Abstract
There is little information on gender differences in cerebral autoregulation. The purpose of this study was to compare autoregulation of the anterior and posterior circulations using the tilt test method in healthy boys and girls who were 10–16 y of age. Transcranial Doppler was used to measure middle cerebral artery and basilar artery flow velocities (Vmca and Vbas). Cerebral autoregulation (ARI) of the middle cerebral (ARImca) and basilar arteries (ARIbas) was examined using the tilt test method. An ARI <0.4 indicates impaired autoregulation. Among the 13 boys and 13 girls, Vmca and Vbas were higher in girls (Vmca 89 ± 16 versus 75 ± 16 cm/s, p = 0.005; Vbas 59 ± 11 versus 51 ± 12, p = 0.005). All children demonstrated intact autoregulation, but boys had higher ARImca than girls (0.98 ± 0.03 versus 0.92 ± 0.10), whereas girls had higher ARIbas than boys (0.92 ± 0.12 versus 0.97 ± 0.06, p = 0.02). Girls demonstrated greater autoregulation in the basilar artery, whereas boys demonstrated greater autoregulation in the middle cerebral artery. Girls had higher flow velocities in both vessels. This study provides normative data on cerebral autoregulation of the posterior circulation in healthy, awake boys and girls.
Abbreviations: ARImca, middle cerebral artery autoregulatory index; ARIbas, basilar artery autoregulatory index; BAS, basilar artery; CBF, cerebral blood flow; LLA, lower limit of autoregulation; MAP, mean arterial pressure; MAPe, mean arterial pressure at the external auditory meatus; MCA, middle cerebral artery; TCD, transcranial Doppler; Vmca, middle cerebral artery flow velocity; Vbas, basilar artery flow velocity
Autoregulation of cerebral blood flow (CBF) is a physiologic and homeostatic process that maintains nearly constant CBF over a range of mean arterial pressures (MAPs). Disease states, including traumatic brain injury, can impair cerebral autoregulation, rendering the brain susceptible to inadequate (cerebral ischemia) or excessive (cerebral hyperemia) CBF (1). Despite its critical role in maintaining CBF, there is limited information on cerebral autoregulation in healthy children.
Little has been published on pediatric cerebral autoregulation in children outside the clinical arena of neurogenic syncope (2–4). In one study that examined dynamic cerebral autoregulation in awake adolescent study participants, the time to return of middle cerebral artery flow velocity (Vmca) to normal after a transient hypotensive stimulus was reported to be more in healthy adolescents compared with their healthy adult counterparts (5). However, in a subsequent evaluation of cerebral autoregulation in children versus adults during general anesthesia using static autoregulation testing, the investigators reported no age-related differences in autoregulatory capacity and no difference in cerebral autoregulation compared with adults (6). Because both of these studies examined cerebral autoregulation of the anterior circulation only, differences in cerebral autoregulation between the anterior and posterior circulation in children could not be evaluated. In addition, to our knowledge, there is no information regarding cerebral autoregulation of the posterior circulation in children, no information on gender differences in cerebral autoregulation in children, and finally no normative data on cerebral autoregulation using the tilt test method. Therefore, the purpose of this study was to 1) provide normative data on cerebral autoregulation using the tilt test method, 2) describe cerebral autoregulation of the posterior cerebral circulation, and 3) examine gender-related differences in autoregulatory capacity in healthy, awake boys and girls.
METHODS
Study participants and setting
Healthy children who were 10 to 16 y of age and had no history of seizures, syncope, dysautonomia, or any neurologic/cardiac disorders were eligible for participation in this study. Eligible study participants were recruited from well-child visits in the general pediatrics clinic at Harborview Medical Center (Seattle, WA). Informed consent for participation was obtained from the parent or guardian, and age-appropriate assent was obtained from each child.
Physiologic testing was conducted in either the Harborview Cerebrovascular Laboratory or the Post-Anesthesia Care Unit at Harborview Medical Center. This study was approved by the University of Washington’s institutional review board.
