Abstract
A/Goose/Guangdong/1/96-like H5N1 influenza viruses now circulating in southeastern China differ genetically from the H5N1 viruses transmitted to humans in 1997 but were their precursors. Here we show that the currently circulating H9N2 influenza viruses provide chickens with cross-reactive protective immunity against the currently circulating H5N1 influenza viruses and that this protective immunity is closely related to the percentage of pulmonary CD8+ T cells expressing gamma interferon (IFN-γ). In vivo depletion of T-cell subsets showed that the cross-reactive immunity was mediated by T cells bearing CD8+ and T-cell receptor (TCR) α/β and that the Vβ1 subset of TCR α/β T cells had a dominant role in protective immunity. The protective immunity induced by infection with H9N2 virus declined with time, lasting as long as 100 days after immunization. Shedding of A/Goose/Guangdong/1/96-like H5N1 virus by immunized chickens also increased with the passage of time and thus may play a role in the perpetuation and spread of these highly pathogenic H5N1 influenza viruses. Our findings indicate that pulmonary cellular immunity may be very important in protecting naïve natural hosts against lethal influenza viruses.
A/Goose/Guangdong/1/96-like H5N1 viruses, which were the precursors of the H5N1 viruses transmitted to humans in Hong Kong in 1997, continue to circulate in geese in southeastern China (5, 28; Y. Guan, M. Peiris, K. F. Kong, K. C. Dyrting, T. M. Ellis, T. Sit, L. J. Zhang, and K. F. Shortridge, unpublished data). These viruses have a hemagglutinin (HA) gene very similar to that of A/Hong Kong (HK)/156/97 (H5N1), but the rest of their genes are of different lineages (29). A/Duck/HK/Y280/97-like H9N2 virus is presently circulating in poultry in southeastern China, and A/Quail/HK/G1/97-like H9N2 virus is endemic in quail of southeastern China (18). H5N1 viruses isolated from poultry in Hong Kong bird markets during outbreaks in 2001 were reassortants of A/Goose/Guangdong/1/96-like H5N1 viruses and avian viruses that are endemic to southeastern China (personal communication from Y. Guan).
In mouse models of influenza virus, CD8+ T cells play an important role in clearing virus from the respiratory tract (1, 2, 3, 13-17, 24). The activity of memory CD8+ T cells in mice enhances virus clearance by only 2 or 3 days (16, 17, 24). When mice were lethally challenged with mouse-adapted A/Equine/London/72 (H7N7) influenza virus, memory CD8+ T cells established by previous infection with A/PR/8/34 (H1N1) virus provided considerable protection, although pulmonary virus titers remained similar to those in naïve control mice for 5 days or more (10). In a study of heterologous protection against lethal A/HK/156/97 (H5N1), C57BL/6 mice immunized with A/Quail/HK/G1/97 (H9N2) virus, which has internal genes very similar to those of A/HK/156/97 (H5N1), were protected from death (23).
The mouse is not a natural host of influenza virus. Generally, mice are susceptible only to influenza viruses that have been adapted to mice. The direct transmission of avian H5N1 viruses from chickens to humans in Hong Kong in 1997 suggests that chickens can be an intermediate host for human infections. Chickens are ideal subjects for the study of cross-reactive protective immunity to highly lethal H5N1 influenza virus, because chickens have an immune system comparable to those of mammals. For example, chicken CD4 is a type I transmembrane glycoprotein and is expressed on 70% of thymocytes, 15% of spleen cells, and 40% of peripheral blood lymphocytes (6). Chicken CD8 exists as a homodimer of two α chains or a heterodimer of an α chain and a β chain and is expressed on 80% of thymocytes, 45% of peripheral blood lymphocytes, and 50% of spleen cells (6, 27).
Our previous study showed that cellular immunity to H9N2 influenza virus could protect chickens from highly pathogenic H5N1 influenza virus (26). However, the currently circulating A/Goose/Guangdong/1/96-like H5N1 influenza viruses contain six internal genes that differ from those of the A/HK/156/97 (H5N1)-like influenza viruses. In this study, we asked three questions: (i) Can currently circulating H9N2 influenza virus protect chickens from A/Goose/Guangdong/1/96-like H5N1 virus recently isolated from geese? (ii) What subsets of T cells (CD4, CD8, T-cell receptor [TCR] α/β, TCR γ/δ, Vβ1 TCR, and Vβ2 TCR) are important in protecting chickens from lethal infection with A/Goose/Guangdong/1/96-like H5N1 influenza virus? (iii) How long does the protective immunity last and by what mechanism does it decline?
