Abstract
While increasing temperatures and altered soil moisture arising from climate change in the next 50 years are projected to decrease yield of food crops, elevated CO2 concentration ([CO2]) is predicted to enhance yield and offset these detrimental factors. However, C4 photosynthesis is usually saturated at current [CO2] and theoretically should not be stimulated under elevated [CO2]. Nevertheless, some controlled environment studies have reported direct stimulation of C4 photosynthesis and productivity, as well as physiological acclimation, under elevated [CO2]. To test if these effects occur in the open air and within the Corn Belt, maize (Zea mays) was grown in ambient [CO2] (376 μmol mol−1) and elevated [CO2] (550 μmol mol−1) using Free-Air Concentration Enrichment technology. The 2004 season had ideal growing conditions in which the crop did not experience water stress. In the absence of water stress, growth at elevated [CO2] did not stimulate photosynthesis, biomass, or yield. Nor was there any CO2 effect on the activity of key photosynthetic enzymes, or metabolic markers of carbon and nitrogen status. Stomatal conductance was lower (−34%) and soil moisture was higher (up to 31%), consistent with reduced crop water use. The results provide unique field evidence that photosynthesis and production of maize may be unaffected by rising [CO2] in the absence of drought. This suggests that rising [CO2] may not provide the full dividend to North American maize production anticipated in projections of future global food supply.
Global climate change, in the form of rising temperature and altered soil moisture, is projected to decrease the yield of food crops over the next 50 years (Thomson et al., 2005). Meanwhile, the simultaneous increase in CO2 concentration ([CO2]) is predicted to stimulate crop production and offset these detrimental components of climate change (Thomson et al., 2005). This encouraging projection results from species-specific “CO2 fertilization” factors in yield models (Phillips et al., 1996; Brown and Rosenberg, 1999; Parry et al., 2004; Thomson et al., 2005). These simulate the enhancements of net CO2 assimilation rate (A) and yield observed, for both C3 (17%–29%) and C4 crops (6%–10%), under elevated [CO2] in controlled environment studies (Kimball, 1983; Allen et al., 1987).
While early projections of “[CO2] fertilization” were based on studies in glasshouses and other protected environments, Free-Air Concentration Enrichment (FACE) experiments are fully open-air trials of crop performance. They provide realistic simulations of future growing conditions and provide perhaps the best opportunity to requantify CO2 fertilization effects and elucidate the mechanism of crop response. FACE experiments on the C3 crops rice (Oryza sativa), wheat (Triticum aestivum), and soybean (Glycine max) have observed smaller increases in yield than were predicted from the early chamber studies (Ainsworth and Long, 2005; Long et al., 2005; Morgan et al., 2005). Yet the primary response mechanisms of C3 crops have not been controversial (Ainsworth and Long, 2005). First, elevated [CO2] directly stimulates A, growth, and yield by decreasing photorespiration and accelerating carboxylation by Rubisco. Second, it decreases stomatal aperture, which can reduce plant water use and indirectly enhance performance by ameliorating water stress. In contrast, the response of C4 crops to future elevated [CO2] is uncertain. In C4 plants, Rubisco is localized in the bundle sheath cell chloroplasts, where [CO2] is 3 to 6 times higher than in the atmosphere (He and Edwards, 1996; Kiirats et al., 2002; von Caemmerer and Furbank, 2003). Thus, C4 crops avoid photorespiration, are CO2 saturated at the current atmospheric [CO2], and should not theoretically display greater A at elevated [CO2]. Yield improvements could still be achieved under elevated [CO2] if reduced stomatal conductance (gs) lowers crop water use and ameliorates short-term drought stress by conserving soil moisture (Ghannoum et al., 2000). At elevated [CO2], gs of C4 plants is typically reduced (Ainsworth and Long, 2005). However, lower gs does not guarantee lower water use in the field, where canopy size, structure, and microclimate also regulate water use (Collatz et al., 1991; Meinzer et al., 1997). Also, lower water use will not typically benefit a crop when sufficient soil moisture is available. Yet current projections of grain production assume a stimulation of maize (Zea mays) production by elevated [CO2] in all situations (Phillips et al., 1996; Brown and Rosenberg, 1999; Parry et al., 2004; Thomson et al., 2005). The need for accuracy in these projections is significant because global demand for the C4 crop maize is expected to exceed that for wheat and rice by 2020, making it the world's most important crop (Pingali, 2001).
Some controlled environment studies of well-watered plants suggest that growth at elevated [CO2] can directly impact C4 photosynthesis by a number of mechanisms (for review, see Ghannoum et al., 2000). As examples, intercellular [CO2] (ci) below the saturation point of the photosynthetic intercellular CO2 response (A/ci) curve has been reported under ambient [CO2], allowing direct stimulation of photosynthesis under elevated [CO2] (Wong, 1979; Watling and Press, 1997; Ziska and Bunce, 1997). Bundle sheath leakiness increased under elevated [CO2], reducing the initial slope and CO2-saturated photosynthetic rate of the A/ci curve in sorghum (Sorghum bicolor; Watling et al., 2000). In developing Flaveria trinervia leaves, 10% of CO2 fixation occurred directly in the bundle sheath, without involvement of the C4 concentrating mechanism, allowing the possibility that elevated [CO2] could directly stimulate photosynthesis (Moore et al., 1986). Some immature C4 leaves have C3-like photosynthesis and are therefore more sensitive to enhanced photosynthesis under elevated [CO2] (Dai et al., 1995; Ziska et al., 1999). Enzymes of both the C4 cycle and Calvin cycle in maize were consistently lower under elevated [CO2], with malate dehydrogenase (−37%) and glyceraldehyde-3-phosphate dehydrogenase activities (−29%) declining to the greatest extent in young leaves (Maroco et al., 1999). Such acclimation was interpreted to potentially benefit maize growth by improving nitrogen (N) use efficiency while maintaining rates of photosynthesis. Of these studies, those investigating crop species at elevated [CO2] predicted for 2050 to 2100 reported that the light-saturated rate of photosynthesis (Asat) was stimulated by an average of 23%. Such direct effects of CO2 fertilization on photosynthesis suggest a more optimistic future for food production from C4 crops in the face of increasing temperatures and water stress. Conversely, theoretical treatment of C4 photosynthesis suggested that differences in either leakiness or direct CO2 fixation are unlikely to play a significant role in the responsiveness of C4 photosynthesis to high CO2 (Ghannoum et al., 2000). Also, young C4 leaves in Panicum antidotale and Panicum coloratum are not C3-like (Ghannoum et al., 1998). Unfortunately, growth in chambers and the confinement of the rooting system to pots could generate substantial and surprising artifacts, and responses might not therefore reflect the response of crops in open-air field situations (Arp, 1991; Thomas and Strain, 1991; McLeod and Long, 1999; Ainsworth et al., 2002). Sorghum grown under FACE in Arizona displayed some sensitivity to [CO2] in young, C3-like leaves (Cousins et al., 2001). However, the primary effect of elevated [CO2] on sorghum performance was reported to be improved water relations and amelioration of drought stress (Wall et al., 2001). At SoyFACE in 2002, A of maize under field conditions was episodically stimulated under elevated [CO2] (Leakey et al., 2004). While CO2 effects on photosynthesis were limited to periods of low rainfall, the mechanistic basis for the episodic response was not demonstrated.
The North American Corn Belt is the largest single area of global maize production and is characterized by high growing season rainfall and deep fertile soils capable of substantial water storage. This region accounted for more than 40% of the world's total maize grain production in 2004 (U.S. Department of Agriculture, 2005). The extent of any direct or indirect stimulation of A, growth, and yield of maize in this region by elevated [CO2] has major economic and social implications. The 32-ha FACE facility at the University of Illinois, and an absence of even intermittent water stress in 2004, provided a unique opportunity to test the following three predictions concerning the effects of elevated [CO2] on C4 plants, and maize specifically, under field conditions. In the absence of water stress there is (1) no direct effect of [CO2] on photosynthetic rate, growth, or yield; (2) no [CO2] effect on the development of photosynthetic capacity, as reflected by in vivo and in vitro activities of the key enzymes; and (3) a decrease in gs and water use under elevated [CO2]. This builds upon previous studies to provide a novel mechanistic understanding of the responses to elevated [CO2] of a major food crop, in the major region of production. Most importantly, changes in crop water use under elevated [CO2] are quantified while also testing for direct effects of elevated [CO2] on C4 photosynthesis in the absence of water stress.
