Abstract
Ultrasound was employed to increase the growth rate of bacterial cells attached to surfaces. Staphylococcus epidermidis, Pseudomonas aeruginosa and Escherichia coli cells adhered to and grew on a polyethylene surface in the presence of ultrasound. It was found that low frequency ultrasound (70 kHz) of low acoustic intensity (<2 W/cm2) increased the growth rate of the cells compared to growth without ultrasound. However, at high intensity levels, cells were partially removed from the surface. Ultrasound also enhanced planktonic growth of S. epidermidis and other planktonic bacteria. It is hypothesized that ultrasound increases the rate of transport of oxygen and nutrients to the cells and increases the rate of transport of waste products away from the cells, thus enhancing their growth.
INTRODUCTION
Ultrasound is defined as acoustic energy or sound waves with frequencies above 20 kHz. Ultrasonication is commonly thought to be detrimental to cell growth; however, cells can grow in low intensity insonation due to the following properties of ultrasound: 1) its ability to increase the transport of small molecules in solution, and 2) its inability to completely remove cells (or even non-living particles) from surfaces. Although the former aspect is well known, the latter is not; and in fact its antithesis is commonly accepted – the misconception that ultrasound is very efficient at removing cells and particles from surfaces.
Ultrasound increases transport of small molecules in a liquid solution by increasing the convection in an otherwise stagnant or relatively slow moving fluid (1–4). The boundary layer of stagnant fluid adjacent to a solid surface creates a resistance to the transport of small molecules to the surface. Increased convection reduces the thickness of this boundary layer with a concurrent increase in transport to the surface. To increase the growth rate of cells on a surface, it is often desirable to increase the transport of oxygen and nutrients to the cells as well as to increase the transport of cellular waste products away from the cells.
Ultrasound increases convection in liquid by at least two mechanisms. The first is acoustic streaming flow in which momentum from directed propagating sound waves is transferred to the liquid, causing the liquid to flow in the direction of the sound propagation. Acoustic streaming increases with insonation intensity, and there are reports of acoustic streaming flow at velocities as high as 14 cm/s (5). Thus any amount of ultrasound in a liquid produces additional convective transport from acoustic streaming.
The second and more notable mechanism of enhancing convection is known as micro-streaming, and is produced by cavitating gas bubbles in the liquid (2, 3, 5–8). The cycles of low and high acoustic pressure cause the gas bubbles to expand and shrink, which in turn creates shear flow around the oscillating bubbles (3). Stable cavitation results when the acoustic intensity is sufficiently low that the bubbles do not collapse completely during their contraction cycle. The onset of stable cavitation greatly increases convective transport; such transport increases with increasing acoustic intensity as larger and more numerous cavitation bubbles form and the amplitude of oscillation increases.
A stable cavitation bubble near a bacteria on a surface or near planktonic bacteria interacts with the liquid and bacteria in many ways. If a planktonic bacterium is more dense than the surrounding liquid, there is a radiation pressure which propels the bacterium toward the oscillating bubble (3). As the bacterium approaches the bubble, it experiences a radiation torque that causes the bacterium to rotate. It also enters an oscillating and swirling velocity field with fairly high shear rates (velocity gradients). Table 1 gives some examples of velocities and shear rates experienced by a bacterium near an oscillating bubble. The high shear rates at higher frequencies and displacement amplitudes are indicative of high mass transfer, especially compared to diffusion through a stagnant liquid.
Table 1.
Velocities, shear rate, and shear stress in water at 37° near an oscillating bubble (3).
| Frequency | Ambient bubble radius (μm) | Amplitude of outward surface displacement (μm) | Maximum liquid velocity at surface (m/s) | Velocity gradient at bubble surface (s−1) | Shear Stress at bubble surface (Pa) |
|---|---|---|---|---|---|
| 70 kHz | 10 | 1 | 0.440 | 2.48 x 104 | 17.2 |
| 70 kHz | 10 | 2 | 0.880 | 9.90 x 104 | 68.8 |
| 70 kHz | 10 | 5 | 2.20 | 6.19 x 105 | 430 |
| 70 kHz | 50 | 5 | 2.20 | 1.24 x 105 | 85.9 |
| 70 kHz | 50 | 10 | 4.40 | 4.95 x 105 | 344 |
| 1 MHz | 10 | 1 | 6.28 | 1.34 x 106 | 928 |
| 1 MHz | 50 | 10 | 62.8 | 2.67 x 107 | 1,860 |
An oscillating bubble adjacent to or attached to a solid surface also creates local micro-streaming. Elder shows that oscillating bubbles attached to a surface create strong flows toward or away from the bubble (and surface), depending upon the fluid viscosity and oscillation amplitude (2).
As the acoustic intensity continues to increase, collapse cavitation begins to occur and thus convection increases dramatically. Collapse (also called inertial or transient) cavitation is produced when the bubble radius is reduced to near zero during the contraction cycle (1). The sudden collapse produces a shock wave, and the adiabatic compression of the gas produces temperatures on the order of 5000 K, which in turn can fragment water and other molecules into free radicals.
