Abstract
Ultrasound (US) has an ever-increasing role in the delivery of therapeutic agents including genetic material, proteins, and chemotherapeutic agents. Cavitating gas bodies such as microbubbles are the mediators through which the energy of relatively non-interactive pressure waves is concentrated to produce forces that permeabilize cell membranes and disrupt the vesicles that carry drugs. Thus the presence of microbubbles enormously enhances delivery of genetic material, proteins and smaller chemical agents. Delivery of genetic material is greatly enhanced by ultrasound in the presence of microbubbles. Attaching the DNA directly to the microbubbles or to gas-containing liposomes enhances gene uptake even further. US-enhanced gene delivery has been studied in various tissues including cardiac, vascular, skeletal muscle, tumor and even fetal tissue. US-enhanced delivery of proteins has found most application in transdermal delivery of insulin. Cavitation events reversibly disrupt the structure of the stratus corneum to allow transport of these large molecules. Other hormones and small proteins could also be delivered transdermally. Small chemotherapeutic molecules are delivered in research settings from micelles and liposomes exposed to ultrasound. Cavitation appears to play two roles: it disrupts the structure of the carrier vesicle and releases the drug; it also makes the cell membranes and capillaries more permeable to drugs. There remains a need to better understand the physics of cavitation of microbubbles and the impact that such cavitation has upon cells and drug-carrying vesicles.
Keywords: ultrasound, targeted drug delivery, liposomes, microbubbles, micelles, chemopotentiation, hyperthermia, cavitation, DNA, protein, chemotherapy
1. Mechanisms of Ultrasonic-Enhanced Drug Delivery
Ultrasound (US) has been employed to enhance the delivery and activity of drugs for the past two decades. In the past 6 years, however, research in US-activated drug delivery has blossomed with the introduction of gas bubbles. To understand the mechanisms of US-enhanced drug delivery, one must first understand the physics of ultrasound and of cavitation, the keystone of this novel approach to drug delivery.
1.1 Physics of Ultrasound
Just as audio sound is the transmission of pressure waves through a medium such as air or water, ultrasound is the same type of transmission of pressure waves, but at frequencies above human hearing, or above 20,000 Hz. As with light waves, these ultrasonic waves can be reflected, refracted (bent), focused, and absorbed. Unlike light waves, ultrasonic waves are very physical in nature; they are actual movement of molecules as the medium is compressed (at high pressure) and expanded (at low pressure), and thus ultrasound can act physically upon biomolecules and cells.
Most importantly, unlike visible light waves, ultrasonic waves are absorbed relatively little by water, flesh and other tissues. Therefore, ultrasound can “see” into the body (e.g., diagnostic ultrasound) and can be used to transmit energy into the body at precise locations. This safe, non-invasive and painless transmission of energy into the body is the key to ultrasonic-activated drug delivery.
1.2 Hyperthermia
The traditional use of ultrasound in medicine is for diagnostic imaging (which occurs at low average intensities and high frequencies) and for tissue heating (which occurs at higher intensities and higher frequencies). The intensity (also called power density) of an ultrasonic beam is measured in terms of power carried per cross section area of the beam, typically having units of Watts/cm2. If a beam is focused down to a small size on a target tissue, the power per area becomes very large and significant thermal energy can be absorbed from the beam by the tissue, resulting in heating. Such hyperthermia has been traditionally employed in physical therapy to warm tissues [1], in drug delivery to “melt” drug-containing liposomes [2], and in medical therapy to kill or ablate tissue [3–5]. Thus hyperthermia in targeted drug delivery accomplishes the role of heating the drugs, drug carriers, and/or the tissues receiving the drugs.
1.3 Cavitation
1.3.1 Nature of Cavitation
A relatively recent and novel application in drug delivery takes advantage of the remarkable ability of ultrasound to produce cavitation activity. Cavitation is the formation and/or activity of gas-filled bubbles in a medium exposed to ultrasound [6]. As the pressure wave passes through the media, gas bubbles of any size will expand at low pressure and contract at high pressure. If the resulting oscillation in bubble size is fairly stable (repeatable over many cycles), the cavitation is called “stable” or “non-inertial” cavitation. Such oscillation creates a circulating fluid flow (called microstreaming) around the bubble [7–9] with velocities and shear rates proportional to the amplitude of the oscillation. At high amplitudes the associated shear forces are capable of shearing open red cells [10] and synthetic vesicles such as liposomes [7].
As the ultrasonic intensity increases, the amplitude of oscillation also increases to a point in which the inward moving wall of fluid has sufficient inertia that it cannot reverse direction when the acoustic pressure reverses, but continues to compress the gas in the bubble to a very small volume, creating extremely high pressures and temperatures [11, 12]. This type of cavitation (called transient, inertial or collapse cavitation) can be detrimental to cells or vesicles because of the very high shear stresses in the region of the collapse, the shock wave produced by the collapse, and the free radicals produced by the high temperatures. The collapsed bubble often fragments into smaller bubbles that serve as cavitation nuclei, grow in size, and eventually collapse again [11, 12]. Figure 1 shows a streak optical photograph before, during and after a cavitation collapse [12]. On the left is the original microbubble. The middle frame is a high-speed streak photograph showing the boundaries of the bubble when subjected to a 5-cycle driving pulse at 2.5 MHz and 1.6 MPa; the pressure measurement is recorded at the top of the photograph. The right frame shows the resulting fragments of the bubble. Cavitation is a violent phenomenon that concentrates the energy from ultrasound into a small volume.
Figure 1.
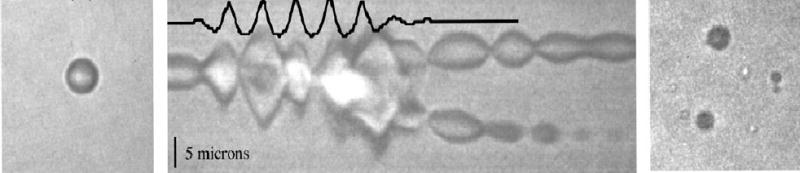
Optical images of a 2.5 μm-radius microbubble exposed to 5 cycles of 2.5 MHz ultrasound at 1.6 MPa pressure amplitude. The left panel shows the bubble before exposure. The central panel shows a streak photograph (an optical M-mode image of a line through the center of the bubble as a function of time) with the measured pressure superimposed at the top of the panel. The right panel shows bubble the fragments produced by the collapse of the cavitating bubble. Taken from [12]. Copyright IEEE.
Furthermore, if the collapse is near a solid surface, an asymmetrical collapse occurs which ejects a liquid jet at sonic speed toward the surface [11, 13]. Figure 2 illustrates this type of collapse. If the rigid surface is a blood vessel wall, skin, a large cell, or semi-rigid vesicle, then the jet can pierce the surface.
Figure 2.
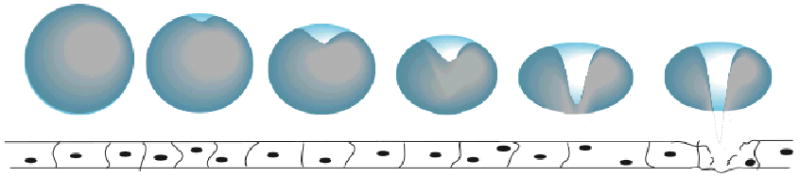
Illustration of an asymmetric collapse of a bubble near a surface, producing a jet of liquid toward the surface.
Since collapse cavitation can be damaging to biological tissues, there has been much research into the conditions under which it is produced. In general the likelihood and intensity of collapse cavitation increases at higher intensities and lower frequencies, as has been demonstrated by experiments [11, 14, 15] and theory [16]. The size of the bubble and its physical properties (gas species, interfacial tension, surface rigidity, etc.) also affect the cavitation process [17–21].
1.3.2 Ultrasound-induced mechanisms of drug delivery
1.3.2.1 Enhanced Transport.
Ultrasonication and cavitation can be involved in drug delivery by several mechanisms. The simplest mechanism derives from the oscillatory motion of the insonated fluid and can occur in the absence of cavitation. The oscillating fluid increases the effective diffusivity of molecules; thus the transport of any drug, whether free or bound to a carrier, will be augmented by the oscillatory motion of the fluid. Such ultrasonic-enhanced transport may occur within blood, cells, or extracellular fluids.
When a strong ultrasonic beam is directed through a partially absorbing liquid, some momentum from the beam is transferred to the fluid, imparting a large-scale convective motion to the fluid that can also increase the overall rate of drug transport [22]. Although this so-called “acoustic streaming” could enhance drug transport in vitro, we opine that it has little, if any application for in vivo systems because fluid convection in the vascular system is already very fast, and in tissues beyond the vascular system, there are few reservoirs of fluid that can be accelerated by an acoustic beam.
In the presence of oscillating bubbles, drug transport is enhanced by orders of magnitude over transport by diffusion alone. At least two mechanisms are reported for creating convection in the presence of a stable oscillating bubble. The first phenomenon, called microstreaming, is the creation of circulating eddies around the oscillating bubble [7–9]. Such eddies can transport drugs at high velocities. For example, the velocity of water near the surface of a 10 micron (diameter) bubble, with a 2 micron oscillation amplitude are on the order of 10 m/s [9]. Even more surprising are the extremely high viscous shear rates near the surface of the bubble, which in this example are on the order of ten million (107) inverse seconds [9]. This shear rate is equivalent to shearing water in a 1 mm gap between parallel plates in which one plate is stationary and the other moving at 1010 m/s. This extremely high shear rate stresses cells and vesicles as will be discussed later.
The second phenomenon related to oscillating bubbles is called acoustic pressure, and is a net force acting on other suspended bodies in the vicinity of an oscillating bubble. If the body is more dense than the suspending liquid, the body is pushed toward the bubble; if it is less dense, the body is repelled from the bubble [23]. Most drug carrying liposomes and micelles are more dense than water, and thus will be convected toward the bubble, thus increasing the dispersive transport of the drug carrier, particularly if the vesicle is drawn into the microstreaming field around the bubble and is sheared open by the high shear rate (thus releasing drug). Marmottant and Hilgenfeldt show a fascinating example of this phenomenon [7]. If the vesicle is another microbubble, it will be dispersed away from the primary oscillating bubble because it is less dense. Thus a field of microbubbles, such as an injected bolus of contrast agent, will tend to spread itself in the ultrasonic field, and at the same time attract and shear more dense vesicles such as suspended cells or introduced liposomes.
1.3.2.2 Perturbation of the drug carrier
This leads to the second major contribution of ultrasound toward drug delivery, the disruption of drug carriers by ultrasound. As mentioned above, vesicles more dense than the surrounding liquid will be sucked into the shear field surrounding an oscillating bubble. If the shear stress exceeds the strength of the vesicle, it will rupture and spill its contents. In the case of a liposome (and perhaps a micelle), the vesicle will probably reform, often at a smaller size than before the encounter with the shear field. The case of a liposome resizing into a number of smaller vesicles with the same amount of surface area (assuming no phospholipids are lost) presents an interesting method of drug release. If a big spherical vesicle is fractured into a number of smaller vesicles and the surface area is conserved, the total volume of the smaller vesicles is less than the original volume. Thus, some interior liquid must be released during the fracture event. If a drug is soluble in the aqueous core of a liposome, some will be released upon fracture, even if the liposomes reform to smaller vesicles. Note that collapse cavitation is not required for this method of drug release; a stable oscillating bubble is sufficient.
Other mechanisms of vesicle perturbation arise from collapse cavitation. The collapse event produces a shock wave that can be envisioned as an expanding thin shell of dense water. As this spike of dense fluid passes over a vesicle, a shear stress is produced at the vesicle surface that can rupture the vesicle if the critical stress is exceeded [24, 25]. As the shock wave expands spherically, its energy density decreases such that beyond a certain distance from the bubble, there is no longer sufficient shear to disrupt vesicles [25].
As mentioned previously, the sonic jet of fluid produced by collapse cavitation near a solid surface also generates extreme shear stresses that can shear open or perhaps pierce nearby vesicles. Some of these methods of drug delivery are illustrated in Figure 3.
Figure 3.
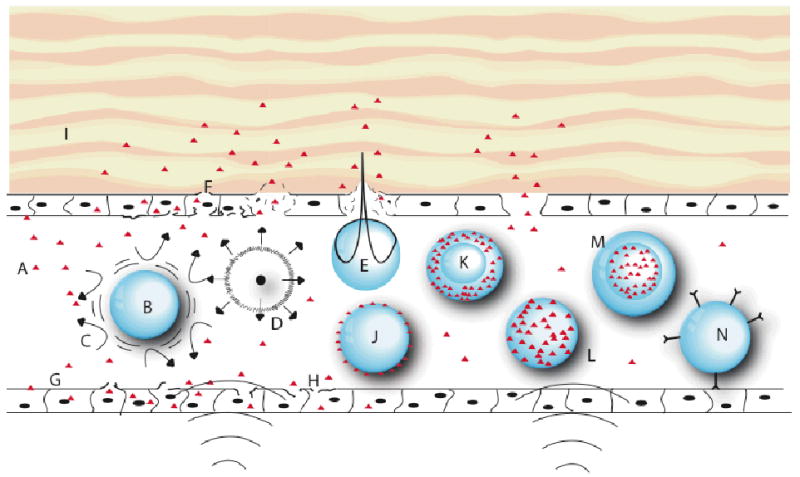
Schematic representation of various modes by which drug delivery can be enhanced by ultrasound. A: therapeutic agent (triangles); B: gas bubble undergoing stable cavitation; C: microstreaming around cavitating bubble; D. collapse cavitation emitting a shock wave; E: asymmetrical bubble collapse producing a liquid jet that pierces the endothelial lining; F: completely pierced and ruptured cell; G: non-ruptured cells with increased membrane permeability due to insonation; H: cell with damaged membrane from microstreaming or shock wave; I: extravascular tissue; J: thin-walled microbubble decorated with agent on surface; K. thick-walled microbubble with agent in lipophilic phase; L: micelle with agent in lipophilic phase; M: liposome with agent in aqueous interior; N: vesicle decorated with targeting moieties attached to a specific target.
It is well known that low frequency high intensity ultrasound (conditions promoting collapse cavitation) reduce the molecular weight of soluble polymers [26–28]. Polymer chain scission is attributed to viscous shear stresses that pull the backbone apart and to the generation of free radicals that may react with and break the chain. In cases employing drugs bound to a polymeric carrier, such reduction in polymer molecular weight will increase the diffusivity of the polymer-drug fragments. In some cases, the drug might be sheared from the polymer backbone. An interesting example is given for the ultrasonically activated delivery of cobalamin (vitamin B12) from a carrier. The release is attributed to free radicals, generated by collapse cavitation, that react with, rearrange, and break the bond connecting the cobalamin to the drug carrier [29].
