Abstract
NF-κB is a transcriptional activator that often regulates inflammatory responses. We demonstrate that human immunodeficiency virus type 1 activates nuclear localization of NF-κB in macrophages in a manner dependent upon virus strain but independent of virus replication. Through the use of an inhibitor, NF-κB activation was found to be responsible for the cytokine and chemokine induction that we recently reported.
It is clear that host cell gene expression can be modulated by human immunodeficiency virus type 1 (HIV-1) infection or exposure (4). The transcriptional factor NF-κB and the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) are among the more frequent participants in activation pathways induced by HIV-1 (12). Recently we reported that within hours of exposure, R5 and X4 HIV-1 induce TNF-α and β-chemokine synthesis in human macrophages in a strain-specific, replication-independent manner (3). In the present study, we investigated the involvement of NF-κB in this potent activation pathway.
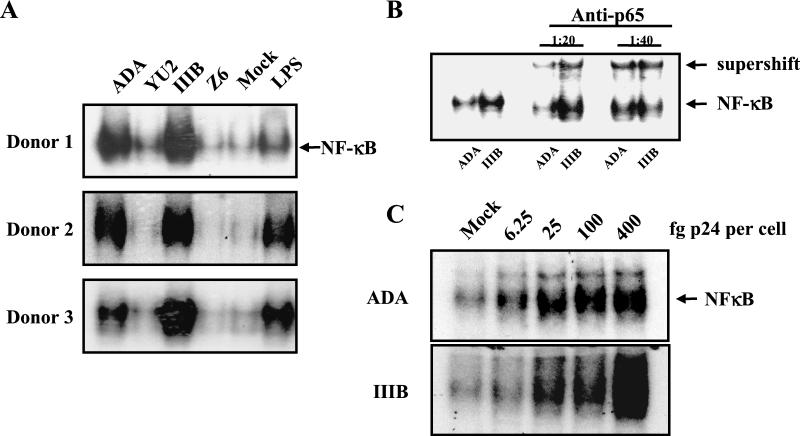
Our general methods for culture of human monocyte-derived macrophages (MDM) and for virus propagation were described previously (3). For the present study, virus stocks were sedimented and resuspended in phosphate-buffered saline (PBS) to minimize the carryover effects of the medium; in addition, they were found to contain less than 0.06 U of endotoxin per ml. Thirty minutes after virus exposure, cells were harvested for assay of NF-κB activation essentially as described previously (2). Briefly, macrophages were incubated on ice for 15 min in buffer A (2) and cells were then harvested, incubated on ice, and pelleted by high-speed centrifugation. The pellet, consisting of nuclei, was resuspended with buffer B (2), spun down, resuspended in buffer C (2), and incubated on ice. The nuclear extract was centrifuged for 15 min at 4°C, and the supernatant was frozen at −80°C. Four micrograms of nuclear extract was incubated for 10 min in a solution containing 10 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 50 μg of bovine serum albumin/ml, 5% glycerol, 2 mM MgCl2, 3 μg of poly(dI:dC) as a nonspecific competitor, and 0.2 ng of [γ-32P]NF-κB oligonucleotide from the HIV-1 long terminal repeat (5′-GATCCGGGACTTTCCGCTGGGGACTTTCCG-3′) radiolabeled as described previously (2). Electrophoretic mobility shift analysis (EMSA) was conducted by electrophoresis of complexes in 4 or 6% native polyacrylamide gels (Fig. 1A). Nuclei from cells exposed to HIV-1 strains ADA or HTLV-IIIB contained NF-κB at high levels, which were comparable to those of cells treated with the positive control inducer, lipopolysaccharide (LPS). By contrast, nuclei from cells exposed to HIV-1 strains YU-2 or Z6 contained no detectable NF-κB. This pattern was reproduced in cells from six donors, and results from three representative donors are shown. Neutralization of potential endotoxin contamination with polymyxin B sulfate had no effect on the ability of HIV-1 to activate NF-κB (data not shown). These findings are consistent with the specificity of response we previously reported: within hours of exposure, ADA and HTLV-IIIB induced macrophage inflammatory protein 1α (MIP-1α), MIP-1β, RANTES, and TNF-α while YU-2 and Z6 did not (3). This strain specificity is independent of the coreceptor phenotype, as ADA and YU-2 employ CCR5 for entry and HTLV-IIIB and Z6 employ CXCR4. To confirm that the oligonucleotide-retarding material in the nuclear extracts was NF-κB, the extracts were incubated with 1 μg of antibody to the p65 subunit of NF-κB (sc-109x; Santa Cruz Biotechnology, Santa Cruz, Calif.) (Fig. 1B). Anti-p65 further retarded migration of the complex, indicating that NF-κB was present in complex with the oligonucleotide probe. To further define the response, the titers of ADA and HTLV-IIIB were determined for their ability to induce NF-κB in MDM (Fig. 1C). A dose per cell of as little as 25 fg of p24 of either virus, or roughly 0.01 infectious units, was able to activate nuclear translocation of NF-κB. This finding suggests that the response to virus is rather sensitive.
