Abstract
Background: Candida albicans is the causative agent of oral and vaginal candidiasis. Innate host defenses against C. albicans are important against each infection. Among these are oral and vaginal epithelial cells that have anti-Candida activity. The mechanism of action includes a requirement for cell contact with no role for soluble factors, and a putative role for carbohydrates based on the sensitivity of the activity to periodic acid.
Methods: Periodic acid treatment of epithelial cells as well as the property of partial resistance of antifungal activity to fixation was used to further dissect the mechanism of action.
Results: The results herein effectively now challenge a role for carbohydrates alone. Firstly, the putative carbohydrate(s) released into supernatants of periodic acid-treated epithelial cells could not compete with fresh epithelial cells for activity, and equivalent abrogation of activity was observed by periodic acid-treated cells irrespective of the amount of carbohydrate released. Instead, the similar abrogation of activity following treatment with other acids or when cocultured under acidic conditions suggests that the activity is acid-labile. Finally, while activity requires intact epithelial cells, it does not require live cells; activity was minimally affected by fixing epithelial cells prior to coculture where the majority of cells remained impermeable to Trypan blue but were defined as non–viable by positive nuclear staining with propidium iodide.
Conclusion: These results suggest that antifungal activity is dependent on contact by intact, but not necessarily live, epithelial cells through an acid-labile mechanism.
Keywords: Candida albicans, epithelial cells, innate immunity, oral mucosa, vaginal mucosa
Candida albicans, the causative agent in the majority of cases of mucosal candidiasis, is a dimorphic fungal organism that is both a commensal of the gastrointestinal and reproductive tracts and an opportunistic pathogen of the same mucosal tissues (11, 12). Oropharyngeal candidiasis (OPC) is a significant problem in immunocompromised individuals and is extremely common during human immunodeficiency virus (HIV) infection, especially when CD4+ T cells are reduced (8, 9). Vulvovaginal candidiasis (VVC) affects 75% of women at least once in their reproductive years, and it is equally common in both immunocompetent and immunocompromised women (11, 12).
Host defense mechanisms against oral and vaginal candidiasis are poorly understood. While cell-mediated immunity by Th1-type CD4+ T cells is considered a critical host defense mechanism against mucosal Candida infections, innate mechanisms are considered to have protective roles as well. We recently reported that epithelial cells from the oral mucosa of humans and from the vaginal mucosa of humans, nonhuman primates, and mice inhibit the growth of C. albicans in vitro at relatively low effector to target (E : T) ratios (6, 13, 15, 16). Compared to vaginal epithelial cells, oral epithelial cells have significantly greater activity at lower E : T ratios (13, 15). Clinically, epithelial cell anti-Candida activity has been found to be reduced in HIV-infected persons with OPC (13) and in women with recurrent VVC (1), indicating that epithelial cells represent a functional/active innate host defense mechanism against C. albicans at the oral and vaginal mucosa.
Studies on the mechanism of the anti-Candida activity demonstrated that both oral and vaginal epithelial cells have a similar strict requirement for cell contact with C. albicans with no demonstrable role for soluble factors (13, 15). Additionally, epithelial cell anti-Candida activity is resistant to gamma-irradiation, partially resistant to fixation, and not mediated by phagocytosis, oxidative mechanisms, or nonoxidative mechanisms such as defensins or calprotectins. In contrast, the activity is sensitive to heat and detergents, suggesting a requirement for intact cells (13-15). Furthermore, the antifungal activity is static, not cidal (10). Studies to identify the effector moiety demonstrated that treatments to remove membrane proteins and lipid moieties had no effect on the epithelial cell antifungal activity. In contrast, the antifungal activity was sensitive to treatment with periodic acid, suggesting a role for a surface carbohydrate (10, 14). To date, the putative membrane carbohydrate moiety or moieties have yet to be identified (10, 14). The purpose of the present study was to further define the mechanism by which epithelial cells exert their anti-Candida activity and the role for carbohydrates.
Materials and methods
Human subjects
Oral epithelial cells were obtained exclusively from healthy volunteers. Informed consent was obtained from each participant, and all procedures were conducted in accordance with the guidelines of the Institutional Review Board at Louisiana State University Health Sciences Center.