High intensity (high power density, > 2 W/cm2) and low frequency (< 100 kHz) ultrasound is commonly used to clean solid surfaces such as the surfaces of glassware, metallic instruments, plastic parts, and more (9–13). Ultrasonic “cleaners” are commonly found in laboratories and industrial settings for such purposes. The mechanism by which dust and particles are removed from these solid surfaces is commonly believed to be related to cavitational events and the related shear forces adjacent to the surface (1, 9, 13, 14). High intensities of ultrasound create cavitation bubbles in the liquid adjacent to the surface, or in the narrow volume between the surface and loosely attached “dirt” particles (15). The rapid expansion and contraction of these bubbles can cause extreme fluid shear forces that can dislodge particles from the surface. For example, Maisonhaute et al. affirm that in their experiments with a sonicating horn, collapsing bubbles reside from 40 to 80 nm from the surface (9). They calculate that the shear stress in the gap between the bubble and surface is on the order of 2.5 to 5 MPa, much higher than the shear stresses presented in Table 1.
During transient cavitation very near a surface, the collapsing bubble is distorted into a non-spherical shape, causing a high velocity jet of liquid to impinge on the surface, shearing off any particles (1).
High intensity ultrasound is commonly used to remove bacterial cells from surfaces (12, 13, 16–21). However, even at very high power levels, not all of the bacteria are removed. One group investigated application of ultrasound to one end of a pipe to remove a biofilm of Proteus mirabilis from the lumen of the pipe (12). They quantified the removal of bacterial mass with infrared absorptiometry and found that the ultrasound propagated axially with sufficient power to partially strip the bacteria from the entire length (50 cm) of the pipe. However, even with frequencies around 100 kHz and intensities approaching 40 W/cm2, they were only able to remove up to 87.5% of the bacteria from 50-centimeter long tubes.
Zips et al. used 38 kHz ultrasound to detach Pseudomonas diminuta biofilms from reverse osmosis membranes (13). They placed a point source of ultrasound at varying distances from a 1-cm2 membrane, and the power of the source was varied. The results indicated that even at their highest power densities, only 95% of the bacteria were removed. They attributed the detachment of the bacteria to collapse cavitation. Another research group found that 40 kHz ultrasound removed only 83% of bacteria from biofilms in a simulated food processing equipment (21).
High intensity (>10 W/cm2) ultrasound is known to lyse bacterial and eucaryotic cells on surfaces and in suspension, which is the principle behind the cell “disrupter” commonly found in laboratories (22–28). Cavitational events are thought to lyse the cells or greatly increase the permeability of their membranes, thus spilling their contents. Therefore high intensity ultrasound can kill cells in addition to partially removing them from surfaces. Because cavitation is usually more intense at low frequencies, low frequency ultrasound is commonly used to perturb or disrupt cell membranes and lyse cells (22, 23, 25, 27, 29–31).
The hypothesis underlying this research is that ultrasound can increase the growth rate of cells on surfaces and in suspension, presumably by increasing the transport of oxygen and nutrients to the cells. Obviously cell removal by ultrasound is not desired, but some degree of cell removal or cell death could be allowed as long as the enhanced rate of cell growth was greater than the rate of cell removal.
This research differs significantly from previous work in our lab that was aimed at killing bacterial cells on surfaces or in suspension using the combination of ultrasound and antibiotics (32–41). In that previous work, antibiotics are required to kill the cells during the exposure to ultrasound.
MATERIALS AND METHODS
Organisms
This work employed three species of bacteria, all of which are known to colonize surfaces. They were Staphylococcus epidermidis (strain RP62A, ATCC #35984), Pseudomonas aeruginosa (ATCC #27853) and Escherichia coli (ATCC #10798). They were stored as frozen cultures and inoculated onto nutrient plates weekly. Tryptic soy broth (TSB) was inoculated with one colony from the plate, and a culture was grown overnight at 37°C with shaking. In some cases involving growth of S. epidermidis, 0.25 wt% glucose was added to the TSB.
Materials and Methods
Polymer rods of high density polyethylene were selected for this study as an exemplary material for the adhesion and growth of bacteria on a surface. The rods had a diameter of 0.12 cm and were approximately 15 cm long. In test tubes filled with 2 ml of TSB, the bottom 0.80 cm of the rod was exposed to the bacterial suspension or sterile nutrient broth.
New rods were prepared by cleaning them with ethanol in an ultrasonic bath. Before the rods were used in these experiments, they were sterilized in an autoclave for twenty minutes. To re-use the rods, the rod surfaces were scrubbed with soap, processed in ethanol in the ultrasonic bath after each use, and then autoclaved just prior to the next experiment. The rods were reused several times during the course of these experiments. SEM micrographs of the rods before and after one ultrasonic exposure showed no discernible difference in the surface morphology.
Ultrasound was delivered with a Sonicor SC100 ultrasonic bath (Copiaque, NY) operating at 70 kHz. A test tube rack inside the bath supported glass test tubes containing the rods, and a hydrophone (Bruel and Kjaer, model 8103, Naerum, DK) placed inside a test tube was used to quantify the intensity applied to each location within the rack in the ultrasonic bath. The ultrasonic intensity inside a test tube was measured before and after each experiment as described previously (42).
