Abstract
Since first identifying two alleles of a rice (Oryza sativa) brassinosteroid (BR)-insensitive mutant, d61, that were also defective in an orthologous gene in Arabidopsis (Arabidopsis thaliana) BRASSINOSTEROID INSENSITIVE1 (BRI1), we have isolated eight additional alleles, including null mutations, of the rice BRI1 gene OsBRI1. The most severe mutant, d61-4, exhibited severe dwarfism and twisted leaves, although pattern formation and differentiation were normal. This severe shoot phenotype was caused mainly by a defect in cell elongation and the disturbance of cell division after the determination of cell fate. In contrast to its severe shoot phenotype, the d61-4 mutant had a mild root phenotype. Concomitantly, the accumulation of castasterone, the active BR in rice, was up to 30-fold greater in the shoots, while only 1.5-fold greater in the roots. The homologous genes for OsBRI1, OsBRL1 and OsBRL3, were highly expressed in roots but weakly expressed in shoots, and their expression was higher in d61-4 than in the wild type. Based on these observations, we conclude that OsBRI1 is not essential for pattern formation or organ initiation, but is involved in organ development through controlling cell division and elongation. In addition, OsBRL1 and OsBRL3 are at least partly involved in BR perception in the roots.
Brassinosteroids (BRs) are plant hormones that have various effects on plant growth and development, including cell elongation, cell division, vascular development, abscission, and stress resistance (Clouse and Sasse, 1998; Sasse, 1999). The study of BRs began much later than that of other classical plant hormones, such as auxin, cytokinin, GA, abscisic acid, and ethylene. The effect of BR was first demonstrated in the 1960s, and the isolation of brassinolide (BL), the most active BR, was accomplished in 1979 (Mandava, 1988; Sasse, 1999). Since then, the study of BRs has rapidly progressed, coupled with successful molecular genetics approaches in Arabidopsis (Arabidopsis thaliana). The cloning of the BR receptor BRASSINOSTEROID INSENSITIVE1 (BRI1), the second plant hormone receptor ever to be cloned (Wang et al., 2001), was typical of the rapid progress in the study of BRs.
Recently, three homologous genes for BRI1, BRL1 to 3, were found in Arabidopsis (Caño-Delgado et al., 2004; Zhou et al., 2004). When these homologous genes, under the control of the BRI1 promoter, were expressed in the Arabidopsis bri1 mutant, the bri1 phenotype was rescued by introduction of BRL1 and BRL3, but not by BRL2 (Caño-Delgado et al., 2004). Chemical experiments support the above observation that BRL1 and BRL3 can interact with BL with high affinity, but BRL2 cannot (Kinoshita et al., 2005). The triple mutant bri1/brl1/brl3 also enhanced the abnormal phenotype of bri1. Based on these observations, Chory and her colleagues concluded that Arabidopsis contains three BR receptors with specific functions in cell growth and vascular differentiation (Wang et al., 2001; Caño-Delgado et al., 2004; Kinoshita et al., 2005).
BR receptors, which are homologous to the Arabidopsis BRI1 gene (AtBRI1), have been isolated from many plant species, including dicots such as tomato (Lycopersicon esculentum) and pea (Pisum sativum), and monocots such as rice (Oryza sativa) and barley (Hordeum vulgare; Yamamuro et al., 2000; Montoya et al., 2002; Chono et al., 2003; Nomura et al., 2003). In rice, the orthologous gene for Arabidopsis BRI1, OsBRI1, is expressed in almost all organs, as in Arabidopsis (Yamamuro et al., 2000). Loss-of-function mutants of OsBRI1, d61-1 and d61-2, are insensitive to BR and have erect leaves, dwarf culms, abnormal skotomorphogenesis, and no organized microtubule arrangement in the cells from nonelongated internodes. Interestingly, several transgenic plants carrying the antisense strand of the OsBRI1 transcript exhibited phenotypes even more severe than those of d61-1 and d61-2 (Yamamuro et al., 2000).
In this study, we screened eight additional d61 alleles that showed various degrees of phenotypic severity. We analyzed a null mutant, d61-4, whose phenotype was the most severe of the 10, to characterize BRI1 function in rice. The phenotypic severity of d61-4 was much stronger and BR accumulation was much greater in shoots than in roots. In rice, the homologous genes for OsBRI1, OsBRL1 and OsBRL3, were preferentially expressed in roots. Based on these observations, we propose that rice has three BR receptors and OsBRI1 dominantly functions in almost all organs, but the other two proteins, OsBRL1 and OsBRL3, also function in the roots.
RESULTS
Phenotypes of Various d61 Alleles in Rice
In addition to two previously reported alleles of the rice OsBRI1 gene (d61-1 and d61-2), which is homologous to Arabidopsis BRI1 (Yamamuro et al., 2000), we isolated eight new Osbri1 alleles. The phenotypic severity of these Osbri1 (d61) alleles varied (Fig. 1). We classified these mutants into three groups according to plant height and fertility. The height of plants with mild and intermediate alleles was approximately 80% to 90% and 60% to 70% of the wild type at the heading stage, respectively (Fig. 2A). Plants with mild and intermediate d61 alleles were fertile and formed dark green, erect leaves. In contrast, plants with severe alleles were severely dwarfed, and plant height 6 months after sowing was only approximately 5 cm (Fig. 2, B and C). These severe alleles resulted in sterile plants, because of the absence of flowers, with malformed dark green leaves (Fig. 2C). These severe mutants could not grow in soil. The overall phenotype of the severe d61 alleles was similar to the knockout mutant of BR C-6 oxidase, brd1-1 (Hong et al., 2002), although the severity of the d61 mutations was stronger.
Figure 1.
The mutation positions of the 10 d61 alleles.
Figure 2.