FIG. 1.
HIV-1 strain-specific activation of NF-κB in macrophages. (A) Primary human MDM were exposed to HIV-1 at 0.2 pg of p24 per cell or LPS at 100 ng per ml for 30 min prior to assay of NF-κB localization by EMSA. (B) Nuclear extracts were treated with anti-p65 as indicated before EMSA. The arrows indicate the mobility of the oligonucleotide probe in complex with NF-κB and anti-p65 immunoglobulin. (C) MDM were exposed to HIV-1 at the indicated concentrations for 30 min prior to assay of NF-κB localization by EMSA. IIIB, HTLV-IIIB.
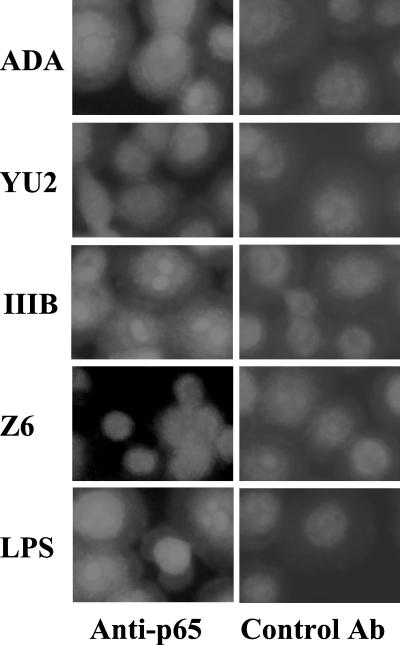
To confirm NF-κB nuclear localization, we stained MDM with anti-p65 after exposure to HIV-1 and performed fluorescence microscopy. Cells were cultured on glass coverslips, infected with HIV-1 for 30 min, washed, fixed in 4% paraformaldehyde-PBS, permeabilized in methanol, and then blocked with 1% fetal bovine serum in PBS. Cells were incubated with rabbit anti-p65 (sc-109; Santa Cruz Biotechnology) or control rabbit immunoglobulin G, washed with PBS-0.1% Tween 20, and then incubated with fluorescein isothiocyanate-labeled goat anti-rabbit immunoglobulin (DAKO, Carpinteria, Calif.) (Fig. 2). Upon exposure to ADA, HTLV-IIIB, or LPS, there was a striking redistribution of NF-κB from a diffuse cytoplasmic stain to intense staining in nuclei. No such redistribution was observed after infection of MDM by YU-2 or Z6. This result validates our interpretation that ADA and HTLV-IIIB specifically activate nuclear translocation of NF-κB.
FIG.2.
