Abstract
Inositol 1,4,5-trisphosphate (InsP3) has been implicated in the early signaling events of plants linking gravity sensing to the initiation of the gravitropic response. However, at present, the contribution of the phosphoinositide signaling pathway in plant gravitropism is not well understood. To delineate the role of InsP3 in plant gravitropism, we generated Arabidopsis (Arabidopsis thaliana) plants constitutively expressing the human type I inositol polyphosphate 5-phosphatase (InsP 5-ptase), an enzyme that specifically hydrolyzes InsP3. The transgenic plants show no significant differences in growth and life cycle compared to wild-type plants, although basal InsP3 levels are reduced by greater than 90% compared to wild-type plants. With gravistimulation, InsP3 levels in inflorescence stems of transgenic plants show no detectable change, whereas in wild-type plant inflorescences, InsP3 levels increase approximately 3-fold within the first 5 to 15 min of gravistimulation, preceding visible bending. Furthermore, gravitropic bending of the roots, hypocotyls, and inflorescence stems of the InsP 5-ptase transgenic plants is reduced by approximately 30% compared with the wild type. Additionally, the cold memory response of the transgenic plants is attenuated, indicating that InsP3 contributes to gravisignaling in the cold. The transgenic roots were shown to have altered calcium sensitivity in controlling gravitropic response, a reduction in basipetal indole-3-acetic acid transport, and a delay in the asymmetric auxin-induced β-glucuronidase expression with gravistimulation as compared to the controls. The compromised gravitropic response in all the major axes of growth in the transgenic Arabidopsis plants reveals a universal role for InsP3 in the gravity signal transduction cascade of plants.
Both gravity and light direct plant growth so that shoots grow upward for efficient photosynthesis and gas exchange and roots grow downward into the soil for anchorage and for water and mineral uptake (Hangarter, 1997). When the orientation of a plant is altered with respect to the gravity vector (i.e. when a plant is blown over by wind or rain), the shoots and roots exhibit differential growth resulting in upward and downward curvature, respectively, which returns the plant to a vertical orientation.
Gravitropism is a universal plant response. All major axes of plant growth, including roots, hypocotyls, coleoptiles, and inflorescence stems, are capable of a gravitropic response, despite differences in their basic structural organization (Tasaka et al., 1999). The presence of mutants defective in the gravity response of either the root or shoot suggests that the mechanisms of gravitropism in roots and shoots have both common and separate steps (Lomax, 1997; Tasaka et al., 1999). Nevertheless, the overall process of gravitropism is well conserved and consists of three major phases—gravity sensing, information transfer from sensing to responding cells (signal transduction and amplification), and the growth response (for review, see Perbal and Driss-Ecole, 2003; Morita and Tasaka, 2004).
One of the primary events in gravity sensing in higher plants involves the displacement or settling of dense starch-containing amyloplasts, which can occur in seconds to minutes depending on the plant tissue (Sack, 1991, 1997). Amyloplasts are found in specialized cells, such as the starch parenchyma surrounding the vascular tissue in plant shoots and grass pulvini, and in the columella of the root cap (Sack, 1991; Chen et al., 1999; Rosen et al., 1999; Perrin et al., 2005). Upon reorientation of a plant shoot or root, the settling of amyloplasts, or the pressure exerted by protoplast settlement (Staves, 1997), is thought to trigger intra- and intercellular signaling, initiating downstream metabolic changes, including a lateral redistribution of auxin, leading to an asymmetric growth response (Lomax et al., 1995; Muday and DeLong, 2001).
Over the past few years, cumulative evidence has supported the role of amyloplasts in gravity sensing (Kiss, 2000; Blancaflor and Masson, 2003), although there is some debate about how the displacement of amyloplasts might trigger a signaling cascade. Some models propose that the amyloplasts are tethered by the actin cytoskeleton, and even small local perturbations in amyloplast movement could disrupt the actin network. This disruption may be transmitted to the plasma membrane (PM) and other endomembranes activating mechanosensitive channels (Volkmann and Baluska, 1999; Yoder et al., 2001) The role of the actin cytoskeleton in gravitropism appears to be more complex, as shown by recent reports by Yamamoto and Kiss (2002) and Hou et al. (2003), and may act to down-regulate gravisignaling (Hou et al., 2004).
Many signaling molecules and second messengers, such as Ca2+ (Plieth and Trewavas, 2002), inositol 1,4,5-trisphosphate (InsP3; Perera et al., 1999, 2001), ionic gradients (Monshausen et al., 1996), and pH (Scott and Allen, 1999; Fasano et al., 2001; Johannes et al., 2001), have been implicated in linking gravity sensing to the initiation of a differential growth response. Very rapid gravity-specific changes in transcript abundance have been documented in gravistimulated root tips of Arabidopsis (Arabidopsis thaliana; Kimbrough et al., 2004) and in whole seedlings (Moseyko et al., 2002). Additionally, Heilmann et al. (2001) have demonstrated that specific transcripts are selectively recruited into polysomes of maize (Zea mays) pulvini within 15 to 30 min of gravistimulation, and Young and Masson (2004) have shown that rapid changes in protein distribution occur within 12 to 30 min of gravistimulation in Arabidopsis roots. These results indicate that transcriptional and translational regulation is also involved in the early signaling events. However, at present, the importance of the different signaling events and their possible interactions is not well understood. It is also unclear whether these multiple components represent parallel or convergent signaling pathways.
The gravitropic response requires differential growth across the gravity-stimulated tissue. As first outlined in the Cholodny-Went hypothesis, lateral gradients of the plant auxin indole-3-acetic acid (IAA) have been suggested to induce this differential growth. More recently, asymmetric distribution of either endogenous free IAA or exogenously applied radiolabeled IAA across gravity-stimulated roots and shoots has been demonstrated in several plant systems, including gravity-stimulated coleoptiles (Parker and Briggs, 1990; Philippar et al., 1999), pulvini tissues (Long et al., 2002), and roots (Young et al., 1990), and has been shown to precede gravitropic bending (Parker and Briggs, 1990) Gradients of IAA have also been indirectly visualized using auxin-responsive promoters to drive the expression of reporter genes, such as β-glucuronidase (GUS) in shoots (Li et al., 1991) and in roots (Rashotte et al., 2001; Buer and Muday, 2004; Hou et al., 2004). A role for auxin transport in the gravitropic response is further supported by the fact that several auxin transport mutants show altered gravitropic responses and the application of IAA transport inhibitors to wild-type roots can abolish the gravitropic response (Muday, 2001; Blancaflor and Masson, 2003).
In previous work, we characterized the involvement of phosphoinositide (PI)-based signaling in the gravitropic response of cereal grass stems. Biphasic changes in InsP3 were detected with gravistimulation of maize and oat (Avena sativa) pulvini (Perera et al., 1999, 2001). These included rapid and transient fluctuations in InsP3 within seconds of gravistimulation in both upper and lower pulvini halves, followed by a long-term sustained increase in InsP3 only in lower pulvini halves. The second increase in InsP3 correlated with the bending response (Perera et al., 1999). Blocking the differential InsP3 gradient with a chemical inhibitor of phospholipase C (PLC) activity (U73122) attenuated the gravitropic response. Furthermore, the biphasic changes in InsP3 only occurred in the sensing and responding tissue (pulvinus) and were not detected in the nonelongating internodal regions of the stem (Perera et al., 2001). These results indicated that InsP3 plays a critical role in gravity signaling in cereal grass pulvini. However, it was not known whether InsP3 played a signaling role in both monocot and dicot plant gravitropism or whether the involvement of InsP3 was unique to the gravitropic response of the pulvinus system.
To test the hypothesis that InsP3 is a universal component involved in establishing the differential growth response in plants, we undertook a molecular approach to alter InsP3 signaling in Arabidopsis. We have generated transgenic Arabidopsis plants expressing the human type I inositol polyphosphate 5-phosphatase (InsP 5-ptase), an enzyme that specifically hydrolyzes the soluble inositol phosphates InsP3 and InsP4 (Laxminarayan et al., 1993). We chose a heterologous enzyme for several reasons. First, the animal type I InsP 5-ptase is well characterized and specifically hydrolyzes InsP3 and not the inositol phospholipids. Arabidopsis contains a family of related inositol 5-phosphatases known as At5PTases (Berdy et al., 2001), which have been shown to have roles in diverse aspects of plant growth and development, including vascular patterning in leaves and cotyledons (Carland and Nelson, 2004; Lin et al., 2005), secondary cell wall and fiber cell development (Zhong et al., 2004), and abscisic acid (ABA)-regulated processes (Sanchez and Chua, 2001; Burnette et al., 2003). However, there does not appear to be a homolog of the animal type I enzyme based on biochemical activity and sequence similarity. Several of the many plant inositol 5-phosphatases characterized to date can hydrolyze both lipid substrates and soluble inositol phosphates and are not specific for InsP3 hydrolysis (Berdy et al., 2001; Ercetin et al., 2004). Finally, the animal type I InsP 5-ptase is associated with the PM and therefore should preferentially dampen InsP3 signals generated at the PM.
We tested the feasibility of this approach by first generating transgenic tobacco (Nicotiana tabacum) cells constitutively expressing the human type I InsP 5-ptase (Perera et al., 2002). The transgenic tobacco cells had normal morphology and growth compared to wild type; however, basal InsP3 and phosphatidylinositol 4,5-bisphosphate (PtdInsP2) levels were greatly reduced in the transgenic cells, resulting from the increased turnover of PtdInsP2 and an increased flux through the PI pathway.
