. 1981. Reactivation of Epstein-Barr virus-specific cytotoxic T cells by in vitro stimulation with the autologous lymphoblastoid cell line. Int. J. Cancer 27:593-601. [DOI] [PubMed] [Google Scholar]
- 43.Rickinson, A. B., M. F. Callan, and N. E. Annels. 2000. T-cell memory: lessons from Epstein-Barr virus infection in man. Philos. Trans. R. Soc. Lond. B 355:391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santoli, D., G. Trinchieri, and H. Koprowski. 1978. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J. Immunol. 121:532-538. [PubMed] [Google Scholar]
- 45.Shimizu, Y., and R. DeMars. 1989. Demonstration by class I gene transfer that reduced susceptibility of human cells to natural killer cell-mediated lysis is inversely correlated with HLA class I antigen expression. Eur. J. Immunol. 19:447-451. [DOI] [PubMed] [Google Scholar]
- 46.Sun, Q., R. L. Burton, K. E. Pollok, D. J. Emanuel, and K. G. Lucas. 1999. CD4+ Epstein-Barr virus-specific cytotoxic T-lymphocytes from human umbilical cord blood. Cell. Immunol. 195:81-88. [DOI] [PubMed] [Google Scholar]
- 47.Tomasello, E., M. Blery, E. Vely, and E. Vivier. 2000. Signaling pathways engaged by NK cell receptors: double concerto for activating receptors, inhibitory receptors and NK cells. Semin. Immunol. 12:139-147. [DOI] [PubMed] [Google Scholar]
- 48.Valiante, N. M., M. Rengaraju, and G. Trinchieri. 1992. Role of the production of natural killer cell stimulatory factor (NKSF/IL-12) in the ability of B cell lines to stimulate T and NK cell proliferation. Cell. Immunol. 145:187-198. [DOI] [PubMed] [Google Scholar]
- 49.Wilson, A. D., and A. J. Morgan. 1998. Indirect measurement of Epstein-Barr virus neutralising antibodies by ELISA. J. Virol. Methods 73:11-19. [DOI] [PubMed] [Google Scholar]
- 50.Wilson, A. D., I. Redchenko, N. A. Williams, and A. J. Morgan. 1998. CD4+ T cells inhibit growth of Epstein-Barr virus-transformed B cells through CD95-CD95 ligand-mediated apoptosis. Int. Immunol. 10:1149-1157. [DOI] [PubMed] [Google Scholar]
- 51.Wilson, A. D., K. Lovgren-Bengtsson, M. Villacres-Ericsson, B. Morein, and A. J. Morgan. 1999. The major Epstein-Barr virus (EBV) envelope glycoprotein gp340 when incorporated into Iscoms primes cytotoxic T-cell responses directed against EBV lymphoblastoid cell lines. Vaccine 17:1282-1290. [DOI] [PubMed] [Google Scholar]
- 52.Wilson, A. D., M. Shooshstari, S. Finerty, P. Watkins, and A. J. Morgan. 1996. Virus-specific cytotoxic T cell responses are associated with immunity of the cottontop tamarin to Epstein-Barr virus (EBV). Clin. Exp. Immunol. 103:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]