Abstract
Neonatal rhesus macaque 95-3 was inoculated with nonpassaged simian-human immunodeficiency virus strain SHIV-vpu+, which encodes env of the laboratory-adapted human immunodeficiency virus (HIV) strain IIIB and is considered nonpathogenic. CD4+ T-cell counts dropped to <200 cells/μl within 4.6 years, and monkey 95-3 died with opportunistic infections 5.9 years postinoculation. Transfer of blood from 95-3 to two naive adult macaques resulted in high peak viral loads and rapid, persistent T-cell depletion. Progeny virus evolved in 95-3 despite high SHIV-vpu+ neutralizing antibody titers and still used CXCR4 but, in contrast to parental SHIV-vpu+, productively infected macrophages and resisted neutralization. Sequence analysis revealed three new potential glycosylation sites in gp120; another two were lost. Strikingly similar mutations were detected in a laboratory worker who progressed to AIDS after accidental HIV-IIIB infection (T. Beaumont et al., J. Virol. 75:2246-2252, 2001), thus supporting the SHIV-vpu+/rhesus macaque system as a relevant model. Similar mutations were also described after rapid passage of chimeric viruses encoding IIIB env in rhesus and pig-tailed macaques (M. Cayabyab et al., J. Virol. 73:976-984, 1999; Z. Q. Liu et al., Virology 260:295-307, 1999; S. V. Narayan et al., Virology 256:54-63, 1999; R. Raghavan et al., Brain Pathol. 7:851-861, 1997; E. B. Stephens et al., Virology 231:313-321, 1997). Thus, HIV-IIIB env evolved similarly in three different species; this selection occurred in chronically infected individuals during disease progression as well as after rapid virus passage. We postulate that evolutionary pressure led to the outgrowth of more aggressive viral variants in all three species.
Simian-human immunodeficiency viruses (SHIVs) contain envelope and accessory genes of HIV type 1 (HIV-1) in a SIV backbone (11, 16, 17, 26, 32, 33, 40). Several chimeric viruses with different pathogenic potentials have been constructed. Whereas some strains are highly pathogenic in rhesus macaques (17, 24, 33, 34), others are thought to be nonpathogenic, such as SHIV-4 and SHIV-vpu+ (20, 21). The latter two chimeras encode env of HXBc2, a molecular clone of the T-cell line-adapted HIV-IIIB (20, 21). In contrast to SHIV-4, SHIV-vpu+ contains an open vpu reading frame (20, 21). Both viruses replicate in rhesus monkeys (2, 25, 36).
Rapid in vivo passage of viruses containing HIV-IIIB env resulted in more aggressive variants that caused acute CD4+ T-cell loss (15, 16, 31, 43). However, thus far, macaques inoculated with the nonpassaged viruses have not developed signs of immune suppression or disease (20, 21). Here, we demonstrate that nonpassaged SHIV-vpu+ can cause CD4+ T-cell depletion and AIDS after prolonged observation, thus approximating the time course of untreated HIV-1 infections in humans.
Nonpassaged SHIV-vpu+ induces AIDS.
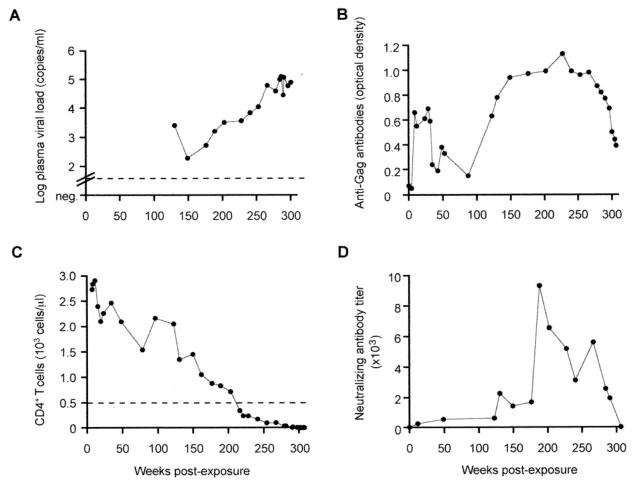
After nontraumatic oral SHIV-vpu+ inoculation (<10 oral 50% animal infectious doses) (1), a neonatal rhesus monkey (Macaca mulatta 95-3) became systemically infected. Virus isolation from peripheral blood mononuclear cells (PBMC) (1, 22) was persistently positive from weeks 2 to 49 postexposure and from week 228 onward. Viral RNA load in plasma measured by real-time reverse transcription-PCR (RT-PCR) (13) continuously increased from week 150 postexposure onward to reach >105 copies/ml (Fig. 1A). After seroconversion, anti-Gag antibodies decreased again (Fig. 1B), a finding that heralds development of immunodeficiency in SIV-infected monkeys and HIV-infected humans (5). Interestingly, a transient rebound of the anti-Gag antibodies was found at 123 weeks postexposure (Fig. 1B). CD4+ T cells steadily declined after week 150 (Fig. 1C) and were <50 cells/μl during the last 7 months. The monkey developed substantial weight loss and diarrhea and was sacrificed at week 307. High viral burdens were detected by cocultivation in lymphoid tissues (data not shown). Gross necropsy and histology demonstrated lymphadenopathy, pneumocystosis, and colitis with cryptitis. Infectious organisms found in this animal are listed in Table 1.