Study design and protocol
Each participant was positioned on a bed with the head and back adjustable for elevation. An appropriately sized noninvasive blood pressure cuff was placed on one arm. Transcranial Doppler (TCD) ultrasonography (Multidop X; DWL Corp., Sipplingen, Germany) was used to measure flow velocities in the middle cerebral (Vmca) and basilar (Vbas) arteries. A hand-held 2-MHz ultrasound probe was used to insonate the desired vessel and positioned for sufficient time to achieve steady-state measurement. Previously age appropriate depths were used to insonate both the middle cerebral artery (MCA) and basilar artery (BAS) (7). During steady-state conditions, Vmca, Vbas, MAP, heart rate, and respiratory rate were recorded in each position.
For examining cerebral autoregulation, change in position proceeded from supine to sitting upright (90 degrees). For the sitting upright position, the vertical distance between the noninvasive blood pressure cuff and the external auditory meatus was used to calculate the estimated MAP (MAPe) at the Circle of Willis. Because MAP decreases by 1 mm Hg for every 1.36-cm increase in vertical height, the change in height from supine to sitting upright was divided by 1.36 cm to calculate the MAPe in the sitting upright position.
Cerebral autoregulation was calculated off-line as previously described (8). Briefly, cerebral autoregulation was quantified using the autoregulatory index (ARI), where ARI = % ΔeCVR/% ΔMAPe; eCVR is the estimated cerebrovascular resistance calculated as the ratio of MAP to Vmca or Vbas as appropriate (8). Two ARIs were calculated for each participant: one for the middle cerebral artery (ARImca) and one for the basilar artery (ARIbas).
Sample size calculations
Sample size calculation was based on previously published ARI data (6). A 30% difference in ARI of boys and girls between the MCA and BAS was considered clinically significant. Accepting an α of 0.05 and a β of 0.8, power analysis indicated the need for a minimum of 24 study participants. Two-factor ANOVA was used to compare 1) ARImca versus ARIbas and 2) Vmca versus Vbas between boys and girls. Significance was set at p < 0.05. Age-related Vmca and Vbas in this study were compared with age-related normative data previously published by Bode (7). All data are presented as mean ± SD.
RESULTS
Twenty-six children (13 boys, 13 girls) between the ages of 10 and 16 y (boys: 12.9 ± 1.7; girls: 12 ± 1.4; p = 0.14) completed the study. Twenty-four of 26 children (12 of 13 boys and 12 of 13 girls) were pubertal (Tanner II–IV). Two boys and one girl were Tanner stage IV. Therefore, 21 of 26 participants were either Tanner II or III. There was no difference in the distribution of Tanner staging between boys and girls. There was no significant difference in MAPe, drop in MAPe with autoregulation testing, Vmca, Vbas, ARImca, ARIbas, or the relationships examined when the two prepubertal children were excluded from the analyses. Therefore, the final analyses include data from all 26 participants.
At baseline (supine), boys had a higher MAPe than girls (79 ± 7 mm Hg for boys versus 75 ± 5 mm Hg for girls; p = 0.004). In the sitting upright position, MAPe decreased in both boys and girls (55 ± 8 mm Hg in boys versus 54 ± 8 mm Hg in girls; p = 0.47). The average drop in MAPe for the whole group was 22 ± 6 mm Hg, from 77 ± 6 to 55 ± 8 mm Hg (p = 0.001). There was no statistically significant difference in drop in MAPe between boys and girls (boys 23 ± 5 mm Hg versus girls 21 ± 6 mm Hg; p = 0.12). All study participants achieved at least a 10-mm Hg drop in MAPe with the position change. Respiratory rate was similar for both boys and girls in the supine (both 20 breaths/min; p = 0.23) and sitting upright positions (boys 20 breaths/min versus girls 19 breaths/min; p = 0.94). Vmca, Vbas, and ARI data for individual participants are given in Tables 1 and 2. For the entire cohort, baseline (supine) Vmca and Vbas were 82 ± 17 and 55 ± 12 cm/s, and upright Vmca and Vbas were 81 ± 18 and 55 ± 12 cm/s (Vmca supine versus upright, p = 0.26; Vbas supine versus upright, p = 0.89). However, baseline Vmca (89 ± 16 cm/s) and Vbas (59 ± 11 cm/s) were higher in girls compared with boys (Vmca 75 ± 16 cm/s and Vbas 51 ± 12 cm/s; p = 0.005; Table 3, Fig. 1). In the sitting upright position, Vmca (87 ± 17 cm/s) and Vbas (60 ± 11 cm/s) were also higher in girls compared with boys (Vmca 75 ± 17 cm/s and Vbas 50 ± 12 cm/s; p = 0.01; Table 3).