1.3.2.3 Cell permeabilization and capillary rupture
The third major contribution of ultrasound to drug delivery relates to the stresses inflicted upon cells and tissues as a result of cavitation events. Ultrasound by itself, in the absence of cavitation, is thought to have little if any effect on cells and tissues apart from some heating that may occur at higher frequencies and intensities [6, 23, 30, 31]. As with vesicles, cells in an environment of cavitation events are subject to shear from microstreaming, shock waves and sonic jets. It is possible that a large semi rigid cell adjacent to a small cavitating bubble could induce an asymmetric bubble collapse, by which a small jet of liquid would shoot directly into the cell at sonic speeds, probably rupturing the cell membrane. Likewise a collapse of a microbubble near a capillary or blood vessel wall will cause the liquid jet to shoot right into the wall. Such a collapse may be the source of the large amount of extravasation that is caused in tissue exposed to ultrasound in the presence of microbubble contrast agents [32–35]. There is some concern that a blood vessel injury by sonic jets, shear stresses, or radicals could generate a nidus for thrombus formation.
1.3.3 Biological and physical consequences of cavitation
Much has been written about the biological consequences of ultrasonic cavitation in living systems, and little if any is positive with respect to the health of the organism or tissue [6, 23, 30, 31]. Obviously a plethora of bubbles undergoing collapse cavitation is not a healthy environment for a cell. Shock waves and shear forces are shearing the cell membrane. Liquid micro-jets at sonic velocities may be shooting right through cells (and lysing them). The free radicals may be interfering with essential biochemical processes. At the other extreme, a field of quivering bubbles experiencing mild stable cavitation probably has no negative biological consequences to most cells, and may even be beneficial in terms of increasing convection of oxygen or nutrients to cells [36].
Targeted drug release is a very active process, and these same energetic cavitational phenomena that may be detrimental to tissues are requisite in promoting the physical or chemical release of drugs from a carrier, or in promoting the transport of drugs into cells. Low intensity stable cavitation may not be adequate to effectively execute drug delivery. Thus some cavitational activity must be tolerated to accomplish the medicinal goal. Somewhere between these harsh and mild extremes lies the desired realm of ultrasonic drug delivery – a cavitational level that produces bubble activity sufficient to permeabilize cell membranes without killing the cells, a level that creates a sufficient number of micro-jets to allow extravasation from capillary walls without killing the endothelial cells or causing thrombosis, or a level that generates sufficient microstreaming to break open liposomes or other vesicles without lysing red cells or other host cells.
1.4 Types of Drug Carriers
The delivery of free drug, not associated with a carrier system, can be enhanced by ultrasound as will be discussed in section 2.3.1. However the disadvantage of free drug is that it often interacts with non-targeted tissues if it is delivered systemically.
To preclude the non-targeted interaction of free drug with tissues, the drug is sometimes attached to a polymeric carrier from which it is released at the target site by degradation of a linker via enzymes or pH at the target site. Ultrasound has been used to release covalently bound cobalamin from a carrier [29], but this was a very specific application and may be difficult to extend to drugs in general.
Liposomes and micelles can sequester hydrophobic drugs within their lipophilic membrane (liposome) or core (micelle), and liposomes can sequester hydrophilic drugs in their aqueous interior. These vesicles appear to prevent general and/or premature release of the drug [37–39]. In theory, liposomes and micelles should not be acoustically active if they do not contain any gas. However, they can be drawn towards and then sheared open by the action of cavitating bubbles. In addition, cell membranes can be sheared by cavitation events, rendering them more permeable toward liposome or free drug uptake. Some studies suggest that even carefully prepared liposomes contain some gas, and thus they can become acoustically active during insonation and release their payload upon cavitation-induced fragmentation [40, 41]. Unger has prepared a hybrid between a liposome and a microbubble, in which gas and stabilizing oil are introduced into the standard liposome [42]. This “liposphere” can be considered a hybrid between a pure liposome and microbubble, and can also be used as an ultrasonic contrast agent.
Microbubbles are distinguished from liposomes in that they contain gas and in general do not have a bilayer structure; they are simply gas bubbles stabilized by a surfactant at their surface. Proteins such as albumin can be used in place of conventional phospholipid surfactants. As mentioned, when microbubbles of gas-carrying lipospheres also carry drugs, they are dually effective drug delivery vehicles, being both the carrier and the activator for the ultrasonic drug delivery.
Some drugs can spontaneously associate with the surface of liposomes and microbubbles. An example is the association of negatively charged DNA and RNA with liposomes and microbubbles composed of cationic surfactants. When the liposome or microbubble is fragmented by cavitation events, the drug can be released, although it may yet retain some association with the cationic surfactant. The free drug can be taken up by normal mechanisms, while the drug associated with fragmented surface could be taken up by pinocytosis or related mechanisms.
Having completed this basic introduction to ultrasound, cavitation and drug carriers, this review is organized according to the general type of drug delivered. First we will discuss the delivery of the largest and most sensitive macromolecules, DNA, to targeted tissue. Then we will focus on smaller and/or globular macromolecules, i.e. proteins. Finally we will present strategies for the ultrasonic delivery of conventional small chemotherapeutic drugs.
2. Ultrasonic-Assisted Delivery of Therapeutic Molecules
2.1 DNA and Gene Delivery
Gene delivery is a topic of intense interest in targeted drug delivery [43–49]. The use of ultrasound in gene delivery has exceptional potential because the beam can be focused on a particular tissue. The trick, as always, is to release the genetic material only at the targeted site. A further complication is that the DNA (or possibly RNA) must enter the targeted cells before it is degraded by DNase or carried to other tissues by the blood. This section will first discuss gene carriers, and then give a review with examples of targeting specific tissues.
2.1.1 Ultrasonically activated gene carriers
2.1.1.1 Microbubbles
Gene delivery using insonated microbubbles was first reported in 2000 [50, 51] and has been a topic of intense study since that time. The popularity of this technique arises from the availability of commercial ultrasound contrast agents, and the versatility and ease of their use. The microbubbles can be injected upstream of the target region, and then that target region is easily imaged at low intensity by the presence of the bubbles. When the imaging demonstrates that the ultrasound is precisely focused on the target tissue, then the ultrasonic intensity can be increased to create collapse cavitation. The collapse events appear to permeabilize the vessel walls and provide pathways for extravasation of DNA that is freely floating along with the bubbles, or that is associated with the bubble surface [47, 52]. Lawrie et al. showed that transfection was not related to free radical production from collapse cavitation, but attributed transfection to transient holes in the cell membrane produced by other cavitation phenomena [53].
A convenient manner to prepare gene-carrying microbubbles is to insonate a mixture of surfactant, such as albumin, in the presence of gas (air or perfluorocarbon) and the plasmid or DNA fragment to be delivered [54]. If commercial contrast agents are available, the genetic material can be mixed directly with the contrast agent and injected [52, 55, 56]. This is often naked DNA, but one can also mix the genetic material with a cationic stabilizing agent such as poly(ethylene imine) or poly(L-lysine) [57].
2.1.1.2 Liposomes
Liposomes have been used for decades as drug carriers, and recently as gene carriers, particularly cationic liposomes. During the past decade ultrasound applied in combination with gene-carrying liposomes has enhanced the transfection rate both in vitro [58–61] and in vivo [62].
Cationic liposomes are particularly effective in delivering DNA because the DNA stays associated with the liposome until it is acoustically activated. Most cationic liposomes contain dioleoylphosphatidylethanolamine, but some contain quaternary ammonium compounds or cationic derivatives of cholesterol [46, 59, 63].
2.1.1.3 Free genetic material
It is noteworthy that in the absence of microbubbles, liposomes, or other acoustically active agents, ultrasound still enhances transfection, although not as much as with those agents [64]. As an in vitro example, US without any extraneous cavitation agents increased transfection of human endothelial cells and vascular smooth muscle cells slightly (compared to a non-US control), whereas when microbubbles were present, the transfection increased by more than 1000-fold [64]. Such transfection is usually attributed to sonoporation of cell membranes by acoustic activity, but the mechanisms leading to sonoporation are not proven in the absence of microbubbles. It is plausible that in vitro, there is sufficiently dissolved gas and enough organic molecules with a surfactant nature that the insonation itself generates cavitation bubbles that subsequently grow, collapse, and shear cell membranes. On the other hand, postulating an in vivo source of cavitation nuclei is more problematic because the lungs are very efficient at clearing out small bubbles from the circulatory system [6, 23].
Another disadvantage of using free DNA is that ultrasound causes DNA fragmentation [28, 65] that reduces the transfection efficiency [57]. Complexing the DNA plasmids with cationic polymers such as poly(ethylene imine) and poly(L-lysine) preserves the integrity of the DNA upon exposure to 20 kHz ultrasound [57]. Another strategy to protect the DNA is to expose the tissues to US and then subsequently perfuse the genetic material into the targeted region [66].
2.1.2 Examples of US-assisted gene delivery
Ultrasonically enhanced gene delivery to cultured cells in vitro has been abundantly reported. However, this review will focus on published examples of gene delivery to tissues in vivo.
2.1.2.1 Cardiac tissue
A major thrust in US-enhanced gene delivery is the combination of visualizing coronary arteries or other heart structures, and then delivering genes or drugs to the diseased tissues [67]. In most published studies a marker gene has been delivered to show efficacy of the concept. One of the long-term goals of cardiac gene delivery is to deliver genes that will inhibit or reverse stenosis of coronary arteries or to regrow missing or diseased tissue.
Bekeredjian et al. imaged and delivered a luciferase transgene marker to the left ventricle of rat hearts using both commercial and custom gas microbubbles mixed with the genetic material [68, 69]. The sonography (1.3 MHz) was triggered by electrocardiographic signals to expose the microbubbles every 4 ventricular contractions. Genetic expression was significantly greater using triggered rather than continuous insonation [70]. The resulting gene expression was limited to the heart, with slight expression in the liver and pancreas, and no expression in the brain, muscles or lung, thus validating their goal of targeted delivery. The microbubble destruction caused very little change in the regulation of host genes in the heart [71].
Shohet et al. mixed an adenovirus delivery system encoding a β-galactosidase gene to albumin microbubbles and injected them into the jugular vein of rats, with or without insonation at 1.3 MHz [50]. Gene expression was enhanced 10-fold when the genetic material was mixed with the microbubbles and insonated. If the virgin microbubbles were insonated, and then the genetic material subsequently infused, gene expression was enhanced only 2-fold compared to gene infusion without any insonation. This experiment indicates that gene delivery is most efficient when applied concomitantly with insonation; apparently the cell permeability to genetic material decreases with time after insonation.
2.1.2.2 Vascular tissue
There are many reports of US-enhanced gene delivery to arteries, with the goal of developing a delivery system that can be used to treat stenosis and other arterial diseases. Both endovascular US and extracorporeal US have been studied. An endovascular ultrasonic catheter was employed in a rabbit femoral artery model of over dilation [72]. An adenovirus delivery system expressing a blue fluorescent protein gene (BFP) or a non-viral plasmid with the BFP was infused with or without application of 2 MHz endovascular ultrasound. Insonation increased plasmid-mediated gene expression 12-fold and viral-mediated gene expression 19-fold over their non-insonated controls.
Beeri et al. mixed an adenovirus encoding a reporter gene with albumin microbubbles and delivered it to the aortic root of rats [55]. Extracorporeal US was employed to both image and disrupt the microbubbles. Genetic expression in the aortic tissue was increased if the blood flow was transiently stopped during insonation. Apparently some incubation time is helpful in allowing transport of the gene into the cells.
Also employing a rat over-dilation model, Taniyama et al. delivered a naked plasmid encoding a luciferase gene to the carotid artery [64]. Neither plasmid DNA alone, nor plasmid DNA with ultrasound induced significant gene expression. However, when albumin microbubbles were mixed with the plasmid and insonated, the data showed more that 1000-fold increase in luciferase activity compared to plasmid DNA alone.
Huber et al. employed a rabbit carotid artery model and used high intensity focused ultrasound (HIFU) at 0.85 MHz to deliver a plasmid reporter gene [73]. Consistent with the above results, they found that gene expression after exposure to plasmid alone or plasmid plus microbubbles was minimal. Although ultrasonication of the vessel with plasmid increased the gene expression, insonation with plasmid and albumin microbubbles increased gene expression even more.
In an ex vivo model, Teupe et al. exposed excised porcine arteries to albumin microbubbles mixed with plasmid DNA with a reporter gene [54]. Ultrasound alone, or microbubbles alone produced a minimal gene expression, whereas the combination of 2.2 MHz diagnostic ultrasound with microbubbles significantly increased gene expression. The perfusion flow rate through the artery affected the expression, with a maximum expression at 2 mL/min (compared to no flow or higher flows).
In a slight twist of the conventional reports above, Du et al. synthesized echogenic gas-filled solid polymer microsphere containing a reporter plasmid [74]. These were injected into the femoral artery of pigs and subjected to diagnostic Doppler imaging. Gene expression was subsequently found (6 days later) in all experiments. Unfortunately there were no control experiments in which genes were delivered without ultrasound. Apparently solid echogenic microspheres can deliver genes, but at this point the transfection efficiency cannot be compared with microbubble delivery systems.
2.1.2.3 Tumor tissue
There is considerable interest in genetic targeting of tumors as an anticancer therapy, and several studies have examined the feasibility of such a therapy. For example, Manome et al. grew MC38 murine colon carcinoma in mice and then injected directly into the tumor a naked plasmid with a reporter gene [75]. Application of 1 MHz transcutaneous insonation increased the reporter activity 3-fold over the non-insonated control. Higher power densities increased reporter activity, as did increasing insonation time up to 30 seconds.
Using a cationic lipid-cholesterol transfection complex, Anwer et al. delivered an IL-12 gene to a mouse tumor model [66]. Insonation significantly increased the gene expression, and the transfected tissue was limited to the tumor vasculature. The expression of IL-12 was sufficient to inhibit tumor growth compared with the control conditions. Although the authors did not expressly introduce microbubbles, or mention their possible existence, it is possible that the synthesis of the DNA-lipid-cholesterol complex created some liposomes, some of which could contain gas and become acoustically active during insonation. McCreery describes a similar procedure for plasmid gene delivery to human adenocarcinoma grown in nude mice, with similar results [62].
Huber et al. delivered a naked plasmid DNA reporter gene into subcutaneous Dunning prostate tumors in rats [76, 77]. Insonation at 0.85 MHz produced a 10-fold to 15-fold increase in reporter activity compared to non-insonated controls.
In a related procedure, Bao reports the use of a lithotripter to deliver a luciferase reporter to B16 melanoma grown in mice [78]. A lithotripter generates a shock wave containing a spectrum of acoustic frequencies and can produce cavitation phenomena. The plasmid was injected directly into the tumor, and in some cases, 10% air (relative to the tumor volume) was also injected. Various regimens of shock waves were subsequently applied, and the luciferase expression evaluated. The results showed that shock waves with direct injection enhanced expression roughly 15-fold relative to direct injection alone, and that application of air produced a further 7-fold increase in expression. More information on in vitro gene delivery by lithotripter shock waves is reviewed elsewhere [49].
2.1.2.4 Skeletal muscle and bone tissue
Skeletal muscle is one of the largest tissue systems in the body, and as such can express a large quantity of therapeutic protein into the circulatory system to eventually find another target tissue, or to produce a “whole body” therapy. Other applications aim at promoting neovascularization for tissue repair. Thus there are several articles written on US-enhanced gene delivery to skeletal muscles.
