Abstract
We reported earlier that engineering of the glyoxalase pathway (a two-step reaction mediated through glyoxalase I and II enzymes) enhances salinity tolerance. Here we report the extended suitability of this engineering strategy for improved heavy-metal tolerance in transgenic tobacco (Nicotiana tabacum). The glyoxalase transgenics were able to grow, flower, and set normal viable seeds in the presence of 5 mm ZnCl2 without any yield penalty. The endogenous ion content measurements revealed roots to be the major sink for excess zinc accumulation, with negligible amounts in seeds in transgenic plants. Preliminary observations suggest that glyoxalase overexpression could confer tolerance to other heavy metals, such as cadmium or lead. Comparison of relative tolerance capacities of transgenic plants, overexpressing either glyoxalase I or II individually or together in double transgenics, evaluated in terms of various critical parameters such as survival, growth, and yield, reflected double transgenics to perform better than either of the single-gene transformants. Biochemical investigations indicated restricted methylglyoxal accumulation and less lipid peroxidation under high zinc conditions in transgenic plants. Studies employing the glutathione biosynthetic inhibitor, buthionine sulfoximine, suggested an increase in the level of phytochelatins and maintenance of glutathione homeostasis in transgenic plants during exposure to excess zinc as the possible mechanism behind this tolerance. Together, these findings presents a novel strategy to develop multiple stress tolerance via glyoxalase pathway engineering, thus implicating its potential use in engineering agriculturally important crop plants to grow on rapidly deteriorating lands with multiple unfavorable edaphic factors.
Environmental factors such as heat, cold, drought, salinity, and heavy metal result in a massive loss of crop yield all over the world. Although metals are required as structural and catalytic components of enzymatic proteins involved in various physiological processes, they can still be toxic to a plant if present at supraoptimal concentrations (Clemens, 2001). Because without intervention heavy metals stay in soil for centuries, efficient remediation strategies are needed. Large parts of agricultural soil are contaminated with zinc by natural and anthropogenic activities, including mining and industrial processes and agricultural practices such as the use of fertilizers containing various heavy metals (Ross, 1994). The pollution of soil by zinc has been a major environmental concern (Dudka et al., 1996; Zarcinas et al., 2004), and the capacity of certain plants to accumulate heavy metals has long been used for phytoremediation. Modulation in the ion transporters for influx or efflux of heavy-metal ions, or chelation and sequestration of excess metal ions by binding with metal-chelating molecules such as metallothioneins, ferritin, or phytochelatins (PCs), are some of the possible strategies for counteracting heavy-metal stress (Clemens, 2001; Clemens et al., 2002; Hall, 2002; Pilon-Smits and Pilon, 2002; Eapen and D'Souza, 2005). This would help to maintain the concentration of essential metal ions in different cellular compartments within the narrow physiological range and to minimize the damage caused by the entry of nonessential metal ions into the cytosol. Zinc plays a critical role in the defense system of cells against reactive oxygen species (ROS) and thus represents an excellent protective agent against the oxidation of several vital cell components, such as membrane lipids and proteins, chlorophyll, sulfhydryl (SH)-containing enzymes, and DNA (Cakmak, 2000).
Plants use a variety of methods to prevent heavy metals from affecting their growth. Ligands such as PCs and metallothioneins bind heavy metals within the cell, thereby reducing the damage these metals would otherwise cause. PCs, small metal-binding peptides derived from reduced glutathione (GSH), represent one of the main metal-chelation and detoxification mechanisms and play an essential role in heavy-metal detoxification in plants (Grill et al., 1985, 1987; Steffens et al., 1986; Scheller et al., 1987). PCs have been proposed as a potential biomarker for metal toxicity (Sun et al., 2005a). PCs chelate heavy metals, and the resulting PC-metal complexes are translocated to vacuoles (Salt and Rauser, 1995), thus decreasing the toxic level of heavy metal in the cytosol of plant cells. Chelation with ligands such as PCs route metals predominantly to root sequestration (Evans et al., 1992). PCs are a class of thiol-enriched peptides that are synthesized by the polymerization of GSH catalyzed by the transpeptidase PC synthase (PCS; Grill et al., 1989; Chen et al., 1997; Clemens et al., 1999; Ha et al., 1999). Increased PC levels obtained by the overexpression of enzymes involved in the PC biosynthetic pathway, for instance, glutamylcysteine synthase (Xiang and Oliver, 1998; Zhu et al., 1999a), GSH synthase (Zhu et al., 1999b), and PCS (Gisbert et al., 2003; Li et al., 2004), have been reported to increase plant metal content. PC synthesis induced by zinc treatment has been correlated with enhancement of heavy-metal tolerance in the marine alga Dunaliella tertiolecta (Tsuji et al., 2002). Numerous attempts are being made to enhance or reduce PC synthesis by overexpression or inhibition of enzymes involved in synthesis of the PC precursor GSH.
Glyoxalases I and II, zinc-binding enzymes of the glyoxalase pathway, carry out catabolism of methylglyoxal (MG), which is a cytotoxic compound formed primarily as a by-product of carbohydrate and lipid metabolism. We have reported recently that salinity stress leads to accumulation of MG in plants (Yadav et al., 2005a), and tobacco (Nicotiana tabacum) plants overexpressing these glyoxalase genes (singly or together in the same plant) tolerate high salt and MG concentrations (Singla-Pareek et al., 2003). In this article, we provide evidence that these transgenic plants sustained growth and produced normal viable seeds without any yield penalty in zinc-spiked soil under laboratory conditions. Further, enzyme inhibitor studies and other biochemical investigations suggest involvement of GSH homeostasis and PC biosynthesis toward zinc tolerance in these glyoxalase-overexpressing transgenic tobacco plants.
RESULTS AND DISCUSSION
In this study, three independent lines of single-gene transformants (overexpressing either glyoxalase I or II alone) and double transgenics (overexpressing glyoxalase I and glyoxalase II together), which showed significant accumulation of glyoxalase proteins and were used previously for the analysis of salinity tolerance (Singla-Pareek et al., 2003), were employed. All the experiments described below were carried out on all three independent transgenic lines. Statistically similar results were obtained for each of the three different lines; however, data for only one of the lines of each type are presented (line NtBIS-11 for glyoxalase I, line 72 for glyoxalase II, and line K for the double transgenic).
Glyoxalases I and II Are Up-Regulated by Zinc
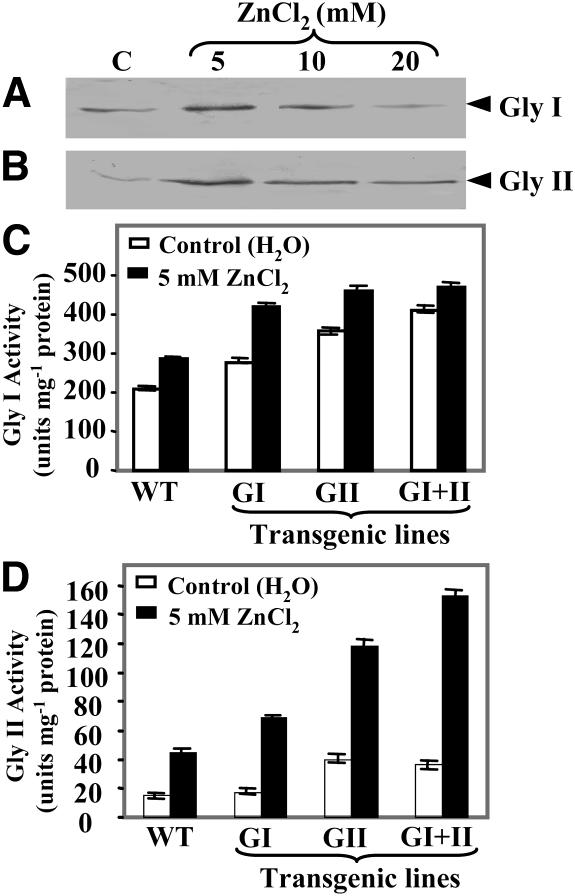
For investigating whether the levels of glyoxalase I and II proteins are regulated by zinc, western-blot analysis of the total proteins isolated from the cotyledonary leaves of Brassica juncea seedlings (source of the glyoxalase I cDNA used in this study), as well as from shoots of rice (Oryza sativa; source of the glyoxalase II cDNA) exposed to different concentrations of ZnCl2, was carried out using anti-glyoxalase I and anti-glyoxalase II antibodies. Significant accumulation of both glyoxalase I and II proteins was noted in response to various concentrations of ZnCl2 (Fig. 1, A and B). To analyze whether the transgenic tobacco lines used in this study retain glyoxalase enzyme activity when exposed to zinc, glyoxalase I and II enzyme activities were measured in seedlings maintained under water or 5 mm ZnCl2 for 24 h. For both enzymes, a higher activity was noted in the transgenic plants grown in water, which showed further enhancement in their activity in response to ZnCl2, indicating the regulation of endogenous glyoxalase enzymes by Zn2+ (Fig. 1, C and D). These transgenic plants showed an overall 15% to 50% enhancement in glyoxalase I activity, whereas glyoxalase II activity increased significantly by 300% to 400%.