FIG. 1.
SHIV-vpu+ RNA load in plasma determined by real-time RT-PCR (A), anti-Gag antibodies (B), peripheral absolute CD4+ T-cell counts (C), and neutralization of SHIV-vpu+ in plasma from animal 95-3 (D). The sensitivity of the RT-PCR assay (A) is indicated by the dotted line (50 copies/ml). During the first 130 weeks postexposure, no plasma samples were available with an anticoagulant suitable for RT-PCR. In panel C, 500 CD4+ T cells/μl are indicated by a dotted line. Anti-Gag antibodies were assessed by enzyme-linked immunodominant assay as described previously (1, 2, 22) and given as optical density. Homologous neutralization of plasma was tested in triplicate in a modified MT-2 cell assay with SHIV-vpu+ (D) as described previously (19). Neutralizing antibody titers are the reciprocal dilution which protected 50% of the MT-2 cells from virus-induced cytotoxicity and correspond to 90% reduction of viral Gag synthesis (6). The lower limit of detection was determined as that giving a titer of 10.
TABLE 1.
Outcome of SHIV-vpu+ or progeny virus infection: infectious organisms
| Monkey | Viral inoculum | Time of sacrifice (weeks after virus exposure) | Infectious agents |
|---|---|---|---|
| Neonate 95-3 | SHIV-vpu+ | 307 | Campylobacter coli |
| Campylobacter jejuni | |||
| Pneumocystis carinii | |||
| Adult RJj-4 | Blood from 95-3 collected at week 291 | 43 | Giardia lamblia |
| Campylobacter coli Adenovirus | |||
| Pneumocystis carinii | |||
| Adult RMk-4 | Blood from RJj-4 collected at week 2 | 58 | Pneumocystis carinii |
Neutralizing activity against parental SHIV-vpu+ was initially low to moderate (Fig. 1D); the titers rose after viral RNA loads had increased and before CD4+ T-cell counts fell below 500 cells/μl. Thus, progeny virus evolved in monkey 95-3 despite high SHIV-vpu+ neutralizing antibody titers.
Parental SHIV-vpu+ evolved into a neutralization-resistant and acutely pathogenic virus in animal 95-3.
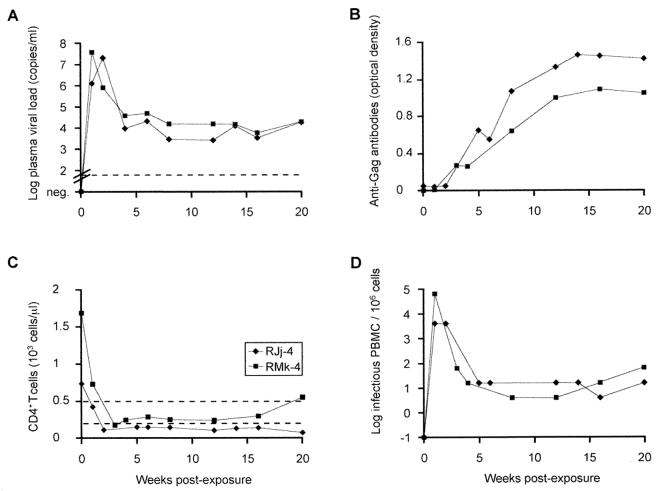
Virus isolated from animal 95-3 at necropsy was highly resistant to neutralization by plasma collected at weeks 180, 200, 225, 270, and 285 postinoculation (titers of <20), despite the presence of high titers of antibodies that neutralized parental SHIV-vpu+ at all time points (data not shown). Animal passage revealed that monkey 95-3 harbored virus with markedly increased virulence after it had progressed to AIDS. Blood (10 ml containing 642 infectious PBMC and 7.4 × 105 RNA copies) was collected at week 291 postinoculation from monkey 95-3 and inoculated intravenously into adult recipient monkey RJj-4. The animal developed high virus loads, rapid CD4+ T-cell losses in blood (Fig. 2) and lymphoid tissue (data not shown), and refractory diarrhea (Table 1). At week 43, RJj-4 had 24 CD4+ T cells/μl and was euthanatized because of Pneumocystis carinii pneumonia. A second blood transfer (10 ml containing 1.3 × 105 infectious PBMC and 1.26 × 108 RNA copies) from RJj-4 at week 2 postexposure to animal RMk-4 also resulted in high viral loads and rapid CD4+ T-cell depletion (Fig. 2). RMk-4 was sacrificed at week 58 due to P. carinii pneumonia.
