SUMMARY
Infection of implanted medical devices by Gram-positive organisms such as Staphylococcus ssp. is a serious concern in the biomaterial community. In this research the application of low frequency ultrasound to enhance the activity of vancomycin against implanted Staphylococcus epidermidis biofilms was examined. Polyethylene disks covered with a biofilm of S. epidermidis were implanted subcutaneously in rabbits on both sides of their spine. The rabbits received systemic vancomycin for the duration of the experiment. Following 24 h of recovery, one disk was insonated for 24 or 48 h while the other was a control. Disks were removed and viable bacteria counted. At 24 h of insonation, there was no difference in viable counts between control and insonated biofilms, while at 48 h of insonation there were statistically fewer viable bacteria in the insonated biofilm. The S. epidermidis biofilms responded favorably to combinations of ultrasound and vancomycin, but longer treatment times are required for this Gram-positive organism than was observed previously for a Gram-negative species.
Keywords: ultrasound, vancomycin, Staphylococcus epidermidis, biofilm, implant infection
INTRODUCTION
As chronically implanted biomaterials become more common, the concern of infection on those medical devices grows. Not only does the infection present serious consequences to the health of the patient, but the infectious bacteria can form biofilms on the surface of the device that can interfere with its performance (e.g. obstruct heart valve movement), or compromise the integration of the device into the body (e.g. septic loosening of orthopedic implants). Although the incidence of such infections may be low and continues to decrease (1), the results are severe and often debilitating to the patient.
Bacteria growing in biofilms on synthetic medical implants are highly resistant to traditional antimicrobial chemotherapy (2, 3). This recalcitrance necessitates the removal and replacement of infected implants to successfully treat the associated infections (4, 5).
In previous articles, we reported enhanced reductions of viable, sessile Escherichia coli in a model implant infection when gentamicin was combined with low-frequency ultrasound (6, 7). Although the success of ultrasound and aminoglycosides in treating an E. coli infection is notable, few clinical implant infections are attributed to E. coli, with the exception of urinary devices or urosepsis (1). Staphylococcus aureus and Staphylococcus epidermidis appear to be most commonly found in orthopedic implant infections. Of the organisms causing prosthetic hip implants, Sanderson reported that 35% were S. aureus, 15% were S. epidermidis, 25% were coliforms, and 25% were anaerobes and others (8). Similarly for prosthetic joint infections, Steckelberg reported that 25% were coagulase-negative staphylococci, 23% were S. aureus, 14% were polymicrobial, 11% were Gram-negative bacilli, 8% were streptococci, 6% were anaerobes, 3% were enterococci, and 10% were unknown or other microorganisms (9). E. coli is a minor player in orthopedic implant infections.
Although aminoglycosides are often recommended for infections of Gram-negative rods, they are not the antibiotics of choice for Gram-positive infectious organisms such as S. epidermidis. Vancomycin was used as an antibiotic of last resort for methicillin-resistant strains of S. epidermidis and other Staphylococcus and Streptococcus ssp. (10). Although Vancomycin is still used for methicillin-resistant infections (11), some bacterial strains are acquiring vancomycin resistance and newer antibiotics may be required (12).
In this research we investigated the combination of low frequency ultrasound and vancomycin in treating S. epidermidis infections in a rabbit model. Ultrasonic irradiation (insonation) has great potential as a medical therapy for drug delivery. Ultrasound consists of acoustic energy in the form of pressure waves with frequency above 20 kHz. Because of its wave nature, ultrasound can be focused through the skin and tissue and directed to the desired target volume in the body. The application is non-invasive; no incisions are required. Low frequency ultrasound (<500 kHz) is not attenuated and does not produce heating to the same levels as the higher frequency (>1 MHz) ultrasound used for medical imaging and physical therapy.
