Abstract
Simian virus 40 (SV40) enters cells by atypical endocytosis mediated by caveolae that transports the virus to the endoplasmic reticulum (ER) instead of to the endosomal-lysosomal compartment, which is the usual destination for viruses and other cargo that enter by endocytosis. We show here that SV4O is transported to the ER via an intermediate compartment that contains β-COP, which is best known as a component of the COPI coatamer complexes that are required for the retrograde retrieval pathway from the Golgi to the ER. Additionally, transport of SV40 to the ER, as well as infection, is sensitive to brefeldin A. This drug acts by specifically inhibiting the ARF1 GTPase, which is known to regulate assembly of COPI coat complexes on Golgi cisternae. Moreover, some β-COP colocalizes with intracellular caveolin-1, which was previously shown to be present on a new organelle (termed the caveosome) that is an intermediate in the transport of SV40 to the ER (L. Pelkmans, J. Kartenbeck, and A. Helenius, Nat. Cell Biol. 3:473-483, 2001). We also show that the internal SV40 capsid proteins VP2 and VP3 become accessible to immunostaining starting at about 5 h. Most of that immunostaining overlays the ER, with some appearing outside of the ER. In contrast, immunostaining with anti-SV40 antisera remains confined to the ER.
Simian virus 40 (SV40) enters host cells by an atypical endocytic process mediated by caveolae (1, 10, 44, 53, 58, 65), rather than by clathrin-coated pits. Caveolae are small invaginations of the plasma membrane that are distinguished from clathrin-coated pits by their size (70 to 100 nm), distinctive flask-like shape, and lack of a visible coat in thin sections. Expression of the caveola marker protein, caveolin-1, causes caveolae to form in sphingolipid and cholesterol-enriched lipid microdomains of the plasma membrane that are also known as lipid rafts (20, 33). The cellular functions of caveolae and rafts are not yet entirely clear. Nevertheless, they have been implicated in a number of cellular processes, including organizing signal transduction pathways and sorting and trafficking through the endocytic and secretory pathways (2, 8, 27, 35, 62).
SV40 entry begins with the virus binding to major histocompatibility complex class I molecules that are distributed over the whole cell surface (4, 7, 10, 65). SV40 then associates with flat regions of raft domains, from which it transmits an extracellular signal-regulated kinase- and mitogen-activated protein kinase-independent intracellular signal that promotes its enclosure within caveolar invaginations (10, 16). The virus then enters in caveolin-1-containing vesicles (53). Thus, in the case of caveola-mediated SV40 entry, ligand-induced signal transmission from raft domains is coupled to subsequent endocytosis of the cargo. In contrast, the clathrin-coated pit-mediated entry of other viruses is generally considered to be a constitutive process (39).
An especially noteworthy feature of the caveola-mediated SV40 entry pathway is that the virus bypasses the endosomal compartment and, instead, is transported to the endoplasmic reticulum (ER) (24, 46, 53). This is particularly unusual since endocytic cargo in general, including other viruses that enter by endocytosis, traffics to the endosomal-lysosomal compartment (39).
How SV40 is transported to the ER is of considerable interest. In a recent study, the pathway of SV40 to the ER was shown to consist of two steps (53). First, caveolin-1-containing vesicles deliver the virus to new larger nonacidic peripheral organelles. As expected, those organelles do not contain ligands that are known to enter by clathrin-coated vesicle endocytosis. Also, they do not contain markers for endosomes, lysosomes, or the ER, nor do they contain the Golgi markers TGN46 or mannosidase II. However, those organelles are rich in caveolin-1 and consequently were dubbed caveosomes. Second, SV40 is transported from caveosomes to the smooth ER in caveolin-1-free tubular membranes.
In general, there are few ligands known that are taken up by non-clathrin vesicle endocytosis, and there are few examples of ligands that traffic to the ER. Included among these are certain bacterial toxins such as cholera toxin (CT). Interestingly, CT enters via caveolae and is then transported to the ER via a retrograde pathway from the Golgi (30, 31, 51). The Golgi-to-ER pathway normally acts to retrieve resident ER proteins that have escaped to the Golgi with the anterograde flux. The retrograde transport of these proteins, and of CT, depends on their sorting into COPI-coated vesicles that mediate trafficking from the Golgi back to the ER (32, 36, 63). This pathway is sensitive to brefeldin A (BFA) (29, 50), which acts by specifically inhibiting the Ras-like GTPase ARF-1, which regulates assembly of COPI coat complexes on Golgi cisternae (54).
The retrieval pathway from the Golgi to the ER provides a precedent for a transport pathway from an internal cellular compartment to the ER. Since the Golgi-to-ER retrieval pathway is mediated by COPI coatamer complexes and is dependent on ARF-1, it is interesting to ask whether the pathway of SV40 from its intermediate compartment, the caveosome, to the ER similarly might display those characteristics. This question was addressed by application of multiparameter confocal immunofluorescence microscopy with antibodies against β-COP, which is a coatamer protein component of COPI coat complexes (17, 52, 57, 68). We also examined the effect of BFA on SV40 transport and infection. We report that SV40 is transported to the ER through an intermediate compartment that contains β-COP. In addition, some β-COP colocalizes with intracellular caveolin-1, completely consistent with the likelihood that this β-COP-containing compartment is the caveosome. Moreover, transport of SV40 from its intermediate compartment to the ER is blocked by BFA and, thus, is also dependent on the ARF-1 GTPase. Finally, this COPI-mediated SV40 pathway to the ER is part of the predominant pathway leading to productive infection, since infection is also blocked in BFA-treated cells.
The SV40 capsid is composed of three viral-encoded proteins, VP1, VP2, and VP3. VP3 translation initiates from an internal AUG within the VP2 coding sequence, using the same translational frame. Thus, both proteins share 234 identical amino acids at their carboxy part. VP1 forms the outer shell of the capsid, while VP2 and VP3 form a bridge between the VP1 shell and the viral double-stranded circular DNA genome, which is complexed in nucleosomes. The VP1 monomers are tightly bound in pentamers, forming structures of fivefold symmetry with an inward-facing cavity (34). A single molecule of VP2 or VP3 is strongly anchored, as a hairpin loop, in the inner cavity of each VP1 pentamer by high-affinity hydrophobic interactions (shown by X-ray crystallograpy for the closely related polyoma virus) (6, 9). The strong association between the VP1 pentamers and VP2 or VP3 monomers suggests that VP1-VP2/VP3 complexes are the building blocks of the viral capsid. The VP1 pentamers are tied together through their carboxy-terminal arms (34). Five arms extend from each pentamer and insert into the neighboring pentamers in three distinct kinds of interactions. This unique type of bonding underlies the variability in contacts between the identical building blocks, allowing the flexibility required for the packing geometry. It has been suggested that correct interpentamer bonding is facilitated by host chaperones (34).