HIV-1 strain-specific activation of NF-κB in macrophages. Primary human MDM were exposed to HIV-1 or LPS, and after 30 min, NF-κB localization was assessed by fluorescence microscopy after fixed cells were stained with anti-p65 or an isotype-matched mouse immunoglobulin and a fluorescently labeled secondary antibody (control). IIIB, HTLV-IIIB; Ab, antibody.
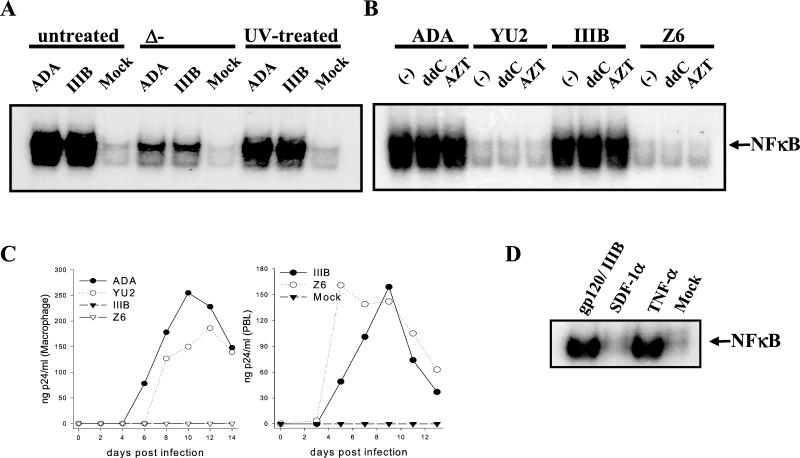
To test the requirement of virus replication for NF-κB activation, HIV-1 stocks and control media were UV irradiated for 30 min, heated to 56°C for 2 h, or left untreated prior to MDM exposure. Alternatively, MDM were treated with the inhibitors of reverse transcription 2′,3′-dideoxycytidine (ddC) or 3′-azido-3′-deoxythymidine (AZT) prior to virus exposure. NF-κB EMSA was performed as described above (Fig. 3A and B). Heat inactivation of HIV-1 detectably reduced nuclear localization of NF-κB, but UV irradiation had little effect. Neither AZT nor ddC affected the ability of ADA and HTLV-IIIB to activate NF-κB. As shown in Fig. 1, YU-2 and Z6 were unable to activate NF-κB. To verify their tropism, viruses were used to infect MDM or peripheral blood lymphocytes (PBL), and extracellular core antigen p24 production was monitored by enzyme-linked immunosorbent assay (ELISA) with the HIV Ag kit (Coulter, Hialeah, Fla.) (Fig. 3C). MDM were productively infected by the R5 viruses ADA and YU-2 but not by the X4 viruses HTLV-IIIB and Z6. However, HTLV-IIIB and Z6 replicated well in PBL. Taken together, these results suggest that viral protein denaturation by heat reduced the ability of the viruses to activate NF-κB but that neither UV irradiation, which principally inactivates nucleic acid (13), nor inhibition of reverse transcription affected viral induction competence. We conclude that the ability of HIV-1 to activate NF-κB in MDM is unrelated to its ability to replicate in these cells. These observations indicate that activation of NF-κB by HIV-1 has requirements similar to those of the pathway of induction of β-chemokines and TNF-α we previously described (3). With these observations in mind, we tested the activation of NF-κB by exposure of MDM to gp120. Previous studies indicate that gp120 can induce nuclear localization of NF-κB in transformed lymphocytes (9). MDM were exposed to HXB-2 gp120, stromal-derived factor 1α (SDF-1α) (Pepro Tech, Rocky Hill, N.J.), the natural ligand for CXCR4 (1), or TNF-α (R & D Systems, Minneapolis, Minn.), and NF-κB activation was tested by EMSA (Fig. 3D). TNF-α and gp120 were potent activators of NF-κB, but SDF-1α did not induce a response in this system. These findings indicate that HIV-1 binding through gp120 can activate NF-κB in MDM but that it is unlikely that ligation of CXCR4 alone mediates the response.