FIG. 2.
Plasma SHIV-vpu+ RNA load determined by real-time RT-PCR (A), anti-Gag antibodies assessed by enzyme-linked immunodominant assay (B), peripheral absolute CD4+ T-cell counts (C), and virus isolation (D) for recipients RJj-4 and RMk-4. The sensitivity of the RT-PCR assay (A) is indicated by a dotted line (50 copies/ml). In panel C, 500 and 200 CD4+ T cells/μl, respectively, are indicated by dotted lines.
Unchanged CXCR4 usage but newly acquired ability to replicate in macrophages.
Acute pathogenicity was also observed in pig-tailed macaques (Macaca nemestrina) after rapid passage of SHIVs encoding HIV-IIIB env (from HXBc2) (16, 43). A molecular clone, SHIVKU-2MC4, isolated after further passage, caused acute CD4+ T-cell loss and disease (23). Unlike the parental SHIV-4, which is not macrophagetropic, these progeny viruses replicated efficiently in macrophages (23, 44), even though they continued to use CXCR4 as coreceptor (23). We characterized coreceptor usage of viruses isolated from 95-3 at week 285 (SHIV-vpu+5.285) and at necropsy (SHIV-vpu+5.307) in U87 cells stably expressing various coreceptors (NIH AIDS Research and Reference Reagent Program). The progeny viruses productively infected CXCR4- but not CCR5-expressing cells. However, virus isolated at necropsy also acquired the capacity to replicate in rhesus macaque macrophages (data not shown). CXCR4 usage and replication in macrophages were also found in virus variants isolated from a laboratory worker who developed AIDS 8 years after accidental infection with HIV-IIIB (4). Thus, all of these virus variants had acquired the ability to productively infect macrophages despite unchanged coreceptor usage.
Sequence analysis of progeny viruses isolated from animal 95-3.
Because earlier work had shown that replacing an env fragment from the acutely pathogenic SHIVKU-1 was sufficient to convert the nonpathogenic parental SHIV-HXBc2 into a virus that caused rapid, profound CD4+ T-cell loss (termed SHIV-HXBc2P3.2) (8), we decided to sequence a large gp120-encoding env segment. We used PCR to clone a 1,226-bp env fragment from PBMC DNA of monkey 95-3 collected at different time points. Two clones from week 2 (SHIV-vpu+5.2) and eight clones from week 285 were sequenced using the following primers: GTAAAACGACGGCCAG, GTTCAATGGAACAGGACCAT, TTGGAGTACTGAAGGGTCAA (sense) and CAGGAAACAGCTATGAC, GTGTCACTTCCTTCAGTGT, ACATTGTACTGTGCTGACAT (antisense).
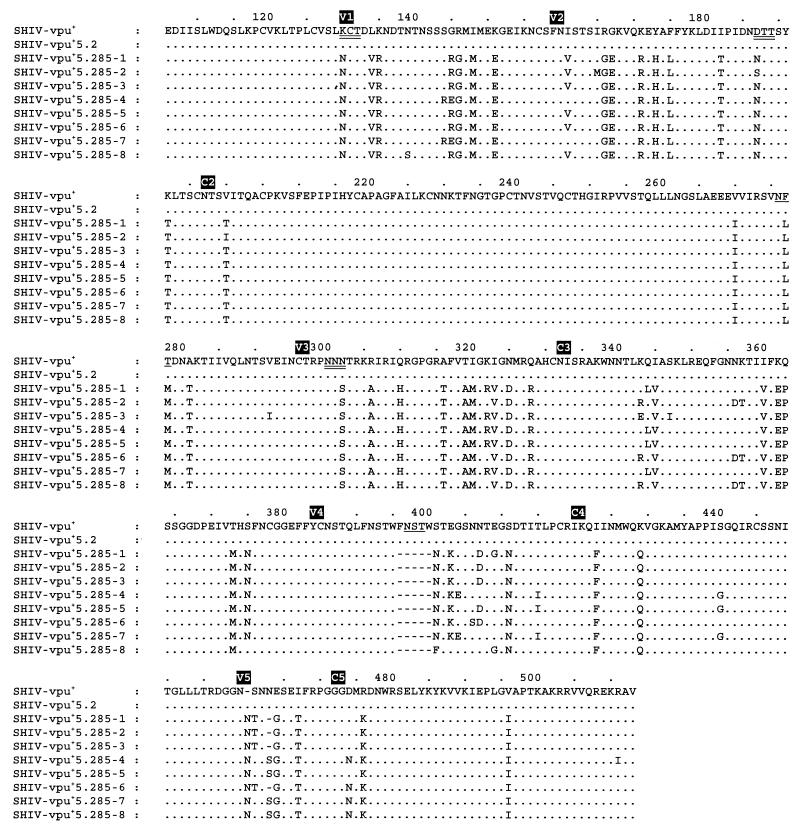
We found a higher number of amino acid substitutions and deletions in SHIV-vpu+5.285 env (Fig. 3) than previously reported for rapidly passaged SHIV encoding HXBc2 env or viruses isolated from the HIV-IIIB-infected laboratory worker (4, 8, 23, 28, 44, 45). From 10 amino acid substitutions in gp120 linked to the conversion of parental SHIV-HXBc2 into the acutely pathogenic SHIV-HXBc2P3.2 (8), 5 were identical in SHIV-vpu+5.285 (Table 2, highlighted in bold). Changes were mainly located in the variable regions of gp120 (Fig. 3). Many mutations were located in V3, a region that contains linear and discontinuous antigenic determinants (9, 18, 39) that can change during disease development and immune escape (37, 38, 41). Some amino acid substitutions led to three new and loss of two potential N-linked glycosylation sites, which in turn might result in changes in conformation and immune recognition of gp120 (3, 12, 14, 29, 30, 35, 47, 50). Two new glycosylation sites were located in V1 and V2 domains that contain epitopes for neutralizing antibodies (10, 27, 46) and thus can modulate neutralization sensitivity (7, 41, 48, 49). There were two FNTSW sequences in V4 of parental SHIV-vpu+; one was lost by a 5-amino-acid deletion.