Table 1.
Middle Cerebral Artery Flow Velocity (Vmca) and Autoregulatory Index (ARI) in Girls and Boys 10 to 16 Years old in the Supine and Sitting Upright Positions
| Girls | |||||||
|---|---|---|---|---|---|---|---|
| Age (y) | Supine MAPe | Supine Vmca | Supine CVRe | Upright MAPe | Upright Vmca | Upright CVRe | ARI |
| 10 | 74 | 90 | 0.82 | 58 | 98 | 0.59 | 1 |
| 11 | 74 | 68 | 1.09 | 55 | 66 | 0.83 | 0.91 |
| 11 | 71 | 88 | 0.81 | 40 | 83 | 0.48 | 0.92 |
| 11 | 85 | 73 | 1.16 | 71 | 73 | 0.97 | 1 |
| 11 | 77 | 97 | 0.79 | 54 | 98 | 0.55 | 1 |
| 12 | 84 | 94 | 0.89 | 68 | 93 | 0.73 | 0.95 |
| 12 | 73 | 100 | 0.73 | 46 | 98 | 0.47 | 0.97 |
| 12 | 73 | 106 | 0.69 | 47 | 103 | 0.46 | 0.95 |
| 12 | 72 | 87 | 0.83 | 52 | 77 | 0.68 | 0.66 |
| 12 | 72 | 99 | 0.73 | 54 | 92 | 0.57 | 0.77 |
| 13 | 78 | 110 | 0.71 | 49 | 110 | 0.45 | 1 |
| 14 | 77 | 90 | 0.86 | 58 | 88 | 0.66 | 0.93 |
| 15 | 78 | 51 | 1.53 | 45 | 48 | 0.94 | 0.91 |
| Boys | |||||||
|---|---|---|---|---|---|---|---|
| Age (y) | Supine MAPe | Supine Vmca | Supine CVRe | Upright MAPe | Upright Vmca | Upright CVRe | ARI |
| 10 | 86 | 83 | 1.04 | 69 | 83 | 0.83 | 1 |
| 10 | 69 | 100 | 0.69 | 46 | 100 | 0.46 | 1 |
| 11 | 80 | 87 | 0.92 | 62 | 86 | 0.72 | 0.96 |
| 12 | 84 | 84 | 1 | 63 | 85 | 0.74 | 1 |
| 13 | 81 | 62 | 1.31 | 43 | 62 | 0.69 | 1 |
| 13 | 89 | 100 | 0.89 | 56 | 104 | 0.54 | 1 |
| 13 | 79 | 76 | 1.04 | 62 | 82 | 0.76 | 1 |
| 14 | 76 | 53 | 1.43 | 50 | 51 | 0.98 | 0.92 |
| 14 | 76 | 76 | 1 | 51 | 77 | 0.66 | 1 |
| 14 | 75 | 48 | 1.56 | 47 | 47 | 1 | 0.96 |
| 14 | 84 | 69 | 1.22 | 59 | 67 | 0.88 | 0.93 |
| 15 | 83 | 64 | 1.30 | 58 | 64 | 0.90 | 1 |
| 15 | 75 | 73 | 1.03 | 50 | 72 | 0.69 | 0.97 |
MAPe = mean arterial pressure at the external auditory meatus, CVRe = estimated cerbrovascular resistance.
Table 2.