MATERIALS AND METHODS
Viruses.
H5N1 (A/Goose/HK/437-4/99 [H5N1]) and H9N2 (A/Chicken/HK/SF3/99 and A/Quail/HK/Ssp10/99) influenza viruses obtained from the St. Jude repository were propagated in the allantoic cavities of 11-day-old embryonated chicken eggs in a U.S. Department of Agriculture-approved biosafety level-3 (BL-3) containment facility. Virus titers in the eggs were quantified as the log 50% egg infective dose (EID50).
Animals.
Outbred 8-week-old SPF White Leghorn chickens were purchased from SPAFAS, Inc. (Norwich, Conn.). The animal experiments were performed in a BL-3 containment facility approved by the U.S. Department of Agriculture and the Centers for Disease Control and Prevention.
Challenge study.
We intranasally inoculated 8-week-old chickens with 104 EID50 (0.3-ml volume) of the A/Duck/HK/Y280/97-like A/Chicken/HK/SF3/99 (H9N2) influenza virus that is prevalent in southeastern China 20 to 100 days before intranasal challenge with 104 EID50 of A/Goose/HK/437-4/99 (H5N1) influenza virus. Chickens were monitored daily for mortality and for signs of illness.
Serologic tests.
Hemagglutination inhibition (HI) and virus neutralization assays were performed as previously described (26). Briefly, sera were collected on various days after infection from 8-week-old chickens immunized with A/Chicken/HK/SF3/99 (H9N2) influenza virus. HI and neutralization antibody assays were performed with H9N2 (A/Chicken/HK/SF3/99, A/Quail/HK/Ssp10/99) and H5N1 (A/Goose/HK/437-4/99) viruses.
Calculation of viral titers.
Viral titers in eggs were determined by 50% endpoint using the method of Reed and Muench. The formula is the following: log dilution above 50% + (proportionate distance × log dilution factor) = log EID50. Proportionate distance = % positive above 50% − 50%/% positive above 50% − % positive below 50%.
In vivo depletion of T-cell subtypes.
Groups of six chickens were immunized with 104 EID50 of A/Chicken/HK/SF3/99 (H9N2) virus 20 days before in vivo depletion. Mouse anti-chicken monoclonal antibodies (CD4, CD8, CD3, TCR γ/δ, and TCR α/β) (Vβ1 and Vβ2), (0.1 mg; Southern Biotechnology Associates, Inc., Birmingham, Ala.) and rabbit complement (1 ml; Cedarlane Laboratories, Ltd., Hornby, Ontario, Canada) were injected into the wing veins of the immunized chicken starting 4 days before and ending 4 days after challenge.
Isolation of CD8+ T cells.
Chickens were immunized with 104 EID50 of A/Chicken/HK/SF3/99 (H9N2) 6 to 100 days before they were euthanatized to collect lymphocytes from the lungs and spleens. Chickens were perfused before lungs and spleens were collected. Tissues were minced and incubated in minimal essential medium supplemented with collagenase D (400 U/ml; Sigma) and brefeldin A (10 mg/ml; Sigma) for 30 min at 37°C to isolate lymphocytes. The lymphocytes were then purified by centrifugation on a Ficoll-Hypaque density gradient (Histopaque 1.0777; Sigma). B lymphocytes were removed by passing the cells through nylon wool columns (Polysciences, Inc., Warrington, Pa.), and macrophages were removed by incubation in tissue culture flasks for 3 h in RPMI 1640 with 10% chicken serum (Sigma). Dynabeads (Dynal; Oslo, Norway) were used as previously described (26) to deplete the purified T cells of CD4+ T cells.
Intracellular staining.