Vaginal epithelial cells that have been shown previously to have antifungal activity (2) were obtained from cervical vaginal lavage samples collected from middle adolescent females (mean age of 15.4 ± 0.9) enrolled as part of the Mid-America Adolescent Sexually Transmitted Disease (STD) Clinical Research Center Young Women's Project. Informed consent was obtained from all participants as well as permission from the accompanying parent/guardian for entry into the study. All procedures were followed in the conduct of clinical research in accordance with the Institutional Review Boards at Louisiana State University Health Sciences Center, New Orleans, LA, and Indiana University Medical Center, Indianapolis, IN. Participants were enrolled and the specimens were collected at Indiana University Medical Center. Specimens were subsequently shipped overnight to Louisiana State University Health Sciences Center, where they were processed and stored. None of the samples used for this study was from subjects with an STD.
Mice
Female CBA/J (H-2K) mice (6–8 weeks old), purchased from the National Cancer Institute, Frederick, MD, were used throughout these studies. All animals were housed and handled according to institutionally recommended guidelines.
Human vaginal epithelial cell line
A human vaginal epithelial cell line (VK2; R. Fichorova, Harvard Medical School, Boston, MA) was used (5). The VK2 cell line, immortalized with human papilloma-virus 16 E6E7, was maintained in keratinocyte serum-free medium (Life technologies, Gaithersburg, MD) supplemented with 50 μg/ml bovine pituitary extract, 0.1 ng/ml epidermal growth factor, 100 U/ml penicillin, and 100 μg/ml streptomycin and passaged every 3–4 days.
Oral epithelial cell isolation
Human oral epithelial cells were isolated as previously described (14, 15). Briefly, 10–15 ml of unstimulated saliva from each participant was expectorated into a polypropylene centrifuge tube and centrifuged at 800 × g for 5 min. The cell pellet was washed with sterile phosphate-buffered saline (PBS), resuspended in Hanks' balanced salt solution (HBSS) (Life technologies), and passed over a 20 μm sterile nylon membrane (Small Parts Inc., Miami Lakes, FL). The epithelial cell-enriched population collected from the membrane was washed, resuspended in cryopreservative solution (50% fetal bovine serum [FBS], 25% RPMI 1640 tissue culture medium, 15% dimethyl sulfoxide), and stored at − 70°C until use. At the time of use, the cells were thawed, washed twice in PBS, and enumerated by Trypan blue dye exclusion. Viability was consistently 60–85% before and after freezing.
Vaginal epithelial cell isolation
Mouse vaginal epithelial cells were isolated as previously described (10). Briefly, vaginae from sacrificed mice were excised and placed into a sterile glass Petri dish containing 10 ml of dispase I-neutral protease (0.25 mg/ml; Roche Diagnostics, Mannheim, Germany) and incubated on a shaking platform (speed, 110 r.p.m.) at 4°C for 8 h. The epithelial sheets were removed from the vaginal tissue with forceps, minced with a sterile scalpel, and placed in a 50 ml sterile tube. The remaining dispase solution was added to the cellular suspension and centrifuged at 800 × g for 5 min. The pellet was resuspended in 2 ml of 10 × Trypsin-EDTA (Sigma, St. Louis, MO), and incubated at 37°C for 10 min. The resulting suspension was sheared using an 18-gauge needle (10 times), followed by a similar procedure using a 21-gauge needle. The cells were washed twice with sterile PBS and enumerated by Trypan blue dye exclusion. Viability was consistently 60–85%.
Human vaginal epithelial cells were isolated as previously described (1). Briefly, a vaginal lavage sample that consisted of 5 ml of sterile physiological saline continuously aspirated for 30–40 s was collected from each subject. The vaginal lavage was processed by centrifugation, and the cell pellet was stored at − 70°C in cryopreservative medium until use. At the time of the assay, the cellular fraction was enriched for epithelioid cells by density gradient centrifugation, as described elsewhere (16). The enriched population was resuspended in PBS and counted by Trypan blue dye exclusion. Viability was generally more than 80%.
Target cells
C. albicans 3153 A from the National Collection of Pathogenic Fungi (London, UK) was grown on Sabouraud dextrose agar (Becton Dickinson, Sparks, MD) at 34°C. One colony was used to incubate 10 ml of phytone-peptone (PP) broth (Becton Dickinson) supplemented with 0.1% glucose for 18 h at 25°C in a shaking water bath. The blastoconidia were collected, washed with PBS, and enumerated on a hemacytometer using Trypan blue dye exclusion.
