Abstract
The existence of nickel (Ni) deficiency is becoming increasingly apparent in crops, especially for ureide-transporting woody perennials, but its physiological role is poorly understood. We evaluated the concentrations of ureides, amino acids, and organic acids in photosynthetic foliar tissue from Ni-sufficient (Ni-S) versus Ni-deficient (Ni-D) pecan (Carya illinoinensis [Wangenh.] K. Koch). Foliage of Ni-D pecan seedlings exhibited metabolic disruption of nitrogen metabolism via ureide catabolism, amino acid metabolism, and ornithine cycle intermediates. Disruption of ureide catabolism in Ni-D foliage resulted in accumulation of xanthine, allantoic acid, ureidoglycolate, and citrulline, but total ureides, urea concentration, and urease activity were reduced. Disruption of amino acid metabolism in Ni-D foliage resulted in accumulation of glycine, valine, isoleucine, tyrosine, tryptophan, arginine, and total free amino acids, and lower concentrations of histidine and glutamic acid. Ni deficiency also disrupted the citric acid cycle, the second stage of respiration, where Ni-D foliage contained very low levels of citrate compared to Ni-S foliage. Disruption of carbon metabolism was also via accumulation of lactic and oxalic acids. The results indicate that mouse-ear, a key morphological symptom, is likely linked to the toxic accumulation of oxalic and lactic acids in the rapidly growing tips and margins of leaflets. Our results support the role of Ni as an essential plant nutrient element. The magnitude of metabolic disruption exhibited in Ni-D pecan is evidence of the existence of unidentified physiological roles for Ni in pecan.
Relatively little is known about the role of nickel (Ni) in plant nutrition, physiology, and metabolism, especially in woody perennial species, such as pecan (Carya illinoinensis [Wangenh.] K. Koch). Ni was suspected of possessing a metabolic role in plants when discovered as a constituent of plant ash in the early 20th century. Evidence for a key metabolic role was strengthened with observation of field-level growth responses to foliar Ni applications to crops as diverse as wheat (Triticum aestivum), potatoes (Solanum tuberosum), and broad beans (Vicia faba; Roach and Barclay, 1946; Dobrolyubskii and Slavvo, 1957; Welch, 1981). The likelihood of an essential role for Ni became evident with the discovery that urease (EC 3.5.1.5; urea amidohydrolase), a ubiquitous enzyme, requires Ni for activation (Dixon et al., 1975). Ni was soon found essential to legumes (Eskew et al., 1983, 1984) and subsequently was found essential to several temperate cereal crops (Brown et al., 1987a, 1987b, 1990). Its essentiality to higher plants was proposed by Brown et al. (1987a), it was generally accepted as a likely essential nutrient element to higher plants (Gerendás et al., 1999; Sirko and Brodzik, 2000; Bloom, 2002; Marschner, 2002), and it was added to the U.S. Department of Agriculture (USDA) list of essential plant nutrient elements (Hull, 2003). Ni was recently recognized as an essential plant nutrient (Association of American Plant Food Control Officials, 2005), thus enabling manufacture and sale of Ni fertilizers in the United States.
Although Ni is a recognized essential mineral nutrient element for higher plants, its agricultural and biological significance is poorly understood. This is largely because of the low levels thought to be needed by plants (about 1–100 ng g−1 dry weight) in relation to the relative abundance of Ni in essentially all soils (>5 kg ha−1). The existence of field-level Ni deficiency in crops was only recently discovered, wherein mouse-ear, a century-old malady of pecan trees, and replant disease were found to be Ni deficiencies (Wood et al., 2004a, 2004b, 2004c). Severe Ni deficiency has subsequently been identified in containerized river birch (Betula nigra; Ruter, 2005) and has putatively been identified in several other crops (Wood, 2006).
The role of Ni in plant metabolism is poorly understood. Whereas many proteins contain Ni (Thomson, 1982), Ni nutrition of higher plants and its physiological significance, especially to woody perennials, have received little attention. There are several enzyme systems (NiFe-hydrogenase, carbon monoxide dehydrogenase, acetyl-CoA decarbonylase synthase, methyl-coenzyme M reductase, superoxide dismutase, Ni-dependent glyoxylase, aci-reductone dioxygenase, and methyleneurease) in bacteria and lower plants that are activated by Ni (Walsh and Orme-Johnson, 1987; Mulrooney and Hausinger, 2003); however, the activation of urease appears, to date, to be the only enzymatic function of Ni in higher plants (Gerendás et al., 1999). Urease contains two Ni ions at the active site (Ciurli, 2001). Ni can also replace Zn or Fe, and other metal ions, in certain other metalloenzymes of lower plants (Mulrooney and Hausinger, 2003). Circumstantial evidence indicates that ureide-transporting species, such as pecan, possess a higher Ni requirement than amide-transporting species (Wood, 2006), thus raising the possibility that ureide transporters might possess enzymes, other than urease, that require Ni for activation or for enhanced activity. Likely candidates are one or more enzymes affecting ureide catabolism.
The metabolic effects of Ni deficiency have, to date, only been reported for a few annual species. For example, Ni-deficient (Ni-D) barley (Hordeum vulgare) exhibited disrupted metabolism of amino acids, malate, and various inorganic anions (e.g. SO4−, Cl−, Pi, and NO3−; Brown et al., 1990). Ni deficiency caused urea accumulation in foliage of soybean (Glycine max) and cowpea (Vigna unguiculata; Eskew et al., 1983, 1984; Walker et al., 1985), affected amino acid metabolism in cowpea (Walker et al., 1985), and reduced urease activity, induced metabolic nitrogen deficiency, and affected amino acids, amides (Gln and Asn), and urea cycle intermediates (Arg, Orn, and citrulline) in several nonwoody species (rye [Secale cereale], wheat, soybean, rape [Brassica tournefortii], zucchini [Cucurbita pepo], and sunflower [Helianthus annuus]; Gerendás and Sattelmacher, 1997).
Morphological symptoms of Ni deficiency in a woody perennial were recently reported (Wood et al., 2004a, 2004c); however, there is a dearth of information regarding the influence of Ni deficiency on metabolism and associated biochemical symptoms of woody perennial species. We hypothesized that Ni deficiency in pecan, a woody perennial, disrupts several metabolic pathways and thus leaves a biochemical signature. Our objectives were to (1) identify the influence of Ni deficiency on ureides, amino acids, and organic acids in young developing pecan foliage; and (2) identify metabolic pathways being influenced by Ni deficiency.
RESULTS
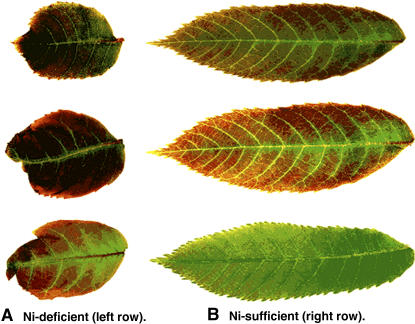
The morphological symptoms in pecan trees with Ni deficiency have already been described in detail (Wood et al., 2004a). One key symptom is dwarfing of leaves and leaflets. Dwarfing of foliage was a primary indicator of Ni deficiency in this study in which young foliage from Ni-D and Ni-sufficient (Ni-S) seedlings was such that same-age young leaflets from deficient trees were considerably smaller and thicker, with blunted and cupped apical tips. Young Ni-D leaflets also exhibited considerably more reddish pigmentation than Ni-S seedlings. As leaflets aged, Ni-D leaflets became darker green, thicker, and remained much smaller in leaf area than Ni-S leaflets (Fig. 1).