Figure 1.
Regulation of glyoxalase I and II protein levels and enzyme activities by zinc. Western blot of total leaf/shoot proteins from seedlings exposed to different concentrations of ZnCl2 for 24 h is shown. A, B. juncea leaf proteins probed with anti-glyoxalase I antibodies. B, Rice shoot proteins probed with anti-glyoxalase II antibodies. The positions of the glyoxalase I and II proteins are marked with arrows. C and D, Histograms showing the activity of glyoxalase I (C) and glyoxalase II (D) enzymes in the selected transgenic tobacco lines exposed to 5 mm ZnCl2 for 24 h. Data represent the mean of three replicates and of three independent experiments ±sd. WT, Wild type; GI, glyoxalase I; GII, glyoxalase II; GI + II, double transgenics. Note that zinc exposure enhances protein levels and enzyme activities for both glyoxalase I and glyoxalase II.
Glyoxalase Transgenic Plants Can Tolerate High Levels of Zinc
The potential of glyoxalase transgenic plants for relative tolerance toward ZnCl2 was assessed employing a rapid bioassay based on detached leaf senescence. Isolated leaf discs of wild-type, glyoxalase I and II, and double-transgenic plants were incubated in various concentrations of ZnCl2. A clear contrast in the tolerance limits of the single- and double-gene transformants was observed at a higher concentration of ZnCl2 (≥10 mm), at which, however, the leaf discs from wild-type plants could not survive. For the purpose of comparing the contribution of individual glyoxalase genes vis-à-vis the combined effect of overexpression of two genes toward this tolerance, it was important to use much higher concentrations of zinc that essentially do not reflect their tolerance capacities under field conditions. Therefore, for all the experiments presented in this study, relatively higher amounts of zinc have been used to impose heavy-metal stress than those reported in previous studies related to metal tolerance.
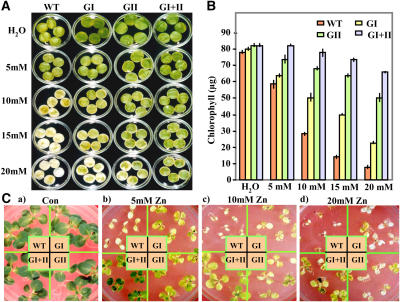
Incubation of the leaf discs in 5, 10, 15, and 20 mm ZnCl2 solution for 5 d showed an early bleaching of wild-type leaf discs compared to those from transgenic plants (Fig. 2A). The double-transgenic plants exhibited significantly more enhanced zinc tolerance than any of the single-gene transformants (Fig. 2, A and B). The wild-type plants exhibited almost a total loss of chlorophyll within 5 d in the presence of zinc (90% loss as compared to its water controls), whereas the double transformants experienced only a 22% decline in chlorophyll at 20 mm ZnCl2 concentration (Fig. 2B). The ability of the transgenic plants to maintain chlorophyll under zinc stress was taken as an index for measuring stress-induced injury. These observations establish a positive relationship between the overexpression of glyoxalase pathway enzymes and zinc stress tolerance in leaf tissues.
Figure 2.
Retardation of zinc promoted senescence in transgenic tobacco plants overexpressing glyoxalase I (GI), glyoxalase II (GII), or both glyoxalase I and glyoxalase II in double transgenics (GI + II), indicating the tolerance at cellular levels toward toxic levels of zinc. Representative image showing phenotypic differences (A) and chlorophyll content (B; μg g−1 fresh weight) from zinc-treated leaf discs of wild-type and various transgenic plants (GI, GII, and GI + II) after incubation in 5, 10, 15, and 20 mm solutions of ZnCl2 for 5 d are shown. Discs floated in water served as the experimental control. The sd in each case is represented by the vertical bar in each graph (n = 3). Note the difference in retention of chlorophyll in wild-type and transgenic plants. C, Zinc tolerance test of representative T1 generation tobacco transgenic seedlings overexpressing glyoxalase enzymes. Seven-day-old seedlings were transferred to 0.5× Murashige and Skoog medium supplemented with various concentrations of zinc and grown for 25 d. a, Control seedlings grown on 0.5× Murashige and Skoog medium; b to d, Seedlings grown on 5 mm, 10 mm, or 20 mm ZnCl2-supplemented medium, respectively. WT, Wild type; GI, glyoxalase I; GII, glyoxalase II; GI + II, double transgenics. Note that the wild-type seedlings show chlorosis and arrested growth under high zinc conditions.
Zinc tolerance of T1 generation transgenic seedlings was further checked by transferring them onto growth media supplemented with various concentrations of ZnCl2 (5, 10, and 20 mm) and compared with growth over a period of 25 d on normal media. All four types of seedlings (i.e. wild type, glyoxalases I and II, and double transgenics) showed similar growth in the absence of ZnCl2 (Fig. 2C, a). On media supplemented with 5 mm ZnCl2, the single-gene transgenics of glyoxalase I or II grew well, but again, the double-transgenic lines showed fewer symptoms of stress. However, at this concentration of zinc, severe chlorosis and stunted phenotype of the wild-type seedlings was observed (Fig. 2C, b). On 10 mm ZnCl2-supplemented media, glyoxalase I and II transgenic seedlings also showed a reduction in growth, whereas the double-transgenic seedlings showed minimal visual symptoms of stress-induced damage (Fig. 2C, c). Although there was a slight reduction in the overall growth of all the transgenic seedlings, growth of double transgenics remained least affected under high levels of ZnCl2 (Fig. 2C, d), thus indicating that overexpression of the entire glyoxalase pathway is better at facilitating enhanced zinc tolerance. Previously, transgenics overexpressing specific heavy-metal transporter proteins have been shown to tolerate zinc in the range of 100 μm to 4 mm (Van der Zaal et al., 1999; Blaudez et al., 2003; Lee et al., 2003; Verret et al., 2004). Engineering of the glyoxalase pathway shows that a previously unexplored metabolic pathway has been implicated to play a role in zinc tolerance.