RESULTS
Palmer Crop Moisture Index and Microclimate
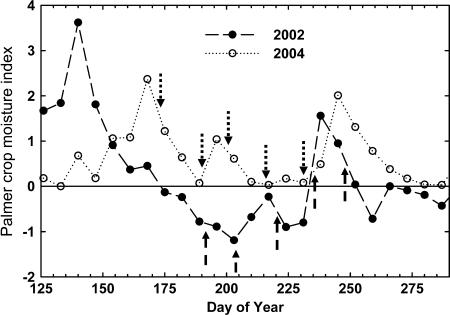
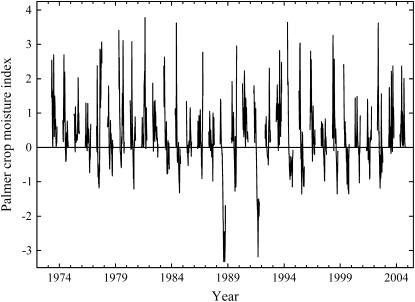
Total rainfall in June, July, and August of 2004 was 347 mm, 11% above the average for the past 50 years of 312 mm. Palmer Crop Moisture Index (PCMI) is a dynamic, meteorological estimate of short-term moisture conditions, based on temperature, precipitation, and modeled soil water content (Palmer, 1968). As PCMI decreases below zero, it indicates progressively greater drought stress conditions. Throughout the 2004 growing season, the PCMI for East Central Illinois was greater than zero (Fig. 1; National Oceanic and Atmospheric Administration [NOAA]/U.S. Department of Agriculture [USDA]; http://www.usda.gov/oce/waob/jawf/). Conditions were rated as favorable for normal growth and field work; moisture adequate for present crop needs for 13 out of the 19 weeks in the growing season, with the remaining weeks rated as some fields too wet; prospects above normal. In other words, 2004 was an ideal growing season in which the crop did not experience drought stress at any time. For comparison, PCMI was often less than zero during a previous experiment at the same site in 2002 (Fig. 1), which indicates that the crop experienced drought stress even though growing season rainfall was also close to average at 321 mm. It is rare in East Central Illinois to have a growing season without any drought stress, in other words, when PCMI is always greater than or equal to zero (Fig. 2). Conditions that were that favorable have only occurred three times since 1973, including 2004 (Fig. 2). However, moderate, episodic drought stress such as in 2002 occurs frequently, with PCMI ≤ −1, occurring roughly one in every three growing seasons.
Figure 1.
PCMI reported weekly during 2002 (•) and 2004 (○) for Illinois Climate Division 5 by the Climate Operation Branch of NOAA (http://www.usda.gov/oce/waob/jawf/). Dates on which diurnal courses of gas exchange were measured are indicated by dashed arrows for 2002 and dotted arrows for 2004.
Figure 2.
Weekly reported PCMI between mid-March and October each year from 1973 to 2004 for Illinois Climate Division 5 (including SoyFACE) by the Climate Operation Branch of the NOAA (http://www.usda.gov/oce/waob/jawf/).
In situ physiological performance was assessed on five dates, corresponding to five discrete and key stages of crop development (Table I). Conditions were predominantly clear and dry on each day except day of year (DOY) 173, when heavy cloud cover and rain affected measurements (Fig. 3). Daily peak values of photosynthetic photon flux density (PPFD; approximately 1,250–2,000 μmol mol−1) covered the range typically experienced in the Midwest United States. The daily mean temperatures (17°C–23°C) were at or slightly below the 40-year average for summer months of 23°C (http://www.sws.uiuc.edu/data/climatedb/).
Table I.
Calendar and DOY of experimental measurements with corresponding crop growth stage described as days after emergence (DAE), developmental stage (defined in Ritchie et al., 1993), and height for maize grown under ambient (370 μmol mol−1) and elevated [CO2] (550 μmol mol−1) during 2004 at SoyFACE, Urbana, IL
| Date
|
DOY
|
DAE
|
Developmental Stage
|
|
|---|---|---|---|---|
| Ambient [CO2] | Elevated [CO2] | |||
| June 21 | 173 | 43 | Tenth leaf | Tenth leaf |
| July 8 | 190 | 60 | Silking | Silking |
| July 19 | 201 | 71 | Blister kernel | Blister kernel |
| August 2 | 215 | 85 | Milky kernel | Milky kernel |
| August 16 | 229 | 89 | Dented kernel | Dented kernel |
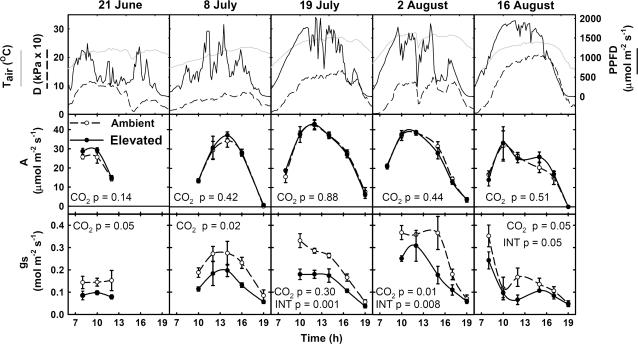
Figure 3.
Diurnal courses of PPFD, Tair, vapor pressure deficit (D), A, and gs of the youngest and uppermost fully expanded leaf of maize grown under ambient (○) and elevated CO2 (•) on five dates during 2004 at SoyFACE. Each point is the mean (±se) of the replicate plots measured at that time (n = 4). P values indicate statistical significance of CO2 and CO2 × time interaction effects.
Diurnal Courses of Leaf Gas Exchange and Chlorophyll Fluorescence
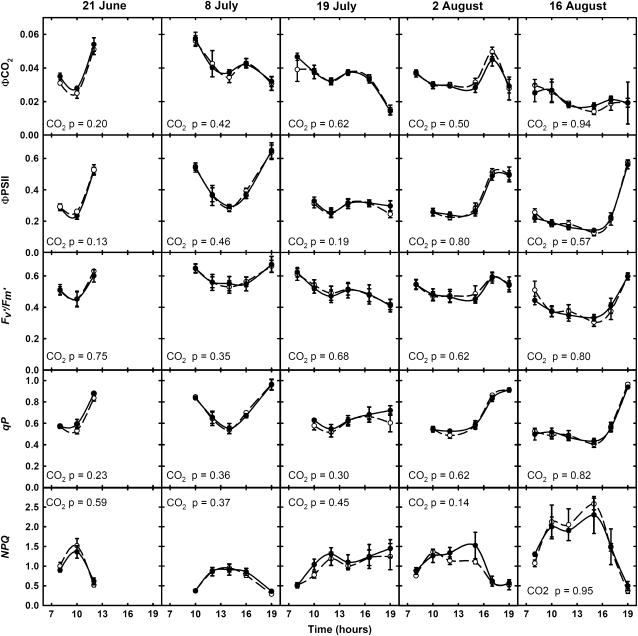
There was no significant effect of CO2 treatment on A at any time on any day (Fig. 3). This lack of difference applied to all photosynthetic parameters measured, including A, quantum yield of photosynthesis (ΦCO2), quantum yield of PSII (ΦPSII), proportion of open PSII reaction centers (qP), intrinsic efficiency of PSII (Fv′/Fm′), and nonphotochemical quenching (NPQ; Figs. 3 and 4). On all dates of measurement and for all photosynthetic parameters investigated, the probability of supporting the null hypothesis was high. As this coincided with relatively small standard errors, the absence of significance is not likely to be the result of high variability leading to a Type II error, but rather because there was no difference. A power test indicated that, with this data set, there was an 88% probability of detecting a 10% stimulation of A by elevated [CO2], even with the Type I error rate of P = 0.05. Under elevated [CO2], gs was 29% lower across the growing season (Fig. 3) and significant for all or part of each day.
Figure 4.
Diurnal courses of ΦCO2, ΦPSII, Fv′/Fm′, qP, and NPQ of the youngest fully expanded leaf of maize grown under ambient (○) and elevated CO2 (•) on five dates during 2004 at SoyFACE, Urbana, IL. Each point is the mean (±se) of the replicate plots measured at that time (n = 4). P values indicate statistical significance of CO2 and CO2 × time interaction effects.
Leaf Midday Gas Exchange; Leaf Photosynthetic Enzyme Activities; and Leaf Carbohydrate, Protein, Amino Acid, Chlorophyll, Specific Leaf Area, Nitrogen, and Water Status
To investigate the basis for any photosynthetic enhancement or acclimation under elevated [CO2], photosynthetic enzyme activities, leaf metabolite pools, and water status were measured at midday alongside gas exchange (Table II). There was no significant [CO2] effect on A at midday across the growing season. Nor was there any significant effect of growth at elevated [CO2] on the activity of the key photosynthetic enzymes phosphoenolpyruvate (PEP) carboxylase (PEPc), pyruvate orthophosphate dikinase (PPDK), or Rubisco, measured at 25°C. In contrast, gs at midday was significantly lower at elevated [CO2], by 34% on average across the season. Therefore, leaf-level transpiration (E) at midday was also significantly lower at elevated [CO2]. Midday ci at elevated [CO2] was significantly greater, by 34% on average across the season. Consequently, there was no significant effect of growth at elevated [CO2] on the ratio of intercellular [CO2] to atmospheric [CO2] (ci/ca) at midday. There was no significant effect of growth at elevated [CO2] on the midday leaf content of total nonstructural carbohydrates (TNC; Table II) or its component pools of starch, Suc, Fru, and Glc (data not shown). There was also no significant effect of growth at elevated [CO2] on the midday leaf content of total protein, total free-amino acids, leaf N, or specific leaf area (SLA). Nor was there a significant [CO2] effect on leaf water status at midday, measured as relative water content (RWC) and total leaf water potential (ψleaf).
