By definition, annual plants complete their life cycle, from germination to seed set, within the course of a year. Summer annuals, the most prevalent, complete their life cycle over a few short months in the summer. Winter annuals germinate in the fall, over winter as seedlings, and then flower in the spring. Although some of the more noxious weeds, including Downey brome (Bromus tectorum), are winter annuals, the most readily recognized winter annual to a plant biologist is Arabidopsis (Arabidopsis thaliana). Examples of both summer and winter annuals can be found among Arabidopsis ecotypes, making it an ideal tool for studying the diversity in flowering time. The lab of Caroline Dean used this to their advantage in their article “Analysis of the molecular basis of flowering time variation in Arabidopsis accessions,” which appeared in our June 2003 issue and had been cited 48 times as of January 2006 (Thompson ISI Web of Science, http://www.isinet.com). It is this month's High Impact article.
BACKGROUND
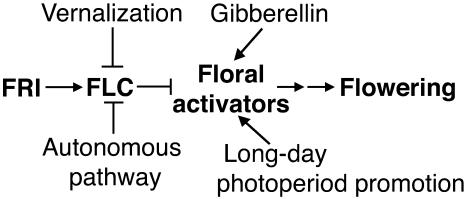
Control of flowering time involves a complex interplay of environmental and developmental factors, the timing of which is crucial to the reproductive success of the plant. If a plant flowers during a time that is unfavorable for seed set or pollination, it will most likely become a genetic dead-end. Four main pathways have been identified to be important for the regulation of key floral regulatory genes, two responding to external stimuli and two to endogenous cues (Fig. 1). The absorption of light by photoreceptors enables plants to detect seasonal changes by daylength, while vernalization signals seasonal changes via cold, a requirement of some plants (i.e. winter annuals) to stimulate flowering. In the absence of long-day promotion, gibberellin has been shown to promote flowering, as suggested by the delayed flowering phenotype of biosynthetic mutants when grown under short days. Finally, the levels of floral repressor FLOWERING LOCUS C (FLC) RNA are reduced by gene products of the autonomous pathway, enabling the plant to flower.
Figure 1.
Regulation of flowering in Arabidopsis. Both vernalization and autonomous pathway genes negatively regulate FLC expression, allowing the plant to transition from a vegetative to flowering state. Gibberellin and long-day photoperiod positively regulate floral activators downstream of FLC.
In Arabidopsis, two genes, FRIGIDA (FRI) and FLC, act synergistically to repress flowering. FRI up-regulates expression of FLC, while FLC, which encodes a MADS-box transcription factor, represses flowering by preventing the expression of floral activators. Vernalization releases the repression of flowering by FLC by decreasing gene expression through histone remodeling of the FLC locus (for review, see Sung and Amasino, 2005).
Not all Arabidopsis ecotypes are winter annuals. The so-called “rapid-cycling” accessions include both Columbia (Col) and Landsberg erecta (Ler), two of the most commonly studied. In both of these accessions, the summer annual flowering behavior is the result of a mutation in FRI. In the instances where FRI is nonfunctional, the autonomous pathway gene products keep FLC levels low, resulting in an early flowering, summer annual phenotype. Another route to the early flowering, summer annual phenotype is through a decrease in FLC expression, which has been found in some accessions (Michaels et al., 2003).
WHAT WAS SHOWN
The summer annual habit of Arabidopsis can arise from the loss of FRI function, resulting in FLC alleles that are no longer up-regulated by FRI or a weak FLC allele. In this work by Gazzani et al. (2003), rapid-cycling accessions of Arabidopsis were analyzed to determine the cause of the early flowering. Initially, the study focused on FRI variation among five accessions. The early flowering accessions Cvi, Shakhdara, Wil-2, Kondara, and Kz-9 were selected since none contains the FRI deletion of Col or Ler. All five were found to contain amino acid substitutions in FRI sequences. Two of these, Cvi and Wil-2, contained identical substitutions in the first intron with Cvi additionally containing an in-frame stop codon. Although the functionality of the Wil-2 FRI allele was unable to be determined, genetic analysis of Cvi suggests that FRI is most likely nonfunctional and the cause of the early flowering phenotype of this ecotype.