Glyoxalase Transgenics Flowered and Produced Viable Seeds under High Zinc Conditions without Affecting Yield and Sequestered More Zinc in Roots
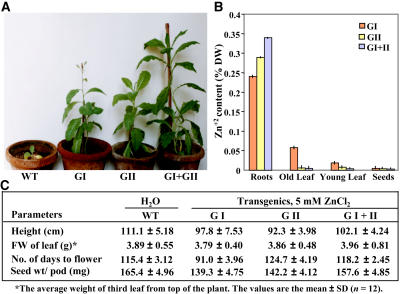
To assess whether the enhanced expression of the glyoxalase enzymes would allow plants to grow, mature, and set seeds in the presence of high zinc, all four types of plants (wild type, glyoxalases I and II, and double transgenics) were grown in the continued presence of 5 mm ZnCl2-spiked soils (Fig. 3A, representative plants are shown). The growth of wild-type plants was severely affected under these conditions as evidenced by their stalled growth and ultimate death. On the other hand, the transgenic plants grew, flowered, and produced normal viable seeds. The growth and survival of double transgenics was much better as compared to the individual transgenic plants. It was found that glyoxalase transgenics that grew well in zinc-spiked soils sequestered the highest amount of zinc in roots (0.34% of dry weight in double transgenics), as compared to leaves, and the lowest amount in seeds (Fig. 3B). Previously, overexpression of a zinc transporter gene in Arabidopsis (Arabidopsis thaliana) led to accumulation of as much as 0.6% of dry weight zinc in roots (Van der Zaal et al., 1999). Zinc content in roots was also reported in a similar range in Arabidopsis overexpressing a P-type ATPase (AtHMA4) involved in root-to-shoot translocation of zinc (Verret et al., 2004). Importantly, in this study, the seeds remained practically free from excess zinc ions (measured using inductively coupled argon-plasma emission spectrometry; see “Materials and Methods”) as has been reported earlier for sodium ions in tomato (Lycopersicon esculentum; Zhang and Blumwald, 2001) by overexpression of the sodium proton antiporter, in rice by overexpression of trehalose (Garg et al., 2002), and also in tobacco by overexpression of glyoxalase enzymes (Singla-Pareek et al., 2003) and a DEAD-box helicase (Sanan-Mishra et al., 2005). Hence, glyoxalase overexpression provides tolerance to a high level of zinc without any effect on seed yield and quality.
Figure 3.
Growth profile and yield parameters of T1 generation transgenic tobacco plants overexpressing glyoxalase I (GI), glyoxalase II (GII), or both glyoxalase I and glyoxalase II in double transgenics (GI + II) in zinc-spiked soils. A, Representative image showing relative growth of wild-type and transgenic tobacco plants in soil pots in the continued presence of 5 mm ZnCl2 for 98 d. B, Total zinc content in various tissues of the glyoxalase transgenic plants (GI, GII, and GI + II) grown under the continued presence of 5 mm ZnCl2 for 150 d (calculated as percent dry weight of the tissue). In the histogram, each of the transgenic types is indicated by different colored bars as shown. For each determination, roots, old leaf (fourth leaf from the bottom), young leaf (second leaf from the top), and seeds were collected from three different plants of each type. Values are the mean ± sd (n = 3). Similar data for wild-type plants could not be obtained because these plants did not grow further in the presence of 5 mm ZnCl2. However, the relative values for zinc in the wild-type plants grown in water were almost negligible: 0.01% in roots; 0.008% in old leaf; 0.005% in young leaf; and 0.005% in seeds. C, Comparison of various growth parameters and seed production of wild-type and glyoxalase transgenic tobacco plants grown in the presence of water and 5 mm ZnCl2, respectively, for 150 d.
Further, it was of interest to evaluate how these transgenic plants perform when grown in the continued presence of zinc. Various growth parameters were scored for T1 generation transgenic plants grown in soils spiked with 5 mm ZnCl2 vis-à-vis wild-type plants grown in normal soil with water (Fig. 3C). It should be noted here that similar data for wild-type plants grown in the presence of zinc could not be obtained because these plants failed to sustain growth in the presence of 5 mm ZnCl2 after 40 d. The overall performance and total seed yield of the double-transgenic plants grown in the presence of 5 mm ZnCl2 were found to be comparable to that of the wild type grown in water, strongly indicating the ameliorating effect of glyoxalase transgenes on seed productivity and yield of transgenic plants. The double transgenics grown in the presence of high zinc were able to produce 95% of the total seeds when compared with wild-type plants grown in water, whereas the glyoxalase I and II transgenic lines yielded 84% and 86%, respectively (Fig. 3C). These data document that seed production during high levels of zinc metal exposure is not severely affected in single-gene transgenics (either glyoxalase I or II overexpressing tobacco) as they yielded relatively fewer seeds per pod and also showed reduced plant height under high zinc conditions as compared to the double-transgenic plants. We observed a similar response in a previous study, where, under 200 mm NaCl, the same lines of double transgenic, glyoxalase I, and glyoxalase II yielded 95%, 80%, and 83%, respectively, of the total seeds when compared with that of wild-type plants grown in water (Singla-Pareek et al., 2003).
Glyoxalase Overexpressors Restrict MG Accumulation, Undergo Similar Degrees of Oxidative Stress as Wild-Type Plants, But Show Less Lipid Peroxidation under High Zinc Conditions
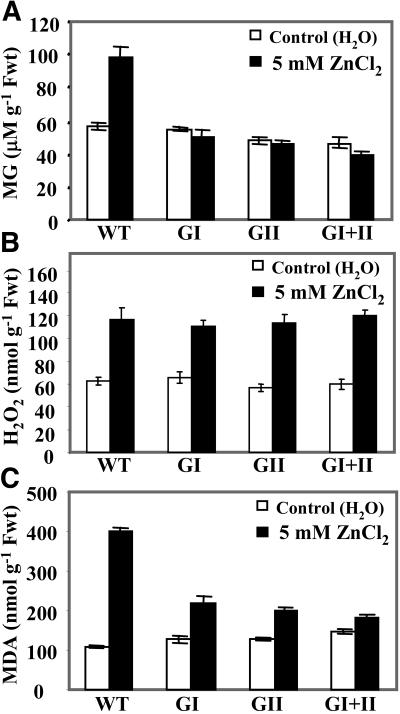
MG is a cytotoxic compound and its level has been reported to increase during various stresses in animal, yeast (Saccharomyces cerevisiae), and bacterial systems (Cooper, 1984; Kalapos et al., 1992; Aguilera and Prieto, 2001), and we recently reported similar observations in plant systems (Yadav et al., 2005a). To investigate the biochemical basis of zinc tolerance in glyoxalase transgenic plants, the level of MG was measured in wild type and transgenics under 5 mm ZnCl2. In unstressed plants, the level of MG was found to be almost similar in wild-type and transgenic plants (Fig. 4A). The glyoxalase-overexpressing transgenic plants do not show an enhanced catabolism of MG as a minimal level of MG is retained in the system under nonstress conditions as well, which is important for normal developmental processes (Yadav et al., 2005a). In response to zinc, wild-type plants exhibited a 69% increase in MG concentration, whereas transgenics maintained MG levels almost similar to those of their unstressed counterparts (Fig. 4A). These results indicated that higher levels of glyoxalase enzymes, as maintained in transgenics, could detoxify MG beyond a certain level, thus preventing its accumulation to a higher toxic level in the presence of zinc. This report documents that MG levels increase in the presence of a heavy metal such as zinc.
Figure 4.
Relative MG levels and the degree of oxidative stress as well as lipid peroxidation in transgenic tobacco plants. A, Accumulation of MG (μm g−1 fresh weight). B, H2O2 (nmol g−1 fresh weight; for measuring oxidative stress). C, MDA (nmol g−1 fresh weight; to reflect the extent of lipid peroxidation) in wild-type (WT) and glyoxalase transgenic plants (GI, glyoxalase I; GII, glyoxalase II; and GI + II, double transgenics) in response to zinc. Second leaf from top of 1-month-old T1 generation plants of each of these types was floated in 5 mm ZnCl2 solution for 24 h. Data represent the mean of three replicates and of three independent experiments ±sd.