Initial Adhesion to the Rods
A 24-h S. epidermidis culture was diluted 1 to 1000 into fresh TSB, and then incubated at 37°C for 4 h, which allowed the cells to grow up to a concentration of about 105 cells/mL. Then 2 ml of cell culture were pipetted into each of 8 test tubes, and a clean polyethylene rod was placed into each test tube. Four of the tubes were placed into a sonicating bath at 37°C at specified power densities, while the other 4 tubes were placed into a 37°C incubator on an orbital shaker set at 70 rpm. The rods were exposed to the culture for 1 h, after which the rods were rinsed with three 2-ml washes of physiological saline solution (PSS) to remove non-adherent bacteria.
Assessment by stripping and plate counting
A standard procedure for stripping bacteria from surfaces followed by plate counting was used in these experiments. It involved the use of ultrasound at higher power densities (2 – 4 W/cm2) and probably did not remove 100% of the bacteria, but the procedure removed a consistent percentage and thus could be used to compare the relative amounts of bacteria adherent under different conditions. After each rod was rinsed with PSS to remove the planktonic bacteria, it was placed into another test tube filled with 2 ml of PSS. The test tubes were placed into an ultrasonic bath and exposed for 30 minutes to power densities set for stripping bacteria (2 – 4 W/cm2). Then the rods were removed and the bacterial concentration of the resulting suspension was measured by standard plate counting techniques in which the suspension was serially diluted and plated on nutrient agar plates. Colonies were counted after 48 h of incubation at 37°C.
Assessment by toluidine blue staining
The presence of bacteria and exopolysaccharides in a biofilm on the rods was measured by using the following modification of a staining procedure described previously (43, 44). After the rods were subjected to the bacterial growth procedure, they were rinsed by submersion into a test tube containing 2 ml of PSS. Then the rod was then placed in 2 ml of Carnoy’s solution (60% ethanol, 30% CHCl3, 10% glacial acetic acid) for 10 minutes. Next the rod was placed in 2 ml of 1% toluidine blue stain for 1 h, followed by a brief rinse in a test tube containing 2 ml of PSS. Then the rod was placed into 1 ml of 0.2 M NaOH at 80ºC for 1 h, after which the rod was removed and the absorbance of the remaining solution was measured at 590 nm in a spectrophotometer. The absorbance generated from a clean rod subjected to the same procedure was used as a control. Because toluidine blue stains both the cells and exopolysaccharides, the difference between the absorbance obtained from a test rod and the control rod was considered proportional to the amount of biofilm on the rod.
In some experiments with S. epidermidis and E. coli, the biofilm was sufficiently dense such that it could not be removed from the rod in the NaOH digestion above. In these cases, the blue stain remained on the rod and was photographed.
Planktonic suspensions
An overnight culture of bacteria in TSB was diluted 1:1000 in fresh TSB and grown at 37°C for 2 h (S. epidermidis) or 3 h (E. coli and P. aeruginosa). The culture was separated into individual test tubes containing 2 ml growing culture. Half of these tubes were placed in the ultrasonicating bath, and the other half were incubated without ultrasound. At regular time intervals, samples were withdrawn, serially diluted, and plate counted.
RESULTS
S. epidermidis
The results of the one-hour exposure of the polyethylene rods to S. epidermidis are detailed in Figure 1. The x-axis of the graph indicates the various intensities of ultrasound under which the rods were exposed to the bacteria. The y-axis indicates the quantity of S. epidermidis adhered to the rod. Data from 4 repeat experiments are presented. Within the scatter inherent in these experiments, the rods showed similar initial adherence under all intensities of ultrasound, including those rods exposed to bacteria in the absence of ultrasound. These data indicate that ultrasound over the range examined (up to 2 W/cm2) did not prevent bacterial adhesion.
Figure 1.

Adherent bacteria after one hour exposure to 105 CFU/ml S. epidermidis as a function of the intensity of 70 kHz ultrasound. The points that were not exposed to ultrasound are represented at the I=0 value on the x-axis.
Subsequent experiments were conducted at 2 W/cm2 (vs. no ultrasound) in which the concentration of bacteria in the suspension was varied from 103 to 105 CFU/ml to see if bacterial concentration made any difference in the amount of adhesion. Although more adhesion was observed at higher concentrations, there were no statistically significant differences (p > 0.1) in adhesion with and without ultrasound at any of these concentrations. Adhesion appears to occur independently of ultrasonic intensity at these low frequencies and low power densities.
To assess S. epidermidis biofilm growth, rods were exposed to S. epidermidis suspended in TSB with 0.25 wt% glucose for 16 h at 2 W/cm2. These experiments allowed for significant growth of the biofilms in the incubated control rods and showed that the rods exposed to the bacteria growing with glucose under ultrasound grew thicker and more uniform biofilms. These biofilms were so thick that the digestion step of the toluidine blue technique did not quantitatively remove all the biofilm, and thus could not be used. However, for bacteria grown in glucose for 16 h, the difference in the biofilms was visually obvious. In three experiments of six rods each, all experiments showed significantly more biofilm on the rods exposed under ultrasound than those incubated without ultrasound. Figure 2 shows some representative photographs of the stained biofilms on the polyethylene rods in two of these experiments. The insonated rods are on the left of each panel, and the incubated rods are on the right of each panel. In these photographs it can be seen that some biofilm grew on the incubated rods. The biofilms on the insonated rods, however, were stained more darkly and uniformly over the exposed surface of the rod.
Figure 2.