The phenotypes of the d61 mutants. A, The gross morphology of the d61 mild (d61-1, middle) and intermediate (d61-2, right) alleles. For comparison, the wild-type (WT) plant (left) is also shown (bar = 20 cm). B, The gross morphology of the wild type (left) and the severe allele (d61-4, right) at 2 months after sowing. A close-up view of the mutant was superimposed at the top right (bar = 5 cm). C, A close-up view of the d61-4 shoot at 6 months after sowing. The plant height was less than 5 cm. The d61-4 leaf was severely stunted and twisted (bar = 1 cm). D, The rice leaf can be divided into the blade (upper) and the sheath (lower) by the lamina joint (arrowhead). The sheath of the d61-4 leaf almost disappeared (bar = 5 cm). E, The first leaves of the wild type (left) and d61-4 (right). The first leaf of rice develops only a sheath and not a blade, while the mutant leaf develops a dominant blade (bar = 1 mm). Arrowheads indicate the lamina joint.
Rice leaves can be separated into two parts, the leaf blade (upper region) and the sheath (lower region), by the lamina joint (Fig. 2D, arrowhead). The rice root system consists of three types of roots: seminal root formed in the embryo, crown (adventitious) root formed from the stem after germination, and lateral root. We measured the length of the leaf blades, leaf sheaths, and seminal root, and counted the number of crown roots and lateral roots in 10-d-old wild-type and d61 plants (Table I). The most dramatic defect was observed in leaf sheath length; the leaf sheaths of d61-4 were shortened to less than one-twenty-fifth the length of wild-type leaf sheaths. Leaf blade length was also shortened in d61-4, but the extent was less than in the sheaths, and no defect was observed in d61-1 or d61-2. Consequently, the ratio of leaf blade length to sheath length increased along with severity. In contrast to the severe defect in leaf sheath elongation, the d61 mutation did not appear to affect seminal root elongation. Seminal root length in d61-4 was almost three-quarters that of the wild type, whereas a slight promotion was observed in d61-1 and d61-2 relative to wild type. The formation of lateral roots in the seminal roots was also not severely affected by the mutations. Although the most plain root phenotype in d61 was the crown root formation in the stem, this may have been caused by defects in stem development, from which crown roots develop, resulting from the d61 mutation. These phenotypic analyses indicate that the d61 mutations preferentially cause defects in leaf formation and elongation and have much less effect on root formation and elongation (see below).
Table I.
Morphology of 10-d-old wild-type and d61 mutantsa
| Wild Type | d61-1 (Mild) | d61-2 (Intermediate) | d61-4 (Severe) | |
|---|---|---|---|---|
| Leaf blade (cm) | 1.15 ± 0.15 | 1.19 ± 0.20 | 1.22 ± 0.38 | 0.69 ± 0.46 |
| Leaf sheath (cm) | 2.70 ± 0.37 | 1.75 ± 0.22 | 1.67 ± 0.23 | 0.10 ± 0.03 |
| Leaf blade/leaf sheath | 0.42 | 0.68 | 0.73 | 7.11 |
| Seminal root length (cm) | 5.65 ± 0.96 | 6.78 ± 1.48 | 8.66 ± 1.46 | 4.36 ± 0.83 |
| No. of lateral roots | 16.9 ± 2.3 | 14.3 ± 2.0 | 11.3 ± 0.9 | 12.6 ± 2.6 |
| No. of crown roots | 7.7 ± 1.7 | 6.4 ± 0.9 | 5.0 ± 0.9 | 3.9 ± 0.9 |
Data represent the means ± sd of 10 plants in each line.
The 10 mutations were located at various domains: Leu-rich repeats (LRRs; four alleles), a 70-amino acid island (ID; two alleles), a transmembrane domain (one allele), and a kinase domain (three alleles; Fig. 1). The mutants d61-4 and d61-6 had a single nucleotide substitution to produce a stop codon at Glu-847 and a 2-bp insertion at Asp-759 to cause a frame shift, respectively. Thus, it was expected that these mutants would have completely lost the OsBRI1 function and exhibited the most severe phenotypes. The phenotypic severity of the alleles causing single nucleotide substitutions at LRR, d61-3, d61-5, d61-7, and d61-2, varied. The amino acid changes of two alleles, d61-3 and d61-5, that were classified in the severe group were predicted to alter the secondary or tertiary structure or both of LRR and severely reduce its function. The intermediate phenotype d61-2 was associated with a Val residue in the LRR just in front of ID substituted with Met. The mutations in d61-8 and d61-9 occurred at Gly-522 and Gly-539 in ID, respectively. Although ID has been considered important for BR binding, both mutants exhibited the mild phenotype. An Arabidopsis bri1 mutation, bri1-113, whose mutation site corresponds to that of d61-9, causes a severe phenotype in Arabidopsis (Li and Chory, 1997). In Arabidopsis, the phenotypic severity of the bri1 mutant somehow depends on the ecotype (Caño-Delgado et al., 2004). Thus, the strong phenotype observed in Arabidopsis bri1-113 may depend on its ecotype. It is also possible that if endogenous active BRs in Arabidopsis and rice differ, the different phenotypic severity reflects the importance of the Gly residue for BR binding. Kinase activity should be essential for BRI1 function, but the amino acid changes of d61-10 and d61-1 at the kinase domain did not cause severe defects in BRI1 function, probably because these amino acid exchanges did not disrupt the BRI1 kinase activity. Thus, these amino acid residues are not considered important for kinase activity (Oh et al., 2000; Wang et al., 2005).
The Phenotype of d61-4
We further analyzed d61-4, which had lost almost all the kinase domain and was therefore considered to be a null allele. Actually, this allele showed the most severe phenotype of all the alleles. Germination of d61-4 was delayed 1 or 2 d relative to the wild type, and it developed severely stunted, rolled, and twisted dark green leaves, while the plastochron of d61-4 was nearly identical to that of the wild type (Fig. 2, B and C; data not shown). In addition to the abnormal leaf structure, the ratio of leaf blade to sheath length was altered in d61-4. The length of the blade and sheath of the fourth leaf were nearly the same as in the wild type, while in d61-4 the length of the leaf blade was slightly less relative to that of the wild type and the sheath length was drastically reduced (Fig. 2D). In 2-month-old d61-4, shoot development was strictly defective and leaves were still rolled and twisted; however, a number of roots were developed, as in the wild type, and unlike the leaves the roots were not rolled (Fig. 2B; see below).
