Abstract
Human rotavirus-specific CD4+ and CD8+ T-cell responses in peripheral blood lymphocytes were studied using a flow cytometric assay that detects the intracellular accumulation of cytokines after short-term in vitro antigen stimulation. The frequencies of virus-specific T cells that secrete gamma interferon and interleukin-13 (IL-13) were determined in adults and children during the acute or convalescent phase of rotavirus-induced diarrhea, in asymptomatically infected adults and laboratory workers who worked with human stool samples containing rotavirus, and in healthy adults. Significantly higher frequencies of rotavirus-specific interferon gamma-secreting CD8+ and CD4+ T cells, but not IL-13-secreting T cells, were detected in symptomatically infected adults and exposed laboratory workers than in healthy adults and children with acute rotavirus diarrhea. The levels of rotavirus-specific T cells returned to levels found in healthy adults by 32 days after the onset of rotavirus diarrhea in most adult subjects. Children with rotavirus diarrhea had undetectable or very low levels of CD4+ and CD8+ T cells that secrete gamma interferon. Adult cytomegalovirus-seropositive individuals had frequencies of cytomegalovirus-specific T cells that secrete gamma interferon that were approximately 20 times the level of rotavirus-specific T cells. This result suggests that rotavirus is a relatively poor inducer of circulating memory T cells that secrete gamma interferon. The frequencies of gamma interferon-secreting CD4+ and CD8+ T cells and the frequencies of IL-13-secreting CD4+ T cells responding to the T-cell superantigen staphylococcal enterotoxin B (SEB) were lower in children than in adults. In both adults and children, the frequencies of CD4+ cells secreting gamma interferon in response to SEB were higher than the frequencies of cells secreting IL-13.
Rotaviruses (RV) are the most important cause of severe dehydrating diarrhea in children worldwide, resulting in an estimated 480,000 to 640,000 deaths annually (5). The first vaccine approved for use in humans has recently been withdrawn by the manufacturer because it was associated with increased numbers of cases of intussusception (1). For these reasons, investigators are exploring alternative vaccination strategies to develop improved second-generation vaccine candidates. A detailed knowledge of the immune response against RV in humans will be useful for the design and/or evaluation of new RV vaccines.
The RV-specific cell-mediated immune response is relatively well studied in the murine model: CD4+ T cells are essential for the development of more than 90% of the RV-specific intestinal immunoglobulin A (IgA) (14). Moreover, the antibody response seems to be the main mechanism that mediates protection against RV reinfection (14, 15, 31). Murine RV-specific CD8+ T cells have a direct antiviral effect, are involved in the timely resolution of primary RV infection, and can mediate partial protection against reinfection (13, 15, 31).
Because RV replication is restricted to enterocytes in vivo, RV-specific T cells are thought to mostly originate in and fulfill their effector function directly at the intestinal mucosa. For this reason, RVs have been used to test the intestinal-homing model (7). According to this model, T cells are activated by intestinally derived antigen in Peyer's patches (PP). After passing through the mesenteric lymph nodes, these T cells gain access to the blood circulatory system through the toraxic duct and then migrate through the postcapillary venules, preferentially to other PP and the lamina propria (7). The preferential homing of lymphocytes stimulated by antigens, first encountered in intestinal PP returning to other PP or the intestinal lamina propria, is mediated in part by interactions between the integrin α4β7, expressed on T lymphocytes, and the cell adhesion molecule MadCAM 1, expressed on the vascular endothelium of the postcapillary venules in the intestine (7). In support of this model, splenic murine RV-specific memory CD8+ T cells that have an in vivo antiviral effect preferentially express the intestinal homing receptor, the integrin α4β7 (35). Nonetheless, the expression of this receptor does not seem to be essential for the migration of the CD8+ T cells to the intestine or the effectiveness of their in vivo antiviral function (25).
Since in humans it is difficult to study intestinal antigen-specific T cells in situ, studies aimed at characterizing these cells have been mostly limited to the identification of circulating T cells that have been induced after intestinal priming (24). Circulating RV-specific T cells in both children and adults have been studied by lymphoproliferation (33, 34). Eight of 11 healthy adults and 10 of 13 healthy children between 6 months and 5 years of age had evidence of RV-specific lymphoproliferative activity (33). Six of eight RV-infected children demonstrated this activity in the convalescent period (2 to 8 weeks after infection) (34). Using lymphoproliferation as a readout, it has been determined that in healthy adults, circulating RV-specific CD4+ cells express the intestinal homing receptor α4β7 (36). This study supports the hypothesis that circulating RV-specific T cells are lymphocytes that have been primed in and are migrating back to the intestine (7, 24, 36). Human RV-specific CD8+-T-cell responses have not been studied to date.
Here, we present results concerning the frequencies of CD8+ and CD4+ RV-specific T cells in healthy adults and recently infected adults and children, as determined using a flow cytometric assay that detects the intracellular accumulation of cytokines after short-term in vitro antigen stimulation.
MATERIALS AND METHODS
Subjects and sample collection.
We studied 12 children (ages 6 months to 7 years) admitted with acute diarrhea of less than 7 days duration to the pediatric emergency service of the San Ignacio Hospital in Bogotá, Colombia. Ten of the 12 children had primary infection with RV, and 2 (see Table 3) had a repeat infection (see “Definitions” below). We also studied 26 adults 21 to 47 years old: 14 recently RV-infected adults, 10 symptomatically and 4 asymptomatically infected adults; 7 healthy adult subjects; and 5 laboratory workers who handled RV+ stool samples on a regular basis. In addition to the risk factor of manipulating RV-infected stool samples, four of these five laboratory workers were recent medical school graduates who could have been infected by RV during their medical training. We collected blood, stool, and saliva (adults) samples from each symptomatic patient at the time of the acute infection (1 to 7 days from the start of symptoms) and in the convalescent period (8 to 42 days from the start of symptoms). The comparison of levels of RV-specific antibodies in the two samples (acute and convalescent) served to establish or confirm the diagnosis of RV infection (see “Definitions” below). One symptomatically infected adult was studied 557 days after the onset of diarrhea (DAOD). The asymptomatically RV-infected adult subjects were identified among parents and guardians of the children with RV-induced diarrhea. From these adult parents and guardians, samples were taken during the acute and the convalescent phases of the diarrhea of the child under their care. Experiments with the T cells from the adult parents and guardians were performed with the samples that corresponded to the convalescent phase of their child, except for one of the subjects, for whom both acute- and convalescent-phase samples were used to perform T-cell experiments. The study was approved by the Ethics Committee of the San Ignacio Hospital, and signed informed consent was obtained from the parents and guardians of the children and from all adults prior to enrollment in the study.
TABLE 3.