Basilar Artery Flow Velocity (Vbas) and Autoregulatory Index (ARI) in Girls and Boys 10 to 16 Years old in the Supine and Sitting Upright Positions
| Girls | |||||||
|---|---|---|---|---|---|---|---|
| Age (y) | Supine MAPe | Supine Vbas | Supine CVRe | Upright MAPe | Upright Vbas | Upright CVRe | ARI |
| 10 | 71 | 65 | 1.09 | 59 | 65 | 0.91 | 1 |
| 11 | 74 | 52 | 1.42 | 60 | 50 | 1.2 | 0.83 |
| 11 | 73 | 66 | 1.11 | 55 | 71 | 0.77 | 1 |
| 11 | 86 | 68 | 1.26 | 67 | 66 | 1.02 | 0.89 |
| 11 | 75 | 50 | 1.5 | 58 | 55 | 1.05 | 1 |
| 12 | 80 | 52 | 1.54 | 62 | 53 | 1.17 | 1 |
| 12 | 65 | 75 | 0.87 | 44 | 72 | 0.61 | 0.91 |
| 12 | 70 | 65 | 1.08 | 43 | 66 | 0.65 | 1 |
| 12 | 73 | 63 | 1.16 | 45 | 65 | 0.69 | 1 |
| 12 | 70 | 70 | 1 | 56 | 70 | 0.8 | 1 |
| 13 | 71 | 57 | 1.25 | 48 | 61 | 0.79 | 1 |
| 14 | 78 | 34 | 2.29 | 63 | 34 | 1.85 | 1 |
| 15 | 73 | 56 | 1.30 | 47 | 55 | 0.85 | 0.97 |
| Boys | |||||||
|---|---|---|---|---|---|---|---|
| Age (y) | Supine MAPe | Supine Vbas | Supine CVRe | Upright MAPe | Upright Vbas | Upright CVRe | ARI |
| 10 | 86 | 61 | 1.40 | 69 | 60 | 1.15 | 0.93 |
| 10 | 67 | 70 | 0.96 | 43 | 70 | 0.61 | 1 |
| 11 | 69 | 50 | 1.38 | 53 | 57 | 0.93 | 1 |
| 12 | 84 | 58 | 1.45 | 63 | 59 | 1.07 | 1 |
| 13 | 74 | 46 | 1.61 | 57 | 42 | 1.36 | 0.68 |
| 13 | 96 | 63 | 1.52 | 68 | 63 | 1.08 | 1 |
| 13 | 76 | 50 | 1.52 | 54 | 48 | 1.13 | 0.9 |
| 14 | 75 | 40 | 1.88 | 58 | 41 | 1.41 | 1 |
| 14 | 76 | 64 | 1.19 | 51 | 64 | 0.80 | 1 |
| 14 | 70 | 31 | 2.26 | 44 | 37 | 1.19 | 1 |
| 14 | 81 | 40 | 2.03 | 52 | 39 | 1.33 | 0.95 |
| 15 | 83 | 41 | 2.02 | 58 | 38 | 1.53 | 0.82 |
| 15 | 71 | 45 | 1.58 | 49 | 39 | 1.26 | 0.66 |
MAPe = mean arterial pressure at the external auditory meatus, CVRe = estimated cerebrovascular resistance.
Table 3.
Supine and Upright Flow Velocities by Gender
| Supine Flow Velocity (cm/s)
|
Upright Flow Velocity (cm/s)
|
|||
|---|---|---|---|---|
| Gender | Vmca | Vbas | Vmca | Vbas |
| Boys | 75 ± 16 | 51 ± 12 | 75 ± 17 | 50 ± 12 |
| Girls | 89 ± 16 | 59 ± 11 | 87 ± 17 | 60 ± 11 |
| Mean ± SD | 82 ± 17 | 55 ± 12 | 81 ± 18 | 55 ± 12 |
| boys vs. girls | p = 0.005 | boys vs. girls | p = 0.01 | |
Vmca = middle cerebral artery flow velocity; Vbas = Basilar artery flow velocity. Girls have higher Vmca and Vbas than boys in both the supine and upright positions.
Figure 1.
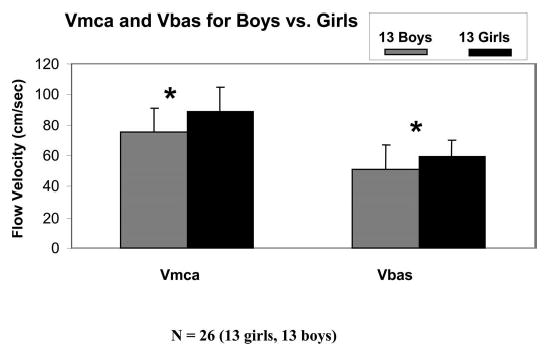
Vmca and Vbas for boys and girls. Girls have higher Vmca and Vbas than boys (*p = 0.005). Vmca is higher than Vbas for both boys and girls. Data are presented as mean ± SD.