In this article, we characterize the transgenic Arabidopsis lines expressing InsP 5-ptase. The plants show no morphological differences compared to wild-type plants under normal growth conditions. Significantly, although vertical growth rates were comparable between wild-type and transgenic seedlings, the reorientation of transgenic roots and hypocotyls in response to gravistimulation was reduced by 30% compared to wild-type seedlings. Furthermore, the gravitropic response of inflorescence stems of transgenic plants was also attenuated compared to wild type. InsP3 levels were found to increase in wild-type inflorescence stems upon gravistimulation, preceding visible bending, consistent with our previous results on cereal grass pulvini. No increases in InsP3 with gravistimulation were detectable in the transgenic plants. In the transgenic roots, basipetal auxin transport was reduced and the development of the lateral auxin asymmetry on the lower side of gravistimulated roots was delayed. Our results indicate that dampening of the InsP3 signal attenuates the gravitropic response and suggest that InsP3-mediated signaling is a necessary component for the full gravitropic response in a dicot system. Additionally, since the PI pathway is implicated in plant responses to many different stresses (Stevenson et al., 2000; Meijer and Munnik, 2003), we anticipate that the transgenic plants will be a useful model system to evaluate the involvement of InsP3-mediated signaling in plant responses to many different abiotic and biotic stresses.
RESULTS
Generation of Transgenic Lines Expressing InsP 5-ptase
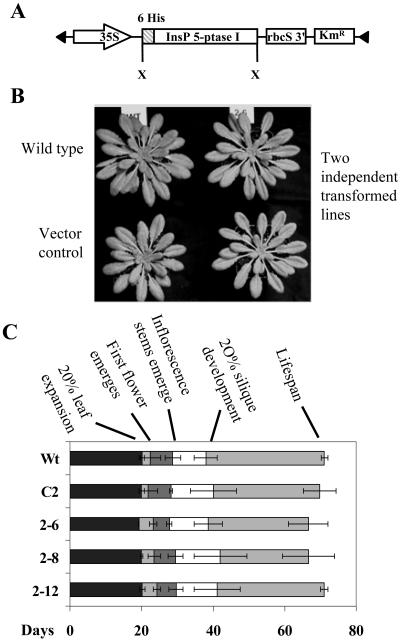
Arabidopsis (Columbia-0) was transformed by Agrobacterium-mediated transformation using vacuum infiltration with the construct shown in Figure 1A. The coding region of the human type I InsP 5-ptase cDNA with a 6-His tag at the N terminus was inserted downstream of the cauliflower mosaic virus 35S promoter for constitutive expression. Four independent homozygous transformed lines were isolated by screening on selective media. The stable integration of a single copy of the transgene was verified by a Southern blot (data not shown). All growth and bending experiments were carried out using T4 generation plants.
Figure 1.
Transgenic plants expressing InsP 5-ptase show no obvious morphological differences compared to the wild type. A, Construct used to transform Arabidopsis plants. 35S, Cauliflower mosaic virus 35S promoter; 6 His, 6-His residues; X, XbaI restriction enzyme sites; rbcS 3′, 3′-untranslated region from the Rubisco small subunit gene; KmR, gene for kanamycin resistance. B, Four-week-old Arabidopsis plants were grown in the phytotron on a short-day cycle (8 h light/16 h dark, 22°C). C, The growth stages of the life cycle were compared between wild type (Wt), vector control (C2), and three transgenic lines. The time (in days) taken for leaves to reach 20% of maximal expansion, for the inflorescence stem and first flower to emerge, 20% silique development, and total life span are shown in the figure. Wt, Wild type; C2, vector control; 2-6, 2-8, and 2-12, three independent InsP 5-ptase transgenic lines.
As seen in Figure 1, B and C, the transgenic plants grow normally and do not exhibit any significant morphological differences compared with the wild-type plants. The growth of the plants was monitored at various developmental stages from seed to seed as described by Boyes et al. (2001). Several vegetative and reproductive features, along with the timing of growth stages and plant yield, were compared between the wild type, vector control, and three independent transgenic lines (Fig. 1C; Table I). No significant differences were observed in any of the parameters measured, indicating the absence of pleiotropic effects of the transgene insertion.
Table I.
Growth-stage measurementsa
| Plant Line | Total No. of Rosette Leavesb | Length of Longest Leafc | Area of Rosetted | Length of Primary Stalke | Seed Production |
|---|---|---|---|---|---|
| mm | cm2 | cm | mg | ||
| Wild type | 8.8 ± 1.8 | 33.7 ± 0.3 | 32.7 ± 3.8 | 41.3 ± 0.7 | 43.8 ± 1.8 |
| C2 | 10.2 ± 2.0 | 33.5 ± 1.1 | 32.7 ± 2.5 | 39.4 ± 2.4 | 34.6 ± 6.7 |
| 2-6 | 10.1 ± 1.6 | 34.6 ± 1.9 | 33.5 ± 5.0 | 35.6 ± 1.2 | 41.7 ± 4.3 |
| 2-8 | 8.9 ± 1.2 | 31.8 ± 1.0 | 28.6 ± 2.0 | 36.2 ± 3.6 | 36.6 ± 10.3 |
| 2-12 | 9.5 ± 1.1 | 31.7 ± 0.1 | 28.6 ± 3.5 | 37.3 ± 2.4 | 36.3 ± 9.2 |
The values are the average of two independent experiments ±sd.
Data taken on day 25.
Data taken on day 33.
Data taken on day 39.
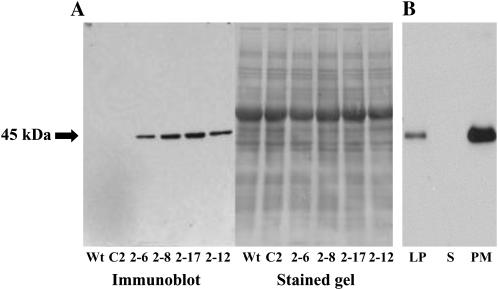
The expression of the transgene was examined by immunoblot of total cellular protein extracts from 2-week-old transgenic and control plants. The InsP 5-ptase gene product is produced in all the transgenic lines tested (Fig. 2A). In addition, the transgene is expressed in all tissues tested, including roots, hypocotyls, mature leaves, and inflorescence stems of the transgenic plants (data not shown). Further fractionation of the cellular proteins by two-phase partitioning into soluble, lower phase, and PM-enriched fractions shows that the InsP 5-ptase protein is primarily associated with the PM fraction (Fig. 2B). We also monitored InsP 5-ptase enzyme activity in the transgenic lines. The specific activity was quantified by measuring InsP3 hydrolysis using the soluble and PM fractions as described previously (Perera et al., 2002). The specific activity in the PM-enriched fraction of transgenic plants is approximately 75-fold higher as compared to the PM fraction of wild-type plants (432.5 pmol min−1 μg−1 protein for transgenic PM and 5.65 pmol min−1 μg−1 protein for wild-type PM). In addition, the PM fraction of the transgenic plants has approximately 5-fold higher specific activity than the soluble fractions from the same plants (data not shown). The localization of InsP 5-ptase protein and enzyme activity are consistent with the PM localization of the InsP 5-ptase protein in animal cells (De Smedt et al., 1996).
Figure 2.
InsP 5-ptase protein is produced in the transgenic plants and is associated with the PM fraction. A, Crude protein extracts (20 μg protein/lane) from 2-week-old wild type (Wt), vector control (C2), and four independent transgenic lines (2-6, 2-8, 2-17, and 2-12) were analyzed by gel electrophoresis and immunoblotting using an antibody that recognizes the 6-His tag. B, Protein extract from the transgenic line 2-8 was further fractionated by high-speed centrifugation and two-phase partitioning to obtain a PM-enriched fraction (PM), lower phase (LP), and soluble (S) fraction (1 μg protein/lane).
Consistent with stable constitutive gene expression and elevated enzyme activity, basal InsP3 levels were found to be drastically reduced in the transgenic lines compared with the wild type and vector control (Table II). InsP3 levels in roots and hypocotyls of 7-d-old seedlings were measured using the receptor-binding assay (Perera et al., 2002). In wild-type seedlings, InsP3 content is typically in the range of 400 to 600 pmol g−1 fresh weight and is reduced by approximately 95% in the transgenic lines to 20 to 30 pmol g −1 fresh weight.
Table II.
Basal InsP3 content is reduced by approximately 95% in the transgenic lines
InsP3 levels were measured in 7-d-old seedlings grown in the dark. Roots and hypocotyls were harvested separately and pooled from 50 individual seedlings. Wild-type levels are typically in the range of 400 to 600 pmol g−1 fresh weight. The InsP 5-ptase transgenic and vector control InsP3 values are presented as a percentage of the wild type.
| Plant Line
|
InsP3 Contenta
|
|
|---|---|---|
| Root | Hypocotyl | |
| % wild type | ||
| Wild type | 100 | 100 |
| C2 | 107 | 106 |
| 2-6 | 3 | 3 |
| 2-8 | 2 | 2 |
| 2-12 | 9 | 5 |
The data presented are the average from two independent experiments.
Growth and Gravitropic Responses of Roots and Hypocotyls
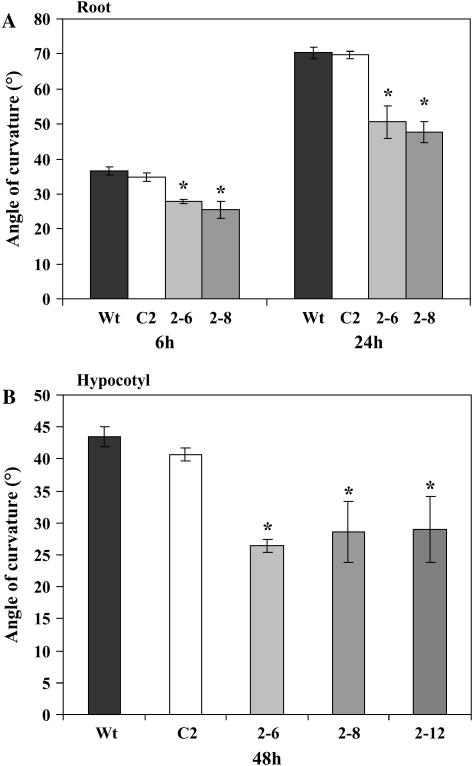
To determine whether the transgenic plants with severely reduced InsP3 levels would show an altered response to gravity, the growth and gravitropic responses were monitored in roots of 4-d-old seedlings. Seedlings were grown vertically on agar plates in the dark and vertical elongation growth was monitored over a 24-h period. There were no significant differences in vertical growth rates between wild-type, vector control, and transgenic lines expressing InsP 5-ptase (Table III). In addition, staining with I2 has shown that starch accumulation in amyloplasts of root cap columella cells is normal in both wild-type and transgenic roots (data not shown). To monitor the gravitropic response, plates were turned by 90° in the dark and images were recorded over a period of 24 h. Bending was first visible after approximately 2 h and, by 6 h of gravistimulation, wild-type and vector control plants exhibit approximately 30° to 35° curvature (Fig. 3A). After 24 h, wild-type and vector control plants show approximately 70° curvature. In contrast, the transgenic lines consistently have a reduced degree of curvature at both 6 and 24 h of gravistimulation. Compared to wild-type roots, the gravitropic response of the transgenic roots is reduced by approximately 30%.