Figure 1.
A comparison of color and shape and size of Ni-D (A) and Ni-S (B) pecan leaves.
Absorbance
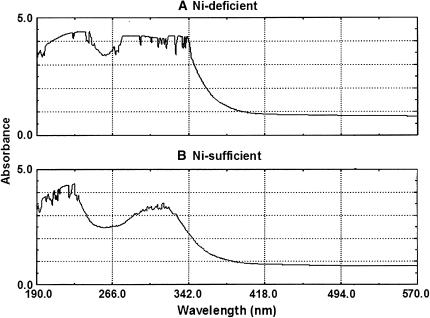
Leaf extraction has been fractioned into a ≤10-kD fraction and a ≥10-kD fraction. The fractions with a molecular mass ≤10 kD exhibited primary UV absorbance at 190- to 230- and 266- to 342-nm spectral zones (Fig. 2). Distinct differences existed in the absorbance curves between the fractions from Ni-D and Ni-S foliage, with absorbance for Ni-D foliage being much greater at 220 nm, but approximately equivalent to Ni-S foliage at 190 nm (Table I).
Figure 2.
Comparative UV absorbance on the fractions with molecular mass ≤10 kD from Ni-D (A) and Ni-S (B) young pecan leaf extracts in water.
Table I.
Comparative UV absorbance of fractions with molecular mass ≤10 kD of Ni-S and Ni-D foliage from young pecan seedling trees
| Sample
|
Dilutiona
|
Absorbance
|
|
|---|---|---|---|
| 190 nm | 220 nm | ||
| Ni-D | 100 | 3.45 | 3.85 |
| Ni-S | 100 | 3.45 | 2.50 |
Each time 0.8 g (fresh weight) of young pecan leaves was dipped into 2 mL of HPLC-grade water and then homogenized and centrifuged. A total of 2 mL of sample solutions was prepared after filtering with a 10-kD filter to remove large molecules. These sample solutions (molecular mass ≤10 kD) were further diluted 100-fold for UV spectrum scanning.
Ureides
Pecan leaves appeared to contain as many as 12 ureides (Table II); however, only eight were identified. These were xanthine, allantoic acid, uric acid, allantoin, ureidoglycolate, urea, Asn, and citrulline. The ureide pool in the ≤10-kD fraction existed at a slightly higher level in Ni-S leaflets than in Ni-D leaflets (Table II, identified ureides). The dominant ureide in Ni-S leaflets was urea, followed by ureidoglycolate, xanthine, allantoic acid, and citrulline, whereas the dominant ureide in Ni-D foliage was ureidoglycolate, followed by allotonic acid, xanthine, citrulline, and urea.
Table II.
A comparative HPLC separationa of ureide composition (based on identical retention time to standard references) and concentration in fractions with molecular mass ≤10 kD of Ni-S and Ni-D foliage from young pecan seedling tree
| Ureide or Unknown Substance
|
Retention Time
|
Concentration X ± sdb
|
Peak Sizec
|
||
|---|---|---|---|---|---|
| Ni-D | Ni-S | Ni-D | Ni-S | ||
| min | μm/mL | ||||
| Identified ureides | |||||
| Allantoic acid | 5.44 | 26.33 ± 0.63a | 13.99 ± 0.16b | ||
| Asn | 5.81 | ≤5.00a | ≤5.00a | ||
| Citrulline | 5.89 | 17.76 ± 0.40a | 12.90 ± 0.58b | ||
| Allantoin | 5.93 | ≤5.00a | ≤5.00a | ||
| Uric acid | 6.06 | ≤5.00a | ≤5.00a | ||
| Urea | 6.14 | 13.46 ± 1.73b | 71.85 ± 17.39a | ||
| Xanthine | 6.90 | 21.13 ± 0.48a | 15.96 ± 0.93b | ||
| Ureidoglycolate | 7.24 | 56.83 ± 0.40a | 43.66 ± 0.66b | ||
| Unknown substances | |||||
| I | 7.82 | +++ | ++ | ||
| II | 9.60 | ++ | ++ | ||
| III | 12.46 | + | ++ | ||
| IV | 14.64 | + | ++ | ||
To further confirm the identification of ureides if their retention time was very close to each other (e.g. Asn anhydrous and l-citrulline), two different HPLC columns were run with two different mobile phases; in this table, the retention time was listed only from the platinum EPS C18 column.
Means (±sd) followed by the same letter are not significantly different (t test, P ≤ 0.05).
+++, Large; ++, medium; +, small.
Ureide catabolism in Ni-S foliage exhibited urea and ureidoglycolate as the dominant ureido nitrogen forms; ureidoglycolate and allantoic acid dominated in Ni-D foliage. Ureidoglycolate accumulated in Ni-D foliage to about 1.3-fold, allantoic acid to 1.9-fold, and xanthine to 1.3-fold greater than in Ni-S foliage. Ureidoglycine, also an intermediate of ureide catabolism, was not detected. Ni-D foliage also exhibited a greater citrulline pool (about 1.4-fold), an Orn cycle intermediate. The urea pool in Ni-D foliage was only 19% of that in Ni-S foliage. Concentrations of uric acid, allantoin, and Asn were very low regardless of Ni nutritional status and appeared to be roughly equal between treatments (Table II, identified ureides). The four ureide-like unknown substances were such that one was greater in Ni-D foliage, two were greater in Ni-S foliage (Table II, unknown substances), and all exhibited relatively long retention times eluting from the HPLC column. The accumulation of xanthine, allotonic acid, and ureidoglycolate in Ni-D foliage indicates catabolic disruption as a consequence of Ni deficiency.
Urease Activity
Ni nutritional status of foliage influenced urease activity (Table III). Urease activity was high in Ni-S foliage, whereas activity of the ≥10-kD protein fraction in Ni-D foliage was only 19% of Ni-S foliage. Ni deficiency, therefore, caused substantial loss of urease activity.
Table III.
Urease specific activitya in the protein solutions with molecular mass ≥10 kD of Ni-S and Ni-D foliage from young pecan seedling trees
| Sample | Activityb | Specific Activity (X ± sd)b |
|---|---|---|
| unit/g fresh leaflet | unit/mg protein | |
| Ni-D | 23.64 ± 5.08b | 5.91 ± 1.27b |
| Ni-S | 121.56 ± 13.92a | 30.39 ± 3.48a |
Enzyme activity was expressed by mean (±sd).
Means (±sd) followed by the same letter are not significantly different (t test; P ≤ 0.05).
Free Amino Acids
The quantitatively prominent amino acids in Ni-S young developing foliage were Gly, Ser/Asn, and Val (Table IV). All other free amino acids were present at relatively low concentrations. Ni nutritional status of foliage quantitatively influenced free amino acid composition but did not qualitatively affect the 20 amino acids measured in this study. The total concentration of measured free amino acids in Ni-D leaflets was 2.1-fold that of Ni-S leaflets, with the increase being largely due to increased relative accumulation of Gly (2.4-fold), Val (3.1-fold), Ile (about 2.8-fold), Tyr (2.1-fold), Trp (2.1-fold), and Arg (21.4-fold). Conversely, Ni-D foliage had only 4% as much His and 45% as much Glu as that of Ni-S foliage. Concentrations of the other 12 free amino acids were roughly equivalent, with no significant differences between Ni-S and Ni-D foliage. The above-described shifts in eight free amino acids indicate that Ni deficiency disrupts the conversion of free amino acids to other products, such as peptides, polypeptides, proteins, and nucleic acids, thus disrupting the nitrogen pathways.