Several biotic and abiotic factors ultimately impose oxidative stress onto the system. Induction of oxidative stress by zinc toxicity has been observed previously (Weckx and Clijster, 1997; Prasad et al., 1999; Rao and Sresty, 2000).To carry out similar investigations in our study, endogenous H2O2 concentration was measured. The H2O2 level was found to be similar in both wild-type and transgenic plants under nonstress conditions that increased by almost a similar degree upon exposure to 5 mm ZnCl2 (Fig. 4B), thereby documenting that plants experience oxidative stress when exposed to high levels of heavy metal. Further, to analyze the damage caused by enhanced levels of H2O2, lipid peroxidation in terms of production of malondialdehyde (MDA) was measured. MDA levels were found to increase drastically (by 300%) in wild-type plants when exposed to zinc, whereas its level in the transgenics increased by 68%, 60%, and 17% in glyoxalases I and II and double transgenics, respectively, indicating that wild-type plants undergo a higher degree of lipid peroxidation, leading to a loss of membrane integrity, than the transgenics (Fig. 4C).
During normal functioning of the electron transport chain in chloroplasts and mitochondria as well as in various enzyme-catalyzed redox reactions, ROS are produced (Dat et al., 2000; Moller, 2001); if not efficiently scavenged and quenched, ROS cause lipid peroxidation, enzyme inactivation, and DNA and membrane damage, leading to the death of plants. However, plants exposed to metals such as cadmium, nickel, and zinc often accumulate ROS and undergo oxidative stress. High levels of metal ions could likely induce disturbance in ROS-generating metabolism and also deactivation of the processes required for ROS destruction (Dietz et al., 1999). MG has a binding site on GSH peroxidase, and its high levels inhibit enzyme activity (Park et al., 2003). Since GSH peroxidation catalyzes the detoxification of H2O2, its inactivation leads to the accumulation of ROS. Upon zinc stress, MG levels also increase, which is cytotoxic, ultimately leading to destruction of organelles and macromolecules (Yadav et al., 2005b). Both MG and ROS have been reported to be harmful to plants, but whether MG leads to the generation of ROS and acts upstream of it is not yet known, although there are indications for similar observations in other systems (Park et al., 2003).
It has been well established that a complex antioxidant system, the ascorbate-GSH cycle (Zhang and Kirkham, 1996), and various other endogenous antioxidants such as thiols and GSH (Foyer et al., 2001), play a major role in H2O2 scavenging in chloroplasts as well as cytosol (Zhang and Kirkham, 1996). Further, it has also been found that maintenance of GSH levels during normal and stress conditions can prevent the generation of free radicals and, hence, tissue damage (Nagalakshmi and Prasad, 2001). Detoxification reactions must involve the fine optimal balance between the formation and detoxification of ROS.
Glyoxalase-Mediated Zinc Tolerance Correlates with Increased PC Biosynthesis and Maintenance of GSH Homeostasis
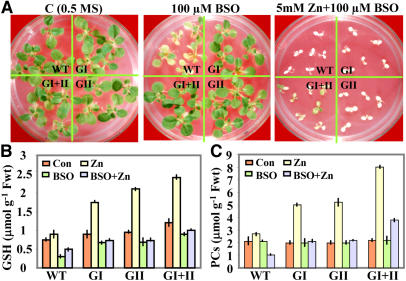
After growth for 25 d on media containing 5 mm ZnCl2, control plants showed zinc toxicity symptoms, including major loss of chlorophyll and significant reduction in shoot growth. In contrast, under similar conditions, the double transgenics showed plant survival of 70% to 80% (see Fig. 2C). Because glyoxalases utilize GSH and recycle it back into the system, it became imperative to analyze whether GSH biosynthesis plays any role in glyoxalase-mediated zinc tolerance. For this, de novo synthesis of GSH was inhibited by transferring wild-type and transgenic seedlings to the medium supplemented with buthionine sulfoximine (BSO), an inhibitor of γ-glutamylcysteine synthetase (Meister, 1988)—the first and rate-limiting enzyme of the GSH biosynthetic pathway (see Fig. 6). It was observed that growth of the wild type was severely inhibited (Fig. 5A, middle), while the glyoxalase transgenics showed almost normal growth in the presence of BSO alone, without any additional stress. This suggests that sufficient levels of GSH must be made available to the system either through efficient recycling of GSH due to glyoxalase overexpression or storing GSH in the form of S-lactoylglutathione (SLG), which could provide a source of GSH, as has been shown in animal systems (Thornalley, 1990a, 1990b), but remains to be deciphered in plants. It was further observed that imposition of zinc stress in the presence of BSO caused significant reduction in the zinc tolerance capacity of glyoxalase transgenic plants (Fig. 5A, right), indicating that, under high zinc conditions, de novo synthesis of GSH is also necessary apart from its homeostasis maintained by overexpression of glyoxalase pathway enzymes.
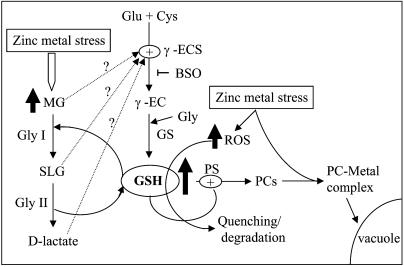
Figure 6.
Demand-driven synthesis and multiple regulation of GSH. Maintenance of GSH homeostasis and increase in PC levels provide tolerance to zinc stress in transgenic tobacco overexpressing glyoxalase genes. Nontransgenic plants exposed to high levels of zinc not only undergo oxidative stress but also show an increase in MG levels. ROS and high MG levels cause damage to the plants. However, glyoxalase transgenics seem to be capable of detoxifying both of these harmful compounds and survive heavy-metal stress. Increased level of MG is directly detoxified through overexpression of glyoxalase pathway enzymes, and maintenance of GSH levels in transgenics could be involved in quenching of ROS generated upon stress. Also, GSH homeostasis helps in increased production of PCs, which might sequester the excess zinc ions through the PC-metal ion complex. γ-EC, (γ-glutamylcysteine); γ-ECS, (γ-glutamylcysteine synthetase); GS, (glutathione synthetase).
Figure 5.
Effect of zinc stress and GSH inhibitor on relative growth of wild-type and glyoxalase transgenic tobacco plants (GI, glyoxalase I; GII, glyoxalase II; and GI + II, double transgenics). A, Representative image of seedlings showing relative growth of wild-type (WT) and transgenic plants under control conditions (C; 0.5× Murashige and Skoog) or in the presence of 100 μm GSH inhibitor BSO or both 5 mm ZnCl2 + 100 μm BSO for 25 d. B and C, Histograms showing levels of GSH (B) and PCs (C) in seedlings of wild-type and glyoxalase transgenics exposed to 5 mm ZnCl2, 100 μm BSO, or both 5 mm ZnCl2 + 100 μm BSO for 24 h. Values are the mean of three independent estimations ±sd. Note the higher content of GSH and PCs in double transgenics.
Further, endogenous levels of GSH were measured in wild-type and transgenic seedlings, and a positive correlation could be established between the levels of GSH and zinc tolerance. The basal levels of GSH were higher by about 64% in double-transgenic plants under nonstress conditions, which further increased by 100% during 5 mm ZnCl2 treatment (Fig. 5B), whereas wild-type plants showed only a marginal increase in the GSH levels under 5 mm ZnCl2. In the presence of BSO alone (under nonstress conditions), there was a significant reduction in the level of GSH, while this decline was minimized in transgenic seedlings. In the presence of 5 mm ZnCl2 and BSO together, even the double transgenics were not able to accumulate very high levels of GSH, although they were still higher than the wild-type levels, indicating that de novo synthesis of GSH under zinc stress also contributes toward total GSH levels in the double-transgenic plants. Further evidence for the correlation between GSH levels and zinc tolerance was provided by the experiment where exogenous application of GSH to growth media could confer zinc tolerance in wild-type plants (data not shown), mimicking the effect of GSH biosynthesis and homeostasis. It has been reported previously that exposure to zinc initially resulted in a severe depletion of GSH (Rao and Sresty, 2000) caused by an increased consumption of GSH for PC production, whereas other studies have shown that heavy metals such as cadmium stimulated production of GSH (Maier et al., 2003; Sun et al., 2005b). Molecular studies have also shown that heavy metals stimulate expression of genes encoding enzymes involved in GSH synthesis (Schafer et al., 1998). Correlative studies have also shown an increased tolerance of plants toward cadmium with elevated levels of GSH, as well as decreased tolerance in plants with diminished levels of GSH (Zhu et al., 1999a, 1999b; Howden et al., 1995). Therefore, GSH synthesis appears to be crucial for the protection of plants from heavy metals, and it has been thought that GSH possibly could serve as a biomarker of metal toxicity in plants.