Whereas the first leaf of the wild type had a unique structure and did not form a leaf blade, in d61-4 the same leaf blade was formed in the first leaf as in the other leaves (Fig. 2E). The formation of a leaf blade in the first leaf was also observed in the intermediate allele, d61-2, although the blade was much smaller than in d61-4 (data not shown). In d61-4, ligule formation was never observed (data not shown). These results indicate that BR is involved in the determination of leaf blade and sheath length ratios and ligule formation.
The Anatomical Characterization of d61-4
To examine the d61-4 phenotype in more detail, we observed its internal structure. One month after sowing, both the wild type and d61-4 developed six leaves, indicating that the leaf extraction rate was approximately the same in d61-4 and the wild type at the young seedling stage, as well as at more developed stages. At this stage, the node-internode structure became clear in the wild-type stem (nodes are indicated by arrowheads in Fig. 3A), but not in d61-4 (Fig. 3B). Instead of developing the node-internode structure, the mutant stem developed randomly oriented vascular bundles in the area below the shoot apical meristem (SAM).
Figure 3.
The anatomical characterization of wild-type and d61-4 plants. A and B, The median longitudinal sections around the SAM of wild type (A) and d61-4 (B). Arrowheads indicate nodes (A). Bidirectional arrows indicate the height (longitudinal length) of the P2 leaf primordium (A and B; bar = 200 μm). C and D, The vertical sections crossing the SAM of wild type (C) and d61-4 (D). P2 to P5 indicate the second to fifth leaf primordia (C and D; bar = 200 μm). E and F, The median longitudinal sections of the crown root tip of the wild type (E) and d61-4 (F). El, Elongating zone; Dv, divisional zone (E and F; bar = 200 μm). G to J, The large vascular bundles of leaf sheaths (G and H) and blades (I and J) in wild type (G and I) and d61-4 (H and J; G–J, bar = 100 μm); px, protoxylem; mx, metaxylem; st, sieve tube; cc, companion cells; MSC, mestome sheath cell; BSC, bundle sheath cell.
The phyllotaxis was not disturbed in d61-4, and leaf initiation occurred in a distichous alternate manner (Fig. 3, C and D). After initiation, however, the vertical elongation of leaf primordia was apparently inhibited (as seen in the heights of P2, indicated by the bidirectional arrows in Fig. 3, A and B). In contrast to the inhibition of vertical elongation, lateral enlargement of leaf primordia was not inhibited in d61-4 (Fig. 3, C and D). The leaf primordia in d61-4 were slightly thicker than those of the wild type at the same stage; the shape of the primordia in d61-4 was more circular (Fig. 3D), while the wild-type primordia were elliptical (Fig. 3C). In the wild type, leaf primordia were densely encircled with younger primordia, whereas some space remained between adjacent primordia in the mutant (Fig. 3D). The large and small vascular bundles were arranged by turns in leaf sheath as a similar manner both in wild-type and mutant plants (Fig. 3C), although the number of large and small vascular bundles was slightly decreased in the mutant (Fig. 3D; data not shown).
We also compared the anatomical organization of crown roots in 1-month-old wild-type and mutant plants. The overall structure around the root tip region was nearly identical in both plants (Fig. 3, E and F). However, the diameter of the d61-4 root was greater than that of the wild-type root, and sudden cell expansion was observed just above the divisional zone (indicated as “Dv”) in the d61-4 root. The longitudinal cell elongation of d61-4 was defective, while the width of cortex cells and, consequently, the diameter of the root were enlarged.
We also examined the vascular vein structure of the leaf sheaths (Fig. 3, G and H) and blades (Fig. 3, I and J) in the wild-type (Fig. 3, G and I) and mutant (Fig. 3, H and J) plants. The phloem tissues of the mutant vascular bundles both in the blades and sheaths appeared to be enlarged, and the number of sieve tubes and companion cells was higher relative to the wild type. Similar abnormal proliferation of phloem regions has been reported in other plants with defective BR signaling (Nagata et al., 2001; Caño-Delgado et al., 2004), indicating that abnormal phloem proliferation is a common phenomenon in both dicots and monocots. The development of xylem tissue was defective in the mutant leaf sheath, and the development of metaxylem cells almost failed (Fig. 3H). However, xylem tissue in the leaf blade of d61-4 developed almost normally, and two well developed metaxylem cells were observed to be the same as those in wild-type leaves (Fig. 3J). These observations indicate that BR signaling is necessary for the normal development of xylem tissue as observed in dicots, whereas in rice the BRI1 contribution is weaker in the leaf blade than in the leaf sheath.
Development of the d61-4 Embryo
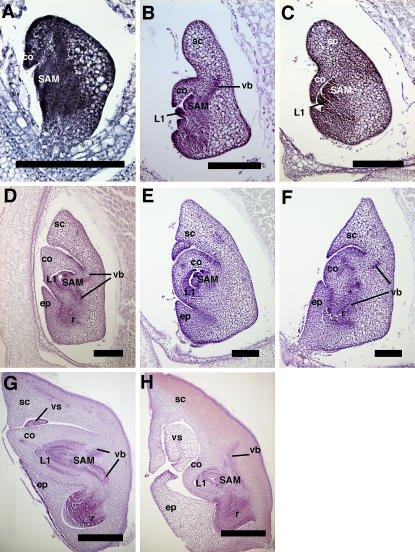
The abnormal phenotype of young seedlings just after germination leads us to speculate the abnormal morphology of embryonic organs. Therefore, we also examined embryo development in d61-4. Because d61-4 plants are sterile, we obtained d61-4 embryos produced by plants carrying the heterozygous alleles of d61-4. The frequency of the mutant embryo fit the normal segregation rate (approximately 25%; data not shown). The wild-type and d61-4 embryos could not be distinguished among more than 30 embryos until 4 d after pollination (DAP; Fig. 4A). At 5 DAP, wild-type and mutant embryos were distinguishable by the overall shape of the embryo; the SAM became clear, and the first leaf (L1) began to develop in both embryos (Fig. 4, B and C). At this stage, the wild-type embryo enlarged in the apical direction to develop the scutellum, and the dorsal side of the embryo facing the endosperm became flattened by the organized division of scutellum cells (Fig. 4B, right side). In mutant embryos, however, enlargement in the apical direction was less active and the leveling of the dorsal side did not occur. Consequently, the mutant embryo became more circular than the wild-type embryo (Fig. 4C). At 7 DAP, the wild-type embryo developed almost all tissues and organs, including three leaf primordia and a well organized root (Fig. 4D). The vascular bundle was also clearly present. At 7 DAP, it was difficult to prepare a section of d61-4 containing both the shoot and root, possibly because the embryo was arranged in a distorted fashion against the endosperm. When we prepared the longitudinal serial section of the embryo ball, one section of the median of the SAM did not contain radicle tissue (Fig. 4E), and the section with the center of the radicle did not contain the SAM (Fig. 4F). Although the coleoptile height was shortened, causing it to be dome-like in shape (Fig. 4, E and F), the d61-4 embryo contained all organs, the SAM, coleoptile, the first and second leaf primordia, scutellum, epiblast, vascular bundles, and radicle, as seen in the wild-type embryo (Fig. 4, E and F).
