Abstract
In flowering plants, fruit morphogenesis is a distinct process following fertilization resulting in the formation of a specialized organ associated with seeds. Despite large variations in types and shapes among species, fleshy fruits share common characteristics to promote seed dispersal by animals such as organ growth and metabolite accumulation to attract animal feeding. The molecular biology of fruit ripening has received considerable attention, but little is known about the determinism of early fruit morphogenesis and why some fruits are fleshy while others lack flesh. We have identified in grapevine (Vitis vinifera) a mutation we have named fleshless berry (flb) that reduces by 20 times the weight of the pericarp at ripening without any effect on fertility or seed size and number. The flb mutation strongly impaired division and differentiation of the most vacuolated cells in the inner mesocarp. The timing of ripening was not altered by the mutation although the accumulation of malic acid in the green stage was noticeably reduced while sucrose content (instead of hexoses) increased during ripening. The mutation segregates as a single dominant locus. These results indicate that the Flb− mutant is suitable material to advance our understanding of the genetic and developmental processes involved in the differentiation of an ovary into a fruit.
Angiosperms produce a great diversity of fruits, from dry single-seeded achenes as in sunflowers (Helianthus annuus) or siliques as in Arabidopsis (Arabidopsis thaliana) to fleshy fruits such as tomato (Solanum esculentum; Knapp, 2002). A perfect fruit, in evolutionary terms, involves tight coordination of seed and pericarp development, the fruit shifting from a repulsive to an attractive status when seeds are able to resist ingestion and unfavorable environmental conditions (Holland et al., 2003). Most fleshy fruits develop from ovary tissues (Eames and MacDaniels, 1947) and exhibit convergent characteristics such as pericarp cell proliferation and enlargement depending on the storage of organic acids in the green stage and on the accumulation of sugars during ripening.
In the past few years, considerable attention has been focused on the molecular events that control fruit ripening, with particular emphasis on the ethylene signal cascade in climacteric fruits like tomato (Seymour et al., 2002; Giovannoni, 2004). In nonclimacteric fruit such as the grapevine (Vitis vinifera) berry, numerous transcripts have been related to ripening, but the determinism of maturation is still not known (Terrier et al., 2005). Important fruit characteristics such as size and some aspects of biochemical composition, e.g. organic acids, are mainly determined during early stages of development (Scorza et al., 1991; Corelli-Grappadelli and Lakso, 2004). The importance of initial cell divisions has been documented in tomato (Bertin et al., 2003). However, data on the specialization of different cellular types inside the ovary remain extremely scarce (Famiani et al., 2000).
During the last two decades, considerable progress in the understanding of various biological processes has resulted from the isolation and study of mutants (Bouché and Bouchez, 2001; Emmanuel and Levy, 2002). In Arabidopsis, the number of genes identified as controlling gynoecium and fruit development has increased significantly (Dinneny and Yanofsky, 2005) and may help our understanding of the evolution of fruit formation in other species. Unfortunately, mutations affecting fruit formation are rare and mutagenesis is difficult to perform in many fruit-bearing species, particularly in woody species, due to their long juvenile period.
We are interested in the domesticated European grapevine, which is currently one of the major fruit crops in the world based on economic value and cultivated area (7.6 million ha; Food and Agricultural Organization of the United Nations, 2004; http://apps.fao.org/). The Vitis genus, which includes many other species (e.g. Vitis labrusca, Vitis amurensis, or Vitis caribaea) share the same developmental characteristics, are present on all continents, and belong to the Vitaceae, a large family including many other genera such as Muscadinia, Cissus, Parthenocissus, and Ampelopsis, with most species producing small fleshy fruits classified as berries. Grapevine is characterized by unique developmental features in both the processes of flowering (Gerrath, 1993; Boss et al., 2003) and fruit development (Terrier et al., 2005). Vitaceae is considered as a basal family of the core eudicots (Judd et al., 1999), and an understanding of the genetic and molecular control of grapevine fruit development could shed light on the evolution of the regulation of fruit development in angiosperms.
At the molecular level, significant advances have recently been made on the identification of genes involved in grapevine flowering (Boss et al., 2001, 2002; Carmona et al., 2002; Calonje et al., 2004) with, in one case, the use of Pinot Meunier and a forward genetic approach to link a phenotype to a mutation in the V. vinifera GA-insensitive (VvGAI) gene (Boss and Thomas, 2002). In grapevine, the study of a white berry mutant with a MYB transcription factor loss of function is, to our knowledge, the only example of forward genetics applied to fruit development (Kobayashi et al., 2004). This study deals with a third grapevine mutant, called fleshless berry (flb), deeply altered in berry morphogenesis and potentially informative to investigate many aspects of fruit growth and development. The effects of the flb mutation on morphological, anatomical, and biochemical development of grapevine berry tissues are described and discussed in a bioevolutionary perspective.
RESULTS
Morphology of the Mutant Flb−
During eight years of observations, the mutant did not display any variation in its vegetative development as compared to the wild type. Throughout the observation period, both genotypes showed synchronous flowering and identical flower set in all tested culture conditions. The earliest mutant phenotype was the presence of a transversal wrinkle at the ovary style base at anthesis when caps detached from flowers (Fig. 1A). After anthesis, longitudinal and transversal sections in mutant berries revealed that seeds developed while ovary wall growth was dramatically impaired (Fig. 1, B and C). As a consequence, the pericarp volume of mutant berries was almost equivalent to seed volume while pericarp represented the major part of the wild-type berries (Figs. 1C and 2). Nevertheless, despite these early and major alterations in pericarp development, mutant berries displayed typical aspects of the ripening process, such as color change (from green to pale yellow) and softening, and also produced seeds with lignified teguments (Fig. 1, D–F). In mutant berries containing three or four developing seeds, we often observed a dramatic phenotype with seeds bursting out of the pericarp, subjecting ovary tissue to desiccation (Fig. 1G). Inflorescence architecture and the number of flowers were identical in mutant and wild-type plants (Fig. 1H).
Figure 1.
Morphology of the ovary and berry of the Flb− mutant. Unless specified, mutant organs are located on the right of pictures. A, Mature ovaries at anthesis, anthers being removed. B and C, Transversal (B) and longitudinal (C) sections of berries before ripening. D, External berry view. E, Mutant fresh berry section. F, Seeds. G, Four developing seeds bursting out of the ovary. H, Clusters at the mature ripening stage. I, Effect of 100 μg mL−1 of GA3, GA4, and GA7 on berry growth with control wild type (left), control mutant (middle), and treated mutant (right). Bars = 2 mm except for H, where bar = 1 cm.