Table III.
The vertical growth of roots and hypocotyls of InsP 5-ptase transgenic plants is similar to wild type
The growth was monitored over a period of 24 h for roots and 48 h for hypocotyls.
| Plant Line
|
Lengtha
|
|
|---|---|---|
| Root | Hypocotyl | |
| mm | ||
| Wild type | 3.5 ± 0.4 | 6.8 ± 0.7 |
| C2 | 3.5 ± 0.6 | 7.1 ± 0.7 |
| 2-6 | 3.4 ± 0.3 | 6.6 ± 1.0 |
| 2-8 | 3.4 ± 0.6 | 6.8 ± 1.0 |
| 2-12 | 3.3 ± 0.5 | 6.5 ± 0.5 |
The data presented are the average of three independent experiments ±sd (n = 25 seedlings/experiment for roots and n = 50 seedlings/experiment for hypocotyls).
Figure 3.
Roots and hypocotyls of transgenic plants show a reduced gravitropic response. A, Root gravitropic bending of wild type (Wt), vector control (C2), and two InsP 5-ptase transgenic lines (2-6 and 2-8) was monitored over a period of 24 h. Seedlings were grown on vertically oriented plates for 3 d in the light followed by 24 h in the dark. Plates were then rotated by 90° in the dark, and bending was monitored at 6 and 24 h after turning. Bending is plotted as the average from four independent experiments ±se (n = 50 roots/experiment). B, Hypocotyl bending of wild type (Wt), vector control (C2), and three independent transgenic lines (2-6, 2-8, and 2-12) was monitored over 48 h. Seedlings were grown on vertically oriented plates for 3 d in the light followed by 24 h in dark. Plates were then rotated by 90° in the dark and bending monitored 48 h after turning. Bending is plotted as the average from three independent experiments ±se (n = 40 hypocotyls/experiment). The asterisks indicate statistically significant differences between the transgenic lines and the wild type (P < 0.05 using Student's t test).
To examine the early stages of the root gravitropic response in more detail, we analyzed curvature using Multi-ADAPT software developed by Ishikawa and Evans (1997). This program provides high spatial and temporal resolution of the gravitropic bending response of a single root by measuring both root tip angle and elongation rates on the upper and lower flanks of the root. Upon gravistimulation, wild-type roots exhibit a lag period of approximately 120 min prior to initiation of curvature (Table IV). This is in good agreement with values previously published (Buer and Muday, 2004). The transgenic roots appear to have a much greater variability in initiation of bending (note the se for this response is twice as large as for the wild type). The transgenic roots also have a slightly longer lag compared with the wild type, although this difference is not statistically significant due to the variability in the response of the transgenic roots. The most pronounced difference between the wild-type and transgenic roots is in the rate of curvature after bending is initiated. Transgenic roots have an approximately 45% reduction in rate compared to wild type, which would help explain the reduced gravitropic response observed in the roots.
Table IV.
Transgenic roots show a slower rate of curvature after initiation of bending
The kinetics of root bending was measured using the Multi-ADAPT softwarea.
| Plant Line | Initiation of Curvature | Rate of Curvature after Initiation | Vertical Elongation Rateb |
|---|---|---|---|
| min | degree min−1 | μm min−1 | |
| Wild type | 124.6 ± 9.2 | 0.165 ± 0.014 | 4.3 ± 0.7 |
| 2-8 | 155.4 ± 17.1 | 0.087 ± 0.014 | 5.3 ± 1.7 |
| P valuec | 0.1168 | 0.0031 | 0.5609 |
The value is the average of six individual roots ±se.
The root elongation rates were measured in 50-μm segments from the root tip.
Wild-type and 2-8 values were compared by a two-tailed Student's t test and the P values are reported.
We also evaluated growth and the gravitropic response of hypocotyls of young seedlings. Arabidopsis seedlings were grown vertically on agar plates in the dark and hypocotyl elongation was measured over a 48-h period. No significant differences were observed in vertical growth of hypocotyls of transgenic and wild-type plants (Table III). In contrast, the gravitropic bending response was reduced in hypocotyls of 4-d-old transgenic seedlings compared to wild type (Fig. 3B). Three independent transgenic lines (2-6, 2-8, and 2-12) showed an approximately 30% reduction in angle of curvature compared with the wild-type and vector control lines. In all plants, hypocotyl reorientation in response to gravistimulation was slower than the root response. After 48 h of gravity stimulation, roots had mostly returned to a vertical orientation. Hypocotyls reached a final bending angle of approximately 45° after 48 h.
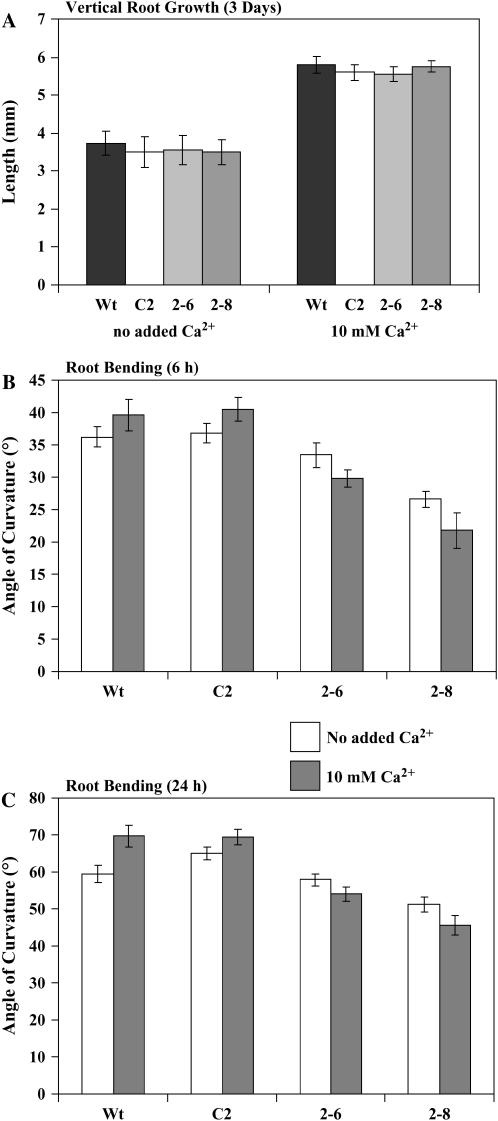
Although changes in InsP3 in plants are associated with various stimuli and stresses, the downstream consequences of the InsP3 changes are virtually unknown. InsP3 is known to trigger the release of Ca2+ from intracellular stores such as the vacuole and endoplasmic reticulum (for review, see Sanders et al., 1999). Furthermore, the generation of InsP3 may require Ca2+ because all known plant PLC enzymes are regulated by Ca2+ (Hunt et al., 2004). Therefore, there seems to be an interdependence and close connection between InsP3 and Ca2+. To investigate the relationship between Ca2+ and InsP3 further, we analyzed the growth and gravitropic responses of roots and hypocotyls under different Ca2+ concentrations. Murashige and Skoog (MS) medium was made by omitting the Ca2+, which will be referred to as “no added Ca2+.” This medium may contain trace amounts of Ca2+ as contaminants in the medium salts and the agar. The highest Ca2+ concentration tested was 10 mm; standard MS medium contains 3 mm Ca2+. The vertical growth of wild-type and transgenic seedlings was first monitored on plates containing the medium described above. As seen with the seedlings grown on regular MS medium, there were no differences in vertical root elongation between wild-type and transgenic seedlings over a 3-d period on both the no added Ca2+ and 10 mM Ca2+ (Fig. 4A). All of the roots (wild type and transgenic) showed decreased growth on the medium with no added Ca2+ (similar growth over a 3-d period as was seen in 24 h on regular MS medium; Table III). All roots (wild type and transgenic) showed increased growth on the 10 mm Ca2+ medium compared to the no added Ca2+medium, suggesting that, in the range of 3 to 10 mm, Ca2+ promoted root growth. We then monitored root bending (Fig. 4, B and C). There was a statistically significant difference in root bending of all the lines examined at the two different Ca2+ concentrations (10 mm or no added Ca2+). With both treatments, the transgenic roots exhibited less bending than the wild type and vector control, consistent with our previous results on regular MS medium. However, when the extent of bending was compared between these two different Ca2+ concentrations, the results were quite intriguing. While root bending was enhanced at 10 mm Ca2+ for the wild type and vector control, the transgenic roots showed the opposite effect and exhibited less bending at 10 mm Ca2+ than on the no added Ca2+ medium. This difference was noticeable at both the 6- and 24-h time points (Fig. 4, B and C). After 48 h, however, the transgenic roots on the 10 mm Ca2+ medium were not significantly different from the roots grown on medium with no added Ca2+. This suggests that the high Ca2+ decreases the reorientation response of the transgenic roots most dramatically within the first few hours.
Figure 4.