Viruses that enter by endocytosis generally disassemble in endosomes as a consequence of the low pH in that compartment (39). However, since the SV40 entry pathway does not intersect endosomes (24, 53), SV40 infection is not dependent on exposure to the low pH of the endosomal compartment. This was shown earlier by an insensitivity to the weakly basic lysosomotropic agent chloroquine (47). Thus, the location and mechanism of virion disassembly and genome release are intriguing. For a number of years it was believed that SV40 virions enter the nucleus and disassemble there. This view was based largely on an early study, from 1970, in which electron-dense SV40 particles were seen in nuclei soon after infection (23). In addition, SV40 virions that were microinjected into the cytoplasm were reported to enter nuclei via nuclear pore complexes (15). However, passage of intact virions through nuclear pore complexes seems unlikely, since the maximum diameter of the gated nuclear pore channel is 28 nm (18), whereas the diameter of SV40 virions is 45 nm. A recent study suggested that some conformational alteration or capsid disassembly occurs in the cytoplasm and that the viral minichromosome enters the nucleus in association with a few capsid proteins (42). To address where disassembly of the virion occurs, we used confocal immunofluorescence microscopy with antibodies specific for the internal capsid proteins VP2 and VP3 (60). Our results imply that SV40 disassembly occurs in the ER.
MATERIALS AND METHODS
Confocal immunofluorescence microscopy assay.
CV-1 cells (from the American Type Culture Collection) were seeded on glass coverslips in Costar 24-well cell culture dishes (Corning). SV40 was adsorbed to cells for 1 h at 4°C at a multiplicity of infection (MOI) of 100 PFU per cell. Cultures then were incubated at 37°C in Dulbecco modified Eagle medium plus 10% newborn calf serum (Atlanta Biologicals). At the times indicated for each experiment, the cultures were washed five times in phosphate-buffered saline and fixed with 70% methanol at −20°C for 10 min. Cells then were immunostained to detect SV40 by using rabbit anti-SV40 antiserum, prepared as previously described (1). Other cell cultures were immunostained to detect exposed epitopes on VP2/3 by using antiserum raised in rabbits against glutathione S-transferase-VP3 produced in Escherichia coli (60). Cultures were simultaneously immunostained for either the marker β-COP, caveolin-1, or the ER marker PDI, using the respective specific monoclonal antisera (Sigma and Transduction Laboratories). The secondary antibodies used were fluorescein-conjugated goat anti-rabbit immunoglobulin G (IgG), and Texas Red-conjugated donkey anti-mouse IgG (Jackson Laboratories, West Grove, Pa.). Cells were incubated with primary antibodies for 1 h and with secondary antibodies for 45 min. All antisera were diluted 1:100. Coverslips were mounted and cells were visualized using a LSM5 PASCAL scanning confocal microscope (Carl Zeiss, Thornwood, N.Y.). Samples were visualized with a 100× objective (unless indicated otherwise) using 488-nm krypton/argon and 633-nm helium/neon laser excitation. Fluorescein isothiocyanate fluorescence was collected using a 505-to-530-nm band-pass emission filter and Texas Red was collected using a 543-to-560-nm band-pass emission filter. For colocalization studies, samples were illuminated sequentially. Images represent a single optical section approximately 1.0 μm in thickness. Images were processed using Adobe Photoshop.
RESULTS
Early transport of SV40 to a β-COP-containing compartment and then to the ER.
We began by asking whether input SV40 might be associated with a compartment that contains β-COP and, if so, whether that association might precede transport of the virus to the ER. SV40-infected CV-1 cell cultures were examined at different times postinfection with double-label confocal microscopy. Parallel samples were immunostained for SV40 and either β-COP or the ER-specific marker protein disulfide isomerase (PDI), which enables proteins to attain their most stable conformations.
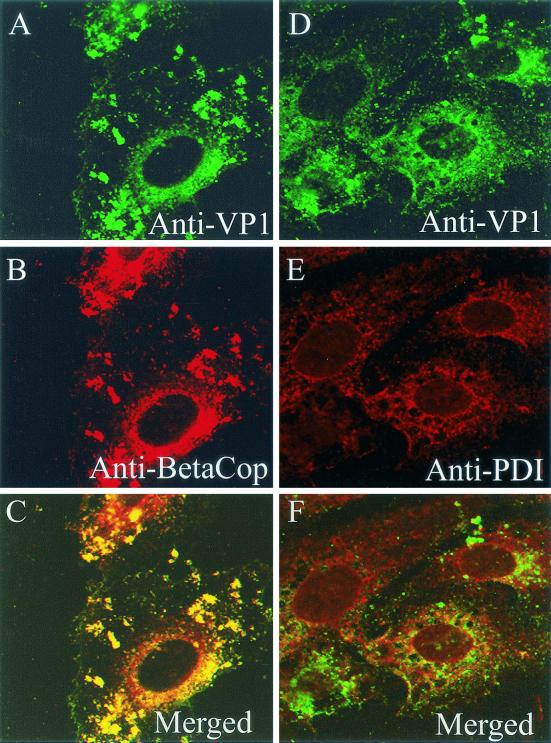
At 3 h, essentially all of the SV40 indeed overlapped the immunostain for β-COP (Fig. 1A to C). Importantly, at that time little, if any, virus associated with the PDI-containing ER (Fig. 1D to F). At 5 h, the immunostain for SV40 still predominantly associated with the immunostain for β-COP (Fig. 2A to C), with little if any SV40 evident in the ER (Fig. 2D to F). However, by 10 h, most of the input virus was located in the PDI-containing ER (Fig. 3). These results demonstrate that SV40 associates with an intermediate compartment that contains β-COP before it accumulates in the ER.
FIG. 1.
