Abstract
Stomatal conductance (gs) typically declines in response to increasing intercellular CO2 concentration (ci). However, the mechanisms underlying this response are not fully understood. Recent work suggests that stomatal responses to ci and red light (RL) are linked to photosynthetic electron transport. We investigated the role of photosynthetic electron transport in the stomatal response to ci in intact leaves of cocklebur (Xanthium strumarium) plants by examining the responses of gs and net CO2 assimilation rate to ci in light and darkness, in the presence and absence of the photosystem II inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), and at 2% and 21% ambient oxygen. Our results indicate that (1) gs and assimilation rate decline concurrently and with similar spatial patterns in response to DCMU; (2) the response of gs to ci changes slope in concert with the transition from Rubisco- to electron transport-limited photosynthesis at various irradiances and oxygen concentrations; (3) the response of gs to ci is similar in darkness and in DCMU-treated leaves, whereas the response in light in non-DCMU-treated leaves is much larger and has a different shape; (4) the response of gs to ci is insensitive to oxygen in DCMU-treated leaves or in darkness; and (5) stomata respond normally to RL when ci is held constant, indicating the RL response does not require a reduction in ci by mesophyll photosynthesis. Together, these results suggest that part of the stomatal response to ci involves the balance between photosynthetic electron transport and carbon reduction either in the mesophyll or in guard cell chloroplasts.
Guard cells respond to light and to intercellular CO2 concentration (ci). Traditionally, the mechanisms for these two responses have been treated independently, but neither response is well understood. Recently, two hypotheses have been proposed by which ci and light responses might be mechanistically linked through photosynthetic processes (Zeiger and Zhu, 1998; Buckley et al., 2003). Both of these hypotheses (discussed in more detail below) predict that the response of stomatal conductance (gs) to ci is controlled by the balance between electron transport capacity and ribulose 1,5-bisphosphate carboxylation capacity. These hypotheses are, however, controversial, and the major goal of this study is to examine the role of photosynthetic processes in the response of gs to ci.
The stomatal response to light has at least two components; the so-called blue light (BL) and red light (RL) responses. The BL response saturates at fluences much lower than photosynthetic saturation (around 50 μmol m−2 s−1; Zeiger, 2000). This response has been shown to involve the activation of a plasma membrane H+-ATPase (Kinoshita and Shimazaki, 1999), although the steps leading up to this activation are not clear. The receptor for this response has yet to be identified unequivocally, and there is evidence supporting both zeaxanthin (Zeiger and Zhu, 1998; Talbott et al., 2003) and phototropins (Kinoshita et al., 2001; Doi et al., 2004) for this role. The RL response is also poorly understood, but many data suggest the involvement of guard cell photosynthetic processes. Specifically, the RL response saturates at fluences similar to those for photosynthetic saturation. In addition, the action spectra for mesophyll photosynthesis and for the stomatal response to RL are similar to one another (Sharkey and Raschke, 1981a), and the RL response is abolished by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), a PSII inhibitor (Sharkey and Raschke, 1981b; Schwartz and Zeiger, 1984; Tominaga et al., 2001; Olsen et al., 2002). For these reasons, chlorophyll is commonly assumed to be the RL receptor (Assmann and Shimazaki, 1999; Zeiger et al., 2002). Despite the accumulation of evidence showing that guard cells respond directly to RL, this conclusion has recently been challenged based on electrophysiological measurements of the guard cell plasma membrane (Roelfsema et al., 2002).
Much less is known about the stomatal response to ci. Malate production by phosphoenolpyruvate carboxylase was once thought to play a role in this response (Raschke, 1975). However, increased ci should enhance malate production, leading to an increase, not a decrease, in guard cell osmotic pressure and thus gs. Therefore, if malate plays a role in the ci response, it must not involve a direct effect on osmotic pressure, but rather an indirect function on guard cell osmoregulation. Along these lines, Hedrich et al. (1994) have proposed that malate plays a role in regulating R-type anion channels and they showed how this regulation could account for stomatal response to CO2. Another possibility is that the ci response involves C3 photosynthetic processes in guard cells. It now seems clear that the Calvin cycle and photosynthetic electron transport are operational in guard cell chloroplasts (see Zeiger et al., 2002; Vavasseur and Raghavendra, 2005) and that these two processes can occur at similar relative rates in guard and mesophyll cells (Cardon and Berry, 1992; Lawson et al., 2002). These results form the basis for two hypotheses that link guard cell photosynthesis with the stomatal responses to light and ci.