The gravitropic response of roots of wild-type and transgenic plants are different on high and low Ca2+ concentrations. A, Vertical growth of 4-d-old wild type (Wt), vector control (C2), and two independent InsP 5-ptase transgenic lines (2-6 and 2-8) was monitored after 24 h in the dark on either medium containing no added Ca2+ or medium containing 10 mm Ca2+. B and C, Gravitropic bending of 4-d-old wild type (Wt), vector control (C2), and two independent transgenic lines (2-6 and 2-8) was monitored after 6 (B) and 24 h (C) of gravistimulation in the dark on either medium containing no added Ca2+ (white bars) or medium containing 10 mm Ca2+ (gray bars). Bending is plotted as the average from two independent experiments ±sd (n = 40 roots/experiment). There were statistically significant differences in the bending of each line between the no added Ca2+ versus the 10 mm Ca2+ medium (P < 0.1 using a Student's t test).
Similar results were obtained with hypocotyl growth and gravitropism on altered Ca2+ medium. Hypocotyls of 4-d-old wild-type and transgenic seedlings grew similarly on both no added Ca2+ and 10 mm Ca2+ with greater elongation at the 10 mm Ca2+. When hypocotyl bending was monitored, wild-type and vector control seedlings showed greater bending at 10 mm Ca2+ than on the no added Ca2+ medium, whereas the transgenic hypocotyls showed more bending on the no added Ca2+medium rather than 10 mm Ca2+ (data not shown).
Basipetal Auxin Transport in Roots
Basipetal transport of auxin from the root tip has been shown to be important for the gravitropic response of Arabidopsis roots (Rashotte et al., 2000). Therefore, we compared basipetal auxin transport in vertically grown root tips of wild-type and transgenic seedlings. In three independent experiments, there was a small, but significant, reduction in basipetal transport in the transgenic line 2-8 compared with the wild type (Table V). In both wild-type and transgenic lines, basipetal transport was similarly inhibited by naphthylphthalamic acid (NPA), suggesting that there are no changes in regulation of auxin transport through its NPA-dependent regulatory mechanisms. Rather, these differences are consistent with a reduction in IAA transport capacity. These results suggest that InsP3-mediated signaling affects basipetal auxin transport. The reduction in basipetal transport could contribute to the reduced gravitropic response in the transgenic roots.
Table V.
Basipetal IAA transport is reduced in the transgenic roots
| Plant Line
|
Basipetal [3H]IAA Transporta
|
Percentage of Inhibition by NPA
|
|
|---|---|---|---|
| −NPA | +NPA | ||
| fmol | |||
| Wild type | 4.2 ± 0.4 | 2.4 ± 0.2 | 57% |
| 2-8 | 3.1 ± 0.2 | 1.6 ± 0.1 | 51% |
| P valueb | 0.010 | 0.00045 | |
The data presented are the average of two independent experiments ±sd (n = 10 roots/treatment/experiment).
Wild-type and 2-8 values were compared by a two-tailed Student's t test and the P values are reported.
Asymmetric Auxin-Induced GUS Expression as a Result of Gravistimulation
The redistribution of auxin has been shown to precede differential growth and the gravity response of plant shoots and roots (Parker and Briggs, 1990; Young et al., 1990). More recently, the lateral redistribution of auxin in gravistimulated roots has been visualized indirectly using the auxin-responsive reporter construct DR5-GUS, as described previously (Rashotte et al., 2001; Buer and Muday, 2004; Hou et al., 2004). We therefore generated transgenic InsP 5-ptase lines expressing DR5-GUS (DR5-GUS InsP 5-ptase). We first compared GUS staining in vertically grown roots. Both control DR5-GUS and DR5-GUS InsP 5-ptase roots showed similar intense staining in the root cap region. We next compared the development of asymmetric auxin-induced GUS expression in response to gravistimulation in the DR5-GUS InsP 5-ptase roots and the control DR5-GUS line. Four-day-old light-grown seedlings were gravistimulated in the dark for 4 and 6 h and stained overnight for visualization of GUS activity. GUS activity (as evidenced by strong blue staining) was visible in the root tips of gravistimulated seedlings in both the control DR5-GUS and DR5-GUS InsP 5-ptase lines. In addition, several roots of both DR5-GUS and DR5-GUS InsP 5-ptase lines also showed a streak of blue staining extending from the tip on the lower side of the root (see Supplemental Fig. 1). This asymmetric expression of DR5-GUS was highly correlated with the angle of bending of the roots. In all roots where a gradient was observed, the angle of bending was between 40° and 45°.
Most significantly, the frequency of the differential GUS staining observed at the 4-h time point was higher in the control DR5-GUS line compared with the DR5-GUS InsP 5-ptase line (Table VI), and the average angle of bending in the control was also higher than the InsP 5-ptase roots (Table VI). By 6 h of gravistimulation, DR5-GUS InsP 5-ptase lines began to catch up. The average angle of bending of the transgenic DR5-GUS InsP 5-ptase lines at 6 h was approximately 44° and more roots showed the asymmetric GUS staining. (Note that for these experiments the seedlings were not pretreated for 24 h with dark before gravistimulation and therefore, on average, exhibit a faster bending response than the wild-type and transgenic root responses shown in Fig. 3.) The data show that the frequency of the asymmetric GUS expression correlates well with the angle of bending and that the development of the asymmetric auxin-induced GUS expression is delayed in the InsP 5-ptase transgenic roots consistent with their delayed bending response.
Table VI.
The development of the asymmetric auxin-induced GUS expression is delayed in the InsP 5-ptase transgenic roots compared with the DR5-GUS control
Histochemical assays and root bending were carried out on DR5-GUS and DR5-GUS InsP 5-ptase roots after 4 and 6 h of bending.
| Time of Bending Lines
|
4 h
|
6 h
|
||
|---|---|---|---|---|
| DR5-GUS | DR5-GUS InsP 5-ptase | DR5-GUS | DR5-GUS InsP 5-ptase | |
| Percent of roots showing gradienta | 66% | 39% | 34% | 61% |
| Average angle of bendingb | 41.9 ± 3.8 | 34.7 ± 2.1 | 52.1 ± 2.9 | 44.0 ± 2.0 |
The data presented are the average of two independent experiments (n = 100 roots/line/experiment). The sd was 1% and 6% for the DR5-GUS and DR5-GUS InsP 5-ptase lines, respectively.
The data presented are the average of three independent experiments ±sd (n = 12 roots/line/experiment).
Bending Response of Inflorescence Stems
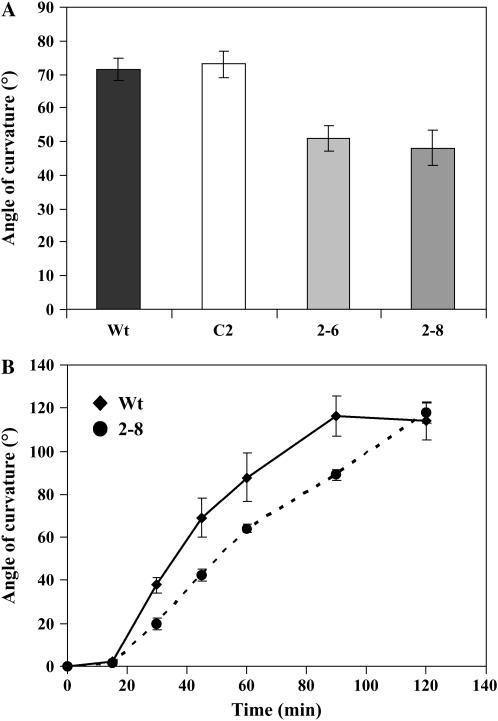
Inflorescence stems of Arabidopsis plants exhibit a fast and robust gravitropic response. We compared the gravitropic response of primary inflorescence stems of 6-week-old wild-type and InsP 5-ptase transgenic plants, which were usually 6 to 9 cm tall and did not contain any developed siliques. After 45 min of gravistimulation, inflorescence stems of wild-type and vector control plants showed a greater degree of bending compared to transgenic plants (approximately 75° curvature in wild type and vector control and approximately 50°curvature in transgenic plants; Fig. 5A). The kinetics of inflorescence bending were monitored over a 2-h period (Fig. 5B). As can be seen in the graph, no significant bending takes place until approximately 15 to 20 min. Between 20 and 60 min, the wild-type stems reach and overshoot the vertical (90°) mark. Wild-type bending peaks around 90 min and starts to return to vertical. The transgenic stems were slower to bend and responded to a lesser degree than the wild type. By about 120 min, the bending is comparable in all lines.
Figure 5.
Inflorescence stems of transgenic plants show a reduced gravitropic bending. A, Gravitropic bending was measured in inflorescence stems of 6-week-old Arabidopsis plants. Plants were first placed upright in the dark for 1 to 2 h and then placed horizontally in the dark. Bending was measured after 45 min. Data plotted are the average of five independent experiments ±se (n = 25/experiment). B, Gravitropic bending of the inflorescence stems was monitored every 15 min for 2 h. The graph shows the average of six representative wild-type and transgenic inflorescence stems ±se. The experiment was repeated twice with similar results.
InsP3 Changes with Gravistimulation of Inflorescence Stems
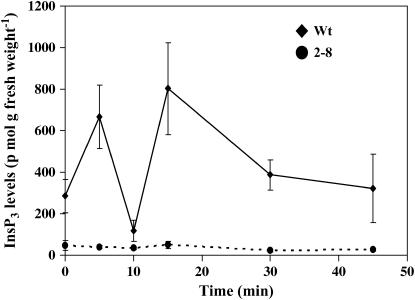
InsP3 levels were monitored during the first 45 min of gravistimulation in inflorescence stems. For each time point, approximately 20 to 30 stems were pooled to obtain sufficient material for the assays. In wild-type inflorescence stems, InsP3 levels increase 2- to 3-fold over the first 5 min, followed by a second increase with a peak around 15 to 20 min after gravity stimulation (Fig. 6). This second InsP3 peak correlates well in timing with the initiation of the bending response. This is in good agreement with our previous results with gravistimulated maize and oat pulvini, where a sustained increase in InsP3 on the lower side of the pulvinus was detected preceding the bending response (Perera et al., 1999, 2001). After approximately 30 min, InsP3 levels return to basal values. The basal InsP3 levels in the transgenic inflorescence stems were reduced by >95% compared to the wild type, and there were no detectable changes in InsP3 in response to gravistimulation in the transgenic inflorescence stems. However, since the InsP3 levels in the transgenic plants are at the lower limit of detection, it is difficult to determine whether or not the slight fluctuations in InsP3 levels detected are representative of true increases in response to gravistimulation.
