Abstract
BACKGROUND
The left ventricular outflow tract (LVOT) malformations aortic valve stenosis (AVS), coarctation of the aorta (CoA), and hypoplastic left heart syndrome (HLHS) contribute significantly to infant mortality due to birth defects. Previous epidemiology data showed rate differences between male and female and white and black ethnic groups. The Texas Birth Defects Registry, an active surveillance program, enables study in a large, diverse population including Hispanics.
METHODS
Records of children up to 1 year old with AVS, CoA, and HLHS born in Texas from 1999 to 2001, were collected from the registry. Those including additional heart defects or a chromosomal anomaly were excluded. Multivariate analysis included: infant sex; United States–Mexico border county residence; and maternal age, race/ethnicity, birthplace, and education.
RESULTS
There were 910 cases among 1.08 million live births, of which 499 met inclusion criteria. Multivariate modeling of all LVOT malformations combined demonstrated lower prevalence rate ratios (PRRs) for black males (0.26) and Hispanic males (0.70). Similar results were found for CoA but not AVS or HLHS. Higher PRRs were noted for increased maternal age for LVOT (1.3 for 24–34 years; 1.7 for >34 years), AVS, and HLHS, but not CoA, and higher PRRs across all diagnoses for males (LVOT PRR, 2.4) were noted. CoA PRRs were higher in border county vs. non–border county residents (PRR, 2.1). Maternal education and birthplace were not significant factors.
CONCLUSIONS
There are rate differences for males among all 3 ethnic groups. Sex and ethnic differences suggest genetic etiologies, where the ethnic differences could be used to find susceptibility loci with mapping by admixture linkage disequilibrium. Increased CoA rates along the U.S.–Mexico border suggest environmental causes that will require further monitoring.
Keywords: congenital heart disease, Hispanic, Poisson distribution, regression analysis, prevalence, south-western United States, genetics
INTRODUCTION
Birth defects are the leading cause of infant mortality, and malformations of the cardiovascular system contribute a disproportionate amount to that mortality compared to malformations in other systems (Anderson, 2002). Obstructions of the left ventricular outflow tract (LVOT) are among the most severe congenital heart defects (CHDs) and include aortic valve stenosis (AVS), bicuspid aortic valve (BAV), coarctation of the aorta (CoA), and hypoplastic left heart syndrome (HLHS). These malformations have been grouped together by a common developmental mechanism of obstructed or altered embryonic blood flow through the left side of the heart, leading to underdevelopment of the valves, aorta, or chambers (Clark, 1996). This is supported by animal studies (Hove et al., 2003) and human prenatal ultrasound observations (Hornberger et al., 1996).
There are several lines of evidence in favor of lumping AVS, BAV, CoA, and HLHS together. CHDs in Turner syndrome (45, X) consists almost exclusively of 1 of the 4 LVOT malformations (Mazzanti and Cacciari, 1998). Epidemiologic studies have found that for LVOT malformations, there is a higher rate of concordant diagnoses in multiple affected family members than for other classes of CHDs (Ferencz, 1997). Multiple case studies have also shown that these defects recur in families (Menahem, 1990; Clementi et al., 1996; Grobman and Pergament, 1996; Stoll et al., 1999). Finally, in recent echocardiography studies of families ascertained by a child affected with AVS, CoA or HLHS, prevalences of BAV were much higher in the first-degree relatives than the background population prevalences (Lewin et al., 2004; Loffredo et al., 2004).
Previous epidemiology studies of the LVOT malformations indicate rate differences for maternal race/ethnicity and infant sex. Data from the Baltimore Washington Infant Study (BWIS) was the first to demonstrate a higher prevalence in whites compared to blacks and confirmed a higher prevalence among males (Ferencz, 1997). A recent study using 3 large registries that included the California Birth Defects Monitoring Program also suggested a slightly lower prevalence of LVOT malformations among Hispanics compared to whites (Pradat et al., 2003).
Environmental elements may also play a role in the etiology of LVOT malformations. Several studies have noted an association between chemical exposures, particularly solvents, and CoA (Tikkanen and Heinonen, 1993; Ferencz, 1997), which may vary depending on the presence or absence of a ventricular septal defect with the CoA (Wollins et al., 2001). Other studies have documented seasonal variations for CoA (Wren et al., 2000). Geographical differences have been noted in Wisconsin (Cronk et al., 2004), where higher prevalences of HLHS have been reported in urban areas, and preliminary Texas Department of Health data have indicated that CoA prevalence in Texas counties bordering Mexico may also be higher (http://www.tdh.texas.gov/tbdmd/index.htm).
This study provides useful comparative data from a large and diverse population, including ethnic Hispanics who make up an increasing percentage of the population in the United States. Ethnic differences could reflect the operation of environmental and genetic influences on the occurrence of these defects. The present data also help to assess possible regional prevalence differences that are most likely to reflect the environmental component of the etiology.