Subcellular location of SV40 at 3 h. CV-1 cells were exposed to SV40 at an MOI of 100 PFU per cell for 1 h at 4°C and then incubated for 3 h at 37°C. (A and D) Cells immunostained for SV40 by using rabbit anti-SV40 polyclonal primary antiserum, followed by a fluorescein-conjugated goat anti-rabbit IgG secondary antiserum. (B) Cells immunostained for β-COP using a monoclonal primary antiserum and a Texas Red-conjugated donkey anti-mouse IgG secondary antiserum. (E) Cells immunostained for the ER using an anti-PDI monoclonal primary antiserum and a Texas Red-conjugated anti-mouse IgG secondary antiserum. (C) Merge of panels A and B; (F) merge of panels D and E.
FIG. 2.
Subcellular location of SV40 at 5 h. CV-1 cells were exposed to SV40 at an MOI of 100 PFU per cell for 1 h at 4°C and then incubated for 5 h at 37°C. (A and D) Cells immunostained for SV40; (B) cells immunostained for β-COP; (E) cells immunostained for the ER marker PDI; (C) merge of panels A and B; (F) merge of panels D and E.
FIG. 3.
Subcellular location of SV40 at 10 h. CV-1 cells were exposed to SV40 at an MOI of 100 PFU per cell for 1 h at 4°C and then incubated for 10 h at 37°C. (A) Cells immunostained for SV40; (B) cells immunostained for the ER marker PDI; (C) merge of panels A and B.
Effect of BFA on intracellular trafficking of SV40 and infection.
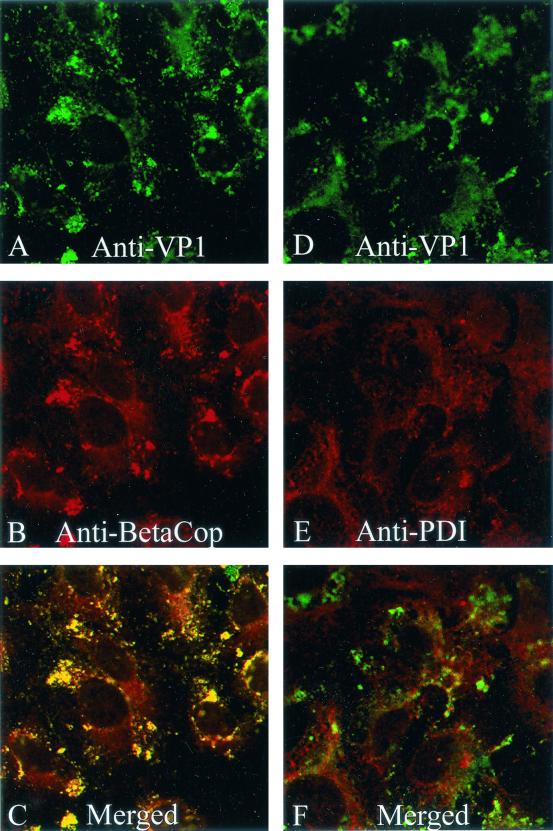
Next, we asked whether the β-COP-containing complexes on the intermediate compartment might be necessary for SV40 transport to the ER. This was accomplished by examining SV40 trafficking in BFA-treated cells. As noted above, BFA acts by specifically inhibiting the Ras-like GTPase ARF-1, which regulates the assembly of membrane-associated COPI coat complexes (54). Treating uninfected cells with BFA caused the rapid redistribution of β-COP into a more diffuse cytosolic pattern (results not shown), in agreement with earlier findings of others (25).
The effect of BFA treatment on the transport of SV40 to the ER was examined at 5 and 10 h postinfection. In BFA-treated cells, SV40 was interspersed with, and close to, the PDI-containing ER compartment, but it did not appear to be in conjunction with that compartment at either 5 h (Fig. 4A to C) or 10 h (Fig. 4D to F). In contrast, in untreated cells at 10 h, most of the virus immunostain did overlay the ER (Fig. 3). These experimental results imply that transport of SV40 to the ER depends on ARF1 GTPase activity and COPI coat complexes. Note that BFA treatment did not prevent SV40 from entering cells per se (Fig. 4).
FIG. 4.
Effect of BFA on subcellular location of SV40 at 5 and 10 h. CV-1 cells were exposed to SV40 at an MOI of 100 PFU per cell for 1 h at 4°C and then incubated for 5 h (A, B, and C) or 10 h (D, E, and F) at 37°C. BFA (0.5 μg/ml) was added to the cell cultures after 30 min at 37°C and was present thereafter. (A and D) Cells immunostained for SV40; (B and E) cells immunostained for the ER marker PDI; (C) merge of panels A and B; (F) merge of panels D and E.
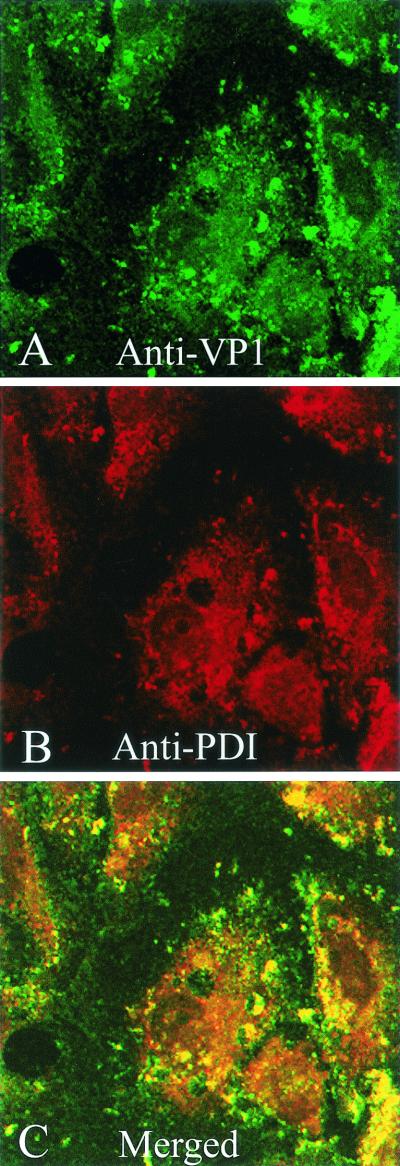
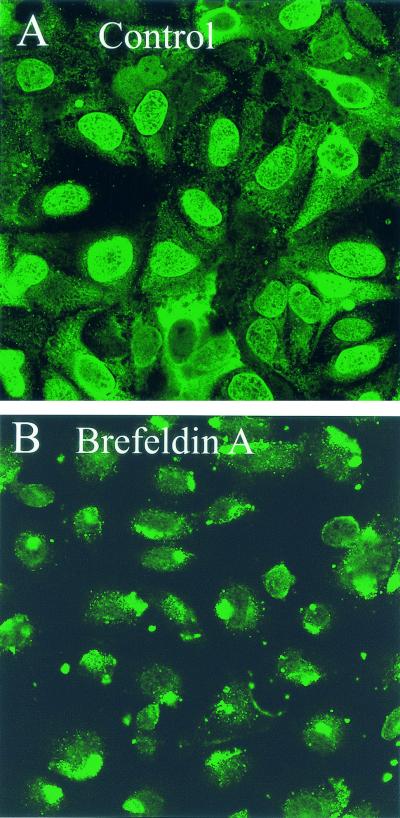
To determine whether the BFA-sensitive pathway of SV40 to the ER is necessary for productive infection, we examined the effect of BFA treatment on infection. BFA treatment essentially prevented infection, as shown by immunofluorescent staining with anti-SV40 antiserum at 24 h (Fig. 5). Indeed, in BFA-treated cells the input virus appeared to accumulate in what is likely the intermediate non-ER compartment (Fig. 5B).