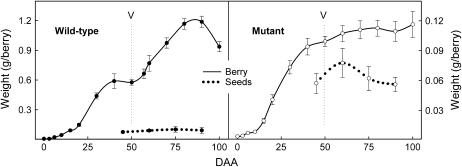
Figure 2.
Berry and seed development of the mutant and wild type. The letter V represents véraison and is the onset of ripening. Bars = confidence interval at P = 95%.
Berry Growth
No growth regulator (gibberellic acid [GA], 6-benzyl-aminopurine [BAP], indole-3-acetic acid [IAA], and 2-isopentenyl adenine) treatments induced any change in the ovary growth of the mutant, regardless of concentration, combination, or the stage of growth regulator application. Growth stimulation of bunch rachis and pedicels was however observed in treatments including GA at 100 or 500 μg mL−1 (Fig. 1I) without any quantitative difference between both concentrations. Other plant regulators, alone or in combination with GA, did not induce significant effects. Growth stimulation of the pedicel by GA varied according to the period of treatment. Application starting at flowering, 7 d after anthesis (DAA), or 14 DAA resulted in an increase of 550%, 310%, or 190% of pedicel diameters, respectively. In addition to the effect on pedicel and rachis growth, treatment at flowering altered the flowering process reducing fruit set by 95% compared to the control.
Wild-type berries displayed a typical synchronized double-sigmoid pattern (Fig. 2) with two distinct phases of rapid growth separated by a lag phase (Coombe, 1992). For wild type, the typical 10-d lag phase II was well marked, indicating synchronous development of individual berries within the population. The mutant berry exhibited altered growth kinetics with a less marked lag phase II compared to the wild type (Fig. 2). During ripening (phase III), the growth was considerably attenuated in the mutant compared to the wild type. However, phase I (green stage) and phase III (ripening) showed similar timing and duration in both wild type and mutant. At the end of maturation, the berry weight remained constant in the mutant while the volume of the wild-type berry decreased by 20%, presumably as a consequence of excess evapotranspiration in respect to a reduction of phloem unloading capacity and berry desiccation. At the end of fruit development, the mutant showed a 90% reduction in final berry volume compared to the wild type.
Seed Development
In both genotypes, seeds displayed a typical growth pattern for grapevine with a maximum weight at the end of the green growth period of the berry. During berry ripening, seed weight was slightly reduced (Fig. 2) as a result of the maturation process. Distributions of seed number per berry were almost similar in the wild type and the mutant, with two main classes represented by berries with one or two seeds (85% of the total) as in other grapevine cultivars (Staudt et al., 1986). In both genotypes, total seed weight per berry was proportional to seed number, but individual seed weight was inversely proportional to seed number per berry (data not shown). Mutant seeds were found to be up to 34% lighter than wild type whatever the number of seeds per berry. In addition, mutant seeds showed a globular shape strongly differing from the typical pyriform shape displayed by the wild type (Fig. 1F). The relative seed contribution to total berry weight was less than 10% in the wild type (Fig. 2). On the contrary, in the mutant, seeds contributed to half of the total berry weight regardless of the number of seeds per berry (data not shown). Despite the changes in mutant seed shape and weight, germination of mutant seeds were normal and gave fully functional zygotic embryos with 70% of seeds giving a viable plant. The germination rate of the mutant was very close to that observed for wild-type seeds (74%; data from 18 wild-type progeny populations).
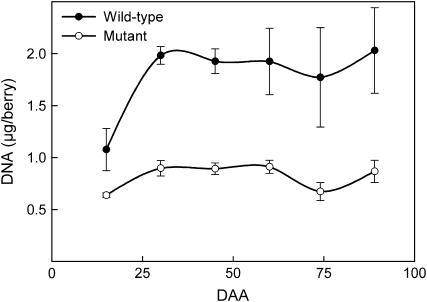
DNA Content
Following fertilization, cell divisions occurred in the wild-type pericarp resulting in a rapid increase of the DNA content per berry compared to the flb mutant (Fig. 3). In both genotypes, pericarp DNA content became constant from 30 DAA and stabilized at 2 μg per wild-type berry and 0.9 μg per mutant berry. The respective developmental profiles of DNA content and berry weight confirmed that berries continued to enlarge whereas cells stopped dividing in both the mutant and the wild type. However, cell enlargement was strongly reduced in the mutant. The relative proportion of pericarp compared to DNA was significantly different in the mutant (0.05 g of fresh pericarp per microgram of DNA) and wild type (0.95 g of fresh pericarp per microgram of DNA). At the maximum of pericarp development, the average cell size was estimated to be 19 times smaller in the mutant than in the wild type.
Figure 3.
DNA content during berry development of both mutant and wild type (bars = sd).
Anatomical Investigation of Ovary Development
Grapevine ovary and berry development have been comprehensively described by Fougère-Rifot et al. (1995) and Hardie et al. (1996), and this section focuses on specific aspects of the Flb− phenotype.
Before anthesis, the formation of the three external whorls of flower organs was identical in the mutant and the wild type (data not shown). During ovary ontogenesis, first differences were visible at the later stage of ovary development, 10 to 15 d before anthesis. While little variations in cell number were found in the wild-type ovary wall (Fig. 4A), alterations in mesocarp and inner epidermis cell number were observed in the upper part of the mutant ovary, leading to irregular wall thickening (Fig. 4B). In longitudinal sections, the mutant ovary assumed a more conical shape than the wild type, sometimes showing a wrinkle at the style base.
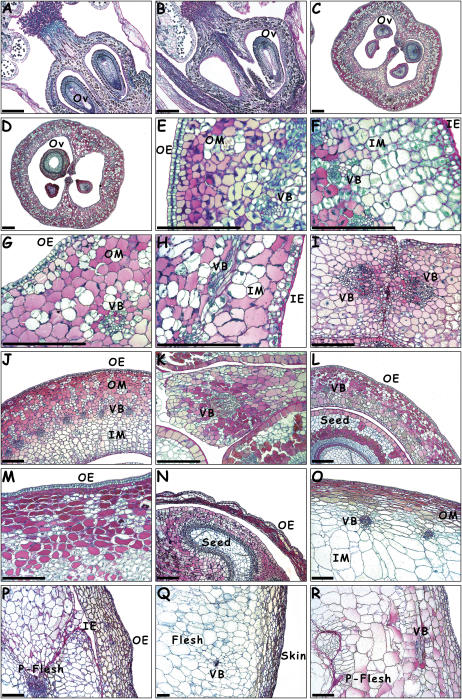
Figure 4.