Our previous research showed that ultrasound enhanced the activity of aminoglycosides against Gram-negative bacteria in both planktonic and biofilm phenotypes (6, 13–18). Ultrasound also increased killing of Gram-positive S. epidermidis and Streptococcus mitis by ampicillin (16). Insonation is hypothesized to increase the effectiveness of antibiotics by increasing the rate of antibiotic transport to the bacteria (19, 20), by increasing the permeability of the cell membrane (21, 22), and by increasing the metabolic activity and growth of the bacteria, perhaps by increasing oxygen and other nutrient transport (19). The research presented
MATERIALS AND METHODS
New Zealand White female rabbits were maintained according to regulations established by the U.S. Department of Agriculture and the Institutional Animal Care and Use Committee of Brigham Young University. The method of surgical biofilm implantation and evaluation of biofilm viability were described in previous reports and are outlined only briefly below (6, 7).
S. epidermidis ATCC 35984 (RP62A) biofilms were grown for 24 h on polyethylene disks (1.88 cm diameter, 0.01 cm thickness, with 2 sewing tabs). The disks were placed in 15 ml tryptic soy broth with 0.01% glucose (TSB). The TSB was then inoculated with 20 μl of an overnight culture that had been centrifuged and resuspended in sterile phosphate buffered saline solution. After eight hours, the disks were removed from the petri dishes, rinsed with 1 ml of sterile physiologic saline solution, and placed in petri dishes containing 15 ml of sterile TSB. This process was repeated for two more 8-h growth periods. After the final 8 h growth period, two disks were rinsed in saline, implanted subcutaneously and sewn into place with one disk positioned on either side of the spine.
A catheter was inserted into a marginal vein in one of the rabbit’s ears. The catheter was used to administer vancomycin (50 mg kg−1) immediately after surgery and 3 times a day thereafter. A second catheter was inserted similarly in the opposite ear to facilitate blood sampling throughout the experiment. Blood was sampled prior to surgery and then every 24 h thereafter just prior to the administration of vancomycin. The sampled blood was cultured to determine any bacteremia.
Twenty-four hours post-surgery, a Tonpilz resonator (EDO Acoustics, Salt Lake City, UT) was fixed over one of the implants with Tensive (Parker Labs, Fairfield, New Jersey), which is both an acoustic conductor and an adhesive. The transducer, operating at 28.48 kHz, delivered pulsed ultrasound in a 1:3 duty cycle at a power density of 500 mW cm−2 during the pulse.
After treatment with ultrasound for 24 or 48 h, the rabbits were euthanized and the implants were removed. The biofilms were stripped from the disks by exposure to 10 ml of a 48 mg l−1 solution of Validase X, a polysaccharase (Valley Research, Inc., South Bend, IN) in an ultrasonic bath. The number of viable bacteria remaining in the biofilms was determined by serial dilution and membrane filtration.
The reduction of bacteria in these experiments is reported in two forms. The “total reduction” reflects the decrease in viable bacteria (on a log basis) based on the average amount of bacteria on the disks before they were implanted in the rabbit; it is the difference between the initial and retrieved counts. The “enhanced reduction” reflects difference in viable counts (on a log basis) between the two implants retrieved from the same rabbit, one of which was insonated; it quantifies the enhancement in killing of vancomycin produced by the addition of ultrasound to the implant. One-tailed paired Student’s t-tests were used to evaluate the differences in mean log10 CFU cm−2 recovered from implanted biofilms treated with vancomycin and implanted biofilms treated with both vancomycin and ultrasound for each rabbit.
In addition to recovering the implanted disks, the heart, kidneys, and skin at the treatment site were collected for independent histopathological examination (ARUP, Salt Lake City, UT).
RESULTS
Figure 1 shows the viable counts of S. epidermidis recovered from the polymer disks. The non-implanted biofilms from six experiments contained 7.67 ± 0.59 log10 CFU/cm2 bacteria on average. Treatment of biofilms of S. epidermidis for 48 h with vancomycin reduced the number of viable bacteria in the biofilm to 2.55 ± 0.20 log10 CFU/cm2, a log10 total reduction of 5.12 CFU/cm2. In the contralateral biofilms, exposed to vancomycin for 48 h and ultrasound for 24 hrs, the number of viable bacteria was reduced to 2.22 ± 0.54 log10 CFU/cm2, a log10 total reduction of 5.45 CFU/cm2. Ultrasonic treatment for 24 h did not significantly enhance the killing of viable bacteria compared to the contralateral non-insonated implant (enhanced reduction = 0.33 log10 CFU/cm2; p = 0.26, n = 3).