Figure 4.
Development of the wild-type and d61-4 embryos. A, An embryo 4 DAP. The wild-type and mutant embryos are indistinguishable at this stage. B and C, Wild-type embryos 5 DAP (B) and d61-4 embryos 5 DAP (C). The overall shape of the mutant embryo was more globular relative to that of the wild type. D to F, Wild-type embryos 7 DAP (D) and d61-4 embryos 7 DAP (E and F). E and F show different sections from the same embryo: median crossings of the SAM region and the root tip, respectively (A to F, bar = 200 μm). G and H, The mature embryos of wild type (G) and d61-4 (H; G and H, bar = 500 μm). co, Coleoptile; vb, vascular bundle; sc, sclerenchyma; L1, the first leaf; ep, epiblast; r, root; vs, ventral scale.
We examined the internal structure of mature embryos. We picked embryo balls from mature seeds and sliced them along the plane connecting the shoot and radicle. Figure 4H shows the typical features of a median longitudinal section of a mature d61-4 embryo. All embryonic organs were arranged in the normal positions and were almost of normal size, with some exceptions, such as the reduced size of the coleoptile and leaf primordia and the enlargement of the ventral scale. The number of cell layers in wild-type and d61-4 coleoptiles was nearly the same, while the number of epidermis cells in the d61-4 coleoptile was approximately two-fifths that in the wild type (Fig. 4, G and H; Table II). These results suggest that cell division toward a lateral direction was normal, while division toward a longitudinal direction was defective in d61-4 embryos. This observation is consistent with that observed in the leaf primordia.
Table II.
The numbers of cells in embryo coleoptilea
| Epidermal | Lateral | |
|---|---|---|
| Wild type | 143.3 ± 5.0 | 8.8 ± 1.5 |
| d61-4 | 56.5 ± 4.7 | 8.8 ± 1.5 |
Data represent the means ± sd of four embryos in each line.
Expression of the Marker Genes in d61-4
We performed in situ hybridization analysis using several molecular markers as probes to further examine the molecular mechanism behind the abnormal morphology in d61-4. The histone H4 gene was used as a marker for the S phase of the cell cycle. OSH1, a rice gene orthologous to maize (Zea mays) KN1 and Arabidopsis STM, is a marker of cells with an undetermined cell fate, including SAMs and young developing vascular bundles (Matsuoka et al., 1995; Sentoku et al., 1999). The rice SCARECROW1, OsSCR1, is a marker of ground tissue (L2 layer of root; Kamiya et al., 2003a). RAmy1A is a marker for the epithelium, the outermost cell of the scutellum (Kaneko et al., 2002; Kamiya et al., 2003b).
In the wild-type shoot apical region 1 month after sowing, the expression of histone H4 was observed in P1 to P4 with preferential localization in the epidermal and subepidermal cells (Fig. 5A). It was also preferentially observed in lateral cells of the stem, just below the SAM. In d61-4, the expression of histone H4 was also observed in P1 to P4 and in the stem, and the frequency of cells expressing H4 was slightly higher than in the wild type because its expression also occurred in internal stem cells, including the developing vascular bundles and leaf founder regions (Fig. 5B). OSH1 was expressed in the SAM, the junction regions between leaf primordia and the stem, and the developing vascular bundles in the wild type (Fig. 5C). The expression pattern was essentially the same in d61-4 as in the wild type (Fig. 5D).
Figure 5.
The expression of the marker genes around the SAM in the vegetative stage and in the developing embryo. A and B, The expression of histone H4 around the SAM of wild-type (A) and d61-4 (B) plants at 1 month after sowing. C and D, The expression of OSH1 around the SAM of wild type (C) and d61-4 (D) at the same stage. The expression patterns of histone H4 and OSH1 in the mutant shoot apex region are similar to those in the wild type (A–D). E, The expression of OSH1 in the 4-DAP embryo. The expression of OSH1 was observed in the SAM and the epiblast (ep). OSH1 expression in wild-type and d61-4 embryos was indistinguishable at 4 DAP. F and G, The expression of OsSCR1 in the 7-DAP embryo in wild type (F) and d61-4 (G). OsSCR1 was expressed in the endodermis layer of the radicle. H and I, The expression of OSH1 in the 7-DAP embryo of wild type (H) and d61-4 (I). OSH1 expression was localized in the SAM, the ventral side of the radicle, and the epiblast of both embryos. J and K, The expression of RAmy1A in the 9-DAP wild-type (J) and d61-4 (K) embryos. RAmy1A expression was localized to the epithelium. L and M, The expression of histone H4 in the wild type (L) and d61-4 (M) embryos at 7 DAP. The signal was sporadically observed at a similar frequency in the both embryos. sc, Sclerenchyma; vb, vascular bundle; r, root. Bar = 200 μm.