MATERIALS AND METHODS
The Texas Birth Defects Registry is operated by the Birth Defects Epidemiology and Surveillance Branch (BDESB) of the Texas Department of State Health Services. The program does not require reporting but uses active surveillance. BDESB staff routinely visit hospitals and other places where children are born or receive medical care. For a child to be included as a case, the mother’s residence must be in an area covered by the registry, the infant or fetus must have a structural defect monitored by the registry, and the defect must be diagnosed prenatally or within 1 year of the infant’s birth. Cases from all pregnancy outcomes are included. Information relevant to birth defects is collected from the medical record, processed, and analyzed by BDESB staff and clinical geneticists. The birth defect is recorded in the database by the British Paediatic Association (BPA) code. Originally, the pilot for 1995 covered 35% of Texas live births, expanding to 80% by 1997, and became statewide since 1999. This registry currently is based on >350,000 births annually. For this study, data from the collection years 1999 –2001 were used (total of 1.08 million births), as those years represented complete data for the whole state of Texas.
Case variables used in this study included year of birth, infant sex, maternal race/ethnicity, maternal education, maternal age at delivery, county residence, maternal birthplace (state or country), and the presence of other birth defects. For denominators, we used the appropriate number of live-born infants for each variable as extracted from the Texas Bureau of Vital Statistics databases.
Case Definition
Cases for this study were defined as matching BPA codes for the diagnoses AVS (746.3), CoA (747.1), and HLHS (746.7). Each infant was assigned only 1 cardiac diagnosis based on a hierarchical scheme of HLHS, CoA, and AVS; for example, if a child had AVS and CoA, the case was classified as CoA, if the child had CoA and HLHS, the case was classified as HLHS.
Inclusions and Exclusions
The registry database was queried using the BPA codes above. Cases were selected if the diagnosis was confirmed and excluded if the diagnosis was considered “trivial” (incidental note of a small flow change through a valve by echocardiography) or “possible.” Each case record was then examined to identify coexisting birth defects. Because we were interested in “noncomplex” heart defects in which the developmental mechanism is more likely to be similar, “complex” heart defects or cases coded with >1 defect (e.g., HLHS and transposition of the great arteries) were removed. Cases of patent foramen ovale, atrial septal defect, ventricular septal defect, or patent ductus arteriosus in addition to their LVOT malformation were included. Cases of subvalvar or supravalvar aortic stenosis, representing different entities from the LVOT malformations, were excluded. Those cases with chromosome anomalies were excluded. Noncardiac malformations were recorded for each case and subsequently analyzed.
Statistical Analysis
All analyses were performed with Stata v8.2 (Stata, College Station, TX). Variables selected were maternal race/ethnicity, infant sex, maternal age at delivery, maternal education level, residence in a border county, and mother’s place of birth. Border counties were defined as those Texas counties that share a border with Mexico (Brewster, Cameron, El Paso, Hidalgo, Hudspeth, Jeff Davis, Kinney, Maverick, Presidio, Starr, Terrell, Val Verde, Webb, and Zapata). Dummy variables were created for race/ethnicity (divided into white, Hispanic and black), maternal education (<12 completed years, high school completion, some postsecondary education) and maternal age at delivery (<25 years of age, 25–34 years of age, and >34 years of age). All variables were screened for differential relative risk first by a univariate analysis using Poisson regression; 95% confidence intervals (CIs) were calculated using the Poisson distribution to obtain exact values.
We used Poisson regression modeling methods in the multivariate analysis. Modeling was performed for all diagnoses together (LVOT malformations) and AVS, COA, and HLHS separately. A minimal model was first created based on previous data associating the variable with congenital heart disease in epidemiology studies (Ferencz, 1997); this included all the variables used in the univariate analysis. The model was then expanded by adding interactive terms. The total number of interactive variables was limited to reduce the number of possible model forms, using only first-order combinations of main effects terms significant at P < 0.05 in the univariate analysis. The full complex models for each of the 4 groups were constructed with all main effects terms and selected interactive terms. Nested models for each of the 4 groups were then assembled using a backward stepwise selection process. Starting with a full model, the least significant term (P < 0.1) was removed, followed by reestimation. This process was repeated until no more terms could be removed. Goodness of fit was determined by comparing the nested model against the full model using a likelihood ratio test, with the appropriate degrees of freedom calculated by the difference in number of terms between the 2 models. A P value >0.2 was used to define a good fit for the model. The main effect terms used to construct the interactive term were arbitrarily retained for any interactive terms included in the model (hierarchical rule). The nested models were inspected to see if any terms could be removed to simplify the model. Any additional nested model was compared to the full model as above, and the most parsimonious nested model was chosen as the final model using Akaike’s Information Criterion. Our approach follows that of other general (Kleinbaum, 1998; Rothman and Greenland, 1998; Agresti, 2002) and specific regression analyses (Ferencz, 1997).