FIG. 3.
Comparison of the gp120 sequence of the parental virus, SHIV-vpu+, and the predicted gp120 amino acid sequences of SHIV-vpu+5.2 isolated from monkey 95-3 at week 2 directly from PBMC and of SHIV-vpu+5.285-1 to SHIV-vpu+5.285-8 isolated from 95-3 285 weeks postexposure. Direct sequencing was not efficient from PBMC collected at week 285; thus, SHIV-vpu+5.285 was amplified beforehand for 6 days in CEMx174/GFP cells. Differences in amino acid residues are indicated, as are the locations of the different regions of gp120 (4). The predicted gp120 amino acid sequence of two clones isolated at week 2 (SHIV-vpu+5.2) was identical to that of the parental virus. The eight clones from week 285 had 39 consistent changes in gp120 when compared with the sequence of the parental virus: 6 in V1, 7 in V2, 9 in V3, 1 in V4, 3 in V5, and the other 13 in various constant regions. New potential N-linked glycosylation sites are double underlined; loss of potential N-linked glycosylation sites are single underlined. The gp120 sequence of SHIV-vpu+ is identical to that of SHIV-4; the GenBank accession number of the latter is AF038399 (20).
TABLE 2.
Similar molecular evolution of HIV-IIIB env sequences in M. mulatta, M. nemestrina, and H. sapiens
| SHIV-vpu+5.285 amino acid changesa (M. mulatta) | Region | Site | SHIVs containing identical changes
|
HIV-IIIB variant with identical and/or similar changes (H. sapiens) | |
|---|---|---|---|---|---|
| M. mulatta | M. nemestrina | ||||
| 130 K→N | V1 | New PGSb | SHIV-HXBc2P3.2c (8), | SHIVKU-1be (28), SHIVKU-PW1, SHIVKU-8124, | |
| SHIVKU-2MC4c (23), | SHIVKU-PEyf (42), SHIVKU-1 Pnbf (44), | ||||
| SHIVKU-2d (23, 31) | SHIVKU-1/105w52, SHIVKU-1/105w98g (45) | ||||
| 145 G→R | V1 | SHIVKU-1 Pnb1 | |||
| 146 R→G | V1 | SHIVKU-1b, SHIVKU-8124, SHIVKU-PW1, | FF3346h (4) | ||
| SHIVKU-1/105w98 | |||||
| 148 I→M | V1 | SHIVKU-1/105w52 | |||
| 151 K→E | V1 | SHIVKU-8124, SHIVKU-1/105w98 | |||
| 161 I→V | V2 | SHIVKU-1/105w52 | |||
| 166 R→G | V2 | FF3346 (R→K)h | |||
| 171 K→R | V2 | FF3346 (K→E) | |||
| 175 F→L | V2 | SHIVKU-1b, SHIVKU-PW1, SHIVKU-PEy | |||
| 187 D→N | V2 | New PGS | |||
| 192 K→T | V2 | SHIV-HXBc2P3.2, | SHIVKU-1b, SHIVKU-PW1, SHIVKU-8124, | FF3346 | |
| SHIVKU-2MC4 | SHIVKU-PEy, SHIVKU-1 Pnb, | ||||
| SHIVKU-1/105w52, SHIVKU-1/105w98 | |||||
| 270 V→I | C2 | SHIVKU-1 Pnb5 | |||
| 278 T→M | C2 | Loss of PGS | SHIV-HXBc2P3.2, | SHIVKU-1b, SHIVKU-8124, SHIVKU-1/105w52, | |
| SHIVKU-2MC4, SHIVKU-2 | SHIVKU-1/105w98, SHIVKU-1 Pnb3+10 | ||||
| 281 A→T | C2 | CD4 binding | SHIVKU-PW1 | FF3346 (A→V) | |
| 302 N→S | V3 | New PGS | |||
| 310 Q→H | V3 | SHIVKU-1b | FF3346 | ||
| 316 A→T | V3 | GPGRAF | FF3346 | ||
| 320 I→M | V3 | SHIV-HXBc2P3.2, | SHIVKU-1b, SHIVKU-PW1, SHIVKU-8124, | ||
| SHIVKU-2MC4, | SHIVKU-PEy, SHIVKU-1 Pnb, | ||||
| SHIVKU-2 | SHIVKU-1/105w52, SHIVKU-1/105w98 | ||||
| 325 N→D | V3 | SHIV-HXBc2P3.2, | SHIVKU-PW1, SHIVKU-8124, SHIVKU-PEy, | ||
| SHIVKU-2 | SHIVKU-1 Pnb, SHIVKU-1/105w52, | ||||
| SHIVKU-1/105w98 | |||||
| 328 Q→R | V3 | SHIVKU-1/105w52, SHIVKU-1/105w98 | |||
| 345 I→V | C3 | FF3346 | |||
| 362 K→E | C3 | FF3346 (K→R) | |||
| 396-400 ΔFNSTW | V4 | Loss of PGS | SHIVKU-PW1, SHIVKU-1 Pnb3+10 | ||
| 429 K→Q | C4 | CD4 binding | FF3346 (K→E) | ||