We also examined the expression of marker genes in developing embryos to analyze whether basic pattern formation and cell-fate determination occurs normally in the mutant embryo. First, we examined the expression of OSH1 to analyze the development of the SAM. At 4 DAP, OSH1 expression was seen in the SAM and the epiblast (Fig. 5E), and the OSH1 expression patterns were the same in more than 20 embryos harvested from plants with the heterozygous allele (D61/d61). This indicates that OSH1 expression in wild-type and d61 embryos was indistinguishable, and therefore the establishment of the SAM occurred normally in d61 embryos. The expression of OsSCR in the wild-type and d61 embryos was also indistinguishable during embryogenesis and in mature embryos (Fig. 5, F and G), indicating that establishment of the L2 layer, including the radicle endodermis layer, occurred normally. The OSH1 expression pattern in both d61 and the wild type was maintained in the mature embryo at 7 DAP, but the expression level was slightly lower in d61 than in the wild type (Fig. 5, H and I), as in the SAM at the vegetative stage. RAmy1A expression was specifically observed in the epithelium in 9-DAP embryos both in the wild type and d61-4 (Fig. 5, J and K). These results demonstrate that fate determination of various cells occurred normally in d61-4 during embryogenesis, but the subsequent morphogenesis or development or both are abnormal. We also observed the expression of histone H4 in 7-DAP wild-type and d61-4 embryos. The frequency of cell division was approximately the same in both wild-type and d61-4 embryos (Fig. 5, L and M).
BR Accumulation in d61-4
We measured the endogenous BR content to determine why the phenotypic severity varied between the shoot and the root. The shoots and roots of 6-week-old d61-4 and wild-type plants were separated and the amounts of BRs were analyzed. So far, we have never detected BL (the most active BR), only castasterone (CS), in rice. As expected, the shoots of d61-4 had large accumulations of bioactive CS, about 30-fold the accumulations in wild-type shoots (Table III), but no BL could be detected even in these materials. This suggests that rice may use CS as a bioactive BR, but not BL, or BL may be rapidly catabolized in rice. Interestingly, such high levels of CS accumulation were not observed in the d61-4 roots; instead, only a 1.5-fold accumulation occurred (Table III), suggesting that there may be different mechanisms in shoots and roots for accumulation of bioactive BR (see below).
Table III.
Endogenous BR contents in wild-type and d61-4 plants
nd, Not detected.
| BRsa
|
Wild Type
|
d61-4
|
||
|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |
| 24-MC | 15,100 | 1,110 | 2,140 | 600 |
| CR | 96,300 | 44,100 | 124,000 | 49,500 |
| CN | 1,790 | 1,360 | 3,550 | 1,280 |
| 6-OxoCN | 74.9 | 27.9 | 59.5 | 58.3 |
| 6-DeoxoCT | 0.89 | 5.79 | 1.16 | 1.44 |
| 6-DeoxoTE | 0.12 | 1.89 | 0.15 | 0.29 |
| 6-Deoxo3DT | 0.39 | 7.97 | 0.92 | 1.66 |
| 6-DeoxoTY | 2.95 | 25.1 | 13.3 | 5.29 |
| 6-DeoxoCS | 0.82 | 1.22 | 3.35 | 1.08 |
| CT | nd | nd | nd | nd |
| TE | 0.02 | 0.29 | 1.02 | 0.26 |
| TY | 0.83 | 1.58 | 10.9 | 0.68 |
| CS | 0.47 | 1.03 | 15.6 | 1.49 |
| BL | nd | nd | nd | nd |
BR contents are expressed as nanogram per gram fresh weight of tissues. MC, Methylenecholesterol; CR, campesterol; CN, campestanol; CT, cathasterone; TE, teasterone; DT, dehydroteasterone; TY, typhasterol.
BRI1 Homologs in Rice
Three homologous genes of BRI1 have been reported in Arabidopsis: BRL1, BRL2, and BRL3. Two of them, BRL1 and BRL3, can compensate for the BRI1 function in bri1 mutant plants (Caño-Delgado et al., 2004). In rice, we also found three homologous genes of OsBRI in silico (Fig. 6A). We compared these rice proteins to the Arabidopsis proteins and other BRI1 proteins, and found that the OsBRI1 protein fell into a group that included Arabidopsis, tomato, pea, and barley BRI1 proteins. OsBRL1 and OsBRL3 were categorized into a group that included Arabidopsis BRL1 and BRL3, while OsBRL2 was independently grouped with Arabidopsis BRL2 (Fig. 6A). Recently, Kinoshita et al. (2005) suggested that a unique sequence in the LRR22 just downstream of ID was important for BR-binding ability. Thus, we compared the LRR just downstream of ID among the BRI1 proteins and the three homologs of Arabidopsis and rice. The unique sequence in the LRR was conserved among all BRI1 proteins from various plants and in BRL1 and BRL3 of Arabidopsis and rice, but was not shared by BRL2 of rice and Arabidopsis (Fig. 6B, underlined). According to complementation experiments using the Arabidopsis bri1 mutant, Arabidopsis AtBRL1 and AtBRL3 could rescue the bri1 phenotype by expression of their cDNAs under the control of the BRI1 promoter, while AtBRL2 could not (Caño-Delgado et al., 2004). Taken together, these observations suggest that OsBRL1 and OsBRL3, but not OsBRL2, have the ability to function as the BR receptor.
Figure 6.
The rice genes homologous to OsBRI1. The phylogenic relationship of BRI1 proteins in various plants and homologous proteins in rice and Arabidopsis is shown. Full-length protein sequences were aligned with ClustalX 1.81. A, The comparison included the following BRI1 family members: for rice, OsBRI1 (Yamamuro et al., 2000; BAB68053), OsBRL1 (BAD34326), OsBRL2 (AAK52544), and OsBRL3 (BAD01717; indicated as OsBRL1 in Caño-Delgado et al., 2004); for Arabidopsis, AtBRI1 (Li and Chory, 1997; AAC49810), AtBRL1 (Caño-Delgado et al., 2004; Zhou et al., 2004; Q9ZWC8), AtBRL2 (Clay and Nelson, 2001; Q9ZPS9), and AtBRL3 (Caño-Delgado et al., 2004; Q9LJF3); for tomato, LeBRI1 (Montoya et al., 2002; AAN85409); for pea, PsBRI1 (Nomura et al., 2003; BAC99050); and for barley, HvBRI1 (Chono et al., 2003; BAD06331). B, Amino acid alignment of the LRR sequence just behind ID. The underlined amino acid sequences are well conserved among all BRI1 and homologous proteins except AtBRI2 and OsBRL2.