Frequencies of RV-specific CD4+ and CD8+ T cells secreting IFN-γ in children with symptomatic RV infection
| Subject | DAOD | Age (mo) | % CD4+ IFN-γ+ | % CD4+ IL-13+ | % CD8+ IFN-γ+ |
|---|---|---|---|---|---|
| C01a | 1 | 30 | 0.01 | 0.01 | 0.01 |
| C02 | 1 | 4 | 0.06 | 0.06 | 0.04 |
| C03 | 1.5 | 11 | 0 | 0.01 | 0.02 |
| C04 | 3 | 12 | 0.04 | 0.05 | 0.03 |
| C05 | 3 | 24 | 0 | 0 | 0.08 |
| C06 | 4 | 18 | 0.02 | 0.06 | 0.04 |
| C07 | 5 | 10 | 0.02 | 0 | 0 |
| C03 | 11 | 11 | 0 | 0 | 0.02 |
| C08 | 13 | 6 | 0.07 | NDb | −0.01 |
| C09 | 14 | 11 | 0 | ND | 0 |
| C10 | 15 | 9 | −0.01 | 0 | 0.01 |
| C11 | 15 | 6 | 0.01 | ND | 0.05 |
| C12a | 22 | 84 | 0.01 | 0 | 0 |
Subject had a secondary RV infection as evidenced by the presence of RV-specific plasma and/or fecal IgA in samples taken during the acute phase of the infection. All other children had primary infection as evidenced by the absence of these antibodies.
ND, not determined.
ELISA for measuring RV antigen in feces.
The presence of RV antigen in feces was determined by using an enzyme-linked immunosorbent assay (ELISA) as previously described (14) with minor modifications (19). Immulon 2 ELISA plates (DYNEX Technologies, Chantilly, Va.) were coated with 70 μl of rabbit anti-RV hyperimmune serum or preimmune serum diluted in phosphate-buffered saline (PBS; Histolab Ltda, Bogotá, Colombia)/well and incubated overnight at 4°C. The wells were then blocked with 150 μl of 5% nonfat powdered milk plus 0.1% Tween-20 in PBS (5% BLOTTO) for 30 min at 37°C. The blocking solution was removed, and 70 μl of 10% stool samples diluted in 5% BLOTTO were added to both the immune- and preimmune-serum-coated wells. After 45 min of incubation at 37°C, the wells were washed five times with PBS-0.1% Tween 20 (PBS-Tween), and 70 μl of guinea pig anti-rhesus RV (RRV) hyperimmune serum, diluted in 2.5% BLOTTO plus 1.5% normal goat serum, was added to each well for 45 min at 37°C. After five washes with PBS-Tween, 70 μl of biotinylated goat anti-guinea pig serum (Vector, Burlingame, Calif.), diluted in 2.5% BLOTTO-1.5% normal goat serum, was added to each well. After 30 min of incubation at 37°C, the wells were washed five times, 70 μl of peroxidase avidin-biotin complex kit (Vector) was added to each well, and the plates were incubated for 30 min at 37°C. After five washes with PBS-Tween, the plates were developed using 70 μl of tetramethyl benzidine substrate (TMB; Sigma, St. Louis, Mo.). The reaction was stopped by the addition of 17.5 μl of 2 M sulfuric acid. Absorbance was read at a wavelength of 450 nm on an ELISA plate reader (MR 2600; Dynatech, McLean, Va.). Serial dilutions of a supernatant from RF virus (bovine RV)-infected MA104 cells (positive control) and a known RV human fecal specimen (negative control) were included in each plate. Samples tested in duplicate were considered positive if the optical density in the experimental well was >0.1 optical-density units and twofold greater than the optical density in the corresponding control wells coated with preimmune serum and incubated with the same RV stool sample suspension. Only plates in which the lowest dilution of the RF virus supernatant known to be positive was positive and the negative stool sample was negative were accepted for analysis.
ELISA for measuring RV-specific antibodies in plasma, saliva, and feces.
For detection of RV-specific IgA and IgG, 96-well Immulon 2 microtiter plates were coated with 70 μl of a 1/10 dilution (in PBS, pH 7.4) of supernatant from RF virus-infected MA104 cells (107 focus-forming units/ml) or the supernatant of mock-infected MA104 cells (negative control) and incubated overnight at 4°C. After these solutions were discarded, 150 μl of 5% BLOTTO was added to the plates, and the plates were incubated at 37°C for 1 h. Then, the BLOTTO was discarded, and 70 μl of the dilutions of plasma, stool, or saliva samples in 2.5% BLOTTO was deposited in each well. After 2 h of incubation at 37°C, the plates were washed five times with PBS-Tween, 70 μl of biotin-labeled goat anti-human IgA or IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) diluted in 2.5% BLOTTO was added to the plates, and the plates were incubated for 1 h at 37°C. After five washes with PBS-Tween, 70 μl of streptavidin-peroxidase (Kirkegaard & Perry Laboratories) diluted in PBS was added, and the plates were incubated for 1 h at 37°C. After five washes with PBS-Tween, the plates were developed using TMB substrate as described above for the antigen ELISA.
For detection of RV-specific IgM, we used a strategy previously described by Coulson et al. (11). ELISA plates were coated overnight with 70 μl of goat F(ab′)2 (0.7 μg) anti-human IgM (Biosource International, Camarillo, Calif.) in PBS (pH 7.4). The plates were then blocked and incubated with dilutions of plasma or stool samples as described above for the IgA ELISA. After two washes with PBS-Tween, 70 μl of a 1/10 dilution of supernatant from RF virus (107 focus-forming units/ml)-infected MA104 cells or mock-infected cell supernatant was added to individual wells in PBS (pH 7.4) and incubated for 1 h at 37°C. The plates were then washed twice with PBS-Tween, guinea pig anti-RRV hyperimmune serum (diluted in 2.5% BLOTTO-1.5% normal goat serum) was added to each well, and the wells were incubated for 1 h at 37°C. After three washes with PBS-Tween, 70 μl of biotin-labeled goat anti-guinea pig serum (Vector) in 2.5% BLOTTO-1.5% normal goat serum was added, and the plates were incubated for 1 h at 37°C. After three washes with PBS-Tween, 70 μl of streptavidin-peroxidase was added to each well. The assay was developed as described above for the RV antigen ELISA. A plasma or stool sample from a non-RV-infected child and serial dilutions from a recently RV-infected adult volunteer were included on each plate. The titer of plasma was defined as the reciprocal of the last dilution exceeding an optical density of 0.1 that was twice the optical density of mock-treated wells. To be accepted for analysis, the titer of the positive control plasma in a plate could not differ by more than 1 dilution from plate to plate.
CMV seroreactivity.
Plasma anti-cytomegalovirus (CMV) IgG was detected using a CMV IgG ELISA system from Zeus Scientific, Inc. (Raritan, N.J.) following the manufacturer's instructions.
Definitions.