RESULTS
A total of 910 cases of LVOT malformations was identified among 1,077,574 live births from 1999 through 2001. This included 245 AVS (26.9%), 452 CoA (49.7%), and 213 HLHS (23.4%) cases. There were 555 noncomplex cases of AVS, CoA, and HLHS, of which 56 included a chromosome anomaly (10.1%). The proportion of chromosome defects (Table 1) in each diagnostic group was increased among cases with CoA (36/276; 13.0%) compared to AVS (5/98; 5.1%) and HLHS (15/181; 8.3%; P = 0.05). After excluding the cases with a chromosome anomaly, there were 499 cases that met the final inclusion criteria for noncomplex AVS, CoA, or HLHS diagnoses. There were 481 live births (96.4%); 9 spontaneous fetal deaths, 8 terminations of pregnancy, and 1 unspecified fetal demise or termination made up the remaining 3.6%. The prevalence of the cardiac defects is enumerated in Table 2.
Table 1.
List of Chromosomal Anomalies in the Noncomplex Cardiac Malformations
| Chromosome anomaly | Number |
|---|---|
| Turner syndrome (full, partial or mosaic) | 20 |
| Trisomy 21 | 12 |
| Trisomy 18 (full or mosaic) | 7 |
| Trisomy 13 (full or mosaic) | 4 |
| Trisomy 8 mosaic | 1 |
| 47XXX | 1 |
| Partial autosome deletiona | 8 |
| Partial autosome trisomy | 3 |
| Balanced translocation | 1 |
| Complex rearrangement | 1 |
| Total | 58 |
Includes 2 velocardiofacial syndrome (del 22q11.2) and 1 Jacobsen syndrome (del 11q23.3-ter).
Table 2.
Prevalence (per 10,000 Live Births) of Noncomplex Left Ventricular Outflow Tract Malformations, Texas, 1999–2001
| AVS
|
CoA
|
HLHS
|
Combined
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Live Births | Cases | Prev. (95% CI) | Cases | Prev. (95% CI) | Cases | Prev. (95% CI) | Cases | Prev. (95% CI) | |
| White | |||||||||
| Male | 216,356 | 44 | 2.03 (1.48–2.73) | 69 | 3.19 (2.48–4.04) | 54 | 2.50 (1.87–3.26) | 167 | 7.72 (6.59–8.98) |
| Female | 205,922 | 10 | 0.49 (0.23–0.89) | 36 | 1.75 (1.22–2.42) | 23 | 1.12 (0.71–1.68) | 69 | 3.35 (2.61–4.24) |
| Total | 422,278 | 54 | 1.28 (0.96–1.67) | 105 | 2.49 (2.03–3.01) | 77 | 1.85 (1.46–2.31) | 236 | 5.68 (4.90–6.35) |
| Hispanic | |||||||||
| Male | 252,584 | 24 | 0.95 (0.61–1.41) | 71 | 2.81 (2.20–3.55) | 49 | 1.94 (1.44–2.56) | 144 | 5.70 (4.81–6.71) |
| Female | 242,975 | 11 | 0.45 (0.23–0.81) | 45 | 1.85 (1.35–2.48) | 22 | 0.91 (0.57–1.37) | 78 | 3.21 (2.54–4.01) |
| Total | 495,559 | 35 | 0.71 (0.49–0.98) | 116 | 2.34 (1.93–2.81) | 71 | 1.45 (1.14–1.83) | 222 | 4.56 (3.91–5.11) |
| Black | |||||||||
| Male | 62,275 | 0 | 0.00 (0.00–0.59) | 6 | 0.96 (0.35–2.10) | 6 | 0.96 (0.35–2.10) | 12 | 1.93 (1.00–3.37) |
| Female | 59,512 | 2 | 0.34 (0.04–1.21) | 10 | 1.68 (0.81–3.09) | 9 | 1.51 (0.69–2.87) | 21 | 3.53 (2.18–5.39) |
| Total | 121,787 | 2 | 0.25 (0.05–0.72) | 16 | 1.31 (0.75–2.13) | 15 | 1.23 (0.69–2.03) | 34 | 2.79 (1.93–3.90) |
| Totala | |||||||||
| Male | 550,816 | 68 | 1.23 (0.96–1.57) | 147 | 2.67 (2.25–3.14) | 110 | 2.00 (1.64–2.41) | 325 | 5.90 (5.28–6.58) |
| Female | 526,758 | 24 | 0.46 (0.29–0.68) | 93 | 1.77 (1.43–2.16) | 54 | 1.03 (0.77–1.34) | 171 | 3.27 (2.78–3.77) |
| Total | 1,077,574 | 93 | 0.86 (0.70–1.06) | 240 | 2.23 (1.95–2.53) | 166 | 1.54 (1.32–1.79) | 499 | 4.63 (4.23–5.06) |
| Residence, Hispanicb | |||||||||
| Border | 128,969 | 4 | 0.31 (0.08–0.79) | 47 | 3.64 (2.68–4.85) | 21 | 1.63 (1.01–2.49) | 72 | 5.58 (4.37–7.03) |