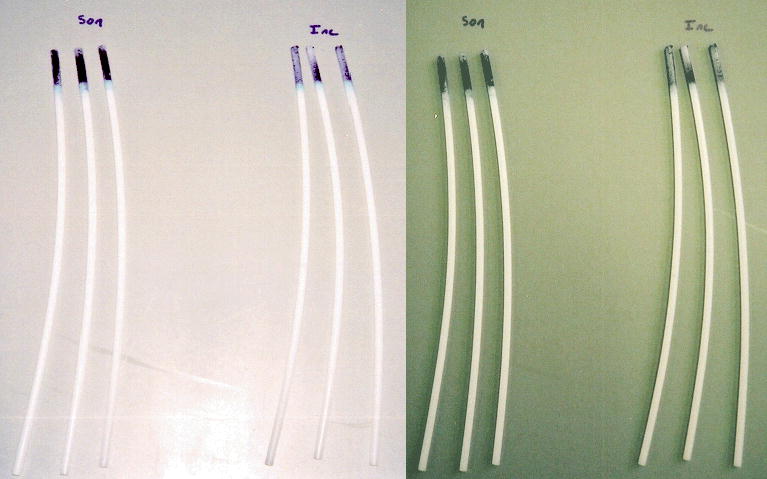
Two sets of S. epidermidis biofilms on polyethylene rods grown for 16 h with and without the presence of 2 W/cm2 ultrasound. The biofilms are stained with toluidine blue. Rods grown under ultrasound are in the left of each photo.
An experiment was designed to insure that outside influences were not causing the incubated rods to grow less biofilm than the insonated rods. Two possibilities for experimental artifacts existed that required examination: 1) perhaps a reduced oxygen supply in the cell incubator apparatus (which is basically a closed box) decreased the rate of biofilm formation compared to the rods in the ultrasonic bath (open to atmosphere); 2) the incubated rods were swirled at 100 rpm on an orbital shaker (to assist in good transport of oxygen), but perhaps the swirling was inhibiting good biofilm growth. These two possibilities were tested by adding two control groups to the experiment. The first possibility was tested by placing another group of rods in the constant temperature water bath that was supplying 37ºC water to the ultrasonic bath, and outside of both the incubator apparatus and the sonicating bath. The second possibility was examined by placing one group of rods in the incubator, but this group was not placed on the orbital shaker. These two control groups allowed analysis of the effects of the incubator’s atmosphere and shaker upon the experiments.
The results of these experiments showed that all of the above groups grown without ultrasound grew similar minimal biofilms, yet rods insonated at 2 W/cm2 grew visually thicker biofilms. The results indicated that neither shaking nor enclosing the incubator apparatus significantly influenced biofilm growth. The observation that these procedures do not produce experimental artifacts improves the reliability of the results indicating that ultrasound enhances biofilm formation.
Other bacterial species
Since the ultrasound enhanced biofilm formation, these experiments were repeated on two other bacterial species. Experiments with E. coli were conducted for 24 h to insure sufficient biofilm formation since E. coli does not form biofilms as quickly as does S. epidermidis RP62A. In triplicate experiments with E. coli, the toluidine blue assay showed a significant increase in biofilm formation for the biofilms grown in the presence of 2 W/cm2 ultrasound.
The P. aeruginosa experiments were extended to 48 h to insure sufficient biofilm formation since P. aeruginosa biofilms grow slower than the other two species. The rods exposed to ultrasound were also exposed to 2 W/cm2 70 kHz ultrasound, but the ultrasound was pulsed in a 1:5 duty cycle. Ultrasound was delivered in a 100 millisecond pulse of 70 kHz ultrasound, and the pulse was repeated each 500 milliseconds for 48 h. The stained rods were not as visually disparate as the other bacterial biofilms, yet some biofilm could be seen on some of the insonated rods, while none could be seen on any of the incubated rods. The results of the toluidine blue assay are shown in Figure 3.
Figure 3.

Absorbance of stained 48-hour P. aeruginosa biofilms from polyethylene rods exposed to 1:5 pulsed 2.2 W/cm2 70kHz ultrasound, 1:5 pulsed 1.5 W/cm2 70 kHz ultrasound, and no ultrasound.
Statistical tests (t-test comparison of means) determined that more stain was associated with biofilm on the insonated rods than the non-insonated rods (p < 0.1, n=8). It should also be noted that the averages of the samples increased with increasing ultrasound, and while large variations existed within the insonated group, all of the values but one were larger than those of the incubated group. These results would indicate that P. aeruginosa biofilm growth is also accelerated by ultrasound.
Planktonic Suspensions
Planktonic cultures of bacteria showed normal growth during the experiments, but consistently more growth was observed when exposed to ultrasound than when incubated without ultrasound. Figure 4 shows the mean and 95% confidence intervals from 4 replicates of S. epidermidis growing in TSB; half of the test tubes were incubated while the others were exposed to 3 W/cm2 at 70 kHz. Experiments with the other two species also showed enhanced planktonic growth under ultrasonic exposure (data not shown). For example, E. coli after growing 3 h in 70 kHz ultrasound had an average planktonic concentration of 8.5 x 107 CFU/ml, whereas without ultrasound the average concentration was 4.8 x 107 CFU/ml. These differences are statistically significant (n=4, p=0.050). Likewise for P. aeruginosa there was an average planktonic concentration of 4.8 x 107 CFU/ml after 3 h of insonation, whereas without ultrasonication there was an average planktonic concentration of 3.5 x 107 CFU/ml after 3 h. These differences are also statistically significant (n=4, p=0.047).