| 464 E→G | V5 | FF3346 | |||
| 467 I→T | V5 | SHIVKU-PEy | |||
| 474 D→N | V5 | SHIVKU-PEy, SHIVKU-PW1 | |||
| 476 R→K | V5 | SHIVKU-PEy, SHIVKU-PW1 | |||
| 496 V→I | C5 | SHIVKU-1b | |||
Comparison with SHIV-4, AF038399 (20). Changes are listed that concerned potential glycosylation sites or had been reported in other viruses also. Bold type indicates 5 of 10 amino acid substitutions in SHIV-HXBc2P3.2 gp120 that were reported to be sufficient to confer disease (8).
PGS, potential glycosylation site.
Neutralization escape variant isolated from a pig-tailed macaque (28).
Immune escape virus isolated from a macaque (45).
Virus isolated from a laboratory worker 7 years after accidental HIV-1 IIIB infection (4). For FF3346, changes are given that are located identically but with different replacing amino acids.
HIV-IIIB and SHIV encoding IIIB env undergo similar gp120 changes during chronic infection in humans or monkeys or during rapid in vivo passage.
Some amino acid changes leading to alterations in potential glycosylation sites were reported also in viruses isolated after rapid passage or chronic infection from monkeys or humans (Table 2). Six amino acid changes found in gp120 of SHIV-vpu+5.285 were identical to those found in gp120 of FF3346 (Table 2), a virus isolated from the HIV-IIIB-infected laboratory worker (4). These substitutions included a mutation in the highly antigenic sequence, GPGRAF, at the tip of the V3 loop (316A→T). An additional five changes were located identically in FF3346 and SHIV-vpu+5.285, but the replacing amino acids were different (details in Table 2).
Summary. We described a general tendency of primate lentiviruses to mutate into more virulent forms over time by using an evolutionary path that was similar within individual hosts of different species. The virus that evolved in monkey 95-3 over several years of infection also resembled, to a certain degree, the viruses selected by rapid serial in vivo passage in pig-tailed and rhesus macaques. These latter viruses also induced acute CD4+ T-cell depletion and AIDS in infected animals. In all three species (M. mulatta, M. nemestrina, and Homo sapiens), progeny viruses became more neutralization resistant (4, 8, 28, 41) and acquired the ability to replicate in macrophages while maintaining CXCR4-restricted coreceptor usage (4, 23, 44). Thus, infection with nonpassaged SHIV-vpu+ in an individual macaque (i) seems to approximate the evolution of HIV-IIIB seen during the course of disease progression in a human individual and (ii) is mirrored by the more rapid evolution that the virus undergoes during rapid serial passages in nonhuman primates.
Our results support the use of SHIV-vpu+ infection of rhesus monkeys as a relevant model that can yield important insights into viral evolution and pathogenesis. Furthermore, since rapid viral passage is less time-consuming than long-term follow-up over years, it seems justifiable to use the former for safety testing of candidate live attenuated virus vaccines to uncover potential virulence.
Nucleotide sequence accession numbers.
The nucleotide sequences of the viral variants isolated 285 weeks after virus inoculation from diseased monkey 95-3 (Fig. 3) are available from GenBank (no. AF384152 through AF384159).