In some in vivo experiments in rats, the triceps brachii or gastrocnemius was exposed to diagnostic ultrasound. A mixture of plasmid encoding for luciferase and commercial microbubble contrast agents was injected into the muscle (IM), followed immediately by insonation. The results showed that insonation in the presence of microbubbles and plasmid resulted in higher luciferase activity than injection of plasmid alone, plasmid plus microbubbles (without insonation), or plasmid lipofection. Virtually no luciferase activity was observed in other muscle or other organs.
In similar experiments, Christiansen et al. injected a luciferase reporter coupled to cationic lipid microbubbles by intra-arterial (IA) or intra-venous (IV) routes [79]. Only the rat hind limb skeletal muscle was insonated. Transfection was 200-fold greater with the IA than with the IV route, and was thus similar to intramuscular injection of plasmid. No transfection was observed if the plasmid was administered without microbubbles. These results indicated that insonation of plasmid-microbubbles at a remote site (away from the injections site) produced sufficient extravascular deposition and DNA incorporation leading to genetic expression. Other groups have also used luciferase and microbubbles to transfect rodent skeletal muscle [80, 81].
Schratzberger et al. investigated the effect of duty cycle and power density on naked DNA transfection in rabbit quadricep muscle [82]. A plasmid reporter gene was injected IM and insonation was immediately applied. Consistent with the above findings, the plasmid reporter gene showed very little expression when DNA but not ultrasound was applied. They concluded that gene expression increased as the duty cycle (the fraction of time that US is activated in a pulse sequence) or the power density increases, consistent with the hypothesis that cavitation is involved in the gene delivery. Apparently no microbubbles were used in these experiments.
2.1.2.5 Fetal tissue
Gene transfection on a fetal mice in utero was done using a plasmid encoding a fluorescent marker [56]. In this procedure, an incision was made on a pregnant mouse and the uterus externalized. Then plasmid mixed with microbubbles was delivered to specific locations by micropipette, the ultrasound applied, the uterus replaced, and the fetus developed for another 24 to 48 hours. Gene expression with naked DNA alone, DNA with microbubbles, or DNA with ultrasound was low and showed no significant difference between these conditions. However, application of 1 MHz ultrasound to plasmid and microbubbles produced about a 1000-fold enhancement in gene expression. Micrographs showed some disruption to the fetal skin under conditions of ultrasound with microbubbles.
2.1.2.6 Brain
It would be advantageous to express genes directly in the brain that may ameliorate debilitating brain diseases such as Alzheimer’s disease. As of this writing, we could find no examples of in vivo enhancement of gene delivery using ultrasound. However, there are some publications of in vitro delivery to neural tissue in culture [63, 83], and reports of employing ultrasound to breach the blood-brain barrier [35, 43, 84–87].
2.1.2.7 Lung
Because lung tissue contains gas, it reflects and scatters ultrasound; thus transcutaneous ultrasound cannot be used to deliver therapeutics to the lungs. An alternative use of ultrasound in gene delivery is to create an aerosol using an ultrasonic nebulizer. Cationic-DNA complexes have been delivered to mice, rats and Guinea pigs, and their lung epithelial cells transfected [88, 89].
2.1.2 Needs in Gene Delivery
Since most of the recent progress in gene delivery involves microbubble cavitation, the most pressing need is a better understanding of cavitation physics and US-microbubble interactions. Of course this will aid in not just gene delivery, but in all aspects of drug delivery.
Another need is to develop better protection for the genetic material so that it is not degraded by fluid shear forces or enzymes before it can be delivered to the cells. In addition, the loading of genetic material needs to be optimized such that the genes are delivered only to the target tissues and not to tissues downstream from the insonated site.
2.2 Protein Delivery
We will differentiate proteins from other macromolecules by their size and characteristic polypeptide backbone. In this review, we will consider proteins as having a molecular weight of over 2000 Daltons. Smaller polypeptides can be treated as low molecular weight drugs. Compared to low molecular weight drugs, the proteins have very different transport and solubility characteristics in tissues, and thus their delivery is usually much more complex. Specifically, proteins do not diffuse easily through solids and most gels. A simple bilayer lipid membrane is sufficient to preclude the transport of a protein. Two systems with large surface area, the skin and the GI tract, are problematic. The skin is designed to be impermeable to protein transport, and the GI tract hydrolyses proteins into smaller peptides and amino acids for absorption. The high surface area of the lungs makes the pulmonary system attractive for protein delivery. However, lung tissue blocks ultrasound as previously mentioned. These limitations relegate most protein delivery to injection with some applications employing inhalation.
Regulatory hormones are the center of focus for controlled protein delivery; specifically insulin delivery for diabetes therapy comprises the vast majority of protein delivery research, with some research effort in growth-related hormones and birth control hormones. With respect to US-assisted protein delivery, nearly all research is focused on insulin delivery, and the great majority of that research is on transdermal delivery. Ultrasonic delivery is ideal because a small transducer can be placed on the skin surface for a painless, non-invasive delivery route.
2.2.1 Transdermal protein delivery
There is a tremendous amount of literature on the use of ultrasound to enhance the permeability of skin for transdermal drug delivery, including several excellent reviews to which the reader is referred [90–97]. Therapeutic levels of ultrasound (1–3 MHz, 1–3 W/cm2) have been used for years to drive small hydrophobic molecules, like steroids, into or through the skin [91, 92, 96–101]. Sometimes chemical enhancers were used to further increase the permeability [102–104]. However, no significant transport of protein could be achieved until 10 years ago when Mitrogotri et al. showed that low frequency ultrasound was much more effective than higher frequencies and provided evidence as to the mechanism involved [102–114]. Skin permeability increased with decreasing frequency, and with increasing time of exposure and intensity (beyond a threshold), thus identifying collapse cavitation as a causative mechanism [105–107, 110, 111, 115].
The current theory is that cavitation events open reversible channels in the lipids layers of the stratum corneum and provide less tortuous paths of transport for proteins such as insulin [90, 105–107, 110]. Electron microscopy on skin exposed to low frequency ultrasound revealed the removal of surface cells and the formation of large pores and pockets (~20 μm), large enough to accomodate transport of proteins and other large molecules [116–119]. Tezel and Mitrogotri have formulated a model of the shock wave and microject cavitation events and their impact upon skin permeability [24, 120]. Although their model can be fit to their data, there are many assumptions and parameters in the model, and more direct evidence is needed to conclusively reveal the mechanisms of US-enhanced transdermal protein delivery.
The future of US-enhanced transdermal protein delivery is brimming with potential, but it has not yet appeared in the clinic. Because large pores and channels are opened through the natural skin barriers, many hormones and proteins could be candidates for transdermal delivery [105, 106, 121–127]. The effect of the ultrasound on the protein conformation and/or activity needs to be addressed in more detail.
2.2.2 Other protein delivery
2.2.2.1 Activated protein depot
Kwok et al. studied insulin-loaded drug depots of poly(2-hydroxyethyl methacrylate) and poly(ethylene glycol) with a surface layer of C-18 alkyl chains [128]. In the absence of insonation, the alkyl chains appeared to form an organized and less permeable barrier to proteins; upon insonation, the surface organization was apparently disrupted and protein within the depot matrix escaped. In their research very little insulin was released until 1.1-MHz ultrasound was applied. Upon termination of insonation the low permeability of the lipid-like surface layer was restored. Such a device is envisioned as a subcutaneous depot with an external transducer positioned over the depot that can be activated either automatically or on demand as insulin is required.
2.2.2.2 Thrombolytic enzymes
The transport of tissue plasminogen activator (tPA) and other lytic proteins such as urokinase into clots is beneficial in increasing the rate of fibrinolysis of clots [129, 130]. In most studies, the protein is delivered to the clot via intravenous catheter, and the ultrasound is applied transdermally.
Francis et al. showed that 1 MHz ultrasound increased the rate of uptake and the depth of penetration of tPA into clots [131]. They speculated that enhanced transport could be due to fluid motion from shock waves, microstreaming, or penetration of the clot by micro-jets. Later studies showed that pulsed low frequency ultrasound (27 kHz) is more effective than higher frequencies in enhancing fibrinolysis by tPA, again supporting a non-thermal cavitation-related transport into the clots [132].
The same group also showed that water permeability through a fibrin gel was increased by ultrasound (in the absence of any lytic enzymes) [133]. This increase was reduced when the fibrin gels were degassed, and thus these authors attributed the increase to cavitational activity. Other authors have shown that high intensity focused US produced echo-dense material in the clot, most probably by creating cavitation bubbles [134].
Although US by itself is beneficial in enhancing thrombolysis, the addition of microbubbles appears to enhance thrombolysis even more [135–139]. As early as 1995, it was shown that application of US with albumin-based contrast agent significantly increased fibrinolysis by urokinase of thrombus in vitro [135–137]. In vivo studies using tPA and other enzymes came to the same conclusions [138, 139].
Still another advance in this technology is to attach receptors for the thrombus material to the microbubbles, thus attaching the bubble to the thrombus surface during the ultrasonic exposure. Often these ligands bind to the GPIIb/IIIa receptor of platelets which are expressed when platelets coalesce into a clot, and are used with or without a thrombolytic protein present [137, 140]. We envision that bubbles cavitating on the thrombus surface will produce micro-jets that can mechanically disrupt the clot.
2.2.2.3 Pulmonary delivery by ultrasonic nebulization
There are several reports of protein delivery to the lungs via inhalation of ultrasonically aerosolized protein solutions. Because nebulization requires rather severe cavitation, it has been found beneficial to protect the proteins by the addition of certain surfactant stabilizers [141, 142]. These additives appear to complex with the proteins and protect them from degradative shear stresses and perhaps from free radical attack. It is possible that the surfactants may form micelles or liposomes that sequester the proteins and protect them from cavitation stresses. A short list of ultrasonically nebulized protein delivery includes interferon [143], platelet-activating factor [144], lactate dehydrogenase [141], superoxide dismutase [145], alpha1 protease inhibitor [146, 147], urokinase plasminogen activator [148], and aviscumine [142].
2.2.3 Needs in Protein Delivery
As mentioned at the beginning of this section, protein delivery is currently fairly limited by very slow diffusion of proteins through skin and polymeric depots, and by the proteinolysis that occurs in the upper GI tract. We foresee a need for technology in which a polymeric depot can be implanted, perhaps in subcutaneous, intraperitoneal, or intramuscular locations. Then timed drug release could by activated by transdermal ultrasound. Polymers in a depot can be degraded by ultrasound [27, 149], but this is a fairly slow process, and the same physicochemical mechanisms that degrade the polymers may degrade the proteins in the depot. What is needed is technology that will open and close the depot to protein transport. The technology of Kwok et al is a good start in this direction, but the depot needs to completely shut off protein delivery when not insonated.
It would also be advantageous if the depot were activated by stable cavitation or some other low intensity phenomenon related to ultrasound. Collapse cavitation may be capable of opening depots, but repeated ultrasonic exposure over long periods may start to have adverse effects on the healthy tissue in the region of the depot. Finally it would be beneficial if the depot were eventually degradable so that it did not need to be surgically removed when it was emptied. A refillable depot would also be useful.
2.3 US-Enhanced Small Chemical Delivery
In the last two decades, ultrasound has been investigated as a delivery mechanism for a variety of therapeutic agents to diseased cells throughout the body. Section 2.3.1 will discuss the use of ultrasound in the delivery of conventional chemotherapy agents for vertebrate tissues while section 2.3.2 will focus on the enhanced activity of antibacterials in the presence of ultrasound.
2.3.1 Traditional Chemotherapy
The use of ultrasound as a drug release and potentiation mechanism in traditional chemotherapy has been studied extensively. In this section, we will discuss ultrasonic chemotherapy delivery in free, micellar and liposomal forms.
2.3.1.1 Free Drug
A synergistic effect between the pharmacological activity of chemotherapeutic drugs and ultrasound has been reported for a variety of agents. Loverock et al. have shown that 1 hr of exposure to ultrasound (2.3 W/cm2 at 2.6 MHz at) rendered Doxorubicin (Dox) significantly more toxic to Chinese hamster lung fibroblasts [150]. Exposure to ultrasound alone did not affect cell viability. Flow cytometry revealed an increase in Dox concentration inside the cells, but the authors did not attribute the increased uptake to any particular mechanism.
Tachibana et al. studied the effect of 0.3 W/cm2 and 48 kHz ultrasound on the cytotoxicity of Cytosine Arabinoside (Ara-C) towards human Leukemia (HL60) cells [151]. Ultrasonication for 120 seconds in the presence of Ara-C, reduced the number of observed colonies 100 fold when compared to cells incubated with the same concentration of the drug. This increase in cell death was not caused by hyperthermia, since the temperature increase was less than 0.2° C throughout the experiments. Scanning electron microscopy of insonated cells revealed a decrease in the total number of microvilli and “a slight disrupted cell surface with flap-like wrinkles” under the action of ultrasound. They concluded that low intensity ultrasound altered the cell membrane, which resulted in the increase of Ara-C cell uptake.
In another study, Tachibana et al. have shown that the exposure of HL60 cells to 255 kHz of ultrasound and MC 540 for 30 seconds formed pores in the cell membrane [152]. They claimed that sonoporation caused the cytoplasm of HL60 cells to extrude through pores formed in their cell membrane. The same effect was not observed when cells were exposed to ultrasound alone, thus implicating a synergism between the drug and US.
Saito et al. reported similar results: exposure to ultrasound increased the permeability of corneal endothelium cells [153]. The increase in permeability appeared to be reversible and the cells regained their membrane integrity after several minutes.
Rapoport et al. investigated the increase in intracellular drug uptake by HL60 cells as a result of ultrasound irradiation (67 kHz and 2.5 W/cm2) using fluorescence techniques [154]. They found that the amount of Dox that intercalated the DNA increased after one hour of insonation. In another related study, Munshi et al. reported that the IC50 for Dox was reduced substantially when HL60 cells were sonicated for one hour at 80 kHz in the presence of Dox [155]. In both studies, the temperature of the cell suspensions was kept at 37 °C using a thermostatic bath and therefore hyperthermia was not part of the mechanism of this ultrasonic enhanced killing.
Yu et al. [156, 157] investigated drug-ultrasound synergism applied to Dox-resistant and cisplatin-resistant human ovarian carcinoma cell. They concluded that the synergism reported was not due to the decrease of the multidrug resistance (mdr1) gene level by insonation. Thus multidrug resistance gene expression is not inhibited by ultrasound. The group did not report whether any changes in drug accumulation occurred.
Some investigators attribute this synergism between drug activity and ultrasound to an increase in the local temperature of the sonicated area (hyperthermia) [158–160]. Saad and Hahn exposed Chinese hamster cells to US (average intensities range between 0.5 and 2 W/cm2) and to several drugs at temperatures ranging between 37° C and 43° C [159]. The study showed that at lower intensities (0.5 W/cm2) the cytotoxicity of Dox was significantly enhanced when the temperature was raised from 37° C to 41° C. At higher ultrasonic power densities (1 W/cm2), the cytotoxic effect of Dox increased three fold when the temperature reached 41° C. The study concluded that the “temperature threshold” decreases as the power intensity of ultrasound increases.