Figure 4.
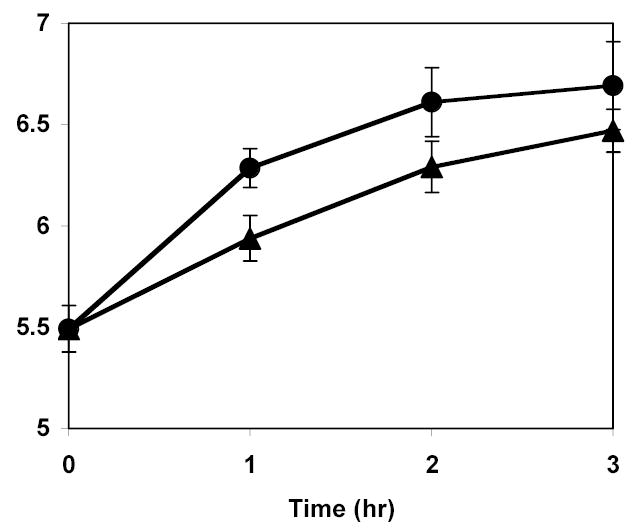
Growth of planktonic S. epidermidis with 70 kHz ultrasound at 3 W/cm2 (circles) and without ultrasound (triangles). The data are the mean and 95% confidence intervals of 4 replicates.
DISCUSSION
The data presented above conclusively show that bacterial biofilms grow better in the presence of the low intensity and low frequency ultrasound explored in these experiments. The growth of planktonic cultures also appears to be enhanced by ultrasound. Bacteria even adhere to surfaces during exposure to ultrasound. To our knowledge, studies of bacterial adhesion under conditions of ultrasound have not been published; therefore, the above results indicating that ultrasound does not prevent initial bacterial adhesion are not necessarily contrary to previous literature, although one report proposed the possibility that ultrasound could prevent adhesion (13). Since ultrasound is commonly used to strip bacteria from surfaces, a common misconception is that ultrasound might also be expected to prevent biofilm formation and even to reduce bacterial growth. Several reasons exist as to why our observation of the opposite does not blatantly contradict past science. The main explanation for our unexpected observation is related to the intensity of the ultrasound. The literature reports that the ultrasound used to strip biofilms employed much higher ultrasonic intensities than our present research used (≤2 W/cm2).
Zips et al. observed partial removal of bacteria from reverse osmosis membranes when they used a 2-W transducer that was placed very close to the membrane (13). Mott et al. used insonation intensities higher than 20 W/cm2 to strip bacteria from pipes (12). Oulahal-Lagsir et al. could only remove 83% of bacteria from food processing equipment with 40 kHz ultrasound (21).
Current thought on ultrasound stripping of bacteria is that the ultrasound simply creates shear forces in the biofilm next to the surface (13). These shear forces are thought to disrupt the interaction of the cells and their exopolysaccharides with the surface. Since 2-W/cm2 ultrasound was incapable of stripping mature S. epidermidis biofilms used in this research, the shear forces created by the ultrasound apparently were not strong enough to disrupt all of the biofilm-surface interactions. The research done by Zips et al. did show that the intensity of the ultrasound determined the amount of the biofilm that was stripped, showing a sort of dose-response effect over the range examined (13). Perhaps higher intensities of ultrasound would have removed the mature biofilm, but increased ultrasonic intensities also may have killed the bacteria. The threshold between promoting growth and killing cells remains to be determined, but we estimate that it is between 1 and 50 W/cm2, depending upon the species of bacteria.
An interesting possibility exists for the mechanism of ultrasound enhancement of biofilm formation. Many researchers have theorized that a nutrient concentration gradient exists within the bacterial biofilms (45–47). The mass-transfer resistance of the exopolymers in the biofilm slows the bacterial growth rate. Using mathematical models, ultrasound is predicted to increase the transport of small molecules within biofilms (41). Subsequent experimental work verified that ultrasound at 100 W/cm2 40 kHz or at 2 W/cm2 70 kHz significantly increased mass transport through biofilms (48, 49).
The fact that ultrasound increased biofilm growth in S. epidermidis, E. coli, and P. aeruginosa indicates that the phenomena may be applicable to many, if not all cells. It is true that the ultrasound apparently increased the biofilm growth to a greater extent with S. epidermidis bacteria than with the other two species, and that the ultrasound increased the biofilm growth more in the E. coli experiments than the P. aeruginosa experiments. While the differences in growth were more pronounced in certain species (such as S. epidermidis), these differences could be due to differences in the normal metabolic growth rates of the bacteria without ultrasound. For example, under our laboratory conditions the biofilm growth rate without ultrasound decreases in the order of S. epidermidis > E. coli > P. aeruginosa. The most significant fact is that all three species demonstrated an increase in biofilm and planktonic growth during application of 2 W/cm2 ultrasound at 70 kHz.