Figure 6.
Biphasic changes in InsP3 with gravistimulation of inflorescence stems. InsP3 levels were measured in inflorescence stems over a time course of gravistimulation. Data plotted are the average of four independent experiments ±se (n = 20–30 stems/time point/experiment).
Sensing and Early Signaling versus Response
When plants are gravistimulated in the cold, they do not respond to gravity; yet, when returned to room temperature, the plants are able to respond to the gravity stimulus received in the cold. In a series of elegant experiments, Fukaki et al. (1996a) demonstrated that inflorescence stems of Arabidopsis plants are able to retain the memory of cold gravistimulation for up to 60 min and respond to that stimulus when returned to room temperature. In previous work with oat pulvini, we showed that InsP3 levels increased in response to gravistimulation in the cold, although the seedlings did not bend until returned to room temperature (Perera et al., 2001). These results indicate that plants can sense the gravistimulus and initiate some signaling events in the cold, although downstream events, including the redistribution of auxin, are blocked at low temperature and the plants will not bend.
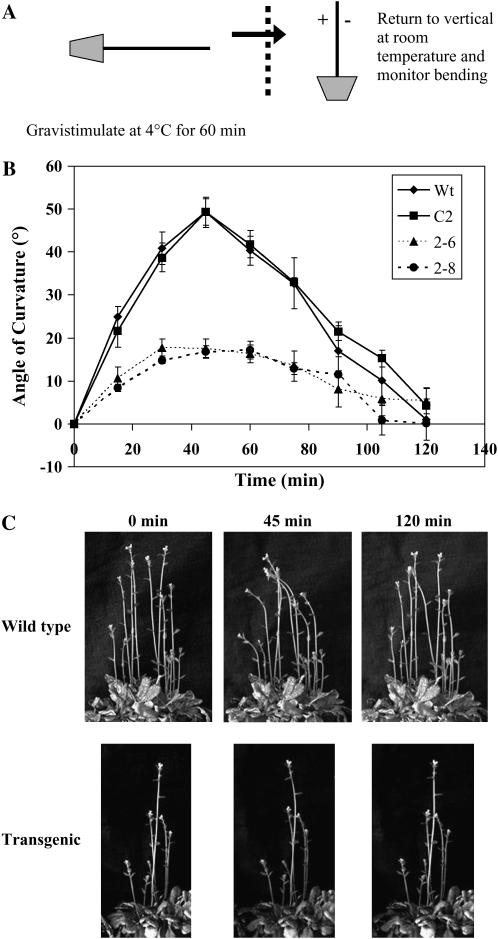
To determine whether the cold memory response of the transgenic plants would be impaired compared to wild type, inflorescence stems were gravistimulated at 4°C for 1 h, the plants were returned to vertical orientation in the dark at room temperature, and the bending response was recorded every 15 min for a period of 2 h (Fig. 7A). Both wild-type and vector control inflorescence stems exhibited a strong bending response, reaching a maximum of approximately 50° curvature at approximately 45 min of being returned to room temperature (Fig. 7, B and C). Between 45 and 90 min, the wild-type and vector control inflorescence stems returned to a vertical orientation. In comparison, the transgenic inflorescence stems exhibited a much attenuated or dampened response, with maximal curvature of approximately 10° to 20° (Fig. 7, B and C). These results indicate that InsP3 is a component of early gravity signaling. The much attenuated gravitropic response of the transgenic plants may be due to their inability to generate and sustain a 2- to 3-fold increase in InsP3 levels.
Figure 7.
Inflorescence bending after gravistimulation in the cold is attenuated in the transgenic plants. A, Arabidopsis plants containing inflorescence stems (6–9 cm long) were gravistimulated in the cold by placing the plants horizontally at 4°C in the dark for 1 h. Plants were returned to room temperature and placed vertically in the dark. B, Bending was monitored every 15 min for 2 h after being returned to room temperature. The graph shows the average of five representative inflorescence stems for each line ±se. The experiment was carried out four times with similar results. C, Representative wild-type and InsP 5-ptase transgenic inflorescence stems photographed immediately after being returned to vertical at room temperature (0 min) and after 45 and 120 min.
DISCUSSION
The PI pathway is implicated in plant responses to various biotic and abiotic stresses (Stevenson et al., 2000; Meijer and Munnik, 2003). Rapid changes in InsP3 have been documented in different plant systems with many different stimuli, including osmotic and cold shock, salinity, light, fungal elicitors, ABA, and gravity. However, it is still not well understood what downstream molecular targets are directly affected by the changes in InsP3 or how these changes lead to a physiological response. To delineate the role of InsP3 in plant responses to stress, we have generated transgenic Arabidopsis plants constitutively expressing the human type I InsP 5-ptase, an enzyme that specifically hydrolyzes InsP3 (Laxminarayan et al., 1993). The transgenic plants have attenuated InsP3 signaling and therefore are a model system to investigate InsP3-mediated responses. In this article, we test the hypothesis that InsP3 is an integral component of plant gravisignaling.
We first monitored expression of the transgene and basal InsP3 levels in the transgenic plants. The InsP 5-ptase transgene is stably expressed and the protein is detectable in all tissues tested. Furthermore, in all transgenic tissues tested, basal InsP3 levels were reduced by >90% compared with wild-type levels (Table II). Despite the drastic reduction in basal InsP3 levels, transgenic InsP 5-ptase plants showed no obvious morphological differences compared to wild-type plants under normal growth conditions. We have monitored the growth and life cycle of at least three independent lines and all parameters investigated were virtually indistinguishable from wild type (Figs. 1 and 2; Table I).
Of particular interest for the gravitropism studies, the InsP 5-ptase transgenic lines showed no defects in elongation growth of the roots or hypocotyls (Table III). However, significantly, the transgenic plants showed a reduced reorientation response upon gravistimulation. This is in contrast to many of the auxin response mutants with altered gravitropism, such as axr3 (Leyser et al., 1996) and axr4 (Hobbie and Estelle, 1995), which have an overall reduced growth phenotype compared to wild type. The data imply that the reorientation response is affected specifically in the InsP 5-ptase transgenic plants and that the increase in InsP3 is one component of gravisignaling. When the root-bending response was monitored more closely using Multi-ADAPT software, it revealed that the transgenic roots have a significantly slower rate of curvature after bending is initiated, leading to a reduced response. In addition, our results suggest that InsP3 levels may modulate polar and lateral IAA transport. Measurement of basipetal [3H]IAA movement in vertical roots indicates that transport in the InsP 5-ptase transgenic lines is reduced relative to the wild-type plants (Table V). Similar reductions in basipetal transport were previously reported for plants with the eir1/pin2/agr1/wav6 and aux1 mutations (Rashotte et al., 2000, 2001), which alter root basipetal IAA transport and abolish root gravitropism. It should be stressed, however, that there are significant differences between the InsP 5-ptase roots and the eir1/pin2/agr1/wav6 mutants. These mutants show a severe agravitropic phenotype along with altered root elongation and an absence of PIN2 protein in the cortical and epidermal cell files of the root (Luschnig et al., 1998; Muller et al., 1998). In contrast, vertical InsP 5-ptase roots have normal root elongation (Table III), and immunolocalization studies reveal normal PIN2 protein distribution compared to wild-type roots (I.A. Paponov and K. Palme, unpublished data). Furthermore, the gravitropic response is delayed, but not absent, in the InsP 5-ptase transgenic, and, usually, after 48 h root bending approaches wild-type plants.
The asymmetric IAA-induced GUS expression at the root tip after gravistimulation was also delayed in the transgenic plants (Table VI). This delayed formation of the GUS asymmetry on the lower side of the gravistimulated roots is consistent with the reduced levels of basipetal IAA transport, making it more difficult for a gradient of IAA to form. The timing of formation of the gradient is still linked to the curvature of the roots, suggesting that the delay in gravity response is linked to the delayed formation of a lateral gradient of IAA. These results suggest that InsP3 may be involved in establishing the timing of the response. Taken together, the fact that basipetal IAA transport was reduced along with the delay in lateral redistribution of auxin-induced GUS expression in the InsP 5-ptase roots with gravistimulation, these data imply that the InsP3-mediated signaling events must occur upstream of polar and lateral auxin transport and that attenuation of InsP3-mediated signaling either directly or indirectly affects the dynamics of auxin translocation.
Although many plant tissues respond to gravity, there are significant differences in how and where these responses are mediated. There are some basic differences in the architecture of roots versus shoots and also in the site of gravity sensing and gravity response in different organs. For example, in plant roots, the columella cells of the root cap are involved in gravity sensing, while the differential growth response takes place primarily in the distal elongation zone of the root (Ishikawa and Evans, 1993; Rosen et al., 1999). In cereal grasses such as oats and maize, both gravity sensing and response take place in a specialized tissue in the stem known as the pulvinus (Kaufman et al., 1995). In herbaceous dicot hypocotyls and floral stems, the gravitropic response occurs throughout the entire elongation zone of the stem (Fukaki et al., 1996a, 1996b). The mechanisms of gravitropism in roots and shoots therefore may have both common and separate steps. The differences are illustrated by some of the altered gravity mutants, which show a gravitropic defect in only a subset of the graviresponding organs. For example, the sgr mutants, which are defective in hypocotyls and/or inflorescence stem gravitropism, show a normal gravitropic response in the roots (Yamauchi et al., 1997; Kato et al., 2002; Yano et al., 2003). Alternatively, several of the auxin response (aux1, axr3, and axr4) mutants have normal hypocotyl and inflorescence bending, although root gravitropism is impaired (Hobbie and Estelle, 1995; Leyser et al., 1996; Marchant et al., 1999). In contrast, the InsP 5-ptase transgenic plants show a reduced gravitropic response in roots, hypocotyls, and inflorescence stems. The transgenic response is slower and reduced by 30% compared with the wild type. These results support the idea that InsP3 is a fundamental conserved component in plant gravisignaling. However, it is clear that multiple parallel or converging signaling pathways must be involved, since the gravitropic response is not completely eliminated in the transgenic plants.