In plants, heavy metals induce the formation of acid-soluble nonprotein thiol-rich peptides made up of (γ-glutamylcysteinyl)n-Gly, with n = 2 to 11 (Grill et al., 1985; Steffens, 1990). These peptides are also known as metal-binding compounds or PCs. It has already been shown that PCs are synthesized from GSH and are involved in the detoxification of heavy metals (Steffens et al., 1986; Scheller et al., 1987; Sun et al., 2005a). PC synthesis is directly related to actual metal stress in plants, and it functions as a biomarker for the evaluation of metal toxicity (Sneller et al., 1999; Sun et al., 2005a). To examine the effect of zinc on PC production, we measured its levels in tobacco exposed to zinc and/or the GSH inhibitor BSO. In a number of previous studies, concentration of PCs has been assessed by subtracting the amount of GSH from the amount of total nonprotein-SH (TNP-SH) compounds (De Knecht et al., 1992; DeVos et al., 1992; Schat and Kalff, 1992) and also adopted in recent years (Hartley-Whitaker et al., 2001, 2002; Sun et al., 2005a, 2005b). Based on these experiments, the amount of TNP-SH compounds other than GSH was taken as a measure of PC production in this study. In previous studies, PCs have been found to be present in considerable amounts under nonstress conditions in plants (Keltjens and van Beusichem, 1998; Maier et al., 2003; Sun et al., 2005a). Present results showed the basal level of PCs to be almost same in the wild-type and glyoxalase transgenic plants. It has been hypothesized that, aside from detoxification, PCs play an important role in intracellular metal homeostasis (Grill et al., 1985; Thumann et al., 1991).
It was further found in this study that PC production is dependent on the presence of heavy metals as exposure to zinc induced about a 280% increase in its levels in double transgenics and about a 155% increase in either of the single-gene transformants (Fig. 5C). However, under similar conditions, this increase was only 32% in wild-type plants. The presence of BSO alone did not affect PC levels, whereas when zinc stress and BSO were applied together, the accumulation of PCs in wild-type plants was significantly reduced, thus leading to their poor survival. However, the double transgenics were able to accumulate some amount of PCs, which possibly imparted a certain degree of tolerance to these plants toward 5 mm ZnCl2. It has been reported that chelation of metal ions with ligands such as PCs or metallothioneins might route metals predominantly toward root sequestration (Evans et al., 1992). Our results are in conformity with this because we also observed higher sequestration of zinc in roots. In Arabidopsis, it has also been reported previously that overexpression of a zinc transporter leads to enhanced zinc tolerance and increased zinc content in the roots under high zinc conditions (Van der Zaal et al., 1999). In this study, we found that zinc-induced PC synthesis was accompanied by enhanced production of GSH, which indicates that glyoxalase-overexpressing transgenic plants are at least partly autonomous in providing the GSH required for PC synthesis and that GSH synthesis did not limit PC production at the exposure levels examined. However, when de novo synthesis of GSH is blocked by the addition of BSO, the production of PCs is highly compromised even in glyoxalase transgenic plants. Hence, we surmise that both PC and GSH concentrations are crucial determinants of tolerance toward toxic levels of zinc.
With a better understanding of the biochemical mechanisms for heavy-metal tolerance in glyoxalase transgenic plants, we speculate on the involvement of some of the potential target sites, which ultimately helps in tolerance. Because GSH is recycled by the glyoxalase system, it was assumed that an increased level of MG is detoxified efficiently in transgenic plants overexpressing glyoxalase enzymes constitutively, thus creating the possibility of up-regulation of GSH levels at least during stress. The role of glyoxalase overexpression in maintaining GSH homeostasis has already been shown to provide enhanced salinity tolerance (Kumar et al., 2003; Yadav et al., 2005c). GSH is a ubiquitous molecule involved in several functions in cell metabolism, such as ROS processing, redox state regulation, etc. (Meister, 1995; Noctor and Foyer, 1998). In plants and yeast, GSH has also been indicated for use in the synthesis of PCs. One of the most-studied mechanisms of heavy-metal accumulation is the GSH-PC-mediated heavy-metal resistance (Mendoza-Cozatl et al., 2005). Although this mechanism has been described for Cd2+, other heavy metals, such as Hg2+, Cu2+, and Zn2+, may also be inactivated and stored (Devars et al., 1998; Vatamaniuk et al., 2000).
We have recently shown the involvement of GSH homeostasis in glyoxalase I transgenics during abiotic stresses. Higher GSH levels are maintained in transgenics and hence survive under stress conditions, whereas wild-type plants cannot maintain GSH levels and die (Yadav et al., 2005c). Therefore, it seems that any of the metabolites of the glyoxalase pathway, MG, SLG, or d-lactate, may be up-regulating GSH biosynthesis. The role of SLG in increasing GSH levels has been shown earlier (Thornalley, 1990a, 1990b). However, the same needs to be deciphered in plants. Once GSH levels are maintained during Zn2+ stress, PCS becomes active in the presence of metal ions and catalyzes the formation of the PC-metal complex (Fig. 6). As has been shown previously, PCS becomes active when two GSH molecules plus a heavy metal form a thiolate (Cd-GS2 or Zn-GS2) and one γ-Glu-Cys moiety is transferred to a free GSH molecule or to a previously synthesized PC (Vatamaniuk et al., 2000). The PC-metal complex can then be transported into the vacuole, and inside the vacuole this complex forms high-Mr complexes, which have been reported to be the ultimate and more stable storage form of heavy metals (Mendoza-Cozatl et al., 2005).
Glyoxalase Transgenic Plants Can Tolerate High Levels of Various Heavy Metals
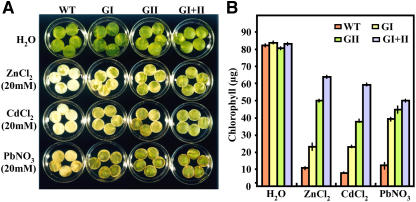
For assessing the potential of glyoxalase transgenic plants for relative tolerance toward other heavy metals, leaf discs from wild-type and various transgenic tobacco plants were floated separately on a range of ZnCl2, CdCl2, or PbNO3 solutions for 5 d. Although various lower concentrations of these heavy metals were also used in this study, data for only relatively higher concentrations (20 mm) have been presented here, where a clear phenotypic difference in the tolerance capacities of the wild-type, single-, and double-transgenic plants was obtained. These experiments revealed that heavy metal-induced loss of chlorophyll was lower in glyoxalase-overexpressing lines compared to wild-type plants (Fig. 7A). The damage caused by heavy-metal stress was reflected visually in the degree of bleaching observed in the leaf discs after 5 d. The leaf discs from the wild type showed an early bleaching as compared to the transgenic plants. Measurement of the chlorophyll content of the leaf discs from different transgenic lines and wild type exposed to various heavy metals (Fig. 7B) provided further support for a positive relationship between overexpression of glyoxalase pathway enzymes and tolerance to heavy-metal stress. Wild-type plants exhibited almost a total loss of chlorophyll within 5 d in the presence of different heavy metals (85%–90% loss as compared to its water controls), whereas the double transformants experienced only a 24% to 38% decline in chlorophyll (Fig. 7B). Together, these observations indicated that glyoxalase transgenic plants could tolerate toxic levels of zinc, cadmium, and lead, thereby suggesting a wider applicability of the glyoxalase pathway for engineering tolerance toward a broader category of edaphic factors.