The purified CD8+ T cells (n = 107) from each chicken were fixed with 2% formaldehyde, permeabilized with 0.3% saponin, and incubated for 1 h on ice with mouse anti-chicken gamma interferon (IFN-γ) monoclonal antibody (kindly provided by B. Lambrecht, Veterinary and Agrochemical Research Center, Brussels, Belgium) diluted in phosphate-buffered saline with 5% normal horse serum. The stained cells were washed three times and incubated for 1 h on ice with diluted horseradish peroxidase-labeled goat anti-mouse immunoglobulin G (1:500) (KPL, Gaithersburg, Md.). Cells were washed three times with phosphate-buffered saline with 5% normal horse serum and 0.1% Tween 20 and were incubated with StableDAB (3,3′-diaminobenzidine) (KPL) color agent. We counted 10,000 cells by light microscopy and used CD8+ T cells from the nonimmunized chickens to control for background staining.
RESULTS
Cross-reactive protection.
Only the HA genes of the currently circulating A/Goose/Guangdong/1/96-like H5N1 viruses are similar to those of the H5N1/97 viruses (29). To determine whether immunity to the currently circulating H9N2 influenza viruses cross-protects chickens from the highly pathogenic H5N1 influenza viruses, we immunized chickens with A/Duck/Y280-like A/Chicken/HK/SF3/99 (H9N2) virus 20 to 100 days before challenge with A/Goose/Guangdong/1/96-like A/Goose/HK/437-4/99 (H5N1). All nonimmunized chickens began to show clinical signs of illness (reddened combs, dyspnea, dropping heads, and difficulty standing) 2 or 3 days after challenge. Four of ten control chickens died (Table 1); the rest recovered within 5 days after infection but lost up to 20% of their body weight (data not shown). Chickens immunized with A/Chicken/HK/SF3/99 (H9N2) virus 20 and 30 days before challenge showed no clinical signs of illness, but signs of illness were seen in chickens immunized 60 days before challenge (Table 1). Two of 10 chickens immunized 60 days in advance showed signs of illness that began 3 days after challenge and recovered by day 5 after challenge. Seven of 10 chickens immunized 100 days before challenge showed clinical signs of illness, and two of these died. These findings showed that the protective immunity declined over time. Chicken immunized 20 days earlier with P/Chicken/HK/QB4/99(Newcastle disease virus) and challenged with A/Goose/Guangdong/1/96-like A/Goose/HK/437-4/99 (H5N1) showed 75% mortality (data not shown).
TABLE 1.
Protection of chickens immunized with circulating H9N2 virus against challenge with H5N1 virusa
| No. of days after infection with A/Chicken/HK/SF3/99 (H9N2) | Protection of chickens from H5N1 challenge (no. ill/no. dead/total)d | No. of chickens shedding H5N1/no. of survivors
|
H5N1 viral titers from surviving chickens (log10 EID50/ml)
|
||
|---|---|---|---|---|---|
| from trachea | from cloaca | from trachea | from cloaca | ||
| 0b | 10/4/10 | 6/6 | 6/6 | 2.5 | 3.75 |
| 20 | 0/0/10 | 7/10 | 0/10 | <1.0e | NDc |
| 30 | 0/0/10 | 9/10 | 3/10 | <1.0 | 1.5 |
| 60 | 2/0/10 | 9/10 | 4/10 | 1.0 | 2.5 |
| 100 | 7/2/10 | 7/8 | 5/8 | 1.75 | 3.5 |
Chickens were infected with 104 50% chicken infectious doses of A/Chicken/HK/SF3/99 (H9N2) influenza virus 20 to 100 days before challenge with 104 EID50 of A/Goose/HK/437-4/99 (H5N1) influenza virus. Chickens were monitored daily for 15 days for death or clinical signs of illness.
Control chickens not immunized with A/Chicken/HK/SF3/99 (H9N2).
ND, no virus was detected.
Signs of illness: dyspnea, reddened comb, dropping head, and difficulty standing.
<1.0, viruses were detectable in undiluted swab samples.
Virus shedding.
We swabbed the tracheae and cloacae of the chickens daily after challenge to determine their viral load. All nonimmunized chickens shed H5N1 virus in the tracheae (2.5 EID50) and cloacae (3.75 EID50) (Table 1). H5N1 influenza viruses were detectable in most immunized chickens 2 days after challenge, but titers were less than 1 log. The number of surviving chickens shedding H5N1 viruses in their cloacae increased with time. Cloacal virus titers ranged from 1.5 to 3.5 logs. The sensitivity of virus isolation from trachea and cloaca was more than 1 log.