In previous work, we demonstrated that biphasic increases in InsP3 are associated with the gravitropic responses of maize and oat pulvini (Perera et al., 1999, 2001); however, until now, it was not clear whether InsP3 signaling was a unique feature of the pulvinus system. Using inflorescence stems of Arabidopsis plants, we have quantified the levels of InsP3 during gravistimulation. Although it was not feasible to separate the inflorescence stems into upper and lower sides, we have detected biphasic increases in InsP3 in the total stems with gravistimulation. These InsP3 changes are similar to the pattern seen with the lower sides of the pulvini and indicate that InsP3 increases with gravistimulation in both monocots and dicots. Taken together with the fact that the transgenic plants with attenuated InsP3 signaling exhibit reduced gravitropic responses, these results are strong evidence for a universal role for polyphosphoinositides and the generation of InsP3 in the gravity signal transduction cascade in plants.
The involvement of InsP3 in gravitropism also implicates calcium. There is much indirect evidence supporting a role for calcium in gravisignaling and response (for review, see Sinclair and Trewavas, 1997; Fasano et al., 2002). However, it has been difficult to directly measure Ca2+ changes in plants in response to gravistimulation. Legue et al. (1997) carried out a careful study of Arabidopsis root tips and were unable to detect changes in Ca2+ upon gravistimulation. More recently, Plieth and Trewavas (2002) have shown a global Ca2+ signal upon reorientation by monitoring the luminescence of a large population of Arabidopsis seedlings expressing aequorin. The signature of the Ca2+ transient with gravistimulation consisted of a rapid spike followed by a longer shoulder lasting about 15 min. Interestingly, mechanical stimulation resulted in only a quick spike as did wind and touch. Based on the results that show that the shoulder exhibits signal adaptation (i.e. the amplitude of the shoulder was affected by the degree of reorientation and also by the number of successive reorientations), whereas the spike was unchanged, the authors infer that the spike and shoulder might have different cellular or subcellular origins (Plieth and Trewavas, 2002).
The biphasic patterns of Ca2+ signals revealed with aequorin are reminiscent of the pattern of InsP3 changes with gravistimulation (Perera et al., 1999, 2001). The initial transient fluctuations in InsP3 detected within seconds of gravistimulation were proposed to be part of a wake-up call, which alerts the plant to a change in its environment (Perera et al., 2001). These initial transients are consistent with the rapid, transient aequorin-sensitive Ca2+ signal and may be common to many different stimuli. The longer sustained increase in InsP3, which only occurs on the lower side of the pulvinus during gravistimulation, precedes and correlates with the bending response and may be more specific for the gravistimulus. This would be analogous to the longer Ca2+ shoulder.
In the classical signal transduction scheme, a localized change in Ca2+ is propagated through the cell and to neighboring cells via the soluble second messenger InsP3 (Tucker and Boss, 1996). In the transgenic cells, InsP3 is constantly being degraded by the InsP 5-ptase enzyme. Therefore, any InsP3-mediated Ca2+ signal generated in response to a stimulus would not spread throughout the cell and to neighboring cells. Additionally, since InsP3-mediated Ca2+ signaling is attenuated, the InsP3-sensitive intracellular Ca2+ stores may always be available in the transgenic plants. This may help explain why the transgenic lines showed a stronger reorientation response on the no added Ca2+ compared to the 10 mm Ca2+ medium.
The cold gravistimulation experiments have also been illuminating. These experiments are based on the fact that plants can sense a gravistimulus in the cold and respond to that stimulus when returned to room temperature. Fukaki et al. (1996a) showed that the memory or stored signal of the cold gravistimulus is retained for up to 60 min in Arabidopsis inflorescence stems. Wyatt et al. (2002) have utilized this phenomenon to devise a creative screen for mutants impaired in some aspect of gravity signaling. We have shown previously that increases in InsP3 can occur at 4°C in gravistimulated oat pulvini (Perera et al., 2001). Furthermore, since the sedimentation of statoliths is not impaired by cold temperature (Wyatt et al., 2002), it is clear that gravity sensing and some of the early signaling can occur in the cold. In contrast, auxin transport is dramatically decreased in the cold (Morris, 1979; Wyatt et al., 2002), and cold temperature also affects cytoskeleton and membrane dynamics (Mizuno, 1992; Orvar et al., 2000; Sangwan et al., 2001).
When wild-type Arabidopsis plants were gravistimulated in the cold and returned to room temperature, within the first 15 to 30 min there was a rapid positive response to the cold gravistimulus, which was followed by a return to the vertical. In some instances, the wild-type inflorescence stems display an overshooting response (bending beyond the vertical) following the return to room temperature. In contrast, the transgenic plants show a dampened response, and the rate of the bending response is significantly slower compared to wild type. In fact, the transgenic response to gravistimulation in the cold is reduced by 50% to 60% compared with the wild type, which is a greater reduction than seen when plants were gravistimulated at room temperature. These data imply that the contribution of InsP3-mediated signaling is magnified in the cold, possibly because other signaling mechanisms are inhibited by low temperature.
An important feature of the InsP 5-ptase transgenic plants is that, although InsP3 does not accumulate to wild-type levels, InsP3 synthesis via PLC is not inhibited (Perera et al., 2002), and, therefore, there is no buildup of lipid precursor (PtdInsP2) or a block in phosphatidic acid production via diacylglycerol (DAG). The expression of the highly active recombinant InsP 5-ptase results in the rapid hydrolysis of InsP3 and possibly an increased flux through the PI pathway. However, the transgenic plants grow normally and show no obvious phenotype under normal conditions. In contrast, a PI PLC knockout (plc1) in the moss Physcomitrella, while exhibiting an altered gravitropic response, also shows several pleiotropic growth defects, such as reduced gametophore formation, reduced levels of chlorophyll, and insensitivity to cytokinin (Repp et al., 2004). This is not surprising, as a loss of PLC activity would lead to reduced InsP3 levels as well as reduced DAG and DAG-mediated phosphatidic acid levels and would also affect PtdInsP2 accumulation. We suspect that, for these reasons, using antisense or knockout strategies of the PLC enzymes will have profound effects on plant growth beyond affecting InsP3 levels. Because the InsP 5-ptase transgenic Arabidopsis plants have altered InsP3 turnover and a dampened InsP3 signal, but no blockage in the pathway, they provide a good model system for specifically analyzing downstream InsP3-mediated responses.
In summary, we have shown that dampening InsP3-mediated signaling delays the timing and reduces the magnitude of the gravitropic response of Arabidopsis roots, hypocotyls, and inflorescence stems, and we propose that InsP3 is a fundamental component of plant gravisignaling that is upstream of auxin redistribution. Future work will focus on understanding the interaction of the InsP3 signaling pathway with other early components of the gravity signal transduction cascade.
MATERIALS AND METHODS
Plant Transformation and Selection of Transgenic Lines
The cDNA encoding the human type I InsP 5-ptase (accession no. X77567) was subcloned into the XbaI site of the pKYL71-35S2 binary vector (Schardl et al., 1987). The pKYL71-35S2 vector contains a modified 35S promoter, 3′-untranslated region of the pea (Pisum sativum) small subunit of Rubisco E-9 gene and a plant kanamycin resistance cassette. The coding region of the InsP 5-ptase gene, along with the 5′-end His tag, was amplified from the bacterial expression vector pQE31 using forward and reverse primers engineered to contain XbaI sites. The orientation of the resulting plasmid, pKYL71-35S2-InsP 5-ptase, was verified by restriction enzyme analysis and DNA sequencing. The binary plasmids pKYL71-35S2-InsP 5-ptase and pKYL71-35S2 (vector control) were electroporated into Agrobacterium tumefaciens strain GV, using a Bio-Rad Gene Pulser system. Arabidopsis (Arabidopsis thaliana Columbia-0) plants were grown to maturity under long-day conditions until primary inflorescence stems had emerged. Plants were transformed by vacuum infiltration with Agrobacterium containing the appropriate plasmid as described by Bechtold and Pelletier (1998). Plants were grown until seed set. Seeds were harvested and dried and stored at −20°C. For selection, surface-sterilized seeds were plated on round petri plates containing 1× MS salts, 1% Suc, MES buffer, pH 5.7, 0.8% type M agar (Sigma), and 50 μg/mL kanamycin. Plants were grown for 2 weeks, and kanamycin-resistant seedlings (from lines segregating 3:1 for kanamycin resistance) were transferred to soil, grown to maturity, and allowed to self-fertilize to produce T2 generation seed. Leaf samples were harvested from plants and DNA was isolated as described (Weigel and Glazebrook, 2002). DNA was digested by restriction enzymes and analyzed by gel electrophoresis, followed by Southern blotting, using the InsP 5-ptase gene as a probe. Four independent transformed lines were further selected for two more generations. Stable expression of the transgene was monitored by immunoblotting as described below.
Seed Germination and Plant Growth
Arabidopsis seeds were surface sterilized by first prewetting for 2 min in 70% ethanol and then incubating in a mixture of 30% (v/v) commercial bleach and 0.1% Triton X-100, with occasional agitation for 10 to 15 min. The seeds were then rinsed several times with sterile deionized water and resuspended in sterile 0.1% type M agar in water (Sigma). The seeds in agar were stored at 4°C for 48 h for stratification and germinated on square 9-cm petri plates containing 1× MS salts (GIBCO-BRL), 1% Suc, MES buffer, pH 5.7, and 0.8% type M agar). Plates were incubated vertically in a growth chamber under short-day conditions (8 h light/16 h dark) at 21°C with light intensity of approximately 150 μmol m−2 s−1. For root and hypocotyl elongation measurements, 4 d after germination plates were covered and placed in the dark and growth was monitored every 24 h for a 3- to 4-d period. For morphometric analysis, surface-sterilized seeds were sown on soil (PGX soil mix; Hummert), incubated in a growth chamber under long-day conditions (16 h light/8 h dark), and four to six plants of each line were monitored over an 8-week period as described by Boyes et al. (2001). Briefly, every 2 to 3 d plants were observed and leaf number, leaf length, and rosette size were measured. Across development, the timing of leaf expansion, emergence of the primary inflorescence stem, flower development, and silique formation were also measured, and seeds were collected to determine yield.