A child was considered infected by RV if (i) viral antigen was detected by ELISA in either of its stool samples (acute or convalescent phase), (ii) stool virus-specific IgM had a titer greater than 1/2 in either sample, (iii) a serum titer greater than 1/400 for RV-specific IgM was found in either sample, (iv) the child copro IgA converted (a fourfold increase in virus-specific titers between the acute- and convalescent-phase samples), or (v) the child's plasma demonstrated a seroconversion in IgA or IgG (a fourfold increase in virus-specific titers between the acute- and convalescent-phase samples) (11, 12). A child was considered to have diarrhea of another origin if he or she lacked all of the above criteria for acute RV infection. All healthy adults and healthy parents and guardians of non-RV-infected children were considered not to be recently RV infected. Adults with acute diarrhea or asymptomatic parents and guardians of children with acute RV-induced diarrhea were classified as recently infected or not recently infected with RV by using the same criteria as for children with diarrhea but replacing measurements of virus-specific stool IgM and IgA with the measurement of saliva-specific IgA.
Antigen stimulation of PBMC.
Peripheral blood mononuclear cells (PBMC) were purified from heparinized whole-blood samples by Ficoll-Hypaque gradients (Histolab Ltda). The cells were washed twice with PBS (Histolab Ltda) plus 2% fetal calf serum (Gibco BRL, Gaithersburg, Md.) and resuspended in RPMI containing 20 mM HEPES, 100 U of penicillin/ml, and 100 mg of streptomycin (Histolab Ltda)/ml plus 10% fetal calf serum (complete medium). PBMC (2 × 106 cells in a final volume of 2 ml of complete medium) were stimulated with either 200 μl of the supernatant of RRV (titer, 7 × 107 focus-forming units per ml)-infected MA104 cells, 200 μl of the supernatant of mock-infected MA104 cells (negative control), or the superantigen staphylococcal enterotoxin B (SEB; Sigma) as a positive control (1.25 μg/ml). Anti-CD28 (0.5 μg/ml) and anti-CD49d (0.5 μg/ml) monoclonal antibodies (Pharmingen, San Diego, Calif.) were added to each sample as costimulators (42). Antigen stimulation was done in 15-ml polystyrene Falcon tubes (Becton Dickinson Labware, Franklin Lakes, N.J.) incubated with a 5° slant for 10 h at 37°C with 5% CO2. The last 5 h of the incubation included brefeldin A (10 μg/ml; Sigma) to block the secretion of cytokines from the cells (41, 42). At the end of the incubation, the cells were washed once with PBS-0.5% bovine serum albumin (Sigma)-0.02% sodium azide (Mallinckrodt Chemicals, Paris, Ky.) (staining buffer [SB]). Then, a 2 mM final concentration of EDTA (Sigma) in PBS was added for 10 min to detach plastic-adherent cells, and the samples were washed once more with SB. The cells were fixed with 1% paraformaldehyde (Carlo ERBA Reagenti, Rodano, Italy) for 5 min, washed with SB, and then frozen at −70°C in SB with 10% dimethyl sulfoxide (Sigma). PBMC from a subset of healthy CMV-seropositive and -seronegative adults were also stimulated with appropriately titered CMV antigen and a control antigen made from mock-infected cells used to grow the CMV, both obtained from BioWhittaker, Inc., Walkersville, Md.
Inmunofluorescent staining and flow cytometric analysis.
Cells were thawed, washed once with SB, incubated with fluorescence-activated cell sorter permeabilization solution (BD Immunocytometry Systems, San Jose, Calif.) for 10 min, washed with SB, and then stained for 30 min in the dark with the following monoclonal antibodies: anti-CD8 and -CD4-peridinin chlorophyl protein (PerCP; BD Immunocytometry Systems), anti-CD69-allophycocyanin (APC), anti-CD69-phycoerytherin (PE), anti-gamma interferon (IFN-γ)-fluorescein isothiocyanate (FITC), and anti-interleukin-13 (IL-13)-PE and isotype-matched control monoclonal antibodies labeled with FITC, PE, and APC obtained from Pharmingen. All monoclonal antibodies were titrated and used at optimal concentrations. We chose to study IFN-γ and IL-13 as representative Th1 and Th2 cytokines, respectively (28). CD69 is an early activation antigen, and its presence was used in combination with the secretion of cytokines to detect antigen-activated T cells. Only cells that were positive for CD69 and the cytokine studied were considered antigen specific. Samples were stained with two combinations of antibodies: (i) CD69-PE/CD8-PerCP/IFN-γ-FITC and (ii) CD69-APC/CD4-PerCP/IFN-γ-FITC/IL-13-PE. In a small subset of experiments, the last combination of antibodies was substituted for CD69-PE/CD4-PerCP/IFN-γ-FITC, and secretion of IL-13 was not measured. Analysis of the stained cells was performed using a FACSCalibur flow cytometer (BD Immunocytometry Systems) equipped with a second 635-nm red diode laser and using Cell Quest software. From 30,000 to 60,000 gated CD8high+ or CD4+ events were acquired. Only CD8high T cells were analyzed to exclude NK cell populations from the analysis. Dead cells and debris were excluded by forward and side scatter gating. The frequencies of antigen-specific T cells (either the CD4+ or CD8+ subset) are expressed as percentages of total CD4+ and CD8+ T cells.
Statistical analysis.
Statistical analysis was performed with SPSS (Chicago, Ill.) software version 8.0 using nonparametric tests. Differences between groups were evaluated with the Kruskal-Wallis test followed by Mann-Whitney tests. Differences between paired results were compared with the Wilcoxon test. Significance was established if P was <0.05. Data are shown as mean, standard error of the mean (SEM), and range unless otherwise noted.
RESULTS
Frequencies of RV-specific T cells in healthy adults and recently RV-infected adults and children.
We used the measurement of intracellular cytokines to quantitate the frequencies of T cells producing IFN-γ and IL-13 in response to RV infection in adults and children with acute- or convalescent-phase RV-induced diarrhea, asymptomatically infected adults, laboratory workers with frequent exposure to human stool samples containing RV, and healthy nonexposed adults (41, 42). The frequencies of CD4+ T cells responding to RV with production of IFN-γ and IL-13 and CD8+ T cells responding with production of IFN-γ are shown in Table 1. To calculate the means from this table, we took into consideration eight repeat experiments with symptomatic adults (Table 2) and one repeat experiment with one child (Table 3) and one asymptomatically RV-infected adult with recent RV infection.
TABLE 1.