Table IV.
A comparative HPLC separation of free amino acid composition (based on identical retention time to standard references) and concentration in the fractions with molecular mass ≤10 kD of Ni-S and Ni-D foliage from young pecan seedling trees
| Amino Acid
|
Retention Time
|
Concentration X ± sda
|
|
|---|---|---|---|
| Ni-D | Ni-S | ||
| min | μm/mL | ||
| Gly | 3.64 | 30.83 ± 3.75a | 12.63 ± 0.93b |
| Ser/Asn | 3.86 | 3.10 ± 0.45a | 3.30 ± 0.17a |
| Asp | 4.47 | 0.20 ± 0.06a | 0.20 ± 0.06a |
| Gln | 5.20 | 0.20 ± 0.06a | 0.08 ± 0.02a |
| Ala/Thr | 5.57 | 0.10 ± 0.02a | 0.07 ± 0.03a |
| Glu | 7.08 | 0.03 ± 0.00b | 0.07 ± 0.01a |
| Cys/Lys | 7.51 | 0.20 ± 0.03a | 0.29 ± 0.01a |
| His | 8.56 | 0.06 ± 0.00b | 0.15 ± 0.03a |
| Pro | 10.39 | 0.00 ± 0.00a | 0.01 ± 0.00a |
| Arg | 12.75 | 0.15 ± 0.03a | 0.07 ± 0.03b |
| Val | 13.38 | 3.23 ± 0.64a | 1.03 ± 0.04b |
| Met | 14.05 | 0.08 ± 0.01a | 0.06 ± 0.02a |
| Tyr | 14.64 | 0.48 ± 0.09a | 0.23 ± 0.00b |
| Ile | 15.86 | 0.96 ± 0.21a | 0.35 ± 0.00b |
| Leu | 16.51 | 0.27 ± 0.09a | 0.04 ± 0.02a |
| Phe | 18.22 | 0.06 ± 0.00a | 0.07 ± 0.01a |
| Trp | 22.66 | 0.30 ± 0.03a | 0.14 ± 0.00b |
Means (±sd) followed by the same letter are not significantly different (t test; P ≤ 0.05).
Organic Acids
The quantitatively prominent organic acids in young Ni-S foliage were lactic acid, oxalic acid, citric acid, and diglycolic acid (Table V). Tartaric, formic, malic, and acetic acid pools were very low in Ni-S foliage. Total concentration of organic acids was about 2.0-fold greater in Ni-D foliage than in Ni-S foliage. Ni deficiency increased lactic acid (3.2-fold) and oxalic acid (2.4-fold), but reduced maleic acid (about 27%) and citric acid (only 15%). There were no detectable differences in tartaric, formic, malic, acetic, and diglycolic acids between Ni-D and Ni-S foliage. These shifts in organic acids indicate that Ni deficiency disrupts the conversion of certain organic acids to other products.
Table V.
A comparative HPLC separation of organic acid (based on identical retention time to standard references) and concentration in the fractions with molecular mass ≤10 kD of Ni-S and Ni-D foliage from young pecan seedling trees
| Organic Acids
|
Retention Time
|
Concentration X ± sda
|
|
|---|---|---|---|
| Ni-D | Ni-S | ||
| min | μm/mL | ||
| Oxalic acid | 1.18 | 6.56 ± 0.94a | 2.68 ± 0.26b |
| Tartaric acid | 1.32 | ≤0.50a | ≤0.50a |
| Formic acid | 1.45 | ≤0.50a | ≤0.50a |
| Malic acid | 1.86 | ≤0.50a | ≤0.50a |
| Lactic acid | 2.25 | 29.80 ± 0.22a | 9.27 ± 1.77b |
| Acetic acid | 2.60 | ≤0.50a | ≤0.50a |
| Diglycolic acid | 2.86 | 2.33 ± 0.88a | 2.16 ± 0.44a |
| Maleic acid | 3.74 | ≤0.50b | 1.83 ± 0.33a |
| Citric acid | 3.95 | ≤0.50b | 3.33 ± 0.33a |
Means (±sd) followed by the same letter are not significantly different (t test; P ≤ 0.05).
DISCUSSION
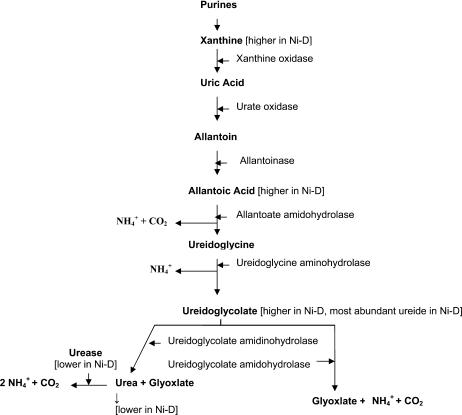
The UV scanning graph provides a generalized assessment of gross changes in biochemical components of young pecan leaves. Ni-S foliage contains greater amounts of organic compounds, as implied by higher absorbance in the 190- to 230-nm spectra. Ni-D foliage exhibited two zones (190–230 and 266–342 nm) showing relatively high absorbance, thus indicating a substantial difference in organic components as compared to Ni-S foliage. Ni-S foliage contained a slightly greater concentration of total ureides, but a lower concentration of total free amino acids and total organic acids, than Ni-D foliage. Thus, there are quantitative differences in ureides, free amino acids, organic acids, and probably other organic substances between Ni-S and Ni-D foliage. Overall, pecan leaflets exhibit a considerably higher concentration of ureides compared with free amino acids and organic acids. Thus, the influence on ureide catabolism is of special interest (Fig. 3).
Figure 3.
Varied allantoic acid amidohydrolase pathway for metabolism of allantoin and allantoic acid in Ni-D pecan leaves (based on proposed pathway by Schubert and Boland, 1990).
Ureides
Higher plants primarily transport nitrogen compounds as either amides or ureides (Schubert and Boland, 1990). Those primarily transporting ureides tend to be hydrophiles (e.g. pecan, river birch) or tropical legumes (e.g. soybean, cowpea, Phaseolus beans, pigeon pea [Cajanus cajan], and mungbean [Vigna radiata]), although much is unknown about ureide anabolism or catabolism in plant tissues. Ureides appear to be primarily metabolized in foliage and fruit (Pate and Atkins, 1983). Ureide catabolism eventually results in cleavage into urea and glyoxylate (with CO2 and NH4 as by-products; Fig. 3). Because urease is ubiquitous in plant organs and tissues, and because Ni is a necessary cofactor, Ni deficiency would be expected to influence ureide catabolism and therefore influence the availability of nitrogen from nitrogen-storage compounds for growth and development processes. Ureide concentrations are relatively high in dormant roots of ureide-transporting perennials, where they are exported via ascending xylem sap to emerging foliage, catabolized, and assimilated into amino acids, peptides, polypeptides, proteins, purines, and nucleic acids (Schubert and Boland, 1990). The catabolic pathway from purine nucleotides and ureides to urea or glyoxylate and NH4 and CO2 via xanthine, uric acid, allantoin, allantoate, ureidoglycine, and ureidoglycolate is well known (Fig. 3; Mathews et al., 2000). Urea is further broken down by urease (which is Ni dependent) to form CO2 and 2 NH4 (Gerendás et al., 1999). Ni-D foliage exhibited relatively high levels of three intermediates of the ureide catabolic chain. The accumulation of these intermediates (xanthine, allantoic acid, and ureidoglycolate, plus urea accumulation was greatly reduced) indicates reduced enzyme activity in at least three ureide catabolic steps. The nature of this study does not allow determination as to whether these, or possibly other, ureide pathway enzymes are being directly limited by Ni deficiency or indirectly via feedback inhibition by an accumulation of downstream intermediates, such as ureidoglycolate or allantoate, or for some other reason.