Ovary and berry anatomy of the Flb− mutant and Flb+ wild type (DAA). A and B, Wild-type (A) and mutant (B) flowers at 11 days before anthesis. C and D, Wild-type (C) and mutant (D) mature ovaries at flowering. E and F, Outer (E) and inner (F) mesocarp in the wild type at 3 DAA. G and H, Outer (G) and inner (H) mesocarp in the mutant at 3 DAA. I and J, Wild-type septum (I) and ovary wall (J) transverse sections at 7 DAA. K and L, Mutant septum (K) and ovary wall transverse sections (L) at 7 DAA. M and N, Wild-type (M) and mutant (N) transverse sections of the pericarp at 14 DAA with a seed visible on the mutant section. O and P, Wild-type (O) and mutant (P) pericarp view at 28 DAA with mesocarp and pseudo pericarp visible in mutant. Q and R, Wild-type (Q) and mutant (R) organization of skin and pericarp in ripe berries at 90 DAA with pseudo flesh visible in the mutant. Ov, Ovule; OE, outer epidermis; OM, outer mesocarp; VB, vascular bundle; IE, inner epidermis; IM, inner mesocarp; P-Flesh, pseudo flesh (bars represent 100 μm).
At flowering, both genotypes had the same ovary tissue organization, with 12 to 16 cells across the ovary wall with a circle of 25 to 40 vascular bundles in the middle (Fig. 4, C and D). No abnormality was noticeable in cell shape and aspect, either in parenchyma or vascular tissue in both genotypes. At this stage the ovary wall was from 120 to 150 μm thick.
At 3 DAA, the wild type showed intense cell divisions in the inner and outer epidermis and in the mesocarp, with the main division plane being anticlinal in the outer epidermis, periclinal in the inner epidermis, and nonoriented in the mesocarp (Fig. 4, E and F). At this stage, the wild-type ovary wall was 250 to 300 μm thick with 25 to 30 isodiametric cells. In the mutant, cell divisions were also visible in the epidermis with predominantly anticlinal divisions in the outer epiderm and periclinal divisions in the inner epiderm (Fig. 4, G and H). In the mesocarp, divisions were much rarer than in wild type and only visible in some cell layers beneath the epidermis (hypoderm). At this stage, the mutant pericarp was 150 to 200 μm thick with less than 20 cells.
At 7 DAA, strong developmental differences were visible between the two genotypes. In the wild type, cell divisions were intense in the ovary septum and wall, which was 350 to 400 μm thick with 35 to 40 cells (Fig. 4, I and J). Differentiation of pericarp cells increased with inner mesocarp cells being less colored than the outer mesocarp cells that contained higher phenolic contents. In the mutant, only a few divisions were visible in the epidermis and in the septum with the same division planes as in the wild type (Fig. 4, K and L). The ovary wall, which was only 150 to 200 μm thick with 20 cells, did not show the same type of differentiation as wild type with the outer mesocarp showing similar staining properties to the inner mesocarp.
At 14 DAA, in wild-type ovaries, intense cell divisions were still observed all across the pericarp, and tissue differentiation increased. Inner mesocarp cells stained less than outer mesocarp cells (Fig. 4M), about half of which were rich in polyphenols. Cells began to enlarge isodiametrically in the inner mesocarp while in the outer hypoderm some cells elongated tangentially. In mutant mesocarp, no dramatic changes were visible at the cellular level, most cells looked similar to the group of cells without polyphenolic compounds in the wild type. In mutant berries with three or four developing seeds, the ovary often cracked and the pericarp collapsed due to drying (Fig. 4N).
At 28 DAA, in wild-type pericarp, cell division rate slowed down as tissue differentiation increased, with strong differences between inner and outer mesocarp (Fig. 4O). In the mutant pericarp however, the differentiation level remained low with little difference between inner and outer mesocarp. By comparison to the ovary wall, the septum was relatively developed with cells larger than in the inner mesocarp. Some endoderm cells divided to produce cell masses inside the ovary locules (Fig. 4P). The septum and inner epidermis cells divided and expanded, progressively filling up the ovary together with the developing seeds. As in the wild type, cells of the outer hypoderm appeared compressed by mechanical pressure due to seed growth, but outer epidermis cells remained isodiametric and undifferentiated.
At 90 DAA, the ripe berry of the wild type showed an organization typical for grapevine with a skin composed of cells from epiderm and hypoderm tangentially elongated (Fig. 4Q). A large fleshy pericarp was formed with cells from the outer and inner mesocarp, as well as from inner epiderm and in the deeper zone, cells from the septum. In the flesh, the largest cells reached 300 to 400 μm in size. In the mutant, cells of the skin and hypoderm appeared less elongated than in the wild type, the skin being less differentiated compared to deeper zones of the pericarp. In the mutant mesocarp, cells enlarged significantly less than in the wild type, with cells in the inner mesocarp and septum reaching a maximum size of 150 μm. The mutant pericarp was up to 1,000 μm thick in the region derived from the ovary wall and 2 to 3 mm wide in the central zone derived from the ovary septum with both tissues forming a pseudo flesh that represented a significant volume of the pericarp (Fig. 4R).
Biochemical Analysis
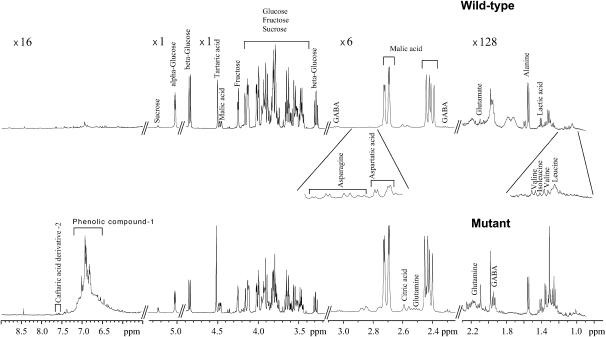
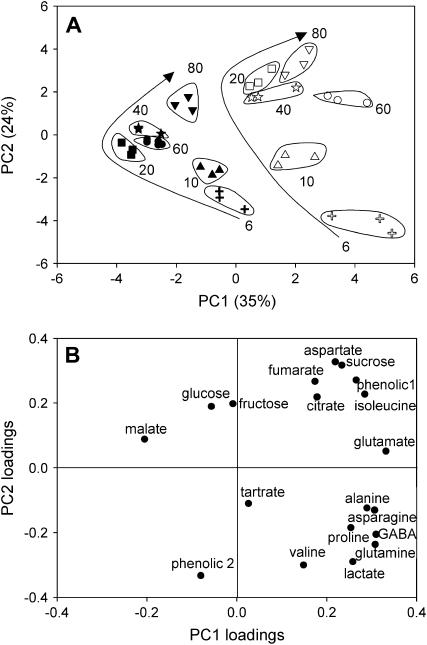
To evaluate putative biochemical changes related to the mutation throughout berry development, major solutes and osmotica were analyzed in a nontargeted approach based on quantitative profiling using one-dimensional 1H-NMR profiles with individual quantification of the major metabolites. Nineteen metabolites were identified and quantified including three sugars, five organic acids, nine amino acids, and two phenolic compounds. The phenolic compounds were assumed to be caftaric derivatives with confirmation required. The results were expressed as concentration or as content per berry. The visual inspection of NMR spectra revealed concentration differences between mutant and wild type for all stages of development as shown for the mature stage in Figure 5. At 80 DAA, the mutant pericarp clearly had lower Glc and Fru and higher concentrations of Suc and phenolic compound 1. Principal component (PC) analysis was used on the concentrations of all metabolites of all pericarp samples to give an overall view of the differences between genotypes and between stages. The first two PC analysis scores explained 59% of the total variability (Fig. 6A). The first PC (PC1), explaining 35% of the total variability, clearly separated the mutant from the wild type. Examination of PC1 loadings (Fig. 6B) suggested that the differences between the wild-type and the mutant samples involved Suc, lactate, Asp, Asn, Ala, Gln, Glu, γ-aminobutyrate, Ile, Pro, and phenolic compound 1 on the positive side, and malate on the negative side. Univariate analyses for each stage of development (data not shown) confirmed these tendencies. The second PC (PC2), explaining 24% of the total variability, separated early from late stages of development. Examination of PC2 loadings (Fig. 6B) suggested that this difference between stages involved Suc, citrate, fumarate, Asp, Ile, and phenolic compound 1 on the positive side, and lactate, Gln, Val, and phenolic compound 2 on the negative side.