Growth inhibition
[3H]-glucose uptake
The growth inhibition assay was performed as previously described (13-15). Briefly, stationary-phase blastoconidia were added to individual wells of a 96-well microtiter plate (Costar, Cambridge, MA) at 1 × 105 cells/ml in a volume of 100 μl of PP broth supplemented with 10% FBS and 1% penicillin (100 U/ml) and streptomycin (100 μg/ml). Epithelial cells were added to triplicate wells in a volume of 100 μl of PP broth at an effector to target ratio of 5 : 1, which was found to be an optimal E : T ratio for this assay (13). Controls included effector cells and target cells cultured alone. The cultures were incubated for 9 h at 37°C in 5% CO2 in the presence of 1 μCi [3H]-glucose (ICN, Costa Mesa, CA). Following the incubation, 100 μl of sodium hypochlorite solution (bleach) was added to all wells and left for 5 min, and the cell extracts were harvested onto glass fiber filters using a PHD cell harvester (Cambridge Technologies, Watertown, MA). The incorporated [3H]-glucose was measured by liquid scintillation. The incorporation of glucose by Candida and epithelial cells during the 9 h incubation was generally 15,000–30,000 cpm and 500–2000 cpm, respectively. The percent growth inhibition was calculated as follows: % growth inhibition = 1 − [(mean experimental cpm − mean effector cpm)/mean Candida cpm] × 100.
Quantitative plate count
Experiments involving periodic acid-treated epithelial cells require a quantitative plate count method to measure the growth inhibition of C. albicans due to high constitutive [3H]-glucose uptake by periodic acid-treated epithelial cells (13-15). Briefly, effector and target cell cocultures were prepared as described above in the absence of [3H]-glucose. Following the 9-h incubation, 100 μl of 0.3% Triton X-100 was added to each well, and adherent Candida cells were scraped from the bottom surface of the wells with a pipette tip. The contents of each well were serially diluted (1 : 10) and plated on Sabouraud dextrose agar. CFU counts were determined after 48 h incubation at 34°C. Wells containing C. albicans alone were included as controls. The percent growth inhibition was calculated as follows: % growth inhibition = (1 − experimental CFU/Candida CFU) × 100.
Epithelial cell treatments
For examination of carbohydrate moieties, epithelial cells were pretreated with periodic acid (Fisher Scientific, Fair Lawn, NJ) (5 mm, 15 min at 37°C). Controls included epithelial cells incubated in PBS alone. Following the incubation, the cells were washed with PBS and cell viability was assessed by Trypan blue dye exclusion and confirmed by propidium iodide (PI) and fluorescein diacetate (FDA) vital staining. For PI/FDA staining, FDA (50 μg/ml, stains live cells) and PI (1 μg/ml, stains dead cells) (Sigma) (3, 8, 10) were added simultaneously to the epithelial cell pellets and placed in the dark for 30 min at room temperature. Following the incubation, the epithelial cells were washed with PBS followed by a second wash with 20% FBS and then a final wash with PBS. The pellets were resuspended with 100 μl of PBS, and 5 μl of each cell suspension were added to a slide and examined under fluorescent microscopy (Nikon Instruments, Melville, NY). Viable cells (5 × 105 cells/ml) were used in the quantitative plate count assay to measure growth inhibition activity. To confirm carbohydrate release, the supernatants from acid-treated and control cells were assayed for total carbohydrate content by the Dubois assay (4). Briefly, 60 μl of 5% phenol and 540 μl of concentrated sulfuric acid (both from Fisher) were added to undiluted supernatants and standards (640 μg/ml of mannose serially diluted 1 : 2) for 5 min at room temperature. The absorbance values were determined at 490 nm by a Ceres 900 automated microplate reader (Bio-Tek, Winooski, VT).
Competitive inhibition studies
To examine whether the putative carbohydrate moiety could compete with fresh epithelial cells for binding to Candida, blastoconidia (1 × 105 cells/ml) were incubated with supernatants from epithelial cells treated with periodic acid as above. The supernatants were dialyzed against PBS (Slide-A-lyzer Dialysis Cassette, 7000 molecular weight cutoff; Pierce, Rockford, IL) before incubation with blastoconidia. The incubation with Candida was either conducted for 30 min at 37°C, the cells washed, and added to fresh epithelial cells in the standard [3H]-glucose assay (pretreatment), or remained with Candida to where fresh epithelial cells were added along with [3H]-glucose assay (direct treatment). Controls included Candida similarly treated with dialyzed periodic acid alone or supernatants from epithelial cells treated with PBS instead of periodic acid.