The application of ultrasound to enhance the growth of cells has numerous possible applications. Of obvious application is the more rapid growth of bacteria and other cells in the lab for research purposes. However, perhaps the most beneficial applications are found in the production of pharmaceuticals, medicines, and tissues from cell culture. Currently E. coli containing recombinant DNA is used to produce medicinal proteins such as growth hormones and other regulatory factors. Eucaryotic cells are also cultured and harvested to obtain viruses, hormones, proteins, and other biomolecules used in medicine or industry. An increased rate of cellular growth will increase the production and lower the costs of such naturally produced biomolecules.
Another medical application of this technology would be its extension to promoting growth in eucaryotic cells such as the growth of human cells for replacement tissues. Replacement tissues of current interest are skin cells (for burn patients), chondrocytes (for cartilage replacement in joints, nose, ears, etc.), nerve cells and other neural tissues (to replace or reconnect damages nerves), cardiac tissue cells (for victims of heart attack or heart valve failure), endothelial cells (to line artificial or bioartificial blood vessels), liver and pancreatic cells (to replace diseased organs), muscle cells (to replace damaged or lost muscle), and more. In nearly all these examples of replacement tissue growth, the cells are harvested from the donor and seeded onto a solid substrate such as degradable fibers and initially grown in vitro without a blood supply to provide nourishment. We postulate that ultrasound can increase the diffusion of nutrients and oxygen into the cellular aggregate, allowing tissue cultures to be grown thicker and faster. In many systems the nutrient penetration into the tissue is the limiting factor in the number of layers of cells that can be grown on the substrate. Therefore ultrasound can be used to enhance this transport and increase the growth rate of these tissues, allowing burn patients, accident victims, and heart attack patients, and even children with birth defects to be healed faster.
Another application may be in biocultures of yeasts, bacteria and other higher organisms that transform one chemical compound into another. Such an example is ethanol production from corn to provide a fuel source. Another example is bioremediation in which the organisms metabolize toxic chemicals into harmless substances. In bioremediation the bacteria are often found in biofilms on solid particles in soil or water. Their enhanced growth via ultrasound would increase the rate of removal of harmful chemicals.
CONCLUSIONS
Contrary to the common belief that ultrasound will completely clean bacteria from surfaces, this research found that the initial adhesion of bacteria is unaffected by the presence of ultrasound. Furthermore, the net biofilm growth is enhanced by low frequency, low intensity ultrasound. This research found that ultrasound enhanced biofilm growth for E. coli, S. epidermidis, and P. aeruginosa, and we postulate that other bacterial species would also behave similarly. The species with normally faster growth rates were more affected by ultrasonic exposure. Since this effect could likely be caused by increased nutrient and waste transport, there are several interesting applications for these finding. It is possible that ultrasound could be used to significantly enhance growth rates of procaryotic and eucaryotic cells on surfaces and in suspension.
Acknowledgments
The authors gratefully acknowledge funding from the Center for Biopolymers at Interfaces and the National Institutes of Health grant HL 59923. We also thank Nathan D. Price and Rachel L. Robison for assistance with the planktonic data.
References
- 1.Brennen, C. E. Cavitation and Bubble Dynamics. Oxford University Press New York, 1995; pp 282.
- 2.Elder SA. Cavitation Microstreaming. J Acoust Soc Amer. 1959;31(1):54–64. [Google Scholar]
- 3.Nyborg WL. Ultrasonic Microstreaming and Related Phenomena. Br J Cancer. 1982;45(Suppl V):156–160. [PMC free article] [PubMed] [Google Scholar]
- 4.Nyborg WL. Biological Effects of Ultrasound: Development of Safety Guidelines. Part II: General Review. Ultrasound Med Biol. 2001;27(3):301–333. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 5.Starritt HC, Duck FA, Humphrey VF. An experimental investigation of streaming in pulsed diagnostic ultrasound beams. Ultrasound Med Biol. 1989;15(4):363–373. doi: 10.1016/0301-5629(89)90048-3. [DOI] [PubMed] [Google Scholar]
- 6.Dyson M. Non-Thermal Cellular Effects of Ultrasound. Br J Cancer. 1982;45(Suppl V):165–171. [PMC free article] [PubMed] [Google Scholar]
- 7.Martin CJ, Pratt BM, Watmough DJ. A Study of Ultrasound-Induced Microstreaming in Blood Vessels of Tropical Fish. Br J Cancer. 1982;45(Suppl V):161–164. [PMC free article] [PubMed] [Google Scholar]
- 8.Rooney, J. A. Other Nonlinear Acoustic Phenomena. In Ultrasound Its Chemical, Physical, and Biological Effects; Suslick, K. S., Ed.; VCH: New York, 1988, pp 65–96.