Table II.
A (μmol m−2 s−1), PEPc activity (μmol m−2 s−1), PPDK activity (μmol m−2 s−1), Rubisco activity (μmol m−2 s−1), gs (mol m−2 s−1), transpiration (T; mmol m−2 s−1), ci (μmol mol−1), ratio of intercellular to atmospheric CO2 (ci/ca), TNC (mmol m−2), total protein content (g m−2), RWC (%), total water potential (ψ; MPa), N (%) content, and SLA (mm2 mg−1) of the youngest fully expanded leaf of maize grown under ambient (370 μmol mol−1) and elevated [CO2] (550 μmol mol−1) at noon on five dates in 2004 at SoyFACE, Urbana, IL
There was no effect of CO2 on A (P = 0.81), PEPc activity (P = 0.26), PPDK activity (P = 0.76), Rubisco activity (P = 0.59), ci/ca (P = 0.52), TNC (P = 0.25), protein (P = 0.80), RWC (P = 0.60), total ψ (P = 0.86), N (P = 0.21), and SLA (P = 0.48). Asterisk (*), There was a significant CO2 effect on gs (P = 0.037), T (P = 0.049), and ci (P = 0.05).
| Parameter
|
DOY 173
|
DOY 190
|
DOY 201
|
DOY 215
|
DOY 229
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| [CO2] 370 | [CO2] 550 | [CO2] 370 | [CO2] 550 | [CO2] 370 | [CO2] 550 | [CO2] 370 | [CO2] 550 | [CO2] 370 | [CO2] 550 | |
| A | 14.3 ± 2.8 | 15.0 ± 2.8 | 29.4 ± 2.8 | 30.6 ± 2.8 | 42.4 ± 2.8 | 42.9 ± 2.8 | 38.4 ± 2.8 | 38.8 ± 2.8 | 26.4 ± 2.8 | 25.1 ± 2.8 |
| PEPc | 222 ± 39 | 213 ± 18 | 259 ± 32 | 248 ± 13 | 283 ± 19 | 243 ± 27 | 230 ± 12 | 222 ± 18 | 152 ± 19 | 174 ± 19 |
| PPDK | 32.5 ± 1.7 | 32.4 ± 5.0 | 33.0 ± 2.0 | 34.5 ± 3.3 | 39.1 ± 2.7 | 37.9 ± 1.1 | 25.0 ± 3.0 | 27.6 ± 2.2 | 37.6 ± 1.9 | 32.8 ± 2.6 |
| Rubisco | 21.1 ± 3.1 | 17.9 ± 1.8 | 17.7 ± 3.1 | 19.2 ± 2.9 | 22.4 ± 2.3 | 18.9 ± 1.2 | 18.0 ± 2.2 | 19.6 ± 1.8 | 13.2 ± 1.0 | 12.9 ± 1.0 |
| gs* | 0.15 ± 0.04 | 0.08 ± 0.04 | 0.27 ± 0.04 | 0.18 ± 0.04 | 0.29 ± 0.04 | 0.18 ± 0.04 | 0.36 ± 0.04 | 0.31 ± 0.04 | 0.17 ± 0.04 | 0.07 ± 0.04 |
| T* | 1.6 ± 0.6 | 0.9 ± 0.6 | 2.9 ± 0.6 | 2.2 ± 0.6 | 5.7 ± 0.6 | 3.9 ± 0.6 | 4.8 ± 0.6 | 3.6 ± 0.6 | 4.7 ± 0.6 | 1.9 ± 0.6 |
| ci* | 167 ± 31 | 226 ± 31 | 187 ± 31 | 241 ± 31 | 118 ± 31 | 141 ± 31 | 187 ± 31 | 292 ± 31 | 126 ± 31 | 154 ± 31 |
| ci/ca | 0.45 ± 0.13 | 0.41 ± 0.13 | 0.49 ± 0.13 | 0.43 ± 0.13 | 0.31 ± 0.13 | 0.25 ± 0.13 | 0.50 ± 0.13 | 0.52 ± 0.13 | 0.33 ± 0.13 | 0.19 ± 0.13 |
| TNC | 14.2 ± 1.1 | 13.0 ± 0.9 | 11.4 ± 1.1 | 11.9 ± 0.9 | 18.3 ± 0.9 | 18.1 ± 0.9 | 20.1 ± 0.9 | 17.7 ± 0.9 | 20.4 ± 0.9 | 18.4 ± 1.1 |
| Protein | 10 ± 1 | 10 ± 1 | 11 ± 1 | 12 ± 2 | 12 ± 1 | 12 ± 1 | 13 ± 1 | 12 ± 1 | 11 ± 1 | 11 ± 1 |
| RWC | 93.5 ± 0.7 | 90.8 ± 2.0 | 93.7 ± 0.5 | 92.6 ± 1.4 | 94.1 ± 0.8 | 94.6 ± 1.0 | 90.2 ± 2.4 | 91.2 ± 0.9 | 94.0 ± 1.5 | 96.4 ± 1.2 |
| Total ψ | −1.0 ± 0.1 | −1.0 ± 0.1 | −1.5 ± 0.1 | −1.4 ± 0.1 | −1.5 ± 0.1 | −1.7 ± 0.1 | −2.1 ± 0.1 | −1.9 ± 0.1 | −2.1 ± 0.1 | −2.1 ± 0.2 |
| N | 4.0 ± 0.1 | 4.0 ± 0.1 | 3.2 ± 0.2 | 3.2 ± 0.1 | 3.5 ± 0.1 | 3.7 ± 0.1 | 3.0 ± 0.1 | 3.0 ± 0.1 | 2.6 ± 0.1 | 2.8 ± 0.1 |
| SLA | 27 ± 2 | 30 ± 2 | 23 ± 1 | 23 ± 1 | 18 ± 1 | 21 ± 1 | 17 ± 1 | 19 ± 2 | 17 ± 1 | 18 ± 1 |
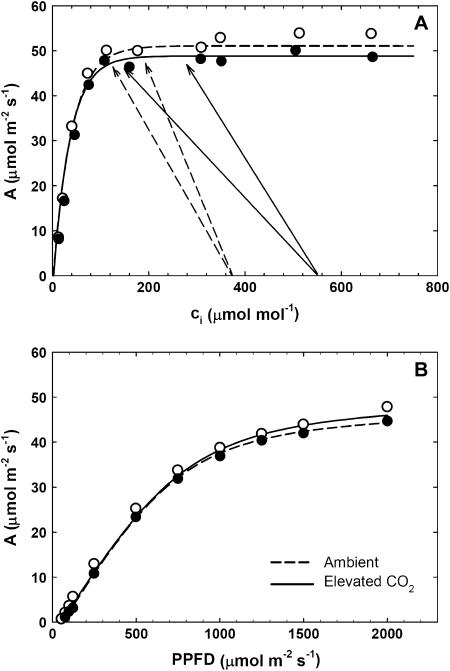
A/ci and Light Response Curves
Leaves cut predawn, maintained hydrated and measured at 30°C in the laboratory, had rates of A equal or higher to fluxes measured in situ, suggesting that the photosynthetic capacity of these leaves was unaffected by this short-term detachment. The A/ci and light response (A/Q) curves (Fig. 5) showed the classical C4 patterns, and the parameter values were close to theoretical expectations (von Caemmerer, 2000). There was no significant effect of growth at elevated [CO2] on maximum apparent rate of PEPc (Vpmax), CO2-saturated rate of photosynthesis (Vpr), Asat, or maximum apparent quantum yield as determined from these response curves.
Figure 5.
Photosynthetic gas-exchange analysis of the youngest fully expanded leaf of maize growing under ambient (○) and elevated CO2 (•). A, Representative A/ci curves fitted with model equations for C4 photosynthesis (von Caemmerer, 2000) of maize grown at ambient (dashed) and elevated [CO2] (solid). Arrows indicate the range of ci measured at midday during in situ measurements of maize grown at ambient (dashed) and elevated [CO2] (solid). There was no significant CO2 effect on Vpmax (DOY 180: ambient 139 ± 16, elevated [CO2] 122 ± 13; DOY 212: ambient 109 ± 3, elevated [CO2] 112 ± 5) or Vpr (DOY 180: ambient 51.2 ± 1.8, elevated [CO2] 48.6 ± 1.2; DOY 212: ambient 44.0 ± 4.6, elevated [CO2] 40.1 ± 3.0). B, Representative A/Q curves fitted with nonrectangular hyperbolas for maize grown at ambient (dashed) and elevated CO2 (solid). There was no significant CO2 effect on maximum apparent quantum yield of photosynthesis (DOY 180: ambient 0.07 ± 0.01, elevated [CO2] 0.07 ± 0.01) or Asat (DOY 180: ambient 58.9 ± 1.3, elevated [CO2] 60.8 ± 1.4).