The enzymes catabolizing ureidoglycolate are likely candidates for either a direct or indirect metabolic role for Ni. The accumulated ureidoglycolate is potentially degraded by two enzymes (Wells and Lees, 1991; Munoz et al., 2001). One is via ureidoglycolate amidinohydrolase (UGL; EC 4.3.2.3) to produce urea and glyoxylate. The second is via ureidoglycolate amidohydrolase (UGAH; EC 3.5.3.19) to produce NH4, CO2, and glyoxylate (Wells and Lees, 1991; Munoz et al., 2001), and requires manganese as a cofactor (Lukaszeski et al., 1992; Vadez and Sinclair, 2000).
Ureide-transporting plants appear to possess the potential to catabolize ureides by at least two pathways (i.e. UGL or UGAH), with one predominating over the other. For example, in soybean, ureidoglycolate catabolism was approximately 95% via UGAH and approximately 5% via UGL; thus, urease did not appear to be essential for ureide catabolism in this species (Stebbins and Polacco, 1995), whereas in chickpea (Cicer arietinum) UGL appears to be the predominant path (Munoz et al., 2001). The accumulation of ureidoglycolate and quantitative reduction in urea from Ni-D foliage suggests that the UGL activity is being impaired. Additionally, the accumulation of ureidoglycolate also suggests either that pecan might not possess the second pathway for the catabolism of ureidoglycolate or that the activity of UGAH is also being impaired by Ni deficiency. Ureidoglycolase is a metalloenzyme whose activity is inhibited by EDTA and enhanced by several different bivalent cations (Munoz et al., 2001). It is possible that one of these metal components is Ni. It is also possible that UGAH is a metalloenzyme requiring Ni to stimulate activity because it is also inhibited by EDTA and activity is enhanced by Mn2+ (Wells and Lees, 1991), of which Ni2+ is likely present as a trace contaminant. An investigation of the relevance of Ni to both UGL and UGAH enzymes is therefore warranted.
The low urea pool in Ni-D foliage of pecan, a woody perennial, is contrary to that observed in nonwoody zucchini and cowpea, where Ni-D plants accumulate urea due to low urease activity (Walker et al., 1985; Gerendás and Sattelmacher, 1997). Similarly, ureide levels were low and unaffected by Ni deprivation in cowpea (Walker et al., 1985) grown with nitrogen being supplied as mineral nitrogen, whereas in pecan ureides were substantially higher in Ni-D foliage. The accumulation of urea and reduced urease activity in zucchini and cowpea is attributed to insufficient Ni for sufficient activation of urease. In the case of pecan, in addition to a likely, but putative, role for Ni in urease, it appears that Ni may potentially play a key role in the activity of either UGL or UGAH, thus possibly requiring Ni ions for activation. The relatively low level of urea in Ni-D pecan foliage may also be due to reduced UGL activity, which is possibly limited by Ni. The low urea level in Ni-D pecan foliage indicates that urea toxicity is not the cause of leaflet blunting, or mouse-ear, in pecan.
The adverse influence of Ni deficiency on ureide catabolism in pecan indicates that similar effects can occur in other ureide-transporting species. Examples of ureide-transporting genera are Acer, Alnus, Annona, Betula, Carpinus, Carya, Cercis, Chamaecyparis, Cornus, Corylus, Diospyros, Juglans, Nothofagus, Ostrya, Platanus, Populus, Pterocarya, Salix, and Vitis (Schubert and Boland, 1990). Additionally, tropical legumes (e.g. soybean, Phaseolus beans, mungbean, and cowpea) are also candidates for disrupted nitrogen metabolism by Ni deficiency. Because the equilibrium between production of ureides and amides in certain crops is influenced by factors such as shade and soil nitrogen sources (nitrate favors amides and ammonium favors ureides), the expression of Ni deficiency could also be partially dependent on orchard or nursery management strategies (Schubert and Boland, 1990). Abnormal elevation of certain ureides is a biochemical symptom of Ni deficiency in pecan and is also likely to be symptomatic in other ureide-transporting species.
Amino Acids
Amino acids serve a wide range of functions in plants and are also the structural units by which proteins are made; thus, any disruption in free amino acid pools potentially disrupts a multitude of growth and developmental processes, plus the production of secondary products that influence plant pest resistance, toxicity, appearance, and taste. Little is known about the influence of Ni deficiency on amino acid metabolism. Ni deficiency in barley increased the pool of total free amino acids and nonprotein nitrogen compounds in shoots and seeds by 20% to 40% (Brown et al., 1990). For shoots, the increase in the various amino acid pools was attributed to Asp, Glu, Val, Lys, His, Arg, Tyr, and Phe, whereas in seeds Glu, Gly, Ala, Val, Leu, Lys, His, Tyr, and Phe increased when plants were supplied with mineral nitrogen (except that Ala was decreased). The same general net accumulation of free amino acids occurred in Ni-D pecan foliage, except the accumulation was much greater than that of Ni-D barley (20%–40% versus 210% in Ni-D pecan), with six amino acids (Gly, Val, Ile, Tyr, Trp, and Arg) increasing and two (His and Glu) decreasing. The Ala pool was not depressed in Ni-D pecan, as was previously observed in barley. Conversely, urea-grown plants subjected to Ni deprivation accumulated urea and exhibited a generalized depletion of free amino acids (Gerendás and Sattelmacher, 1997; Gerendás et al., 1999). In our study with pecan, the nitrogen source was mineral rather than urea-nitrogen, and the nitrogen sources being metabolized were from the dormant season nitrogen pool.
It appears that for crops as diverse as barley and pecan, when the nitrogen source is either mineral or stored, most free amino acid pools increase under conditions of Ni deficiency. This disruption of the various amino acid pools and associated apparent disruption in usage is potentially serious in that these amino acids are the building blocks of proteins needed for canopy deployment and other key growth processes. While end-product inhibition usually appears to be the principal mechanism regulating amino acid pools, it appears that the normal metabolism of free amino acid pools of several such monomers is being blocked by Ni deficiency.