Figure 7.
Relative tolerance of transgenic tobacco plants overexpressing glyoxalase I (GI), glyoxalase II (GII), or both glyoxalase I and glyoxalase II in double transgenics (GI + II), toward toxic levels of zinc, cadmium, and lead. Representative images showing phenotypic differences (A) and chlorophyll content (B; μg g−1 fresh weight) from heavy metal-treated leaf discs of wild-type and various transgenic plants (GI, GII, and GI + II) after incubation in 20 mm solution of ZnCl2, CdCl2, or PbNO3 for 5 d are shown. Discs floated in water served as the experimental control. The sd in each case is represented by the vertical bar in each graph (n = 3). Note the difference in retention of chlorophyll in wild-type and transgenic plants in response to various heavy metals.
CONCLUSION
This study suggests a novel strategy for ameliorating heavy-metal, especially zinc, stress in plants. We have reported here the functional validation of enzymes of the glyoxalase pathway in enhanced accumulation and tolerance of toxic levels of zinc in the transgenic system. The glyoxalase transgenics were able to grow, flower, and set seeds in the presence of 5 mm ZnCl2 and sequestered excess zinc in roots. An increase in the level of PCs and maintenance of GSH homeostasis in transgenics during exposure to high Zn2+ levels seem to be the mechanisms behind this tolerance. The high accumulation of Zn2+ in the roots and the low accumulation of Zn2+ in the seeds of transgenic plants under high zinc conditions suggest the potential use of this engineering strategy in agriculture of crop plants on zinc-contaminated soil. The role of this pathway in ameliorating heavy-metal toxicity has not been shown previously. Together with our earlier observations on salinity tolerance, we suggest that glyoxalase pathway engineering can be an effective strategy for developing multiple stress tolerance in plants.
MATERIALS AND METHODS
Generation of Transgenic Tobacco Plants
The single-gene transformants of tobacco (Nicotiana tabacum) carrying the glyoxalase I open reading frame (ORF) from Brassica juncea (Y13239) or the glyoxalase II ORF from rice (Oryza sativa; AY054407) and the double transgenics of tobacco carrying both glyoxalase I or II ORFs were raised in our previous study (Singla-Pareek et al., 2003). In this study, we employed three independent transgenic lines of these single or double transgenics that have been analyzed previously for salinity tolerance (Singla-Pareek et al., 2003) and for characterization of zinc tolerance. For growth of T1 generation seedlings, glyoxalase I or II transformants were selected on either kanamycin (50 mg L−1) or hygromycin (25 mg L−1), respectively, while the double transformants were selected on the mixture of both antibiotics.
Western Blotting and Enzyme Assays
For western blotting and enzyme assays, 7-d-old seedlings of B. juncea and rice were exposed to 5 mm ZnCl2 for 24 h. Extraction of soluble proteins was essentially carried out as described previously (Singla-Pareek et al., 2003). The amount of protein was estimated by the Bradford method (Bradford, 1976). Twenty micrograms of soluble proteins from Brassica and rice were resolved on one-dimensional SDS-PAGE, transferred onto nitrocellulose membrane, and probed with either anti-glyoxalase I antibodies (Veena et al., 1999) or anti-glyoxalase II antibodies, respectively, as described previously (Singla-Pareek et al., 2003). For enzyme assays, protein extract was prepared by homogenizing tobacco leaf tissue in liquid nitrogen and then resuspending the powder in 2 vol (w/v) of extraction buffer (0.1 m sodium phosphate buffer, pH 7.0, 50% glycerol, 16 mm MgSO4, 0.2 mm phenylmethylsulfonyl fluoride, 0.2% polyvinyl polypyrrolidone). The enzyme activity for glyoxalases I and II was determined as described previously (Ramaswamy et al., 1983; Maiti et al., 1997). Three different enzyme extractions were done per sample for three independent transgenic lines of each of the single (glyoxalase I or II) and double transformants (glyoxalase I + II). The specific activity for both enzymes is expressed in units per milligram of protein.
Leaf Disc Assay for Tolerance under High Levels of Zinc, Cadmium, and Lead
To compare relative stress tolerance between wild-type and transgenic tobacco plants, a rapid bioassay based on detached leaf senescence was performed. For this, fully expanded leaves (60 d old) were briefly washed in deionized water. Leaf discs of 1 cm in diameter were punched out and floated on a 6-mL solution of ZnCl2 (5–20 mm, 5 d) or sterile distilled water (which served as the experimental control). For a separate experiment, leaf discs were floated on a 6-mL solution of either ZnCl2, CdCl2, or PbNO3 for 5 d (here data for only a 20 mm concentration of each have been presented that show clear visible phenotypic difference between tolerance of wild-type, single-gene transformants, and double transgenics). The chlorophyll content was measured as described previously (Singla-Pareek et al., 2003). The experiment was repeated three times with three different transgenic lines.
Transgenic Plants and Zinc Tolerance
To assess the relative zinc tolerance of various plants, wild-type and T1 generation transgenic seeds overexpressing glyoxalase I and glyoxalase II or both were germinated on one-half-strength (0.5×) Murashige-Skoog medium in the presence of appropriate antibiotics. The surviving seedlings (7 d old) were transferred to 0.5× Murashige and Skoog medium supplemented with 5, 10, or 20 mm ZnCl2 for imposing heavy-metal stress or onto plain 0.5× Murashige and Skoog medium that served as the experimental control. The seedlings were maintained under culture room conditions, and their growth was monitored for 25 d under stress. For analyzing the effect of the GSH biosynthesis inhibitor BSO, 7-d-old surviving seedlings were transferred to 0.5× Murashige and Skoog medium supplemented with either 5 mm ZnCl2 or 100 μM BSO or both (5 mm ZnCl2 + 100 μM BSO), and their growth was monitored for 25 d under culture room conditions. In addition to the experiments with seedlings, we carried out the assessment of the transgenic plants for their tolerance toward high levels of zinc throughout their life cycle. For this purpose, wild-type and T1 transgenic seeds were germinated on Murashige and Skoog medium containing appropriate antibiotics. The surviving seedlings were transferred to earthen pots and grown in a greenhouse (16 h light, 8 h dark, 25°C ± 2°C). Starting 2 weeks after transfer, plants were watered biweekly with a 5 mm ZnCl2 solution. Three wild-type and three independent transgenic lines of each type (i.e. glyoxalases I and II and double transformants) with three plants each were distributed in two groups, and each group was watered with either 5 mm ZnCl2 solution or water.
Endogenous Zn2+ Ion Content Determination
Mature wild-type and transgenic plants grown under water or in 5 mm ZnCl2 in a greenhouse for 150 d were used. Roots, old leaves, young leaves, and seeds were collected from three different plants of each type and thoroughly rinsed in deionized water and the fresh weight of each sample was determined. The samples were then processed for estimation of zinc (Zn2+) content using simultaneous inductively coupled argon-plasma emission spectrometry as described earlier (Singla-Pareek et al., 2003).
MG, H2O2, and MDA (Lipid Peroxidation) Estimation
MG was extracted from leaf tissue (0.3 g) by homogenizing in 3 mL of 0.5 m perchloric acid. One milliliter of total reaction mixture contained 250 μL 7.2 mm 1,2-diaminobenzene, 100 μL 5 m perchloric acid, and 650 μL of the sample, which was added last, and the absorbance of the derivative was read at 336 nm as suggested (Yadav et al., 2005a). Global leaf H2O2 was measured using the chromogenic peroxidase-coupled oxidation of 3-methyl-2-benzothiazoline hydrazone and 3-dimethyl aminobenzoic acid as described (Veljovic-Jovanovic et al., 2002). Lipid peroxidation was measured in terms of MDA value by the reaction with thiobarbituric acid as suggested (Heath and Packer, 1968).