To summarize, ultrasound has been used in combination with chemotherapeutic agents for increased efficacy. Insonation appears to enhance the transport of drugs into cells and tissues. Considering the physics of cavitation processes, we opine that ultrasound produces transient micropores in the cell membrane, which would increase the passive accumulation of the drugs in the cells and tissues. Although the cytotoxic effect of chemotherapeutic agents has been shown to increase with insonation, this effect has not been shown to be independent of any increase in drug uptake.
Most studies attribute ultrasonic enhanced killing of cancer cells in the presence of drugs to a phenomena called chemopotentiation. The use of the term chemopotentiation is rather misleading, since it suggests that ultrasound alters the structure of the drug and renders the chemical more potent. Most data reported in literature support the hypothesis that ultrasound permeabilizes the membrane so that more chemotherapeutic drug molecules are able to diffuse into the cells. In some cases, the permeabilization appears truly synergistic in that both drug and ultrasound are required simultaneously to render the cell membrane more permeable. In general, though, at sufficiently high intensities the ultrasound permeabilizes the membrane, most probably through shear stresses in the membrane from microstreaming or shock waves.
A major problem associated with whole body chemotherapy is not totally alleviated by US; the drug is still delivered systemically thus causing systemic side effects. For this reason, research in recent years has focused on developing molecular vehicles that can sequester the drug inside a package and then release it using ultrasound stimulus at the tumor site. Two types of drug delivery molecules have been developed for this purpose: polymeric micelles and liposomes.
2.3.1.2 Micelles
Polymeric micelles have been used to improve site-specific drug delivery in cancer therapy. The technique relies on these carriers’ small size to extravasate at the tumor site where the drug can diffuse into the tumor and carry out its therapeutic effect [161–164]. Although several groups have investigated the use of polymeric carriers to deliver chemotherapeutic as well as other drugs, the only group that has reported the use of polymeric micelles in conjunction with ultrasound is Rapoport, Pitt and colleagues at the University of Utah and Brigham Young University. Their micelles are made from the Pluronic™ family of block copolymers.
Pluronic™ polymers are triblock copolymers of poly(ethylene oxide) (PEO) –poly(propylene oxide) (PPO) – poly(ethylene oxide) (PEO). At sufficiently high aqueous concentrations they form micelles [165–170]. These micelles have several advantages as drug deliver vehicles. They are stable in blood and other biological fluids. These micelles are large enough to escape renal excretion while being small enough to extravasate at the tumor site. Antineoplastic agents can be easily sequestered inside the core of these polymeric micelles by the simple act of mixing [154, 171], this avoiding the complexities involved with covalently boding the drug to the polymeric carrier [172]. Several studies have reported the effect of Pluronic surfactants in overcoming multidrug resistance (MDR) [173–175]. Furthermore the PEO chains on the micelle exterior prevent its recognition by cells of the reticulo endothelial system.
The feasibility of using ultrasound with Pluronic micelles to deliver anti-cancer agents in vitro was first reported by Munshi et al. [155] and Husseini et al. [39]. They reported that a combination of 70 kHz ultrasound and Pluronic P105-encapsulated Dox substantially increased the cytotoxicity of the drug. The enhanced toxicity upon insonation was attributed to the release of the agent from micelles under the action of ultrasound [176, 177].
Using fluorescent microscopy and flow cytometry, thy also reported that insonation enhanced the intracellular uptake of Pluronic micelles and its internalization into the nucleus of HL60 cells [178–183].
The main challenge facing the use of micelles to deliver chemotherapy drugs is that the concentration of the polymer must remain above the critical micellar concentration (CMC) to guarantee that the micellar structures remain intact and do not dissolve and prematurely release the drug before reaching the target site. Pruitt et al. have stabilized Pluronic micelles with an interpenetrating network using N, N-diethylacrylamide to form Plurogels™ [184]. The network expands at room temperature, allowing the drug to accumulate inside its hydrophobic core, and contracts above 31°C, thus trapping the drug at body temperature. The network-micellar structure is eventually degraded after a few days (the half life is approximately 17 hours [185]). Plurogel™ micelles have also been shown to release Dox after exposure to ultrasound [186].
Recently, in vivo studies examined the feasibility of acoustically activated drug delivery from Plurogel™. Nelson et al. [187] showed that exposure to 70-kHz ultrasound of Dox encapsulated in Plurogel™ significantly decreased the size of colorectal cancer tumors in rats. When unencapsulated Dox was administered, the same dose was lethal to the rats within two weeks of injection. Gao et al. [188] studied the intracellular distribution of fluorescently labeled non-stabilized Pluronic P105 and Pluronic P105 stabilized using PEG-diacylphospholipid. The study showed that insonation at 1 MHz was able to enhance the accumulation of these labeled micelles at the tumor site in ovarian cancer-bearing nu-nu mice. Using the same in vivo mouse model, Rapoport et al. [189] showed that micelle accumulation was significantly higher in the ultrasonicated tumor than in the non-insonated tumor in the same mouse.
The mechanisms of this acoustically activated micellar drug delivery system are still being investigated, and there is a strong correlation with insonation frequency and power density that suggests a strong role of stable and transient cavitation.
2.3.1.3 Liposomes
Unlike micelles, liposomes can sequester both hydrophilic and hydrophobic drugs in their aqueous interior and lipid bilayer membrane respectively. Liposomes are also larger than the previously discussed polymeric micelles; the average diameters of liposomes range between 150 and 200 nm compared to 5–30 nm for micelles. Herman et al. [190] showed that liposomes are able to encapsulate Dox and reported a decrease in the cardiotoxicity of the drug. Zvi et al. [191] reported that Liposome-encapsulated Dox accumulates preferentially in cancerous muscle tissues when compared to tumor free muscles. The studies mentioned above rely on the increased accumulation of liposomes at the tumor site due to increased extravasation (passive targeting). Recently, there have been reports in literature where ultrasound is used as an active targeting mechanism to release drugs from liposomes. [32, 42, 101, 192]
Ning et al. demonstrated that ultrasound-induced hyperthermia, in addition to enhancing drug anti-tumor activity, accelerated the release of Dox from long-circulating liposomes [37]. The group reported that by increasing the temperature from 37° C to 41° C, the rate of release of Dox was increased six-fold after one hour of sonication at 2 W/cm2. The accumulation of Dox in RIF-1 tumor cells was 10 times higher when introduced in liposomes at 42° C compared to when introduced as free drug at 37° C. Several others reports have shown that other drugs can be released from liposomes using ultrasonic hyperthermia [2, 193–195].
2.3.2 Antibacterial Chemotherapy
Rediske et al. showed that ultrasound increased the killing of bacteria both in planktonic suspension [196, 197] and biofilm forms [198–200] in the presence of antibiotics. This synergistic killing effect was most pronounced at lower frequencies and decreased as the frequency of insonation increased [201, 202]. The effect was more pronounced in E. coli and P. aeruginosa (gram negative bacteria) than in S. epidermidis (gram positive bacteria). They found that this enhanced killing synergistic effect is prevalent with certain antibiotics (the aminoglycosides), but does not exist when others are used. They hypothesized that stable cavitation or sonoporation might be involved in increasing the transport of antibiotics into the bacteria either by reducing the mass transfer boundary layer around the cells, or by altering the cell membrane, thus allowing the antibiotics to diffuse through newly formed membrane pores.
In vivo experiments in a rabbit model of an implant infection confirmed the increased toxicity of gentamicin against E. coli biofilms in the presence of low frequency ultrasound (28.48 kHz and 0.3 W/cm2) [203]. Pulsed US applied for 48 hours with gentamicin was effective in eliminating E. coli infections [204], but was less effective against P. aeruginosa [205]. Vancomycin combined with US was somewhat effective against S. epidermidis infections in the same rabbit model [206].
2.3.3 Needs in Ultrasonic Enhanced Small Chemical Delivery
The role of ultrasound in small chemical delivery involves permeabilization of the cell membrane such that these molecules can enter more easily. Currently there is very little known about the details of how US permeabilizes the cell membrane. Are small transient holes formed for microsecond timescales, allowing diffusive entry? Or are larger more permanent holes or disorganized regions formed? Is a different treatment needed for molecules of different sizes?
Neither is much known about how US may regulate gene expression and protein production. Does US cause a stress response (similar to heat shock) that may enhance or interfere with the action of drugs? Is this response similar for all cells and does it differ for various ultrasonic frequencies and intensities? These and other questions about cell physiology need to be addressed.
Finally, liposomes used in drug delivery are thermodynamically stable, but are cleared by the reticulo-endothelial system (RES). Alternatively, micelles may not be cleared by the RES, but they are thermodynamically unstable when diluted in blood. Neither system is ideal, and thus there is a need to make the liposomes more stealthy and the micelles more stable.
3. Opinions in Ultrasonic Drug Delivery
3.1 The Future of Ultrasonic-Activated Drug Delivery
Obviously the field of ultrasonic-enhanced drug delivery has expanded tremendously during the past decade, and we expect that this trend will continue as our understanding and technology increases. Furthermore, the use of microbubbles to assist drug delivery has exploded in the past 5 years, and we expect that much of the technological growth in the next decade will be in the clever use of microbubbles and ultrasonic pulse sequences.
The published literature regarding US and microbubble in gene delivery is solid, makes important contributions, and is definitely worth studying. This review has cited many innovations, and more are sure to come. We posit that US-enhanced gene delivery will experience the greatest growth and provide the greatest medical contribution because ultrasound and microbubbles allow genes to be delivered through non-viral technologies to specific locations. Such genetic therapy can make monumental contributions to the treatment of heart disease, vascular disease, cancer, autoimmune diseases, and much more. As microbubble collapse is controlled and fine-tuned, we speculate that it can be applied to deliver genetic therapy to the brain to treat neurological diseases.
The area of protein delivery has been limited in the past to insulin delivery technology, and will probably remain so, with some small expansion into delivery of a few other small regulatory hormones. Despite 10 years of solid research on transdermal delivery of proteins, there are still limitations in the rate at which a protein can pass through the skin without inflicting permanent damage. Transport can be increased by increasing the surface area, but a larger treatment area and heavy transducers may lead to difficulty with patient compliance. We foresee some growth in this area, but not wide clinical application until the size of the transducer is reduced such that it can be easily attached and carried at work or at home.
Technological developments for small chemical delivery will follow those of gene delivery. For cancer chemotherapy or antibiotic therapy, delivery to specific tissues will be targeted by decorating the carriers or microbubbles with antibodies or other site-specific adhesive molecules.
3.2 What are the Critical Needs?
The basis of future technological advancement requires a better understanding of the behaviour of microbubbles in ultrasonic fields. This will require more modeling and experimental understanding of the physics of microbubble oscillation and collapse as a function of microbubble size, internal gas composition, membrane or wall thickness and mechanical properties (modulus, shear strength, viscosity, etc.), acoustic frequency, and pressure amplitude. Currently most US/microbubble drug delivery is done with imaging transducers at high frequencies that are not necessarily optimized for cavitation or drug delivery. What are the optimal bubble sizes and ultrasonic frequencies for drug delivery? The goal of such research should be to develop the acoustic parameters and perhaps the pulse sequences that can excite a bubble to cavitate without imposing mechanical or thermal damage to tissues. Some modeling effort has commenced [17, 207], but much more is needed.
If bubble physics dictate that the optimal frequencies are different than those frequencies available in imaging transducers, then a new generation of transducers must be developed for use in US/microbubble drug delivery. Even better would be a multi-frequency transducer that can perform three functions, perhaps all at different frequencies: target imaging, bubble destruction, and cell membrane permeabilization.
As mentioned in conjunction with transdermal drug delivery, small-sized low-frequency transducers need to be developed so that patients can wear them for continuous insulin delivery. Although some intra-luminal transducers are available in a catheter format, these are currently designed for imaging; catheter-based transducers also need to be developed for drug delivery.
Finally, we must not forget the issue of biological response to these new applications of US in drug delivery. Is the frequency for optimum drug release from a carrier also the optimum frequency for permeabilizing a cell membrane without destroying the cell? It is very probable that frequencies that optimize membrane permeability are different that frequencies that optimize drug release. Thus the response of cells and their membranes to US must be studied so that in our efforts to optimize drug release we do not produce collateral damage to the target or adjacent tissues.
3.3 What are the Best Technologies?
In our opinion the best current technologies are those that employ gas bubbles that have the therapeutic agent in or on the bubble, such as DNA decorating the exterior of a surfactant-stabilized microbubble, or a thick-shelled microbubble [12, 42] that can carry the drug inside, either in an oil or aqueous phase. In these systems the drug and cavitation agent are intimately mixed and drug is therefore released at the location where tissues are stressed by cavitation. Micelles and liposomes without gas are less useful because the cavitation is not necessarily produced at the same time or place as is the drug. Application of free drug or free DNA is least efficient because it is not sequestered, and thus can interact with non-targeted tissue or be cleared (or degraded) before it can reach therapeutic concentrations. Gas liposomes or microbubbles constructed from native proteins (such as albumin) or having poly(ethylene oxide) chains on the surface may have an advantage in that they will not be recognized and cleared as fast from the circulatory system [208, 209].
Another current and innovative technology in which we anticipate more growth is the attachment of targeting molecules to microbubbles or drug carriers. Because the targeting molecules may be different for each application (such as unique of individualized antibodies), it may be advantageous to attach a generic binder to the microbubbles. Then the specific antibodies could be attached to a complementary binder. The target-specific antibodies could then be mixed with the generic microbubbles to create custom drug delivery systems with the drug attached to the bubbles. Although biotin and streptavidin have been suggested for such a system [210], these proteins may elicit an antigenic response. Thus other systems should be investigated such as a self-assembled biological system or a chemical system such as maleimide-thiol bond formation.
Without a crystal ball, one cannot predict what specific technology will be in place 10 years from now. However, we can predict that ultrasonic-activated drug delivery will play an ever-increasing role in targeted drug therapies.
Acknowledgments
This research was supported by grants from the National Institutes of Health (R01 CA 98138) and from Philips Oral Healthcare.