The first of these hypotheses (Zeiger et al., 2002; Outlaw, 2003) is an extension of the zeaxanthin hypothesis for the BL response (Zeiger and Zhu, 1998). Zeaxanthin functions in nonphotochemical quenching. As a result, increased dissipation of photosynthetic reducing power by the Calvin cycle—promoted by increased ci at a given level of photosynthetically active radiation (PAR)—decreases zeaxanthin concentration (Zhu et al., 1998). Conversely, at a given ci, zeaxanthin rises with PAR because greater photon supply demands more nonphotochemical quenching. According to the hypothesis, PAR- and ci-induced variations in the concentration of zeaxanthin regulate stomatal aperture by modulating guard cell sensitivity to BL. The second hypothesis builds on the observation that photosynthetically derived ATP is shuttled from guard cell chloroplasts to the cytosol, where it is used to drive proton pumping and hence cation uptake at the plasmalemma (Tominaga et al., 2001). The abundance of ATP, like zeaxanthin, should increase with the thylakoid pH gradient and hence PAR, and should decrease with Calvin cycle activity and hence ci (Buckley et al., 2003). It is worth noting the existence of a third hypothesis concerning guard cell photosynthesis and gs. This hypothesis holds that the Calvin cycle generates Suc or other osmotica within guard cells, driving osmotic swelling and stomatal opening in RL, or more generally in PAR (Zeiger et al., 2002; Outlaw, 2003). However, in contrast to observations, this mechanism predicts greater stomatal opening under elevated ci.
A key feature of the zeaxanthin and ATP hypotheses is that the ci and RL responses depend on the balance between photosynthetic electron transport and Calvin cycle activity in guard cells. However, several recent studies have questioned the role for guard cell photosynthetic processes in stomatal control. For example, Roelfsema et al. (2002) concluded that the RL response results from reductions in ci caused by mesophyll photosynthesis. In addition, studies on antisense plants with suppressed expression of Rubisco (von Caemmerer et al., 2004) and Rieske FeS protein (Price et al., 1998) have found little or no difference in stomatal function between wild-type and antisense plants, suggesting neither mesophyll nor guard cell photosynthesis plays a role in the ci response. Finally, it has also been argued for many years that the existence of a CO2 response in darkness and of functional nonchlorophyllous guard cells in some orchids (Nelson and Mayo, 1975) disproves any necessary role for photosynthesis.
In this study, we used several techniques to explore the roles of both mesophyll and guard cell photosynthetic electron transport and Calvin cycle activity in the response of gs to ci. We monitored spatial and temporal changes in photosynthetic capacity and gs concurrently after treating a leaf with DCMU, a compound known to interrupt photosynthetic electron transport. We also compared the stomatal response to ci between a leaf in the dark and a leaf treated with DCMU in the light. Finally, we examined the responses of gs and photosynthesis to ci using O2 and light to alter the balance between electron transport and Calvin cycle activity.
RESULTS
Effects of DCMU on Photosynthesis and gs
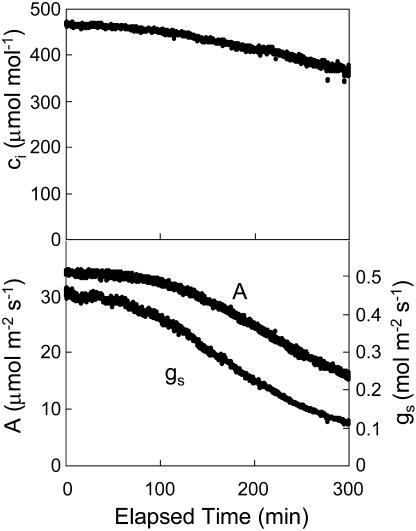
When 100 μm DCMU was applied to a leaf via the transpiration stream, photosynthetic CO2 uptake declined gradually over several hours. This decline in photosynthetic rate was accompanied by a decline in gs and a slight decline in ci (Fig. 1). Fluorescence images (Fig. 2, top row) were taken concurrently with the gas-exchange measurements to assess the spatial distribution of photosynthesis in response to DCMU. These images show distinct areas with near-zero quantum efficiency for PSII (φPSII) spreading out from the veins. The proportion of the leaf with near-zero φPSII was directly proportional to the percent reduction in photosynthesis as measured by gas exchange (Fig. 3). This supports the conclusion that the areas with near-zero φPSII had near-zero photosynthesis. These results show that the gradual decline in photosynthesis observed in gas-exchange data was caused by an increase in the proportion of the leaf for which photosynthesis was severely inhibited, rather than a slow uniform decline in photosynthesis for the entire leaf. This experiment was repeated three times with similar results.
Figure 1.
Responses of ci, A, and gs to DCMU (100 μm) fed through the transpiration stream of a detached leaf. DCMU was applied at time zero. Other conditions were as described in “Materials and Methods.”
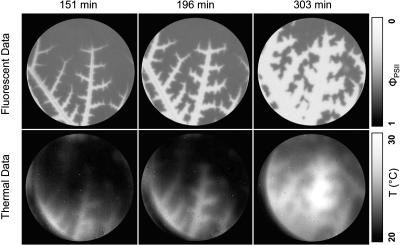
Figure 2.
Fluorescence and thermal images at three times following DCMU application. Images we taken of the leaf for which data are shown in Figure 1.
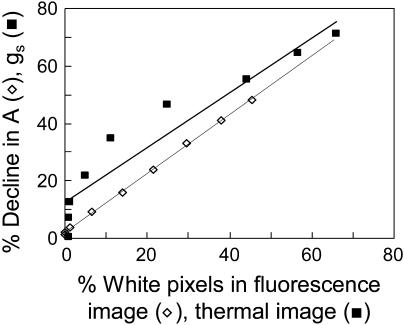
Figure 3.