The FRI alleles from the remaining three accessions, Shakhdara, Kondara, and Kz-9, differed from a known active FRI allele by only a few amino acids, and genetic analysis demonstrated FRI to be functional in these plants. When crossed with Col (nonfunctional FRI and strong FLC locus), these three reverted to long flowering times but remained rapid cycling when crossed with a plant containing a functional FRI and a nonfunctional FLC. Taken together, this suggests that, unlike what was found for Cvi, the early flowering phenotypes of Shakhdara, Kondara, and Kz-9 are not due to a nonfunctioning FRI but instead more likely to a weakly functioning FLC allele.
To further look into FLC involvement in flowering time, promoter and intron sequences from the strong FLC allele of Col and the weak FLC allele of C24 were examined. Single nucleotide variations were found between them along with a 30-bp repeat in the first intron of Col. This insert is located in a region of the protein required for the repression of FLC by an autonomous pathway gene (Sheldon et al., 2002) and upon further examination was found in five out of 23 other accessions. It was concluded that this insertion does not interfere with regulation of flowering time because the accessions containing this insertion have both strong and weak FLC alleles. Ler, which has a weak FLC allele yet encodes a protein identical to Col, was also investigated. Within Ler intron 1, a large 1.2-kb insert was found with many characteristics reminiscent of a transposable element, including 9-bp direct repeats delimiting the sequence. A paper examining the contribution of FLC to early flowering habit by Michaels et al. (2003) demonstrated that the insert in the first intron of Ler was indeed a transposable element and the cause of the weak allele. This insert was not found in the other early flowering accessions examined, and Gazzani et al. (2003) hypothesized that the insertion could be potentially reducing transcription of FLC in Ler and the cause of the FLC weak allele in this ecotype.
THE IMPACT
As discussed by Gazzani et al. (2003), the rapid-cycling phenotype of Arabidopsis can arise from the loss of FRI function or a weak FLC allele. A study by Michaels et al. (2004) identified another mutation that can cause the shift from winter to summer annual phenotype. FRIGIDA LIKE 1, a member of the same gene family as FRI, was found to be required for the FRI-mediated up-regulation of FLC. Mutations in the FRL1 locus did not affect flowering time of autonomous-pathway mutants, a photoperiod-pathway mutant, or those lacking FRI activity, indicating that it is specific for FRI activity and adding another piece to the puzzle of flowering time regulation.
How much of the variation found in flowering time of wild accessions is not due to an alteration FRI? An investigation into this launched by Werner et al. (2005) involved observing the time to flowering in 145 accessions grown in long-day photoperiods both with and without vernalization. These accessions were then genotyped for the FRI lesions found in either Col or Ler. Among these accessions, 40% of the variation in flowering times was found to be due to a FRI deletion as found in either Col or Ler. In the process of determining why the remaining were late flowering, late-flowering alleles that map to neither FRI nor FLC, along with new FRI and FLC loss-of-function alleles, were uncovered. The large number of accessions examined revealed that disruptions of FLC alleles are not very common, possibly due to greater selective pressure.
CONCLUSION
The acquisition of the early flowering phenotype in Arabidopsis appears to have arisen multiple times during evolution and by different pathways. The article by Gazzani et al. and numerous others that have followed have helped in the elucidation of these pathways and have furthered our understanding of the regulation of flowering time.
References
- Gazzani S, Gendall AR, Lister C, Dean C (2003) Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol 132: 1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Bezerra IC, Amasino RM (2004) FRIGIDA-related genes are required for the winter-annual habit in Arabidopsis. Proc Natl Acad Sci USA 101: 3281–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, He Y, Scortecci KC, Amasino RM (2003) Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis. Proc Natl Acad Sci USA 100: 10102–10107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon CC, Conn ES, Dennis ES, Peacock WJ (2002) Different regulatory regions are required for the vernalization-induced repression of FLOWERING LOCUS C and for the epigenetic maintenance of repression. Plant Cell 14: 2527–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Amasino RM (2005) Remembering winter: toward a molecular understanding of vernalization. Annu Rev Plant Biol 56: 491–508 [DOI] [PubMed] [Google Scholar]
- Werner JD, Borevitz JO, Uhlenhaut NH, Ecker JR, Chory J, Weigel D (2005) FRIGIDA-independent variation in flowering time of natural Arabidopsis thaliana accessions. Genetics 170: 1197–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]