All study participants had intact cerebral autoregulation of the MCA and BAS (Tables 1 and 2). For the whole cohort, there was no difference between ARImca and ARIbas (ARImca: 0.95 ± 0.08, ARIbas: 0.94 ± 0.10; p = 0.78). There was also no overall difference in ARI for both MCA and BAS by gender (0.95 ± 0.09 for boys versus 0.95 ± 0.08 for girls; p = 0.86). However, boys had significantly higher ARImca than girls, whereas girls had higher ARIbas than boys (ARImca: boys 0.98 ± 0.03 versus girls 0.92 ± 0.1; ARIbas: boys 0.92 ± 0.12 versus girls: 0.97 ± 0.06; p = 0.02; Table 4, Fig. 2).
Table 4.
Cerebral Autoregulation by Gender
| Autoregulatory Index
|
|||
|---|---|---|---|
| Gender | ARImca | ARIbas | Mean ± SD |
| Boys | 0.98 ± 0.03 | 0.92 ± 0.12 | 0.95 ± 0.09 |
| Girls | 0.92 ± 0.10 | 0.97 ± 0.06 | 0.95 ± 0.08 |
| Mean ± SD | 0.95 ± 0.08 | 0.94 ± 0.10 | |
| p = 0.02 | |||
Girls have higher ARImca than boys whereas boys have higher ARIbas than girls. ARI = autoregulatory index; MCA = middle cerebral artery; BAS = basilar artery.
Figure 2.
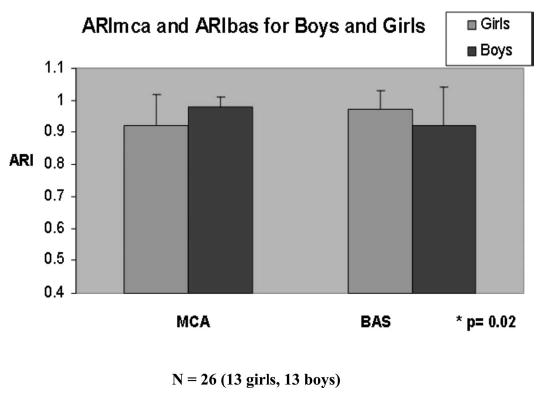
ARImca and ARIbas for boys and girls. Girls had higher ARIbas, whereas boys had better ARImca (p = 0.02). Data are presented as mean ± SD
DISCUSSION
In this study of healthy children who were 10 to 16 y of age, boys demonstrated greater autoregulation of the MCA than girls, whereas girls had greater autoregulation of the BAS than boys. Girls also had higher flow velocities of both the MCA and the BAS than boys. The present study provides normative data on cerebral autoregulation of both the anterior and posterior circulations in healthy children using the tilt test method. To the best of our knowledge, this is the first description of cerebral autoregulation of the posterior circulation and the first description of gender-related differences in CBF velocity and cerebral autoregulation in children.
We examined the effect of gender on cerebral autoregulation because there are known differences between the central nervous systems and the hormonal milieu of growing children of different genders (9). In addition, estrogen has been shown to correlate directly with CBF velocity (10). Clinically, the overall higher flow velocities in both the MCA and the BAS in pubertal girls between 10 and 16 y of age in this study are consistent with similar gender differences reported by Bakker et al. (11) in adults. We chose to study children who were 10 to 16 y of age because Bode (7) grouped Vmca and Vbas data for older children in this age range, thereby allowing us to compare our data with this referenced information. However, Bode did not analyze flow velocities according to gender. The higher flow velocities in girls in the MCA and BAS suggest that the gender-related differences in Vmca reported in adults are present in both the anterior and the posterior circulations before adulthood.
When examined without reference to gender, the present study is consistent with previous findings in adults of equivalent autoregulatory capacity in the anterior and posterior circulations (12). Although we report that pubertal boys demonstrated greater autoregulation of the MCA, whereas pubertal girls demonstrated greater autoregulation of the BAS, the present differences are small and the clinical relevance is not yet known. However, differences in the hormonal milieu may account for the observed differences in autoregulation. For example, estradiol has been shown to exhibit tissue selectivity in its vasodilation in rabbit arteries (13). For example, one might expect, from a teleologic perspective, greater autoregulation in all humans in a vessel that supplies the brainstem. One might also expect a global change in autoregulation as a result of the cerebral vasodilatory properties of estrogen. In this study, gender-related differences in flow velocities (higher flow velocities and lower cerebrovascular resistance) do not seem to account solely for regional differences in autoregulatory capacity patterns between boys and girls. Like the reported gender-related differences in flow velocities, gender differences in autoregulatory patterns seem to exist before adulthood.