Crop Biomass, Development, and Yield
There was no significant effect of growth at elevated [CO2] on stover biomass, grain biomass, kernel number, individual kernel weight, total leaf area, anthesis date, or silking date (Table III). The yields of approximately 10.5 t (seed) ha−1 and approximately 20.3 t (total biomass) ha−1 are among the higher yields for the Corn Belt, showing that the crop was representative of current agriculture.
Table III.
Biomass of stover and grain, kernel number, individual kernel weight, total leaf area, and DOY of anthesis and silking for maize grown at ambient (370 μmol mol−1) or elevated [CO2] (550 μmol mol−1) upon harvest at the end of the growing season in 2004 at SoyFACE in Urbana, IL
| Parameter | [CO2] 370 | [CO2] 550 | P |
|---|---|---|---|
| Stover biomass R6 (g plant−1) | 134 ± 11 | 131 ± 9 | 0.68 |
| Grain biomass R6 (g plant−1) | 140 ± 6 | 142 ± 6 | 0.8 |
| Kernel number (plant−1) | 598 ± 38 | 609 ± 29 | 0.37 |
| Kernel weight (mg) | 248 ± 7 | 247 ± 5 | 0.83 |
| Total leaf area (cm2 plant−1) | 6,280 ± 471 | 6,304 ± 365 | 0.48 |
| Anthesis date | 188.9 ± 0.3 | 188.7 ± 0.2 | 0.53 |
| Silking date | 188.3 ± 0.3 | 188.1 ± 0.3 | 0.63 |
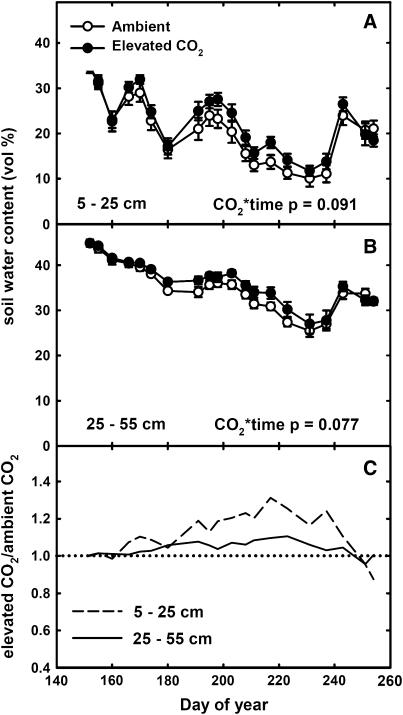
Soil Water Content
There was no difference in volumetric soil water content (H2O%) between treatments at the beginning of the season (Fig. 6, A and B). Over the growing season, H2O% decreased due to crop water use but was regularly replenished by rain. The ratio of H2O% in elevated [CO2] compared to ambient [CO2] plots gradually increased to reach 1.31 between 5 and 25 cm depth on DOY 215, and 1.11 between 25 and 55 cm depth on DOY 223 (Fig. 6C). This greater H2O% under elevated [CO2] reflected a significant interaction between the [CO2] and time in both the upper and lower soil layers.
Figure 6.
Soil H2O% at depths of 5 to 25 cm (A) and 25 to 55 cm (B) in plots of maize growing under ambient (○) and elevated CO2 (•) during 2004 at SoyFACE. Each point is the mean (±se) of the replicate plots measured at that time (n = 4). C, The ratio of H2O% in elevated [CO2] compared to ambient [CO2] treatments, at 5 to 25 cm (dashed line) and 25 to 55 cm (solid line). P values indicate statistical significance of CO2 × time interaction effects.
DISCUSSION
In 2004, the climate of Central Illinois was particularly favorable for crop growth, as reflected in the high yield in both control and elevated [CO2] plots. The absence of water stress throughout the season provided a rare opportunity to test for direct effects of elevated [CO2] on photosynthesis and water relations in a major C4 crop, under field conditions. In accordance with our first and second predictions, growth at elevated [CO2] did not stimulate A at any time of the day and at any of the developmental stages investigated. Nor did it impact photosynthetic development by altering in vivo or in vitro activities of key enzymes. However, in accordance with our third prediction, growth at elevated [CO2] did significantly decrease gs, corresponding to improved soil water availability by midseason. In the same genotype on the same site in 2002, A was transiently stimulated by elevated [CO2] during periods of intermittent drought stress, probably as a result of improved water relations (Leakey et al., 2004). Early chamber studies (Rudorff et al., 1996; Samarakoon and Gifford, 1996) found no increase in A and production in well-watered maize grown in elevated [CO2], but many other studies of well-watered plants have reported an increase in A and acclimation in the amounts of key photosynthetic enzymes (Wong, 1979; Moore et al., 1986; Dai et al., 1995; Watling and Press, 1997; Ziska and Bunce, 1997; Maroco et al., 1999; Ziska et al., 1999; Watling et al., 2000). It is hard to compare the results presented here with this previous work because most previous studies examined Asat at only one time in the day and at one or two stages in the plant life cycle. Such data cannot be easily extrapolated to field conditions where photosynthetic regulation and flux vary substantially over the diurnal course and with development of the crop. Additionally, most prior work was carried out in chambers rather than the field. Plants in chambers do not experience normal atmospheric coupling and have limited rooting volume, both of which might impact whole-plant water relations, carbon gain, and growth. Uniquely, this study of field-grown maize, at a site very typical of the major crop production area, found no evidence of any direct effect of elevated [CO2] on photosynthetic rate, photosynthetic enzymes, development, dry matter production, or harvestable yield in the absence of water deficit. This work will enable the updating of models projecting future crop yields and food supply.
No Direct Effect of Elevated [CO2] on Photosynthesis
If any of the proposed mechanisms for direct CO2 effects on C4 photosynthesis (Wong, 1979; Moore et al., 1986; Dai et al., 1995; Watling and Press, 1997; Ziska and Bunce, 1997; Maroco et al., 1999; Ziska et al., 1999; Watling et al., 2000) occurred in this experiment, it was not evident in either net photosynthetic CO2 assimilation or any chlorophyll fluorescence parameter at any time of day or at any of the five developmental stages assessed. If responses occurred at a developmental stage not assessed by the physiological analyses performed (e.g. C3-like photosynthesis in very young leaves), then they were not of sufficient significance to alter biomass accumulation, development, or yield. In addition, there was no effect of growth under FACE on midday photosynthesis or final yield of two additional cultivars (FR1064 × LH185 and FR1064 × IHP; M. Uribelarrea, unpublished data), which have previously been shown to differ in grain yield and composition (Uribelarrea et al., 2004).
Rising temperature can stimulate CO2-saturated photosynthesis on the plateau of the C4 A/ci curve while having little effect on the initial slope (Sage and Kubien, 2003). This means that at high temperatures the CO2-saturation point increases and C4 photosynthesis could be more sensitive to direct enhancement by elevated [CO2]. However, this appears unlikely to regulate the responses to elevated [CO2] observed at SoyFACE. First, temperatures were relatively low (maximum approximately 26°C) on July 11, 2002, when the greatest [CO2] effect on photosynthesis was observed (Leakey et al., 2004). The crop experienced higher temperatures on all other measurement dates in 2002 and also on several dates in this study without a [CO2] effect on photosynthesis. Second, the initial slopes of the A/ci curves for maize in this study were steep (at 30°C, CO2-saturation point <150 μmol mol−1) compared to those for Amaranthus retroflexus reported by Sage and Kubien (2003; at 32°C, CO2-saturation point >200 μmol mol−1). Therefore, in maize grown at SoyFACE, any increase in the Vpr with temperature would have less effect on the CO2-saturation point. Notably, the CO2-saturation point of the A/ci curve is reported to increase with increasing PPFD, N, and water supplies as a result of changes in PEPc/Rubisco activity ratio and bundle sheath leakiness (Leegood and von Caemmerer, 1989; Ghannoum et al., 2000). This would make C4 photosynthesis more sensitive to direct stimulation by elevated [CO2] and suggests that the favorable growing conditions at SoyFACE in 2004 make this study a relatively conservative test for direct CO2 effects on C4 photosynthesis. Nonetheless, it only infers that elevated [CO2] will not directly stimulate the large fraction of global maize supply produced in the U.S. Corn Belt. Projecting the future performance of maize crops grown in tropical latitudes, where stress is more severe and elevated [CO2] might provide greater benefits, requires further study.