A and gs plotted versus the percent of image with near-zero φPSII and percent of image with temperatures above approximately 27°C. Data were calculated at 30-min intervals as DCMU was added to the leaf via the transpiration stream.
Thermal images were used to assess the spatial distribution of gs in response to DCMU treatment. Leaf temperature, and hence infrared emission, should be higher in areas of lower conductance as a result of reduced evaporative heat loss. These images (Fig. 2, bottom row) show areas of high temperature spreading out from the veins in a very similar pattern to that observed in the fluorescence images. The area of the leaf with higher than average temperatures was proportional to the percent reduction in gs (Fig. 3), and the pixel intensity of the bright areas did not increase appreciably as the gs of the entire leaf approached zero. These results show that, similar to photosynthesis, the gradual decline in gs observed with gas exchange was caused by an increase in the proportion of the leaf with near-zero conductance rather than by a slow uniform decrease in conductance. Furthermore, the spatial pattern of photosynthesis inhibition was very similar to the pattern for conductance inhibition.
The Responses of Assimilation Rate and gs to ci Change Slope Concurrently
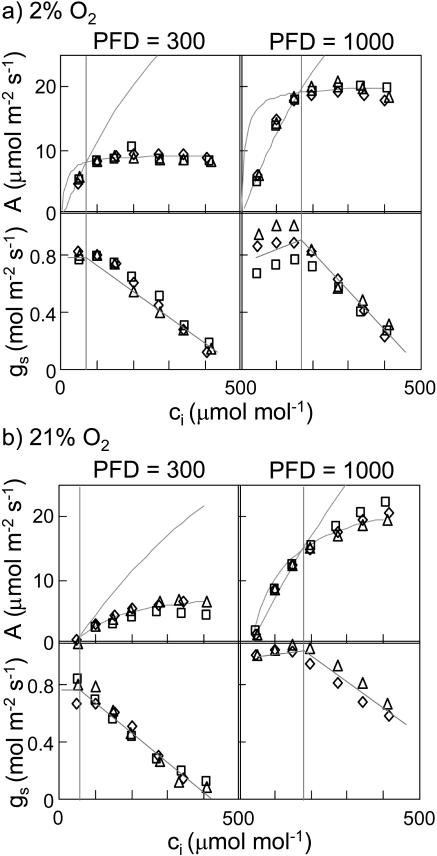
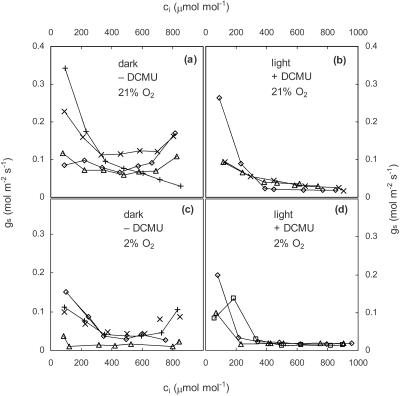
The relationship between gs and electron transport was further explored by examining the responses of gs and photosynthesis to CO2. These experiments were carried out at 21% and 2% O2 using a high photon flux density (PFD; 1,000 μE m−2 s−1) and a low PFD (300 μE m−2 s−1), yielding four separate treatments. In all four treatments, conductance declined steeply with increasing CO2 at high values of ci (Fig. 4). However, in the two high-PFD treatments, the response of conductance to CO2 was less steep at low ci than at high ci and, in some cases, the slope was actually positive at low ci. This was not true for the low-PFD treatments. Furthermore, the transition from a shallow slope at low ci to a steep slope at high ci under high PFD occurred at approximately the same ci values as the transition from Rubisco-limited to electron transport-limited mesophyll photosynthesis, as determined by fitting the photosynthesis model of Farquhar et al. (1980) to the assimilation rate (A) versus ci curves (Table I). At low PFD, CO2 assimilation was limited by electron transport at all but the lowest ci value, and gs declined more or less steadily with increasing ci. Although there were differences in gs between the upper and lower surfaces, the stomatal responses of the two surfaces to all treatments were qualitatively similar.
Figure 4.
Responses of A and gs to ci at 2% (a) and 21% (b) O2. Lines drawn through the A data are best fits to the photosynthesis model of Farquhar et al. (1980) as described in “Materials and Methods.” Lines drawn through the gs data are hand-drawn approximations. Different symbols represent different replicate experiments. Parameters for the fitted A versus ci curves are given in Table I.
Table I.