Extraction and Determination of TNP-SH Compounds
TNP-SH compounds were extracted and assayed according to De Vos et al. (1992). Essentially, TNP-SH compounds were extracted by homogenizing 0.2 g of frozen leaf tissue in 2 mL of 5% (w/v) sulfosalicylic acid with 6.3 mm diethylenetriaminepentaacetic acid (pH < 1) with mortar and pestle at 4°C. The homogenate was centrifuged at 4°C at 10,000g for 10 min. The clear supernatants were collected and used immediately for the assay of TNP-SH. The concentrations of TNP-SH were determined using Ellman's reagent (Ellman, 1959). One milliliter of reaction mixture contained 630 μL of 0.5 m K2HPO4, 30 μL of 10 mm 5,5′-dithiobis(2-nitrobenzoic acid), and 300 μL of supernatant, final pH 7.0. Absorbance was read after every 2 min at 412 nm, and values were corrected for the absorbance of supernatant and 5,5′-dithiobis(2-nitrobenzoic acid) as suggested (De Vos et al., 1992).
Determination of GSH Concentration
Total and oxidized glutathione (GSSG) analysis was carried out as described previously (Griffith, 1980). GSH was calculated by subtracting GSSG from total glutathione.
Calculation of PC Production
The level of PCs was estimated in terms of PC-SH levels calculated by subtracting the amount of GSH from that of TNP-SH compounds. The validity of this method has been investigated (De Knecht et al., 1992; De Vos et al., 1992; Schat and Kalff, 1992) and widely employed in studies (Gupta et al., 1998; Keltjens and van Beusichem, 1998; Hartley-Whitaker et al., 2001, 2002; Sun et al., 2005a, 2005b).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers Y13239 and AY054407.
Acknowledgments
We thank Dr. B. Porter and Dr. F. White, Kansas State University, for the initial glyoxalase II clone, and Dr. V. Rajamani and Dr. J.K. Tripathi, Jawaharlal Nehru University, New Delhi, for helping with the work related to ionic content measurements. We also thank Dr. M.V. Rajam, University of Delhi South Campus, New Delhi, for a critical reading of the manuscript.
This work was supported by internal grants from the International Centre for Genetic Engineering and Biotechnology, New Delhi; the Department of Biotechnology (DBT) Network Project; the International Foundation for Science, Sweden (research grant to S.L.S.-P.); and a DBT postdoctoral fellowship (to S.K.Y.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sneh L. Singla-Pareek (sneh@icgeb.res.in).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.073734.
References
- Aguilera J, Prieto JA (2001) The Saccharomyces cerevisiae aldose reductase is implied in the metabolism of methylglyoxal in response to stress conditions. Curr Genet 39: 273–283 [DOI] [PubMed] [Google Scholar]
- Blaudez D, Kohler A, Martin F, Sanders D, Chalot M (2003) Poplar metal tolerance protein 1 confers zinc tolerance and is an oligomeric vacuolar zinc transporter with an essential leucine zipper motif. Plant Cell 15: 2911–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MN (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146: 185–205 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou J, Goldsbrough PB (1997) Characterization of phytochelatin synthase from tomato. Physiol Plant 101: 165–172 [Google Scholar]
- Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 475–486 [DOI] [PubMed] [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI (1999) Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J 18: 3325–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Palmgren M, Kramer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7: 309–315 [DOI] [PubMed] [Google Scholar]
- Cooper RA (1984) Metabolism of methylglyoxal in microorganism. Annu Rev Microbiol 38: 49–68 [DOI] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57: 779–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Knecht JA, Koevoets PLM, Verkleij JAC, Ernst WHO (1992) Evidence against a role for phytochelatins in naturally selected increased cadmium tolerance in Silene vulgaris (Moench) Garcke. New Phytol 122: 681–688 [Google Scholar]
- De Vos CHR, Vonk MJ, Vooijs R, Schat H (1992) Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 98: 853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devars S, Hernández R, Moreno-Sánchez R (1998) Enhanced heavy metal tolerance in two strains of photosynthetic Euglena gracilis by preexposure to mercury or cadmium. Arch Environ Contam Toxicol 34: 128–135 [DOI] [PubMed] [Google Scholar]
- Dietz K-J, Baier M, Krämer U (1999) Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In MNV Prasad, J Hagemeyer, eds, Heavy Metal Stress in Plants. Springer, Berlin, pp 73–97
- Dudka S, Piotrowska M, Terelak H (1996) Transfer of cadmium, lead, and zinc from industrially contaminated soil to crop plants: a field study. Environ Pollut 94: 181–188 [DOI] [PubMed] [Google Scholar]
- Eapen S, D'Souza SF (2005) Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol Adv 23: 97–114 [DOI] [PubMed] [Google Scholar]
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70–77 [DOI] [PubMed] [Google Scholar]
- Evans KM, Gatehouse JA, Lindsay WP, Shi J, Tommey AM, Robinson NJ (1992) Expression of the pea metallothionein-like gene PsMTA in Escherichia coli and Arabidopsis thaliana and analysis of trace metal ion accumulation; implications for PsMTA function. Plant Mol Biol 20: 1019–1028 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Theodoulou F, Delrot S (2001) The functions of inter- and intracellular glutathione transport systems in plants. Trends Plant Sci 6: 486–492 [DOI] [PubMed] [Google Scholar]
- Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99: 15898–15903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert C, Ros R, De Haro A, Walker DJ, Pilar Bernal M, Serrano R, Navarro-Avino J (2003) A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochem Biophys Res Commun 303: 440–445 [DOI] [PubMed] [Google Scholar]
- Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106: 207–212 [DOI] [PubMed] [Google Scholar]
- Grill E, Loffler S, Winnacker EL, Zenk MH (1989) Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86: 6838–6842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Winnacker E-L, Zenk MH (1985) Phytochelatins: the principal heavy metal complexing peptides of higher plants. Science 230: 674–676 [DOI] [PubMed] [Google Scholar]
- Grill E, Winnacker E-L, Zenk MH (1987) Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA 84: 439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Tripathi RD, Rai UN, Chandra P (1998) Role of glutathione and phytochelatin in Hydrilla verticillata (l.f.) Royle and Vallusneria spiralus L. under mercury stress. Chemosphere 37: 785–800 [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsbrough PB, Cobbett CS (1999) Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53: 1–11 [PubMed] [Google Scholar]
- Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper- and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ 24: 713–722 [Google Scholar]
- Hartley-Whitaker J, Woods C, Meharg AA (2002) Is differential phytochelatin production related to decreased arsenate influx in arsenate tolerant Holcus lanatus? New Phytol 155: 219–225 [Google Scholar]
- Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125: 189–198 [DOI] [PubMed] [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobett CS (1995) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalapos MP, Garzo T, Antoni F, Mandl J (1992) Accumulation of S-D-lactoylglutathione and transient decrease of glutathione level caused by methylglyoxal load in isolated hepatocytes. Biochim Biophys Acta 1135: 159–164 [DOI] [PubMed] [Google Scholar]
- Keltjens WG, van Beusichem ML (1998) Phytochelatins as biomarker for heavy metal stress in maize (Zea mays L.) and wheat (Triticum aestivum L.): combined effects of copper and cadmium. Plant Soil 203: 119–126 [Google Scholar]
- Kumar S, Singla-Pareek SL, Reddy MK, Sopory SK (2003) Glutathione: biosynthesis, homeostasis and its role in abiotic stresses. J Plant Biol 30: 179–187 [Google Scholar]