Frequencies of CD4+ IFN-γ+ IL-13+ and CD8+ IFN-γ+ T cells responding to RRV and mock preparations in five subject groupsa
| Subject groupb | % CD4+ IFN-γ+
|
% CD4+ IL-13+
|
% CD8+ IFN-γ+
|
|||
|---|---|---|---|---|---|---|
| RRV | Mock | RRV | Mock | RRV | Mock | |
| Healthy adults (n = 7, 5, 6) | 0.040** (0.007) | 0.014 (0.003) | 0.046 (0.01) | 0.024 (0.004) | 0.065* (0.01) | 0.033 (0.008) |
| Asymptomatic adults (n = 5, 3, 5) | 0.068* (0.01) | 0.018 (0.001) | 0.006 (0.002) | 0.006 (0.002) | 0.188* (0.07) | 0.034 (0.008) |
| Symptomatic adults (n = 18, 9, 18) | 0.255*** (0.07) | 0.034 (0.001) | 0.040 (0.01) | 0.030 (0.001) | 0.333*** (0.07) | 0.031 (0.002) |
| RV-exposed lab workers (n = 5, 4, 5) | 0.100* (0.02) | 0.018 (0.004) | 0.032 (0.005) | 0.017 (0.004) | 0.528* (0.17) | 0.034 (0.004) |
| Infected children (n = 13, 10, 13) | 0.037** (0.005) | 0.020 (0.002) | 0.041* (0.01) | 0.022 (0.003) | 0.065** (0.008) | 0.043 (0.005) |
Values are given as mean (SEM). P value for comparison between the responses to RRV and the mock preparation with the Wilcoxon test are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Numbers of experiments (n) for each T-cell determination are shown in the same order as the columns. For one healthy adult, the response in CD8+ T cells was not determined. For one to three subjects from each group, IL-13 responses were not studied.
TABLE 2.
Frequencies of RRV-specific CD4+ and CD8+ T cells secreting IFN-γ in adults with symptomatic RV infection
| Subject | DAOD | % CD4+ | % CD8+ |
|---|---|---|---|
| A01 | 4 | 0.02 | 0.1 |
| A02 | 5 | 0.12a | 0.03 |
| A03 | 6 | 0.03 | 0.35 |
| A04 | 7 | 0.26 | 0.35 |
| A05 | 8 | 0.18 | 0.65 |
| A04 | 14 | 0.94 | 0.51 |
| A06 | 15 | 0.11 | 0.12 |
| A07 | 15 | 0.03 | 0.04 |
| A08 | 21 | 0.05 | 0.1 |
| A05 | 23 | 0.09 | 0.13 |
| A04 | 28 | 0.71 | 0.91 |
| A09 | 32 | −0.01 | 0.06 |
| A05 | 33 | 0.03 | 0.01 |
| A08 | 35 | 0.01 | 0.03 |
| A10 | 42 | 0.01 | 0.04 |
| A04 | 60 | 0.48 | 0.48 |
| A04 | 105 | 0.42 | 0.79 |
| A04 | 557 | 0.5 | 0.74 |
Values above 0.1% are shown in boldface.
We used the Wilcoxon test to evaluate differences between the percentages of donor T cells responding to the RV antigen and the mock preparation. Statistically significant responses to RV were found in all subject groups for both CD8+ and CD4+ cells responding with the production of IFN-γ (Table 1). Small but statistically significant differences for CD4+ cells that responded with production of IL-13 were found in symptomatically infected children but not in any of the four groups of adults studied (Table 1). Thus, CD4+ T cells from adult subjects that responded with the production of IL-13 were considered to be non-RV specific. For all groups of responding T cells except the CD4+ cells producing IL-13, the percentage of RV-specific T cells was obtained by subtracting the percentage of T cells responding to the mock preparation from the percentage of T cells responding to the viral antigen.
Frequencies of responding T cells similar to those obtained using unpurified RRV antigen as a stimulant (Table 1) were also obtained using cesium chloride density gradient-purified bovine RV, RF virus (3.5 μg/test), as an antigen in a subset of five adults and three children with symptomatic RV infection, one adult with asymptomatic infection, and three healthy adults (data not shown).
Kinetics of detection of RV-specific CD8+ and CD4+ cells expressing IFN-γ in adults with RV diarrhea.
The frequencies of CD4+ and CD8+ RV-specific T cells in individual adults with RV-induced diarrhea are shown in Table 2 and Fig. 1. Seven of eight symptomatically infected adults studied between 4 and 21 DAOD had frequencies of CD4+ and/or CD8+ IFN-γ-secreting T cells of 0.1% (a value not attained by the T cells of healthy adult subjects) (Tables 1 and 2). After 28 days, four of five symptomatically infected adults had frequencies below 0.1%. T-cell experiments were performed at different DAOD with three symptomatically infected adult subjects (Fig. 1, A04, A05, and A08). Long-term persistence (up to 557 DAOD) of RV-specific T cells with frequencies above 0.1% was detected in subject A04. Subject A04 did not have any identifiable persistent or recurrent source of RV reinfection during the 557 days he was followed. In contrast, RV-specific T cells were identified only transiently after infection in subject A05 (until 23 DAOD) and subject A08 (until 21 DAOD). In A09 and A10, who were studied only on days 32 and 42, respectively, frequencies of CD4+ or CD8+ T cells above 0.1% were not detected. Based on this data, and since the level of RV-specific T cells in healthy adults is quite low (Table 1) and it is known that these subjects had experienced several RV infections during their lives, we conclude that following acute infection in adults, RV-specific T cells secreting IFN-γ rise acutely by day 4 or 5 and then return to levels similar to those of healthy adult subjects after approximately 1 month in most cases (Table 2).
FIG. 1.
Frequencies of RV-specific CD4+ CD69+ IFN-γ+ and CD8+ CD69+ IFN-γ+ T cells in adults with RV diarrhea as a function of DAOD. The data are taken from Table 2 excluding (for clarity) the responses of subject A04 at 105 and 557 DAOD. Results from subjects with whom only one experiment was performed are represented by individual solid diamonds. The results from four, three, and two experiments at different DAOD with PBMC from subjects A04, A08, and A05 are represented by bars, triangles, and squares, respectively. The results from experiments with the same individual are joined by lines.
Comparative frequencies of RV-specific CD8+ T cells in recently infected adults and children and in uninfected adults.
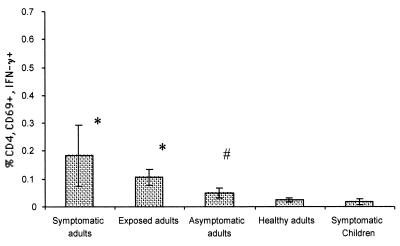
In order to compare the mean frequencies of RV-specific T cells secreting IFN-γ among the five subject groups, mean responses for each group of subjects were calculated using only one result (the one with the highest RV-specific T-cell frequencies) from subjects for whom the assay was performed more than once (Fig. 2 and 3). For the calculation of the mean frequencies of T cells in symptomatically infected adults, we excluded the results of subjects A09 and A10, because these subjects were only studied late, >1 month after the onset of diarrhea. After this time point, most symptomatically infected adults had frequencies of RV-specific T cells similar to those of healthy adults (Table 2). The mean percentage of CD8+ IFN-γ+ cells detected in healthy, not recently exposed, adult subjects was 0.03% (SEM, 0.03%; range, 0 to 0.09%) (Fig. 2). Significantly higher percentages of reactive CD8+ T cells were observed in exposed laboratory workers (mean, 0.49%; SEM, 0.17%; range, 0.2 to 1.13%; P < 0.01) and symptomatically infected adults (mean, 0.28%; SEM, 0.11%; range, 0.03 to 0.91%; P < 0.05). Asymptomatically infected adults did not have a significantly higher (P > 0.05) percentage of CD8+ IFN-γ+ cells (mean, 0.15%; SEM, 0.06%; range, 0.03 to 0.37%) than healthy adults. RV-infected children had a mean percentage of RV-specific CD8+ T cells of 0.02% (SEM, 0.007%; range, −0.01 to 0.08%), which was similar to levels detected in healthy adults and significantly lower than those of all other groups of adults (P < 0.05) (Fig. 2 and Table 3).