Figure 5.
Representative example of NMR spectra of freeze-dried mature pericarp (80 DAA) extract from the Flb− mutant and Flb+ wild type.
Figure 6.
PC analysis on 1H-NMR data (absolute quantification of 19 compounds from the flb mutant and the wild type for extracts at six stages of berry development). A, Scores obtained on PC1 and PC2 for the wild type (black symbols) and the mutant (white symbols). Arrows represent changes during berry development. Individual values are given for each biological triplicate. Each number corresponds to DAA. B, PC1 and PC2 loadings for each analyzed compound.
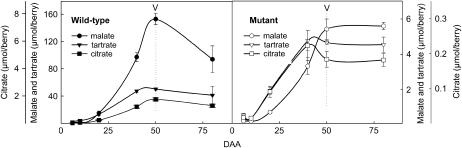
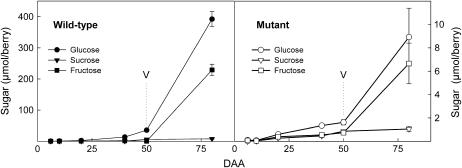
When the data were expressed as content per berry, wild-type berries displayed the shift from acid to sugar accumulation typically encountered in the grape berry and other fleshy fruits during the transition from the green stage to ripening (Figs. 7 and 8). The first significant osmolyte appearing in berries was tartaric acid, synthesized during the first weeks of growth, and the amount per berry remained constant throughout fruit development thereafter (Fig. 7). Malic acid accumulation was delayed 1 week as compared to tartaric acid and exceeded 3-fold the tartaric acid amount at the end of the green stage. The sum of both acids represented 450 mEq/L (data not shown), a typical value in green grapevine berries (Rüffner, 1982). The second growth period was initiated with the onset of hexose storage. It was accompanied by an intense malate breakdown and an acidity decrease with pH values at 2.7 and 3.3, respectively, at the beginning and end of the maturation period.
Figure 7.
Changes in organic acids during berry development (bars = sd). The letter V represents véraison and is the onset of ripening.
Figure 8.
Changes in sugar content during berry development (bars = sd). The letter V represents véraison and is the onset of ripening.
In the mutant, tartaric acid concentration reached a similar value (80 mm) and malic acid accumulation also occurred with a 1-week delay. However, malic acid did not accumulate in excess to tartaric acid during the green stage as observed in the wild type. The lower content of malic acid in mutant berries was not counterbalanced by another organic acid (data not shown); as a consequence fruit pH value was significantly higher (3.7 compared to 2.7 at 20 DAA; and 4.4 compared to 3.3 at maturity). In both genotypes, citric acid accumulation almost paralleled that of tartaric acid and did not contribute to the osmotic pressure.
The kinetics of sugar accumulation were not affected by the mutation: Noticeably, the strong acceleration in sugar storage at the onset of ripening was also observed in the mutant. However the sugar concentration at 80 DAA in the mutant reached a low value (0.33 m, 17 μmol/berry) as compared to 0.54 m (628 μmol/berry) in the wild type (Fig. 8). Moreover, the relative contribution of Suc was unexpectedly higher in the mutant (up to 27%) as compared to the wild type (less than 1.5% during maturation).
Genetics of the flb Mutation
The flb mutation was inherited as a single, dominant locus in progenies produced either through selfing or crossing (Table I). The observed phenotypic segregation fitted that expected under a Mendelian model involving a single, dominant allele with the original mutant being heterozygous for the flb mutation (χ2 tests with 1 degree of freedom not significant at the 5% level).
Table I.
Segregation of the Flb phenotype
| Population | Plants | Wild Type | Mutant | Tested Ratio | χ2 |
|---|---|---|---|---|---|
| MU-S1 | 54 | 20.6% | 79.4% | 1:3 | 0.61a |
| MUWT-F1 | 79 | 49.4% | 50.6% | 1:1 | 1.00a |
Not significant.
DISCUSSION
Within the domesticated grapevine, we have identified a loss-of-function mutation called flb that has a central role in fruit morphogenesis and development. In this study, we have described the phenotype of the corresponding grapevine mutant that has altered ovary development and produces very small fleshless fruits.