Effects of other acids on epithelial cell activity
To further examine the issue of an effector carbohydrate moiety, epithelial cells (5 × 105 cells/ml) were pretreated with trifluoromethane sulfonic acid (TFMS) (Sigma) or hydrochloric acid (HCl) (Fisher), (5, 1, 0.1 mM, 15 min at 37°C). Controls included epithelial cells incubated in PBS alone. Cell viability by Trypan blue dye exclusion, growth inhibition by quantitative plate count, and carbohydrate release were measured in parallel following the periodic acid-treatment. In addition, the growth inhibition assay was performed in an acidic medium to examine the effects of acidity on epithelial cells during the 9 h coculture period. PP broth was adjusted to pH 4.2 with HCl. The acidified PP broth was used for the [3H]-glucose uptake assay as described above. Controls included the standard PP broth (pH 7.2).
Viability requirement of epithelial cell activity
To examine viability requirements for epithelial cell activity, epithelial cells were fixed with 1% paraformaldehyde for 15 min at 37°C (Sigma). Controls included epithelial cells treated with PBS alone (unfixed). After fixation, epithelial cells were washed 3 times with PBS and cell viability was measured by Trypan blue dye exclusion and vital staining with PI/FDA. The standard [3H]-glucose uptake assay was conducted on fixed and unfixed cells. In other studies, fixed and unfixed epithelial cells were left in culture medium (PP broth) for 4 days at 37°C before [3H]-glucose uptake assay was conducted. Cell viability as measured by Trypan blue dye exclusion and PI/FDA vital staining was used to monitor viability before and after culture.
Statistical analysis
The unpaired Student's t-test was used to analyze most data. A correlation coefficient was determined for the scatter plot together with the P-value by a regression analysis. Significant differences were defined at a confidence level where P was < 0.05. All statistics were evaluated using Prism Software (Graph Pad, San Diego, CA).
Results
Role of the putative effector moiety in the growth inhibition activity
Based on the fact that epithelial cells potentially mediate their inhibitory activity against Candida through a putative carbohydrate moiety, we sought to further investigate the properties of this cell surface moiety in the anti-Candida activity.
We first examined whether the periodic acid-derived moiety could compete with fresh oral epithelial cells to inhibit Candida. Results in Fig. 1 show that fresh epithelial cells had similar activity against Candida treated with periodic acid-treated cell supernatants, PBS supernatants or periodic acid alone, both pretreated and treated throughout the coculture period (P > 0.05). Carbohydrates in the supernatants of treated epithelial cells included 1 ± 1 μg/ml for PBS-treated cell supernatants vs. 22 ± 1 μg/ml for periodic acid-treated cell supernatants. Periodic acid treatment was confirmed to reduce the epithelial cell activity (78 ± 9% for PBS treatment vs. 3 ± 3% inhibition for periodic acid treatment). Similar results were demonstrated for primary vaginal epithelial cells or a vaginal epithelial cell line (data not shown). Together, these results began to challenge a role for carbohydrate as the effector moiety.
Fig. 1.
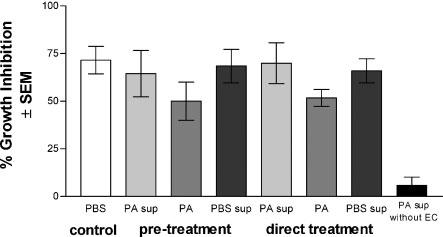
The putative carbohydrate cannot compete with fresh epithelial cells for anti-Candida activity. Whole unstimulated saliva was collected from healthy volunteers (n = 3), and epithelial-enriched populations were isolated by nylon membrane retention. Candida was either pretreated with epithelial cell supernatants containing putative carbohydrates (PA sup) prior to the coculture with fresh epithelial cells (pretreatment), or treated throughout the coculture period (direct treatment). Controls included similar treatment with supernatants from PBS-treated cells (PBS sup) and periodic acid alone (PA). Figure shows cumulative data from three separate experiments. EC, epithelial cells. SEM, standard error of the mean.