In the present study, we measured flow velocities and calculated ARI for tilt from supine to the sitting upright position, which resulted in a 22-mm Hg average drop in MAPe. We chose to use the sitting upright position (90 degrees) because we were concerned that the potentially large drop in MAPe with standing may approach the lower limit of autoregulation (LLA). The decrease in MAPe from supine to sitting upright in this age group corroborates our previous work that the LLA of the MCA in older children without neurologic disease is <55 mm Hg and that the autoregulatory reserve in children this age group is large (14,15).
Comparatively, little is known about cerebral autoregulation of the posterior circulation. In one study of 16 healthy, awake adults, Park et al. (12) reported no difference in ARI between the BAS and the MCA. Unlike Park’s study, we used a static autoregulation testing method, in which the decrease in blood pressure is sustained. In addition, other studies compared the MCA with the posterior cerebral artery but not the BAS (16,17). We examined the BAS primarily because it supplies the posterior fossa contents as opposed to the posterior cerebral artery, which supplies the occipital cortex (18).
It is important to understand cerebral autoregulation of the posterior circulation because certain disease processes may affect the posterior circulation independent of the anterior circulation. Although strokes in children are uncommon, posterior circulation strokes are more common in previously healthy boys (19). The results from this study question whether regional differences in cerebral autoregulation (less well-developed cerebral autoregulatory mechanisms of the BAS) may play a role in this pattern. In addition, conditions such as traumatic dissection, sickle cell disease, and prothrombotic states may engender symptomatic lesions and impair cerebral autoregulation in one distribution and not the other (20–23).
Cerebral autoregulation can be evaluated quantitatively using several different techniques. Although static cerebral autoregulation testing, using TCD, has been validated and can be performed using pharmacologic intervention to increase MAPe, it may be too invasive for awake, healthy children (6). Tilt testing allows cerebral autoregulation to be studied at the bedside noninvasively, is well tolerated by children, and has been used for many years in the evaluation of children and adults with syncope (24,25). It has also been combined with TCD ultrasonography to evaluate changes in cerebral vascular resistance in patients with dysautonomia (26).
There are some limitations to this study. First, we measured CBF velocity, not CBF. The study was conducted during steady state by investigators with experience in using TCD technology, and the flow velocities in this study correlate well with the normal values previously reported by Bode (7). The advantage of the present study over the previous study is the removal of potential confounders, such as the effect of volatile anesthetic on cerebrovascular tone (27–29). Although we could estimate the drop in MAPe with tilt testing, we could not control the blood pressure to ensure a specified drop in MAPe. However, the range of MAPe tested (77–55 mm Hg) was within physiologic norms and sufficiently broad to precipitate an autoregulatory response. We are unable to comment on potential differences in LLA between the anterior and the posterior circulations between boys and girls because we could not match the LLA. In addition, we evaluated only the MCA and the BAS, not the other cerebral arteries. Although we did not use capnography to measure end-tidal CO2 and cannot exclude the influence of changes in tidal volume (surrogate for partial pressure of arterial CO2) on change in Vmca and Vbas, we measured respiratory rate during steady state and found the rate to be similar whether supine or sitting upright. We measured respiratory rate and MAPe in each position to examine whether changes in minute ventilation were dependent on position, because partial pressure of arterial CO2 and sympathetic tone both potentially influence Vmca and Vbas. Similarly, we could not control cerebral metabolic rate or flow-metabolism coupling in our awake study participants. To maintain steady-state conditions, we made attempts to minimize distractions during the study, and we ensured that flow velocities reached steady state before we recorded it. We did not use invasive arterial blood pressure monitoring. However, because the study participants were healthy children without vascular disease, we believe our results to be valid. Finally, we did not obtain hormone levels from our participants, precluding us from definitively attributing our observations of cerebrovascular physiology on hormonal rather than genetic influences.