Humoral immunity.
To ascertain whether antibody was involved in the protective effect against H5N1 influenza virus, we immunized chickens with A/Chicken/HK/SF3/99 (H9N2) influenza virus before testing their sera for cross-reactivity with H9N2 and H5N1 viruses by HI and viral neutralization assays (Table 2). The range of HI titers against a homologous H9N2 virus (A/Chicken/HK/SF3/99) was 360 to 1,344, whereas that against a heterologous H9N2 virus (A/Quail/HK/Ssp10/99) was 120 to 240. The ranges of neutralization antibody titers were log10 1.5 to 3.0 against a homologous virus and log10 1.0 to 1.5 against a heterologous virus. In contrast, no HI or neutralization activity against A/Goose/HK/437-4/99 (H5N1) was observed.
TABLE 2.
Titers of HI and virus neutralization after infection with H9N2 influenza virusa
| No. of days after infection | HI antibody titersb
|
Neutralization antibody titersc
|
||||
|---|---|---|---|---|---|---|
| H9N2
|
H5N1 437-4 | H9N2
|
H5N1 437-4 | |||
| SF3 | Ssp10 | SF3 | Ssp10 | |||
| 20 | 360 | 120 | NRd | 1.5 | 1.0 | NR |
| 30 | 1,344 | 256 | NR | 3.5 | 1.5 | NR |
| 60 | 1,200 | 260 | NR | 3.0 | 1.0 | NR |
| 100 | 1,250 | 240 | NR | 3.0 | 1.0 | NR |
Ten chickens per group were infected with 104 50% chicken infectious doses of A/Chicken/HK/SF3/99 (H9N2), and sera were collected from wing veins 20 to 100 days later. Abbreviations: SF3, A/Duck/Y280-like A/Chicken/HK/SF3/99 (H9N2); Ssp10, A/Quail/GI-like A/Quail/HK/Ssp10/99 (H9N2); and 437-4, A/Goose/HK/437-4/99 (H5N1).
Mean titers expressed as the reciprocal of the highest dilution of serum that inhibited the hemagglutination of 4 HA units of virus.
Mean titers expressed as the reciprocal of the highest dilution of serum (log10) that neutralized 100 50% tissue culture infective doses in 50% of virus-infected St. Jude porcine lung epithelial cells.
NR, no reaction detected.
Cellular immunity.
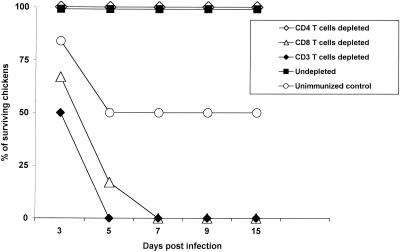
The absence of cross-reactivity between antibodies to H9N2 and H5N1 influenza viruses indicated that cellular immunity played a role in protecting chickens from the lethal H5N1 influenza virus. To understand which subtypes of T cells were involved, we depleted immunized chickens in vivo of specific T-cell subsets by inoculating them with mouse anti-chicken monoclonal antibody and rabbit complement. The continuous treatment was essential for depletion since T cells could quickly be reconstituted when treatment stopped. We first wanted to know whether memory CD4+ or CD8+ T cells were crucial in the protective effect. Chickens were immunized with A/Chicken/HK/SF3/99 (H9N2) 20 days before depletion of CD4+, CD8+, or CD3+ T cells (Fig. 1). All chickens depleted of CD8+ or CD3+ T cells died, but chickens depleted of CD4+ T cells and control immunized chickens that did not undergo depletion showed no signs of illness. Most of the nonimmunized chickens showed signs of severe illness, and 50% died.
FIG. 1.
The role of memory CD8+ T cells in protecting chickens from highly pathogenic H5N1 influenza virus. Groups of six outbred chickens were immunized with 104 EID50 of A/Chicken/HK/SF3/99 (H9N2) influenza virus 20 days before in vivo depletion of T-cell subsets by injection of mouse anti-chicken CD4, CD8, or CD3 with rabbit complement. Chickens were challenged with 104 EID50 of A/Goose/HK/437-4/99 (H5N1) virus and were monitored for survival daily for 15 days. Six nonimmunized chickens were used as a control.