Parameters of the photosynthesis model of Farquhar et al. (1980) fitted to the relationships shown in Figure 4 between net CO2 A and ci
Values of the parameters Γ* and K′ (photorespiratory compensation point and effective Km for Rubisco, respectively) were calculated from expressions given by Farquhar et al. (1980) using values of Rubisco Km and turnover rates for carboxylation and oxygenation given by de Pury and Farquhar (1997) for 25°C.
| Parameter
|
Symbol
|
Units
|
PFD/[O2]
|
|||
|---|---|---|---|---|---|---|
| 300/2 | 1,000/2 | 300/21 | 1,000/21 | |||
| μEm−2s−1/% | ||||||
| Maximum velocity of carboxylation | Vc,max | μmol m−2 s−1 | 68 ± 6 | 90 ± 6 | 105 ± 38 | 109 ± 6 |
| Potential electron transport rate | J | μmol e− m−2 s−1 | 40 ± 1 | 84 ± 3 | 39 ± 7 | 105 ± 5 |
| Mitochondrial respiration rate | Rd | μmol m−2 s−1 | 0.7 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.4 | 1.1 ± 0.1 |
| Intercellular CO2 mole fraction at transition point | ci | μmol mol−1 | 79 ± 10 | 145 ± 20 | 35.9 | 181 ± 33 |
| Photorespiratory compensation point | Γ* | μmol mol−1 | 3.4 | 35.9 | ||
| Effective Km for carboxylation | K′ | μmol mol−1 | 502.3 | 811.9 | ||
The change in the slope of the A versus ci curve associated with the transition between Rubisco and electron transport limitation was larger at 2% O2 than at 21% O2, which makes it easier to identify unambiguously the transition point between Rubisco and electron transport limitation. It is noteworthy that the slope change in the gs versus ci curve was also more pronounced at 2% O2 (Fig. 4A) and conductance actually increased slightly as the ci values approached the transition point from below.
Photosynthetic Electron Transport Alters the Stomatal Response to ci
Finally, we investigated the response of gs to CO2 in darkness and following the application of DCMU. Both treatments should reduce photosynthetic electron transport to near zero and CO2 uptake rates were negative for both treatments at all ci (data not shown). The response of gs to CO2 was essentially identical for leaves in darkness (Fig. 5A) and DCMU-treated leaves (Fig. 5B). Furthermore, there was no effect of O2 on the response of conductance to CO2 in darkness (Fig. 5C) or in DCMU (Fig. 5D).
Figure 5.
Responses of gs to ci at 21% oxygen in darkness (a) and for a DCMU-treated leaf at a PFD of 1,000 μE m−2 s−1 white light (b); and at 2% oxygen in darkness (c) and for a DCMU-treated leaf at a PFD of 1,000 μE m−2 s−1 white light (d). Different symbols represent different replicate experiments.
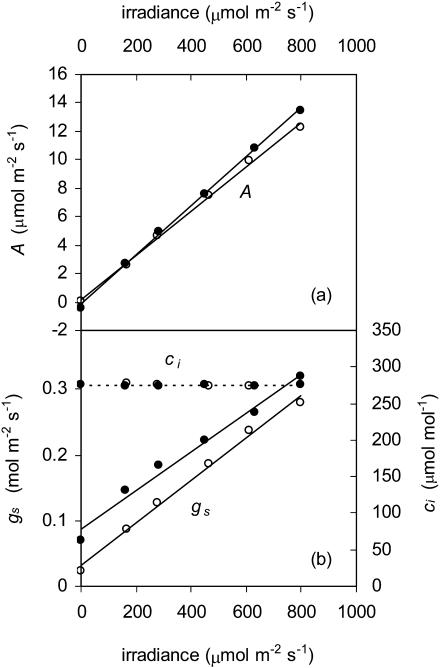
To demonstrate that the difference in gs in light and darkness was not the result of lowered ci, as suggested by Roelfsema et al. (2002), we examined the response of photosynthesis and gs to wavelengths greater than 500 nm while holding ci constant by adjusting ambient CO2 mole fraction. Both A and gs increased approximately linearly with increasing PFD in the steady state despite a constant value of ci (Fig. 6). Results with only wavelengths greater than 600 nm were similar to those shown in Figure 6.
Figure 6.
Responses of A (a) and gs (b; solid lines), and ci (dashed lines) to PFD with wavelengths above 500 nm. Ambient CO2 was manipulated to keep ci constant. Different symbols represent different replicates.
DISCUSSION
Many previous studies have examined the role of photosynthesis in guard cell processes. However, most of these have centered on establishing the presence of photosynthetic CO2 fixation in guard cells (Gotow et al., 1988) or the involvement of guard cell photosynthesis in providing Suc as an osmoticum (Talbott and Zeiger, 1998). In this study, we used several approaches to investigate the role of photosynthetic processes in determining the response of gs to CO2.
Our first approach was simply to compare stomatal responses to ci with photosynthetic responses to ci at two PFD values and two O2 concentrations. These data (Fig. 4) show that the stomatal response to ci in cocklebur (Xanthium strumarium) changes slope at the same ci value as the transition from Rubisco to electron transport limitation in photosynthesis. Specifically, the stomatal response is steeper at ci values above the transition (i.e. when mesophyll photosynthesis is limited by the supply of ATP and NADPH). The relationship holds true as the transition ci changes with PFD, and it is clearer at 2% O2 than at 21% O2 because the transition between the Rubisco- and electron transport-limited portions of the curve is more distinct at 2% O2. It is noteworthy in this regard that both the gs versus ci curve and the A versus ci curve show a larger change in slope at 2% O2 than at 21% O2, making the changes in slopes less ambiguous. Collectively, these data strongly suggest that the stomatal response to ci is somehow influenced by the balance between the light reactions and the carbon reactions in photosynthesis.