CONCLUSION
In this study of children who were 10 to 16 y of age, girls demonstrated greater autoregulation of the BAS than boys, whereas boys demonstrated greater autoregulation of the MCA than girls. Like their adult counterparts, girls in this age group had higher CBF velocities of both the MCA and the BAS. It is possible that as we recognize age-related changes in pediatric CBF, CBF and cerebral autoregulation may now have to be considered with respect to both age and gender. This study provides new information on gender differences in CBF velocities and cerebral autoregulation of the anterior and posterior circulations, as well as normative data on autoregulatory indices using the tilt test method in healthy children. This information may be useful to researchers who study and clinicians who manage children with and without neurologic disease.
Acknowledgments
We thank the University of Washington’s Department of Pediatrics, the nurses of the Harborview Medical Center Post-Anesthesia Care Unit, the staff of the Harborview Medical Center Children and Teens Clinic, Domonique Calhoun, and, most important, the children and their families who participated in this study for support of this research.
Footnotes
This research was supported by the NIH K23 HD044632-02 (to M.S.V.).
References
- 1.Obrist WD, Gennarelli TA, Segawa H, Dolinskas CA, Langfitt TW. Relation of cerebral blood flow to neurological status and outcome in head-injured patients. J Neurosurg. 1979;51:292–300. doi: 10.3171/jns.1979.51.3.0292. [DOI] [PubMed] [Google Scholar]
- 2.Albina G, Fernandez Cisneros L, Laino R, Nobo UL, Ortega D, Schwarz E, Barja L, Lagos R, Giniger A, Ameriso SF. Transcranial Doppler monitoring during head upright tilt table testing in patients with suspected neurocardiogenic syncope. Europace. 2004;6:63–69. doi: 10.1016/j.eupc.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Sung RY, Du ZD, Yu CW, Yam MC, Fok TF. Cerebral blood flow during vasovagal syncope induced by active standing or head up tilt. Arch Dis Child. 2000;82:154–158. doi: 10.1136/adc.82.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Nunez A, Couceiro J, Alonso C, Eiris J, Fuster M, Sanchez L, Martinon JM. Cerebral oxygenation in children with syncope during head-upright tilt test. Pediatr Cardiol. 1997;18:406–409. doi: 10.1007/s002469900216. [DOI] [PubMed] [Google Scholar]
- 5.Vavilala MS, Newell DW, Junger E, Douville CM, Aaslid R, Rivara FP, Lam AM. Dynamic cerebral autoregulation in healthy adolescents. Acta Anesthesiol Scand. 2002;46:393–397. doi: 10.1034/j.1399-6576.2002.460411.x. [DOI] [PubMed] [Google Scholar]
- 6.Vavilala MS, Lee LA, Lee M, Graham A, Visco E, Lam AM. Cerebral autoregulation in children during sevoflurane anaesthesia. Br J Anaesth. 2003;90:636–641. doi: 10.1093/bja/aeg119. [DOI] [PubMed] [Google Scholar]
- 7.Bode H 1988 Pediatric Applications of Transcranial Doppler Sonography. Springer-Verlag, New York, pp 114
- 8.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 9.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 10.Shamma FN, Fayad P, Brass L, Sarrel P. Middle cerebral artery blood velocity during controlled ovarian hyperstimulation. Fertil Steril. 1992;57:1022–1025. [PubMed] [Google Scholar]
- 11.Bakker SL, de Leeuw FE, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. Cerebral haemodynamics in the elderly: the Rotterdam study. Neuroepidemiology. 2004;23:178–184. doi: 10.1159/000078503. [DOI] [PubMed] [Google Scholar]
- 12.Park CW, Sturzenegger M, Douville CM, Aaslid R, Newell DW. Autoregulatory response and CO2 reactivity of the basilar artery. Stroke. 2003;34:34–39. doi: 10.1161/01.str.0000047122.42591.b3. [DOI] [PubMed] [Google Scholar]