Comparison of Phenotypes and Gene Expression in d61-4 and brd1-1
The phenotypic severity of d61-4 in the shoots was stronger than that of the strongest BR-deficient mutant, brd1-1 (Fig. 7A). In contrast, the severity in the roots was stronger in brd1-1 than in d61-4 (Fig. 7B). This type of severe phenotype in shoots and a mild phenotype in roots of d61-4 corresponded well with the accumulation pattern of CS (Table III). We observed in detail the internal structure of the fourth leaf primordium (P4) and the root elongation zone. The cell arrangement in the d61-4 leaf primordia was severely disturbed, but was organized in the wild-type leaf primordia (Fig. 7, C and E). In contrast to the leaf primordia, the cell layer in the d61-4 roots was maintained and the number of layers was approximately the same as in the wild type (Fig. 7, D and F). These internal cell structures corresponded well with the gross morphologies of the shoots and roots, that is, the longitudinal growth of the roots may be maintained by the cell layer (Fig. 7, B and F), while the rolled abnormal growth of leaves may be caused by the disturbed cell arrangement in leaf primordia (Fig. 7, A and E). When we observed the internal structure of brd1-1, the cell arrangement in the leaf primordia was almost maintained, while that in the roots was disturbed, also corresponding to the gross morphology of brd1-1 (Fig. 7, G and H).
Figure 7.
The phenotypic comparison between d61-4 and brd1-1 and the expression of BR-related genes in d61-4 and brd1-1 plants. A, The gross morphology of the shoots of 1-month-old d61-4 (left) and brd1-1 (right) plants (bar = 5 cm). B, The gross morphology of the roots of 2-month-old wild-type (left), d61-4 (middle), and brd1-1 (right) plants (bar = 5 cm). C to H, Longitudinal section of the P4 leaf primordia (C, E, and G) and root elongation zone (D, F, and H) of the wild-type (C and D), d61-4 (E and F), and brd1-1 (G and H) plants at 1 month after sowing (C–H, bar = 100 μm). I and J, The expression levels of OsBRI1, OsBRL1, OsBRL3, D2, D11, and OsDWARF were analyzed using RNA extracted from 6-week-old wild-type and d61-4 plants (I), and wild-type and brd1 plants (J). The mRNA level for each gene was quantified by semiquantitative reverse transcription-PCR (25 cycles for OsBRI1, OsBRL1, OsBRL3, and OsDWARF; 30 cycles for D2 and D11). The expression of the OsACT1 gene was used as a control (25 cycles). ep, Epidermis; sc, sclerenchyma; cor, cortex; S, shoot; R, root.
We compared the expression level of BR-related genes between the wild type and d61-4 (Fig. 7I). In 6-week-old seedlings, the expression of both OsBRL1 and OsBRL3 was higher in d61-4 roots than in wild-type roots, whereas slightly increased, but still low, levels of expression occurred in the shoots (Fig. 7I). In d61-4 roots, the expression of OsBRI1 was also observed and was slightly higher relative to wild-type roots, although this transcript contained a stop codon in the kinase domain (Fig. 1). We also examined the expression of rice BR biosynthetic genes D2 (Hong et al., 2003), D11 (Tanabe et al., 2005), and OsDWARF (Hong et al., 2002). The expression of the D2 gene was only detected in the shoots and was more expressed in d61-4 than in the wild type. D11 and OsDWARF gene expression was higher in d61-4 than in the wild type in both shoots and roots; however, the increase was higher in shoots than in roots (Fig. 7I). The increased expression of these BR biosynthetic genes in shoots relative to root expression may be correlated with the higher levels of CS in shoots (Table III). Taken together, these expression results suggest that the mild phenotype of d61-4 roots may be a result of compensation by the BRI1 homologous genes OsBRL1 and OsBRL3, and that consequently the BR signal can be transduced in the roots, while the phenotype cannot be rescued by the small increase in OsBRL1 and OsBRL3 expression in the shoots.
We also compared the expression of these BR-related genes between the wild type and brd1-1. As seen in d61-4, the expression of the OsBRI1, D2, and OsDWARF genes was higher in the brd1-1 mutant; however, the increase of OsBRL1 and OsBRL3 was not observed either in shoots or in roots in brd1 (Fig. 7J). These results suggest that BR-deficient phenotypes should be partly ameliorated by the increased BRI1 expression in brd1 shoots, but not in roots, because the increased expression of BRI1 or BRI1 homologs did not occur in roots.
DISCUSSION
In this study, we isolated eight alleles of the rice bri1 mutant, in addition to the already reported d61-1 and d61-2 alleles. Four of the 10 mutant alleles caused much more severe dwarf phenotypes than what was reported for d61-1 and d61-2 (Yamamuro et al., 2000). We analyzed in detail the null allele of rice bri1, d61-4, which caused the most severe phenotype among the 10 alleles, and discussed the role of BR and BR receptors in rice.
BRI1 Is Involved in the Maintenance of the Normal Direction of Cell Division
Although d61-4 resulted in a severe dwarf phenotype accompanied by malformed leaves and the absence of flowers, the phyllotaxis, plastochron, and vascular patterning were almost normal (Figs. 2 and 3; data not shown). Embryo development also progressed normally, except for changes in the sizes of some organs (Fig. 4). In addition, the expression of all molecular markers we used, OSH1, SCR1, Ramy1A, and histone H4, showed nearly identical patterns around the SAM and in the embryo in the mutant as observed in the wild type (Fig. 5). The sole defect in mutant organ development, that is, node formation failure, may have been a secondary effect of the incomplete stem development in the dwarf phenotype; node-internode differentiation is triggered by the transition from the juvenile to the adult phase (Itoh et al., 2005), and the mutant stem was in a juvenile state even at the 11th leaf stage, judging by the arrangement of the vascular bundle (data not shown). All these observations suggest that OsBRI1 is not essential for either pattern formation or organ initiation in rice embryo and juvenile plants, even though its loss-of-function mutant formed malformed and grotesque shoots.