Gravitropic Bending Measurements
For root bending, seeds were germinated as described above and grown on vertically oriented plates for 3 d in the light and 1 d in the dark. Plates were then rotated by 90° and incubated in the dark. Plates were photographed prior to turning and at 2, 6, and 24 h after turning. Images were captured using a Hamamatsu color camera attached to a Leica stereo dissecting microscope or a Nikon Coolpix 4500 digital camera. Photographs were analyzed and bending angles were measured using Adobe Photoshop and analyzed using Microsoft Excel. Root bending was also monitored using Multi-ADAPT software (Ishikawa and Evans, 1997) as described by Rashotte et al. (2001).
For hypocotyl bending, seeds were grown on vertically oriented plates for days in the light followed by 1 d in the dark. Plates were then rotated by 90° and incubated in the dark for 48 h. Images were captured prior to turning the plates and 24 and 48 h after turning using a Hamamatsu color camera attached to a Leica stereo dissecting microscope. Bending angles were measured using Adobe Photoshop and analyzed using Microsoft Excel.
For inflorescence bending, Arabidopsis plants were grown in soil in pots (20 seeds/pot) for 4 weeks under short-day conditions followed by 2 weeks under long-day conditions. Experiments were carried out when primary inflorescence stems were 6 to 9 cm long. Plants were first incubated in the dark for 2 h prior to turning and then the pots were oriented horizontally in the dark. Bending was monitored every 15 min for 2 h using a Nikon Coolpix 4500 digital camera. For cold bending experiments, plants were incubated horizontally in the dark at 4°C for 1 h. Plants were then returned to room temperature and placed vertically in the dark and monitored every 15 min for 2 h.
Statistical Analysis
For all growth and bending measurements, data were subjected to statistical analysis by one-way ANOVA. The mean values for each line were compared using Student's t test assuming equal variance.
Root Basipetal Transport Assays
Root auxin transport measurements were made on 5-d-old vertically grown seedlings, using a previously published procedure (Rashotte et al., 2000, 2001). A mixture containing 1% agar, 5 mm MES buffer/1% (w/v) Suc, pH 5.5, and 100 nm 3H-IAA was prepared in a 3-mL scintillation vial. A narrow stem transfer pipette was carefully inserted into the hardened agar such that a long 1-mm-diameter cylinder of agar was removed. Seedlings were transferred to control plates or plates containing 10 μm NPA and oriented vertically such that the root tips were aligned. One hour after transfer to new plates, a cylinder containing the radioactive auxin mixture was applied to roots so that the cylinder was placed in contact with the root tips. Auxin transport was measured after 5 h by first removing and discarding the 1 mm of tissue in contact with the agar cylinder, then cutting a 5-mm segment back from the tip. Root tips were counted for 2 min and the disintegrations per minute were converted to the femtomole IAA transported using the specific activity of the tritiated IAA.
Histochemical Assays for Expression of the Auxin Reporter DR5-GUS
InsP 5-ptase lines expressing DR5-GUS were generated by crossing T5 generation InsP 5-ptase transgenic plants 2-6-5d and 2-8-5c (female parent plants) with DR5-GUS (male parent plants) as described in Weigel and Glazebrook (2002). F1 DR5-GUS InsP 5-ptase seedlings were checked by western blotting for InsP 5-ptase expression and by staining for GUS activity. For histochemical assays, 4-d-old seedlings grown on MS plates (1× MS, 1% Suc, 0.8% type M agar, pH 5.7) under a short-day cycle were covered and turned by 90° for 4 or 6 h. Roots were first washed with 100 mm sodium phosphate buffer, pH 7, and then incubated in staining solution (2 mm X-glucuronide, 100 mm sodium phosphate, pH 7.0, 0.5 mm potassium ferrocyanide, 0.5 mm potassium ferricyanide, and 0.5% [v/v] Triton X-100) overnight at room temperature in the dark. After incubation, the staining solution was removed and roots were rinsed carefully with 100 mm sodium phosphate buffer, pH 7.0, followed by 70% ethanol. Roots were observed under a dissecting microscope (Nikon SMZ1500) and images were collected using a MicroPublisher 5.0 cooled RTV camera (QImaging) and processed in Adobe Photoshop.
InsP3 Assays
Root and shoot samples were harvested from 5-d-old seedlings grown on plates and frozen immediately in liquid N2. For inflorescence-bending time courses, inflorescence stems (15–20/time point) were harvested without any flowers and siliques and frozen immediately in liquid N2. Frozen tissue (approximately 0.05 g) was ground to powder in liquid N2 and incubated with 150 μL of 10% perchloric acid on ice for 15 min. Samples were centrifuged to remove the precipitate and the supernatant was transferred to a new tube and the pH adjusted to 7.5 using 1.5 m KOH/3 mm HEPES. InsP3 assays were carried out using the TRK1000 InsP3 assay kit (Amersham-Pharmacia Biotech) as described previously (Perera et al., 1999, 2001, 2002).
Protein Isolation and Immunoblotting
Root and shoot samples were harvested from 2-week-old Arabidopsis seedlings grown on plates and extracted in buffer as described by Weigel and Glazebrook (2002). Protein concentration was estimated using Bio-Rad reagent. PM-enriched fractions were obtained by two-phase partitioning as described previously (Perera et al., 2002). Protein was separated by SDS-PAGE on 10% (w/v) polyacrylamide gels. For immunoblotting, proteins were transferred to polyvinylidene difluoride membrane, and blots were incubated with RGS-His primary antibody (at 1:1,500 dilution) from Qiagen followed by incubation in the secondary antibody (horseradish peroxidase-conjugated anti-mouse). Immunoreactivity was visualized by incubating the blot in SuperSignal West Pico Chemiluminescent substrate (Pierce) and exposure to x-ray film. Following chemiluminescence detection, total protein was visualized by staining the blots with Amido black (Sigma).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number X77567.
Supplementary Material
Acknowledgments
We thank Dr. Sarah E. Wyatt (Ohio University, Athens, OH) and Dr. John Love (University of Exeter, UK) for advice with the plant transformation and selection of primary transformants. We acknowledge Matthew Keefe, Beth Stapperfenne, Katie Kovac, and Candace Randall for their hard work and help in selecting and characterizing the transformants and carrying out the growth studies, and Kelly Althaus for carrying out the GUS histochemical assays. Thanks to “Jeff” Yue Xu, Dr. Eva Johannes, and Dr. Nina Allen of the Cell and Molecular Imaging Facility at North Carolina State University for help with microscopy, Dr. Linda Hanley-Bowdoin (North Carolina State University) for use of the dissecting microscope, and Peter Aspesi Jr. for the digital photography. We also thank Dr. Ingo Heilmann (University of Göttingen, Germany) and the members of the Boss lab (North Carolina State University) for helpful suggestions and discussion.
This work was supported by the National Aeronautics and Space Administration (grant no. NAGW–4984 awarded to the Specialized Center of Research and Training in Gravitational Biology at North Carolina State University and grant no. NAG2–1502 to I.Y.P.)
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Imara Y. Perera (imara_perera@ncsu.edu).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.075119.