FIG. 3.
HIV-1 replication is dispensable for activation of NF-κB in macrophages. (A and B) HIV-1 was inactivated by heat or UV irradiation, or MDM were pretreated with AZT or ddC at 1 μM for 12 h prior to cell exposure to the virus and assay of NF-κB localization by EMSA. (C) The progress of HIV-1 infection in MDM (left panel) and PBL (right panel) was monitored by ELISA for extracellular p24. (D) MDM were exposed to 10 nM HTLV-IIIB gp120, 1.25 nM SDF-1α, 200 U of TNF-α per 106 cells, or normal medium prior to assay of NF-κB localization by EMSA. IIIB, HTLV-IIIB.
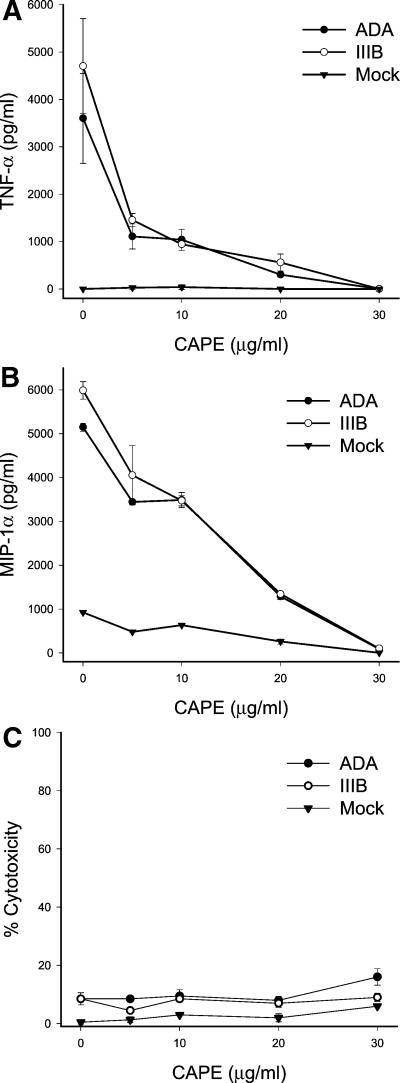
Transcription of TNF-α and MIP-1α can be activated by NF-κB (5, 6). Based upon the shared strain specificity and independence from virus replication, the induction of synthesis of TNF-α and the β-chemokines by HIV-1 seems to parallel NF-κB activation. To test the linkage of these responses, we employed caffeic acid phenethyl ester (CAPE; Calbiochem, San Diego, Calif.), which specifically blocks activation of NF-κB but not other transcriptional factors tested (7). MDM were treated with CAPE in dose response prior to exposure to HIV-1, and cell supernatants were harvested 3 h after virus exposure and assayed for levels of TNF-α and MIP-1α by ELISA as described previously (3) (Fig. 4). There was a dose-dependent inhibition of synthesis of both TNF-α and MIP-1α by CAPE. At concentrations fully inhibiting TNF-α or MIP-1α synthesis, CAPE was not toxic to MDM, as determined by assay of lactate dehydrogenase release with the CytoTox 96 assay (Promega, Madison, Wis.). Our results demonstrate that within minutes of the exposure of macrophages to HIV-1 strains ADA or HTLV-IIIB, nuclear translocation of NF-κB was activated, resulting in induction of transcription and synthesis of MIP-1α and TNF-α.
FIG. 4.
Inhibition of NF-κB activation by HIV-1 also inhibits induction of TNF-α and MIP-1α by HIV-1. MDM were pretreated with CAPE at the indicated concentrations for 2 h prior to exposure to HIV-1 at 0.1 pg of p24 per cell. Cells and supernatants were harvested 3 h after infection. The levels of TNF-α (A) and MIP-1α (B) in cell supernatants were measured by ELISA. (C) Cell viability was measured by lactate dehydrogenase release. IIIB, HTLV-IIIB.