In this study, production of Gly in both Ni-D and Ni-S foliage indicates that the Gly pathway is functioning in pecan foliage regardless of Ni nutritional status. The free Gly pool was the greatest of any of the free amino acids measured in foliage of either Ni class. The free Ser pool was also abundant and did not appear to be influenced by Ni nutrition. Gly and Ser are precursors for phospholipids and purine synthesis and are the main sources of one-carbon units in higher plants (Morot-Gaudry et al., 2001). The free Gly pool is normally much lower than that of Ser in green plants, with the ratio being dependent on the degree of irradiance and photorespiration, although the ratio is typically in the 3- to 10-fold range. In this study, the relative abundance of these two interconvertible amino acids (via Ser hydroxymethyl transferase; EC 2.1.2.1) was reversed, with the Gly pool being about 10-fold greater in foliage than the Ser pool (Morot-Gaudry et al., 2001). This drastic equilibrium shift suggests either a major influence of Ni on photorespiration processes that produce Gly or the conversion of Gly to Ser. The impressive accumulation of free Gly raises the possibility that Ni depredation is limiting the activity of either Gly decarboxylase or Ser hydroxymethyl transferase, which acts to convert Gly to Ser in the mitochondria. These two enzyme systems comprise about 40% to 50% of the soluble proteins in the mitochondria, with their buildup potentially being dependent on leaf development (Vauclare et al., 1996). Alternatively, the incorporation of Gly into purines could also be blocked.
The branched-chain amino acid family contains Ile, Val, and Leu. The Asp-derived Ile pool increased to about 3-fold in Ni-D foliage, whereas the pyruvate-derived Val and Leu pools increased to about 3- and 8-fold, respectively, in Ni-D foliage. These amino acids share several common enzymes in their synthesis and their pathways are reported to be localized in chloroplasts (Bryan, 1990).
The disruption in the aromatic amino acid pool by Ni deficiency indicates a likely disruption of the shikimate pathway existing in chloroplasts and cytosol. Several secondary metabolites depend upon this pathway for precursors; also, other key metabolites, such as indole-3-acetic acid (IAA) and lignin, are similarly linked to the shikimate pathway (Heldt, 2005). This pathway produces aromatic amino acids via three different routes, with the synthesis of chorismate being the last common link for all three routes (Morot-Gaudry et al., 2001). The increase of Trp and Tyr pools in Ni-D foliage indicates the possibility of a Ni-associated disruption of enzyme activity or substrate availability downstream of these two aromatic amino acids. Such a disruption likely explains certain prominent symptoms of Ni deficiency in which dwarfed foliage and shoots and loss of apical dominance and rosetting suggest an auxin deficiency and exceedingly brittle wood suggests poor lignification (Wood et al., 2004a). In the case of IAA, conversion from Tyr to either indole-3-pyruvic acid (via Trp transaminase and indole pyruvate decarboxylase) or tryptamine (via Trp decarboxylase and amino oxidase) or indole-3-acetaldehyde (via indole aldehyde dehydrogenase) appears to be directly or indirectly disrupted by Ni deficiency, although it appears that plants have different pathways for IAA biosynthesis at different developmental stages, tissues, and environmental conditions (Bartel, 1997). While lignin biosynthesis is complex, it also appears likely that its synthesis or incorporation into cell walls is being influenced by Ni, perhaps in the shikimate pathway downstream of Phe ammonia lyase in the phenylpropanoid pathway because Phe did not accumulate in Ni-D foliage. This apparent influence of Ni deficiency on the shikimate pathway, from which many defense-related biochemicals are derived, indicates that disease resistance is likely to be influenced by Ni deficiency.
Although the Arg pool of Ni-D foliage was not great relative to that of many other amino acids, the percentage increase in Arg (21.4-fold), an increase of about 10-fold that of other amino acids, indicates that Ni deficiency substantially influences Arg catabolism and likely affects the Orn cycle because Arg is an intermediate. The citrulline pool, a nonprotein nitrogen compound, also increased, and is an upstream intermediate between Orn and Arg. A similar increase in Arg was reported by Shimada et al. (1980) for tomato (Lycopersicon esculentum) growing under Ni depredation, where it has been suggested that urea accumulation may have interfered with Orn cycle intermediates via feedback inhibition (Gerendás et al., 1999).
The His pool was affected by Ni depredation more so than any other measured amino acid, with the pool being only about 4% of that in Ni-S foliage. Histidine synthesis is an extremely energy-consuming process and thus appears to take place in chloroplasts (Morot-Gaudry et al., 2001). The notably low level of His raises the possibility that (1) chloroplasts were poorly developed at this stage in Ni-D foliage; (2) Ni deficiency somehow reduces the availability of energy needed for synthesis (i.e. 41 ATPs/His molecule) because the necessary Gln and Glu substrates appeared to be unaffected by Ni deficiency; or (3) the activity of one or more enzymes of the His biosynthetic pathway was being reduced because of Ni deficiency.
The synthesis of amino acids is complex, with several amino acids being made by more than one synthetic pathway, as influenced by environment, age, tissue, etc. This study indicates that plant Ni nutritional physiology influences either directly or indirectly the pools of several essential amino acids as well as potentially several different amino acid pathways, but does not elucidate the nature of this Ni-amino acid interaction. However, results indicate a possible role of Ni in certain aspects of amino acid metabolism.
Organic Acids
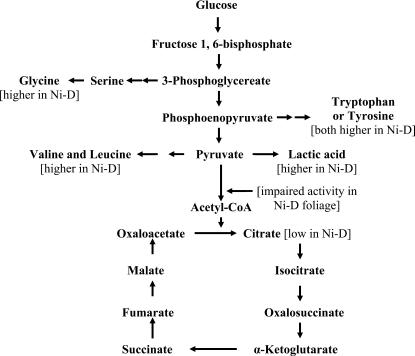
There is a dearth of information on the influence of Ni deficiency on organic acids in plant tissues. In Ni-deprived barley, malate decreased in shoots but was unchanged in seeds (Brown et al., 1990). In the case of Fe-deficient sugar beets (Beta vulgaris), certain TCA organic acids (i.e. citrate and malate) increased in moderately chlorotic leaves (López-Millán et al., 2001). The increase in lactic acid is especially puzzling in that it is normally a product of glycolysis under anaerobic conditions and the Ni-D foliage of this study was not growing in water-saturated soil and did not appear to experience oxygen depredation. It is possible that the accumulation of these acids poisons cells and tissues of Ni-D foliage rather than the toxic accumulation of urea (Walker et al., 1985). The concentration drop in citric acid, a TCA cycle acid, is evidence that Ni deficiency may be influencing the availability of acetyl-CoA, which is key to the conversion of oxaloacetate to citric acid in the mitochondria. The accumulation of lactic acid, Val, Leu, Try, Trp, and Gly, all potentially derived from intermediates in glycolysis, is additional evidence of impaired respiration (Fig. 4). Thus, Ni depredation can adversely influence carbohydrate oxidation via indirect effects on glycolysis and a direct effect on the TCA cycle. Further evidence for impairment of acetyl-CoA is the fact that the three nitrogen-containing components (adenine, panthothenic acid, and β-mercaptoethylamine) of CoA are likely to be influenced by low Ni-associated disruption of normal nitrogen metabolism as a consequence of ureide and amino acid metabolism. It is noteworthy that a slowdown of the TCA cycle results in considerable reduction in the ATP, NADH, and FADH2 available for the electron transfer/oxidative phosphorylation stage of respiration. Thus, the low vegetative growth vigor of Ni-D plants is likely due in part to a disruption of respiration by Ni deficiency. Because Ni deficiency symptoms in pecan are first apparent at bud break as poor growth vigor, when Suc and other carbohydrates are translocating from roots to the developing canopy (Wood, 1987), Ni-deprived cells might therefore potentially experience a disruption in carbohydrate oxidation sufficient to alter growth. This raises the possibility that disrupted carbohydrate oxidation and metabolism, and not just nitrogen-associated metabolism, contribute to the morphological symptoms of Ni-D plants (Wood et al., 2004a). The wide range of morphological and growth symptoms exhibited by Ni-D woody perennials suggests that Ni might affect plant metabolism by influencing a key metabolic intermediate. A viable candidate is acetyl-CoA, as it is probably the most central intermediate in cellular metabolism, provides a link between several pathways (e.g. glycolysis, fatty acid oxidation, amino acids, flavonoids, sterols, isoprenoid derivatives, TCA cycle), does not appear to cross membranes, and appears to be influenced by Ni.