T cells bear one of two types of TCRs: TCR α/β or γ/δ (7, 8, 11). Therefore, we investigated the roles of these two T-cell subtypes (Fig. 2). In vivo depletion of TCRγ/δ T cells did not cause mortality or morbidity, but depletion of TCRα/β (Vβ1) or CD3+ T cells resulted in 100% mortality of immunized chickens. Nonimmunized control chickens had 75% mortality.
FIG. 2.
Protective role of T cells bearing TCR α/β (Vβ1), TCR α/β (Vβ2), or TCR γ/δ. Groups of six outbred chickens were immunized with A/Chicken/HK/SF3/99 (H9N2) influenza virus and after 20 days were depleted in vivo of T-cell subsets by injection with mouse anti-chicken monoclonal antibodies to TCR α/β (Vβ1), TCR α/β (Vβ2), or TCR γ/δ with rabbit complement. Chickens were then challenged with 104 EID50 of A/Goose/HK/437-4/99 (H5N1) influenza virus. Six nonimmunized chickens or six chickens depleted of CD3+ T cells were used as a control.
In chickens, there are two kinds of TCR α/β, Vβ1 and Vβ2, which represent two distinct Vβ families (7, 11). We tested which TCR α/β T cells were involved in protecting chickens (Fig. 2). Chickens depleted of TCR α/β (Vβ2) experienced no mortality, but chickens depleted of TCR α/β (Vβ1) had 100% mortality. Nonimmunized control chickens had 50% mortality.
Mechanism of declining protective immunity.
The cross-reactive protective immunity declined over time (Table 2). To understand the mechanism of this decline, we measured the number of CD8+ T cells expressing IFN-γ in the lungs and spleens of chickens at different times after immunization with A/Chicken/HK/SF3/99 (H9N2) virus. We decided to use the modified enzyme-linked immunospot assay since we could not take lymphocytes out of the highly restricted BL-3 facility. Memory CD8+ T cells are known to express IFN-γ and tumor necrosis factor alpha (19, 20). Although a high proportion of primary CD8+ T cells in both lungs and spleens expressed IFN-γ 6 days after immunization, most did not (Fig. 3). The proportion of memory CD8+ T cells declined in spleens after day 20 but then leveled off and remained similar until day 100. The expression level of IFN-γ was determined. The proportion of memory CD8+ T cells in spleens was 13.3% at 20 days postinfection (p.i.), 5% at 30 days p.i., 4.5% at 60 days p.i., and 3.7% at 100 days p.i. The memory CD8+ T cells were antigen specific (26). In the lungs, the proportion of CD8+ T cells expressing IFN-γ began to decline 30 days after immunization and continued to decline until day 100. The purity of depletion of CD4+ or CD8+ T cells from lungs and spleens was more than 90% (26).
FIG. 3.
Immunocytochemical detection of IFN-γ in CD8+ T cells. Outbred chickens that had been immunized with 104 EID50 of A/Chicken/HK/SF3/99 (H9N2) influenza virus 6 to 100 days previously were euthanatized (four chickens per time point), and CD8+ T cells were isolated from their lungs and spleens. The purified CD8+ T cells were stained with mouse anti-chicken IFN-γ monoclonal antibody. Data points represent the mean values derived from four chickens. Error bars represent the standard deviations. INF-gamma, IFN-γ.
DISCUSSION
We have shown that inoculation with circulating H9N2 influenza virus provides cross-protective immunity to circulating A/Goose/Guangdong/1/96-like H5N1 influenza virus. Although the immunized chickens were protected, most of the chickens shed virus tracheally and cloacally. Such shedding may perpetuate and spread the lethal H5N1 viruses in poultry in the Hong Kong bird markets. We also found that CD8+ T cells or TCR α/β (Vβ1) T cells, rather than TCR γ/δ T cells, protected these natural hosts of influenza virus from lethal H5N1 influenza in the absence of neutralizing antibody. The protective immunity lasted as long as 100 days.