It is possible that A might not change in situ if counteracting acclimations to elevated [CO2] occur, e.g. direct stimulation of A by elevated [CO2] offset by a decrease in capacity for PEP carboxylation or PEP regeneration. However, there was no effect of growth at elevated [CO2] on these activities in vivo or in vitro. Neither PEP carboxylation or PEP regeneration capacity calculated from the A/ci response, nor the in vitro activity at 25°C of the key photosynthetic enzymes Rubisco, PEPc, and PPDK, were altered by elevated [CO2]. Similarly, the A/Q responses suggest a complete absence of acclimation in both light-limited and light-saturated photosynthetic capacity. The in vitro photosynthetic enzyme activities did not match the photosynthetic rates measured in situ. In vitro conditions did not mimic in vivo temperatures, and it is likely that some activity or protein was lost during the extraction and in vitro assay procedure. However, these effects should impact samples from each treatment to the same degree and should not prevent comparisons between treatments on a relative basis. Previously, incomplete extraction of Rubisco protein from pine needles reduced in vitro measures of activity below estimates from in vivo assays but did not alter the magnitude of CO2 treatment effects (Rogers et al., 2001). There was also no change in ci/ca, suggesting an absence of any acclimation of stomatal response. Therefore, the lower gs in elevated [CO2] likely resulted from an instantaneous response and not any long-term response to growth at elevated [CO2]. Increased activities of enzymes involved in Suc and starch synthesis have been reported in maize grown at elevated [CO2] with stimulated photosynthetic rates (e.g. Maroco et al., 1999). There was no evidence of increased starch or Suc content at elevated [CO2] in this study even during the vegetative stage of crop development, when sink capacity in maize is relatively low compared to sink capacity during grain filling. It is also possible that lower water use, indicated by consistently lower gs, might reduce nitrate uptake by mass flow, reduce leaf N, and counteract any enhancement of A by elevated [CO2]. Total leaf N, soluble protein, and chlorophyll contents are markers of leaf N status, while total leaf free-amino acid content reflects whole-plant N status (Hirel et al., 2005). Total free-amino acid content, which is the hub around which the processes of N assimilation and associated carbon metabolism revolve (Foyer et al., 2003), was higher in young vegetative plants, as reported previously (Hirel et al., 2005). Nonetheless, there was no [CO2] effect on markers of plant or leaf N status. In summary, despite a consistent decrease in gs, there was no evidence of the stimulation of A and acclimation in the photosynthetic apparatus in response to the elevated [CO2] that has been observed in some studies within chambers.
A number of factors may explain this difference in results; these include genotype, developmental stage, and the treatment [CO2]. Maize has been grown at approximately 3 times current [CO2] (Maroco et al., 1999) and sorghum at approximately 2 times current [CO2] (Watling et al., 2000), compared to approximately 1.5 times current [CO2] in this experiment. But there was no evidence of the physiological acclimation to elevated [CO2] observed in these chamber studies in this field study. Therefore, the difference could only be explained by a threshold effect, i.e. acclimation occurring when a threshold concentration is exceeded. The cultivar used in this experiment is a major production line currently used in the Corn Belt and closely related to the germ plasm used in most of the region's current production lines. Although effects could be specific to developmental stages not covered by this study, this has included vegetative and reproductive stages of the crop, including those where any effect on A would have its maximum impact on yield (Ritchie et al., 1993). Alternatively, maize crops in the deep soils of the Corn Belt can root to 2 m. Therefore, their root systems extend far beyond that allowed by even the largest pots. Compared to the field, the root system of any potted plant will be highly restricted, and this would slow water uptake of even the best watered pots. As a result, subtle improvements in plant water status may occur in container-grown maize under elevated [CO2] that would be absent in the open field under ample water conditions.
Only one other FACE experiment has assessed the photosynthetic response of a C4 crop to elevated [CO2]. Sorghum was grown under elevated [CO2] in Arizona, with irrigation. These plants were suggested to display C3-like photosynthesis in young leaves and some suppression of photorespiration, along with increases in energy use efficiency (Cousins et al., 2001). The analysis of A/ci curves indicated that this phenomenon did not occur in maize at SoyFACE. In addition, it was concluded that the direct effects of CO2 enrichment on A of sorghum under FACE in Arizona were minor, and indirect enhancement of A by improved water relations was cited as the primary mechanism of response (Wall et al., 2001).
Direct Effects of Elevated [CO2] on Water Use
Given no change in leaf area, the decrease in gs at elevated [CO2] would favor reduced whole-plant water use. This is consistent with the observation that, at soil depths of 5 to 55 cm, soil in the elevated [CO2] plots retained progressively more moisture compared to ambient plots until maximum leaf area was reached. Water conservation under elevated [CO2] has been observed in chamber experiments on C4 species (Owensby et al., 1997; Nelson et al., 2004). However, in these cases, forced canopy-atmosphere coupling, caused by fumigation with forced air circulation, may have artificially increased the extent to which plant water use was controlled by gs. Sorghum canopy evapotranspiration, measured across two growing seasons by energy balance techniques in the FACE experiment in Arizona, was reduced by elevated [CO2] under both ample water supply (−10%) and severe drought stress (−4%; Conley et al., 2001). However, in the “wet” treatment, with ample water supply, the soil was drier under elevated [CO2] throughout both growing seasons (Wall et al., 2001), creating some ambiguity as to the basis for the result.
The growing conditions of 2004 in Central Illinois were so close to ideal that the observed improvements in water use efficiency did not alter plant water status. However, greater soil water would in most growing seasons be expected to delay or prevent the onset of drought stress during the periods of low rainfall. The episodic enhancement of A in maize during periods of drought at SoyFACE in 2002 is consistent with this phenomenon (Leakey et al., 2004).
Should an increase in A be expected due to the reduced evaporative cooling caused by lower gs at elevated [CO2]? The season-long average gs over the five diurnal cycles of measurement was 0.21 mmol m−2 s−1 for ambient [CO2] and 0.15 mmol m−2 s−1 for elevated [CO2], with a daytime average air temperature (Tair) of 22.7°C and PPFD of 880 μmol m−2 s−1, approximating to a solar radiation flux of 420 J m−2 s−1. Assuming an absorptance of 0.9, typical of healthy leaves, mean daytime relative humidity of 70%, and wind speed of 4.1 m s−1, the average increase in temperature caused by the lower gs in elevated [CO2] would be 0.26°C, calculated from the energy balance equations of Grace (1983). Using the relationship of A to leaf temperature for maize defined by Hofstra and Hesketh (1969), this would cause an increase in leaf photosynthesis of 0.3 μmol m−2 s−1. Even this may be an overestimate since it is based on a mean temperature (22.7°C) below the optimum; during periods when the optimum (approximately 34°C) is approached or reached, there will be no increase in A. Therefore, growth at elevated [CO2] probably favors greater photosynthesis due to increased leaf temperature, but the effect is small relative to Asat. As a result, the effect had no detectable impact on biomass accumulation and yield over the growing season.
CONCLUSION
Maize is predicted to become the world's most important crop, in terms of human food supply, by 2050 (Pingali, 2001). While the results of our study are limited to one location and one hybrid line, farming practice and crop performance at SoyFACE are typical of the surrounding area and the genotype shares lineage with many other production lines. Champaign County is centrally located in the U.S. Corn Belt and is consistently high yielding (http://www.usda.gov/nass/graphics/county04/crpmap04.htm#corn). So the results of this study should at least relate to the Corn Belt, which generates 40% of global maize production (USDA, 2005). The absence of any photosynthetic, growth, or yield response of maize to elevated [CO2] in 2004 at SoyFACE is inconsistent with some earlier cabinet studies, and suggests that including a direct and consistent CO2 fertilization effect on C4 crop performance is currently a significant source of error in estimating future food security. It appears that elevated [CO2] will only enhance performance by reducing crop water use. Therefore, improvements in A, growth, and yield will only occur if stress is ameliorated in times or places of drought. Unfortunately, the indirect nature of this mechanism, combined with considerable uncertainty regarding future soil water availability (Cubasch et al., 2001), makes predicting future crop performance difficult. Total precipitation in North America is projected to increase slightly this century (Giorgi et al., 2001), but there is also predicted to be an increase in the frequency and magnitude of droughts as climate becomes more variable (Gregory et al., 1997; Beersma and Buishand, 1999). Therefore, projections of crop performance will need to explicitly deal with water stress, and its interaction with elevated [CO2], if they are to be reliable. In 2002, A of maize at SoyFACE was stimulated, on average, by 10% (Leakey et al., 2004). Years with episodic droughts, such as 2002, occur every 2 to 3 years in Central Illinois. Therefore, future elevated [CO2] may often indirectly enhance A and possibly yield. However, the impact on growth and yield will vary with the duration and timing of water stress in the growing season. Additionally, FACE studies of C3 crops indicate that the benefits of growth at elevated [CO2] are greatest for A, lower for productivity, and least for yield (Ainsworth and Long, 2005; Long et al., 2005; Morgan et al., 2005). Likewise, in a FACE experiment on amply irrigated sorghum, elevated [CO2] stimulated A by 9%, but did not enhance total biomass or grain yield (Ottman et al., 2001). Therefore, it appears that elevated [CO2] will increasingly have a role in determining C4 crop performance via amelioration of drought stress. However, in the absence of any direct stimulation of photosynthesis, it is unclear that this will be sufficient to override, or even negate, the detrimental effects of increasing temperature and drought on yield.