The data do not, however, indicate how or where this balance might be sensed. The photosynthesis model of Farquhar et al. (1980), as extended by Farquhar and Wong (1984), predicts that mesophyll chloroplastic [ATP] should decline as ci increases, with a steeper slope at high ci—as reported here for the response of gs to ci—so variations in ATP associated with guard cell photosynthesis are one possible sensory mechanism (Buckley et al., 2003). Zeaxanthin, one of the putative BL receptors in guard cells is another possibility. The equilibrium for interconversion of violaxanthin and zeaxanthin depends on thylakoid pH, which in turn depends on the balance between the light reactions and the carbon reactions in photosynthesis (Zeiger et al., 2002). Our data do not appear to rule out guard cell chloroplastic zeaxanthin, ATP, or any other photosynthetic intermediary, nor do they indicate clearly that the putative sensor is located in mesophyll or guard cells.
If the response of gs to ci is affected by the balance between electron transport and Rubisco limitations to photosynthesis, then the cessation of electron transport should markedly change the response of stomata to ci. We tested this idea by inhibiting electron transport with DCMU. Using high ci to ensure that photosynthesis was electron transport limited, we found that photosynthesis and gs declined in parallel over time as DCMU spread throughout the leaf. This confirms the earlier finding of Wong et al. (1979). It is possible that this parallel decline was caused by a slow uniform decrease in photosynthesis and gs over the entire leaf. However, previous studies using chlorophyll fluorescence imaging have shown that DCMU inhibition of photosynthesis spreads slowly from the veins into the mesophyll, with affected areas having near-zero photosynthesis and nonaffected areas retaining high photosynthesis rates (Daley et al., 1989; Genty and Meyer, 1994).
To resolve this issue, we mapped both electron transport and gs by imaging chlorophyll fluorescence and thermal emission simultaneously. Fluorescence images confirmed previous studies showing that areas in which photosynthetic electron transport was completely inhibited spread slowly from the veins. More importantly, thermal images clearly show that gs had also dropped to nearly zero in areas for which electron transport was reduced to zero (see “Results”). Thus, as DCMU spread throughout the leaf, it inhibited electron transport and caused gs to decline to zero. These two effects were simultaneous within the spatiotemporal resolution of the images. It is unlikely that the decline in conductance was caused by an increase in ci resulting from the decline in mesophyll photosynthesis, because the average ci for the leaf changed only slightly as both A and gs declined by more than 50% (if mesophyll photosynthesis had declined before gs in leaf regions affected by DCMU, the ratio of A to gs would have declined in those regions, causing the whole-leaf estimate of ci to increase).
DCMU was fed to leaves until the photosynthesis rate was constant and negative. At that point, we assumed that electron transport was uniformly inhibited throughout the leaf. Fluorescence images confirmed this (data not shown). Under these conditions, stomata retained a small, but measurable, response to ci (Fig. 5B). However, several lines of reasoning suggest that this represents a separate response of stomata to CO2 that is independent, and perhaps complementary, to the response that occurs in the presence of photosynthetic electron transport. First, the response in the presence of DCMU was not altered by the presence or absence of light and it was similar to the responses that occur in darkness and in the absence of DCMU. All responses in the absence of photosynthetic electron transport (either in darkness or after DCMU treatment) were unaffected by oxygen concentration and were qualitatively and quantitatively different from the stomatal response in the presence of photosynthetic electron transport. Thus, our data suggest that there are two mechanisms by which stomata respond to CO2: one that depends on photosynthetic electron transport and one that does not. The idea of multiple CO2 response mechanisms has been suggested before (Assmann, 1999) and it explains numerous previous reports that stomata can respond to ci in the dark (Heath and Russell, 1954; Dale, 1961; Mansfield and Heath, 1961).
Although DCMU is well known to inhibit photosynthetic electron transport, its effects on gs have not been studied in detail. It has been shown to inhibit sugar production in guard cells (Poffenroth et al., 1992) and to inhibit opening under RL in epidermal peels (Schwartz and Zeiger, 1984; Olsen et al., 2002). More recently, it has been shown to inhibit the effect of RL on H+ pumping at the plasma membrane of guard cells of Vicia faba and Commelina benghalensis (Tominaga et al., 2001). These results suggest that DCMU should cause stomata to close in intact leaves and are therefore consistent with our data. Our results appear contradictory to those of Sharkey and Raschke (1981b), who found little effect of DCMU and cyanazine (a herbicide that blocks photosynthetic electron transport at PSII) on gs in cocklebur. However, in Gossypium hirsutum, Sharkey and Raschke (1981b) found a reduction in gs and a change in the response of conductance to ci in response to cyanazine, and these effects were qualitatively similar to our results (compare their Fig. 1 with our Figs. 4 and 5B). Part of the discrepancy between their results and ours may be because they fed the photosynthetic inhibitor only until whole-leaf photosynthesis reached the compensation point, which would suggest that at least some areas of the leaf were still photosynthesizing. In our study, CO2 uptake was negative and constant at all ci values, indicating that photosynthetic electron transport was completely blocked in all areas of the leaf.