FIG. 2.
Mean (±SEM) frequencies of CD8+ IFN-γ+ RV-specific T cells in the five subject populations: symptomatic RV-infected adults (n = 8), RV-exposed laboratory workers (n = 5), asymptomatic RV-infected adults (n = 4), healthy nonexposed adults (n = 6), and symptomatic RV-infected children (n = 12). For the adults and children with diarrhea for whom we had more than one sample, the means were calculated using the sample with the highest response (Tables 2 and 3). We excluded from the calculation of symptomatic adults subjects A09 and A10, who were only studied 32 and 42 DAOD (Table 2). *, statistically significant differences between the responses of symptomatic children and healthy adults (P < 0.01; Mann-Whitney); #, statistically significant differences compared to the responses of symptomatic children (P < 0.01; Mann-Whitney).
FIG. 3.
Mean (±SEM) frequencies of CD4+ IFN-γ+ RV-specific T cells in the five subject populations: symptomatic RV-infected adults (n = 8), RV-exposed laboratory workers (n = 5), asymptomatic RV-infected adults (n = 4), healthy nonexposed adults (n = 7), and symptomatic RV-infected children (n = 12). For the adults and children with diarrhea for whom we had more than one sample, the means were calculated using the sample with the highest response (Tables 2 and 3). We excluded from the calculation of symptomatic adults subjects A09 and A10, who were only studied 32 and 42 DAOD (Table 2). *, statistically significant differences between the responses of children and healthy adults (P < 0.01; Mann-Whitney); #, statistically significant differences with the response of children (P < 0.01; Mann-Whitney).
Using an assay similar to the one used here, the frequency of Epstein-Barr virus-specific CD8+ IFN-γ+ T cells in individuals with an acute infection has been shown to be approximately 25 to 50% of total CD8+ T cells (8, 22). These percentages are clearly much greater than the frequencies of RV-specific CD8+ T cells we have identified in adults with RV-induced diarrhea. The percentages observed in recently RV-infected adults seem to be even lower than those detected in long-term healthy Epstein-Barr virus carriers, with frequencies ranging between 0.63 and 1.29% (26). In healthy individuals with serologic evidence of past viral infection, Asanuma et al. did not detect circulating varicella-zoster or herpes simplex virus-specific CD8+ IFN-γ+ T cells (4). This result seems comparable to the low frequencies of RV-specific CD8+ IFN-γ+ T cells observed in healthy adults (Fig. 2).
Comparative frequencies of RV-specific CD4+ T cells in recently infected adults and children and healthy adults.
The mean percentage of RV-specific CD4+ IFN-γ+ T cells identified in healthy adults was 0.02% (SEM, 0.01%; range, 0.01 to 0.06%) (Fig. 3). These values are lower than the percentages of other virus-specific CD4+ T cells detected in seropositive adults: frequencies of antigen responsive IFN-γ+ CD4+ T cells are higher in seropositive donors for mumps virus (mean, 0.18%; range, 0.02 to 0.23%) (28, 42), herpes simplex virus (mean, 0.22%; range, 0.05 to 0.52%), and varicella-zoster virus (mean, 0.11%; range, 0 to 0.28%) (4). We did not find an increase in RV-specific CD4+ IFN-γ+ T cells in the asymptomatic RV-infected adult group (mean, 0.05%; SEM, 0.01%; range, 0.01 to 0.09%; P > 0.05) (Fig. 3). In contrast, significantly greater responses (P < 0.05) were observed in exposed laboratory workers (mean, 0.1%; SEM, 0.02%; range, 0.02 to 0.19%) and symptomatically infected adults (mean, 0.18%; SEM, 0.10%; range, 0.02 to 0.94%) than in healthy adults. To our knowledge, this is the first characterization of the frequencies of cytokine-secreting human RV-specific CD4+ T cells studied during an acute viral infection.
Very low percentages of RV-specific CD4+ IFN-γ+ (mean, 0.02%; SEM, 0.007%; range, −0.01 to 0.07%) or CD4+ IL-13+ (mean, 0.02%; SEM, 0.009%; range, 0 to 0.06%) T cells were detected in infected children (Fig. 3 and Table 3). These frequencies are significantly lower than those from all adult groups except healthy adults (Fig. 3). Children's CD4+ T cells showed no predominance in secretion of either IFN-γ or IL-13 (P > 0.05; Wilcoxon). Thus, our results indicate a Th1 cytokine response in adults and a lack of CD4+ T-cell response in most of the children, with a very low mixed Th1-Th2 response in a few children. The last result needs to be confirmed by future studies.
Frequencies of CD8+ and CD4+ T cells specific for CMV in adults.
To compare the frequencies of RV-specific T cells observed in healthy adults with the T-cell response against a persistent systemic virus, we studied the T-cell response to CMV in CMV-seropositive and -seronegative healthy subjects. In CMV-seropositive individuals, the response to CMV antigen was significantly different from the response to mock antigen (P < 0.03; Wilcoxon). The mean frequencies (response after subtraction of the response to the mock preparation; mean, 0.03%) of virus-specific CD4+ IFN-γ+ T cells in CMV-seropositive adult subjects was 0.48% (SEM, 0.25%; range, 0 to 2.02%; n = 8). The mean frequencies (response after subtraction of the response to mock antigen; mean, 0.02%) of CD8+ IFN-γ+ T cells in CMV-seropositive adult subjects was 0.53% (SEM, 0.32%; range, 0 to 2.72%; n = 8). In CMV-seronegative subjects, the mean frequencies of CD4+ and CD8+ T cells responding to the CMV antigen (means, 0.02% [SEM, 0.01%; n = 5] and 0.006% [SEM, 0.01%; n = 3], respectively) were not significantly different from the mean responses to the mock preparation (P > 0.14; Wilcoxon). In both seropositive (n = 6) and seronegative (n = 4) subjects, no statistically significant differences were found between the frequencies of IL-13-secreting CD4+ T cells responding to the CMV and mock antigens (P > 0.13). These results are similar to those published by other investigators (4, 42). We conclude that in our study population, memory RV-specific T cells in healthy seropositive adult subjects are approximately 20-fold lower in frequency than T cells reactive to the systemic persistent virus CMV.
Frequencies of CD8+ and CD4+ T cells stimulated by SEB in adults and children.