MATERIALS AND METHODS
Field Site, Cultivation, and FACE System
The study was conducted in a 16-ha field of maize (Zea mays) at the SoyFACE facility in Champaign, IL. The facility operational procedures and crop cultivation were repeated from a previous experiment (Leakey et al., 2004). Maize cv 34B43 (Pioneer Hi-Bred International) was planted on April 29, 2004, emerged on May 9, 2004, and was harvested on September 10, 2004. The infrastructure for CO2 enrichment was installed immediately after planting in four experimental blocks (n = 4 for statistical tests). In each block, one plot was at current ambient [CO2] of 376 μmol mol−1, while a second plot was fumigated during daylight hours to an average elevated [CO2] of 542 μmol mol−1. The target [CO2] for simulating the conditions in 2050 was 550 μmol mol−1, midpoint of different projections varying in assumptions about population and economic development (Prentice et al., 2001). The [CO2] enrichment achieved during the growing season was within ±20% of the target 93% of the time.
Meteorological and Soil Water Data
An on-site weather station measured Tair, relative humidity, incident PPFD, and rainfall throughout the season. H2O% was measured in 10-cm increments between depths of 5 and 105 cm using a capacitance probe (Diviner-2000; Sentek Sensor Technologies). Measurements were taken every 3 to 7 d at four positions in a 1-m2 area near the center of each plot. Weekly records of the PCMI from 1973 to 2004 for East Central Illinois were provided by the Climate Operation Branch of NOAA (http://www.usda.gov/oce/waob/jawf/).
In Situ Gas Exchange and Tissue Sampling
The diurnal course of gas exchange and chlorophyll fluorescence of the youngest fully expanded leaf in each plot was measured on five dates across the season, using four open gas-exchange systems with integrated modulated chlorophyll fluorometers (LI-6400 and LI-6400-40; LI-COR). Full expansion was judged by emergence of the ligule. The dates corresponded to five discrete stages of crop development, including vegetative growth, silking, and grain filling (Table I). On each date, four gas-exchange systems were used simultaneously at intervals of approximately 2 h from early morning to sunset. At each interval, one gas-exchange system was operated within each of the four experimental blocks. Each block consisted of one ambient and one elevated [CO2] plot. Two gas-exchange systems were first used in ambient [CO2] plots, while the other two gas-exchange systems were first used in elevated [CO2] plots. Each gas-exchange system was then moved to the alternate [CO2] treatment within the block. The gas-exchange systems were rotated among blocks and starting [CO2] treatment at each time point. These procedures ensured that measurements were not biased by differences in microclimate over time, or differences between gas-exchange systems. Three plants were measured in each plot at each time interval. Measurements of chlorophyll fluorescence and gas-exchange parameters on all plants were made at growth [CO2], Tair, and PPFD. Leaf A, gs, and ci were calculated using the equations of von Caemmerer and Farquhar (1981). The formation of dew on leaves precluded measurement of gs at dawn and dusk and at other periods of the day on occasion. Transpiration per unit leaf area (E), measured by the gas-exchange system, is affected by chamber humidity, which may differ from that of the external atmosphere. Therefore, a better measure of transpiration was calculated as the product of leaf conductance and the leaf vapor pressure deficit, which was determined from measured leaf temperature and the external ambient air humidity. Chlorophyll fluorescence parameters (qP, ΦPSII, Fv′/Fm′, NPQ, and ΦCO2) were defined and calculated as described by Naidu and Long (2004).
Directly after the photosynthetic measurements, leaf discs (approximately 1.2 cm2) were excised, plunged immediately into liquid N, and then stored at −80°C until analyzed for carbohydrate, protein, free-amino acid, and chlorophyll contents. Additional discs were removed and sealed into scintillation vials for RWC analysis (approximately 3.6 cm2 per plant) or sealed in stainless steel psychrometer chambers (approximately 2.4 cm2 per plant; C-30; Wescor) for water potential analyses. Finally, leaf discs (approximately 3.6 cm2 per plant) were removed and dried in an oven at 70°C to constant weight and weighed for calculation of SLA.
Foliar Biochemical, Nitrogen, and Water Analyses
Foliar contents of carbohydrates, protein, and total free-amino acids were determined from 80% (v/v) ethanol extracts as by Geigenberger et al. (1996). Glc, Fru, and Suc were determined using a continuous enzymatic substrate assay (Rogers et al., 2004). For protein and starch determination, pellets of the ethanol extraction were solubilized by heating to 95°C in 0.1 m NaOH. Protein content was determined using a commercial kit (Protein assay kit; Pierce) with bovine serum albumin as a standard. The NaOH solution containing the dissolved pellet was then acidified to pH 4.9 and the starch content was determined as by Hendriks et al. (2003). Total free-amino acid contents were determined using a fluorescamine assay (Bantan-Polak et al., 2001).
The in vitro activities of Rubisco, PPDK, and PEPc were all measured indirectly as the rate of oxidation of NADH (specific absorption coefficient of 6.22 mm−1) using linked enzyme assays in a dual-beam spectrophotometer (Cary I; Varian) at 340 nm and 25°C. The extraction of Rubisco followed the procedure outlined by Sharkey et al. (1991), with the following modifications. One protease inhibitor cocktail tablet (Roche Applied Science) per 10 mL of extraction solution was added to inhibit enzyme degradation. Leaf tissue was rapidly ground (60–120 s) in 2.0 mL of extraction solution at 0°C using an ice-chilled glass tissue homogenizer. The extract was then centrifuged for 15 s at 15,000g. To fully activate Rubisco, a 1-mL aliquot of the supernatant was added to 20 mm MgCl2 and 10 mm NaHCO3 (Sharkey et al., 1991). The crude extract was incubated at room temperature until maximum activity was stable (approximately 8 min). A 50-μL aliquot of the crude extract was added to a cuvette containing 700 μL of assay medium and assayed for 1 min. The assay medium was prepared according to Sharkey et al. (1991) with the following modifications: 1.8 units (2.6 units mL−1) of creatine phosphokinase, 1.8 units (2.6 units mL−1) of phosphoglycerate kinase, and 9.2 units (13.1 units mL−1) of glyceraldehyde-3-P dehydrogenase were used. Leaf tissue was prepared as for the Rubisco assay, before PPDK was extracted as previously described (Crafts-Brandner and Salvucci, 2002). The crude extract was allowed to incubate in the assay medium for 5 min at 25°C, and the reaction was initiated by the addition of 15 μL of 100 mm pyruvate (2.2 mm final concentration) and 12 μL (6 units) of purified maize PEPCase (Bio-Research Products) and then assayed for 1 min. Incubation in this manner was found to increase the in vitro activity by 10% to 20%. Leaf tissue was prepared as for the Rubisco assay, before PEPCase was extracted by the method of Crafts-Brandner and Salvucci (2002), with the exception of using 5 mm dithiothreitol in place of β-mercaptoethanol. A 35-μL aliquot of the supernatant was added to a cuvette containing 665 μL of assay medium and assayed for 1 min. The assay medium was prepared as described previously (Giglioli-Guivarc'h et al., 1996) with the addition of 5 mm Glc-6-P and 2 mm dithiothreitol (Ashton et al., 1990). Malate dehydrogenase was increased to 6.5 units per assay (9.4 units mL−1).
Dried leaf material was powdered and analyzed for N content using an elemental combustion system (model 4010; Costech Analytical Technologies). RWC was measured as by Ghannoum et al. (2002). A dew point microvoltmeter (HR-33T; Wescor) measured ψleaf after psychrometer chambers (C-30; Wescor) containing leaf discs (2.4 cm2) were equilibrated in a controlled environment cabinet at 25°C.