References
- 1.DRAPER DO, CASTEL JC, CASTEL D. Rate of Temperature Increase in Human Muscle During 1 MHz and 3 MHz Continuous Ultrasound. J Orthop Sports Phys Ther. 1995;22(4):142–150. doi: 10.2519/jospt.1995.22.4.142. [DOI] [PubMed] [Google Scholar]
- 2.TACKER JR, ANDERSON RU. Delivery of Antitumor Drug to Bladder Cancer by Use of Phase Transition Liposomes and Hyperthermia. Journal of Urology. 1982;127:1211–121214. doi: 10.1016/s0022-5347(17)54299-8. [DOI] [PubMed] [Google Scholar]
- 3.KENNEDY JE, TER HAARGR, CRANSTON D. High intensity focused ultrasound: surgery of the future? Br J Radiol. 2003;76(909):590–599. doi: 10.1259/bjr/17150274. [DOI] [PubMed] [Google Scholar]
- 4.MADERSBACHER S, MARBERGER M. High-energy shockwaves and extracorporeal high-intensity focused ultrasound. J Endourol. 2003;17(8):667–672. doi: 10.1089/089277903322518680. [DOI] [PubMed] [Google Scholar]
- 5.HUBER PE, JENNE JW, RASTERT R, et al. A New Noninvasive Approach in Breast Cancer Therapy Using Magnetic Resonance Imaging-guided Focused Ultrasound Surgery. Cancer Res. 2001;61:8441–8447. * Seminal paper regarding high intensity ultrasound for tumor destruction. [PubMed] [Google Scholar]
- 6.BARNETT SB, TER HAARGR, ZISKIN MC, NYBORG WL, MAEDA K, BANG J. Current Status of Research on Biophysical Effects of Ultrasound. Ultrasound Med Biol. 1994;20(3):205–218. doi: 10.1016/0301-5629(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 7.MARMOTTANT P, HILGENFELDT S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature. 2003;423(6936):153–156. doi: 10.1038/nature01613. ** Excellent paper showing the shearing of vesicles by cavitating bubbles. The downloadable supplement is superb. [DOI] [PubMed] [Google Scholar]
- 8.ELDER SA. Cavitation Microstreaming. J Acoust Soc Amer. 1958;31(1):54–64. [Google Scholar]
- 9.NYBORG WL. Ultrasonic Microstreaming and Related Phenomena. Br J Cancer. 1982;45(Suppl V):156–160. * Excellent paper on the forces and stresses around a cavitating bubble. [PMC free article] [PubMed] [Google Scholar]
- 10.ROONEY JA. Hemolysis Near an Ultrasonically Pulsating Gas Bubble. Science. 1970;169:869–871. doi: 10.1126/science.169.3948.869. [DOI] [PubMed] [Google Scholar]
- 11.BRENNEN CE: Cavitation and Bubble Dynamics Oxford University Press; New York: (1995), An excellent book on cavitation phenomena.
- 12.MAY DJ, ALLEN JS, FERRARA KW. Dynamics and fragmentation of thick-shelled microbubbles. . Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2002;49(10):1400–1410. doi: 10.1109/tuffc.2002.1041081. ** This article presents excellent images of cavitating microbubbles. [DOI] [PubMed] [Google Scholar]
- 13.JENNE J. Kavitation in biologischem Gewebe. Ultraschall in Med. 2001;22:200–207. doi: 10.1055/s-2001-17913. [DOI] [PubMed] [Google Scholar]
- 14.URICK RJ: Principles of Underwater Sound 3 Ed McGraw-Hill Book Company; San Francisco: (1983)
- 15.HILL CR. Ultrasonic Exposure Thresholds for Changes in Cells and Tissues. J Acoust Soc Am. 1971;52(2):667–672. [Google Scholar]
- 16.APFEL RE, HOLLAND CK. Gauging the Liklihood of Cavitation from Short-pulse, Low-duty Cycle Diagnostic Ultrasound. Ultrasound Med Biol. 1991;17(2):179–185. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- 17.LEIGHTON TG. Transient excitation of insonated bubbles. Ultrasonics. 1989;27:50–53. [Google Scholar]
- 18.ALLEN JS, KRUSE DE, DAYTON PA, FERRARA KW. Effect of coupled oscillations on microbubble behavior. . Journal of the Acoustical Society of America. 2003;114(3):1678–1690. doi: 10.1121/1.1600721. * Excellent analysis of the behaviour of microbubbles subjected to ultrasound. [DOI] [PubMed] [Google Scholar]
- 19.SOETANTO K, CHAN M. Study on the lifetime and attenuation properties of microbubbles coated with carboxylic acid salts. Ultrasonics. 2000;38(10):969–977. doi: 10.1016/s0041-624x(00)00027-5. [DOI] [PubMed] [Google Scholar]
- 20.SBOROS V, MORAN CM, PYE SD, MCDICKEN WN. The behaviour of individual contrast agent microbubbles. Ultrasound in Medicine and Biology. 2003;29(5):687–694. doi: 10.1016/s0301-5629(03)00027-9. [DOI] [PubMed] [Google Scholar]
- 21.KVIKLIENE A, JURKONIS R, RESSNER M, et al. Modelling of nonlinear effects and the response of ultrasound contrast micro bubbles: simulation and experiment. Ultrasonics. 2004;42(1–9):301–307. doi: 10.1016/j.ultras.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 22.STARRITT HC, DUCK FA, HUMPHREY VF. An experimental investigation of streaming in pulsed diagnostic ultrasound beams. Ultrasound Med Biol. 1989;15(4):363–373. doi: 10.1016/0301-5629(89)90048-3. [DOI] [PubMed] [Google Scholar]
- 23.NYBORG WL. Biological Effects of Ultrasound: Development of Safety Guidelines. Part II: General Review. Ultrasound Med Biol. 2001;27(3):301–333. doi: 10.1016/s0301-5629(00)00333-1. ** This is an excellent review of the bioeffects of ultrasound, and their mechanisms. [DOI] [PubMed] [Google Scholar]
- 24.SUNDARAM J, MELLEIN BR, MITRAGOTRI S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys J. 2003;84(5):3087–101. doi: 10.1016/S0006-3495(03)70034-4. * Excellent analysis of damage to cells by collapse cavitation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GUZMAN HR, MCNAMARA AJ, NGUYEN DX, PRAUSNITZ MR. Bioeffects caused by changes in acoustic cavitation bubble density and cell concentration: A unified explanation based on cell-to-bubble ratio and blast radius. Ultrasound in Medicine and Biology. 2003;29(8):1211–1222. doi: 10.1016/s0301-5629(03)00899-8. [DOI] [PubMed] [Google Scholar]
- 26.KUIJPERS MWA, VAN ECKD, KEMMERE MF, KEURENTJES JTF. Cavitation-induced reactions in high-pressure carbon dioxide. Science. 2002;298(5600):1969–1971. doi: 10.1126/science.1078022. [DOI] [PubMed] [Google Scholar]
- 27.KOST J, LEONG K, LANGER R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc Natl Acad Sci USA. 1989;86:7663–7666. doi: 10.1073/pnas.86.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PRITCHARD NJ, HUGHES DE, PEACOCKE AR. The Ultrasonic Degradation of Biological Macromolecules under Conditions of Stable Cavitation. I. Theory, Methods, and Application to Deoxyribonucleic Acid. Biopolymers. 1966;4(3):259–273. doi: 10.1002/bip.1968.360060414. * Classic paper on the degradation of DNA by ultrasonic phenomena. [DOI] [PubMed] [Google Scholar]
- 29.HOWARD WAJ, BAYOMI A, NATARAJAN E, et al. Sonolysis promotes indirect Co-C bond cleavage of alkylcob(III)alamin bioconjugates. Bioconjug Chem. 1997;8(4):498–502. doi: 10.1021/bc970077l. [DOI] [PubMed] [Google Scholar]
- 30.BARNETT SB, ROTT H-D, HAAR GRT, ZISKIN MC, MAEDA K. The Sensitivity of Biological Tissue to Ultrasound. Ultrasound Med Biol. 1997;23(6):805–812. doi: 10.1016/s0301-5629(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 31.ZISKIN MC, BARNETT SB. Ultrasound and the Developing Central Nervous System. Ultrasound in Med & Biol. 2001;27(7):875–876. doi: 10.1016/s0301-5629(01)00368-4. [DOI] [PubMed] [Google Scholar]
- 32.KRUSKAL J, GOLDBERG S, KANE R: Novel in Vivo Use of Conventional Ultrasound to Guide and Enhance Molecular Delivery and Uptake into Solid Tumors. Annual Meeting of the Radiological Society of North America. Chicago, IL RSNA; (2001) 804. * See also Science News, vol 160, p. 391, Dec 22&29, 2001
- 33.SONG J, CHAPPELL JC, QI M, VANGIESON EJ, KAUL S, PRICE RJ. Influence of injection site, microvascular pressure and ultrasound variables on microbubble-mediated delivery of microspheres to muscle. Journal of the American College of Cardiology. 2002;39(4):726–731. doi: 10.1016/s0735-1097(01)01793-4. [DOI] [PubMed] [Google Scholar]
- 34.SKYBA DM, PRICE RJ, LINKA AZ, SKALAK TC, KAUL S. Direct In Vivo Visualization of Intravascular Destruction of Microbubbles by Ultrasound and its Local Effects on Tissue. Circulation. 1998;98:290–293. doi: 10.1161/01.cir.98.4.290. [DOI] [PubMed] [Google Scholar]
- 35.SCHLACHETZKI F, HOLSCHER T, KOCH HJ, et al. Observation on the integrity of the blood-brain barrier after microbubble destruction by diagnostic transcranial color-coded sonography. J Ultrasound Med. 2002;21(4):419–429. doi: 10.7863/jum.2002.21.4.419. [DOI] [PubMed] [Google Scholar]
- 36.PITT WG, ROSS SA. Ultrasound increases the rate of bacterial cell growth. Biotechnol Prog. 2003;19(3):1038–44. doi: 10.1021/bp0340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NING S, MACLEOD K, ABRA R, HUANG AH, HAHN GM. Hyperthermia Induces Doxorubicin Release From Long-Circulating Liposomes and Enhances Their Anti-tumor Efficacy. Int J Radiation Oncology Phys. 1994;29(4):827–834. doi: 10.1016/0360-3016(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 38.GABIZON A, CATANE R, UZIELY B, et al. Prolonged Circulation Time and Enhanced Accumulation in Malignant Exudates of Doxorubicin Encapsulated in Polyethylene-glycol Coated Liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- 39.HUSSEINI GA, EL-FAYOUMI RI, O'NEILL KL, RAPOPORT NY, PITT WG. DNA damage induced by micellar-delivered doxorubicin and ultrasound: comet assay study. Cancer Lett. 2000;154:211–216. doi: 10.1016/s0304-3835(00)00399-2. * Cells are protected from sequestered drug, but die rapidly upon application of ultrasound. [DOI] [PubMed] [Google Scholar]
- 40.COUSSIOS CC, HOLLAND CK, JAKUBOWSKA L, et al. In vitro characterization of liposomes and Optison (R) by acoustic scattering at 3.5 MHz. Ultrasound in Medicine and Biology. 2004;30(2):181–190. doi: 10.1016/j.ultrasmedbio.2003.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.HUANG S, HAMILTON AJ, TIUKINHOY SD, et al. Liposomes as Ultrasond Imaging Contrast Agents and as Ultrasound-Sensitive Drug Delivery Agents. Cell Mol Biol Lett. 2002;7(2):233–235. [PubMed] [Google Scholar]
- 42.UNGER EC, MCCREERY TP, SWEITZER RH, CALDWELL VE, WU Y. Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest Radiol. 1998;33(12):886–892. doi: 10.1097/00004424-199812000-00007. * The use of a gas-containing liposome directed to ultrasonic drug delivery. [DOI] [PubMed] [Google Scholar]
- 43.UNGER E, PORTER T, CULP W, LABELL R, MATSUNAGA TO, ZUTSHI R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56(9):1291–1314. doi: 10.1016/j.addr.2003.12.006. ** Excellent review of the use of ultrasound and microbubbles in drug delivery. [DOI] [PubMed] [Google Scholar]
- 44.STRIDE E, SAFFARI N. The potential for thermal damage posed by microbubble ultrasound contrast agents. Ultrasonics. 2004;42(1–9):907–13. doi: 10.1016/j.ultras.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 45.SCHUTT EG, KLEIN DH, MATTREY RM, RIESS JG. Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: The key role of perfluorochemicals. Angewandte Chemie-International Edition. 2003;42(28):3218–3235. doi: 10.1002/anie.200200550. [DOI] [PubMed] [Google Scholar]
- 46.NIIDOME T, HUANG L. Gene therapy progress and prospects: Nonviral vectors. Gene Therapy. 2002;9(24):1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 47.PRICE RJ, KAUL S. Contrast Ultrasound Targeted Drug and Gene Delivery: An Update on a New Therapeutic Modality. J Cardiovasc Pharmascol Therapeut. 2002;7(3):171–180. doi: 10.1177/107424840200700307. * Good review of various modes and mechanisms of gene delivery using microbubbles. [DOI] [PubMed] [Google Scholar]
- 48.LINDNER LH, EICHHORN ME, EIBL H, et al. Novel temperature-sensitive liposomes with prolonged circulation time. Clinical Cancer Research. 2004;10(6):2168–2178. doi: 10.1158/1078-0432.ccr-03-0035. [DOI] [PubMed] [Google Scholar]
- 49.MILLER DL, PISLARU SV, GREENLEAF JE. Sonoporation: Mechanical DNA Delivery by Ultrasonic Cavitation. Somatic Cell Molec Genetics. 2002;27(1):115–134. doi: 10.1023/a:1022983907223. ** Excellent review of DNA delivery using ultrasound. [DOI] [PubMed] [Google Scholar]
- 50.SHOHET RV, CHEN S, ZHOU Y-T, et al. Echocardiographic Destruction of Albumin Microbubbles Directs Gene Delivery to the Myocardium. Circulation. 2000;101:2554–2556. doi: 10.1161/01.cir.101.22.2554. ** Seminal paper showing that US and microbubbles can deliver a transgene to myocardium in vivo. [DOI] [PubMed] [Google Scholar]
- 51.LAWRIE A, BRISKEN AF, FRANCIS SE, CUMBERLAND DC, CROSSMAN DC, NEWMAN CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7(23):2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 52.MIURA S, TACHIBANA K, OKAMOTO T, SAKU K. In vitro transfer of antisense oligodeoxynucleotides into coronary endothelial cells by ultrasound. Biochem Biophys Res Commun. 2002;298(4):587–590. doi: 10.1016/s0006-291x(02)02467-1. [DOI] [PubMed] [Google Scholar]
- 53.LAWRIE A, BRISKEN AF, FRANCIS SE, et al. Ultrasound-enhanced transgene expression in vascular cells is not dependent upon cavitation-induced free radicals. Ultrasound Med Biol. 2003;29(10):1453–1461. doi: 10.1016/s0301-5629(03)01032-9. [DOI] [PubMed] [Google Scholar]
- 54.TEUPE C, RICHTER S, FISSLTHALER B, et al. Vascular Gene Transfer of Phosphomimetic Endothelial nitric Oxide Synthase (S1177D) Using Ultrasound-Enhanced Destruction of Plasmid-Loaded Microbubbles Improves Vasoreactivity. Circulation. 2002:1104–1109. doi: 10.1161/hc0902.104720. [DOI] [PubMed] [Google Scholar]
- 55.BEERI R, GUERRERO JL, SUPPLE G, SULLIVAN S, LEVINE RA, HAJJAR RJ. New efficient catheter-based system for myocardial gene delivery. Circulation. 2002;106(14):1756–1759. doi: 10.1161/01.cir.0000035240.92015.e4. [DOI] [PubMed] [Google Scholar]
- 56.ENDOH M, KOIBUCHI N, SATO M, et al. Fetal gene transfer by intrauterine injection with microbubble-enhanced ultrasound. Molecular Therapy. 2002;5(5):501–508. doi: 10.1006/mthe.2002.0577. [DOI] [PubMed] [Google Scholar]
- 57.KUO JHS, JAN MS, SUNG KC. Evaluation of the stability of polymer-based plasmid DNA delivery systems after ultrasound exposure. International Journal of Pharmaceutics. 2003;257(1–2):75–84. doi: 10.1016/s0378-5173(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 58.UNGER EC, FRITZ TA, MATSUNAGA T, RAMASWAMI VR, YELLOWHAIR D, WU G: Therapeutic drug delivery systems. In: U. S. Patent Database ImaRx Pharmaceutical Corp; United States of America: (1996):48.