FIG. 5.
Effect of BFA on SV40 productive infection. CV-1 cells were exposed to SV40 at an MOI of 100 PFU per cell for 1 h at 4°C, incubated for 24 h at 37°C, and then immunostained for SV40. (A) Untreated infected cells; (B) treated infected cells, in which BFA (0.5 μg/ml) was added to the cell cultures after 30 min at 37°C and was present thereafter. Cells were viewed using a 40× objective.
β-COP and intracellular SV40 colocalize with caveolin-1.
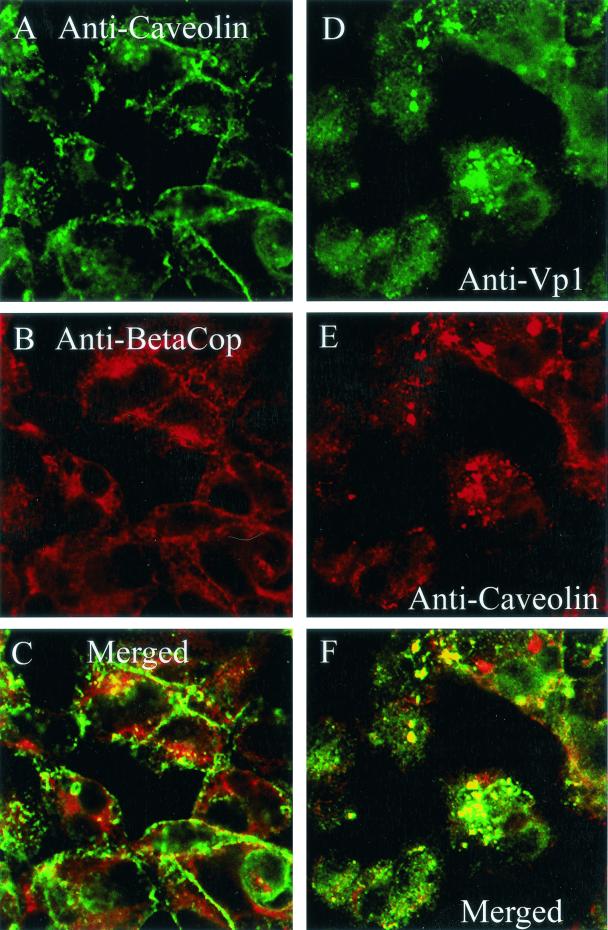
To determine whether the intermediate β-COP-containing compartment might be the caveosome, cells were immunostained for β-COP and caveolin-1 at 5 h postinfection. Parallel samples were immunostained for SV40 and caveolin-1. The confocal micrographs show that some of the β-COP indeed colocalized with intracellular caveolin-1 at 5 h and that most of that caveolin-1 was associated with β-COP (Fig. 6A to C). Additionally, most of the intracellular SV40 was associated with caveolin-1 at 5 h (Fig. 6D to F). Since SV40 also is associated predominantly with its β-COP-containing intermediate compartment at that time (Fig. 2), these results are completely consistent with the likelihood that the β-COP-containing intermediate compartment is the caveosome.
FIG. 6.
Colocalization of β-COP and SV40 with caveolin-1 at 5 h. (A) Cells immunostained for caveolin-1; (B) cells immunostained for β-COP; (C) merge of panels A and B; (D) cells immunostained for SV40; (E) cells immunostained for caveolin-1; (F) merge of panels D and E.
Disassembly of virions in the ER.
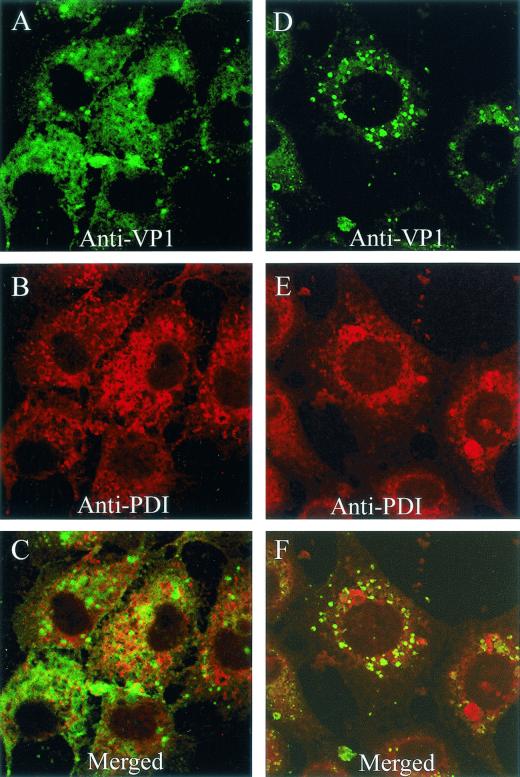
SV40 disassembly was monitored by immunostaining with antibodies specific for epitopes on the internal capsid proteins VP2 and VP3 (60). Since VP2 and VP3 are not exposed on the surface of virus particles (9, 34), the availability of epitopes on these proteins for immunostaining is indicative of particle rearrangement or disassembly. Because the antibodies we used are reactive with VP2 as well as VP3, the immunostained entity is referred to here as VP2/3.