A/ci and A/Q Curves
Predawn on DOY 180 and 212, the youngest fully expanded leaf of two plants per plot were cut from the plant and then immediately recut under water and kept immersed. The objective was to reveal any effect of elevated [CO2] on the potential photosynthetic capacity of the leaves, through measurement of A/ci and A/Q curves. Sampling leaves predawn and performing measurements under controlled conditions avoided the short-term decreases in water potential, chloroplast inorganic phosphate concentration, and maximum PSII efficiency that can occur in the field and may transiently limit photosynthetic performance. Using the gas-exchange and fluorescence apparatus described above, A/ci curves were determined in the laboratory at a PPFD of 1,750 μmol m−2 s−1 and A/Q curves were determined at growth [CO2], as by Bernacchi et al. (2005). All measurements were performed at 30°C. The response of A to ci at ci <50 μmol mol−1 was used to solve for Vpmax (von Caemmerer, 2000). Vpr was estimated from the horizontal asymptote of a nonrectangular hyperbolic function for each A/ci curve. From A/Q curves, ΦCO2 and Asat were calculated as by Naidu and Long (2004).
Crop Development, Biomass, and Yield
Silking dates were defined at the point in time when 50% of plants had visible silks and anthesis dates when 50% of plants shed pollen. At flowering, the area of every leaf on four randomly sampled plants from each plot was determined from the linear dimensions and a pre-established relationship with area (McKee, 1964). At the end of the growing season, four plants were sampled from each plot and separated into grain and stover (i.e. the remainder of the shoot). These fractions were oven dried at 75°C to constant weight and their mass determined.
Statistics
In all cases, statistics were performed on plot means using the MIXED procedure of SAS, with the Satterthwaite option (SAS Institute, Cary, NC). The Akaike's criterion was used to choose the best model of variance-covariance. In all tests, [CO2] treatment was a fixed effect and block a random effect. In tests of physiological processes, time of day and DOY were fixed effects. For the overall comparison of H2O% between treatments over the growing season, a mixed model was fitted to repeated measures of time.
Acknowledgments
We thank Carl Bernacchi, Joe Castro, Katie Ciccodicola, Emily Doherty, Ryan Goodling, Mark Harrison, Lindsey Heady, Emily Heaton, Kevin Hollis, Justin Mcgrath, David Marshak, Amy Peterson, Kelly Ramig, John Szarejko, Tony Watson, Richard Webster, Meagan Wells, and Victoria Wittig for assistance with field measurements and sampling; Tim Mies for operating and managing the SoyFACE experimental facility; Tom Heddinghaus at NOAA for PCMI data; and the Illinois State Water Survey for climate data.
This work was supported by the Illinois Council for Food and Agricultural Research, by the Archer Daniels Midland Company, by the International Arid Land Consortium, and by the U.S. Department of Agriculture Agricultural Research Service. A.R. was supported by the U.S. Department of Energy Office of Science (contract no. DE–AC02–98CH10886 to Brookhaven National Laboratory and a Laboratory Directed Research and Development award).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Andrew D.B. Leakey (leakey@life.uiuc.edu).
Open Access articles can be viewed online without a subscription.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.073957.
References
- Ainsworth EA, Davey PA, Bernacchi CJ, Dermody OC, Heaton EA, Moore DJ, Morgan PB, Naidu SL, Ra HSY, Zhu XG, et al (2002) A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Glob Change Biol 8: 695–709 [Google Scholar]
- Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165: 351–371 [DOI] [PubMed] [Google Scholar]
- Allen LH, Boote KJ, Jones JW, Jones PH, Valle RR, Acock B, Rogers HH, Dahlman RC (1987) Response of vegetation to rising carbon dioxide: photosynthesis, biomass and seed yield of soybean. Global Biogeochem Cycles 1: 1–14 [Google Scholar]
- Arp WJ (1991) Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ 14: 869–875 [Google Scholar]
- Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD (1990) Enzymes of C4 photosynthesis. In PJ Lea, ed, Methods in Plant Biochemistry, Vol 3. Academic Press, New York, pp 39–72
- Bantan-Polak T, Kassai M, Grant KB (2001) A comparison of fluorescamine and naphthalene-2,3-dicarboxaldehyde fluorogenic reagents for microplate-based detection of amino acids. Anal Biochem 297: 128–136 [DOI] [PubMed] [Google Scholar]
- Beersma JJ, Buishand TA (1999) A simple test for equality of variances in monthly climate data. J Clim 12: 1770–1779 [Google Scholar]
- Bernacchi CJ, Morgan PB, Ort DR, Long SP (2005) The growth of soybean under free air CO2 enrichment (FACE) stimulates photosynthesis while decreasing in vivo Rubisco capacity. Planta 220: 434–446 [DOI] [PubMed] [Google Scholar]
- Brown RA, Rosenberg NJ (1999) Climate change impacts on the potential productivity of corn and winter wheat in their primary United States growing regions. Clim Change 41: 73–107 [Google Scholar]
- Collatz GJ, Ball JT, Grivet C, Berry JA (1991) Physiological and environmental-regulation of stomatal conductance, photosynthesis and transpiration: a model that includes a laminar boundary-layer. Agr Forest Meteorol 54: 107–136 [Google Scholar]
- Conley MM, Kimball BA, Brooks TJ, Pinter PJ, Hunsaker DJ, Wall GW, Adam NR, LaMorte RL, Matthias AD, Thompson TL, et al (2001) CO2 enrichment increases water-use efficiency in sorghum. New Phytol 151: 407–412 [Google Scholar]
- Cousins AB, Adam NR, Wall GW, Kimball BA, Pinter PJ, Leavitt SW, LaMorte RL, Matthias AD, Ottman MJ, Thompson TL, et al (2001) Reduced photorespiration and increased energy-use efficiency in young CO2-enriched sorghum leaves. New Phytol 150: 275–284 [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129: 1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubasch U, Meehl GA, Boer GJ, Stouffer RJ, Dix M, Noda A, Senior CA, Raper S, Yap KS, Abe-Ouchi A, et al (2001) Projections of future climate change. In JT Houghton, Y Ding, DJ Griggs, M Noguer, PJ van der Linden, X Dai, K Maskell, CA Johnson, eds, Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York, pp 525–582
- Dai ZY, Ku MSB, Edwards GE (1995) C4 photosynthesis. The effects of leaf development on the CO2-concentrating mechanism and photorespiration in maize. Plant Physiol 107: 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Parry M, Noctor G (2003) Markers and signals associated with nitrogen assimilation in higher plants. J Exp Bot 54: 585–593 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Lerchl J, Stitt M, Sonnewald U (1996) Phloem-specific expression of pyrophosphatase inhibits long-distance transport of carbohydrates and amino acids in tobacco plants. Plant Cell Environ 19: 43–55 [Google Scholar]
- Ghannoum O, Siebke K, Von Caemmerer S, Conroy JP (1998) The photosynthesis of young Panicum C4 leaves is not C3-like. Plant Cell Environ 21: 1123–1131 [Google Scholar]
- Ghannoum O, von Caemmerer S, Conroy JP (2002) The effect of drought on plant water use efficiency of nine NAD-ME and nine NADP-ME Australian C4 grasses. Funct Plant Biol 29: 1337–1348 [DOI] [PubMed] [Google Scholar]
- Ghannoum O, von Caemmerer S, Ziska LH, Conroy JP (2000) The growth response of C4 plants to rising atmospheric CO2 partial pressure: a reassessment. Plant Cell Environ 23: 931–942 [Google Scholar]
- Giglioli-Guivarc'h N, Pierre J-N, Brown S, Chollet R, Vidal J, Gadal P (1996) The light-dependent transduction pathway controlling the regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase in protoplasts from Digitaria sanguinalis. Plant Cell 8: 573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi F, Hewitson B, Christensen J, Hulme M, Von Storch H, Whetton R, Jones R, Mearns L, Fu C, Arritt R, et al (2001) Regional climate information: evaluation and projections. In JT Houghton, Y Ding, DJ Griggs, M Noguer, PJ van der Linden, D Xiaosu, eds, Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York, pp 583–639
- Grace J (1983) Plant-Atmosphere Relationships. Chapman & Hall, London
- Gregory JM, Mitchell JFB, Brady AJ (1997) Summer drought in northern midlatitudes in a time-dependent CO2 climate experiment. J Clim 10: 662–686 [Google Scholar]
- He DX, Edwards GE (1996) Estimation of diffusive resistance of bundle sheath cells to CO2 from modeling of C4 photosynthesis. Photosynth Res 49: 195–208 [DOI] [PubMed] [Google Scholar]