References
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82: 259–266 [DOI] [PubMed] [Google Scholar]
- Berdy SE, Kudla J, Gruissem W, Gillaspy GE (2001) Molecular characterization of At5PTase1, an inositol phosphatase capable of terminating inositol trisphosphate signaling. Plant Physiol 126: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Masson PH (2003) Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol 133: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Muday GK (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16: 1191–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette RN, Gunesekera BM, Gillaspy GE (2003) An Arabidopsis inositol 5-phosphatase gain-of-function alters abscisic acid signaling. Plant Physiol 132: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland FM, Nelson T (2004) Cotyledon vascular pattern2-mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs. Plant Cell 16: 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson PH (1999) Gravitropism in higher plants. Plant Physiol 120: 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt F, Boom A, Pesesse X, Schiffmann SN, Erneux C (1996) Post-translational modification of human brain type I inositol-1,4,5-trisphosphate 5-phosphatase by farnesylation. J Biol Chem 271: 10419–10424 [DOI] [PubMed] [Google Scholar]
- Ercetin M, Gunesekera BM, Burnette RN, Kanter D, Robinson J, Gillaspy G (2004) A functional genomic approach for understanding inositol phosphate and phosphatidylinositol phosphate breakdown in plants (abstract no. 440). In Plant Biology 2004, July 24–July 28, 2004, Lake Buena Vista, FL. American Society of Plant Biologists, Rockville, MD
- Fasano JM, Massa GD, Gilroy S (2002) Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul 21: 71–88 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13: 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M (1996. a) Gravitropic response of inflorescence stems in Arabidopsis thaliana. Plant Physiol 110: 933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M (1996. b) How do plant shoots bend up? The initial step to elucidate the molecular mechanisms of shoot gravitropism using Arabidopsis thaliana. J Plant Res 109: 129–137 [DOI] [PubMed] [Google Scholar]
- Hangarter RP (1997) Gravity, light and plant form. Plant Cell Environ 20: 796–800 [DOI] [PubMed] [Google Scholar]
- Heilmann I, Shin J, Huang J, Perera IY, Davies E (2001) Transient dissociation of polyribosomes and concurrent recruitment of calreticulin and calmodulin transcripts in gravistimulated maize pulvini. Plant Physiol 127: 1193–1203 [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J 7: 211–220 [DOI] [PubMed] [Google Scholar]
- Hou G, Kramer VL, Wang YS, Chen R, Perbal G, Gilroy S, Blancaflor EB (2004) The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinization of the columella cytoplasm and a persistent lateral auxin gradient. Plant J 39: 113–125 [DOI] [PubMed] [Google Scholar]
- Hou G, Mohamalawari DR, Blancaflor EB (2003) Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton. Plant Physiol 131: 1360–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L, Otterhag L, Lee JC, Lasheen T, Hunt J, Seki M, Shinozaki K, Sornmarin M, Gilmour DJ, Pical C, et al (2004) Gene-specific expression and calcium activation of Arabidopsis thaliana phospholipase C isoforms. New Phytol 162: 643–654 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML (1993) The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol 102: 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML (1997) Novel software for analysis of root gravitropism: comparative response patterns of Arabidopsis wild-type and axr1 seedlings. Plant Cell Environ 20: 919–928 [DOI] [PubMed] [Google Scholar]
- Johannes E, Collings DA, Rink JC, Allen NS (2001) Cytoplasmic pH dynamics in maize pulvinal cells induced by gravity vector changes. Plant Physiol 127: 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Morita MT, Tasaka M (2002) Role of endodermal cell vacuoles in shoot gravitropism. J Plant Growth Regul 21: 113–119 [DOI] [PubMed] [Google Scholar]
- Kaufman PB, Wu L-L, Brock T, Kim D (1995) Hormones and the orientation of growth. In P Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 547–571
- Kimbrough JM, Salinas-Mondragon R, Boss WF, Brown CS, Sederoff HW (2004) The fast and transient transcriptional network of gravity and mechanical stimulation in the Arabidopsis root apex. Plant Physiol 136: 2790–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ (2000) Mechanisms of the early phases of plant gravitropism. CRC Crit Rev Plant Sci 19: 551–573 [DOI] [PubMed] [Google Scholar]
- Laxminarayan KM, Matzaris M, Speed CJ, Mitchell CA (1993) Purification and characterization of a 43-kDa membrane-associated inositol polyphosphate 5-phosphatase from human placenta. J Biol Chem 268: 4968–4974 [PubMed] [Google Scholar]
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S (1997) Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol 114: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser HMO, Pickett FB, Dharmasiri S, Estelle M (1996) Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J 10: 403–413 [DOI] [PubMed] [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ (1991) An auxin-responsive promoter is differentially induced by auxin gradients during tropisms. Plant Cell 3: 1167–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WH, Wang Y, Mueller-Roeber B, Brearley CA, Xu ZH, Xue HW (2005) At5PTase13 modulates cotyledon vein development through regulating auxin homeostasis. Plant Physiol 139: 1677–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL (1997) Molecular genetic analysis of plant gravitropism. Gravit Space Biol Bull 10: 75–82 [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH (1995) Auxin transport. In P Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 509–530
- Long JC, Zhao W, Rashotte AM, Muday GK, Huber SC (2002) Gravity-stimulated changes in auxin and invertase gene expression in maize pulvinal cells. Plant Physiol 128: 591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18: 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer HJ, Munnik T (2003) Phospholipid-based signaling in plants. Annu Rev Plant Biol 54: 265–306 [DOI] [PubMed] [Google Scholar]
- Mizuno K (1992) Induction of cold stability of microtubules in cultured tobacco cells. Plant Physiol 100: 740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Zieschang HE, Sievers A (1996) Differential proton secretion in the apical elongation zone caused by gravistimulation is induced by a signal from the root cap. Plant Cell Environ 19: 1408–1414 [DOI] [PubMed] [Google Scholar]
- Morita MT, Tasaka M (2004) Gravity sensing and signaling. Curr Opin Plant Biol 7: 712–718 [DOI] [PubMed] [Google Scholar]
- Morris DA (1979) Effect of temperature on the velocity of exogenous auxin transport in intact chilling-sensitive and chilling-resistant plants. Planta 146: 603–605 [DOI] [PubMed] [Google Scholar]
- Moseyko N, Zhu T, Chang HS, Wang X, Feldman LJ (2002) Transcription profiling of the early gravitropic response in Arabidopsis using high-density oligonucleotide probe microarrays. Plant Physiol 130: 720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK (2001) Auxins and tropisms. J Plant Growth Regul 20: 226–243 [DOI] [PubMed] [Google Scholar]
- Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6: 535–542 [DOI] [PubMed] [Google Scholar]
- Muller A, Guan C, Galweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvar BL, Sangwan V, Omann F, Dhindsa RS (2000) Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J 23: 785–794 [DOI] [PubMed] [Google Scholar]
- Parker KE, Briggs WR (1990) Transport of indole-3-acetic-acid during gravitropism in intact maize coleoptiles. Plant Physiol 94: 1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal G, Driss-Ecole D (2003) Mechanotransduction in gravisensing cells. Trends Plant Sci 8: 498–504 [DOI] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Boss WF (1999) Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA 96: 5838–5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Chang SC, Boss WF, Kaufman PB (2001) A role for inositol 1,4,5-trisphosphate in gravitropic signaling and the retention of cold-perceived gravistimulation of oat shoot pulvini. Plant Physiol 125: 1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IY, Love J, Heilmann I, Thompson WF, Boss WF (2002) Up-regulation of phosphoinositide metabolism in tobacco cells constitutively expressing the human type I inositol polyphosphate 5-phosphatase. Plant Physiol 129: 1795–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RM, Young LS, Murthy UMN, Harrison BR, Wang Y, Will JL, Masson PH (2005) Gravity signal transduction in primary roots. Ann Bot (Lond) 96: 737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Lüthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Böttger M, et al (1999) Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA 96: 12186–12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth C, Trewavas AJ (2002) Reorientation of seedlings in the earth's gravitational field induces cytosolic calcium transients. Plant Physiol 129: 786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13: 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repp A, Mikami K, Mittmann F, Hartmann E (2004) Phosphoinositide-specific phospholipase C is involved in cytokinin and gravity responses in the moss Physcomitrella patens. Plant J 40: 250–259 [DOI] [PubMed] [Google Scholar]
- Rosen E, Chen R, Masson PH (1999) Root gravitropism: a complex response to a simple stimulus? Trends Plant Sci 4: 407–412 [DOI] [PubMed] [Google Scholar]
- Sack FD (1991) Plant gravity sensing. Int Rev Cytol 127: 193–252 [DOI] [PubMed] [Google Scholar]
- Sack FD (1997) Plastids and gravitropic sensing. Planta 203: S63–S68 [DOI] [PubMed] [Google Scholar]
- Sanchez JP, Chua NH (2001) Arabidopsis PLC1 is required for secondary responses to abscisic acid signals. Plant Cell 13: 1143–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF (1999) Communicating with calcium. Plant Cell 11: 691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan V, Foulds I, Singh J, Dhindsa RS (2001) Cold-activation of Brassica napus BN115 promoter is mediated by structural changes in membranes and cytoskeleton, and requires Ca2+ influx. Plant J 27: 1–12 [DOI] [PubMed] [Google Scholar]
- Schardl CL, Byrd AD, Benzion G, Altschuler MA, Hildebrand DF, Hunt AG (1987) Design and construction of a versatile system for the expression of foreign genes in plants. Gene 61: 1–11 [DOI] [PubMed] [Google Scholar]
- Scott AC, Allen NS (1999) Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol 121: 1291–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair W, Trewavas AJ (1997) Calcium in gravitropism. A re-examination. Planta 203: S85–S90 [DOI] [PubMed] [Google Scholar]
- Staves MP (1997) Cytoplasmic streaming and gravity sensing in Chara internodal cells. Planta 203: S79–S84 [DOI] [PubMed] [Google Scholar]
- Stevenson JM, Perera IY, Heilmann I, Persson S, Boss WF (2000) Inositol signaling and plant growth. Trends Plant Sci 5: 252–258 [DOI] [PubMed] [Google Scholar]
- Tasaka M, Kato T, Fukaki H (1999) The endodermis and shoot gravitropism. Trends Plant Sci 4: 103–107 [DOI] [PubMed] [Google Scholar]
- Tucker EB, Boss WF (1996) Mastoparan-induced intracellular Ca2+ fluxes may regulate cell-to-cell communication in plants. Plant Physiol 111: 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann D, Baluska F (1999) Actin cytoskeleton in plants: from transport networks to signaling networks. Microsc Res Tech 47: 135–154 [DOI] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J (2002) Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Wyatt SE, Rashotte AM, Shipp MJ, Robertson D, Muday GK (2002) Mutations in the gravity persistence signal loci in Arabidopsis disrupt the perception and/or signal transduction of gravitropic stimuli. Plant Physiol 130: 1426–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Kiss JZ (2002) Disruption of the actin cytoskeleton results in the promotion of gravitropism in inflorescence stems and hypocotyls of Arabidopsis. Plant Physiol 128: 669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Fukaki H, Fujisawa H, Tasaka M (1997) Mutations in the SGR4, SGR5 and SGR6 loci of Arabidopsis thaliana alter the shoot gravitropism. Plant Cell Physiol 38: 530–535 [DOI] [PubMed] [Google Scholar]
- Yano D, Sato M, Saito C, Sato MH, Morita MT, Tasaka M (2003) A SNARE complex containing SGR3/AtVAM3 and ZIG/VTI11 in gravity-sensing cells is important for Arabidopsis shoot gravitropism. Proc Natl Acad Sci USA 100: 8589–8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder TL, Zheng HQ, Todd P, Staehelin LA (2001) Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol 125: 1045–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LM, Evans ML, Hertel R (1990) Correlations between gravitropic curvature and auxin movement across gravistimulated roots of Zea mays. Plant Physiol 92: 792–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L-S, Masson PH (2004) Proteomics approach identifies adenosine kinase as a component in Arabidopsis root gravitropism (abstract no. 59). Gravit Space Biol Bull 18: 23 [Google Scholar]
- Zhong R, Burk DH, Morrison WH III, Ye ZH (2004) FRAGILE FIBER3, an Arabidopsis gene encoding a type II inositol polyphosphate 5-phosphatase, is required for secondary wall synthesis and actin organization in fiber cells. Plant Cell 16: 3242–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.