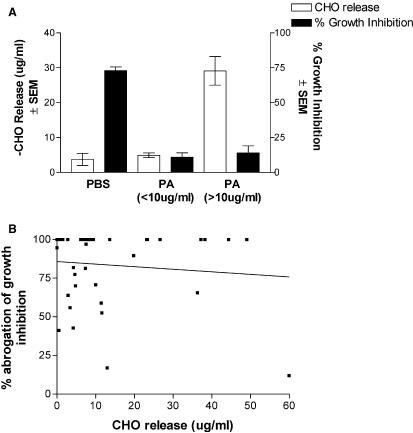
Periodic acid-treatment and moiety stripping vs. growth inhibition
To continue to address the role of carbohydrate and the amounts of carbohydrates released by periodic acid on anti-Candida activity, stratified data of carbohydrates released by periodic acid-treated oral epithelial cells were compared with results in growth inhibition. The results in Fig. 2A show that anti-Candida activity of periodic acid-treated epithelial cells was equally abrogated under low (< 10 μg/ml, n = 25 experiments) or high (> 10 μg/ml, n = 14 experiments) carbohydrate release compared to PBS-treated cells (P < 0.0001) that had minimal carbohydrate release. This was confirmed further by a scatter plot and regression analysis, showing a lack of a positive correlation between carbohydrate release and abrogation of growth inhibitory activity (r2 = 0.010, P = 0.53) (Fig. 2B).
Fig. 2.
Abrogation of growth inhibition is independent of the amount of carbohydrate release. A retrospective study was conducted from experiments using whole unstimulated saliva collected from healthy volunteers, and the resulting oral epithelial-enriched populations were treated with periodic acid (PA) and examined for in vitro growth inhibition. Carbohydrate release was measured by the Dubois assay. A) Percent growth inhibition for high (> 10 μg/ml) and low (< 10 μg/ml) carbohydrate release. B) Scatter plot of carbohydrate release vs. percent abrogation of growth inhibition. Figure shows cumulative data from 39 separate experiments. SEM, standard error of the mean; CHO, carbohydrate.
Effect of other acids on antifungal activity
We next evaluated whether the abrogation of growth inhibition was generally acid-labile rather than specific to periodic acid. The results in Fig. 3 show that anti-Candida activity of periodic acid-, TFMS- or HCl-treated epithelial cells was equally abrogated compared to PBS-treated epithelial cells (P < 0.0001). In these experiments, epithelial cell viability was similar under each acid treatment (65–90%), but neither TFMS nor HCl released significant amounts of carbohydrates from the cells as per the standard carbohydrate assay. Similar results were observed for vaginal epithelial cells (data not shown). As an additional means to examine the effects of acidity on epithelial cells, the [3H]-glucose uptake assay was performed in an acidified PP broth (pH 4.2). The results showed that the growth inhibition of Candida by oral epithelial cells was significantly reduced under the acidic condition (69 ± 7% for pH 7.2 vs. 33 ± 8% for pH 4.2 at a 10 : 1 E : T ratio, P < 0.05). Although Candida was restricted to blastoconidia in the acidic environment, growth of Candida alone was not affected by the acidic medium as detected by [3H]-glucose uptake (data not shown).
Fig. 3.
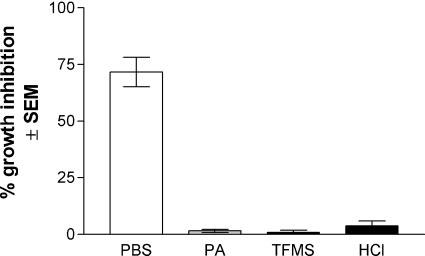
Effect of several acids on epithelial cell anti-Candida activity. Whole unstimulated saliva was collected from healthy volunteers (n = 4), and epithelial-enriched populations were treated with periodic acid (PA), trifluoromethane sulfonic acid (TFMS) or hydrochloric acid (HCl) and examined for in vitro growth inhibition of C. albicans by quantitative plate count. Figure shows cumulative data from four separate experiments. SEM, standard error of the mean.