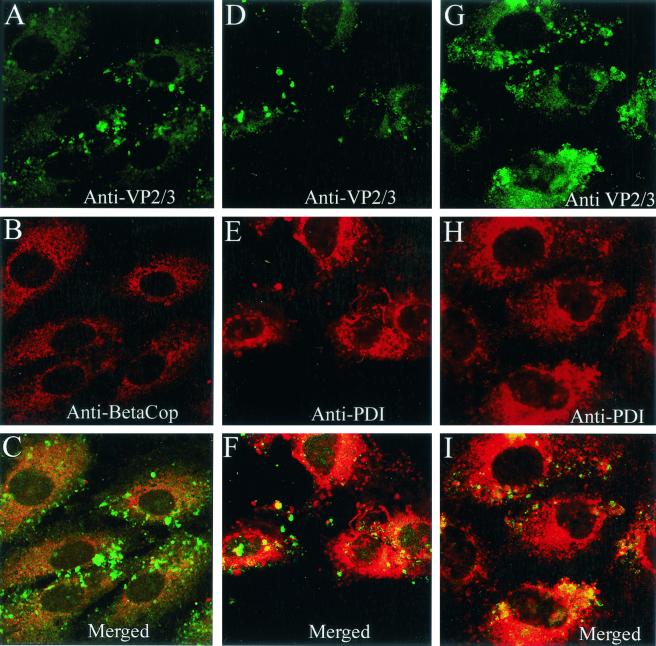
Epitopes on VP2/3 already were exposed for immunostaining at 5 h postinfection (Fig. 7A and D). At that time, internalized SV40 virions still were mostly seen in conjunction with β-COP (Fig. 2). In contrast, the immunostain for VP2/3 at 5 h mostly overlapped the ER, with lesser amounts seen outside the ER (Fig. 7D to F). Virtually no VP2/3 was evident in association with the β-COP-containing compartment at that time (Fig. 7A to C). By 10 h, more VP2/3 was exposed for immunostaining (Fig. 7G). Most of that VP2/3 still was associated with the ER, but some VP2/3 again was seen outside the ER (Fig. 7G to I). Since no VP2/3 was seen in the β-COP-containing compartment, the VP2/3 that is external to the ER is either in the cytosol or some other cellular compartment. Its precise location is not yet clear. In contrast, we have not observed immunostaining for input virus particles or disassociated VP1 outside of the ER, as late as 20 h (Fig. 8). Importantly, immunostaining for VP2/3 first was visible in the ER and was not evident anywhere else at times preceding the arrival of virions in the ER, supporting the premise that SV40 disassembly occurs in the ER.
FIG. 7.
Location of immunostaining for VP2/3 at 5 and 10 h. (A and D) Cells immunostained for VP2/3 at 5 h; (G) cells immunostained for VP2/3 at 10 h; (B) cells immunostained for β-COP; (E and H) cells immunostained for PDI; (C) merge of panels A and B; (F) merge of panels D and E; (I) merge of panels G and H.
FIG. 8.
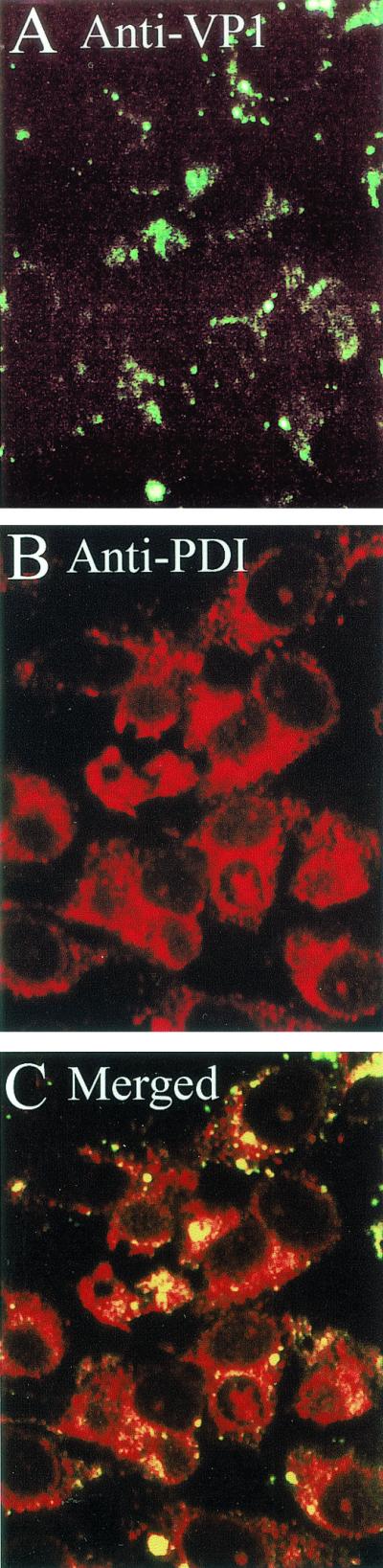
SV40 and the ER at 20 h. CV-1 cells were exposed to SV40 at an MOI of 500 PFU per cell for 1 h at 4°C and then incubated for 20 h at 37°C. Cycloheximide was present to prevent synthesis of progeny virions. (A) Cells immunostained for SV40; (B) cells immunostained for the ER marker PDI; (C) merge of panels A and B. Identical results were obtained in the absence of cycloheximide.
DISCUSSION
SV40 is the first virus that has unequivocally been shown to enter cells by endocytosis mediated by caveolae (1, 10, 53, 58, 65). A particularly noteworthy feature of SV40 endocytosis mediated by caveolae is that the virus is transported to the ER rather than to the usual endosomal-lysosomal compartment (24, 46, 53).
SV40 transport to the ER was recently reported to occur by a two-step process (53). In the first step, the virus enters in caveolin-1-containing vesicles that transport it to a new organelle, the caveolin-1-containing caveosome, which is distinct from endosomes and the Golgi. SV40 is then transported from the caveosome to the ER in caveolin-1-free tubular membranes.
The experimental results presented here also show that transport of SV40 to the ER is a two-step process. First, the virus is transported to a compartment that contains β-COP. That protein is best known as a component of the COPI coatamer complexes that mediate the recycling pathway from the Golgi to the ER (17, 52, 57, 68). SV40 is then transported from the β-COP-containing compartment to the ER. Moreover, transport of SV40 to the ER, and productive infection, are blocked by BFA. This drug is known to impede the Golgi-to-ER recycling pathway by specifically blocking the ARF-1 GTPase, which regulates assembly of COPI coat complexes on membranes (54).