Acknowledgments
We thank T. Graf (Informatics Core, Dana-Farber Cancer Institute) for support with sequence analyses and C. Gallegos and S. Sharp for help in preparing the manuscript.
This work was supported in part by NIH grants RO1 AI34266, RR14180, and DE12937 awarded to R.M.R., AI85343 awarded to D.C.M., and RR00165 awarded to the Yerkes Primate Research Center (H.M.M. and D.C.A.). This work was also aided by Center for AIDS Research (CFAR) core grant IP3028691 awarded to the Dana-Farber Cancer Institute in support of AIDS research efforts by the Institute and by Swiss National Science Foundation grant 823A-50315 to R.H.-L. T.W.B. was a recipient of an NIH Clinical Investigator Development Award (5 KO8-A101327).
REFERENCES
- 1.Baba, T. W., J. Koch, E. S. Mittler, M. Greene, M. Wyand, D. Pennick, and R. M. Ruprecht. 1994. Mucosal infection of neonatal rhesus monkeys with cell-free SIV. AIDS Res. Hum. Retrovir. 10:351-357. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 3.Back, N. K., L. Smit, J. J. De Jong, W. Keulen, M. Schutten, J. Goudsmit, and M. Tersmette. 1994. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 199:431-438. [DOI] [PubMed] [Google Scholar]
- 4.Beaumont, T., A. van Nuenen, S. Broersen, W. A. Blattner, V. V. Lukashov, and H. Schuitemaker. 2001. Reversal of human immunodeficiency virus type 1 IIIB to a neutralization-resistant phenotype in an accidentally infected laboratory worker with a progressive clinical course. J. Virol. 75:2246-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binley, J. M., P. J. Klasse, Y. Cao, I. Jones, M. Markowitz, D. D. Ho, and J. P. Moore. 1997. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J. Virol. 71:2799-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored gp120 followed by soluble gp160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 7.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayabyab, M., G. B. Karlsson, B. A. Etemad-Moghadam, W. Hofmann, T. Steenbeke, M. Halloran, J. W. Fanton, M. K. Axthelm, N. L. Letvin, and J. G. Sodroski. 1999. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2). J. Virol. 73:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etemad-Moghadam, B., Y. Sun, E. K. Nicholson, G. B. Karlsson, D. Schenten, and J. Sodroski. 1999. Determinants of neutralization resistance in the envelope glycoproteins of a simian-human immunodeficiency virus passaged in vivo. J. Virol. 73:8873-8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung, M. S., C. R. Sun, W. L. Gordon, R. S. Liou, T. W. Chang, W. N. Sun, E. S. Daar, and D. D. Ho. 1992. Identification and characterization of a neutralization site within the second variable region of human immunodeficiency virus type 1 gp120. J. Virol. 66:848-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayami, M., and T. Igarashi. 1997. SIV/HIV-1 chimeric viruses having HIV-1 env gene: a new animal model and a candidate for attenuated live vaccine. Leukemia 11(Suppl. 3):95-97. [PubMed] [Google Scholar]
- 12.Hemming, A., G. J. Gram, A. Bolmstedt, B. Losman, J. E. Hansen, A. Ricksten, and S. Olofsson. 1996. Conserved N-linked oligosaccharides of the C-terminal portion of human immunodeficiency virus type 1 gp120 and viral susceptibility to neutralizing antibodies. Arch. Virol. 141:2139-2151. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann-Lehmann, R., R. K. Swenerton, V. Liska, C. M. Leutenegger, H. Lutz, H. M. McClure, and R. M. Ruprecht. 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- vs. two-enzyme systems. AIDS Res. Hum. Retrovir. 16:1247-1257. [DOI] [PubMed] [Google Scholar]
- 14.Huang, X., J. J. Barchi, Jr., F. D. Lung, P. P. Roller, P. L. Nara, J. Muschik, and R. R. Garrity. 1997. Glycosylation affects both the three-dimensional structure and antibody binding properties of the HIV-1IIIB gp120 peptide RP135. Biochemistry 36:10846-10856. [DOI] [PubMed] [Google Scholar]
- 15.Joag, S. V., Z. Li, L. Foresman, D. M. Pinson, R. Raghavan, W. Zhuge, I. Adany, C. Wang, F. Jia, D. Sheffer, J. Ranchalis, A. Watson, and O. Narayan. 1997. Characterization of the pathogenic KU-SHIV model of acquired immunodeficiency syndrome in macaques. AIDS Res. Hum. Retrovir. 13:635-645. [DOI] [PubMed] [Google Scholar]