Our in vivo depletion study showed that memory CD8+ T cells were crucial in controlling the highly pathogenic H5N1 influenza virus in outbred chickens. CD4+ T cells were apparently not involved in protecting chickens from H5N1 influenza virus. In the mouse model, both CD4+ and CD8+ T cells are reportedly required for optimal control of influenza virus replication (15). Mice devoid of antibodies and mature B cells were able to clear influenza B virus, and mice were protected against challenge with a homologous virus 1 month later. The protective immunity was partially abrogated by in vivo depletion of memory CD4+ or CD8+ T cells and was completely abrogated by depletion of both subtypes. The difference in the CD4+ T cell's roles in protecting mice and chickens from the lethal infection may be due to three possible factors: the difference in virulence between H5N1 and mouse-adapted influenza B virus, the functional difference between mouse and chicken memory CD4+ T cells, and the different anatomical origins of mouse and chicken memory CD4+ T cells. However, we speculate that the difference in lethality between H5N1 and mouse-adapted influenza B viruses is best accounted for by the different roles of CD4+ T cells. To date, the role of CD4+ T cells in protecting mice against highly pathogenic H5N1 influenza viruses is not known.
The protective immunity of chickens to H5N1 influenza viruses was abrogated by in vivo depletion of TCR α/β T cells but not of TCR γ/δ T cells. TCR γ/δ T cells comprise a larger proportion of T lymphocytes in chickens (20 to 25% of circulating T cells) than in humans and mice (8, 9, 11). Most TCR γ/δ T cells express CD8 only after migration to the spleen and intestine; they are CD4− CD8− in the thymus and circulating blood. A study of the pulmonary inflammatory response of mice infected with influenza A virus showed a high frequency of cells with TCR γ/δ mRNA 7 days after inoculation; the predominant phenotype of these cells was CD3+ CD4− CD8− TCR α/β− (4). However, this study did not provide evidence that TCR γ/δ T cells were involved in clearing the lungs of influenza virus. In another study, the adoptive transfer of TCR α/β T cells protected chickens from acute infectious bronchitis virus infection, whereas transfer of TCR γ/δ T cells did not (25). The role of TCR γ/δ T cells in immunity against influenza virus in chickens remains unclear.
Our results showed that TCR α/β (Vβ1) T cells and not TCR α/β (Vβ2) T cells protected chickens from the lethal H5N1 influenza virus. It appears that chicken T cells predominantly use TCR α/β (Vβ1) to recognize the major histocompatibility complex class I glycoproteins associated with T-cell epitopes. Previous studies showed that mouse CD8+ T cells also use a dominant Vβ TCR subset for recognizing major histocompatibility complex class I glycoproteins on target cells expressing influenza virus epitopes. An analysis of virus-specific CD8+ T cells in C57BL/6 mice infected acutely with H3N2 (A/HKx31) virus or in immune C57BL/6 mice secondarily challenged with A/PR/8/34 (H1N1) virus found that 20 to 50% of CD8+ T cells in the mediastinal lymph nodes and in bronchoalveolar lavage specimens were Vβ 8.3 TCR+ (12). In BALB/c mice infected with A/PR/8/34 (H1N1) virus, most lytic activity against cells expressing viral epitopes was shown to be mediated by Vβ8.1/2+ TCR+ CD8+ T cells (9).
Our results showed that protective immunity against the highly pathogenic H5N1 virus was closely related to the percentage of CD8+ T cells expressing IFN-γ in the lung rather than in the spleen. These findings indicate that memory CD8+ T cells in the lung, which is the major target organ of influenza virus, play a crucial role in protecting chickens from the lethality of H5N1 influenza virus. Similarly, in a mouse model of viral infection, Marshall et al. (21) showed that a high percentage of memory CD8+ T cells reside in nonlymphoid organs (lungs, liver, and kidneys). In C57BL/6J mice primed with A/PR/8/34 (H1N1), a higher percentage of CD8+ Db NP366+ T cells was detected in the lungs and bronchoalveolar lavage specimens than in the mediastinal lymph nodes. Infection of C57BL/6J mice with vesicular stomatitis virus led to the appearance of virus-specific CD8+ T cells in lymphoid and nonlymphoid organs (22). However, a higher percentage of CD8+ T cells specific for vesicular stomatitis virus nucleoprotein epitopes appeared in nonlymphoid organs (kidney, lung, and liver) than in lymphoid organs (spleen and lymph nodes).