The simplest interpretation of our findings is that the β-COP-containing compartment that SV40 traffics through is indeed the caveosome. This interpretation is supported by our observation that most intracellular caveolin-1 is seen in conjunction with β-COP. In addition, at a time when SV40 was associated with its β-COP-containing intermediate compartment, it also was associated with caveolin-1. The interpretation that this β-COP-containing intermediate compartment is the caveosome, rather than the Golgi, also is supported by the fact that SV40 particles have not been seen in the Golgi, either in thin-section electron micrographs or when entry was followed by dual-color live fluorescence microscopy (24, 53). In the latter study, TGN46 and ManII were used as markers for the trans Golgi network and the cis and medial Golgi, respectively (53). Note that caveolin-1 is preferentially found at the cell surface, where it is associated with caveolae (56). In addition, intracellular caveolin-1 also has been observed and is best known as a component of post-Golgi transport vesicles (26).
Our finding that BFA blocks transport of SV40 to the ER implies that transport from the caveosome to the ER, like retrograde transport from the Golgi to the ER, is dependent on COPI coat complexes and ARF-1 GTPases. Moreover, since productive SV40 infection is blocked by BFA, even under conditions of high MOI, the entry pathway of SV40 via the β-COP-containing compartment indeed is the pathway that leads to productive infection.
As noted above, β-COP is best known for its association with the lateral rims of the cis and medial Golgi cisternae and also for its presence on the buds and vesicles derived from them (17, 21, 40, 49, 68). Our finding that β-COP also is present on the intermediate compartment of the SV40 pathway to the ER, and moreover mediates that pathway, is not the first instance in which the presence and activity of β-COP has been noted on organelles other than the Golgi. In an earlier report, β-COP was found on endosomes, where it appears to function with ARF1 GTPase for delivery of cargo from early endosomes to late endosomes and lysosomes (3, 67). The finding of β-COP on endosomes is not relevant to the entry pathway of SV40, since this virus does not intersect the endosome-lysosome compartment (24, 53). Regardless, β-COP and ARF1 appear to function in several intracellular trafficking pathways, including the pathway utilized by SV40, which involves transport from caveosomes to the ER.
Our results, based on immunostaining for the internal SV40 capsid proteins VP2 and VP3, show that SV40 disassembly occurs in the ER. Whereas dissociation of the carboxy-terminal VP1 arms may be sufficient to expose VP2 and VP3 for immunostaining, we suggest that at least some VP2 and VP3 disassociates from virus particles. This intimation is based on immunostaining for VP2/3 located outside of the ER, whereas immunostaining using anti-SV40 antiserum, which recognizes particles and VP1, remains confined to the ER. Together, these findings are consistent with the premise that some of the VP2/3 molecules completely separate from the VP1 pentamers in the ER and then selectively leave the ER.
The exact location of the non-ER VP2/3 is not yet certain. Nevertheless, we believe it may be in the cytosol. This is consistent with the findings that little, if any, SV40 traffics to endosomes (24, 53) and that no immunostaining for VP2/3 was seen in the intermediate compartment.
The mechanism by which SV40 disassembly occurs is not known. We propose that disassembly, which requires dissociation of high-affinity hydrophobic interactions, might be facilitated by ER chaperones. Chaperone activity was implicated in the formation of the intricate bonding between the C-terminal arms of neighboring VP1 pentamers during SV40 assembly, which occurs in the nucleus (34). The SV40 T antigen might provide that nuclear chaperone activity (64). It is attractive to hypothesize that a chaperone activity likewise is required for the reverse process of disassembly of the VP1 and VP2/3 building blocks, which occurs in the chaperone-rich ER.
Our findings raise the question of how the SV40 minichromosome might be released from the ER. Our working hypothesis is that the myristylated N terminus of VP2 has a role in coupling release of the genome to its transport out of the ER (9), perhaps by forming a membrane channel. Myristylated proteins are found in a variety of other nonenveloped virus families, including picornaviruses (11), rotaviruses (12), reoviruses (43), and polyomaviruses (59, 66), and indeed are associated with viral entry in each instance. Both VP2 and VP3 of SV40 have a DNA binding domain (13) and a nuclear transport signal (14), which together may provide the functions required for nuclear delivery of the SV40 minichromosome. This model is supported by the findings that antibodies against VP2 and VP3 in the cytosol block infection and that microinjected minichromosomes alone do not enter the nucleus (42).
Although SV40 is the first virus shown to enter cells via caveolae (1, 10, 53, 58, 65), evidence is accumulating which indicates that other viruses may also enter in this way (e.g., see references 38 and 55). Human immunodeficiency virus can enter cells using the related lipid raft microdomains (37), and the example of secreted CT was noted above. In addition, a variety of other obligate intracellular pathogens, including protozoa and bacteria, are now known to enter cells via caveolae or caveolae-like raft domains (reviewed in reference 45). Those pathogens include the protozoa Toxoplasma gondii (41) and Plasmodium falciparum (28) and the bacteria Campylobacter jejuni (69), FimH-expressing E. coli (5, 61), and serovar K of Chlamydia trachomatis (48). Each of these intracellular protozoan and bacterial pathogens must find an appropriate niche within the host cell in which they can survive and propagate. Importantly, the endocytic vesicles or inclusions that contain these pathogens avoid intersecting the endosomal-lysosomal compartment and the potentially lethal effects of acidification and lysosomal hydrolases. Together, these findings suggest that caveola-mediated entry may indeed be a determinant of the atypical intracellular targeting of these parasites, bacteria, viruses, and secreted toxins (45). Furthermore, this targeting provides an advantage in each instance. It facilitates intracellular survival of the protozoan and bacterial pathogens (19, 45), activation of CT (31), dissemination of human immunodeficiency virus by transcytosis across the vascular endothelia (22), disassembly of SV40, and perhaps nuclear transport of the viral minichromosome, as discussed here. Thus, clarification of the entry pathways of the several pathogens noted above should provide important new insights into the interactions of these microbes with their host cells and the development of rational therapies. Moreover, SV40 will be a valuable probe for the study of what is apparently a unique new pathway for endocytosis mediated by caveolae.
Acknowledgments
This work was supported by a Faculty Research Grant from the University of Massachusetts and by the Cooperative State Research Extension, U.S. Department of Agriculture, Massachusetts Agricultural Experiment Station. A.O. was supported by the Israeli Science Foundation, founded by the Israeli Academy of Science and Humanities. The University of Massachusetts Central Microscopy Facility is supported by a grant from the National Science Foundation (NSF BBS 8714235).
We are grateful to Lukas Pelkmans for communicating the results of his research prior to publication.