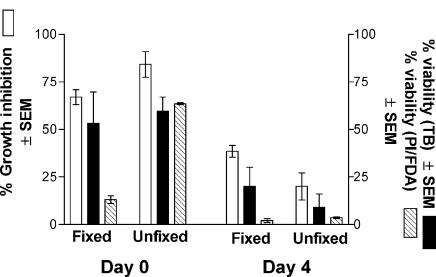
Viability requirement of epithelial cell activity
Previous studies showed that the epithelial cell anti-Candida activity was partially resistant to fixation (14). This property was examined more closely to elucidate the viability (permeability) requirement for the antifungal activity. For this, oral epithelial cells were fixed with 1% paraformaldehyde as previously described (14), and cell viability was determined by PI/FDA vital staining in addition to Trypan blue dye exclusion. As the images in Fig. 4 show, the majority (∼ 75%) of both fixed and unfixed cells were impermeable to Trypan blue and thus deemed viable by definition, whereas by PI/FDA staining, 16% of fixed and 82% of unfixed cells were viable by definition. Irrespective, significant anti-Candida activity was observed for both fixed (77%) and unfixed (97%) epithelial cells. In a subsequent study, fixed and unfixed cells were tested immediately after fixation and following 4 days of culture. Results in Fig. 5 show that equally high anti-Candida activity was observed by fixed and unfixed epithelial cells on day 0 when permeability by Trypan blue was low, irrespective of that by PI, whereas the activity was significantly reduced on day 4, when cell permeability measured by Trypan blue dye exclusion was substantially increased for both fixed and unfixed cells.
Fig. 4.
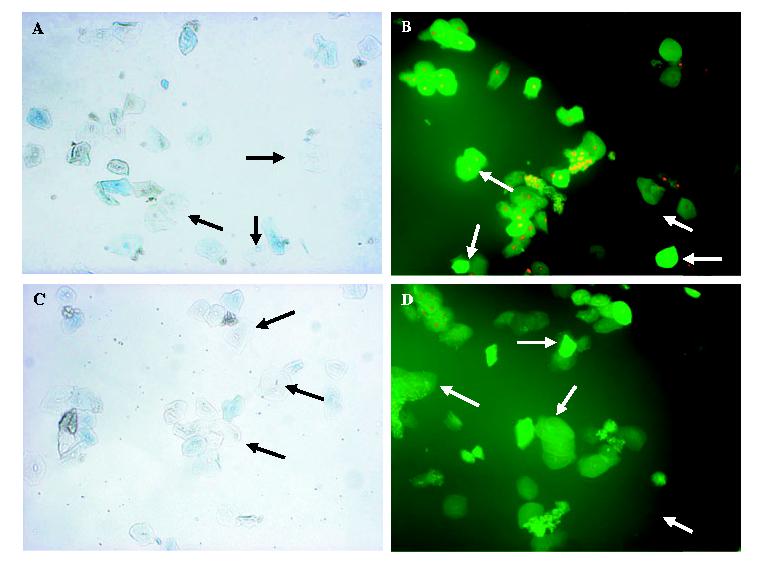
Viability requirement of epithelial cell antifungal activity. Whole unstimulated saliva was collected from healthy volunteers (n = 10), and epithelial-enriched populations were fixed with 1% paraformaldehyde, and stained with fluorescein diacetate (FDA, 50 μg/ml) and propidium=iodide (PI, 1 μg/ml) or Trypan blue. Controls included staining of unfixed cells. A) fixed cells stained with Trypan blue; B) fixed cells stained with FDA and PI; C) unfixed cells stained with Trypan blue; D) unfixed cells stained with FDA and PI. Arrows point to representative live cells. Magnification, 100×.
Fig. 5.
Effects of fixation on cell viability evaluation and antifungal activity. Whole unstimulated saliva was collected from healthy volunteers (n = 10), and epithelial-enriched populations were fixed with 1% paraformaldehyde and examined for in vitro growth inhibition of C. albicans by [3H]-glucose uptake immediately or after 4 days in culture medium at 37°C. Cell viability was determined by Trypan blue (TB) dye exclusion and vital staining with FDA and PI. SEM, standard error of the mean.
Discussion
Studies to date show that vaginal and oral epithelial cell anti-Candida activity requires cell contact and a putative carbohydrate moiety (10, 14). Although a specific effector carbohydrate had yet to be identified, the role for a carbohydrate comes from the sensitivity of the antifungal activity to periodic acid that cleaves carbohydrates to aldehydes and thus releases the majority of the sugar moieties from the cell surface. The present study focused on a better understanding of the properties and mechanisms of this potentially important innate host defense activity. The vast majority of data were obtained from oral epithelial cells, although vaginal epithelial cells were employed as well in many of the designs to confirm the similar action by both types of cells.