| Nonborder | 366,590 | 31 | 0.85 (0.57–1.20) | 69 | 1.88 (1.46–2.38) | 51 | 1.39 (1.04–1.83) | 151 | 4.12 (3.49–4.83) |
| Birthplace, Hispanic | |||||||||
| U.S.–born | 247,398 | 18 | 0.73 (0.43–1.15) | 59 | 2.38 (1.82–3.08) | 34 | 1.37 (0.95–1.92) | 111 | 4.49 (3.69–5.40) |
| Mexican–born | 218,585 | 16 | 0.73 (0.42–1.19) | 50 | 2.29 (1.70–3.02) | 32 | 1.46 (1.00–2.07) | 98 | 4.48 (3.64–5.46) |
| Maternal age | |||||||||
| <25 yrs | 469,081 | 21 | 0.45 (0.28–0.68) | 98 | 2.09 (1.70–2.55) | 58 | 1.24 (0.94–1.60) | 177 | 3.80 (3.24–4.37) |
| 25–34 yrs | 497,525 | 56 | 1.13 (0.85–1.46) | 112 | 2.25 (1.85–2.71) | 84 | 1.69 (1.35–2.09) | 252 | 5.17 (4.46–5.73) |
| 35+ yrs | 110,844 | 16 | 1.44 (0.83–2.34) | 30 | 2.71 (1.83–3.86) | 24 | 2.17 (1.39–3.22) | 70 | 6.41 (4.92–7.98) |
| Maternal education | |||||||||
| <12 yrs | 348,004 | 20 | 0.57 (0.35–0.89) | 69 | 1.98 (1.54–2.51) | 53 | 1.52 (1.14–1.99) | 142 | 4.14 (3.44–4.81) |
| 12 yrs | 330,789 | 28 | 0.85 (0.56–1.22) | 72 | 2.17 (1.70–2.74) | 52 | 1.57 (1.17–2.06) | 152 | 4.62 (3.89–5.39) |
| >12 yrs | 377,698 | 42 | 1.11 (0.80–1.50) | 90 | 2.38 (1.92–2.93) | 50 | 1.32 (0.98–1.75) | 182 | 4.92 (4.14–5.57) |
Includes unknown sex and other ethnicities.
Residence, maternal county residence (see text for definition).
AVS, aortic valve stenosis; CoA, coarctation of the aorta; HLHS, hypoplastic left heart syndrome.
Of the 499 cases without chromosomal anomalies, malformations outside the cardiovascular system were found in 101 (20.2%). Noncardiac malformations were equally likely among cases of AVS 14/93 (15.1%), CoA 49/240 (20.4%), or HLHS 38/166 (22.9%; P = 0.32). Genitourinary malformations as a group were the most common associated malformation for all LVOT groups (Table 3).
Table 3.
Additional Congenital Malformations Among the Noncomplex Cases of AVS, CoA, and HLHS
| Congenital malformation | Count |
|---|---|
| Urinary system | 62 |
| Other CNS malformations | 45 |
| Other musculoskeletal | 30 |
| Unspecified other | 25 |
| Other GI | 25 |
| Other limb anomalies | 23 |
| Genital organs | 19 |
| Respiratory | 17 |
| Cleft lip and palate | 17 |
| Musculoskeltal | 16 |
| Other upper GI | 8 |
| Eye anomalies | 6 |
| DiGeorge syndrome (clinical diagnosis) | 4 |
| Ear, face, and neck | 3 |
| Pierre Robin sequence | 2 |
| Amniotic bands (constricting bands, amniotic cyst) | 2 |
| Other specified disorders of lymphatics (including chylothorax) | 1 |
| Spina bifida cystica, lumbar, with unspecified hydrocephalus | 1 |
| Total | 306 |
CNS, central nervous system; GI, gastrointestinal.
The cases consisted of 236 whites (47.2%), 222 Hispanics (44.4%), 34 blacks (6.8%), and 7 unspecified or from other ethnic groups (1.4%). Hispanics consisted of 197 Mexican (88.7%), 8 Central–South American (3.6%), 14 other or unknown Hispanic (6.3%), and 3 without data (1.5%). None were specified as Cuban or Puerto Rican. Race/ethnic comparisons were only made between the 3 major groups. Prevalence rate ratio differences existed between white and black total, but not between white and Hispanic ethnic groups (Table 4; Fig. 1). Rates for LVOT malformations as a group and by individual diagnosis (AVS, CoA, and HLHS) differed significantly by infant sex. Rates by race/ethnicity did not vary for females but were significantly different across groups for males.
Table 4.