Figure 1.
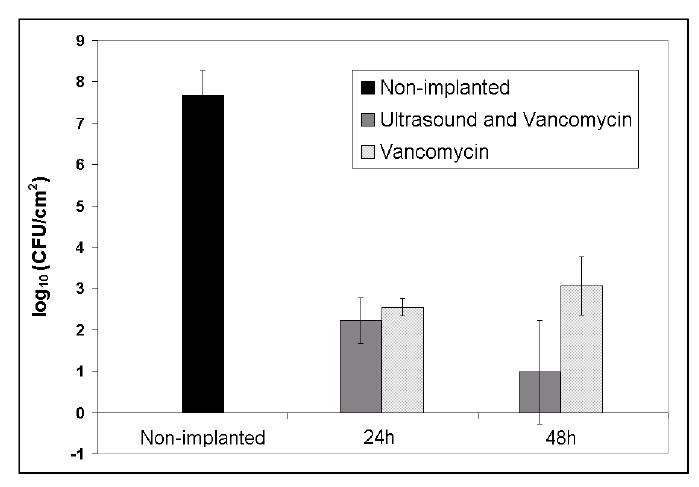
Viable Staphylococcus epidermidis recovered from non-implanted and implanted biofilms treated with vancomycin or with vancomycin and ultrasound for 24 or 48 h. The error bars represent 95% confidence intervals.
Treatment of S. epidermidis biofilms with only vancomycin for 72 h post-surgery reduced the number of viable bacteria to 3.06 ± 0.70 log10 CFU/cm2, a log10 total reduction of 4.61 CFU/cm2. When the contralateral biofilms were treated for 72 h with vancomycin, the final 48 h of which also received ultrasound, the number of viable bacteria was reduced to 0.98 ± 1.25 log10 CFU/cm2, a log10 total reduction of 6.69 CFU/cm2 (Fig. 2.5). Treatment of S. epidermidis biofilms with vancomycin and 48 h of ultrasound significantly enhanced the reduction of viable bacteria in the biofilm, an enhanced reduction of 2.08 log10 CFU/cm2 (p = 0.01, n = 3).
Histopathology indicated neither abnormalities nor infection in the heart or kidneys removed from euthanized rabbits. The skin at the implant site appeared healthy. In addition, blood cultures revealed no bacteria in any of the daily blood samples taken.
DISCUSSION
Previously, our lab has reported the use of low-frequency ultrasound to enhance the activity of antibiotics against planktonic and sessile Gram-negative bacteria in vitro and in vivo (6, 7, 16, 18, 23, 24). The combination of gentamicin and low-frequency ultrasound was very effective in reducing viability of E. coli biofilms. Because of the prevalence of Gram-positive organisms in vascular biofilm infections, it was important to determine whether or not combined ultrasound/antibiotic therapy enhanced the reduction of viable Gram-positive bacteria in a biofilm in vivo.
Following the implantation of heavily colonized biofilms of S. epidermidis, the rabbits’ immune system in combination with vancomycin reduced the number of viable bacteria by 5 orders of magnitude in 48 hrs. However, there was no further reduction by 72 hrs post-surgery. Even with this high level of vancomycin, the implant infection could not be resolved. When ultrasound was applied for 24 hrs in combination with vancomycin, the viable counts were not different than those without insonation. However, insonation of implanted biofilms for 48 h along with the presence of systemic vancomycin reduced the average number of viable bacteria to below 10 CFU cm−2, which was significantly lower than the viable counts without insonation. A similar observation was made for E. coli implant infections in which 48-h insonation was more effective than 24-h insonation. This ultrasonic treatment increased the killing of viable bacteria without causing bacteremia or tissue damage. We do not know whether or not this reduction will allow the host immune system to eliminate the remaining viable bacteria. However, it is probable that longer treatments would further increase the amount of killing. A combination of antibiotics such as vancomycin plus rifampin may be more effective (1).
