Abstract
Objective: To determine whether mutations in the optineurin gene contribute to susceptibility to adult-onset primary open-angle glaucoma.
Methods: The optineurin gene was screened in 86 probands with adult-onset primary open-angle glaucoma and in 80 age-matched control subjects. Exons 4 and 5, containing the recurrent mutations identified in patients with normal-tension glaucoma, were sequenced in all individuals studied, while the remaining exons were screened for DNA sequence variants with denaturing high-performance liquid chromatography.
Results: The recurrent mutation, Met98Lys, previously found to be associated with an increased risk of disease was found in 8 (9%) of 86 probands. We also found the Met98Lys mutation in 10% of individuals from a control population of similar age, sex, and ethnicity. Consistent segregation of the mutation with the disease was not demonstrated in any of the 8 families. No other DNA changes altering the amino acid structure of the protein were found.
Conclusion: The mutations in the optineurin gene associated with normal-tension glaucoma are not associated with adult-onset primary open-angle glaucoma in this patient population.
Clinical Relevance: Genetic abnormalities that render the optic nerve susceptible to degeneration are excellent candidates for genetic factors that could contribute to adult-onset primary open-angle glaucoma. Mutations in optineurin have been associated with normal-tension glaucoma, but are not associated with disease in patients with adult-onset primary open-angle glaucoma. This result may indicate that normal-tension glaucoma is not necessarily part of the phenotypic spectrum of adult open-angle glaucoma.
GLAUCOMA IS the third leading cause of blindness in the United States.1. Of the various forms of glaucoma, adult-onset primary open-angle glaucoma (adult-onset POAG) is the most common.2 Typically, adult-onset POAG is associated with an elevation of intraocular pressure and a characteristic degeneration of the optic nerve. Patients may have optic nerve damage without an increase in intraocular pressure, and this form of open-angle glaucoma is called "low-tension" or "normal-tension" glaucoma. Normal-tension glaucoma may be part of a spectrum of phenotypes extending from patients with only ocular hypertension to those with optic nerve disease without significant elevation of intraocular pressure. Patients with adult-onset POAG fall in the middle of this spectrum and exhibit both ocular hypertension and optic nerve degeneration.3-5
Recently, mutations in a novel gene, optineurin, have been described in patients with normal-tension glaucoma.6,7 Two mutations were observed in multiple probands: a G-to-A change in codon 50 resulting in the replacement of the glutamate in that position with a lysine, and a T-to-A change in codon 98 changing the methionine at that position to a lysine. The Glu5OLys change was described as "disease-causing" because it was found in 7 (13.5%) of 52 patients with normal-tension glaucoma and in none of 540 control subjects. The Met98Lys was determined to be a "risk-associated" alteration because it was found in 23 (13.6%) of 169 patients with normal-tension glaucoma and in 9 (2.1%) of 422 controls.
The optineurin gene contains 3 non-coding exons in the 5′ untranslated region and 13 coding exons that produce a 577-amino acid protein. The protein has previously been reported as FIP-2 (Gen-bank accession No. AH00971 1) and has been shown to be expressed in many nonocular tissues, including brain, heart, liver, skeletal muscle, kidney, and pancreas.8 In the eye, the protein has been detected by reverse transcriptase polymerase chain reaction in human trabecular meshwork, nonpigmented ciliary epithelium, and retina.6 The protein does not have significant homology to any known protein, but it may participate in the tumor necrosis factor α signaling pathway.6 Tumor necrosis factor α has been proposed to be one factor that could induce apopotosis in retinal ganglion cells in patients with normal-tension glaucoma and in patients with POAG.9,10 It has been speculated that the optineurin protein may function to protect the optic nerve from tumor necrosis factor a-mediated apopotosis, and that the loss of function of this protein may decrease the threshold for ganglion cell apopotosis in patients with glaucoma.6
Adult-onset POAG is inherited as a complex disease, suggesting that multiple genes may contribute to the phenotype. One gene, TIGR/Myocilin, has been associated with POAG,11 and the locations of a number of other genes have been indicated from genetic linkage studies.12-15 Genes that predispose to POAG may influence intraocular pressure or optic nerve degeneration or both. As part of a genome scan to identify chromosomal regions harboring POAG susceptibility genes, we have collected data from 86 families with multiple members affected by adult-onset POAG with elevated intraocular pressure and optic nerve degeneration.12 The observation that mutations in optineurin predispose to normal-tension glaucoma suggests that mutations in this gene may also contribute to the degeneration of the nerve in patients with adult-onset POAG. To investigate this hypothesis, we screened the optineurin gene for mutations in our collection of patients with adult-onset POAG.