Previous studies showed that adherence of epithelial cells to Candida was not affected by periodic acid treatment (10, 14), indicating that the putative carbohydrate moiety was directly involved in the anti-Candida activity rather than a secondary effect of adherence (10, 14). Despite the effective cleavage of sulfated polysaccharides, sialic acid residues, or glucose- and mannose-containing carbohydrates, a specific carbohydrate moiety was not identified (10, 14).
Recognizing the difficulty in identifying a specific effector carbohydrate, we reasoned that supernatants containing the putative carbohydrate(s) could be used to better understand the properties of the moiety and the mechanism of action. Results showed that supernatants containing the putative carbohydrates incubated with Candida could not compete for the inhibitory activity by fresh epithelial cells, using both a pretreatment or direct treatment design. The moiety equivalence to that on epithelial cells (5 : 1 ratio equivalence) was used in principle in these assays and as such reduces the possibility of a role for released carbohydrate. However, we recognize that the released moiety may not resemble the intact moiety such that it was unable to bind to receptors on Candida in the same manner as that present on the epithelial cells. Alternatively, the concentration of the released moiety may not have been saturating. In any event, similar results were seen for both oral and vaginal cells confirming, as with all previous studies (10, 13-15), that oral and vaginal epithelial cells function similarly.
Taken together, results suggest that the effector moiety on oral and vaginal epithelial cells may not be a carbohydrate and that periodic acid is simply inactivating the cells instead. Support for this comes from a retrospective analysis of a large number of experiments conducted over several years where it was determined that no matter what level of carbohydrate was stripped from the cells by periodic acid, the same abrogation of growth inhibition was observed. Thus, an acid-labile property of the epithelial cell antifungal activity became a distinct alternative possibility. This was confirmed by a final series of studies that evaluated the effects of two additional acids. In one experiment, treatment of oral or vaginal epithelial cells with either TFMS or HCl abrogated the anti-fungal activity without liberating appreciable carbohydrates or affecting epithelial cell viability. In a second design, performing the standard [3H]-glucose uptake assay under acidic conditions resulted in reduced oral epithelial cell antifungal activity. Therefore, future mechanistic studies will focus on the interaction of Candida and epithelial cells in the presence or absence of acid treatment. These studies also provided a possible explanation for the weaker activity of vaginal epithelial cells compared to oral epithelial cells (14). The reduction of the oral cell activity in an acidic environment suggests that the lower activity by human vaginal epithelial cells may be due to the general acidic microenvironment of the vaginal cavity. An alternative explanation is that the restriction of Candida to blastoconidia at the lower pH influenced the epithelial cell activity. However, this is unlikely since a similar study performed at room temperature that restricted Candida to the blastoconidia had no effect on the activity (13), and the acidic pH in these studies had no effect on the growth of Candida alone despite being confined to blastoconidia.
Additional information regarding the mechanism of action was garnered from studies using fixed epithelial cells. Previously we reported that the anti-Candida activity was partially resistant to fixation (14). In those studies, Trypan blue was used as the indicator of viability. As such, the small numbers of positively stained cells suggested that the fixed cells were still viable when tested, or at least impermeable to Trypan blue. However, the present study clearly shows that fixed cells that had antifungal activity were indeed non–viable, as evidenced by nuclear staining by propidium iodide. Together, these results suggest that the antifungal activity requires intact (impermeable to Trypan blue) but not necessarily live epithelial cells. The requirement for Trypan blue impermeability had been suggested for some time as growth inhibition was never observed if the cells were not deemed viable by Trypan blue dye exclusion (15). This was confirmed in the present study by the positive correlation of antifungal activity with Trypan blue dye exclusion from fixed and unfixed cells tested immediately after fixation compared to after 4 days in culture. But obviously the requirement for Trypan blue impermeability does not infer a requirement for live cells. Of note, the lack of a requirement for live cells is consistent with the lack of any evidence for intracellular signals (tyrosine kinases or phospholipase C) in the epithelial cell antifungal activity, as well as a lack of involvement by microtubules and microfilaments (Fidel, unpublished data).