Univariate Prevalence Relative Risk Ratios for Noncomplex Left Ventricular Outflow Tract Malformations, Texas 1999 –2001
| AVS
|
COA
|
HLHS
|
LVOT combined
|
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | PRR | 95% CI | PRR | 95% CI | PRR | 95% CI | PRR | 95% CI |
| Sex | ||||||||
| Female | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Male | 2.71** | (1.70–4.31) | 1.51** | (1.17–1.96) | 1.95** | (1.40–2.70) | 1.77*** | (1.48–2.13) |
| Ethnicity | ||||||||
| White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Hispanic | 0.74 | (0.49–1.10) | 1.05 | (0.84–1.31) | 0.83 | (0.62–1.09) | 0.94 | (0.81–1.09) |
| Black | 0.57 | (0.29–1.11) | 0.71* | (0.54–0.93) | 0.75 | (0.51–1.08) | 0.74** | (0.61–0.90) |
| Maternal age | ||||||||
| <25 years | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| 25–34 years | 2.51*** | (1.52–4.15) | 1.08 | (0.82–1.41) | 1.37 | (0.98–1.91) | 1.36** | (1.12–1.65) |
| >34 years | 3.23*** | (1.68–6.18) | 1.30 | (0.86–1.95) | 1.75* | (1.09–2.82) | 1.69*** | (1.28–2.22) |
| Maternal education | ||||||||
| <12 years | 0.52* | (0.30–0.88) | 0.83 | (0.61–1.13) | 1.15 | (0.78–1.69) | 0.84 | (0.68–1.04) |
| 12 years | 0.76 | (0.47–1.23) | 0.91 | (0.67–1.24) | 1.19 | (0.80–1.75) | 0.84 | (0.68–1.04) |
| >12 years | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| County residence | ||||||||
| Border | 0.54 | (0.25–1.17) | 1.84*** | (1.35–2.50) | 1.07 | (0.69–1.66) | 1.29* | (1.02–1.63) |
| Nonborder | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Birthplace | ||||||||
| US born | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Mexican born | 0.86 | (0.51–1.46) | 1.12 | (0.83–1.51) | 0.96 | (0.65–1.40) | 1.01 | (0.81–1.25) |
P < 0.05.
P < 0.01.
P < 0.001.
PRR, prevalence relative risk ratio.
Figure 1.
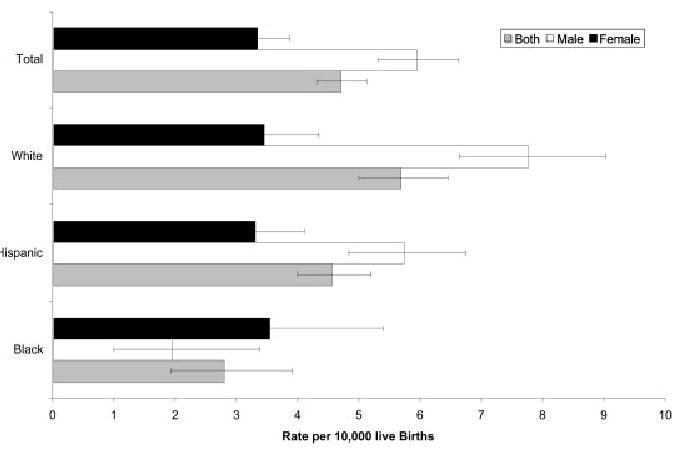
Prevalence rates and 95% CIs of LVOT malformations by sex and race/ethnicity, Texas, 1999 –2001.
Increases in maternal age were associated with higher rates of LVOT malformations. These increases were similar for all diagnoses except CoA (Fig. 2; Table 4). Maternal education level and maternal birthplace were not significant factors, with the exception of a lower PRR for mothers with <12 years of education in the AVS group.
Figure 2.
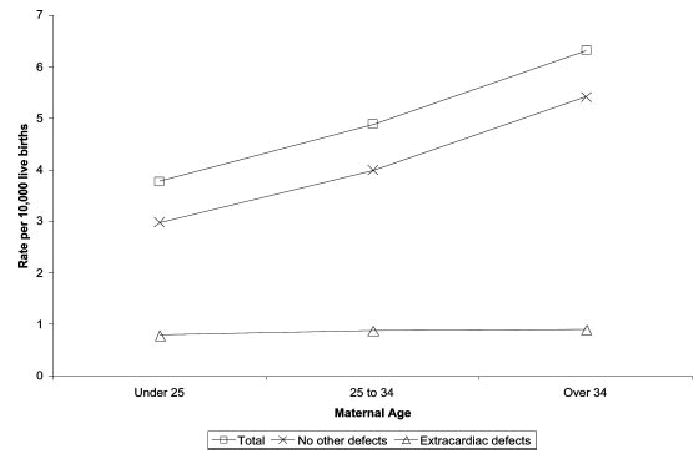
Prevalence rates of noncomplex LVOT malformations by maternal age, Texas, 1999 –2001, by total group, presence of noncardiac birth defect, and no other birth defect, plotted by maternal age.
The prevalence of LVOT malformations as a group was higher in the border counties compared to the nonborder counties, primarily due to increased prevalence of CoA. The CoA cases were further divided into CoA with and without a ventricular septal defect. Prevalence comparisons of CoA cases with and without VSD in border counties were not statistically different (data not shown). The unadjusted prevalences for Mexican-born Hispanics living in a border county (5.63/10,000 live births; 95% CI, 3.9–7.8), U.S.-born Hispanics living in a border county (5.63/10,000 live births; 95% CI, 4.0–7.9), and U.S.-born whites living in a nonborder county (5.47/10,000 live births; 95% CI, 4.8–6.3) were not statistically different (data not shown).
The final Poisson multivariate regression model for the total LVOT group contained sex, race/ethnicity, maternal age, border county residence, and the interactive variable sex by race/ethnicity (Table 5). AVS modeling used white and Hispanic cases only, due to the paucity of black AVS cases. The final model for AVS included sex, maternal age, race/ethnicity, border county residence, and the border county by race/ethnicity interactive term. Other AVS models without the interactive term, with or without the main effects race/ethnicity and border county residence were rejected (P < 0.01) when compared to the full model. The final model for CoA included sex, race/ethnicity, border county residence, and sex by race/ethnicity, whereas the HLHS final model included sex and maternal age variables.