There are numerous studies showing that guard cells respond to RL directly (Heath and Russell, 1954; Sharkey and Raschke, 1981a; Zeiger, 1990). However, this finding has recently been called into question by Roelfsema et al. (2002), who concluded that the RL response of stomata in intact leaves was strictly indirect and was mediated by a reduction in ci caused by mesophyll photosynthesis. It is critical to the central arguments of this study that guard cells respond directly to PAR independent of the BL response and independent of mesophyll-induced changes in ci. We verified this by examining the gs response to RL while holding ci constant by adjusting ambient CO2 concentration. Our results show that, in our leaves, the major RL response was not caused by mesophyll-induced reductions in ci (Fig. 6), but instead either by a ci-independent signal from the mesophyll (Lee and Bowling, 1995) or by processes located within guard cells.
It is unclear from our data whether guard cells are responding directly to electron transport in the guard cells or indirectly to mesophyll electron transport through an unknown signaling mechanism. If the former, our data suggest that the balance between electron transport and Rubisco capacities is similar for guard cells and mesophyll cells. This is supported by data showing that fluorescence responses to CO2 and O2 are similar in guard and mesophyll cells (Cardon and Berry, 1992; Lawson et al., 2002, 2003).
The suggestion that a large part of the stomatal response to ci is closely linked to photosynthetic processes within guard cells contrasts with the findings of several studies on antisense plants with impaired photosynthetic functioning, which have generally found little difference in gs or its response to ci between normal and antisense plants. Price et al. (1998) found similar gss in wild-type plants and antisense plants with up to a 90% reduction in Rieske FeS protein (a component of the chloroplast cytochrome b6f complex). Since these plants presumably had reduced electron transport relative to Rubisco capacity, they should have shown reduced gs to be consistent with our results. However, although fluorescence data suggest guard and mesophyll cells possess similar photosynthetic competency on a chlorophyll basis (Lawson et al., 2002), guard cells have much less chlorophyll per cell and hence lower content and activity of photosynthetic components than mesophyll cells on a per-cell basis. As a result, it is possible that systemic reductions in the expression of Rieske FeS protein may have little or no effect in guard cells except at very low expression levels. In any event, the C3-type fluorescence responses of guard cells in the C4 species Amaranthus caudatus (Lawson et al., 2003) suggest that photosynthetic coordination may be regulated by entirely different means in guard and mesophyll cells.
Von Caemmerer et al. (2004) likewise found no effect of reduced Rubisco content on either gs or its response to ci. Rubisco antisense plants possess increased electron transport capacity relative to Rubisco capacity, which should increase both gs and the value of ci at which the transition from Rubisco to electron transport occurs. However, our data suggest that these effects will be subtle and limited to conductances at low values of ci. Since the experiments by von Caemmerer et al. (2004) covered a wide range of ci values and included relatively few points at low ci, it is unclear whether their data show a transition point effect similar to that reported in this study.
CONCLUSION
This study has shown that the response of steady-state gs to ci in intact leaves of cocklebur is qualitatively different when photosynthetic electron transport is eliminated, either by removal of light or by addition of DCMU, a PSII inhibitor. In the presence of photosynthetic electron transport, the response changes slope markedly at values of ci very close to the transition of whole-leaf photosynthesis from Rubisco limitation to photosynthetic electron transport limitation: The response is shallow (small slope) at low ci and steeper at high ci. In contrast, the response in darkness or under DCMU is relatively small and does not show a distinct change in slope.
These data suggest there are at least two mechanisms by which stomata respond to CO2. One of these depends on photosynthetic electron transport and is therefore sensitive to the balance between the light and dark reactions of photosynthesis; the other is independent of photosynthetic electron transport and is therefore present in darkness. Both mechanisms may contribute to normal stomatal responses to CO2 in the light.
MATERIALS AND METHODS
Cocklebur (Xanthium strumarium) plants were grown in a controlled-environment greenhouse as described previously (West et al., 2005). Gas-exchange measurements were made using a standard, single-pass gas-exchange system that has also been described previously (West et al., 2005). For all experiments, leaf temperature was maintained at 25°C ± 0.2°C by changing air temperature, and the water vapor mole fraction gradient between the leaf and air was maintained at 15 mmol mol−1 by changing the mole fraction of water in the chamber air.