The next question is why d61-4 exhibits such a severe phenotype. As OsBRI1 is not involved in pattern formation or organ initiation, BRI1 should be involved in the developmental process of organs. An essential reason for the abnormal phenotype of d61-4 was the defect in cell elongation, as observed in many BR-related mutants (e.g. Fig. 7F). In addition, the arrangement of cells in the mutant was severely affected by disordered cell division that caused disturbed cell files in leaf primordia (Fig. 7E), followed by decreased longitudinal elongation and increased lateral expansion of leaf primordia (Fig. 3, B and D). The number of epidermal cells (longitudinal proliferation) in the coleoptile of the d61-4 embryo was approximately two-fifths the number in the wild type, suggesting that cell division was also affected in the d61-4 embryo (Table II). A similar phenomenon was seen in the Arabidopsis det2 mutant, in which the number of cells in a longitudinal section was approximately one-half that of the wild type (Nakaya et al., 2002). These observations suggest that BR functions in the fine-tuning of the direction and rate of cell division.
BR Signaling Is Regulated Differently in Shoots and Roots
In d61-4, we noticed a difference in the phenotypic severity between shoots and roots. A clear difference between shoots and roots in d61-4 was in the accumulation of BRs. The level of CS in the mutant shoot was about 30-fold higher than that in the wild type, whereas there was only a 1.5-fold accumulation in the root (Table III). This corresponded to the high and low increases in the expression of BR biosynthetic genes, such as D2, D11, and OsDWARF, in shoots and roots, respectively, expression that was negatively regulated by BRs and BR signals (Hong et al., 2002, 2003; Tanabe et al., 2005; Fig. 7I). In d61-4, the functional homologs of OsBRI1, OsBRL1, and OsBRL3 were preferentially expressed in the roots, and their expression was very low in shoots (Fig. 7I). These results suggest that, in roots of d61-4, the BRI1 homologs compensated for the lack of BR signal caused by the loss of BRI1 function. Interestingly, the pattern of phenotypic severity of shoots and roots in brd1-1, one of the most severe rice BR-deficient mutants (Hong et al., 2002), was opposite that seen in d61-4 (Fig. 7, A and B). In fact, the shoot phenotype in brd1-1 was much milder than that in d61-4, and the root phenotype in brd1-1 was more affected than that in d61-4. This phenomenon could also be explained by the expression patterns of OsBRI1, OsBRL1, and OsBRL3, as follows. In brd1-1, the expression of OsBRI1 was increased in shoots and partially compensated for the severe phenotype, whereas OsBRI1 and OsBRLs were not up-regulated and there was no compensation in the roots. The reason for the increased expression of OsBRL1 and OsBRL3 in d61-4 and the decreased expression in brd1-1 is not clear. Perhaps there is an unknown BR signaling pathway in the roots distinct from that in the shoots.
Why does the expression of OsBRL1 and OsBRL3 in the roots not completely compensate for the abnormal root phenotype in d61-4? There are two possible answers to this question. The function of OsBRL1 or OsBRL3 may not completely overlap that of OsBRI1, and, consequently, the expression of OsBRL1 and OsBRL3 would not completely compensate for the loss of OsBRI1 function. Alternatively, OsBRI1 and its homologous genes could have different expression patterns in terms of tissue or cell specificity or both. At present, we cannot determine which answer is correct. In Arabidopsis, AtBRL1 was expressed in roots and stems, and AtBRL1 and AtBRL3 were also preferentially expressed in vascular tissues. In rice, OsBRL1 and OsBRL3 were preferentially expressed in roots (Fig. 7), and OsBRL3 was also expressed in embryos (data not shown). Such preferential expression of rice and Arabidopsis BRL1 and BRL3 leads us to speculate that three BR receptors, BRI1, BRL1, and BRL3, function in an organ- or tissues-specific manner both in monocot (rice) and dicot (Arabidopsis) plants. Even such case, it is no wonder that BRI1 plays a dominant and essential function in both plants and BRLs have a subordinate function. Why then do both rice and Arabidopsis BRL1 and BRL3 retain a similar structure and a similar expression pattern? Further analysis of the OsBRL genes is needed to clarify the understanding of BR signaling in rice roots.
MATERIALS AND METHODS
Plant Materials and Growth Condition
Wild-type rice plants (Oryza sativa L. cv Taichung 65) and plants with mutant alleles of d61 were grown in the field, a greenhouse at 30°C and 24°C (day/night), or a plant box containing half-strength Murashige and Skoog medium with 1.5% (w/v) Suc at 30°C.
Semiquantitative Reverse Transcription-PCR
Total RNA was isolated using the standard SDS-phenol method. The first strand of cDNA was synthesized from 2 μg of total RNA using an Omniscript RT kit (Qiagen). The primer sequences used for analysis were 5′-CTCGGCAGCGTCGAGGTGC-3′ and 5′-AGGAATTGTTGCTGAGCTTC-3′ for OsBRI1, 5′-GGTGTCCGTGAGCCGCTAAG-3′ and 5′-TGCAGTGAATTCCCGGTCACCTTGG-3′ for OsBRL1, and 5′-CCGGTGAGATACCAGACAAG-3′ and 5′-GAGGTTGCTGCAGCTGCCAAGCTCT-3′ for OsBRL3. The primer sets used for the OsDWARF, OsACT1, D2, and D11 gene analyses were described previously (Hong et al., 2002, 2003; Tanabe et al., 2005). The products amplified using these primers were separated on a 1.2% (w/v) agarose gel.
Histological Analysis and in Situ Hybridization
Tissues were fixed overnight at 4°C in 4% (w/v) paraformaldehyde and 0.25% (v/v) glutaraldehyde in 0.1 m sodium phosphate buffer, dehydrated through a graded ethanol series followed by a t-butanol series, and finally embedded in Paraplast Plus (Oxford Labware) and sectioned to 8-μm thicknesses. For histological analysis, microtome sections were stained with Delafield's hematoxylin. In situ hybridization with digoxigenin-labeled RNA was performed following the methods of Kouchi and Hata (1993). Digoxigenin-labeled RNA was produced from the cording region without the poly(A) of OSH1, OsSCR, RAmy1A, and histone H4.
Quantification of Endogenous BRs
Six-week-old wild-type and d61-4 plants were separated into shoots and roots and lyophilized immediately at −80°C. To analyze the endogenous BRs, lyophilized samples were extracted twice with 250 mL of methanol:CHCl3 (4:1, v/v). BR purification and quantification were performed following the methods of Fujioka et al. (2002) and He et al. (2003).