- 9.Maisonhaute E, Prado C, White PC, Compton RG. Surface acoustic cavitation understood via nanosecond eletrochemistry. Part III: shear stress in ultrasonic cleaning. Ultrasonics Sonochem. 2002;9:297–303. doi: 10.1016/s1350-4177(02)00089-5. [DOI] [PubMed] [Google Scholar]
- 10.Crawford AH. Large Scale Ultrasonic Cleaning. Ultrasonics. 1968;6(10):211–216. [Google Scholar]
- 11.Bulat TJ. Macrosonics in industry 3. Ultrasonic cleaning. Ultrasonics. 1972;12(3):59–68. [Google Scholar]
- 12.Mott IEC, Stickler DJ, Coakley WT, Bott TR. The removal of bacterial biofilm from water-filled tubes using axially propagated ultrasound. J Appl Microbiol. 1998;84:509–514. [Google Scholar]
- 13.Zips A, Schaule G, Flemming HC. Ultrasound as a Means of Detaching Biofilms. Biofouling. 1990;2:323–333. [Google Scholar]
- 14.Li JX, Sanderson RD, Jacobs EP. Ultrasonic cleaning of nylon microfiltration membranes fouled by kraft paper mill effluent. J Membrane Sci. 2002;205(1–2):247–257. [Google Scholar]
- 15.Atchley, A. A.; Crum, L. A. Acoustic Cavitation and Bubble Dynamics. In Ultrasound Its Chemical, Physical, and Biological Effects; Suslick, K. S., Ed.; VCH Publishers: New York, 1988, pp 1–64.
- 16.Chang CC, Merritt K. Microbial adherence on poly(methyl methacrylate) (PMMA) surfaces. J Biomed Mater Res. 1992;26:197–207. doi: 10.1002/jbm.820260206. [DOI] [PubMed] [Google Scholar]
- 17.Epstein SS, Rossel J. Enumeration of sandy sediment bacteria: search for optimal protocol. Mar Ecol Prog Ser. 1995;117:289–298. [Google Scholar]
- 18.Stickler D, Hewett P. Activity of Antiseptics against Biofilms of Mixed bacterial Species Growing on Silicone Surfaces. Eur J Clin Microbiol Infect Dis. 1991;10(5):416–421. doi: 10.1007/BF01968021. [DOI] [PubMed] [Google Scholar]
- 19.Dewhurst E, Rawson DM, Steele GC. The use of a model system to compare the efficiency of ultrasound and agitation in the recovery of Bacillus subtilis spores from polymer surfaces. J Appl Bacteriology. 1986;61:357–363. doi: 10.1111/j.1365-2672.1986.tb04297.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuwae T, Hosokawa Y. Determination of abundance and biovolume of bacteria in sediments by dual staining with 4',6-diamidino-2-phenylindole and acridine orange: relationship to dispersion treatment and sediment characteristics. Appl Environ Microbiol. 1999;65(8):3407–3412. doi: 10.1128/aem.65.8.3407-3412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oulahal-Lagsir N, Martial-Gros A, Boistier E, Blum LJ, Bonneau M. The development of an ultrasonic apparatus for the noninvasive and repeatable removal of fouling in food processing equipment. Lett Appl Microbiol. 2000;30(1):47–52. doi: 10.1046/j.1472-765x.2000.00653.x. [DOI] [PubMed] [Google Scholar]
- 22.Scherba G, Weigel RM, O'Brien WD. Quantitative assessment of the germicidal efficacy of ultrasonic energy. Appl Environ Microbiol. 1991;57:2079–2084. doi: 10.1128/aem.57.7.2079-2084.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raso J, Pagan R, Condon S, Sala FJ. Influence of Temperure and Pressure on the Lethality of Ultrasound. Appl Env Micro. 1998;64(2):465–471. doi: 10.1128/aem.64.2.465-471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Malo A, Guerrero S, Alzamora SM. Saccharomyces cerevisiae thermal inactivation kinetics combined with ultrasound. J Food Protect. 1999;62(10):1215–1217. doi: 10.4315/0362-028x-62.10.1215. [DOI] [PubMed] [Google Scholar]
- 25.Cochran SA, Prausnitz MR. Sonoluminescence as an Indicator of Cell Membrane Disruption by Acoustic Cavitation. Ultrasound in Med & Biol. 2001;27(6):841–850. doi: 10.1016/s0301-5629(01)00382-9. [DOI] [PubMed] [Google Scholar]
- 26.Chandler DP, Brown J, Bruckner-Lea CJ, Olson L, Posakony GJ, Stults JR, Valentine NB, Bond LJ. Continuous spore disruption using radially focused, high-frequency ultrasound. Anal Chem. 2001;73(15):3784–3789. doi: 10.1021/ac010264j. [DOI] [PubMed] [Google Scholar]
- 27.Belgrader P, Hansford D, Kovacs GT, Venkateswaran K, Mariella RJ, Milanovich F, Nasarabadi S, Okuzumi M, Pourahmadi F, Northrup MA. A minisonicator to rapidly disrupt bacterial spores for DNA analysis. Anal Chem. 1999;71(19):4232–4236. doi: 10.1021/ac990347o. [DOI] [PubMed] [Google Scholar]
- 28.Guzman HR, Nguyen DX, Kahn S, Prausnitz MR. Ultrasound-mediated disruption of cell membranes. I. Quantification of molecular uptake and cell viability. J Acoust Soc Am. 2001;110(1):588–596. doi: 10.1121/1.1376131. [DOI] [PubMed] [Google Scholar]
- 29.Singer AJ, Coby CT, Singer AHHCT, Jr, Tortora GT. The Effects of Low-Frequency Ultrasound on Staphylococcus epidermidis. Current Microbiology. 1999;38:194–196. doi: 10.1007/pl00006786. [DOI] [PubMed] [Google Scholar]