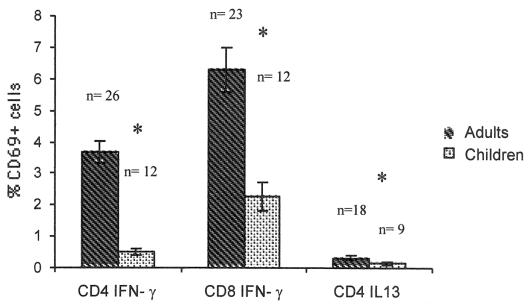
Frequencies of CD4+ and CD8+ T cells responding to SEB with the production of cytokines were also studied in children and adults (Fig. 4). For the analysis of these results, only one experiment per volunteer was evaluated. No significant differences in the responses to SEB were observed among the four groups of adults (P > 0.05; Mann-Whitney). Based on this result, we averaged the results from the four groups of adults and compared this value to the response of children to SEB. The mean percentage of CD4+ IFN-γ+ T cells in children (mean age, 18.75 months) was 0.51% (SEM, 0.10%; range, 0.16 to 1.59%; n = 12), while in adults it was 3.67% (SEM, 0.37%; range, 1.05 to 8.81%; n = 26). The mean frequency of CD4+ IL-13+ T cells in children was 0.13% (SEM, 0.04%; range, 0.01 to 0.46%; n = 9), and in adults it was 0.32% (SEM, 0.06%; range, 0.01 to 0.91%; n = 18). Finally, the mean percentage of CD8+ IFN-γ+ T cells in children was 2.27% (SEM, 0.43%; range, 0.37 to 5.48%; n = 12), and in adults it was 6.3% (SEM, 0.7%; range, 1.12 to 13.51%; n = 23). Significantly lower percentages of SEB-stimulated CD4+ IFN-γ+, CD4+ IL-13+, and CD8+ IFN-γ+ cells were observed in children than in adults (P < 0.01; Mann-Whitney) (Fig. 4).
FIG. 4.
Mean (±SEM) frequencies of CD4+ IFN-γ+ IL-13+ and CD8+ IFN-γ+ T cells in children and adults (including individuals from all of the adult groups studied) responding to SEB. *, statistically significant differences were detected between the responses of children and adults (P < 0.01; Mann-Whitney).
DISCUSSION
We have quantified the frequencies of human T cells producing IFN-γ and IL-13 in response to RV, a prototypical viral intestinal pathogen that replicates almost exclusively in enterocytes (20). To our knowledge, this is the first report that has identified human RV-specific CD8+ T cells and determined the frequencies of RV-specific CD4+ IFN-γ+, CD4+ IL-13+, and CD8+ IFN-γ+ cells in children and adults. The frequencies of RV-specific T cells secreting IFN-γ in recently and not recently infected adults seem to be reduced compared to the T-cell frequencies for several other viruses. Moreover, the most striking result is the very low levels of RV-specific IFN-γ+ or IL-13+ T cells in children with acute RV-induced diarrhea.
The relatively low frequencies of RV-specific T cells we have detected in adults with RV diarrhea and in healthy adults could be characteristic of intestinal immune responses or attributable specifically to RV infection. Concerning the first hypothesis, since the peripheral blood T cells tested were originally stimulated in PP and are theoretically on their way back to the intestine (7), it is possible that an important proportion of RV-specific T cells remain in the intestine or are localized to other organs and are not present for detection in the circulation, as has been recently suggested in a murine model (30). An alternative hypothesis to explain the low frequencies of RV-specific T cells detected in peripheral blood is that only a small percentage of circulating RV-specific T cells secrete cytokines, reflecting an unusually inefficient Th1-Th2 polarization process. In both mice (37) and humans (38), it has been shown that stimulation of T cells in vitro in the presence of transforming growth factor β (TGF-β) and antibodies that favor a Th2 differentiation (antibodies against IFN-γ or IL-12) results in the selective expansion of T cells that do not display immediate cytokine production capacity. In mice, these nonpolarized cells retain the capacity to differentiate to either Th1 or Th2 when stimulated under appropriate polarizing conditions and to proliferate after antigen stimulation (37). Murine PP dendritic cells appear to be more prone to produce TGF-β and IL-10 than spleen dendritic cells (23). This difference seems to influence the pattern of cytokines secreted by T cells stimulated by each type of dendritic cell (23). Although it is not known if human PP dendritic cells are prone to secrete TGF-β and IL-10, it is possible that RV-specific T cells have differentiated under the influence of these or other cytokines that favor the development of a nonpolarized T-cell response. Alternatively, the RV-specific T cells we have identified could have been induced by an intestinal priming environment to secrete certain Th1 or Th2 cytokines not measured in our experiments, or the Th3 cytokine TGF-β, as has been shown for some intestinally derived human T cells (17). Future experiments with major histocompatibility complex tetramers (bound to RV-derived peptides that are recognized by RV-specific T cells) will be useful to identify and quantitate non-cytokine-secreting RV-specific T cells. Alternatively, experiments that measure TGF-β and/or other cytokines in RV-stimulated T cells would test the hypothesis that RV infection induces T cells that secrete a unique cytokine repertoire.
Concerning the hypothesis that the low levels of circulating RV T cells are dependent on the nature of the RV as immunogen, it should be born in mind that RVs are lytic, nonpersistent viruses (20). It is probable that the higher levels of memory-specific T cells that we and others have detected to CMV, Epstein-Barr virus, varicella-zoster virus, and herpes simplex virus are related to the fact that these are persistent viruses. In individuals infected with some, but not all, persistent viruses, continuous restimulation of T cells with antigen could assure the persistence of memory T cells (4). The particularly high levels of memory T-cell responses to CMV could be explained, in addition, by the fact that CMV persists in cells of monocyte progenitor lineage, thus favoring antigen presentation (4). Laboratory workers who manipulated RV+ stool samples on a regular basis had frequencies of RV-specific T cells close to those found in symptomatic adults rather than those detected in healthy adults or asymptomatic subjects (Fig. 2 and 3). Laboratory workers could have been exposed repeatedly to low doses of antigen, which might explain the persistence of RV-specific T cells.
We found lower frequencies of RV-specific T cells secreting IFN-γ in children than in adults with RV diarrhea (Fig. 2 and 3). It is probable that other reasons, in addition to the simple fact that adults and not children have accumulated substantial pools of RV-specific memory T cells due to repeated RV infections, can explain this difference. For example, in agreement with our results, it has been reported that children's T cells respond less efficiently in producing IFN-γ (measured in the supernatants of cell cultures) to herpesvirus (6) and measles virus (18) than the T cells of adults. The lower frequencies of RV-specific T cells secreting IFN-γ in children could be due to at least two non-mutually exclusive reasons: (i) lower virus-specific precursor frequencies could be generated in children than in adults and (ii) a qualitative difference could exist between adult's and children's RV-specific T cells in their capacity to produce IFN-γ (see below). The generation of lower numbers of precursors of RV-specific T cells could be explained by the fact that children's T cells seem to have lower levels of T-cell receptors and adhesion molecules (reviewed in reference 2). Antigen-presenting cells of neonates and children (10, 39) and of neonatal mice and mice <4 weeks old (32) have been reported to be less efficient than the antigen-presenting cells of adults and could thus also explain the decreased responses observed in this study. In agreement with the last hypothesis, addition of IL-12 and/or other cytokines to the cultures of antigen-stimulated or polyclonally stimulated T cells of children or neonates can partially overcome their hyporesponsiveness in production of IFN-γ (reviewed in reference 2).