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, R. G. W. 1998. The caveolae membrane system. Annu. Rev. Biochem. 67:199-225. [DOI] [PubMed] [Google Scholar]
- 3.Aniento, F., F. Gu, R. G. Parton, and J. Gruenberg. 1996. An endosomal βCOP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 133:29-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atwood, W. J., and L. C. Norkin. 1989. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J. Virol. 63:4474-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baorto, D. M., Z. Gao, R. Malaviya, M. L. Dustin, A. van der Merwe, D. M. Lublin, and S. N. Abraham. 1997. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389:636-639. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., and Harrison, S. C. 1994. Interactions among the major and minor coat proteins of polyomavirus. J. Virol. 68:3982-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breau, W. C., W. J. Atwood, and L. C. Norkin. 1992. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J. Virol. 66:2037-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 9.Chen, X. S., T. Stehle, and S. C. Harrison. 1998. Interaction of polyomoavirus internal protein VP2 with the major capsid protein VP1 and implications for participation in viral entry. EMBO J. 17:3233-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y., and L. C. Norkin. 1999. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp. Cell Res. 246:83-90. [DOI] [PubMed] [Google Scholar]
- 11.Chow, M., J. F. E. Newman, D. Filman, J. M. Hogle, B. J. Rowlands, and F. Brown. 1987. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature 327:484-486. [DOI] [PubMed] [Google Scholar]
- 12.Clark, B., and U. Desselberger. 1988. Myristylation of rotavirus proteins. J. Gen. Virol. 69:2681-2686. [DOI] [PubMed] [Google Scholar]
- 13.Clever, J., D. Dean, and H. Kasamatsu. 1993. Identification of a DNA binding domain in simian virus 40 capsid proteins VP2 and VP3. J. Biol. Chem. 268:20877-20883. [PubMed] [Google Scholar]
- 14.Clever, J., and H. Kasamatsu. 1991. Simian virus 40 VP2/3 small structural proteins harbor their own nuclear transport signals. Virology 181:78-90. [DOI] [PubMed] [Google Scholar]
- 15.Clever, J., M. Yamada, and H. Kasamatsu. 1991. Import of simian virus 40 virions through nuclear pore complexes. Proc. Natl. Acad. Sci. USA 88:7333-7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dangoria, N. S., W. C. Breau, H. A. Anderson, D. A. Cishek, and L. C. Norkin. 1996. Extracellular simian virus 40 induces an ERK/MAPK-independent signaling pathway that activates primary response genes and promotes virus entry. J. Gen. Virol. 77:2173-2182. [DOI] [PubMed] [Google Scholar]
- 17.Duden, R., G. Griffiths, R. Frank, P. Argos, and T. E. Kreis. 1991. β-COP, a 110 kD protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to β-adaptin. Cell 64:649-655. [DOI] [PubMed] [Google Scholar]
- 18.Feldherr, C. M., and D. Akin. 1990. The permeability of the nuclear envelope in dividing and nondividing cell culture. J. Cell Biol. 111:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fra, A. M., E. Williamson, K. Simons, and R. G. Parton. 1995. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc. Natl. Acad. Sci. USA 92:8655-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths, G., R. Pepperkok, J. K. Locker, and T. E. Kreis. 1995. Immunocytochemical localization of β-COP to the ER-Golgi boundary and the TGN. J. Cell Sci. 108:2839-2856. [DOI] [PubMed] [Google Scholar]
- 22.Gujulova, C., A. R. Burns, T. Pushkarsky, W. Popik, O. Berger, M. Bukrinsky, M. C. Graves, and M. Fiala. 2001. HIV-1 penetrates coronary artery endothelial cells by transcytosis. Mol. Med. 7:169-176. [PMC free article] [PubMed] [Google Scholar]
- 23.Hummeler, K., N. Tomassini, and F. Sokol. 1970. Morphological aspects of the uptake of simian virus 40 by permissive cells. J. Virol. 6:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kartenbeck, J., H. Stukenbrok, and A. Helenius. 1989. Endocytosis of simian virus 40 into the endoplasmic reticulum. J. Cell Biol. 109:2721-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klausner, R. D., J. G Donaldson, and J. Lippincott-Schwartz. 1992. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116:1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurzchalia, T. V., P. Dupree, R. G. Parton, R. Kellner, H. Virta, N. Lehnert, and K. Simons. 1992. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J. Cell Biol. 118:1003-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurzchalia, T. V., and R. G. Parton. 1999. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11:424-431. [DOI] [PubMed] [Google Scholar]
- 28.Lauer, S., J. VanWye, T. Harrison, H. McManus, B. U. Samuel, N. L. Hiller, N. Mohandas, and K. Haldar. 2000. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lencer, W. I., J. B. de Almeida, S. Moe, J. L. Stow, D. A. Ausiello, and J. L. Madara. 1993. Entry of cholera toxin into polarized human intestinal epithelial cells: identification of an early brefeldin A-sensitive event required for peptide A1 generation. J. Clin. Investig. 92:2941-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lencer, W. I., C. Constable, S. Moe, M. G. Jobling, H. M. Webb, S. Ruston, J. L. Madara, T. R. Hirst, and R. K. Holmes. 1995. Targeting of cholera toxin and Escherichia coli heat-labile toxin in polarized epithelia: role of COOH-terminal KDEL. J. Cell Biol. 131:951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lencer, W. I., T. R. Hirst, and R. K. Holmes. 1999. Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta 1450:177-190. [DOI] [PubMed] [Google Scholar]
- 32.Letourneur, F., E. C. Gaynor, S. Hennecke, C. Demolliere, S. D. Emre, H. Riezman, and P. Crosson. 1994. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 79:1199-1207. [DOI] [PubMed] [Google Scholar]
- 33.Li, S., K. S. Song, S. S. Koh, A Kikuchi, and M. P. Lisanti. 1996. Baculovirus-based expression of caveolin in Sf21 insect cells. A model system for the biochemical and morphological study of caveolae biogenesis. J. Biol. Chem. 271:28647-28654. [DOI] [PubMed] [Google Scholar]
- 34.Liddington, R. C., Y. Yan, H. C. Zhao, R. Sahli, T. L. Benjamin, and S. C. Harrison. 1991. Structure of simian virus 40 at 3.8 Å resolution. Nature 354:278-284. [DOI] [PubMed] [Google Scholar]
- 35.Lisanti, M. P., P. E. Scherer, L. Z. Tang, and M. Sargiacomo. 1994. Caveolae, caveolin, and caveolin-rich membrane domains: a signaling hypothesis. Trends Cell Biol. 123:595-604. [DOI] [PubMed] [Google Scholar]
- 36.Majoul, I., K. Sohn, T. H. Wieland, R. Pepperkok, M. Pizza, J. Hilleman, and H.-D. Soling. 1998. KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit involves COP1, p23, and the COOH-terminus of Erd2p. J. Cell Biol. 143:601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and C. Martinez-A. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of Echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh, J., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36:107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Menarguez, J. A., H. J. Geuze, J. W. Slot, and J. Klumperman. 1999. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell 98:81-90. [DOI] [PubMed] [Google Scholar]
- 41.Mordue, D. G., N. Desai, M. Dustin, and L. D. Sibley. 1999. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med. 190:1783-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakanishi, A., J. Clever, M. Yamada, P. P. Li, and H. Kasamatsu. 1996. Association with capsid proteins promotes nuclear targeting of simian virus 40. Proc. Natl. Acad. Sci. USA 93:96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nibert, M. L., L. A. Schiff, and B. N. Fields. 1991. Mammalian reoviruses contain a myristylated structural protein. J. Virol. 65:2372-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norkin, L. C. 1999. Simian virus 40 infection via MHC class I molecules and caveolae. Immunol. Rev. 168:13-22. [DOI] [PubMed] [Google Scholar]
- 45.Norkin, L. C. 2001. Caveolae in the uptake and targeting of infectious agents and secreted toxins. Adv. Drug Deliv. Rev. 49:301-315. [DOI] [PubMed] [Google Scholar]
- 46.Norkin, L. C., and H. A. Anderson. 1996. Multiple stages of virus receptor interactions as shown by simian virus 40, p. 159-167. In I. Kahane and I. Ofek. (ed.), Toward anti-adhesion therapy for microbial diseases. Plenum Press, New York, N.Y.