It is known from work in other species, such as tomato, that ethylene, auxins, cytokinins, gibberellins, and abscisic acid levels change after fertilization in relation to fruit development (Gillaspy et al., 1993). The role of plant growth regulators in controlling fruit growth has also been documented for grapevine (Matsui et al., 1986). Increasing berry size by hormonal treatment is a common agronomic practice in table grape production, particularly in seedless cultivars (Winkler et al., 1974). It is also known that the number of fertilized ovules and developing seeds can influence the final volume of grapevine fruit. Seeds are supposed to modulate pericarp cell division and enlargement via growth regulator synthesis (Geelen et al., 1987). In tomato, Balbi and Lomax (2003) have reported that a single-gene diageotropica mutation involved in auxin metabolism could reduce fruit weight from between 29% to 81%. Consequently, among the multiple possible causes of pericarp growth inhibition in the flb mutant, plant growth regulators have to be considered. However, spraying flowers and young berries with various combinations and levels of growth regulators, classically used to stimulate berry growth, failed to reverse the mutant phenotype, although they triggered a significant response in the inflorescence rachis and flower pedicel. In addition, the mutant phenotype did not show any obvious relationship with the number of seeds per berry. The mutant berries produced the normal number of perfect seeds fully tegumented and containing viable zygotic embryos. Although greatly reduced in volume, the amount of pseudo flesh per mutant berry was well correlated with the number of perfect seeds per berry. These observations indicate that the defect in the mutant is not simply a deficiency in plant growth regulator levels. While a lack in hormone signal reception or transduction in the mutant might be a cause, the positive response of the rachis and pedicel to applied growth regulators suggests that this is unlikely. Finally, the flb mutation had little effect on fertility and seed size or number. Similar features were observed in Arabidopsis ful-1 mutants (Gu et al., 1998) and in tomato lines bearing the fw2.2 allele (Liu et al., 2003) and indicate that the effects of the mutation on fruit phenotype is specifically due to an alteration in the maternal tissue of developing fruit and not mediated through fertility or seed set-related processes.
The final size of an organ is dependant on cell number and/or size (Ho, 1992; Cowan et al., 2001; Rapoport et al., 2004). Despite the fact that cell expansion may account for the greatest increase in volume, cell division is also an essential factor of fruit organogenesis because it determines the final number of cells within the fruit (Cong et al., 2002). It has been shown that the variation in the cell number of the fruit is a major determinant of fruit size variability between cultivars in Solanum (Bonher and Bangerth, 1988) and Prunus (Yamaguchi et al., 2004). In tomato, fw2.2, a major quantitative trait locus, was found to account for as much as 30% of fruit size through an effect on cell division (Frary et al., 2000). In the Flb− mutant, flower organization conformed to the wild type of the domesticated grapevine (Fougère-Rifot et al., 1995; Hardie et al., 1996). At later stages of flower development, irregular multiplications slightly modified the ovary shape, without impairing the formation of fertile ovules, fecundation, or development of perfect seeds and skin tissues up to 3 months after fruit set. This very early phenotype may indicate that cells specific for later flesh development may be lacking in mature ovaries, with the consequences on cell division becoming obvious following pollination. In the mutant and its wild-type counterpart, cell division kinetics are consistent with previous studies in grapevine showing that ovary wall cells divide only during the green growth stage. The total DNA amount in the wild type was found to be slightly higher than the value reported by Ojeda et al. (1999), but this change probably resulted from variations in cultivars or environmental growth conditions. In grapevine, late endoreduplications that are observed in tomato flesh during the maturation period (Bertin et al., 2003) can be excluded, since DNA content was found to be very stable from 30 DAA.
At maturity, the mutant pericarp volume was reduced by 20 times, resulting in a 10-times reduction in fruit weight. The effect of the flb mutation reduced the DNA content by 50% and the pericarp volume by 95%, suggesting that the more vacuolated cells are specifically impaired. Microscopic observations confirmed that such inhibition of cell division and growth predominantly concerns the mesocarp, with the septum and epidermis being less disturbed by the flb mutation. Consequently, septum tissues or derived cells can represent an important proportion of a fruit, especially in the mutant berry. Developmental pattern difference between septum and ovary wall cells after fertilization has been similarly reported by Gu et al. (1998) in the Arabidopsis ful-1 mutant and also by Müller et al. (2001) studying the overexpressed Antirrhinum DEFH28 FUL ortholog gene in Arabidopsis. Cong et al. (2002) investigated a similar effect of the fw2.2 allele in different tomato near-isogenic lines. These observations across several species suggest that the septum follows a separate developmental pathway to the mesocarp and there is a conserved mechanism of development for the central ovary tissues in angiosperms.
In grapevine, fruits enlarge in two phases, green stage and maturation, the first one being associated with organic acid synthesis and the second one coupled with sugar accumulation (Possner and Kliewer, 1985; Davies and Robinson, 1996). Although severely reduced in size and organization, the pseudo flesh of the flb mutant showed the transition from acid to sugar storage typically observed during grapevine fruit ripening, with the same timing in the mutant and the wild type. However, the mutant showed variations in the concentration of some osmotica. The most striking difference was the failure of the mutant berries to accumulate 3-fold more malate than tartrate during the green stage. The distribution inside the berry of tartrate and oxalate synthesis from ascorbate has been recently documented (Debolt et al., 2004). Organic acids are nonuniformly distributed in the wild-type berry, with tartaric acid being highest toward the outside of the developing berry and malic acid being highest in the core of the flesh (Iland and Coombe, 1988). Changes in organic acid patterns during berry development are possibly related to cell specialization and developmental changes in the relative proportion of the cell layers forming the pseudo flesh. In addition, the mutant exhibited a unique variation in the mesocarp tissue differentiation pattern compared to wild type. In the mutant the inner and outer mesocarp maintained cells with high phenolic compounds throughout the green growth stage, which could be related to the higher concentration of a putative caftaric acid derivative observed in the pericarp. Conversely, in the wild type, a few days following anthesis, cells of the inner mesocarp lost their phenols and developed into highly vacuolated cells leading to enormous differences between the inner and outer mesocarp. To explain the variations in organic acid balance observed in the mutant, it could be hypothesized that the cell layers of the inner mesocarp are specialized for malate storage during berry development.
Another biochemical difference was the high relative contribution of Suc to total sugar during berry development. To our knowledge, in grapevine, the relative proportion of Suc in the berry has never been reported to be more than 7% (Ageorges et al., 2000). A 20% relative contribution of Suc was previously reported in the Steuben grapevine hybrid as a consequence of a reduction in vacuolar invertase activity; however this inhibition did not preclude berry growth and flesh differentiation (Takayanagi and Yokotsuka, 1997). Invertase is preferentially located in the inner mesocarp (Famiani et al., 2000), and these tissues are apparently lacking or reduced in the Flb− mutant. Recent transcriptomic and proteomic data indicated a predominate role of the apoplastic versus symplastic pathway during grapevine flesh development (Sarry et al., 2004; Terrier et al., 2005). The observation that ovule development is not impaired by the flb mutation demonstrates that phloem conductance is not a limiting factor during mutant berry development. In addition, histological analysis did not reveal any alteration of vascular bundles in the flb ovary. More likely, cell layers playing a key role in the apoplastic pathway of sugar uploading in the pericarp are lacking, affecting the biochemical development of the remaining tissues. The above strongly suggests that specialized cells that differentiate very early in the inner mesocarp, characterized by the loss of phenolic compounds, are possibly those overaccumulating malic acid, sugars, and those showing the greatest enlargement (Hardie et al., 1996). The mutant is apparently unable to differentiate these highly vacuolated cells.