Mouse pups immunized with RV have been shown to develop a mixed Th1-Th2 response (16), while immunization of adult mice seems to lead to a predominant Th1 response (40). These results are in agreement with several studies that show that immunization of neonatal mice frequently leads to a Th2-biased profile, while adult mice respond preferentially with Th1 responses (2, 3). The few available studies of neonates and children seem to suggest that they can develop both Th2- and Th1-type responses and are not prone to the development of Th2 responses, like mice (2, 29). In children, but not in adults, we found a small but statistically significant difference between the IL-13 production of RV-specific CD4+ T cells in response to the RV antigen and the mock preparation (Table 1). This result suggests that, at least for a few of the children studied, the RV-specific T-cell responses resemble those of neonatal Th2-prone mice. As introduced above, this conclusion would support the hypothesis that qualitative differences between the RV-specific T cells of adults and children exist. Nonetheless, because of the very low-level or negative response in production of both IL-13 and IFN-γ in most of the children studied (Table 3), no firm conclusion on this point can be reached.
In agreement with reports of healthy children by other investigators (9), we found lower percentages of SEB-stimulated CD4+ IFN-γ+ and CD8+ IFN-γ+ cells in RV-infected children than in infected and uninfected adults (Fig. 4). In healthy children, a reduced production of the Th2 cytokine IL-4 in response to SEB has been shown (21). Here, we have shown a decreased frequency of T cells from RV-infected children that respond with the production of IL-13, another Th2 cytokine (Fig. 4). In children, the frequencies of T cells responding to SEB with the production of IL-13 are significantly lower (P < 0.01) than the frequencies of T cells responding with the production of IFN-γ (Fig. 4). Nonetheless, the ratios (adult to children) of cells producing IFN-γ and IL-13 in response to SEB are 7.19 and 2.46, respectively, suggesting that in children the IFN-γ response is more reduced than the IL-13 response. Taken together, these findings suggest that in children with RV diarrhea, if a tendency to a Th2 response exists, it is a minor effect.
It has been shown that in response to polyclonal stimulators, CD45RA+ T cells (mostly naive), coming from either adults or children, secrete lower levels of IFN-γ than memory CD45RO+ T cells from adults (27). CD45RA+ T cells from adults also respond to SEB with lower levels of proliferation than unfractioned PBMC of adults (21). The RA/RO proportion in adults is smaller, however, than in children, so that SEB proliferation of unfractioned T cells is greater in adults. Thus, the principal factor to explain the differences in response to SEB between children and adults is likely to be the higher levels of hyporesponsive CD45RA T cells present in children (27). However, this factor does not play a role in the diminished responses to RV we have observed in children, because the RV-specific T cells of both adults and children are likely to be memory CD45RO+ cells.
Other investigators have studied T-cell lymphoproliferative responses to RV antigen in PBMC of recently RV-infected children (34). Although it is difficult to compare these results with ours because of differences in the populations and the methods used, it seems that the number of children responding with RV-specific T cells secreting cytokines (Table 3) is lower than the number of children responding in the lymphoproliferation assay. This divergence could be explained by the hypothesis presented above, which suggests that RV-specific T cells are enriched in nonpolarized (non-cytokine-secreting) T cells that nonetheless are capable of proliferating in response to antigen in vitro (37). Another factor that could be influencing this difference is the fact that the lymphoproliferation assay lasts 5 days compared to the 10 h in our protocol. The greater time used in the proliferation assay could permit the children's antigen-presenting cells to mature and thus to perform their function more efficiently (39). Direct detection of RV-reactive T cells, via tetramer staining, for example, could shed light on the hypothesis that RV T cells in children are nonpolarized.
Acknowledgments
This work was supported by funds from the Pontificia Universidad Javeriana, Colciencias grant 1203-04-151-98, Fundación para la Promoción de la Investigación y la Tecnología, Banco de la República grant 1103, and NIH grant AI21362 and by a VA merit review grant to H.B.G.
We thank the subjects who participated in this study for their generosity, the personnel of the Pediatrics Department of the Hospital San Ignacio for their help in identifying children with diarrhea, Mauricio Calvo for his support, and Sara Patricia Bonilla for help in administrating the funds from the grants.
REFERENCES
- 1.Abramson, J. S., C. J. Baker, M. C. Fisher, M. A. Gerber, H. C. Meissner, D. L. Murray, G. D. Overturf, C. G. Prober, M. B. Rennels, T. N. Saari, L. B. Weiner, R. J. Whitley, et al. 1999. Possible association of intussusception with rotavirus vaccination. Pediatrics 104:575.. [PubMed] [Google Scholar]
- 2.Adkins, B. 1999. T-cell function in newborn mice and humans. Immunol. Today 20:330-335. [DOI] [PubMed] [Google Scholar]
- 3.Adkins, B., Y. Bu, and P. Guevara. 2001. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J. Immunol. 166:918-925. [DOI] [PubMed] [Google Scholar]
- 4.Asanuma, H., M. Sharp, H. T. Maecker, V. C. Maino, and A. M. Arvin. 2000. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J. Infect. Dis. 181:859-866. [DOI] [PubMed] [Google Scholar]
- 5.Bresee, J. S., R. I. Glass, B. Ivanoff, and J. R. Gentsch. 1999. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine 17:2207-2222. [DOI] [PubMed] [Google Scholar]
- 6.Burchett, S. K., L. Corey, K. M. Mohan, J. Westall, R. Ashley, and C. B. Wilson. 1992. Diminished interferon-gamma and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J. Infect. Dis. 165:813-818. [DOI] [PubMed] [Google Scholar]
- 7.Butcher, E. C., M. Williams, K. Youngman, L. Rott, and M. Briskin. 1999. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72:209-253. [DOI] [PubMed] [Google Scholar]