The results of this study provide a considerable amount of new information regarding the requirements and mechanism for the epithelial cell antifungal activity. The static antifungal activity, while not having a strict requirement for live cells, does require that the putative effector moiety be adequately present on intact cells. Furthermore, the activity appears to be acid-labile rather than mediated by an effector carbohydrate, although we understand that the two may not be mutually exclusive. In this regard, it is possible that the experiments designed and conducted involved a carbohydrate that was not able to be evaluated in the absence of the epithelial cells. However, our most recent data suggest that surface proteins (or glycoproteins) extracted from the epithelia cells inhibit Candida growth (unpublished data). Thus, while carbohydrates may be involved at some level, it does not appear that they are involved exclusively. We propose that the static activity by epithelial cells may represent a sophisticated symbiotic relationship between the host and Candida, where the host benefits by Candida staying ‘in check’, and Candida benefits by not being killed. As for the growth inhibition mechanism, inasmuch as he moiety on the epithelial cells can exert some action on Candida, it is equally possible that, as a result of attachment to the epithelial cells, Candida elicits a selfcontrolling response that halts its growth. Studies are in progress to further investigate the effector moiety and to evaluate the interaction between epithelial cells and Candida at the molecular level.
Acknowledgments
This work was supported by National Institutes of Health Public Health Service grants DE 12178 (Fidel) from the National Institute of Dental and Craniofacial Research and AI 32556 (Fidel) from the National Institute of Allergy and Infectious Diseases.
References
- 1.Barousse MM, Steele C, Dunlap K, Espinosa T, Boikov D, Sobel JD, Fidel PL., Jr. Growth inhibition of Candida albicans by human vaginal epithelial cells. J Infect Dis. 2001;184:1489–1493. doi: 10.1086/324532. [DOI] [PubMed] [Google Scholar]
- 2.Barousse M, Van Der Pol BJ, Fortenberry D, Orr D, Fidel PL., Jr. Vaginal yeast colonization, prevalence of vaginitis, and associated local immunity in adolescents. Sex Trans Infect. 2004;80:48–53. doi: 10.1136/sti.2002.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darzynkiewicz Z, Li X. Measurements of cell death by flow cytometry. In: Cotter TG, Martin SJ, editors. Techniques in apoptosis. A user's guide to cytometry. Portland Press; London: 1996. pp. 71–106. [Google Scholar]
- 4.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 5.Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 6.Fidel PL, Jr, Cutright JL, Steele C. Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun. 2000;68:651–657. doi: 10.1128/iai.68.2.651-657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilbault GG, Kramer DN. Fluorometric determination of lipase, acylase, alpha and gamma chymotrypsin, and inhibitors of these enzymes. Anal Chem. 1964;36:409–412. [Google Scholar]
- 8.Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–357. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 9.Macher AM. The pathology of AIDS. Public Health Rep. 1988;103:246–254. [PMC free article] [PubMed] [Google Scholar]
- 10.Nomanbhoy F, Steele C, Yano J, Fidel PL., Jr. Vaginal and oral epithelial cell anti-Candida activity. Infect Immun. 2002;70:7081–7088. doi: 10.1128/IAI.70.12.7081-7088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992;14:S148–S153. doi: 10.1093/clinids/14.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 12.Sobel JD. Pathogenesis of recurrent vulvovaginal candidiasis. Curr Infect Dis Rep. 2002;4:514–519. doi: 10.1007/s11908-002-0038-7. [DOI] [PubMed] [Google Scholar]
- 13.Steele C, Leigh JE, Swoboda RK, Fidel PL., Jr. Growth inhibition of Candida by human oral epithelial cells. J Infect Dis. 2000;182:1479–1485. doi: 10.1086/315872. [DOI] [PubMed] [Google Scholar]
- 14.Steele C, Leigh JE, Swoboda RK, Ozenci H, Fidel PL., Jr. Potential role for a carbohydrate moiety in anti-Candida activity of human oral epithelial cells. Infect Immun. 2001;69:7091–7099. doi: 10.1128/IAI.69.11.7091-7099.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steele C, Ozenci H, Luo W, Scott M, Fidel PL., Jr. Growth inhibition of Candida albicans by vaginal cells from naive mice. Med Mycol. 1999;37:251–260. [PubMed] [Google Scholar]
- 16.Steele C, Ratterree M, Fidel PL., Jr. Differential susceptibility to experimental vaginal candidiasis in macaques. J Infect Dis. 1999;180:802–810. doi: 10.1086/314964. [DOI] [PubMed] [Google Scholar]