- Hendriks JHM, Kolbe A, Gibon Y, Stitt M, Geigenberger P (2003) ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133: 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel B, Andrieu B, Valadier MH, Renard S, Quillere I, Chelle M, Pommel B, Fournier C, Drouet JL (2005) Physiology of maize. II. Identification of physiological markers representative of the nitrogen status of maize (Zea mays) leaves during grain filling. Physiol Plant 124: 178–188 [Google Scholar]
- Hofstra G, Hesketh JD (1969) Effects of temperature on the gas exchange of leaves in the light and dark. Planta 85: 228–237 [DOI] [PubMed] [Google Scholar]
- Kiirats O, Lea PJ, Franceschi VR, Edwards GE (2002) Bundle sheath diffusive resistance to CO2 and effectiveness of C4 photosynthesis and refixation of photorespired CO2 in a C4 cycle mutant and wild-type Amaranthus edulis. Plant Physiol 130: 964–976; erratum Kiirats O, Lea PJ, Franceschi VR, Edwards GE (2003) Plant Physiol 132: 400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball BA (1983) Carbon-dioxide and agricultural yield: an assemblage and analysis of 430 prior observations. Agron J 75: 779–788 [Google Scholar]
- Leakey ADB, Bernacchi CJ, Dohleman FG, Ort DR, Long SP (2004) Will photosynthesis of maize (Zea mays) in the US Corn Belt increase in future [CO2] rich atmospheres? An analysis of diurnal courses of CO2 uptake under free-air concentration enrichment (FACE). Glob Change Biol 10: 951–962 [Google Scholar]
- Leegood RC, Von Caemmerer S (1989) Some relationships between contents of photosynthetic intermediates and the rate of photosynthetic carbon assimilation in leaves of Zea mays L. Planta 178: 258–266 [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Morgan PB (2005) Global food insecurity. Treatment of major food crops with elevated carbon dioxide and ozone under large-scale fully open-air conditions suggests models may seriously over-estimate future yields. Philos Trans R Soc Lond B Biol Sci 360: 2011–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroco JP, Edwards GE, Ku MSB (1999) Photosynthetic acclimation of maize to growth under elevated levels of carbon dioxide. Planta 210: 115–125 [DOI] [PubMed] [Google Scholar]
- McKee GW (1964) A coefficient for computing leaf area in hybrid corn. Agron J 56: 240–241 [Google Scholar]
- McLeod A, Long SP (1999) FACE in global change research: a review. Adv Ecol Res 28: 1–55 [Google Scholar]
- Meinzer FC, Andrade JL, Goldstein G, Holbrook NM, Cavelier J, Jackson P (1997) Control of transpiration from the upper canopy of a tropical forest: the role of stomatal, boundary layer and hydraulic architecture components. Plant Cell Environ 20: 1242–1252 [Google Scholar]
- Moore BD, Cheng SH, Edwards GE (1986) The influence of leaf development on the expression of C4 metabolism in Flaveria trinervia, a C4 dicot. Plant Cell Physiol 27: 1159–1167 [Google Scholar]
- Morgan PB, Bollero GA, Nelson RL, Dohleman FG, Long SP (2005) Smaller than predicted increase in aboveground net primary production and yield of field-grown soybean under fully open-air CO2 elevation. Glob Change Biol 11: 1856–1865 [Google Scholar]
- Naidu SL, Long SP (2004) Potential mechanisms of low-temperature tolerance of C-4 photosynthesis in Miscanthus x giganteus: an in vivo analysis. Planta 220: 145–155 [DOI] [PubMed] [Google Scholar]
- Nelson JA, Morgan JA, LeCain DR, Mosier A, Milchunas DG, Parton BA (2004) Elevated CO2 increases soil moisture and enhances plant water relations in a long-term field study in semi-arid shortgrass steppe of Colorado. Plant Soil 259: 169–179 [Google Scholar]
- Ottman MJ, Kimball BA, Pinter PJ, Wall GW, Vanderlip RL, Leavitt SW, LaMorte RL, Matthias AD, Brooks TJ (2001) Elevated CO2 increases sorghum biomass under drought conditions. New Phytol 150: 261–273 [Google Scholar]
- Owensby CE, Ham JM, Knapp AK, Bremer D, Auen LM (1997) Water vapour fluxes and their impact under elevated CO2 in a C4-tallgrass prairie. Glob Change Biol 3: 189–195 [Google Scholar]
- Palmer WC (1968) Keeping track of crop moisture conditions, nationwide: the new Crop Moisture Index. Weatherwise 21: 156–161 [Google Scholar]
- Parry ML, Rosenzweig C, Iglesias A, Livermore M, Fischer G (2004) Effects of climate change on global food production under SRES emissions and socio-economic scenarios. Glob Environ Change 14: 53–67 [Google Scholar]
- Phillips DL, Lee JJ, Dodson RF (1996) Sensitivity of the US corn belt to climate change and elevated CO2. 1. Corn and soybean yields. Agric Syst 52: 481–502 [Google Scholar]
- Pingali PL, editor (2001) CIMMYT 1999–2000 Facts and Trends. Meeting World Maize Needs: Technological Opportunities and Priorities for the Public Sector. CIMMYT, Mexico City
- Prentice IC, Farquhar GD, Fasham MJR, Goulden ML, Heimann M, Jaramillo VJ, Kheshgi HS, Le Quere C, Scholes RJ, Wallace DWR, et al (2001) The carbon cycle and atmospheric carbon dioxide. In JT Houghton, Y Ding, DJ Griggs, M Noguer, PJ van der Linden, D Xiaosu, eds, Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York, pp 183–239
- Ritchie SW, Hanway JJ, Benson GO (1993) How a Corn Plant Develops. Special Report Number 48. Iowa State University of Science and Technology, Ames, IA
- Rogers A, Allen DJ, Davey PA, Morgan PB, Ainsworth EA, Bernacchi CJ, Cornic G, Dermody O, Dohleman FG, Heaton EA, et al (2004) Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under Free-Air Carbon dioxide Enrichment. Plant Cell Environ 27: 449–458 [Google Scholar]
- Rogers A, Ellsworth DS, Humphries SW (2001) Possible explanation of the disparity between the in vitro and in vivo measurements of Rubisco activity: a study in loblolly pine grown in elevated pCO(2). J Exp Bot 52: 1555–1561 [DOI] [PubMed] [Google Scholar]
- Rudorff BFT, Mulchi CL, Lee EH, Rowland R, Pausch R (1996) Effects of enhanced O3 and CO2 enrichment on plant characteristics in wheat and corn. Environ Pollut 94: 53–60 [DOI] [PubMed] [Google Scholar]
- Sage RF, Kubien DS (2003) Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynth Res 77: 209–225 [DOI] [PubMed] [Google Scholar]
- Samarakoon AB, Gifford RM (1996) Elevated CO2 effects on water use and growth of maize in wet and drying soil. Aust J Plant Physiol 23: 53–62 [Google Scholar]
- Sharkey TD, Savitch LV, Butz ND (1991) Photometric method for routine determination of kcat and carbamylation of Rubisco. Photosynth Res 28: 41–48 [DOI] [PubMed] [Google Scholar]
- Thomas RB, Strain BR (1991) Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon-dioxide. Plant Physiol 96: 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Brown RA, Rosenberg NJ, Izaurralde RC, Benson V (2005) Climate change impacts for the conterminous USA: an integrated assessment. Part 3. Dryland production of grain and forage crops. Clim Change 69: 43–65 [Google Scholar]
- Uribelarrea M, Below FE, Moose SP (2004) Grain composition and productivity of maize hybrids derived from the Illinois protein strains in response to variable nitrogen supply. Crop Sci 44: 1593–1600 [Google Scholar]
- U.S. Department of Agriculture (2005) World Agricultural Production: World Grains by Commodity for December 2004. Production Estimates and Crop Assessment Division, Foreign Agricultural Service, U.S. Department of Agriculture, Washington, DC
- von Caemmerer S (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Collingwood, Australia
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Furbank RT (2003) The C-4 pathway: an efficient CO2 pump. Photosynth Res 77: 191–207 [DOI] [PubMed] [Google Scholar]
- Wall GW, Brooks TJ, Adam R, Cousins AB, Kimball BA, Pinter PJ, LaMorte RL, Triggs L, Ottman MJ, Leavitt SW, et al (2001) Elevated atmospheric CO2 improved Sorghum plant water status by ameliorating the adverse effects of drought. New Phytol 152: 231–248 [Google Scholar]
- Watling JR, Press MC (1997) How is the relationship between the C4 cereal Sorghum bicolor and the C3 root hemi-parasites Striga hermonthica and Striga asiatica affected by elevated CO2? Plant Cell Environ 20: 1292–1300 [Google Scholar]
- Watling JR, Press MC, Quick WP (2000) Elevated CO2 induces biochemical and ultrastructural changes in leaves of the C4 cereal sorghum. Plant Physiol 123: 1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC (1979) Elevated atmospheric partial pressure of CO2 and plant growth. I. Interactions of nitrogen nutrition and photosynthetic capacity in C3 and C4 plants. Oecologia 44: 68–74 [DOI] [PubMed] [Google Scholar]
- Ziska LH, Bunce JA (1997) Influence of increasing carbon dioxide concentration on the photosynthetic and growth stimulation of selected C4 crops and weeds. Photosynth Res 54: 199–208 [Google Scholar]
- Ziska LH, Sicher RC, Bunce JA (1999) The impact of elevated carbon dioxide on the growth and gas exchange of three C4 species differing in CO2 leak rates. Physiol Plant 105: 74–80 [Google Scholar]