More research remains to be done to determine why the combination of ultrasound and gentamicin is more effective against E. coli in vivo than the combination of ultrasound and Vancomycin against S. epidermidis. The particular strain of S. epidermidis used herein (RP62A) is a notorious clinical isolate (25–27) and is resistant to aminoglycosides, and thus we did not attempt to use gentamicin in the in vivo model. In planktonic systems, ultrasonic enhancement of antibiotic activity is most pronounced with systems employing aminoglycosides and Gram-negative bacteria. We speculate that the normal stress on Gram-negative membranes due to aminoglycoside binding (28, 29) is further accentuated by the ultrasonic exposure and leads to increased antibiotic uptake, and that such a mechanism may be missing in Gram-positive species. This hypothesis remains to be tested in biofilms.
Acknowledgments
The research was funded by NIH grant HL59923.
References
- 1.Zimmerli W, Ochsner PE. Management of infection associated with prosthetic joints. Infection. 2003;31(2):99–108. doi: 10.1007/s15010-002-3079-9. [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M. Bacterial biofilms in nature and disease. Ann. Rev. Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 3.Donlan RM. Role of Biofilms in Antimicrobial Resistance. ASAIO J. 2000;46(6):S47–S52. doi: 10.1097/00002480-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP. Bacterial Biofilms: A Common Cause of Persistent Infections. Science. 1999 21 May;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Costerton JW, Stewart PS. Battling Biofilms. Sci. Amer. 2001;285(1):74–81. doi: 10.1038/scientificamerican0701-74. [DOI] [PubMed] [Google Scholar]
- 6.Rediske AM, Roeder BL, Brown MK, Nelson JL, Robison RL, Draper DO, et al. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob. Agents Chemother. 1999;43(5):1211–1214. doi: 10.1128/aac.43.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rediske AM, Roeder BL, Nelson JL, Robison RL, Schaalje GB, Robison RA, et al. Pulsed ultrasound enhances the killing of E. coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob. Agents Chemother. 2000;44:771–772. doi: 10.1128/aac.44.3.771-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanderson PJ. Infection in orthopaedic implants. J Hosp Infect. 1991;18(Suppl A):367–75. doi: 10.1016/0195-6701(91)90043-8. [DOI] [PubMed] [Google Scholar]
- 9.Steckelberg JM, Osmon DR. Prosthetic Joint Infections. In: Bisno AL, Waldvogel FA, editors. Infections Associated with Indwelling Medical Devices. 2nd ed. Washington, DC: Amer. Soc. Microbiology; 1994. p. 259–289.
- 10.Sieradzki K, Roberts RB, Serur D, Hargrave J, Tomasz A. Heterogeneously vancomycin-resistant Staphylococcus epidermidis strain causing recurrent peritonitis in a dialysis patient during vancomycin therapy. J Clin Microbiol. 1999;37(1):39–44. doi: 10.1128/jcm.37.1.39-44.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotozono C, Inagaki K, Fujita A, Koizumi N, Sano Y, Inatomi T, et al. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections in the cornea. Cornea. 2002;21(7 Suppl):S94–101. doi: 10.1097/01.ico.0000263127.84015.3f. [DOI] [PubMed] [Google Scholar]
- 12.Strahilevitz J, Rubinstein E. Novel agents for resistant Gram-positive infections--a review. Int J Infect Dis. 2002;6(Suppl 1):S38–46. doi: 10.1016/s1201-9712(02)90153-0. [DOI] [PubMed] [Google Scholar]
- 13.Qian Z, Sagers RD, Pitt WG. The Effect of Ultrasonic Frequency upon Enhanced Killing of P. aeruginosa Biofilms. Annals Biomed. Eng. 1997;25(1):69–76. doi: 10.1007/BF02738539. [DOI] [PubMed] [Google Scholar]