- 59.UNGER EC, MCCREERY TP, SWEITZER RH. Ultrasound Enhances Gene Expression of Liposomal Transfection. Investigative Radiology. 1997;32(12):723–727. doi: 10.1097/00004424-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 60.LAWRIE A, BRISKEN AF, FRANCIS SE, et al. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation. 1999;99(20):2617–2620. doi: 10.1161/01.cir.99.20.2617. [DOI] [PubMed] [Google Scholar]
- 61.MCCREERY TP, SWEITZER RH, UNGER EC: DNA Delivery to Cells in Culture Using Ultrasound. In: Methods in Molecular Biology Vol. 245. HEISER WC, ed. Humana Press; Totowa, New Jersey: (2004):287–291. [DOI] [PubMed]
- 62.MCCREERY TP, SWEITZER RH, UNGER EC: DNA Delivery to Cells In Vivo by Ultrasound. In: Methods in Molecular Biology Vol. 245. HEISER WC, ed. Humana Press; Totowa, New Jersey: (2004):293–298. [DOI] [PubMed]
- 63.KOCH S, POHL P, COBET U, RAINOV NG. Ultrasound enhancement of liposome-mediated cell transfection is caused by cavitation effects. Ultrasound Med Biol. 2000;26(5):897–903. doi: 10.1016/s0301-5629(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 64.TANIYAMA Y, TACHIBANA K, HIRAOKA K, et al. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105(10):1233–1239. doi: 10.1161/hc1002.105228. * Excellent paper comparing gene delivery with and without microbubbles, in vitro and in vivo. [DOI] [PubMed] [Google Scholar]
- 65.RIESZ P, KONDO T. Free-Radical Formation Induced by Ultrasound and Its Biological Implications. Free Radical Biology and Medicine. 1992;13(3):247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 66.ANWER K, KAO G, PROCTOR B, et al. Ultrasound enhancement of cationic lipid-mediated gene transfer to primary tumors following systemic administration. Gene Therapy. 2000;7(21):1833–1839. doi: 10.1038/sj.gt.3301302. [DOI] [PubMed] [Google Scholar]
- 67.LINDNER JR. Microbubbles in medical imaging: current applications and future directions. Nature Reviews Drug Discovery. 2004;3(6):527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 68.BEKEREDJIAN R, CHEN SY, GRAYBURN PA, SHOHET RV. Delivery of luciferase enzyme to the heart using ultrasound targeted microbubble destruction. Journal of the American College of Cardiology. 2004;43(5):374a–374a. [Google Scholar]
- 69.BEKEREDJIAN R, CHEN SY, FRENKEL PA, GRAYBURN PA, SHOHET RV. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108(8):1022–1026. doi: 10.1161/01.CIR.0000084535.35435.AE. [DOI] [PubMed] [Google Scholar]
- 70.CHEN SY, SHOHET RV, BEKEREDJIAN R, FRENKEL P, GRAYBURN PA. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. Journal of the American College of Cardiology. 2003;42(2):301–308. doi: 10.1016/s0735-1097(03)00627-2. [DOI] [PubMed] [Google Scholar]
- 71.BEKEREDJIAN R, CHEN SY, PAN WT, GRAYBURN PA, SHOHET RV. Effects of ultrasound-targeted microbubble destruction on cardiac gene expression. Ultrasound in Medicine and Biology. 2004;30(4):539–543. doi: 10.1016/j.ultrasmedbio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 72.AMABILE PG, WAUGH JM, LEWIS TN, ELKINS CJ, JANAS W, DAKE MD. High-efficiency endovascular gene delivery via therapeutic ultrasound. Journal of the American College of Cardiology. 2001;37(7):1975–1980. doi: 10.1016/s0735-1097(01)01253-0. [DOI] [PubMed] [Google Scholar]
- 73.HUBER PE, MANN MJ, MELO LG, et al. Focused ultrasound (HIFU) induces localized enhancement of reporter gene expression in rabbit carotid artery. Gene Therapy. 2003;10(18):1600–1607. doi: 10.1038/sj.gt.3302045. [DOI] [PubMed] [Google Scholar]
- 74.DU XY, YANG YS, LE VISAGE C, et al. In vivo US monitoring of catheter-based vascular delivery of gene microspheres in pigs: Feasibility. Radiology. 2003;228(2):555–559. doi: 10.1148/radiol.2282020539. [DOI] [PubMed] [Google Scholar]
- 75.MANOME Y, NAKAMURA M, OHNO T, FURUHATA H. Ultrasound facilitates transduction of naked plasmid DNA into colon carcinoma cells in vitro and in vivo. Human Gene Therapy. 2000;11(11):1521–1528. doi: 10.1089/10430340050083252. [DOI] [PubMed] [Google Scholar]
- 76.HUBER PE, DEBUS J. Tumor cytotoxicity in vivo and radical formation in vitro depend on the shock wave-induced cavitation dose. Radiation Research. 2001;156(3):301–309. doi: 10.1667/0033-7587(2001)156[0301:tcivar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 77.HUBER PE, PFISTERER P. In vitro and in vivo transfection of plasmid DNA in the Dunning prostate tumor R3327-AT1 is enhanced by focused ultrasound. Gene Therapy. 2000;7(17):1516–1525. doi: 10.1038/sj.gt.3301242. [DOI] [PubMed] [Google Scholar]
- 78.BAO S, THRALL BD, GIES RA, L MD. In vivo transfection of melanoma cells by lithotripter shock waves. Cancer Res. 1998;58(2):219–221. [PubMed] [Google Scholar]
- 79.CHRISTIANSEN JP, FRENCH BA, KLIBANOV AL, KAUL S, LINDNER JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound in Medicine and Biology. 2003;29(12):1759–1767. doi: 10.1016/s0301-5629(03)00976-1. [DOI] [PubMed] [Google Scholar]
- 80.TANIYAMA Y, TACHIBANA K, HIRAOKA K, et al. Development of safe and efficient novel nonviral gene transfer using ultrasound: enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Therapy. 2002;9(6):372–380. doi: 10.1038/sj.gt.3301678. [DOI] [PubMed] [Google Scholar]
- 81.LU QL, LIANG HD, PARTRIDGE T, BLOMLEY MJK. Microbubble ultrasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene Therapy. 2003;10(5):396–405. doi: 10.1038/sj.gt.3301913. [DOI] [PubMed] [Google Scholar]
- 82.SCHRATZBERGER P, KRAININ JG, SCHRATZBERGER G, et al. Transcutaneous ultrasound augments naked DNA transfection of skeletal muscle. Molecular Therapy. 2002;6(5):576–583. [PubMed] [Google Scholar]
- 83.BARBARESE E, HO S-Y, D'ARRIGO JS, SIMON RH. Internalization of microbubbles by tumor cells in vivo and in vitro. Journal of Neuro-Oncology. 1995;26:25–34. doi: 10.1007/BF01054766. [DOI] [PubMed] [Google Scholar]
- 84.CHO C-W, LIU Y, COBB WN, et al. Ultrasound-Induced Mild Hyperthermia as a Novel Approach to Increase Drug Uptake in Brain Microvessel Endothelial Cells. Pharm Res. 2002;19(8):1123–1129. doi: 10.1023/a:1019837923906. [DOI] [PubMed] [Google Scholar]
- 85.MESIWALA AH, FARRELL L, WENZEL HJ, et al. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med Biol. 2002;28(3):389–400. doi: 10.1016/s0301-5629(01)00521-x. * High intensity US disruption of the blood brain barrier is reversible after 72 h. [DOI] [PubMed] [Google Scholar]
- 86.CHO CW, LIU Y, COBB WN, et al. Ultrasound-induced mild hyperthermia as a novel approach to increase drug uptake in brain microvessel endothelial cells. Pharmaceutical Research. 2002;19(8):1123–1129. doi: 10.1023/a:1019837923906. [DOI] [PubMed] [Google Scholar]
- 87.HYNYNEN K, MCDANNOLD N, MARTIN H, JOLESZ FA, VYKHODTSEVA N. The threshold for brain damage in rabbits induced by bursts of ultrasound in the presence of an ultrasound contrast agent (Optison (R)) Ultrasound in Medicine and Biology. 2003;29(3):473–481. doi: 10.1016/s0301-5629(02)00741-x. ** Seminal paper showing that ultrasound can breach the blood brain barrier with minimal tissue damage. [DOI] [PubMed] [Google Scholar]
- 88.GUILLAUME C, DELEPINE P, DROAL C, MONTIER T, TYMEN G, CLAUDE F. Aerosolization of cationic lipid-DNA complexes: lipoplex characterization and optimization of aerosol delivery conditions. Biochem Biophys Res Commun. 2001;286(3):464–471. doi: 10.1006/bbrc.2001.5418. [DOI] [PubMed] [Google Scholar]
- 89.PILLAI R, PETRAK K, BLEZINGER P, et al. Ultrasonic nebulization of cationic lipid-based gene delivery systems for airway administration. Pharm Res. 1998;15(11):1743–1747. doi: 10.1023/a:1011964813817. [DOI] [PubMed] [Google Scholar]
- 90.MITRAGOTRI S, KOST J. Low-frequency sonophoresis. A review. Adv Drug Deliv Rev. 2004;56:589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 91.BARRY BW. Novel mechanisms and devices to enable successful transdermal drug delivery. European Journal of Pharmaceutical Sciences. 2001;14:101–114. doi: 10.1016/s0928-0987(01)00167-1. * Good review on mechanisms of transdermal drug delivery. [DOI] [PubMed] [Google Scholar]
- 92.PRAUSNITZ MR. Reversible Skin Permeabilization for Transdermal Delivery of Macromolecules. Critical Reviews in Therapeutic Drug Carrier Systems. 1997;14(4):455–483. * Excellent review of transdermal drug delivery by various routes. [PubMed] [Google Scholar]
- 93.GUY RH. Current Status and Future Prospects of Transdermal Drug Delivery. Pharm Res. 1996;13(12):1765–1769. doi: 10.1023/a:1016060403438. [DOI] [PubMed] [Google Scholar]
- 94.KASSAN DG, LYNCH AM, J SM. Physical Enhancement of Dermatologic Drug Delivery — Iontophoresis and phonophoresis. J Amer Acad Dermatology. 1996;34(4):657–666. doi: 10.1016/s0190-9622(96)80069-7. [DOI] [PubMed] [Google Scholar]
- 95.BYL NN. The Use of Ultrasound as an Enhancer for Transcutaneous Drug Delivery: Phonophoresis. Physical Therapy. 1995;75(6):539–553. doi: 10.1093/ptj/75.6.539. [DOI] [PubMed] [Google Scholar]
- 96.TYLE P, AGRAWALA P. Drug Delivery by Phonophoresis. Pharm Res. 1989;6(5):355–361. doi: 10.1023/a:1015967012253. [DOI] [PubMed] [Google Scholar]
- 97.SKAUEN DM, ZENTNER GM. Phonophoresis. Int J Pharmaceutics. 1984;20:235–245. [Google Scholar]
- 98.BOMMANNAN D, OKUYAMA H, STAUFFER P, GUY RH. Sonophoresis. I The Use of High-Frequency Ultrasound to Enhance Transdermal Drug Delivery. Pharm Res. 1992;9(4):559–564. doi: 10.1023/a:1015808917491. [DOI] [PubMed] [Google Scholar]
- 99.BOMMANNAN D, MENON GK, OKUYAMA H, ELIAS PM, GUY RH. Sonophoresis. II Examination of the Mechanism(s) of Ultrasound-Enhanced Transdermal Drug Delivery. Pharm Res. 1992;9(8):1043–1047. doi: 10.1023/a:1015806528336. [DOI] [PubMed] [Google Scholar]
- 100.MENON GK, BOMMANNAN DB, ELIAS PM. High-Frequency Sonophoresis: Permeation Pathways and Structural Basis for Enhanced Permeability. Skin Pharmacol. 1994;7:130–139. doi: 10.1159/000211287. [DOI] [PubMed] [Google Scholar]
- 101.VYAS SP, SINGH R, ASATI RK. Liposomally encapsulated diclofenac for sonophoresis induced systemic delivery. J Microencapsul. 1995;12(2):149–154. doi: 10.3109/02652049509015285. [DOI] [PubMed] [Google Scholar]
- 102.MITRAGOTRI S. Synergistic Effect of Enhancers for Transdermal Drug Delivery. Pharm Res. 2000;17(11):1354–1357. doi: 10.1023/a:1007522114438. [DOI] [PubMed] [Google Scholar]
- 103.JOHNSON ME, MITRAGOTRI S, PATEL A, BLANKSCHTEIN D, LANGER R. Synergistic Effects of Chemical Enhancers and Therapeutic Ultrasound on Transdermal Drug Delivery. Journal of Pharmaceutical Sciences. 1996;85(7):670–677. doi: 10.1021/js960079z. [DOI] [PubMed] [Google Scholar]
- 104.TEZEL A, SENS A, TUCHSCHERER J, MITRAGOTRI A. Synergistic Effect of Low-Frequency Ultrasound and Surfactants on Skin Permeability. J Pharm Sci. 2002;91(1):91–100. doi: 10.1002/jps.10000. [DOI] [PubMed] [Google Scholar]
- 105.MITRAGOTRI S, BLANKSCHTEIN D, LANGER R. Ultrasound-Mediated Transdermal Protein Delivery. Science. 1995;269:850–853. doi: 10.1126/science.7638603. ** Seminal paper showing that low frequency US enhances skin permeability sufficiently to deliver insulin. [DOI] [PubMed] [Google Scholar]
- 106.MITRAGOTRI S, EDWARDS DA, BLANKSCHTEIN D, LANGER R. A Mechanistic Study of Ultrasonically-Enhanced Transdermal Drug Delivery. Journal of Pharmaceutical Sciences. 1995;84(6):697–706. doi: 10.1002/jps.2600840607. [DOI] [PubMed] [Google Scholar]
- 107.MITRAGOTRI S, BLANKSCHTEIN D, LANGER R. Transdermal drug delivery using low-frequency sonophoresis. Pharmaceut Res. 1996;13(3):411–420. doi: 10.1023/a:1016096626810. [DOI] [PubMed] [Google Scholar]
- 108.KOST J, PLIQUETT U, MITRAGOTRI S, YAMAMOTO A, LANGER R, WEAVER J. Synergistic Effect of Electric Field and Ultrasound on Transdermal Transport. Pharm Res. 1996;13(4):633–638. doi: 10.1023/a:1016070710397. [DOI] [PubMed] [Google Scholar]
- 109.MITRAGOTRI S, BLANKSCHTEIN D, LANGER R. An Explanation for the Variation of the Sonophoretic Transdermal Transport Enhancement from Drug to Drug. J of Pharm Sci. 1997;86(10):1190–1192. doi: 10.1021/js960528v. [DOI] [PubMed] [Google Scholar]
- 110.MITRAGOTRI S, KOST J. Low-Frequency Sonophoresis: A Noninvasive Method of Drug Delivery and Diagnostics. Biotechnol Prog. 2000;16:488–492. doi: 10.1021/bp000024+. [DOI] [PubMed] [Google Scholar]
- 111.TEZEL A, SENS A, TUCHSCHERER J, MITRAGOTRI S. Frequency Dependence of Sonophoresis. Pharmaceutical Research. 2001;18(12):1694–1700. doi: 10.1023/a:1013366328457. * Threshold for skin permeabilization is lower at lower frequencies, and permeability correlates with energy delivered. [DOI] [PubMed] [Google Scholar]