Interestingly, the mutant produces a type of berry and seed unknown in the domesticated European grapevine. Analysis of previous ampelography and botanic studies (Viala and Vermorel, 1910; Galet, 1988) revealed a correlation between berry size and seed morphology in Vitaceae. Most Vitis species with large berries (grapevine, Vitis lincecumii, and Vitis coriacea) develop pear-shaped seeds in contrast with other Vitis species with small berries (Vitis berlandieri, Vitis riparia, Vitis rupestris, and V. caribaea) that develop orbicular seeds as seen in the mutant. This correlation is more obvious if we consider other Vitaceae such as Ampelopsis, Parthenocissus, or Muscadinia. In addition, wild progenitors of the domesticated European grapevine commonly identified as grapevine subsp. sylvestris, are also characterized by very small berries with orbicular seeds (Levadoux, 1956; Marinval, 1997). The phenotype changes caused by the flb mutation could correspond to a reverse evolution of the domesticated European grapevine type toward an ancestral type of grapevine fruit.
Genetic analysis of progeny populations showed that the original mutant is heterozygous for the flb mutation. The mutation segregated as a single dominant allele indicating that a single locus is involved and that the mutation affects either a single gene or a number of genes at this locus. Further analysis and mapping are currently being undertaken to better understand whether the flb locus encompasses one or several genes.
In conclusion, this study has shown that the formation of viable grapevine seeds does not require complete fruit development after anthesis. Conversely, development of an ovary into a berry without the need of seeds has been widely documented in stenospermocarpic and parthenocarpic grapevine cultivars. These two lines of evidence indicate that the synchronous production of functional zygotic embryos for sexual propagation with the development of the fruit are physiologically independent processes that need to be strictly coordinated to form a mature fleshy fruit with seeds ready for dispersal. Another important observation is related to the relationship between maturation processes and fruit growth. The study of the effects of the flb mutation showed that the storage of organic compounds (i.e. organic acid and sugar) is not sufficient to induce fruit enlargement. To our knowledge, no similar extreme mutation with such specific effects on fleshy fruit growth has previously been described. We expect that molecular genetic analysis of the grapevine mutant will provide a unique opportunity to investigate key gene(s) involved in fruit morphogenesis in higher plants and lead to a better understanding of differences between fleshy and fleshless fruit.
MATERIALS AND METHODS
Plant Material
The flb mutation was identified in 1996 in a vineyard located at Prades-le-Lez (France) on a grapevine (Vitis vinifera L. cv Ugni-Blanc) plant later characterized as a genetic anticlinal chimera. The original plant was maintained in situ and the two genotypes (wild type and mutant) were propagated in containers from woody canes taken from the original plant. Flowers and berries from the Flb− mutant and the Flb+ wild type were collected at different developmental stages from 1999 to 2004 and used in various experiments. All flowers and berries were carefully cut at the pedicel base and either used as fresh material or frozen and powdered in liquid nitrogen.
Morphological and Physiological Aspects of Berry Growth
Developmental patterns were obtained from plants cultivated either in the field (1999) or in the greenhouse (2004) by weighing 60 berries from anthesis to maturity. Seed data was collected from 20 berries from anthesis to maturity. At maturity (90 DAA), berry, pericarp, and seed weights were evaluated from 300 berries for each genotype. Two hundred seeds were collected, dried, and tested for germination rate. The effect of several plant growth regulators on the expression of the flb mutation was evaluated by immersing the lower half of inflorescences once (14 DAA) or twice (7 and 14 DAA) or 3 times (flowering, 7 and 14 DAA) in solutions containing 10, 20, 100, or 500 μg mL−1 of GA3, BAP, IAA alone, or combinations of GA3 + BAP, GA3 + IAA, GA3 + GA4 + GA7, or GA3 + GA7 + 2-isopentenyl adenine with water as control. Each treatment was applied to two clusters from two separate plants and repeated twice, once in the field (2001) and once in the greenhouse (2002). The berry response was evaluated at 20 and 40 DAA by recording flower set and growth of the stalk, pedicels, and berries.
DNA Quantification
For each genotype, DNA was extracted from 20 berries as in Ojeda et al. (1999) at 15, 30, 45, 60, 75, and 90 DAA and after seed removal. To reduce possible artifacts due to berry position, samples were randomly taken from previously tagged inflorescences with synchronous flowering. DNA was quantified through technical triplicates with 33 ng mL−1 4,6-diamidino-2-phenylindol in 0.01 m Tris-Hcl, 0.1 m NaCl, and 0.01 m EDTA by reference to salmon sperm DNA standards.
Anatomical Analysis
Mutant and wild-type flowers and berries were collected from field or greenhouse-grown vines at different intervals: −21, −11, −3, 0, +3, +7, +11, +14, +18, +21, +25, +28, +32, +35, +39, +43, +47, +60, and +90 d from anthesis. Samples were vacuum infiltrated for 1 h with cold 4% paraformaldehyde solution (1× phosphate-buffered saline, pH 7) and maintained in the same fixative solution on ice for 7 to 10 h. After a 0.85% NaCl rinse for 30 min (4°C), samples were dehydrated in a 15% to 70% ethanol series and maintained in 70% ethanol at 4°C until use. Developmental stages were compared by examining 7-μm sections paraffin embedded and stained with periodic acid-Schiff reaction/Naphthol-blue-black reaction with 2-min regression with 7% acetic acid. This staining combination revealed polysaccharides such as starch or hemicelluloses as pink, proteins and nucleoproteins as blue-black, and polyphenol compounds as gray-brown.
Variation in Organic Compounds
Analyses were carried out on berries collected at 6, 10, and 20 DAA and on berries with seeds removed for later stages (40, 50, and 80 DAA). Sample extracts were prepared from pericarp (pulp and skin) as follows: 5 g of fresh tissue (6–140 berries) was mixed with 25 mL of water, immediately boiled for 5 min to inhibit vacuolar invertase, crushed, and filtered (100 μm). Biological triplicates were prepared for each stage. The pH of the extract was raised to 6 with NaOH to prevent potassium hydrogen tartrate precipitation. Extracts were centrifuged for 15 min (8,000 rpm) at 20°C and the supernatant was frozen in nitrogen and lyophilized. Quantitative metabolic profiling on main sugars, organic acids, amino acids, and two phenolic compounds were determined on dried extracts solubilized in 200 mm phosphate buffer in D2O (with 2.5 mm EDTA for 6 and 20 DAA) by one-dimensional 1H-NMR analyses with a 5-mm inverse probe according to Moing et al. (2004). Results were expressed as concentration or content per berry of individual metabolites. The data for all compounds and all samples were analyzed simultaneously using multivariate analysis to give an overall view of the data during berry development. PC analysis, an unsupervised method, was chosen as a useful visualization method to observe sample groupings within the 1H-NMR data. PC analysis was performed on mean-centered data using SAS software (SAS 496 Institute, version 8.1). Data were represented in an n-dimensional space (n is the number of original variables, i.e. concentrations of 19 individual metabolites), and then reduced into a few PCs that described the maximum variation within the data. The coefficients by which the original variables were multiplied to obtain the PCs were called loadings. Loading plots were used to detect the metabolites responsible for the separation between development stages or genotypes in the data.