- 16.Joag, S. V., Z. Li, L. Foresman, E. B. Stephens, L. J. Zhao, I. Adany, D. M. Pinson, H. M. McClure, and O. Narayan. 1996. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J. Virol. 70:3189-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson, G. B., M. Halloran, J. Li, I. W. Park, R. Gomila, K. A. Reimann, M. K. Axthelm, S. A. Iliff, N. L. Letvin, and J. Sodroski. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 71:4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langedijk, J. P., G. Zwart, J. Goudsmit, and R. H. Meloen. 1995. Fine specificity of antibody recognition may predict amino acid substitution in the third variable region of gp120 during HIV type 1 infection. AIDS Res. Hum. Retrovir. 11:1153-1162. [DOI] [PubMed] [Google Scholar]
- 19.Li, A., H. Katinger, M. R. Posner, L. Cavacini, S. Zolla-Pazner, M. K. Gorny, J. Sodroski, T. C. Chou, T. W. Baba, and R. M. Ruprecht. 1998. Synergistic neutralization of simian-human immunodeficiency virus SHIV-vpu+ by triple and quadruple combinations of human monoclonal antibodies and high-titer anti-human immunodeficiency virus type 1 immunoglobulins. J. Virol. 72:3235-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, J., C. I. Lord, W. Haseltine, N. L. Letvin, and J. Sodroski. 1992. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J. Acquired Immune Defic. Syndr. 5:639-646. [PubMed] [Google Scholar]
- 21.Li, J. T., M. Halloran, C. I. Lord, A. Watson, J. Ranchalis, M. Fung, N. L. Letvin, and J. G. Sodroski. 1995. Persistent infection of macaques with simian-human immunodeficiency viruses. J. Virol. 69:7061-7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liska, V., A. H. Khimani, R. Hofmann-Lehmann, A. N. Fink, J. Vlasak, and R. M. Ruprecht. 1999. Viremia and AIDS in rhesus macaques after intramuscular inoculation of plasmid DNA encoding full-length SIVmac239. AIDS Res. Hum. Retrovir. 15:445-450. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Z. Q., S. Muhkerjee, M. Sahni, C. McCormick-Davis, K. Leung, Z. Li, V. H. Gattone, 2nd, C. Tian, R. W. Doms, T. L. Hoffman, R. Raghavan, O. Narayan, and E. B. Stephens. 1999. Derivation and biological characterization of a molecular clone of SHIVKU-2 that causes AIDS, neurological disease, and renal disease in rhesus macaques. Virology 260:295-307. [DOI] [PubMed] [Google Scholar]
- 24.Lu, Y., C. D. Pauza, X. Lu, D. C. Montefiori, and C. J. Miller. 1998. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J. Acquired Immune Defic. Syndr. 19:6-18. [DOI] [PubMed] [Google Scholar]
- 25.Lu, Y., M. S. Salvato, C. D. Pauza, J. Li, J. Sodroski, K. Manson, M. Wyand, N. Letvin, S. Jenkins, N. Touzjian, C. Chutkowski, N. Kushner, M. LeFaile, L. G. Payne, and B. Roberts. 1996. Utility of SHIV for testing HIV-1 vaccine candidates in macaques. J. Acquired Immune Defic. Syndr. 12:99-106. [DOI] [PubMed] [Google Scholar]
- 26.Luciw, P. A., E. Pratt-Lowe, K. E. Shaw, J. A. Levy, and C. Cheng-Mayer. 1995. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc. Natl. Acad. Sci. USA 92:7490-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKeating, J. A., C. Shotton, J. Cordell, S. Graham, P. Balfe, N. Sullivan, M. Charles, M. Page, A. Bolmstedt, S. Olofsson, S. Kayman, Z Wu, A. Pinter, C. Dean, J. Sodroski, and R. Weiss. 1993. Characterization of neutralizing monoclonal antibodies to linear and conformation-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J. Virol. 67:4932-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayan, S. V., S. Mukherjee, F. Jia, Z. Li, C. Wang, L. Foresman, C. McCormick-Davis, E. B. Stephens, S. V. Joag, and O. Narayan. 1999. Characterization of a neutralization-escape variant of SHIVKU-1, a virus that causes acquired immune deficiency syndrome in pig-tailed macaques. Virology 256:54-63. [DOI] [PubMed] [Google Scholar]
- 29.Overbaugh, J., and L. M. Rudensey. 1992. Alterations in potential sites for glycosylation predominate during evolution of the simian immunodeficiency virus envelope gene in macaques. J. Virol. 66:5937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papandreou, M. J., and E. Fenouillet. 1998. Effect of changes in the glycosylation of the human immunodeficiency virus type 1 envelope on the immunoreactivity and sensitivity to thrombin of its third variable domain. Virology 241:163-167. [DOI] [PubMed] [Google Scholar]
- 31.Raghavan, R., E. B. Stephens, S. V. Joag, I. Adany, D. M. Pinson, Z. Li, F. Jia, M. Sahni, C. Wang, K. Leung, L. Foresman, and O. Narayan. 1997. Neuropathogenesis of chimeric simian/human immunodeficiency virus infection in pig-tailed and rhesus macaques. Brain Pathol. 7:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reimann, K. A., J. T. Li, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Paritz, Y. Lu, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 70:3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reimann, K. A., A. Watson, P. J. Dailey, W. Lin, C. I. Lord, T. D. Steenbeke, R. A. Parker, M. K. Axthelm, and G. B. Karlsson. 1999. Viral burden and disease progression in rhesus monkeys infected with chimeric simian-human immunodeficiency viruses. Virology 256:15-21. [DOI] [PubMed] [Google Scholar]