Table 5.
Multivariate Analysis of Noncomplex Left Ventricular Outflow Tract Malformations in Texas, 1999 –2001: Final Models
| AVS
|
COA
|
HLHS
|
LVOT Combined
|
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | PRR | 95% CI | PRR | 95% CI | PRR | 95% CI | PRR | 95% CI |
| Sex (male) | 3.56 | (2.11–5.98) | — | — | 1.97 | (1.40–2.77) | — | — |
| Maternal age | ||||||||
| <25 years | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||||
| 25–34 years | 2.58 | (1.54–4.33) | — | — | 1.44 | (1.02–2.04) | 1.35 | (1.10–1.65) |
| >34 years | 2.96 | (1.47–5.95) | — | — | 1.85 | (1.11–3.07) | 1.66 | (1.24–2.23) |
| Border county | — | — | 2.17 | (1.52–3.09) | — | — | 1.42 | (1.09–1.86) |
| Sex by race/ethnicity | ||||||||
| White male | 1.00 (ref) | 1.00 (ref) | ||||||
| Hispanic male | — | — | 0.70 | (0.39–1.25) | — | — | 0.73 | (0.49–1.11) |
| Black male | — | — | 0.26 | (0.08–0.85) | — | — | 0.21 | (0.09–0.46) |
| Border county by race/ethnicity | ||||||||
| Border county white | 1.00 (ref) | — | — | — | — | — | — | |
| Border county Hispanic | 0.16 | (0.03–0.52) | — | — | — | — | — | — |
PRR, prevalence relative risk factor.
DISCUSSION
This study examined population-based data on noncomplex diagnoses of AVS, CoA, and HLHS as part of a grouping based on developmental mechanisms. This grouping, LVOT obstruction malformations, was studied for prevalence differences to help elucidate possible genetic and environmental etiologies. The strength of this study is the Texas Birth Defects Registry, which uses an active ascertainment of cases derived from multiple sources, extending over the first year of an infant’s life.
Overall prevalences for AVS, CoA, and HLHS appear similar to those reported other studies in U.S. populations from different geographical locations and time points (Fixler et al., 1990; Storch and Mannick, 1992; Gillum, 1994; Ferencz, 1997; Loffredo, 2000; Pradat et al., 2003). Prevalence ratios were significantly higher for male sex and increased maternal age for all diagnoses. The racial difference of higher rates for whites compared to blacks is confirmed for CoA and LVOT malformations as a group in this study. Rate differences between white and black groups were not significant for AVS and HLHS cases, likely due to insufficient case numbers for blacks. Previous suggestions of an intermediate (but not significantly different) prevalence of Hispanics between whites and blacks (Pradat et al., 2003) could not be confirmed here.
The increased prevalences for LVOT malformations and CoA in males have a distinct racial/ethnic pattern, which has not been reported in previous studies. Compared to the white male reference group, a statistically significant difference was evident for black males, and although present, the difference for Hispanic males was not significant in the multivariate analysis. This suggests an ethnic susceptibility difference manifest only in males. Many birth defects, including neural tube defects (NTDs), pyloric stenosis, and cleft lip and palate, have deviations in the sex ratio (Shaw et al., 2003). Differences in sex ratios between ethnic groups have been observed for cleft lip, with a male preponderance in whites but a female preponderance among Japanese (Gorlin et al., 2001). No satisfactory explanation for altered sex ratios has been found.
Increasing maternal age was associated with higher prevalence rates for the combined LVOT group, AVS, HLHS, but not CoA. Increased prevalences of LVOT malformations with increasing maternal age in this study were not related to chromosomal malformations or occurrence with multiple congenital malformations (Fig. 2). Many studies have noted increased prevalences of LVOT malformations among older mothers (Ferencz, 1997; Pradat et al., 2003). In contrast with the data from the California Birth Defects Monitoring Program where the increased prevalences at older maternal ages were from cases with multiple congenital anomalies, the current study noted a flat prevalence for multiple anomaly cases and increasing prevalences with increasing age in the isolated heart defect cases. The reason for this phenomenon is unknown but may reflect a surrogate for advanced paternal age, with the possibility of a Mendelian inheritance from increased frequency of new mutations.
The increased risk for CoA among border county residents did not appear to be related to maternal education or the mother’s birthplace, suggesting a geographical environmental factor. The effect of border county residence for CoA but not the other LVOT malformations suggests a different etiology or susceptibility factor in CoA. Previous data have shown that CoA with a ventricular septal defect (VSD) may be a distinct group from CoA without VSD (Wollins et al., 2001). The possibility of differences by CoA subgroup accounting for the higher border county prevalence does not appear likely, as this study did not reveal a difference between CoA with and without a VSD when compared by border county residence. The decreased rate of AVS among Hispanics in border counties may be chance effect from small sample size (4 Hispanic cases).