- 47.Norkin, L. C., and K. H. Eink. 1978. Cell killing by simian virus 40: protective effect of chloroquine. Antimicrob. Agents Chemother. 14:930-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norkin, L. C., S. A. Wolfrom, and E. S. Stuart. 2001. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp. Cell Res. 266:229-308. [DOI] [PubMed] [Google Scholar]
- 49.Oprins, A., R. Duden, T. E. Kreis, H. J. Geuze, and J. W. Slot. 1993. β-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J. Cell Biol. 121:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orlandi, P. A., P. K. Curran, and P. H. Fishman. 2016. 1993. Brefeldin A blocks the response of cultured cells to cholera toxin: implications for intracellular trafficking in toxin action. J. Biol. Chem. 268:12010-12011. [PubMed] [Google Scholar]
- 51.Orlandi, P. A., and P. H. Fishman. 1998. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141:905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pelham, H. R. B. 1998. Getting through the Golgi complex. Trends Cell Biol. 8:45-49. [DOI] [PubMed] [Google Scholar]
- 53.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a novel two-step vesicular transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 54.Peyroche, A., B. Antonny, S. Robineau, J. Acker, J. Cherfils, and C. L. Jackson. 1999. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol. Cell 3:275-285. [DOI] [PubMed] [Google Scholar]
- 55.Richterova, Z., D. Lieble, M. Horak, Z. Palkova, J. Stokrova, P. Hozak, J. Korb, and J. Forstova. 2001. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VPI pseudocapsids toward cell nuclei. J. Virol. 75:10880-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothberg, K. G., J. E. Heuser, W. C., Donzell, Y. S. Ying, J. R. Glenney, and R. G. Anderson. 1992. Caveolin, a protein component of caveolae protein coats. Cell 68:673-682. [DOI] [PubMed] [Google Scholar]
- 57.Rothman, J. E., and L. Orci. 1992. Molecular dissection of the secretory pathway. Nature 355:409-415. [DOI] [PubMed] [Google Scholar]
- 58.Roy, S., R. Luetterforst, A. Harding, A. Apolloni, M. Etheridge, E. Stang, B. Rolls, J. F. Hancock, and R. G. Parton. 1999. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1:98-105. [DOI] [PubMed] [Google Scholar]
- 59.Sahli, R., R. Freund, T. Dubensky, R. Garcea, R. Bronson, and T. Benjamin. 1993. Defect in entry and altered pathogenicity of a polyoma virus mutant blocked in VP2 myristylation. Virology 192:142-153. [DOI] [PubMed] [Google Scholar]
- 60.Sandalon, Z., and A. Oppenheim. 1997. Self-assembly and protein-protein interactions between the SV40 capsid proteins produced in insect cells. Virology 237:414-421. [DOI] [PubMed] [Google Scholar]
- 61.Shin, J.-S., Z. Gao, and S. N. Abraham. 2000. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785-788. [DOI] [PubMed] [Google Scholar]
- 62.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 63.Sonnichsen, B., R. Watson, H. Clausen, T. Misteli, and G. Warren. 1996. Sorting by COP1-coated vesicles under interphase and mitotic conditions. J. Cell Biol. 134:1411-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Srinivasen, A., A. J. McClellan, J. Vartikar, I. Marks, P. Cantalupo, Y. Li, P. Whyte, K. Rundell, J. L. Brodsky, and J. M. Pipas. 1997. The amino terminal transforming region of simian virus 40 large T antigen and small t antigens functions as a J domain. Mol. Cell. Biol. 17:4761-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stang, E., J. Kartenbeck, and R. G. Parton. 1997. Major histocompatibility class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell 8:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Streuli, C. H., and B. E. Griffin. 1987. Myristic acid is coupled to a structural protein of polyoma virus and SV40. Nature 326:619-622. [DOI] [PubMed] [Google Scholar]
- 67.Whitney, J. A., M. Gomez, D. Sheff, T. E. Kreis, and I. Mellman. 1995. Cytoplasmic coat proteins involved in endosome function. Cell 83:703-713. [DOI] [PubMed] [Google Scholar]
- 68.Wieland, F., and C. Harter. 1999. Mechanisms of vesicle formation: insights from the COP system. Curr. Opin. Cell Biol. 11:440-446. [DOI] [PubMed] [Google Scholar]
- 69.Wooldridge, K., P. H. Williams, and J. M. Ketley. 1996. Host signal transduction and endocytosis of Campylobacter jejuni. Microb. Pathog. 21:299-305. [DOI] [PubMed] [Google Scholar]