METHODS
PATIENTS
Eighty-six families with 2 or more individuals affected by adult-onset POAG were collected from the New England area and from the southeast area of the United States. After informed consent was obtained, all patients and appropriate family members had a complete ocular examination including visual acuity, refraction, tonometry, slit-beam evaluation, gonioscopy, and funduscopic evalution. Optic nerve evaluation (usually including photography) and visual field testing were done on all affected individuals. For most patients, visual field testing was done with the automated Humphrey perimeter. Some patients had manual visual fields using the Goldmann perimeter or other automated methods. The following criteria were used to assign affected status: intraocular pressure greater than 22 mm Hg in both eyes on 2 occasions or intraocular pressure greater than 19 mm Hg in both eyes during treatment with 2 or more glaucoma medications; evidence of optic nerve damage in both eyes, characterized by increased cup-disc ratios above 0.7, and/or focal notching of the neural-retinal rim; and visual field defects consistent with the clinical evidence of optic nerve damage. All affected patients had at least 1 of the following visual field defects in at least 1 eye: paracentral scotoma, central scotoma, nasal step, or generalized loss in 1 or more quadrants (corresponding to changes in the optic nerve). All affected patients had onset of the disease after age 35 years. Control patients underwent a complete eye examination, including slitlamp evaluation, tonography, and funduscopic evaluation of the optic nerve. Most controls did not have a visual field test. Age, sex, and race of the affected and control patients used in this study are shown in the Table.
Age, Sex, and Race of Probands and Control Subjects
| Probands (n = 86) | Controls (n = 80) | |
|---|---|---|
| Age at study entry, mean ± SD, y | 77 ± 4 | 74 ± 4 |
| Sex, No (%) | ||
| Male | 37(44) | 35(44) |
| Female | 49(57) | 45(56) |
| Race, No,(%) | ||
| White | 74(87) | 74(92) |
| Black | 10(12) | 4(5) |
| Asian | 0 | 2(2) |
| Hispanic | 1(1) | 0 |
DNA ANALYSIS
Blood specimens were obtained from all affected members and appropriate unaffected family members. Genomic DNA was extracted by standard procedures. All of the translated exons of the gene (exons 4-16) were selectively amplified by means of oligonucleotide primers located in the 5′ and 3′ introns flanking each exon. The exons containing the recurrent mutations (exons 4 and 5) were sequenced bidirectionally with nested primers and BigDye chemistry (Applied Biosystems Tnc, Foster City, Calif). The reaction products were analyzed on an automated sequencer. The remaining exons were screened with the Transgenomic WAVE denaturing high-performance liquid chromatography system (Transgenomic, Tnc, Omaha, Neb). Pools of genomic DNA from 3 individuals were subjected to denaturing high-performance liquid chromatography at several different temperatures. Pools displaying altered column retention time were sequenced to confirm and identify sequence variants.
RESULTS
We have collected DNA and clinical information from 86 families with adult-onset POAG for genetic linkage studies. The optineurin gene was screened for DNA sequence variants in all 86 probands and in 80 controls with similar age, sex, and ethnicity. We found the previously identified disease risk-associated Met98Lys mutation in 8 (9%) of 86 probands and 8 (10%) of 80 controls. We did not find any other DNA sequence variants resulting in a change in amino acid sequence in the probands or controls, including the recurrent disease-associated Glu5OLys mutation in exon 4.