To examine the effects of DCMU on photosynthesis and gs, a leaf was detached under water and the petiole was placed in distilled, degassed water. The leaf was brought to steady state in a clamp-on gas-exchange chamber that enclosed a circular area of leaf (diameter = 2.54 cm). PFD was maintained at 1,000 μE m−2 s−1, O2 concentration at 21%, and ambient CO2 concentration at 600 μmol mol−1 to ensure that photosynthesis was electron transport limited. At time zero, the petiole was removed from the water and quickly placed in 100 μm DCMU (or in water for control experiments). Gas-exchange data were recorded every 10 s, and fluorescence and thermal images were captured every 3 min. The details of the methods for fluorescence and thermal images have been described previously (West et al., 2005).
The responses of gs and A to ci were determined by placing a leaf (attached to the plant) in a clamp-on chamber that enclosed a square area of the leaf (2.54 × 2.54 cm). The leaf was brought to steady state at an ambient CO2 concentration of 360 μmol mol−1, O2 concentration of either 2% or 21%, and a PFD of either 1,000 or 300 μE m−2 s−1. PFD was gradually increased to these levels over a period of several hours to avoid damaging the leaf. After gas exchange reached steady state (usually within 2–3 h), ci was manipulated by varying ambient CO2 concentration.
To determine the response of gs to ci in darkness, a detached leaf was enclosed in the square gas-exchange chamber and allowed to reach steady state at 2% or 21% O2 at the lowest CO2 concentration. After steady state was achieved, ambient CO2 concentration was increased in steps, allowing the leaf to reach steady state at each concentration. DCMU experiments were done as above, except that 100 μm DCMU were fed to the leaf the day before the experiment.
A long-pass filter with a cutoff at 500 nm was used to investigate the response of stomata to RL. An attached leaf was allowed to reach steady state in the square chamber at a PFD of 800 μE m−2 s−1 of RL, 21% O2, and 360 μmol mol−1 CO2. The PFD was then lowered in steps, allowing the leaf to reach steady state at each value. As gs and photosynthesis changed in response to the new PFD value, ambient CO2 was adjusted to maintain ci constant at the value observed at the highest PFD.
The A versus ci response curves obtained from the light and oxygen experiments were analyzed to estimate the transition point between Rubisco-limited and electron transport-limited photosynthesis. The datapoints on the A versus ci response curves were identified as electron transport limited or Rubisco limited by fitting them with the biochemical photosynthesis model of Farquhar et al. (1980). In that model, the shape of the A versus ci response curve and, consequently, the transition point, depends sensitively on the values of two parameters—the maximum carboxylation rate of Rubisco, Vc,max, and the maximum potential electron transport rate, Jmax. While the other model parameters can be decided a priori, the values of Vc,max and Jmax must be estimated from the A versus ci response curve. For each dataset, the values of Vc,max and Jmax were estimated by minimizing the sum of square error of the model. For each of the four treatments, the parameter values were averaged to estimate the transition point.
Acknowledgments
We thank Rand Hooper for excellent technical assistance and Steve Long for helpful comments on an earlier version of this manuscript.
This work was supported by the National Science Foundation (grant no. 0416600 to K.A.M.) and by the Cooperative Research Centre for Greenhouse Accounting at the Research School of Biological Sciences, Australian National University (T.N.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Keith A. Mott (kmott@biology.usu.edu).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.073676.
References
- Assmann SM (1999) The cellular basis of guard cell sensing of rising CO2. Plant Cell Environ 22: 629–637 [Google Scholar]
- Assmann SM, Shimazaki K (1999) The multisensory guard cell: stomatal responses to blue light and abscisic acid. Plant Physiol 119: 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, Mott KA, Farquhar GD (2003) A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ 26: 1767–1786 [Google Scholar]
- Cardon ZG, Berry JA (1992) Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2, and humidity. Plant Physiol 128: 52–62 [PMC free article] [PubMed] [Google Scholar]
- Dale JE (1961) Investigations into the stomatal physiology of upland cotton. I. The effect of hour of day, solar radiation, temperature and leaf matter content on stomatal behavior. Ann Bot (Lond) 25: 39–52 [Google Scholar]
- Daley PF, Raschke K, Ball JT, Berry JA (1989) Topography of photosynthetic activity of leaves obtained from video images of chlorophyll fluorescence. Plant Physiol 90: 1233–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pury DGG, Farquhar GD (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ 20: 537–557 [Google Scholar]
- Doi M, Shigenaga A, Emi T, Kinoshita T, Shimazaki K (2004) A transgene encoding a blue-light receptor, phot1, restores blue-light responses in the Arabidopsis phot1 phot2 double mutant. J Exp Bot 55: 517–523 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Wong SC (1984) An empirical model of stomatal conductance. Aust J Plant Physiol 11: 191–209 [Google Scholar]