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: OsBRI1, BAB68053; OsBRL1, BAD34326; OsBRL2, AAK52544; OsBRL3, BAD01717; AtBRI1, AAC49810; AtBRL1, Q9ZWC8; AtBRL2, Q9ZPS9; AtBRL3, Q9LJF3; LeBRI1, AAN85409; PsBRI1, BAC99050; and HvBRI1, BAD06331.
Acknowledgments
We thank Ms. Masayo Sekimoto and Mr. Makoto Kobayashi (both RIKEN) for their technical assistance. We thank Dr. Toshinori Kinoshita (Kyusyu University), Dr. Jianming Li (University of Michigan), and Dr. Zhiyong Wang (Department of Plant Biology) for helpful discussion.
This work was supported in part by the Japan Society for the Promotion of Science (research fellowship to A.N.), the Center of Excellence (Grant-in-Aid to M.M.), and the Japan Rice Genome Project of the Ministry of Agriculture, Forestry and Fisheries (grant no. IP1003 to M.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Makoto Matsuoka (makoto@nuagr1.agr.nagoya-u.ac.jp).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.072330.
References
- Caño-Delgado A, Yin Y, Yu C, Vafeados D, Mora-Garcia S, Cheng J-C, Nam HG, Li J, Chory J (2004) BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131: 5341–5351 [DOI] [PubMed] [Google Scholar]
- Chono M, Honda I, Zeniya H, Yoneyama K, Saisho D, Takeda K, Takatsuto S, Hoshino T, Watanabe Y (2003) A semidwarf phenotype of barley uzu results from a nucleotide substitution in the gene encoding a putative brassinosteroid receptor. Plant Physiol 133: 1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay NK, Nelson T (2001) VH1, a provascular cell-specific receptor kinase that influences leaf cell patterns in Arabidopsis. Plant Cell 14: 2707–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49: 427–451 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Takatsuto S, Yoshida S (2002) An early C-22 oxidation branch in the brassinosteroid biosynthetic pathway. Plant Physiol 130: 930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J-X, Fujioka S, Li TS, Kang SG, Seto H, Takatsuto S, Yoshida S, Jang JC (2003) Sterols regulate development and gene expression in Arabidopsis. Plant Physiol 131: 1258–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S, Shimada Y, Takatsuto S, Agetsuma M, Yoshida S, Watanabe Y, et al (2002) Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J 32: 495–508 [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M (2003) A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell 15: 2900–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh J-I, Nonomura K-I, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y (2005) Rice plant development: from zygote to spikelet. Plant Cell Physiol 46: 23–47 [DOI] [PubMed] [Google Scholar]
- Kamiya N, Nagasaki H, Morikami A, Sato Y, Matsuoka M (2003. a) Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem. Plant J 35: 429–441 [DOI] [PubMed] [Google Scholar]
- Kamiya N, Nishimura A, Sentoku N, Takabe E, Nagato Y, Kitano H, Matsuoka M (2003. b) Rice globular embryo 4 (gle4) mutant is defective in radial pattern formation during embryogenesis. Plant Cell Physiol 44: 875–883 [DOI] [PubMed] [Google Scholar]
- Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M (2002) The alpha-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol 128: 1264–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Caño-Delgado A, Seto H, Hiranuma S, Fujioka S, Yoshida S, Chory J (2005) Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433: 167–171 [DOI] [PubMed] [Google Scholar]
- Kouchi H, Hata S (1993) Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol Gen Genet 238: 106–119 [DOI] [PubMed] [Google Scholar]
- Li JM, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938 [DOI] [PubMed] [Google Scholar]
- Mandava NB (1988) Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol 39: 23–52 [Google Scholar]
- Matsuoka M, Tamaoki M, Tada Y, Fujimura T, Tagiri A, Yamamoto N, Kano-Murakami Y (1995) Expression of a rice OSH1 gene is localized in developing vascular strands and its ectopic expression in transgenic rice causes altered morphology of leaf. Plant Cell Rep 14: 555–559 [DOI] [PubMed] [Google Scholar]
- Montoya T, Nomura T, Farrar K, Kaneta T, Yokota T, Bishop GJ (2002) Cloning the tomato Curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell 14: 3163–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N, Asami T, Yoshida S (2001) Brassinazole, an inhibitor of brassinosteroid biosynthesis, inhibits development of secondary xylem in cress plants (Lepidium sativum). Plant Cell Physiol 42: 1006–1011 [DOI] [PubMed] [Google Scholar]
- Nakaya M, Tsukaya H, Murakami N, Kato M (2002) Brassinosteroids control the proliferation of leaf cells of Arabidopsis thaliana. Plant Cell Physiol 43: 239–244 [DOI] [PubMed] [Google Scholar]
- Nomura T, Bishop GJ, Kaneta T, Reid JB, Chory J, Yokota T (2003) The LKA gene is a BRASSINOSTEROID INSENSITIVE 1 homolog of pea. Plant J 36: 291–300 [DOI] [PubMed] [Google Scholar]
- Oh MH, Ray WK, Huber SC, Asara JM, Gage DA, Clouse SD (2000) Recombinant brassinosteroid insensitive 1 receptor-like kinase autophosphorylates on serine and threonine residues and phosphorylates a conserved peptide motif in vitro. Plant Physiol 124: 751–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse J (1999) Physiological actions of brassinosteroids. In A Sakurai, T Yokota, SD Clouse, eds, Brassinosteroids: Steroidal Plant Hormones. Springer-Verlag, Tokyo, pp 137–161
- Sentoku N, Sato Y, Kurata N, Ito Y, Kitano H, Matsuoka M (1999) Regional expression of the rice KN1-type homeobox gene family during embryo, shoot and flower development. Plant Cell 11: 1651–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, et al (2005) A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17: 776–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Goshe MB, Soderblom EJ, Phinney B, Kuchar JA, Li J, Asami T, Yoshida S, Huber SC, Clouse SD (2005) Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell 17: 1685–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-Y, Seto H, Fujioka S, Yoshida S, Chory J (2001) BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410: 380–383 [DOI] [PubMed] [Google Scholar]
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12: 1591–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Wang H, Walker JC, Li J (2004) BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J 40: 399–409 [DOI] [PubMed] [Google Scholar]