Cross-reactive cellular immunity seems to have two important potential consequences: the protection of hosts from disease and the perpetuation of viruses in these hosts. Some protected chickens shed H5N1 viruses in their tracheae and cloacae. H9N2 viruses similar to A/Chicken/HK/SF3/99 are endemic in chickens in southeastern China (18), and while this prior immunity may mask the pathogenicity of subsequent H5N1 infections, it does not completely suppress infection. Replication of H5N1 virus in H9N2-immunized chickens may provide an opportunity for reassortment between A/Goose/Guangdong/1/96-like viruses and other endemic avian viruses in the Hong Kong bird markets, thus increasing the odds that more virulent H5N1 viruses will emerge. The outbreaks of H5N1 viruses in Hong Kong poultry markets in 2001 support this premise. The isolates from these outbreaks are reported to be multiple reassortant viruses between A/Goose/Guangdong/1/96-like H5N1 viruses and other avian viruses endemic to southeastern Asia (personal communication with Y. Guan). We are now conducting a study of cross-reactive protection against H5N1 influenza viruses isolated from poultry during outbreaks in Hong Kong in 2001. In conclusion, our findings demonstrate that memory CD8+ T cells and TCR α/β T cells primed by exposure to H9N2 influenza virus are key elements in cross-reactive immune control of the highly pathogenic H5N1 influenza virus in chickens and that protective immunity is correlated with the percentage of memory CD8+ T cells expressing IFN-γ in the lungs.
Acknowledgments
This work was supported by Public Health Service grants AI29860, AI795357, and CA-21765; by the American Lebanese Syrian Associated Charities (ALSAC); and by WellcomeTrust grant 057476/2/99/Z.
We thank Scott Krauss, David Walker, Jennifer Humberd, and Patrick Seiler for excellent technical support; Alice Herren and Laurie Twit for manuscript preparation; and Sharon Naron for editorial assistance.
REFERENCES
- 1.Bennink, J. R., and J. W. Yewdell. 1988. Murine cytotoxic T lymphocyte recognition of individual influenza virus protein. High frequency of nonresponder MHC class I alleles. J. Exp. Med. 168:1935-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennink, J. R., J. W. Yewdell, and W. Gerhard. 1982. A viral polymerase involved in recognition of influenza virus-infected cells by a cytotoxic T-cell clone. Nature 296:75-76. [DOI] [PubMed] [Google Scholar]
- 3.Bennink, J. R., J. W. Yewdell, G. L. Smith, C. Moller, and B. Moss. 1984. Recombination vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. 1984. Nature 311:578-579. [DOI] [PubMed] [Google Scholar]
- 4.Carding, S. R., W. Allan, S. Kyes, A. Hayday, K. Bottomly, and P. C. Doherty. 1990. Late dominance of the inflammatory process in murine influenza by γ/δ+ T cells. J. Exp. Med. 172:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauthen, A. N., D. E. Swayne, S. Schultz-Cherry, M. L. Perdue, and D. L. Suarez. 2000. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J. Virol. 74:6592-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, M. M., C. L. Chen, L. L. Ager, and M. D. Cooper. 1988. Identification of the avian homologues of mammalian CD4 and CD8 antigens. J. Immunol. 140:2133-2138. [PubMed] [Google Scholar]
- 7.Char, D. P., P. Sanchez, C. H. Chen, R. P. Bucy, and M. D. Cooper. 1990. A third sublineage of avian T cells can be identified with a T cell receptor-3-specific antibody. J. Immunol. 145:3547-3555. [PubMed] [Google Scholar]
- 8.Chen, C. H., T. W. Gobel, T. Kubota, and M. D. Cooper. 1994. T cell development in the chicken. Poult. Sci. 73:1012-1018. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W., L. C. Anton, J. R. Bennink, and J. W. Yewdell. 2000. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 12:83-93. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, J. P., P. C. Doherty, K. C. Branum, and J. M. Riberdy. 2000. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8+ T-cell memory. J. Virol. 74:11690-11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cihak, J., U. Losch, G. Hoffman-Fezer, C. H. Chen, M. D. Cooper, and H. W. Ziegler-Heitbrock. 1993. In vivo depletion of chickens T-cell subsets. Scand. J. Immunol. 38:123-129. [DOI] [PubMed] [Google Scholar]