- Genty B, Meyer S (1994) Quantitative mapping of leaf photosynthesis using chlorophyll fluorescence imaging. Aust J Plant Physiol 22: 277–284 [Google Scholar]
- Gotow K, Taylor S, Zeiger E (1988) Photosynthetic carbon fixation in guard cell protoplasts of Vicia faba: evidence from radiolabel experiments. Plant Physiol 86: 700–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath OVS, Russell J (1954) Studies in stomatal behaviour. VI. An investigation of the light responses of wheat stomata with the attempted elimination of control by the mesophyll. Part I. Effects of light independent of carbon dioxide. J Exp Bot 5: 1–15 [Google Scholar]
- Hedrich R, Marten I, Lohse G, Dietrich P, Winter H, Lohous G, Heldt H-W (1994) Malate-sensitive anion channels enable guard cells to sense changes in the ambient CO2 concentration. Plant J 6: 741–748 [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Oxborough K, Morison JIL, Baker NR (2002) Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2, and humidity. Plant Physiol 128: 52–62 [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Oxborough K, Morison JIL, Baker NR (2003) The responses of guard and mesophyll cell photosynthesis to CO2, O2, light and water stress in a range of species are similar. J Exp Bot 54: 1743–1752 [DOI] [PubMed] [Google Scholar]
- Lee J-S, Bowling DJF (1995) Influence of the mesophyll on stomatal opening. Aust J Plant Physiol 22: 357–363 [Google Scholar]
- Mansfield TA, Heath OVS (1961) Photoperiodic effects on stomatal behavior in Xanthium pennsylvanicum. Plant Physiol 69: 273–277 [Google Scholar]
- Nelson SD, Mayo JM (1975) The occurrence of functional non-chlorophyllous guard cells in Paphiopedilum spp. Can J Bot 53: 1–7 [Google Scholar]
- Olsen RL, Pratt RB, Gump P, Kemper A, Tallman G (2002) Red light activates a chloroplast-dependent ion uptake mechanism for stomatal opening under reduced CO2 concentrations in Vicia spp. New Phytol 153: 497–508 [DOI] [PubMed] [Google Scholar]
- Outlaw WH (2003) Integration of cellular and physiological function of guard cells. CRC Crit Rev Plant Sci 22: 503–529 [Google Scholar]
- Poffenroth M, Green DB, Tallman G (1992) Sugar concentrations in guard cells of Vicia faba illuminated with red or blue light—analysis by high performance liquid chromatography. Plant Physiol 98: 1460–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Siebke K, Anderson JM, Badger MR (1998) Photosynthesis is strongly reduced by antisense suppression of chloroplastic cytochrome bf complex in transgenic tobacco. Aust J Plant Physiol 25: 445–452 [Google Scholar]
- Raschke K (1975) Stomatal action. Annu Rev Plant Physiol 26: 309–340 [Google Scholar]
- Roelfsema MRG, Hanstein S, Felle H, Hedrich R (2002) CO2 provides an intermediate link in the red light response of guard cells. Plant J 32: 65–75 [DOI] [PubMed] [Google Scholar]
- Schwartz A, Zeiger E (1984) Metabolic energy for stomatal opening: role of photophosphorylation and oxidative phosphorylation. Planta 161: 129–136 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Raschke K (1981. a) Effect of light quality on stomatal opening in leaves of Xanthium strumarium L. Plant Physiol 68: 1170–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Raschke K (1981. b) Separation and measurement of direct and indirect effects of light on stomata. Plant Physiol 68: 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Shmayevich IJ, Chung Y, Hammad JW, Zeiger E (2003) Blue light and phytochrome-mediated stomatal opening in the npq1 and phot1 phot2 mutants of Arabidopsis. Plant Physiol 133: 1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E (1998) The role of sucrose in guard cell osmoregulation. J Exp Bot 49: 329–337 [Google Scholar]
- Tominaga M, Kinoshita T, Shimazaki K-i (2001) Guard-cell chloroplasts provide ATP required for H+ pumping in the plasma membrane and stomatal opening. Plant Cell Physiol 42: 795–802 [DOI] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS (2005) Guard cell metabolism and CO2 sensing. New Phytol 165: 665–682 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA (2004) Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J Exp Bot 55: 1157–1166 [DOI] [PubMed] [Google Scholar]
- West JD, Peak D, Peterson J, Mott KA (2005) Dynamics of stomatal patches for a single surface of Xanthium strumarium L. leaves observed with fluorescence and thermal images. Plant Cell Environ 28: 633–641 [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426 [Google Scholar]
- Zeiger E (1990) Light perception in guard cells. Plant Cell Environ 13: 739–747 [Google Scholar]
- Zeiger E (2000) Sensory transduction of blue light in guard cells. Trends Plant Sci 5: 183–185 [DOI] [PubMed] [Google Scholar]
- Zeiger E, Talbott LD, Frechilla S, Srivastava A, Zhu J (2002) The guard cell chloroplast: a perspective for the twenty-first century. New Phytol 153: 415–424 [DOI] [PubMed] [Google Scholar]
- Zeiger E, Zhu JX (1998) Role of zeaxanthin in blue light photoreception and the modulation of light-CO2 interactions in guard cells. J Exp Bot 49: 433–442 [Google Scholar]
- Zhu J, Talbott LD, Jin X, Zeiger E (1998) The stomatal response to CO2 is linked to changes in guard cell zeaxanthin. Plant Cell Environ 21: 813–820 [Google Scholar]