- 112.TANG H, MITRAGOTRI S, BLANKSCHTEIN D, LANGER R. Theoretical Description of Transdermal Transport of Hydrophilic Permeants: Application to Low-Frequency Sonophoresis. J Pharm Sci. 2001;90(5):545–568. doi: 10.1002/1520-6017(200105)90:5<545::aid-jps1012>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 113.TERAHARA T, MITRAGOTRI S, KOST J, LANGER R. Dependence of low-frequency sonophoresis on ultrasound parameters; distance of the horn and intensity. International Journal of Pharmaceutics. 2002;235:35–42. doi: 10.1016/s0378-5173(01)00981-4. [DOI] [PubMed] [Google Scholar]
- 114.TEZEL A, SENS A, MITRAGOTRI S. Incorporation of lipophilic pathways into the porous pathway model for describing skin permeabilization during low-frequency sonophoresis. Journal of Controlled Release. 2002;83:183–188. doi: 10.1016/s0168-3659(02)00177-3. [DOI] [PubMed] [Google Scholar]
- 115.LANGER R. Biomaterials in drug delivery and tissue engineering: one laboratory's experience. Acc Chem Res. 2000;33:94–101. doi: 10.1021/ar9800993. [DOI] [PubMed] [Google Scholar]
- 116.WU J, CHAPPELOW J, YANG J, WEIMANN L. Defects Generated in Human Stratum Corneum Specimens by Ultrasound. Ultrasound in Med & Biol. 1998;24(5):705–710. doi: 10.1016/s0301-5629(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 117.YAMASHITA N, TACHIBANA K, OGAWA K, TSUJITA N, TOMITA A. Scanning Electron Microscopic Evaluation of the Skin Surface After Ultrasound Exposure. Anatom Rec. 1997;247:455–461. doi: 10.1002/(SICI)1097-0185(199704)247:4<455::AID-AR3>3.0.CO;2-Q. * SEM micrographs show skin disruption causes by ultrasound. [DOI] [PubMed] [Google Scholar]
- 118.TEZEL A, MITRAGOTRI S. On the origin of size-dependent tortuosity for permeation of hydrophilic solutes across the stratum corneum. Journal of Controlled Release. 2003;86(1):183–186. doi: 10.1016/s0168-3659(02)00375-9. [DOI] [PubMed] [Google Scholar]
- 119.TEZEL A, SENS A, MITRAGOTRI S. Description of transdermal transport of hydrophilic solutes during low-frequency sonophoresis based on a modified porous pathway model. 2003;92(2):381–393. doi: 10.1002/jps.10299. [DOI] [PubMed] [Google Scholar]
- 120.TEZEL A, MITRAGOTRI S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophysical Journal. 2003;85(6):3502–3512. doi: 10.1016/S0006-3495(03)74770-5. ** Excellent paper of theory and experiment of various modalities of how cavitation events permeabilized skin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.HIPPIUS M, UHLEMANN C, SMOLENSKI U, SCHREIBER U, REIBIG S, HOFFMANN A. In Vitro investigations of drug release and penetration -- enhancing effect of ultrasound on transmembrane transport of flufenamic acid. Int J Clin Pharmacol Therapeutics. 1998;36:107–111. [PubMed] [Google Scholar]
- 122.ASANO J, SUISHA F, TAKADA M, KAWASAKI M, MIYAZAKI S. Effects of Pulsed Output Ultrasound on the Transdermal Absorption of Indomethacin from an Ointment in Rats. Biol Pharm Bull. 1997;20(3):288–291. doi: 10.1248/bpb.20.288. [DOI] [PubMed] [Google Scholar]
- 123.FANG J-Y, HWANG T-L, HUANG Y-B, TSAI Y-H. Transdermal iontophoresis of sodium nonivamide acetate V. Combined effect of physical enhancement methods. International Journal of Pharmaceutics. 2002;235:95–105. doi: 10.1016/s0378-5173(01)00972-3. [DOI] [PubMed] [Google Scholar]
- 124.BOUCAUD A, GARRIGUE MA, MACHET L, VAILLANT L, PATAT F. Effect of sonication parameters on transdermal delivery of insulin to hairless rats. Journal of Controlled Release. 2002;81:113–119. doi: 10.1016/s0168-3659(02)00054-8. [DOI] [PubMed] [Google Scholar]
- 125.LEVY D, KOST J, MESHULAM Y, LANGER R. Effect of Ultrasound on Transdermal Drug Delivery to Rats and Guinea Pigs. J Clin Invest. 1989;83:2074–2078. doi: 10.1172/JCI114119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.MIYAZAKI S, MIZUOKA H, KOHATA Y, TAKADA M. External Control of Drug Release and Penetration. VI Enhancin g Effect of Ultrasound on the Transdermal Absorption of Indomethacin from an Ointment in Rats. Chem Pharm Bull. 1992;40(10):2826–2830. doi: 10.1248/cpb.40.2826. [DOI] [PubMed] [Google Scholar]
- 127.ZHANG I, SHUNG KK, EDWARDS DA. Hydrogels with Enhanced Mass Transfer for Transdermal Drug Delivery. J Pharm Sci. 1996;85(12):1312–1316. doi: 10.1021/js9601142. [DOI] [PubMed] [Google Scholar]
- 128.KWOK CS, MOURAD PD, CRUM LA, RATNER BD. Self-assembled molecular structures as ultrasonically-responsive barrier membranes for pulsatile drug delivery. J Biomed Mater Res. 2001;57:151–164. doi: 10.1002/1097-4636(200111)57:2<151::aid-jbm1154>3.0.co;2-5. * Excellent paper describing an insulin depot activated by ultrasound. [DOI] [PubMed] [Google Scholar]
- 129.FRANCIS CW, ONUNDARSON PT, CARSTENSEN EL, et al. Enhancement of Fibrinolysis In Vitro by Ultrasound. J Clin Invest. 1992;90(11):2063–2068. doi: 10.1172/JCI116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.LAUER CG, BURGE R, TANG DB, BASS BG, GOMEZ ER, ALVING BM. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation. 1992;86:1257–1264. doi: 10.1161/01.cir.86.4.1257. * Good paper showing that US transports enzymes into clots, leading to fibrinoylsis. [DOI] [PubMed] [Google Scholar]
- 131.FRANCIS CW, BLINC A, LEE S, COX C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound in Med & Biol. 1995;21(3):419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- 132.SUCHKOVA V, CARSTENSEN EL, FRANCIS CW. Ultrasound enhancement of fibrinolysis at frequencies of 27 to 100 kHz. Ultrasound Med Biol. 2002;28(3):377–382. doi: 10.1016/s0301-5629(01)00522-1. * The best fibrinolysis is at the lowest frequency, supporting cavitation mechanisms. [DOI] [PubMed] [Google Scholar]
- 133.SIDDIQI F, BLINC A, BRAATEN J, FRANCIS CW. Ultrasound Increases Flow through Fibrin Gels. Thromb Haemostasis. 1995;73(3):495–498. [PubMed] [Google Scholar]
- 134.ROSENSCHEIN U, FURMAN V, KERNER E. Ultrasound imaging-guided noninvasive ultrasound thrombolysis: preclinical results. Circulation. 2000;102(2):238–245. doi: 10.1161/01.cir.102.2.238. [DOI] [PubMed] [Google Scholar]
- 135.TACHIBANA K, TACHIBANA S. Albumin Microbubble Echo-Contrast Material as an Enhancer for Ultrasound Accelerated Thrombolysis. Circulation. 1995;92(5):1148–1150. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- 136.PORTER TR, LEVEEN RF, FOX R, KRICSFELD A, XIE F. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. American Heart Journal. 1996;132(5):964–968. doi: 10.1016/s0002-8703(96)90006-x. [DOI] [PubMed] [Google Scholar]
- 137.WU YQ, UNGER EC, MCCREERY TP, et al. Binding and lysing of blood clots using MRX-408. Investigative Radiology. 1998;33(12):880–885. doi: 10.1097/00004424-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 138.SIEGEL RJ, ATAR S, FISHBEIN MC, et al. Noninvasive transcutaneous low frequency ultrasound enhances thrombolysis in peripheral and coronary arteries. Echocardiography-a Journal of Cardiovascular Ultrasound and Allied Techniques. 2001;18(3):247–257. doi: 10.1046/j.1540-8175.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- 139.FRANCIS CW. Ultrasound-enhanced thrombolysis. Echocardiography-a Journal of Cardiovascular Ultrasound and Allied Techniques. 2001;18(3):239–246. doi: 10.1046/j.1540-8175.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- 140.CULP WC, PORTER TR, LOWERY J, ROBERSON PK, XIE F, MCCOWAN TC. Intracranial clot lysis with intravenous platelet targeted microbubbles and transcranial ultrasound. Circulation. 2003;108(17):604–604. [Google Scholar]
- 141.NIVEN RW, WHITCOMB KL, SHANER L, IP AY, KINSTLER OB. The Pulmonary Absorption of Aerosolized and Intratracheally Instilled Rhg-Csf and Monopegylated Rhg-Csf. Pharmaceutical Research. 1995;12(9):1343–1349. doi: 10.1023/a:1016281925554. [DOI] [PubMed] [Google Scholar]
- 142.STECKEL H, ESKANDAR F, WITTHOHN K. Effect of cryoprotectants on the stability and aerosol performance of nebulized aviscumine, a 57-kDa protein. European Journal of Pharmaceutics and Biopharmaceutics. 2003;56(1):11–21. doi: 10.1016/s0939-6411(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 143.IP AY, ARAKAWA T, SILVERS H, RANSONE CM, NIVEN RW. Stability of Recombinant Consensus Interferon to Air-Jet and Ultrasonic Nebulization. Journal of Pharmaceutical Sciences. 1995;84(10):1210–1214. doi: 10.1002/jps.2600841013. [DOI] [PubMed] [Google Scholar]
- 144.KOSUGI T, SAITOH S, TAMAKI N, HANASHIRO K, NAKAHODO K, NAKAMURA M. Inhalation of Platelet-Activating-Factor Increases Respiratory Resistance in Rats - Determination by Means of an Astograph under Nonanesthetized Conditions. Laryngoscope. 1993;103(4):428–430. doi: 10.1002/lary.5541030411. [DOI] [PubMed] [Google Scholar]
- 145.LANGENBACK EG, DAVIS JM, ROBBINS C, SAHGAL N, PERRY RJ, SIMON SR. Improved pulmonary distribution of recombinant human Cu/Zn superoxide dismutase, using a modified ultrasonic nebulizer. Pediatric Pulmonology. 1999;27(2):124–129. doi: 10.1002/(sici)1099-0496(199902)27:2<124::aid-ppul9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 146.FLAMENT MP, LETERME P, GAYOT A. Study of the technological parameters of ultrasonic nebulization. Drug Development and Industrial Pharmacy. 2001;27(7):643–649. doi: 10.1081/ddc-100107320. [DOI] [PubMed] [Google Scholar]
- 147.FLAMENT MP, LETERME P, GAYOT A. Influence of the technological parameters of ultrasonic nebulisation on the nebulisation quality of alpha 1 protease inhibitor (alpha 1I) International Journal of Pharmaceutics. 1999;189(2):197–204. doi: 10.1016/s0378-5173(99)00248-3. [DOI] [PubMed] [Google Scholar]
- 148.MUNSTER AMB, BENDSTRUP E, JENSEN JI, GRAM J. Jet and ultrasonic nebulization of single chain urokinase plasminogen activator (scu-PA) Journal of Aerosol Medicine-Deposition Clearance and Effects in the Lung. 2000;13(4):325–333. doi: 10.1089/jam.2000.13.325. [DOI] [PubMed] [Google Scholar]
- 149.KOST J. Ultrasound for Controlled Delivery of Therapeutics. Clinical Materials. 1993;13:155–161. doi: 10.1016/0267-6605(93)90103-e. [DOI] [PubMed] [Google Scholar]
- 150.LOVEROCK P, TER HAARG, ORMEROD MG, IMRIE PR. The effect of ultrasound on the cytoxicity of adriamycin. Brit J Radiol. 1990;63:542–546. doi: 10.1259/0007-1285-63-751-542. [DOI] [PubMed] [Google Scholar]
- 151.TACHIBANA K, UCHIDA T, TAMURA K, EGUCHI H, YAMASHITA N, OGAWA K. Enhanced cytotoxic effect of Ara-C by low intensity ultrasound to HL-60 cells. Cancer Lett. 2000;149:189–194. doi: 10.1016/s0304-3835(99)00358-4. [DOI] [PubMed] [Google Scholar]
- 152.TACHIBANA K, UCHIDA T, OGAWA K, YAMASHITA N, TAMURA K. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409. doi: 10.1016/S0140-6736(99)01244-1. * Paper shows micropores forming in HL-60 cells as a result of ultrasonication. [DOI] [PubMed] [Google Scholar]
- 153.SAITO K, MIYAKE K, MCNEIL PL, KATO K, YAGO K, SUGAI N. Plasma Membrane Disruption Underlies Injury of the Corneal Endothelium by Ultrasound. Exp Eye Res. 1999;68:421–427. doi: 10.1006/exer.1998.0626. * Flow cytometry shows that US permeablizes the corneal cell membrane. [DOI] [PubMed] [Google Scholar]
- 154.RAPOPORT NY, HERRON JN, PITT WG, PITINA L. Micellar delivery of doxorubicin and its paramagnetic analog, ruboxyl, to HL-60 cells: effect of micelle structure and ultrasound on the intracellular drug uptake. J Controlled Rel. 1999;58(2):153–162. doi: 10.1016/s0168-3659(98)00149-7. [DOI] [PubMed] [Google Scholar]
- 155.MUNSHI N, RAPOPORT N, PITT WG. Ultrasonic activated drug delivery from Pluronic P-105 micelles. Cancer Letters. 1997;117:1–7. doi: 10.1016/s0304-3835(97)00218-8. [DOI] [PubMed] [Google Scholar]
- 156.YU TH, HU K, BAI J, WANG ZB. Reversal of adriamycin resistance in ovarian carcinoma cell line by combination of verapamil and low-level ultrasound. Ultrasonics Sonochemistry. 2003;10(1):37–40. doi: 10.1016/s1350-4177(02)00106-2. [DOI] [PubMed] [Google Scholar]
- 157.YU T, WANG W, MASON TJ. A review of research into the uses of low level ultrasound in cancer therapy. Ultrasonics Sonochem. 2004;11:95–103. doi: 10.1016/S1350-4177(03)00157-3. [DOI] [PubMed] [Google Scholar]
- 158.SAAD AH, HAHN GM: Ultrasound enhances adriamycin toxicity in vitro In: Heat transfer in bioengineering and medicine Vol. 7. CHATO JC, DILLER TE, DILLER KR, ROEMER RB, eds. Am. Soc. Mech. Engr. Press; New York: (1987):28–31.