- 12.Deckhut, A. M., W. Allan, A. McMickle, M. Eichelberger, M. A. Blackman, P. C. Doherty, and D. L. Woodland. 1993. Prominent usage of V beta 8.3 T cells in the H-2Db-restricted response to an influenza A virus nucleoprotein epitope. J. Immunol. 151:2658-2666. [PubMed] [Google Scholar]
- 13.Doherty, P. C., W. Allan, M. Eichelberger, and S. R. Carding. 1992. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu. Rev. Immunol. 10:123-151. [DOI] [PubMed] [Google Scholar]
- 14.Eichelberger, M., W. Allan, M. Zijlstra, R. Jaenisch, and P. C. Doherty. 1991. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J. Exp. Med. 174:875-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein, S. L., C. Y. Lo, J. A. Misplon, and J. R. Bennink. 1998. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J. Immunol. 160:322-327. [PubMed] [Google Scholar]
- 16.Flynn, K. J., G. T. Belz, J. D. Altman, R. Ahmed, D. L. Woodland, and P. C. Doherty. 1998. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8:683-691. [DOI] [PubMed] [Google Scholar]
- 17.Graham, M. B., and T. J. Braciale. 1997. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J. Exp. Med. 186:2063-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan, Y., K. F. Shortridge, S. Krauss, P. S. Chin, K. C. Dyrting, T. M. Ellis, R. G. Webster, and M. Peiris. 2000. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 74:9372-9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagi, D., F. Vignaux, B. E. A. Ledermann, K. Burki, V. Depraetere, S. Nagata, H. Hengartner, and P. Golstein. 1994. Fas and perforin pathways as major mechanisms of T-cell-mediated cytotoxicity. Science 265:528-530. [DOI] [PubMed] [Google Scholar]
- 20.Lowin, B., M. Hahne, C. Mattmann, and J. Tschopp. 1994. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 370:650-652. [DOI] [PubMed] [Google Scholar]
- 21.Marshall, D. R., S. J. Turner, G. T. Belz, S. Wingo, S. Andreansky, M. Y. Sangster, J. M. Riberdy, T. Liu, M. Tan, and P. C. Doherty. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA 98:6313-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill, E., S. L. Krauss, J. M. Riberdy, R. G. Webster, and D. L. Woodland. 2000. Heterologous protection against lethal A/Hong Kong/156/97 (H5N1) influenza virus infection in C57BL/6 mice. J. Gen. Virol. 81:2689-2696. [DOI] [PubMed] [Google Scholar]
- 24.Riberdy, J. M., K. J. Flynn, J. Stech, R. G. Webster, J. D. Altman, and P. C. Doherty. 1999. Protection against a lethal avian influenza A virus in a mammalian system. J. Virol. 73:1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo, S. H., J. Pei, W. E. Briles, J. Dzielawa, and E. W. Collisson. 2000. Adoptive transfer of infectious bronchitis virus primed αβ T cells bearing CD8 antigen protects chicks from acute infection. Virology 269:183-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo, S. H., and R. G. Webster. 2001. Cross-reactive, cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. J. Virol. 75:2516-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tregaskes, C. A., F. Kong, E. Paramithiotis, C. L. Chen, M. J. H. Ratcliffe, T. F. Davison, and J. R. Young. 1995. Identification and analysis of the expression of CD8 alpha beta and CD8 alpha alpha isoforms in chickens reveals a major TCR-gamma delta CD8 alpha beta subset of intestinal intraepithelial lymphocytes. J. Immunol. 154:4485-4494. [PubMed] [Google Scholar]
- 28.Webster, R. G., Y. Guan, M. Peiris, D. Walker, S. Krauss, N. N. Zhou, E. A. Govorkova, T. M. Ellis, K. C. Dyrting, T. Sit, D. R. Perez, and K. F. Shortridge. 2002. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J. Virol. 76:118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, X., K. Subbarao, N. J. Cox, and Y. Guo. 1999. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261:15-19. [DOI] [PubMed] [Google Scholar]