To determine whether the Met98Lys mutation segregated with the disease in the 8 families carrying this DNA sequence change, we sequenced all the affected and unaffected members of each family. In these 8 families, we found that this DNA sequence variant was equally distributed between the affected and unaffected individuals, with 14 (56%) of the 25 affected individuals and 9 (69%) of the 13 unaffected individuals heterozygous for the methionine and lysine alleles. Consistent segregation of the mutation with the disease was not demonstrated in any of these 8 families, suggesting that in these families the Met98Lys change is not associated with disease risk (Figure). We did not find any individuals who were homozygous for the lysine allele.
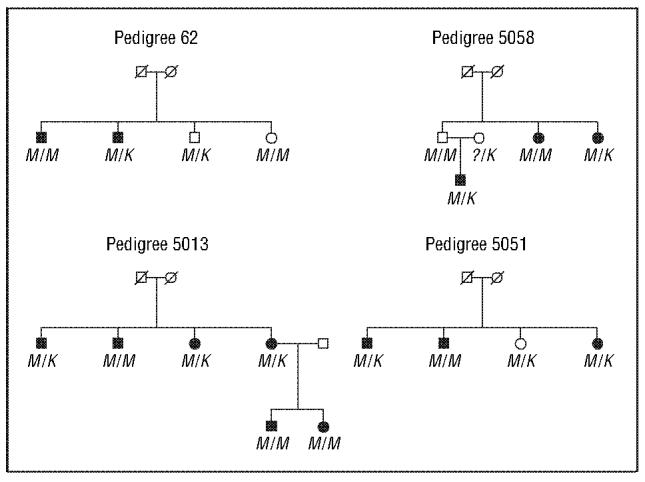
Representative pedigrees showing the segregation of disease and the Met98Lys DNA sequence variant genotypes. M/M is homozygous for methionine at codon 98, while M/K is heterozygous for the methionine/lysine at codon 98. Square indicates male; circle, female; diagonal line, deceased; solid symbol, affected individual; and question mark, unknown allele.
COMMENT
Primary open-angle glaucoma is a complex disorder that is likely to be the result of multiple genetic and/or environmental defects. Normal-tension glaucoma may represent a subset of POAG that is characterized by extensive deterioration of the optic nerve in response to normal or even low-normal intraocular pressure.16 Certainly genes that cause the optic nerve to degenerate in the setting of low or normal pressure would be excellent candidates for genes that could potentially contribute to optic nerve degeneration associated with elevated pressure.
In this study, we did not find any of the mutations in optineurin reported to be "disease-causing" in the 86 probands with POAG screened. We did find the "disease risk-associated" mutation, Met98Lys, in 9% of the probands and in 10% of the control individuals. However, we did not find that the sequence variant segregated with the disease in any of the families in which it was present. Indeed, approximately half of the affected individuals and more than half of the unaffected individuals carried the sequence variant. In a complex disease with multiple genetic causes, a risk-associated gene defect may not segregate perfectly with the disease; however, an overall association of the gene defect with the disease should be evident. Our results would not support a conclusion that the Met98Lys mutation confers a significantly increased risk of disease in adult-onset POAG.
Optineurin has been shown to be associated with disease in families with at least 1 member affected by normal-tension glaucoma.6 We hypothesized that mutations in optineurin would also contribute to optic nerve degeneration in patients with elevated intraocular pressure. Surprisingly, our results demonstrate that mutations in optineurin are not associated with adult-onset POAG in the patient population we have studied. Possibly defects in other genes that are more commonly associated with optic nerve disease will participate to a larger extent in adult-onset POAG. Alternatively, normal-tension glaucoma may be a genetically distinct disease entity that is not a major component of the phenotypic spectrum of adult-onset POAG.
Footnotes
This work was supported by the Barkhauser Glaucoma Trust, Durham, NC; the Massachusetts Lions Research Fund, Boston, Mass; and grant EY10886 from the National Institutes of Health, Bethesda, Md.