Figure 4.
Influence of Ni-D on amino acid biosynthesis and organic acid metabolism in pecan leaves (modified from Buchanan et al., 2000).
Aerobic respiration is the most common form of energy release in organisms, with glycolysis of Glc resulting in pyruvate. The converse is anaerobic respiration, in which glycolysis yields lactic acid or ethanol instead of water. During anaerobic glycolysis, if glycolysis is proceeding at a high rate (or in anaerobic organisms), there is substantial oxidation of NADH. The large quantity of NADH produced is oxidized by reducing pyruvate to lactate, leading to a buildup of lactic acid (King, 2005). Thus, pyruvate undergoes oxidation with products entering the TCA cycle via acetyl-CoA, whereas lactic acid is a product of anaerobic glycolysis. If the energy level in these cells is sufficiently high, the carbons of pyruvate can be diverted back to Glc via the gluconeogenesis pathway (King, 2005). The high concentration of lactic acid is puzzling because it is typically associated with anaerobic respiration, yet the plants used in this study did not appear to be anaerobic, nor were soil conditions anaerobic. Although not measured in this study, it is possible that the activity of pyruvate dehydrogenase (EC 1.2.1.51) is being suppressed as a consequence of Ni deficiency. It is an iron-requiring enzyme (Inui et al., 1987) and thus there is the possibility of it also being activated by Ni.
The occurrence of oxalic acid is typically greatest in leaves and lowest in roots. Oxalic acid content is related to photosynthesis and carbohydrate metabolism and varies according to tissue age, season of the year, climatic stresses, and soil type. Oxalic acid can be harmful to plants because it is produced by certain pathogenic fungi and often plays an essential role in pathogenicity (Caliskan, 2000). Botrytis cinerea, a phytopathogenic fungus, secretes oxalic acid as a pathogenicity factor suspected of facilitating degradation of cell walls and repressing plant defenses. Thus, degradation of oxalic acid during host-pathogen interaction potentially interferes with the infection process. Sclerotinia minor is the causal agent of Sclerotinia blight, a highly destructive disease of peanut (Arachis hypogaea). Evidence indicates that oxalic acid serves as a pathogenicity factor during Sclerotinia infection. Direct application of oxalic acid to stem or leaf tissue causes tissue injury and wilting, similar to plant responses to fungal infection by Sclerotinia sclerotiorum (Livingstone et al., 2005). The double amount of oxalic acid present in Ni-D foliage compared to Ni-S foliage, in addition to lactic acid, is a potential marker for identifying Ni-D foliage and is thus a potential indicator of cellular damage. It appears that the relatively high levels of lactic and oxalic acids in young foliage is likely a contributing factor to leaflet dwarfing (i.e. mouse-ear), a key identifying factor of Ni deficiency in woody perennials.
Metabolism of free amino acids and organic acids is linked to ureide metabolism. For example, ureidoglycolate can be converted to glyoxylate, which is then converted to Gly or urea and pyruvate. The pyruvate can then be converted to Val or lactic acid. In plants, two main reactions involving Gly are potential sources of C1 units (Mouillon et al., 1999). The greater level of Val and lactic acid content in Ni-D foliage may possibly be linked to a shift in pyruvate metabolism.
CONCLUSION
Ni deficiency in pecan, a woody perennial, substantially disrupts several metabolic pathways (Figs. 3 and 4), thus presenting distinct biochemical-based symptoms of Ni deficiency. Such symptoms may potentially enable recognition of Ni deficiency prior to the development of morphological symptoms associated with disruption of vegetative growth processes. It appears that both carbon respiration and nitrogen metabolism are sensitive to Ni nutrition. The associated degree of metabolic disruption raises the possibility that Ni exerts an active role in pecan metabolism other than that of being a cofactor of urease; thus, the role of Ni in the activation of key enzymes merits greater investigation. Circumstantial evidence therefore points to the distinct possibility of undiscovered roles of Ni in plant nutritional physiology.
MATERIALS AND METHODS
Plant Materials
The source of either Ni-D or Ni-S foliage was from third-leaf seedlings originating from open-pollinated Desirable cv. Seedlings were grown in plastic pots containing low-Ni soil (<2 kg ha−1) from a commercial pecan (Carya illinoinensis [Wangenh.] K. Koch) orchard possessing trees exhibiting severe Ni deficiency. Seedlings were grown in a greenhouse with temperatures maintained between 20°C and 30°C during the growing season and at 5°C to 20°C during the dormant season. Seedlings did not exhibit phenological symptoms of Ni deficiency during the first year of growth; however, soon after spring bud break of the second growing season, roughly 50% (about 500 seedlings) of the seedling population exhibited various degrees of Ni deficiency recognizable by distinct morphological distortions to foliage, thus providing specimens covering a wide range of severity of Ni-deficiency symptoms. A similar pattern was also exhibited during the third growing season, at which time same-age newly expanding foliage was collected from randomly selected Ni-D (those exhibiting morphological symptoms of severe deficiency) and Ni-S (i.e. those exhibiting normal growth and not exhibiting any morphological symptoms of Ni deficiency) trees. Fully illuminated foliage was collected in triplicate at midmorning about 7 to 10 d after bud break using a Ni-free zirconium oxide ceramic blade, with samples initially stored on ice while being collected and subsequently being stored at −70°C. Atomic absorption spectroscopic analysis of leaflet Ni concentration revealed that the young leaflets form the Ni-S trees typically possessed a Ni concentration of approximately 0.86 to 1.5 μg g−1 dry weight, whereas those from Ni-D seedlings generally contained Ni at approximately 0.011 to 0.068 μg g−1 dry weight.
Preparation of Pecan Leaflet Extraction and Fractionation
Both Ni-D and Ni-S foliage (0.8 g fresh weight/sample) were homogenized in HPLC-grade water (2 mL) using a 15-mL glass tissue grinder for 40 revolutions. The tissue extract was then centrifuged twice (20,000g) for 20 min each to collect the supernatant. The supernatant was further purified by removing molecules ≥10 kD by twice filtering using a Centricon-10 filter (Millipore filter units) after centrifugation (5,000g) for 75 min. After completion of the above-described processes, samples were maintained at 4°C and partially purified by retaining components with a molecular mass ≤10 kD. Purified samples were then brought up to equal volumes using HPLC-grade water, and subsamples were analyzed for ureides, free amino acids, and organic acids. Meanwhile, with a similar procedure, protein solutions from 20 g foliage tissues were prepared. The protein was precipitated with chilled acetone and dissolved in buffer E (Bai et al., 1999). The resulting protein solutions (molecular mass ≥10 kD) after filtration to remove small molecules were used for enzyme assay. The smaller molecules were removed because they contain many phenolics that would interfere with the enzyme activity.