- 35.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 36.Ruprecht, R. M., T. W. Baba, V. Liska, N. B. Ray, L. N. Martin, M. Murphey-Corb, T. A. Rizvi, B. J. Bernacky, M. E. Keeling, H. M. McClure, and J. Andersen. 1999. Oral transmission of primate lentiviruses. J. Infect. Dis. 179(Suppl. 3):S408-S412. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber, M., H. Muller, C. Wachsmuth, T. Laue, F. T. Hufert, M. D. Van Laer, and H. Schmitz. 1997. Escape of HIV-1 is associated with lack of V3 domain-specific antibodies in vivo. Clin. Exp. Immunol. 107:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber, M., C. Wachsmuth, H. Muller, C. Hagen, H. Schmitz, and J. van Lunzen. 1996. Loss of antibody reactivity directed against the V3 domain of certain human immunodeficiency virus type 1 variants during disease progression. J. Gen. Virol. 77:2403-2414. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber, M., C. Wachsmuth, H. Muller, S. Odemuyiwa, H. Schmitz, S. Meyer, B. Meyer, and J. Schneider-Mergener. 1997. The V3-directed immune response in natural human immunodeficiency virus type 1 infection is predominantly directed against a variable, discontinuous epitope presented by the gp120 V3 domain. J. Virol. 71:9198-9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362-373. [DOI] [PubMed] [Google Scholar]
- 41.Si, Z., M. Cayabyab, and J. Sodroski. 2001. Envelope glycoprotein determinants of neutralization resistance in a simian-human immunodeficiency virus (SHIV-HXBc2P 3.2) derived by passage in monkeys. J. Virol. 75:4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverstein, P. S., G. A. Mackay, S. Mukherjee, Z. Li, M. Piatak, Jr., J. D. Lifson, O. Narayan, and A. Kumar. 2000. Pathogenic simian/human immunodeficiency virus SHIVKU inoculated into immunized macaques caused infection, but virus burdens progressively declined with time. J. Virol. 74:10489-10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephens, E. B., S. V. Joag, D. Sheffer, Z. Q. Liu, L. Zhao, S. Mukherjee, L. Foresman, I. Adany, Z. Li, D. Pinson, and O. Narayan. 1996. Initial characterization of viral sequences from a SHIV-inoculated pig-tailed macaque that developed AIDS. J. Med. Primatol. 25:175-185. [DOI] [PubMed] [Google Scholar]
- 44.Stephens, E. B., S. Mukherjee, M. Sahni, W. Zhuge, R. Raghavan, D. K. Singh, K. Leung, B. Atkinson, Z. Li, S. V. Joag, Z. Q. Liu, and O. Narayan. 1997. A cell-free stock of simian-human immunodeficiency virus that causes AIDS in pig-tailed macaques has a limited number of amino acid substitutions in both SIVmac and HIV-1 regions of the genome and has altered cytotropism. Virology 231:313-321. [DOI] [PubMed] [Google Scholar]
- 45.Stipp, H. L., A. Kumar, and O. Narayan. 2000. Characterization of immune escape viruses from a macaque immunized with live-virus vaccine and challenged with pathogenic SHIVKU-1. AIDS Res. Hum. Retrovir. 16:1573-1580. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan, N., M. Thali, C. Furman, D. D. Ho, and J. Sodroski. 1993. Effect of amino acid changes in the V1/V2 region of the human immunodeficiency virus type 1 gp120 glycoprotein on subunit association, syncytium formation, and recognition by a neutralizing antibody. J. Virol. 67:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, Z., S. C. Kayman, W. Honnen, K. Revesz, H. Chen, S. Vijh-Warrier, S. A. Tilley, J. McKeating, C. Shotton, and A. Pinter. 1995. Characterization of neutralization epitopes in the V2 region of human immunodeficiency virus type 1 gp120: role of glycosylation in the correct folding of the V1/V2 domain. J. Virol. 69:2271-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyatt, R., J. Moore, M. Accola, E. Desjardin, J. Robinson, and J. Sodroski. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69:5723-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 67:4557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye, Y., Z. H. Si, J. P. Moore, and J. Sodroski. 2000. Association of structural changes in the V2 and V3 loops of the gp120 envelope glycoprotein with acquisition of neutralization resistance in a simian-human immunodeficiency virus passaged in vivo. J. Virol. 74:11955-11962. [DOI] [PMC free article] [PubMed] [Google Scholar]