We thank the patients for their participation.
Contributor Information
Janey L. Wiggs, Department of Ophthalmology, Harvard Medical School, Boston, Mass.
Josette Auguste, Department of Ophthalmology, Harvard Medical School, Boston, Mass.
R. Rand Allingham, Department of Ophthalmology, Duke School of Medicine, Durham, NC.
Jason D. Flor, Center for Human Genetics, Duke School of Medicine, Durham, NC.
Margaret A. Pericak-Vance, Center for Human Genetics, Duke School of Medicine, Durham, NC.
Kathryn Rogers, Department of Ophthalmology, Harvard Medical School, Boston, Mass.
Karen R. LaRocque, Center for Human Genetics, Duke School of Medicine, Durham, NC.
Felicia L. Graham, Center for Human Genetics, Duke School of Medicine, Durham, NC.
Bob Broomer, Department of Ophthalmology, Duke School of Medicine, Durham, NC.
Elizabeth Del Bono, Department of Ophthalmology, Harvard Medical School, Boston, Mass.
Jonathan L. Haines, Program in Human Genetics, Vanderbilt School of Medicine, Nashville, Tenn.
Michael Hauser, Center for Human Genetics, Duke School of Medicine, Durham, NC.
REFERENCES
- Munoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–825. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variation in the prevalence of primary open-angle glaucoma. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans: the Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–1095. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- Bonomi L, Marchini G, Marraffa M, Morbio R. The relationship between intraocular pressure and glaucoma in a defined population: data from the Egna-Neumarkt Glaucoma Study. Ophthalmologica. 2001;215:34–38. doi: 10.1159/000050823. [DOI] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Sarfarazi M, Child A, Stoilova D, et al. Localization of the fourth locus (GLC1 E) for adult-onset primary open-angle glaucoma to 1Op15-p14. Am J Hum Genet. 1998;62:641–652. doi: 10.1086/301767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kang J, Horwitz MS. Interaction of an adenovirus E3 14.7-kilodalton protein with a novel tumor necrosis factor alpha-inducible cellular protein containing leucine zipper domains. Mol Cell Biol. 1998;18:1601–1610. doi: 10.1128/mcb.18.3.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Neufeld AH. Tumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32:42–50. [PubMed] [Google Scholar]
- Yan X, Tezel G, Wax MB, Edward DP. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol. 2000;118:666–673. doi: 10.1001/archopht.118.5.666. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Wiggs JL, Allingham RR, Hossain A, et al. Genome-wide scan for adult onset primary open angle glaucoma. Hum Mol Genet. 2000;9:1109–1117. doi: 10.1093/hmg/9.7.1109. [DOI] [PubMed] [Google Scholar]
- Wirtz MK, Samples JR, Kramer PL, et al. Mapping a gene for adult-onset primary open-angle glaucoma to chromosome 3q. Am J Hum Genet. 1997;60:296–304. [PMC free article] [PubMed] [Google Scholar]
- Stoilova D, Child A, Trifan OC, Crick RP, Coakes RL, Sarfarazi M. Localization of a locus (GLC1 B) for adult-onset primary open angle glaucoma to the 2cen-ql 3 region. Genomics. 1996;36:142–150. doi: 10.1006/geno.1996.0434. [DOI] [PubMed] [Google Scholar]
- Trifan OC, Traboulsi El, Stoilova D, et al. The third locus (GLC1D) for adult-onset primary open-angle glaucoma maps to the 8q23 region. Am J Ophthalmol. 1998;126:17–28. doi: 10.1016/s0002-9394(98)00073-7. [DOI] [PubMed] [Google Scholar]
- Shigeeda T, Tomidokoro A, Araie M, Koseki N, Yamamoto S. Long-term follow-up of visual field progression after trabeculectomy in progressive normal-tension glaucoma. Ophthalmology. 2002;109:766–770. doi: 10.1016/s0161-6420(01)01009-0. [DOI] [PubMed] [Google Scholar]