Genetic Analysis of the flb Mutation
For genetic analysis, progeny populations involving the original mutant were produced. Plants were first grown in the greenhouse for 1 year and then planted in the field at the Institut National de la Recherche Agronomique experimental station of Chapitre (Montpellier, France). A total of 133 progeny plants were phenotyped; 54 individuals from selfing the mutant (MU; MU-S1 progeny) and 79 from F1 reciprocal crosses between the wild type (WT) and the mutant (MUWT-F1). Occurrence of ovary alterations corresponding to the flb phenotype in progenies was recorded during two successive years (2003 and 2004), first at flowering and with further confirmation 10 d later.
Acknowledgments
We would like to thank Dr. Françoise Dosba and Dr. Guy Albagnac for encouraging the project and for helpful discussions, Dr. Catherine Deborde for her expertise in NMR spectra interpretation and Drs. Agnes Ageorges, Thierry Lacombe, and Philippe Chatelet for critical reading of the manuscript. We would also like to thank the Vassal staff for the excellent technical assistance for seed germination assays. This work would not have been possible without the initial observation of Mr. Hubert Pioch, who discovered the flb mutant in his vineyard at Prades-le-Lez (France).
This work was supported by the Languedoc-Roussillon region, Commonwealth Scientific and Industrial Research Organization Plant Industry, and the Institut National de la Recherche Agronomique departments of Caractérisation et Elaboration des Produits Issus de l'Agriculture and Génétique et Amélioration des Plantes.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Laurent Torregrosa (laurent.torregrosa@ensam.inra.fr).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.067488.
References
- Ageorges A, Issaly R, Picaud S, Delrot S, Romieu C (2000) Identification and functional expression in yeast of a grape berry sucrose carrier. Plant Physiol Biochem 38: 177–185 [Google Scholar]
- Balbi V, Lomax TL (2003) Regulation of early tomato fruit development by the diageotropica gene. Plant Physiol 131: 186–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin N, Borel C, Brunel B, Cheniclet C, Causse M (2003) Do genetic make-up and growth manipulation affect tomato fruit size by cell number, or cell size and DNA endoreduplication? Ann Bot (Lond) 92: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonher J, Bangerth F (1988) Cell number, cell size and hormone levels in semi-isogenic mutants of Lycopersicon pimpinellifolim differing in fruit size. Physiol Plant 72: 316–320 [Google Scholar]
- Boss PK, Buckeridge EJ, Poole A, Thomas MR (2003) New insights into grapevine flowering. Funct Plant Biol 30: 593–606 [DOI] [PubMed] [Google Scholar]
- Boss PK, Sensi E, Hua C, Davies C, Thomas MR (2002) Cloning and characterisation of grapevine (Vitis vinifera L.) MADS-box genes expressed during inflorescence and berry development. Plant Sci 162: 887–895 [Google Scholar]
- Boss PK, Thomas MR (2002) Association of dwarfism and floral induction with a grape “green revolution” mutation. Nature 416: 847–850 [DOI] [PubMed] [Google Scholar]
- Boss PK, Vivier M, Matsumoto S, Dry IB, Thomas MR (2001) A cDNA from grapevine (Vitis vinifera L.), which shows homology to AGAMOUS and SHATTERPROOF, is not only expressed in flowers but also throughout berry development. Plant Mol Biol 45: 541–553 [DOI] [PubMed] [Google Scholar]
- Bouché N, Bouchez D (2001) Arabidopsis gene knockout: phenotypes wanted. Curr Opin Plant Biol 4: 111–117 [DOI] [PubMed] [Google Scholar]
- Calonje M, Cubas P, Martinez-Zapater JM, Carmona MJ (2004) Floral meristem identity genes are expressed during tendril development in grapevine. Plant Physiol 135: 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona MJ, Cubas P, Martinez-Zapater JM (2002) VFL, the grapevine FLORICAULA/LEAFY ortholog, is expressed in meristematic regions independently of their fate. Plant Physiol 130: 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong B, Liu JP, Tanksley SD (2002) Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proc Natl Acad Sci USA 99: 13606–13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe BG (1992) Research on development and ripening of the grape berry. Am J Enol Vitic 43: 101–110 [Google Scholar]
- Corelli-Grappadelli L, Lakso AN (2004) Fruit development in deciduous tree crops as affected by physiological factors and environmental conditions. Acta Hortic 557: 377–383 [Google Scholar]
- Cowan AK, Cripps RF, Richings EW, Taylor NJ (2001) Fruit size: towards an understanding of the metabolic control of fruit growth using avocado as a model system. Physiol Plant 111: 127–136 [Google Scholar]
- Davies C, Robinson SP (1996) Sugar accumulation in grape berries: cloning of two putative vacuolar invertase cDNAs and their expression in grapevine tissues. Plant Physiol 111: 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debolt S, Hardie J, Tyerman S, Ford CM (2004) Composition and synthesis of raphide crystals and druse crystals in berries of Vitis vinifera L. cv. Cabernet Sauvignon: ascorbic acid as precursor for both oxalic and tartaric acids as revealed by radiolabelling studies. Aust J Grape Wine Res 10: 134–142 [Google Scholar]
- Dinneny JR, Yanofsky MF (2005) Drawing lines and borders: how the dehiscent fruit of Arabidopsis is patterned. Bioessays 27: 42–49 [DOI] [PubMed] [Google Scholar]
- Eames AJ, MacDaniels LH (1947) An Introduction to Plant Anatomy, Ed 2. MacGraw-Hill, New York
- Emmanuel E, Levy AA (2002) Tomato mutants as tools for functional genomics. Curr Opin Plant Biol 5: 112–117 [DOI] [PubMed] [Google Scholar]
- Famiani F, Walker RP, Tecsi L, Chen ZH, Proietti P, Leegood RC (2000) An immunohistochemical study of the compartmentation of metabolism during the development of grape (Vitis vinifera L.) berries. J Exp Bot 51: 675–683 [PubMed] [Google Scholar]