UV Absorbance
Partially purified fractions with molecular mass ≤10 kD were evaluated for differences in UV absorbance. The fractions were diluted in water (HPLC grade) and UV absorbance performed with a DU-640 spectrophotometer (Beckman-Coulter). Sample fractions were scanned for absorbance in the 190- to 800-nm or 190- to 380-nm spectra at a sample temperature of 25°C. The scanning was carried out using a blank composed of HPLC-grade water.
HPLC Analysis of Partially Purified Fractions with Molecular Mass ≤10 kD
Ureide analysis was performed by the HPLC Spectra System SCM 1000 linked with the Spectra System UV 1000 detector (Thermo Electron Corporation) using a platinum EPS C18 column, 5 μm, 250 mm × 4.6 mm (Alltech Associates). Mobile phase was acetonitrile 0.03 m potassium phosphate, pH 3.2 (20:80), 0.5 mL/min at 30°C. Flow rate was 0.5 mL/min. Sample injection volume was 20 μL of the above-described partially purified fractions. Column temperature was 30°C. Detection was at 190 or 220 nm. Tentative ureide identification was based on identical retention times compared to ureide standards. Ureide references included xanthine, uric acid, allantoin, allantoic acid, glyoxylic acid, and urea (Sigma). The l-Asn (anhydrous) was from Fluka. The standards and internal standards were used at concentrations ranging from 100 to 400 μm/mL (in acetonitrile, 0.03 m potassium phosphate monobasic, pH 3.2 [20:80]). Ureidoglycolate was synthesized by the reaction of urea with glyoxylic acid. The absorbencies of these chemicals are much greater at 190 nm than at 220 nm. Thus, the absorbance was then evaluated at 190 nm. There were three replicates for each Ni sample class. In addition, to confirm the identification of ureides (e.g. allantoic acid, Asn anhydrogenase, and ureidoglycolate), samples were also run on a ZORBAX Rx-SIL (Agilent Technologies) column (5 μm, 4.6 mm × 150 mm) with a mobile phase consisting of acetonitrile, 20 mm potassium phosphate, pH 7.2 (90:10).
Amino acid analysis was by performed by the HPLC Spectra System SCM 1000 linked with an ELSD 800 detector using a Prevail C18 column (5 μm, 250 × 4.6 mm; Alltech Associates). Mobile phases were as follows: A, 5 mm heptafluorobutyric acid, pH 1.0, with 0.7% trifluoroacetic acid; B, acetonitrile. The mobile phase gradient was (time: %B) (0, 0), (6, 0), (8, 15), (25, 35), 1 mL/min at 25°C. Amino acids were tentatively identified based on retention times of nonderivatized amino acid standards. The standards (20 mixed amino acids; Sigma) and internal standards, such as Gly, Arg, and Trp (Sigma) in 0.1 n HCl at a concentration of 2.5 μm/mL (except for l-cystine at 1.25 μm/mL), were used. The profiles for separation of underivatized amino acids were also used as a reference for free amino acid identification. For purposes of statistical analysis, three samples were run for each of the two Ni classes.
Organic acid analysis was performed by the above-described HPLC system using a Spectra System UV 1000 detector. Separation of organic acids was by a Prevail organic acid column, 5 μm, 150 × 4.6 mm. The mobile phase was 25 mm KH2PO4, pH 2.5, and the flow rate was 1.5 mL/min. Sample injection volume was 20 μL. Column temperature was 30°C. The UV detector operated at 210 nm. Tentative identification of organic acids was based on identical retention times of organic acid standards. Organic acid standards were dl-tartaric acid, fumaric acid, succinic acid, dl-malic acid, cis-aconitic acid, and oxalic acid dehydrate (Sigma). Standards were dissolved in 25 mm KH2PO4, pH 2.5 (pH adjusted with 1 n HCl). The profiles for separation of organic acids (Alltech) were used as references for organic acid identification. Three replicates were measured per Ni sample class.
Assay of Urease Activity in Protein Solutions with Molecular Mass ≥10 kD
Urease catalyzes the hydrolysis of urea. Urease activity was determined based on the methods of Kaltwasser and Schlegel (1966), but with slight modification. The assay is carried out in a coupled enzyme with Glu dehydrogenase. All chemical reagents used for enzyme assay were dissolved in 0.1 m potassium phosphate buffer (pH 7.6). The assay mix was 0.37 mL of 0.1 m potassium phosphate buffer (pH 7.6), 0.1 mL of 1.8 m urea, 0.1 mL of 0.025 m ADP, 0.2 mL of 0.008 m NADH, 0.1 mL of 0.025 m α-ketoglutarate; then 0.1 mL of 50 units/mL Glu dehydrogenase and 5 μL of enzyme solution were added. The change in A340 at 25°C was recorded at 0.5, 1, 3, and 5 min. Urease of Jack beans (Canavalia ensiformis; 29.5 units/mg; Sigma) was used as a reference. A unit is defined as the amount of urease that causes the oxidation of 1 μm of NADH/min at 25°C, pH 7.6, in a coupled reaction using Glu dehydrogenase. Protein concentration was determined with the Bio-Rad protein assay with bovine serum albumin as standard.
Statistical Analyses
Concentrations of organic molecules in Ni-D and Ni-S samples were square root-transformed prior to analysis. Differences in concentrations in two classes were compared by t test (P ≤ 0.05; SAS software, version 8; SAS Institute). Mean values were analyzed with three tissue replicates.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Cheng Bai (cbai@saa.ars.usda.gov).
Open Access articles can be viewed online without a subscription.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.072983.