- 14.Qian Z, Sagers RD, Pitt WG. The role of insonation intensity in acoustic-enhanced antibiotic treatment of bacterial biofilms. Colloids and Surfaces B: Biointerfaces. 1997;9:239–245. [Google Scholar]
- 15.Rediske AM, Rapoport N, Pitt WG. Reducing bacterial resistance to antibiotics with ultrasound. Lett. Appl. Microbiol. 1999;28(1):81–84. doi: 10.1046/j.1365-2672.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- 16.Rediske AM, Hymas WC, Wilkinson R, Pitt WG. Ultrasonic enhancement of antibiotic action on several species of bacteria. J. Gen. Appl. Microbiol. 1998;44:283–288. doi: 10.2323/jgam.44.283. [DOI] [PubMed] [Google Scholar]
- 17.Williams RG, Pitt WG. In Vitro Response of Escherichia coli to Antibiotic and Ultrasound at Various Insonation Intensities. J Biomaterials Applications. 1997;12:20–30. doi: 10.1177/088532829701200102. [DOI] [PubMed] [Google Scholar]
- 18.Peterson RV, Pitt WG. The effect of frequency and power density on the ultrasonically-enhanced killing of biofilm-sequestered Escherichia coli. Colloids and Surfaces B: Biointerfaces. 2000;17:219–227. [Google Scholar]
- 19.Pitt WG, Ross SA. Ultrasound increases the rate of bacterial cell growth. Biotechnol Prog. 2003;19(3):1038–44. doi: 10.1021/bp0340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmen JC, Nelson JL, Beckstead BL, Runyan CM, Robison RA, Schaalje GB, et al. Ultrasonic-Enhanced Gentamicin Transport through Colony Biofilms of Pseudomonas aeruginosa and Escherichia coli J Antimicrob Chemother 2003;submitted. [DOI] [PMC free article] [PubMed]
- 21.Rapoport N, Pitt WG, Smirnov AI, Timoshin AI. Bioreduction of Tempone and Spin-Labeled Gentamicin by Gram-Negative Bacteria: Kinetics and Effect of Ultrasound. Arch. Biochem. Biophys. 1998;362(2):233–241. doi: 10.1006/abbi.1998.1020. [DOI] [PubMed] [Google Scholar]
- 22.Rapoport N, Smirnov AI, Timoshin A, Pratt AM, Pitt WG. Factors Affecting the Permeability of P. aeruginosa Cell Walls toward Lipophilic Compounds: Effects of Ultrasound and Cell Age. Archives Biochem. Biophys. 1997;344(1):114–124. doi: 10.1006/abbi.1997.0176. [DOI] [PubMed] [Google Scholar]
- 23.Qian Z, Sagers RD, Pitt WG. Investigation of the mechanism of the bioacoustic effect. J. Biomed. Mater. Res. 1999;44:198–205. doi: 10.1002/(sici)1097-4636(199902)44:2<198::aid-jbm10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Pitt WG, McBride MO, Lunceford JK, Roper RJ, Sagers RD. Ultrasonic Enhancement of Antibiotic Action on Gram-Negative Bacteria. Antimicrob. Agents and Chemother. 1994;38(11):2577–2582. doi: 10.1128/aac.38.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baddour LM, Christensen GD, Hester MG, Bisno AL. Production of Experimental Endocarditis by Coagulase-Negative Staphylococci: Variability in Species Virulence. J. Inf Diseases. 1984;150(5):721–727. doi: 10.1093/infdis/150.5.721. [DOI] [PubMed] [Google Scholar]
- 26.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of Slime-Producing Strains of Staphylococcus epidermidis to Smooth Surfaces. Inf. Immun. 1982;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen GD, Parisi JT, Bisno AL, Simpson WA, Beachey EH. Characterization of Clinically Significant Strains of Coagulase-Negative Staphylococci. J Clinical Microbiology. 1983;18(2):258–269. doi: 10.1128/jcm.18.2.258-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrobial Agents and Chemotherapy. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