NTDs are also more prevalent among border county residents, particularly Hispanics, and specifically for children born to Mexican-born Hispanic women compared to U.S.-born Hispanic women (Hendricks et al., 1999). Possible connections between the increased prevalences of NTDs and LVOT malformations may be folate or exposure to solvents (Suarez et al., 2000; Brender et al., 2002; Felkner et al., 2002); however, folate may play less of a role in the etiology of LVOT malformations (McBride et al., 2004).
Recent geographic prevalence differences for HLHS have also been observed in large urban areas in Wisconsin (Cronk et al., 2004). The increased prevalences along the Texas–Mexico border noted above and the independent observations in Wisconsin lend support to an environmental etiology for the LVOT malformations.
Multivariate analysis for LVOT malformations highlights the importance of maternal age, border county residence and the interacting effect of sex and ethnicity, as these variables remained significant at the 5% level. CoA mirrored the total group, except for absence of maternal age effect. Sex and maternal age alone were significant for AVS and HLHS without any ethnic/ethnic difference. Whether the difference between AVS and HLHS groups and CoA reflects a difference in etiologies or is due to an effect of smaller cell numbers cannot be determined.
One possible limitation of this study is the ascertainment of pregnancy terminations under 20 weeks of gestation. Terminations are not actively screened in Texas, and terminations with birth defects account for only 2% of all birth defects identified in all categories. However, there does not appear to be an excess of AVS, CoA, or HLHS among early terminations, suggesting that terminations do not occur due to the presence of these defects, in contrast to NTDs, trisomies, and abdominal wall defects (Special Report from the Texas Birth Defects Registry; http://www.tdh.state.tx.us/tbdmd/monitor/terminations.htm). Thus the overall impact of pregnancy terminations on rates of LVOT malformations is probably minimal.
These findings have several practical and research implications. The presence of increased prevalences of CoA along the Texas–Mexico border is cause for concern. Possible environmental causes for the increased prevalence among Texas–Mexico border county residents need confirmation with further data collection over time. The decreased LVOT prevalences in blacks compared to whites raises the possibility of using mapping by admixture linkage disequilibrium techniques (Patterson et al., 2004) to identify susceptibility loci. Further exploration of the increased rates for white males (compared to black males), and the lack of difference for females, will be required to identify the etiology behind the sex differential. The suggestion of the intermediate rates for Hispanic males should be explored, perhaps by combining information from several databases.
Footnotes
Grant sponsor: March of Dimes (to J.W.B.); Grant sponsor: National Institutes of Health (NIH); Grant numbers: K23 HL70823; K12 HD43372; K12 HD41648 (to K.L.M.); R01 HD39056 (to J.W.B.); Grant sponsor: Centers for Disease Control and Prevention via Texas Center for Birth Defects Research and Prevention, Birth Defects Epidemiology and Surveillance Branch, Texas Department of State Health Services; Grant number: U50/CCU613232.
References
- Agresti A. 2002. Categorical data analysis. 2nd ed. Hoboken, N.J.: Wiley-Interscience. 710 p.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep. 2002;50:1– 85. [PubMed] [Google Scholar]
- Brender J, Suarez L, Hendricks K, et al. Parental occupation and neural tube defect-affected pregnancies among Mexican Americans. J Occup Environ Med. 2002;44:650 – 656. doi: 10.1097/00043764-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Clark EB. Pathogenetic mechanisms of congenital cardiovascular malformations revisited. Semin Perinatol. 1996;20:465– 472. doi: 10.1016/s0146-0005(96)80062-0. [DOI] [PubMed] [Google Scholar]
- Clementi M, Notari L, Borghi A, Tenconi R. Familial congenital bicuspid aortic valve: a disorder of uncertain inheritance. Am J Med Genet. 1996;62:336 –338. doi: 10.1002/(SICI)1096-8628(19960424)62:4<336::AID-AJMG2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Cronk CE, Pelech AN, Malloy ME, McCarver DG. Excess birth prevalence of Hypoplastic Left Heart syndrome in eastern Wisconsin for birth cohorts 1997–1999. Birth Defects Res Part A Clin Mol Teratol. 2004;70:114 –120. doi: 10.1002/bdra.20007. [DOI] [PubMed] [Google Scholar]
- Felkner M, Suarez L, Hendricks K, Gunter EW. Blood folate levels on the Texas-Mexico border. Tex Med. 2002;98:58 – 60. [PubMed] [Google Scholar]
- Ferencz C. 1997. Genetic and environmental risk factors of major cardiovascular malformations: the Baltimore-Washington infant study, 1981–1989. Armonk, NY: Futura. 463 p.
- Fixler DE, Pastor P, Chamberlin M, et al. Trends in congenital heart disease in Dallas County births. 1971–1984. Circulation. 1990;81:137–142. doi: 10.1161/01.cir.81.1.137. [DOI] [PubMed] [Google Scholar]
- Gillum RF. Epidemiology of congenital heart disease in the United States. Am Heart J. 1994;127:919 –927. doi: 10.1016/0002-8703(94)90562-2. [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Cohen MM, Hennekam RCM. 2001. Syndromes of the head and neck. 4th ed. Oxford: Oxford University Press. 1283 p.