A circuit was described in culture in which HIV-1, NF-κB, and TNF-α each exert positive feedback upon the others, amplifying virus replication and inducing changes in cellular gene expression (8, 10). Analogous observations were recently reported for the brains of HIV-1-infected patients (11). Nuclear translocation of NF-κB was demonstrated in macrophages and microglial cells, and this activation was correlated to the expression of TNF-α and the degree of dementia observed. The results presented here introduce the possibility that independent of its replication, HIV-1 induces NF-κB activation and cellular gene expression in macrophages that contribute to functional impairment of the brain. Further studies are required to determine the impact of this pathway on HIV-1 pathogenesis.
Acknowledgments
We thank R. Banerjee for helpful discussions and I. Totillo for manuscript preparation. HIV-1 gp120 was obtained from DAIDS, NIAID, through the AIDS Research Reagent Program.
This work was supported by Public Health Service grants to M.J.P. and D.J.V.
REFERENCES
- 1.Bleul, C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, J. Sodroski, and T. A. Springer. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. [DOI] [PubMed] [Google Scholar]
- 2.Caruccio, L., and R. Banerjee. 1999. An efficient method for simultaneous isolation of biologically active transcription factors and DNA. J. Immunol. Methods 230:1-10. [DOI] [PubMed] [Google Scholar]
- 3.Choe, W., D. J. Volsky, and M. J. Potash. 2001. Induction of rapid and extensive β-chemokine synthesis in macrophages by human immunodeficiency virus type 1 and gp120, independently of their coreceptor phenotype. J. Virol. 75:10738-10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbeil, J., D. Sheeter, D. Genini, S. Rought, L. Leoni, P. Du, M. Ferguson, D. R. Masys, J. B. Welsh, J. L. Fink, R. Sasik, D. Huang, J. Drenkow, D. D. Richman, and T. Gingeras. 2001. Temporal gene regulation during HIV-1 infection of human CD4+ T cells. Genome Res. 11:1198-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grove, M., and M. Plumb. 1993. C/EBP, NF-κB, and c-Ets family members and transcriptional regulation of the cell-specific and inducible macrophage inflammatory protein 1α immediate-early gene. Mol. Cell. Biol. 13:5276-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mogensen, T. H., and S. R. Paludan. 2001. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 65:131-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natarajan, K., S. Singh, T. R. Burke, Jr., D. Grunberger, and B. B. Aggarwal. 1996. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc. Natl. Acad. Sci. USA 93:9090-9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poli, G., A. Kinter, J. S. Justement, J. H. Kehrl, P. Bressler, S. Stanley, and A. S. Fauci. 1990. Tumor necrosis factor α functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc. Natl. Acad. Sci. USA 87:782-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popik, W., J. E. Hesselgesser, and P. M. Pitha. 1998. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J. Virol. 72:6406-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg, Z. F., and A. S. Fauci. 1989. Induction of expression of HIV in latently or chronically infected cells. AIDS Res. Hum. Retrovir. 5:1-4. [DOI] [PubMed] [Google Scholar]
- 11.Rostasy, K., L. Monti, C. Yiannoutsos, J. Wu, J. Bell, J. Hedreen, and B. A. Navia. 2000. NFκB activation, TNF-α expression, and apoptosis in the AIDS-dementia-complex. J. Neurovirol. 6:537-543. [DOI] [PubMed] [Google Scholar]
- 12.Roulston, A., R. Lin, P. Beauparlant, M. A. Wainberg, and J. Hiscott. 1995. Regulation of human immunodeficiency virus type 1 and cytokine gene expression in myeloid cells by NF-κB/Rel transcription factors. Microbiol. Rev. 59:481-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spire, B., F. Barré-Sinoussi, D. Dormont, L. Montagnier, and J. C. Chermann. 1985. Inactivation of lymphadenopathy-associated virus by heat, gamma rays, and ultraviolet light. Lancet i:188-189. [DOI] [PubMed]