- Lee J, Bae H, Jeong J, Lee JY, Yang YY, Hwang I, Martinoia E, Lee Y (2003) Functional expression of a bacterial heavy metal transporter in Arabidopsis enhances resistance to and decreases uptake of heavy metals. Plant Physiol 133: 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dhankher OP, Carreira L, Lee D, Chen A, Schroeder JI, Balish RS, Meagher RB (2004) Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol 45: 1787–1797 [DOI] [PubMed] [Google Scholar]
- Maier EA, Matthews RD, McDowell JA, Walden RR, Ahner BA (2003) Environmental cadmium levels increase phytochelatin and glutathione in lettuce grown in a chelator-buffered nutrient solution. J Environ Qual 32: 1356–1364 [DOI] [PubMed] [Google Scholar]
- Maiti MK, Krishnasamy S, Owen HA, Makaroff CA (1997) Molecular characterization of glyoxalase II from Arabidopsis thaliana. Plant Mol Biol 35: 471–481 [DOI] [PubMed] [Google Scholar]
- Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263: 17205–17208 [PubMed] [Google Scholar]
- Meister A (1995) Glutathione metabolism. Methods Enzymol 251: 3–13 [DOI] [PubMed] [Google Scholar]
- Mendoza-Cozatl D, Loza-Tavera H, Hernández-Navarro A, Moreno-Sánchez R (2005) Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol Rev 29: 653–671 [DOI] [PubMed] [Google Scholar]
- Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52: 561–591 [DOI] [PubMed] [Google Scholar]
- Nagalakshmi S, Prasad MNV (2001) Reponses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160: 291–299 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279 [DOI] [PubMed] [Google Scholar]
- Park YS, Koh YH, Takahashi M, Miyamoto Y, Suzuki K, Dohmae N, Takio K, Honke K, Taniguchi N (2003) Identification of the binding site of methylglyoxal on glutathione peroxidase: methylglyoxal inhibits glutathione peroxidase activity via binding to glutathione binding sites Arg 184 and 185. Free Radic Res 37: 205–211 [DOI] [PubMed] [Google Scholar]
- Pilon-Smits E, Pilon M (2002) Phytoremediation of metals using transgenic plants. CRC Crit Rev Plant Sci 21: 439–456 [Google Scholar]
- Prasad KVSK, Paradha Saradhi P, Sharmila P (1999) Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ Exp Bot 42: 1–10 [Google Scholar]
- Ramaswamy O, Guha-Mukherjee S, Sopory SK (1983) Presence of glyoxalase I in pea. Biochem Int 7: 307–318 [Google Scholar]
- Rao KVM, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157: 113–128 [DOI] [PubMed] [Google Scholar]
- Ross SM (1994) Sources and forms of potentially toxic metal in soil-plant systems. In SM Ross, ed, Toxic Metals in Soil-Plant Systems. John Wiley & Sons, New York, pp 3–25
- Salt DE, Rauser WE (1995) MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol 107: 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanan-Mishra N, Pham XH, Sopory SK, Tuteja N (2005) Pea DNA helicase 45 overexpression in tobacco confers high salinity tolerance without affecting yield. Proc Natl Acad Sci USA 102: 509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer HJ, Haag-kerwer A, Rausch T (1998) cDNA cloning and expression analysis of genes encoding GSH synthesis in roots of the heavy-metal accumulator Brassica juncea L.: evidence for Cd-induction of a putative mitochondrial γ-glutamylcysteine synthetase isoform. Plant Mol Biol 37: 87–97 [DOI] [PubMed] [Google Scholar]
- Schat H, Kalff MMA (1992) Are phytochelatins involved in differential metal tolerance or do they merely reflect metal-imposed strain? Plant Physiol 99: 1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller HV, Huang B, Hatch E, Goldsbrough PB (1987) Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol 85: 1031–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla-Pareek SL, Reddy MK, Sopory SK (2003) Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci USA 100: 14672–14677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller FEC, Noordover ECM, Bookum WMT, Schat H, Bedaux JJM, Verkleij JAC (1999) Quantitative relationship between phytochelatin accumulation and growth inhibition during prolonged exposure to cadmium in Silene vulgaris. Ecotoxicology 8: 167–175 [Google Scholar]
- Steffens JC (1990) The heavy metal-binding peptides of plants. Annu Rev Plant Physiol Plant Mol Biol 41: 553–575 [Google Scholar]
- Steffens JC, Hunt DF, Williams BG (1986) Accumulation of non-protein metal binding polypeptides (γ-glutamyl-cysteinyl)n-glycine in selected cadmium-resistant tomato cells. J Biol Chem 261: 13879–13882 [PubMed] [Google Scholar]
- Sun Q, Wang XR, Ding SM, Yuan XF (2005. a) Effects of exogenous organic chelators on phytochelatin production and its relationship with cadmium toxicity in wheat (Triticum aestivum L.) under cadmium stress. Chemosphere 60: 22–31 [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang XR, Ding SM, Yuan XF (2005. b) Effects of interactions between cadmium and zinc on phytochelatin and glutathione production in wheat (Triticum aestivum L.). Environ Toxicol 20: 195–201 [DOI] [PubMed] [Google Scholar]
- Thornalley PJ (1990. a) The glyoxalase system: towards functional characterization and a role in disease processes. In J Via, ed, CRC Handbook on Glutathione Metabolism. CRC Press, Boca Raton, FL, pp 135–144
- Thornalley PJ (1990. b) The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 269: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumann J, Grill E, Winnacker EL, Zenk MH (1991) Reactivation of metal-requiring apoenzymes by phytochelatin-metal complexes. FEBS Lett 284: 66–69 [DOI] [PubMed] [Google Scholar]
- Tsuji N, Hirayanagi N, Okada M, Miyasaka H, Hirata K, Zenk MH, Miyamoto K (2002) Enhancement of tolerance to heavy metals and oxidative stress in Dunaliella tertiolecta by Zn-induced phytochelatin synthesis. Biochem Biophys Res Commun 293: 653–659 [DOI] [PubMed] [Google Scholar]
- Van der Zaal BJ, Neuteboom LW, Pinas JE, Chardonnens AN, Schat H, Verkleij JA, Hooykaas PJ (1999) Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol 119: 1047–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu Y, Rea PA (2000) Mechanism of heavy metal ion activation of phytochelatin (PC) synthase. J Biol Chem 275: 31451–31459 [DOI] [PubMed] [Google Scholar]
- Veena, Reddy VS, Sopory SK (1999) Glyoxalase I from Brassica juncea: molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J 17: 385–395 [DOI] [PubMed] [Google Scholar]
- Veljovic-Jovanovic S, Noctor G, Foyer H (2002) Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol Biochem 40: 501–507 [Google Scholar]
- Verret F, Gravot A, Auroy P, Leonhardt N, David P, Nussaume L, Vavasseur A, Richaud P (2004) Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett 576: 306–312 [DOI] [PubMed] [Google Scholar]
- Weckx JEJ, Clijster HMM (1997) Zn phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol Biochem 35: 405–410 [Google Scholar]
- Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10: 1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2005. a) Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun 337: 61–67 [DOI] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005. b) Methylglyoxal detoxification by glyoxalase system: a survival strategy during environmental stresses. Physiol Mol Biol Plants 11: 1–11 [Google Scholar]
- Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005. c) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett 579: 6265–6271 [DOI] [PubMed] [Google Scholar]
- Zarcinas BA, Pongsakul P, McLaughlin MJ, Cozens G (2004) Heavy metals in soils and crops in Southeast Asia. 2. Thailand. Environ Geochem Health 26: 359–371 [DOI] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19: 765–768 [DOI] [PubMed] [Google Scholar]
- Zhang J, Kirkham MB (1996) Enzymatic responses of the ascorbate-glutathione cycle to drought in sorghum and sunflower plants. Plant Sci 113: 139–147 [Google Scholar]
- Zhu YL, Pilon-Smits EA, Tarun AS, Weber SU, Jouanin L, Terry N (1999. a) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol 121: 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smits E, Jouanin L, Terry N (1999. b) Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol 119: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]