- Fougère-Rifot M, Benharbit El-Alami N, Brun O, Bouard J (1995) Ontogenesis of the gynoecium of Vitis vinifera L. var. Chardonnay in relation to the appearance of tannic vacuoles. J Int Sci Vigne Vin 29: 105–130 [Google Scholar]
- Frary A, Nesbitt TC, Grandillo S, van der Knaap E, Cong B, Liu JP, Meller J, Elber R, Alpert KB, Tanksley SD (2000) fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289: 85–88 [DOI] [PubMed] [Google Scholar]
- Galet P (1988) Cépages et vignobles de France, Tome I, Les vignes américaines. Charles Dehan, Montpellier, France
- Geelen TAM, Varga A, Bruinsma J (1987) Cell division and elongation in the exocarp of tomato fruits grown in systems in vitro and on the vine. J Plant Physiol 130: 343–349 [Google Scholar]
- Gerrath JM (1993) Developmental morphology and anatomy of grape flowers. Hortic Rev (Am Soc Hortic Sci) 13: 315–337 [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5: 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell (Suppl) 16: S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Ferrandiz C, Yanofsky MF, Martienssen R (1998) The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 125: 1509–1517 [DOI] [PubMed] [Google Scholar]
- Hardie WJ, O'Brien TP, Jaudzems VG (1996) Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust J Grape Wine Res 2: 97–142 [Google Scholar]
- Ho LC (1992) Fruit growth and sink strength. In C Marshall, J Grace, eds, Fruits and Seed Production: Aspects of Development, Environmental Physiology and Ecology. Cambridge University Press, Cambridge, UK, pp 101–124
- Holland JN, Wyatt R, Bronstein JL, Ness JH (2003) Relating the biology of flower-to-fruit survivorship to the ecology and evolution of fruit-to-flower ratios. In SG Padalai, ed, Recent Research Developments in Plant Science, Vol. 1. Research Signpost, Trivandrum, India, pp 75–84
- Iland PG, Coombe BG (1988) Malate, tartrate, potassium, and sodium in flesh and skin of Shiraz grapes during ripening: concentration and compartmentation. Am J Enol Vitic 39: 71–76 [Google Scholar]
- Judd WS, Campbell CS, Kellogg E, Stevens FF (1999) Plant Systematics: A Phylogenetic Approach. Sinauer Associates, Sunderland, MA
- Knapp S (2002) Tobacco to tomatoes: a phylogenetic perspective on fruit diversity in the Solanaceae. J Exp Bot 53: 2001–2022 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Goto-Yamamoto N, Hirochika H (2004) Retrotransposon-induced mutations in grape skin color. Science 304: 982. [DOI] [PubMed] [Google Scholar]
- Levadoux L (1956) Les populations sauvages et cultivées de Vitis vinifera L. Ann Amel Plantes (Paris) 6: 59–118 [Google Scholar]
- Liu JP, Cong B, Tanksley SD (2003) Generation and analysis of an artificial gene dosage series in tomato to study the mechanisms by which the cloned quantitative trait locus fw2.2 controls fruit size. Plant Physiol 132: 292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinval P (1997) Vigne sauvage et vigne cultivée dans le bassin méditerranéen: emergence de la viticulture, contribution archéo-botanique. In OIV, ed, Histoire du Vin, une histoire de rites. OIV, Paris, pp 137–172
- Matsui S, Ryugo K, Kliewer WM (1986) Effects of heat stress and plant hormone treatments on growth of young Napa Gamay (Vitis vinifera L.) berries. Res Bull Fac Agric Gifu Univ 51: 227–234 [Google Scholar]
- Moing A, Maucourt M, Renaud C, Gaudillere M, Brouquisse R, Lebouteiller B, Gousset-Dupont A, Vidal J, Granot D, Denoyes-Rothan B, et al (2004) Quantitative metabolic profiling by 1-dimensional H-1-NMR analyses: application to plant genetics and functional genomics. Funct Plant Biol 31: 889–902 [DOI] [PubMed] [Google Scholar]
- Müller BM, Saedler H, Zachgo S (2001) The MADS-box gene DEFH28 from Antirrhinum is involved in the regulation of floral meristem identity and fruit development. Plant J 28: 169–179 [DOI] [PubMed] [Google Scholar]
- Ojeda H, Deloire A, Carbonneau A, Ageorges A, Romieu C (1999) Berry development of grapevines: relations between the growth of berries and their DNA content indicate cell multiplication and enlargement. Vitis 38: 145–150 [Google Scholar]
- Possner DRE, Kliewer WM (1985) The localisation of acids, sugars, potassium and calcium in developing grape berries. Vitis 24: 229–240 [Google Scholar]
- Rapoport HF, Manrique T, Gucci R (2004) Cell division and expansion in the olive fruit. Acta Hortic 636: 461–465 [Google Scholar]
- Rüffner HP (1982) Metabolism of tartaric and malic acids in Vitis: a review, Part B. Vitis 21: 346–358 [Google Scholar]
- Sarry JE, Sommerer N, Sauvage FX, Bergoin A, Rossignol M, Albagnac G, Romieu C (2004) Grape berry biochemistry revisited upon proteomic analysis of the mesocarp. Proteomics 4: 201–215 [DOI] [PubMed] [Google Scholar]
- Scorza R, May LG, Purnell B, Upchurch B (1991) Differences in number and area of mesocarp cells between small- and large-fruited peach cultivars. J Am Soc Hortic Sci 116: 861–864 [Google Scholar]
- Seymour GB, Manning K, Eriksson EM, Popovich AH, King GJ (2002) Genetic identification and genomic organization of factors affecting fruit texture. J Exp Bot 53: 2065–2071 [DOI] [PubMed] [Google Scholar]
- Staudt G, Schneider W, Leidel J (1986) Phases of berry growth in Vitis vinifera. Ann Bot (Lond) 58: 789–800 [Google Scholar]
- Takayanagi T, Yokotsuka K (1997) Relationship between sucrose accumulation and sucrose-metabolizing enzymes in developing grapes. Am J Enol Vitic 48: 403–407 [Google Scholar]
- Terrier N, Glissant D, Grimplet J, Barrieu F, Abbal P, Couture C, Ageorges A, Atanassova R, Léon C, Renaudin J-P, et al (2005) Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta 222: 832–847 [DOI] [PubMed] [Google Scholar]
- Viala P, Vermorel V (1910) Ampélographie, Tome 1. Masson et Cie, Paris
- Winkler AJ, Cook JA, Kliewer WM, Lider LA (1974) General Viticulture. University of California Press, Los Angeles
- Yamaguchi M, Haji T, Yaegaki H (2004) Differences in mesocarp cell number, cell length and occurrence of gumming in fruit of Japanese apricot (Prunus mume Sieb. et Zucc.) cultivars during their development. J Jpn Soc Hortic Sci 73: 200–207 [Google Scholar]