References
- Association of American Plant Food Control Officials (2005) Model for Fertilizer Regulation in North America. http://www.aapfco.org/aapfcorules.html (December 20, 2005)
- Bai C, Fernandez E, Chen R (1999) Purification and stabilization of a monomeric isocitrate dehydrogenase from Corynebacterium glutamicum. Protein Expr Purif 15: 344–348 [DOI] [PubMed] [Google Scholar]
- Bartel B (1997) Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 48: 51–66 [DOI] [PubMed] [Google Scholar]
- Bloom AJ (2002) Mineral nutrition. In L Taiz, E Zeiger, eds, Plant Physiology. Sinauer Associates, Sunderland, MA, pp 67–86
- Brown PH, Welch RM, Cary EE (1987. a) Nickel: a micronutrient essential for higher plants. Plant Physiol 85: 801–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Welch RM, Cary EE, Checkai RT (1987. b) Beneficial effects of nickel on plant growth. J Plant Nutr 10: 2125–2135 [Google Scholar]
- Brown PH, Welch RM, Madison JT (1990) Effect of nickel deficiency on soluble anion, amino acid, and nitrogen levels in barley. Plant Soil 125: 19–27 [Google Scholar]
- Bryan JK (1990) Advances in the biochemistry of amino acid biosynthesis. In BJ Miflin, PJ Lea, eds, The Biochemistry of Plants, Vol 16. Academic Press, New York, pp 161–195
- Buchanan BB, Gruissem W, Jones R (2000) Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD
- Caliskan M (2000) The metabolism of oxalic acid. Turk J Zool 24: 103–106 [Google Scholar]
- Ciurli S (2001) Electronic structure of the nickel ions in the active site of urease. Chemistry (Easton) 2001: 99–100 [Google Scholar]
- Dixon NC, Gazzola C, Blakely RL, Zemer R (1975) Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel. J Am Chem Soc 97: 4131–4133 [DOI] [PubMed] [Google Scholar]
- Dobrolyubskii OK, Slavvo AV (1957) Use of trace element nickel for the nutrition of grapes. Dokl Akad Nauk SSSR 112: 347–359 [Google Scholar]
- Eskew DL, Welch RM, Cary EE (1983) Nickel and essential micronutrient for legumes and possibly all higher plants. Science 222: 691–693 [DOI] [PubMed] [Google Scholar]
- Eskew DL, Welch RM, Norvell WA (1984) Nickel in higher plants: further evidence for an essential role. Plant Physiol 76: 691–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerendás J, Polacco J, Freyermuth SK, Sattelmacher B (1999) Significance of nickel for plant growth and metabolism. Z Pflanzenernaehr Bodenkd 162: 241–256 [Google Scholar]
- Gerendás J, Sattelmacher B (1997) Significance of Ni supply for growth, urease activity and concentrations of urea, amino acids and mineral nutrients of urea-grown plants. Plant Soil 190: 153–162 [Google Scholar]
- Heldt HW (2005) Plant Biochemistry, Ed 3. Elsevier Academic Press, Burlington, MA
- Hull RJ (2003) How do turfgrasses use nickel? www.turfgrass.com (August 22, 2005)
- Inui H, Ono K, Miyatake K, Nakano Y, Kitaoka S (1987) Purification and characterization of pyruvate:NADP+ oxidoreductase in Euglena gracilis. J Biol Chem 262: 9130–9135 [PubMed] [Google Scholar]
- Kaltwasser H, Schlegel HG (1966) NADH-dependent coupled enzyme assay for urease and other ammonia-producing systems. Anal Biochem 16: 132–138 [DOI] [PubMed] [Google Scholar]
- King MW (2005) Glycolysis. The Medical Biochemistry Page, Indiana University, Terre Haute, IN, http://web.indstate.edu/thcme/mwking/glycolysis.html (August 22, 2005)
- Livingstone DM, Hampton JL, Phipps PM, Grabau EA (2005) Enhancing resistance to Sclerotinia minor in peanut by expressing a barley oxalate oxidase gene. Plant Physiol 137: 1354–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Millán AF, Morales F, Abadía A, Abadía J (2001) Changes induced by Fe deficiency and Fe supply in the organic acid metabolism of sugar beet (Beta vulgaris L.) leaves. Physiol Plant 112: 31–38 [DOI] [PubMed] [Google Scholar]
- Lukaszeski KM, Blevins DG, Randall DD (1992) Asparagine and boric acid cause allantoate accumulation in soybean leaves by inhibiting manganese-dependent allantoate amidohydrolase. Plant Physiol 99: 1670–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H (2002) Mineral Nutrition of Higher Plants, Ed 2. Academic Press, New York
- Mathews CK, van Holde KE, Ahern KG (2000) Biochemistry. Addison-Wesley, New York
- Morot-Gaudry JF, Job D, Lea PJ (2001) Amino acid metabolism. In PJ Lea, JF Morot-Gaudry, eds, Plant Nitrogen. Springer-Verlag, New York, pp 167–211
- Mouillon J-M, Aubert S, Bourguignon J, Gout E, Douce R, Rébeillé F (1999) Glycine and serine catabolism in non-photosynthetic higher plant cells: their role in C1 metabolism. Plant J 20: 197–205 [DOI] [PubMed] [Google Scholar]
- Mulrooney SB, Hausinger RP (2003) Nickel uptake and utilization by microorganisms. FEMS Microbiol Rev 27: 239–261 [DOI] [PubMed] [Google Scholar]
- Munoz A, Piedras P, Aguilar M, Pineda M (2001) Urea is a product of ureidoglycolate degradation in chickpea: purification and characterization of the ureidoglycolate urea-lyase. Plant Physiol 125: 828–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate JS, Atkins CA (1983) Nitrogen uptake, transport and utilization. In WJ Broughton, ed, Nitrogen Fixation. Oxford University Press, London, pp 245–298
- Roach WA, Barclay C (1946) Nickel and multiple trace-element deficiencies in agricultural crops. Nature (Lond) 157: 696 [Google Scholar]
- Ruter JM (2005) Effect of nickel applications for the control of mouse ear disorder on river birch. J Environ Hort 23: 17–20 [Google Scholar]
- Schubert KR, Boland MJ (1990) The ureides. In BJ Miflin, PJ Lea, eds, The Biochemistry of Plants, Vol 16. Academic Press, San Diego, pp 197–283
- Shimada N, Ando T, Tomiyama M, Kaku H (1980) Role of nickel in plant nutrition. Effects of nickel on the growth of tomato and soybean. Japanese J Soil Sci Plant Nutr 51: 493–496 [Google Scholar]
- Sirko A, Brodzik R (2000) Plant ureases: roles and regulation. Acta Biochim Pol 47: 1189–1195 [PubMed] [Google Scholar]
- Stebbins N, Polacco JC (1995) Urease is not essential for ureide degradation in soybean. Plant Physiol 109: 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AJ (1982) Proteins containing nickel. Nature 298: 602–603 [DOI] [PubMed] [Google Scholar]
- Vadez V, Sinclair TR (2000) Ureide degradation pathways in intact soybean leaves. J Exp Bot 51: 1459–1465 [PubMed] [Google Scholar]
- Vauclare P, Diallo N, Bourguignon J, Macherel D, Douce R (1996) Regulation of the expression of the glycine decarboxylase complex during pea leaf development. Plant Physiol 112: 1523–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, Graham RD, Madison JT, Cary EE, Welch RM (1985) Effects of Ni deficiency on some nitrogen metabolites in cowpea (Vigna unguiculata L. Walp). Plant Physiol 79: 474–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CT, Orme-Johnson WH (1987) Nickel enzymes. Biochemistry 26: 4901–4906 [DOI] [PubMed] [Google Scholar]
- Welch RM (1981) The biological significance of nickel. J Plant Nutr 3: 345–356 [Google Scholar]
- Wells XE, Lees EM (1991) Ureidoglycolate amidohydrolase from developing French bean fruits (Phaseolus vulgaris [L.]) Arch Biochem Biophys 287: 151–159 [DOI] [PubMed] [Google Scholar]
- Wood BW (1987) Carbohydrate composition of vascular system exudates and characterization of their uptake by leaf tissue of pecan. J Am Soc Hortic Sci 112: 346–351 [Google Scholar]
- Wood BW (2006) Field deficiency of nickel in trees: symptoms and causes. Acta Hortic (in press)
- Wood BW, Reilly CC, Nyczepir AP (2004. a) Mouse-ear of pecan: I. Symptomology and occurrence. HortScience 38: 87–94 [Google Scholar]
- Wood BW, Reilly CC, Nyczepir AP (2004. b) Mouse-ear of pecan: II. Influence of nutrient applications. HortScience 38: 95–100 [Google Scholar]
- Wood BW, Reilly CC, Nyczepir AP (2004. c) Mouse-ear of pecan: a nickel deficiency. HortScience 39: 1238–1242 [Google Scholar]