- 30.Vollmer AC, Kwakye S, Halpern M, Everbach EC. Bacterial Stress Responses to 1 MHz Pulsed Ultrasound in the Presence of Microbubbles. Appl Envr Microbio. 1998;64(10):3927–3931. doi: 10.1128/aem.64.10.3927-3931.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lillard HS. Decontamination of Puoltry Skin by Sonication. Food Technol-Chicago. 1994;48(12):72–73. [Google Scholar]
- 32.Rediske AM, Hymas WC, Wilkinson R, Pitt WG. Ultrasonic enhancement of antibiotic action on several species of bacteria. J Gen Appl Microbiol. 1998;44:283–288. doi: 10.2323/jgam.44.283. [DOI] [PubMed] [Google Scholar]
- 33.Johnson LL, Peterson RV, Pitt WG. Treatment of bacterial biofilms on polymeric implants using antibiotics and ultrasound. J Biomat Sci Polymer Ed. 1998;9:1177–1185. doi: 10.1163/156856298x00712. [DOI] [PubMed] [Google Scholar]
- 34.Williams RG, Pitt WG. In Vitro Response of Escerichia coli to Antibiotic and Ultrasound at Various Insonation Intensities. J Biomaterials Applications. 1997;12:20–30. doi: 10.1177/088532829701200102. [DOI] [PubMed] [Google Scholar]
- 35.Rapoport N, Smirnov AI, Timoshin A, Pratt AM, Pitt WG. Factors Affecting the Permeability of P. aeruginosa Cell Walls toward Lipophilic Compounds: Effects of Ultrasound and Cell Age. Archives Biochem Biophys. 1997;344(1):114–124. doi: 10.1006/abbi.1997.0176. [DOI] [PubMed] [Google Scholar]
- 36.Qian Z, Stoodley P, Pitt WG. The Effect of Low Intensity Ultrasound upon Biofilm Structure from Confocal Scanning Laser Microscopy Observation. Biomaterials. 1996;17(20):1975–1980. doi: 10.1016/0142-9612(96)00022-1. [DOI] [PubMed] [Google Scholar]
- 37.Qian Z, Sagers RD, Pitt WG. The Effect of Ultrasonic Frequency upon Enhanced Killing of P. aeruginosa Biofilms. Annals Biomed Eng. 1997;25(1):69–76. doi: 10.1007/BF02738539. [DOI] [PubMed] [Google Scholar]
- 38.Huang CT, James G, Pitt WG, Stewart PS. Effects of ultrasonic treatment on the efficacy of gentamicin against established Pseudomonas aeruginosa biofilms. Colloids and Surfaces B: Biointerfaces. 1996;6:235–242. [Google Scholar]
- 39.Pitt WG, McBride MO, Lunceford JK, Roper RJ, Sagers RD. Ultrasonic Enhancement of Antibiotic Action on Gram-Negative Bacteria. Antimicrob Agents and Chemother. 1994;38(11):2577–2582. doi: 10.1128/aac.38.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rediske AM, Rapoport N, Pitt WG. Reducing bacterial resistance to antibiotics with ultrasound. Lett Appl Microbiol. 1999;28(1):81–84. doi: 10.1046/j.1365-2672.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- 41.Peterson RV, Pitt WG. The effect of frequency and power density on the ultrasonically-enhanced killing of biofilm-sequestered Escherichia coli. Colloids and Surfaces B: Biointerfaces. 2000;17:219–227. [Google Scholar]
- 42.Qian Z, Sagers RD, Pitt WG. The role of insonation intensity in acoustic-enhanced antibiotic treatment of bacterial biofilms. Colloids and Surfaces B: Biointerfaces. 1997;9:239–245. [Google Scholar]
- 43.Johnston JB. A Simple, Nondescructive Assay for Bound Hyaluronan. J Biomed Mater Res (Appl Biomater) 2000;53:188–191. doi: 10.1002/(sici)1097-4636(2000)53:2<188::aid-jbm9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Tsai CL, Schurman DJ, Smith RL. Quantitation of glycocalyx production in coagulase-negative staphylococcus. J Orthopaedic Res. 1998;6:666–670. doi: 10.1002/jor.1100060507. [DOI] [PubMed] [Google Scholar]
- 45.Stewart PS. Theoretical Aspects of Antibiotic Diffusion into Microbial Biofilms. Antimicrob Agents Chem. 1996;40(11):2517–2522. doi: 10.1128/aac.40.11.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart PS. A Review of Experimental Measurements of Effective Diffusive Permeabilities and Effective Diffusion Coefficients in Biofilms. Biotech Bioeng. 1998;59(3):261–272. doi: 10.1002/(sici)1097-0290(19980805)59:3<261::aid-bit1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 47.Stewart PS, Griebe T, Srinivasan R, Chen CI, Yu FP, DeBeer D, McFeters GA. Comparison of Respiratory Activity and Culturability during Monochloramine Disinfection of Binary Population Biofilms. Appl Envr Micro. 1994;60(5):1690–1692. doi: 10.1128/aem.60.5.1690-1692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson, L. L., Investigations of the Kinetics and Mechanisms of Ultrasonically Enhanced Killing of Escherichia coli Biofilms, in Chemical Engineering. 1999, Brigham Young University: Provo, UT. p. 56.
- 49.Carmen, J., An Investigation of the Mechanism of the Action of Ultrasound and Antibiotics on Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus epidermidis, in Microbiology. 2001, Brigham Young University: Provo, UT.