- Grobman W, Pergament E. Isolated hypoplastic left heart syndrome in three siblings. Obstet Gynecol. 1996;88:673– 675. doi: 10.1016/0029-7844(96)00178-0. [DOI] [PubMed] [Google Scholar]
- Hendricks KA, Simpson JS, Larsen RD. Neural tube defects along the Texas-Mexico border, 1993–1995. Am J Epidemiol. 1999;149:1119 –1127. doi: 10.1093/oxfordjournals.aje.a009766. [DOI] [PubMed] [Google Scholar]
- Hornberger LK, Need L, Benacerraf BR. Development of significant left and right ventricular hypoplasia in the second and third trimester fetus. J Ultrasound Med. 1996;15:655– 659. doi: 10.7863/jum.1996.15.9.655. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG. 1998. Applied regression analysis and other multivariable methods. 3rd ed. Pacific Grove, CA: Duxbury Press. 798 p.
- Lewin MB, McBride KL, Pignatelli R, et al. Echocardiographic evaluation of asymptomatic parental and sibling cardiovascular anomalies associated with congenital left ventricular outflow tract lesions. Pediatrics. 2004;114:691– 696. doi: 10.1542/peds.2003-0782-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo CA. Epidemiology of cardiovascular malformations: prevalence and risk factors. Am J Med Genet. 2000;97:319 –325. doi: 10.1002/1096-8628(200024)97:4<319::aid-ajmg1283>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Loffredo CA, Chokkalingam A, Sill AM, et al. Prevalence of congenital cardiovascular malformations among relatives of infants with hypoplastic left heart, coarctation of the aorta, and d-transposition of the great arteries. Am J Med Genet. 2004;124A:225–230. doi: 10.1002/ajmg.a.20366. [DOI] [PubMed] [Google Scholar]
- Mazzanti L, Cacciari E. Congenital heart disease in patients with Turner’s syndrome. Italian Study Group for Turner Syndrome (ISGTS) J Pediatr. 1998;133:688 – 692. doi: 10.1016/s0022-3476(98)70119-2. [DOI] [PubMed] [Google Scholar]
- McBride KL, Fernbach S, Menesses A, et al. A family-based association study of congenital left-sided heart malformations and 5,10 methylenetetrahydrofolate reductase. Birth Defects Res Part A Clin Mol Teratol. 2004;70:825– 830. doi: 10.1002/bdra.20049. [DOI] [PubMed] [Google Scholar]
- Menahem S. Familial aggregation of defects of the left-sided structures of the heart. Int J Cardiol. 1990;29:239 –240. doi: 10.1016/0167-5273(90)90227-v. [DOI] [PubMed] [Google Scholar]
- Patterson N, Hattangadi N, Lane B, et al. Methods for high-density admixture mapping of disease genes. Am J Hum Genet. 2004;74:979 –1000. doi: 10.1086/420871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradat P, Francannet C, Harris JA, Robert E. The epidemiology of cardiovascular defects, part I: a study based on data from three large registries of congenital malformations. Pediatr Cardiol. 2003;24:195–221. doi: 10.1007/s00246-002-9401-6. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. 1998. Modern epidemiology. 2nd ed. Philadelphia: Lippincott-Raven. 737 p.
- Shaw GM, Carmichael SL, Kaidarova Z, Harris JA. Differential risks to males and females for congenital malformations among 2.5 million California births, 1989 –1997. Birth Defects Res Part A Clin Mol Teratol. 2003;67:953–958. doi: 10.1002/bdra.10129. [DOI] [PubMed] [Google Scholar]
- Stoll C, Alembik Y, Dott B. Familial coarctation of the aorta in three generations. Ann Genet. 1999;42:174 –176. [PubMed] [Google Scholar]
- Storch TG, Mannick EE. Epidemiology of congenital heart disease in Louisiana: an association between race and sex and the prevalence of specific cardiac malformations. Teratology. 1992;46:271–276. doi: 10.1002/tera.1420460311. [DOI] [PubMed] [Google Scholar]
- Suarez L, Hendricks KA, Cooper SP, et al. Neural tube defects among Mexican Americans living on the US-Mexico border: effects of folic acid and dietary folate. Am J Epidemiol. 2000;152:1017–1023. doi: 10.1093/aje/152.11.1017. [DOI] [PubMed] [Google Scholar]
- Tikkanen J, Heinonen OP. Risk factors for coarctation of the aorta. Teratology. 1993;47:565–572. doi: 10.1002/tera.1420470608. [DOI] [PubMed] [Google Scholar]
- Wollins DS, Ferencz C, Boughman JA, Loffredo CA. A population-based study of coarctation of the aorta: comparisons of infants with and without associated ventricular septal defect. Teratology. 2001;64:229 –236. doi: 10.1002/tera.1069. [DOI] [PubMed] [Google Scholar]
- Wren C, Richmond S, Donaldson L. Temporal variability in birth prevalence of cardiovascular malformations. Heart. 2000;83:414 – 419. doi: 10.1136/heart.83.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]