- 159.SAAD AH, HAHN GM. Ultrasound Enhanced Drug Toxicity on Chinese Hamster Ovary Cells in Vitro. Cancer Res. 1989;49:5931–5934. *Paper attributing enhanced ultrasonic killing in the presence of doxorubicin to hyperthermia. [PubMed] [Google Scholar]
- 160.SAAD AH, HAHN GM. Ultrasound-enhanced effects of adriamycin against murine tumors. Ultrasound Med Biol. 1992;18(8):715–723. doi: 10.1016/0301-5629(92)90122-q. [DOI] [PubMed] [Google Scholar]
- 161.YOKOYAMA M, OKANO T, SAKURAI Y, FUKUSHIMA S, OKAMOTO K, KATAOKA K. Selevtive Delivery of Adriamycin to solid tumor using a polymeric micelle carrier system. Journal of drug targeting. 1999;7(3):171–186. doi: 10.3109/10611869909085500. [DOI] [PubMed] [Google Scholar]
- 162.KWON G, NAITO M, YOKOYAMA M, OKANO T, SAKURAI Y, KATAOKA K. Block copolymer micelles for drug delivery: loading and release of doxorubicin. J Control Release. 1997;48:195–201. [Google Scholar]
- 163.KWON GS, NAITO M, YOKOYAMA M, OKANO T, SAKURAI Y, KATAOKA K. Physical entrapment of Adriamicin in AB block copolymer micelles. Pharm Res. 1995;12:192–195. doi: 10.1023/a:1016266523505. [DOI] [PubMed] [Google Scholar]
- 164.KWON GS, KATAOKA K. Block copolymer micelles as long circulating drug vehicles. Advanced Drug Delivery Reviews. 1995;16:295–309. [Google Scholar]
- 165.ALEXANDRIDIS P, HOLZWARTH JF, HATTON TA. Micellization of Poly(ethylene oxide)- Poly(propylene oxide)- Poly(ethylene oxide) Triblock Copolymer in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules. 1994;27(9):2414–2425. [Google Scholar]
- 166.ALEXANDRIDIS P, NIVAGGIOLI T, HATTON TA. Temperature Effects on Structural Properties of Pluronic P104 and F108 PEO-PPO-PEO Block Copolymer Solutions. Langmuir. 1995;11:1468–1476. [Google Scholar]
- 167.ALEXANDRIDIS P, ATHANASSIOU V, HATTON TA. Pluronic-P105 PEO-PPO-PEO Block Copolymer in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment. Langmuir. 1995;11:2442–2450. [Google Scholar]
- 168.ALEXANDRIDIS P, HATTON TA. Poly(ethyleneoxide)-poly(propyleneoxide)-Poly(ethyleneoxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1995;96:1–46. [Google Scholar]
- 169.RAPOPORT N, CALDWELL K. Structural transitions in micellar solutions of Pluronic P-105 and their effect on the conformation of dissolved Cytochrome C: an electron paramagnetic resonance investigation. Colloids and Surfaces B: Biointerfaces. 1994;3:217–228. * The various phases of Pluronic micelles are described. [Google Scholar]
- 170.YUAN F, LEUNING M, HUANG SK, BERK DA, PAPAHADJOPULOS D, JAIN RK. Microvascular permeability and interstitial penetration of sterically-stabilized (stealth) liposomes in human tumor xenograft. Cancer Res. 1994;54:3352–3356. [PubMed] [Google Scholar]
- 171.RAPOPORT N, PITINA L. Intracellular distribution and intracellular dynamics of a spin-labeled analog of Doxorubicin. J Pharm Sci. 1998;87(3):321–325. doi: 10.1021/js970271g. [DOI] [PubMed] [Google Scholar]
- 172.PECHAR M, ULBRICH K, SUBR V. Poly(ethylene glycol) Multiblock Copolymer as a Carrier of Anti-cancer Drug Doxorubicin. Bioconjugate Chem. 2000;11(2):131–139. doi: 10.1021/bc990092l. [DOI] [PubMed] [Google Scholar]
- 173.ALAKHOV VY, MOSKALEVA EY, BATRAKOVA E, KABANOV AV. Hypersensitization of Multidrug resistant Human Ovarian Carcinoma Cells by Pluronic P85 Block Copolymer. Bioconj Chem. 1996;7:209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- 174.VENNE A, LI S, MANDEVILLE R, KABANOV A, ALAKHOV V. Hypersensitizing Effect of Pluronic L61 on Cytotoxic Activity, Transport, and Subcellular Distribution of Doxorubicin in Multiple Drug-resistant Cells. Cancer Res. 1996;56:3626–3629. [PubMed] [Google Scholar]
- 175.SOMA CE, DUBERNET C, BENTOLILA D, BENITA S, COUVREUR P. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkylcyanoacrylate nanoparticles. Biomaterials. 2000;21:1–7. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 176.HUSSEINI GA, MYRUP GD, PITT WG, CHRISTENSEN DA, RAPOPORT NY. Factors Affecting Acoustically-Triggered Release of Drugs from Polymeric Micelles. J Contr Release. 2000;69:43–52. doi: 10.1016/s0168-3659(00)00278-9. Release of two chemotherapy drugs from micelles increases with decreasing frequencies and increasing intensities. This study focuses on low frequency ultrsound. [DOI] [PubMed] [Google Scholar]
- 177.HUSSEINI GA, RAPOPORT NY, CHRISTENSEN DA, PRUITT JD, PITT WG. Kinetics of ultrasonic release of doxorubin from pluronic P105 micelles. Coll Surf B: Biointerfaces. 2002;24:253–264. [Google Scholar]
- 178.MARIN A, MUNIRUZZAMAN M, RAPOPORT N. Mechanism of the ultrasonic activation of micellar drug delivery. J Control Rel. 2001;75:69–81. doi: 10.1016/s0168-3659(01)00363-7. [DOI] [PubMed] [Google Scholar]
- 179.RAPOPORT N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. International Journal of Pharmaceutics. 2004;227:155–162. doi: 10.1016/j.ijpharm.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 180.RAPOPORT N, MARIN A, LUO Y, PRESTWICH GD, MUNIRZZAMAN M. Intracellular uptake and trafficking of Pluronic micelles in drug-sensitive and MDR cells: Effect on the intracellular drug localization. J Pharm Sci. 2002;91(1):157–170. doi: 10.1002/jps.10006. * This paper describes how US affects the distribution of drug in the cells. [DOI] [PubMed] [Google Scholar]
- 181.MUNIRUZZAMAN MD, MARIN A, LUO Y, et al. Intracellular uptake of Pluronic copolymer: effects of the aggregation state. Colloids and Surfaces B: Biointerfaces. 2002;25(3):233–241. [Google Scholar]
- 182.MARIN A, MUNIRUZZAMAN M, RAPOPORT N. Acoustic activation of drug delivery from polymeric micelles: effect of pulsed ultrasound. J Controlled Rel. 2001;71:239–249. doi: 10.1016/s0168-3659(01)00216-4. [DOI] [PubMed] [Google Scholar]
- 183.HUSSEINI GA, RUNYAN CM, PITT WG. Investigating the mechanism of acoustically activated uptake of drugs from Pluronic micelles. BMC Cancer. 2002;2(20):1–6. doi: 10.1186/1471-2407-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.PRUITT JD, HUSSEINI G, RAPOPORT N, PITT WG. Stabilization of Pluronic P-105 Micelles with an Interpenetrating Network of N,N-Diethylacrylamide. Macromolecules. 2000;33(25):9306–9309. *This paper reports the synthesis of P105 micelles that are stable at low concentration. [Google Scholar]
- 185.PRUITT JD, PITT WG. Sequestration and Ultrasound-Induced Release of Doxorubicin from Stabilized Pluronic P105 Micelles. Drug Delivery. 2002;9(4):253–259. doi: 10.1080/10717540260397873. [DOI] [PubMed] [Google Scholar]
- 186.HUSSEINI GA, CHRISTENSEN DA, RAPOPORT NY, PITT WG. Ultrasonic release of doxorubicin from Pluronic P105 micelles stabilized with an interpenetrating network of N,N-diethylacrylamide. J Controlled Rel. 2002;83(2):302–304. doi: 10.1016/s0168-3659(02)00203-1. *This paper reports on the release of doxorubicin from stabilized micelles. [DOI] [PubMed] [Google Scholar]
- 187.NELSON JL, ROEDER BL, CARMEN JC, ROLOFF F, PITT WG. Ultrasonically Activated Chemotherapeutic Drug Delivery in a Rat Model. Cancer Res. 2002;62:7280–7283. *First paper to report the results of an in vivo model for micellar drug delivery. [PubMed] [Google Scholar]
- 188.GAO Z, FAIN HD, RAPOPORT N: Ultrasound-Enhanced Tumor Targeting of Polymeric Micellar Drug Carriers. Molecular Pharmaceutics (2004) in press [DOI] [PMC free article] [PubMed]
- 189.RAPOPORT N, CHRISTENSEN DA, FAIN HD, BARROWS L, GAO Z. Ultrasound-triggered drug targeting of tumors in vitro and in vivo. Ultrasoincs. 2004;42:943–950. doi: 10.1016/j.ultras.2004.01.087. *Release of doxorubicin from P105 micelles is reported at high frequencies. [DOI] [PubMed] [Google Scholar]
- 190.HERMAN EH, RAHMAN A, FERRANS VJ, VICK JA, SCHEIN PS. Prevention of Chronic Doxorubicin Cardiotoxicity in Beagles by Liposomal Encapsulation. Cancer Res. 1983;43:5427–5432. [PubMed] [Google Scholar]
- 191.SYMON Z, PEYSER A, TZEMACH D. Selective Delivery of Doxorubicin to Patients with Breast Carcinoma Metastases by Stealth Liposomes. Cancer. 1999;86:72–8. [PubMed] [Google Scholar]
- 192.BEDNARSKI MD, LEE JW, CALLSTROM MR, LI KCP. In Vivo Target-specific Delivery of Macromolecular Agents with MR-guided Focused Ultrasound. Radiology. 1997;204:263–268. doi: 10.1148/radiology.204.1.9205257. [DOI] [PubMed] [Google Scholar]
- 193.KODAMA M, MIYATA T, TAKAICHI Y. Calorimetric investigations of phase transitions of sonicated vesicles of dimyristoylphosphatidylcholine. Biochim Biophys Acta. 1993;1169(1):90–97. [PubMed] [Google Scholar]
- 194.MORSE R, MA LD, MAGIN RL, DUNN F. Ultrasound interaction with large unilamellar vesicles at the phospholipid phase transition: perturbation by phospholipid side chain substitution with deuterium. Chem Phys Lipids. 1999;103(1–2):1–10. doi: 10.1016/s0009-3084(99)00068-7. [DOI] [PubMed] [Google Scholar]
- 195.MAYNARD VM, MAGIN RL, DUNN F. Ultrasonic absorption and permeability for liposomes near phase transition. Chem Phys Lipids. 1985;37(1):1–12. doi: 10.1016/0009-3084(85)90070-2. [DOI] [PubMed] [Google Scholar]
- 196.REDISKE AM, RAPOPORT N, PITT WG. Reducing bacterial resistance to antibiotics with ultrasound. Lett Appl Microbiol. 1999;28(1):81–84. doi: 10.1046/j.1365-2672.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- 197.REDISKE AM, HYMAS WC, WILKINSON R, PITT WG. Ultrasonic enhancement of antibiotic action on several species of bacteria. J Gen Appl Microbiol. 1998;44:283–288. doi: 10.2323/jgam.44.283. [DOI] [PubMed] [Google Scholar]
- 198.QIAN Z, STOODLEY P, PITT WG. The Effect of Low Intensity Ultrasound upon Biofilm Structure from Confocal Scanning Laser Microscopy Observation. Biomaterials. 1996;17(20):1975–1980. doi: 10.1016/0142-9612(96)00022-1. [DOI] [PubMed] [Google Scholar]
- 199.QIAN Z, SAGERS RD, PITT WG. The role of insonation intensity in acoustic-enhanced antibiotic treatment of bacterial biofilms. Colloids and Surfaces B: Biointerfaces. 1997;9:239–245. [Google Scholar]
- 200.QIAN Z, SAGERS RD, PITT WG. Investigation of the mechanism of the bioacoustic effect. J Biomed Mater Res. 1999;44:198–205. doi: 10.1002/(sici)1097-4636(199902)44:2<198::aid-jbm10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 201.QIAN Z, SAGERS RD, PITT WG. The Effect of Ultrasonic Frequency upon Enhanced Killing of P. aeruginosa Biofilms. Annals Biomed Eng. 1997;25(1):69–76. doi: 10.1007/BF02738539. [DOI] [PubMed] [Google Scholar]
- 202.JOHNSON LL, PETERSON RV, PITT WG. Treatment of bacterial biofilms on polymeric implants using antibiotics and ultrasound. J Biomat Sci Polymer Ed. 1998;9:1177–1185. doi: 10.1163/156856298x00712. [DOI] [PubMed] [Google Scholar]
- 203.REDISKE AM, ROEDER BL, BROWN MK, et al. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob Agents Chemother. 1999;43(5):1211–1214. doi: 10.1128/aac.43.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.REDISKE AM, ROEDER BL, NELSON JL, et al. Pulsed ultrasound enhances the killing of E. coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob Agents Chemother. 2000;44:771–772. doi: 10.1128/aac.44.3.771-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.CARMEN JC, ROEDER BL, NELSON JL, et al: The Treatment of Implanted Biofilms with Low-Frequency Ultrasound and Gentamicin. Am. J. Infect. Control (2004) accepted [DOI] [PMC free article] [PubMed]
- 206.CARMEN JC, ROEDER BL, NELSON JL, et al. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biomilms in vivo. J Biomater Appl. 2004;18(4):237–245. doi: 10.1177/0885328204040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.STRIDE E, SAFFARI N. On the destruction of microbubble ultrasound contrast agents. Ultrasound in Medicine and Biology. 2003;29(4):563–573. doi: 10.1016/s0301-5629(02)00787-1. [DOI] [PubMed] [Google Scholar]
- 208.LEE JC, BURMUDEZ H, DISCHER BM, et al. Preparation, stability, and in vitro performance of vesicles made with diblock copolymers. Biotechnol Bioeng. 2001;73(2):135–135. doi: 10.1002/bit.1045. [DOI] [PubMed] [Google Scholar]
- 209.SCHIFFELERS RM, KONING GA, TEN HAGEN TLM, et al. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. Journal of Controlled Release. 2003;91(1–2):115–122. doi: 10.1016/s0168-3659(03)00240-2. [DOI] [PubMed] [Google Scholar]
- 210.TAKALKAR AM, KLIBANOV AL, RYCHAK JJ, LINDNER JR, LEY K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J Control Rel. 2004;96(3):473–482. doi: 10.1016/j.jconrel.2004.03.002. * Targetting moieties were attached to microbubbles. [DOI] [PubMed] [Google Scholar]