- 8.Callan, M. F., C. Fazou, H. Yang, T. Rostron, K. Poon, C. Hatton, and A. J. McMichael. 2000. CD8(+) T-cell selection, function, and death in the primary immune response in vivo. J. Clin. Investig. 106:1251-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, D. E., A. S. Fryga, S. Bol, and A. S. Kemp. 1999. Intracellular interferon-gamma (IFN-gamma) production in normal children and children with atopic dermatitis. Clin. Exp. Immunol. 115:377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clerici, M., L. DePalma, E. Roilides, R. Baker, and G. M. Shearer. 1993. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J. Clin. Investig. 91:2829-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulson, B. S., K. Grimwood, R. F. Bishop, and G. L. Barnes. 1989. Evaluation of end-point titration, single dilution and capture enzyme immunoassays for measurement of antirotaviral IgA and IgM in infantile secretions and serum. J. Virol. Methods 26:53-65. [DOI] [PubMed] [Google Scholar]
- 12.Coulson, B. S., K. Grimwood, I. L. Hudson, G. L. Barnes, and R. F. Bishop. 1992. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J. Clin. Microbiol. 30:1678-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco, M. A., and H. B. Greenberg. 1997. CD8+ T cells can mediate almost complete short-term and partial long-term immunity to rotavirus in mice. J. Virol. 71:4165-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco, M. A., and H. B. Greenberg. 1997. Immunity to rotavirus in T cell deficient mice. Virology 338:169-179. [DOI] [PubMed] [Google Scholar]
- 15.Franco, M. A., and H. B. Greenberg. 1995. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J. Virol. 69:7800-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fromantin, C., L. Piroth, I. Petitpas, P. Pothier, and E. Kohli. 1998. Oral delivery of homologous and heterologous strains of rotavirus to BALB/c mice induces the same profile of cytokine production by spleen cells. Virology 244:252-260. [DOI] [PubMed] [Google Scholar]
- 17.Fukaura, H., S. C. Kent, M. J. Pietrusewicz, S. J. Khoury, H. L. Weiner, and D. A. Hafler. 1996. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J. Clin. Investig. 98:70-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gans, H. A., Y. Maldonado, L. L. Yasukawa, J. Beeler, S. Audet, M. M. Rinki, R. DeHovitz, and A. M. Arvin. 1999. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J. Immunol. 162:5569-5575. [PubMed] [Google Scholar]
- 19.Gilchrist, M. J., T. S. Bretl, K. Moultney, D. R. Knowlton, and R. L. Ward. 1987. Comparison of seven kits for detection of rotavirus in fecal specimens with a sensitive, specific enzyme immunoassay. Diagn. Microbiol. Infect. Dis. 8:221-228. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg, H. B., H. F. Clark, and P. A. Offit. 1994. Rotavirus pathology and pathophysiology. Curr. Top. Microbiol. Immunol. 185:255-283. [DOI] [PubMed] [Google Scholar]
- 21.Hayward, A., and M. Cosyns. 1994. Proliferative and cytokine responses by human newborn T cells stimulated with staphylococcal enterotoxin B. Pediatr. Res. 35:293-298. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino, Y., T. Morishima, H. Kimura, K. Nishikawa, T. Tsurumi, and K. Kuzushima. 1999. Antigen-driven expansion and contraction of CD8+-activated T cells in primary EBV infection. J. Immunol. 163:5735-5740. [PubMed] [Google Scholar]
- 23.Iwasaki, A., and B. L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantele, A., J. Zivny, M. Häkkinen, C. O. Elson, and J. Mestecky. 1999. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J. Immunol. 162:5173-5177. [PubMed] [Google Scholar]
- 25.Kuklin, N. A., L. Rott, J. Darling, J. J. Campbell, M. A. Franco, N. Feng, W. Müller, N. Wagner, J. Altman, E. C. Butcher, and H. B. Greenberg. 2000. α4β7 independent pathway for CD8+ T cell mediated intestinal immunity to rotavirus. J. Clin. Investig. 106:1541-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuzushima, K., Y. Hoshino, K. Fujii, N. Yokoyama, M. Fujita, T. Kiyono, H. Kimura, T. Morishima, Y. Morishima, and T. Tsurumi. 1999. Rapid determination of Epstein-Barr virus-specific CD8(+) T-cell frequencies by flow cytometry. Blood 94:3094-3100. [PubMed] [Google Scholar]
- 27.Lewis, D. B., C. C. Yu, J. Meyer, B. K. English, S. J. Kahn, and C. B. Wilson. 1991. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J. Clin. Investig. 87:194-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maino, V. C., and L. J. Picker. 1998. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry 34:207-215. [DOI] [PubMed] [Google Scholar]
- 29.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 30.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 31.McNeal, M. M., K. S. Barone, M. N. Rae, and R. L. Ward. 1995. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virology 214:387-397. [DOI] [PubMed] [Google Scholar]
- 32.Muthukkumar, S., J. Goldstein, and K. E. Stein. 2000. The ability of B cells and dendritic cells to present antigen increases during ontogeny. J. Immunol. 165:4803-4813. [DOI] [PubMed] [Google Scholar]
- 33.Offit, P. A., E. J. Hoffenberg, E. S. Pia, P. A. Panackal, and N. L. Hill. 1992. Rotavirus-specific helper T cell responses in newborns, infants, children, and adults. J. Infect. Dis. 165:1107-1111. [DOI] [PubMed] [Google Scholar]
- 34.Offit, P. A., E. J. Hoffenberg, N. Santos, and V. Gouvea. 1993. Rotavirus-specific humoral and cellular immune response after primary, symptomatic infection. J. Infect. Dis. 167:1436-1440. [DOI] [PubMed] [Google Scholar]
- 35.Rosé, J. R., M. B. Williams, L. S. Rott, E. C. Butcher, and H. B. Greenberg. 1998. Expression of the mucosal homing receptor α4β7 correlates with the ability of CD8+ memory T cells to clear rotavirus infection. J. Virol. 72:726-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rott, L. S., J. R. Rosè, D. Bass, M. B. Williams, H. B. Greenberg, and E. C. Butcher. 1997. Expression of mucosal homing receptor alpha4beta7 by circulating CD4+ cells with memory for intestinal rotavirus. J. Clin. Investig. 100:1204-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sad, S., and T. R. Mosmann. 1994. Single IL-2-secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J. Immunol. 153:3514-3522. [PubMed] [Google Scholar]
- 38.Sallusto, F., D. Lenig, C. R. Mackay, and A. Lanzavecchia. 1998. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiehm, E. R., D. Mann, C. Newland, M. B. Sztein, P. S. Steeg, J. J. Oppenheim, and R. M. Blaese. 1983. Deficient DR antigen expression on human neonatal monocytes: reversal with lymphokines. Birth Defects 19:295-298. [PubMed] [Google Scholar]
- 40.VanCott, J. L., M. A. Franco, H. B. Greenberg, S. Sabbaj, B. Tang, R. Murray, and J. R. McGhee. 2000. Protective immunity to rotavirus shedding in the absence of interleukin-6: Th1 cells and immunoglobulin A develop normally. J. Virol. 74:5250-5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waldrop, S. L., K. A. Davis, V. C. Maino, and L. J. Picker. 1998. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J. Immunol. 161:5284-5295. [PubMed] [Google Scholar]
- 42.Waldrop, S. L., C. J. Pitcher, D. M. Peterson, V. C. Maino, and L. J. Picker. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Investig. 99:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]