- 13.Salom JB, Burguete MC, Perez-Asensio FJ, Torregrosa G, Alborch E. Relaxant effects of 17-beta-estradiol in cerebral arteries through Ca(2+) entry inhibition. J Cereb Blood Flow Metab. 2001;21:422–429. doi: 10.1097/00004647-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Vavilala MS, Lee LA, Lam AM. The lower limit of cerebral autoregulation in children during sevoflurane anesthesia. J Neurosurg Anesthesiol. 2003;15:307–312. doi: 10.1097/00008506-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Ramaekers VT, Casaer P, Daniels H, Marchal G. Upper limits of brain blood flow autoregulation in stable infants of various conceptional age. Early Hum Dev. 1990;24:249–258. doi: 10.1016/0378-3782(90)90032-e. [DOI] [PubMed] [Google Scholar]
- 16.Rosengarten B, Kaps M. Cerebral autoregulation in middle cerebral artery territory precedes that of posterior cerebral artery in human cortex. Cerebrovasc Dis. 2002;13:21–25. doi: 10.1159/000047741. [DOI] [PubMed] [Google Scholar]
- 17.Haubrich C, Wendt A, Diehl RR, Klotzsch C. Dynamic autoregulation testing in the posterior cerebral artery. Stroke. 2004;35:848–852. doi: 10.1161/01.STR.0000120729.99039.B6. [DOI] [PubMed] [Google Scholar]
- 18.Jatuzis D, Zachrisson H, Blomstrand C, Ekholm S, Holm J, Volkmann R. Evaluation of posterior cerebral artery blood flow with transcranial Doppler sonography: value and risk of common carotid artery compression. J Clin Ultrasound. 2000;28:452–460. doi: 10.1002/1097-0096(200011/12)28:9<452::aid-jcu2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Ganesan V, Chong WK, Cox TC, Chawda SJ, Prengler M, Kirkham FJ. Posterior circulation stroke in childhood. Neurology. 2002;59:1552–1556. doi: 10.1212/01.wnl.0000033092.87560.1a. [DOI] [PubMed] [Google Scholar]
- 20.Rommel O, Niedeggen A, Tegenthoff M, Kiwitt P, Botel U, Malin J. Carotid and vertebral artery injury following severe head or cervical spine trauma. Cerebrovasc Dis. 1999;9:202–209. doi: 10.1159/000015956. [DOI] [PubMed] [Google Scholar]
- 21.tk;2Verdu A, Cazorla MR, Granados MA, Alonso JA, Casado LF. Basilar artery thrombosis in a child heterozygous for factor V Leiden mutation. Pediatr Neurol. 2001;24:69–71. doi: 10.1016/s0887-8994(00)00228-9. [DOI] [PubMed] [Google Scholar]
- 22.Riebel T, Kebelmann-Betzing C, Gotze R, Overberg US. Transcranial Doppler ultrasonography in neurologically asymptomatic children and young adults with sickle cell disease. Eur Radiol. 2003;13:563–570. doi: 10.1007/s00330-002-1481-4. [DOI] [PubMed] [Google Scholar]
- 23.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- 24.Lerman-Sagie T, Rechavia E, Strasberg B, Sagie A, Blieden L, Mimouni M. Head-up tilt for the evaluation of syncope of unknown origin in children. J Pediatr. 1991;118:676–679. doi: 10.1016/s0022-3476(05)80025-3. [DOI] [PubMed] [Google Scholar]
- 25.Grossi D, Nozzoli C, Roca ME, Santostasi R, Simone F. Head-up tilt for triggering and diagnosing syncope. Funct Neurol. 1987;2:457–464. [PubMed] [Google Scholar]
- 26.Hilz MJ, Axelrod FB, Haertl U, Brown CM, Stemper B. Transcranial Doppler sonography during head up tilt suggests preserved central sympathetic activation in familial dysautonomia. J Neurol Neurosurg Psychiatry. 2002;72:657–660. doi: 10.1136/jnnp.72.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cremer OL, Diephuis JC, van Soest H, Vaessen PH, Bruens MG, Hennis PJ, Kalkman CJ. Cerebral oxygen extraction and autoregulation during extracorporeal whole body hyperthermia in humans. Anesthesiology. 2004;100:1101–1107. doi: 10.1097/00000542-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Blaha M, Aaslid R, Douville CM, Correra R, Newell Dw. Cerebral blood flow and dynamic cerebral autoregulation during ethanol intoxication and hypercapnia. J Clin Neurosci. 2003;10:195–198. doi: 10.1016/s0967-5868(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 29.Strebel S, Lam AM, Matta B, Mayberg TS, Aaslid R, Newell DW. Dynamic and static autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995;83:66–76. doi: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]


