Abstract
Considerable evidence exists for sex differences in human pain sensitivity. Women typically report a higher incidence of various painful conditions and report that the conditions are more painful when compared to men. In the present study, we sought to determine whether sex differences in pain sensitivity are observed using a lumbar radiculopathy model of low back pain in the rat and whether removal or alteration of gonadal hormones at specific timepoints can modulate these sex differences. Pubertal and adult male and female Sprague—Dawley rats were castrated 2 or 6 weeks prior to L5 nerve root injury to determine the activational hormonal effects. In a separate study, neonatal male and female Sprague—Dawley rats were either castrated or injected with testosterone, respectively, on postnatal day one to determine the organizational effects of gonadal hormones on L5 nerve root injury-induced behavioral hypersensitivity. Our results demonstrate that there was a statistically significant sex difference in the magnitude of mechanical allodynia and thermal hyperalgesia following experimentally induced radiculopathy in the rat: females demonstrated decreased thresholds to tactile and thermal stimuli as compared to males. Furthermore, the enhanced female hypersensitivity was reversed in pubertal and adult animals ovariectomized 6 weeks, but not 2 weeks prior to L5 nerve root injury. Our results demonstrate that the activational effects of gonadal hormones mediate the enhanced female tactile and thermal hypersensitivity following L5 nerve root injury. These results suggest that manipulation of gonadal hormones may be a potential source for novel therapies for chronic pain in women.
Keywords: Low back pain animal model, Sex difference, Sex hormone, Sex differentiation
1. Introduction
A considerable body of research exists demonstrating sex differences in pain perception, with females typically reporting lower thresholds to experimental stimuli and an increased incidence of a number of painful diseases (Berkley, 1997; Giles and Walker, 2000; Yunus, 2002). Furthermore, research has suggested that the sex hormones may have a pivotal role in mediating the sex differences in pain (Aloisi, 2003; Blackburn-Munro and Blackburn-Munro, 2003; Fillingim and Ness, 2000). The complexity and seemingly contradictory actions of gonadal hormones in the CNS have made it difficult to delineate a singular mechanism for their role in mediating sex differences in pain sensitivity.
Sex steroids are classically known to operate through two distinct mechanisms to affect physiology and behavior. The first mechanism, organizational, is a developmental mechanism in which steroids act during critical periods to mediate the generally permanent sexually dimorphic differentiation of brain morphology that gives rise to male and female sexual behavior and physiology in adulthood. The second mechanism, activational, is mediated through the acute effects of gonadal hormones on the fully developed nervous system and is responsible for maintaining male and female sexual and other behaviors in adulthood. For a more thorough review of the full scope of organizational/activational effects of gonadal hormones on male and female physiology refer to excellent reviews by Arnold and Breedlove (1985), Breedlove et al. (1999), and Cooke et al. (1998).
Few studies have systematically examined the activational and organizational effects of gonadal hormones on nociception. It has been observed that manipulation of the activational effects of sex hormones can modulate formalin nociception in the female rat (Gaumond et al., 2002), while only organizational manipulations altered the jump threshold to electric shock in male rats (Beatty and Fessler, 1977). Two independent studies have shown that a sex difference in morphine analgesic potency is organizationally mediated (Cicero et al., 2002; Krzanowska et al., 2002).
We have previously observed enhanced tactile allodynia in female Sprague—Dawley rats following peripheral nerve injury (DeLeo and Rutkowski, 2000) and following L5 nerve root ligation (LaCroix-Fralish et al., in press), as compared to male rats. The aim of the present studies was to systematically determine the organizational and activational effects of gonadal hormones on basal tactile and thermal sensitivity and on sensitivity following L5 nerve root injury, a rodent model of low back pain associated with radiculopathy. We utilized the classic strategy of ‘masculinization’ of female pups within 24 h after birth using large doses of testosterone and ‘feminization’ of male pups by castration within the first 24 h of life. In this way, we were able to determine the organizational role of gonadal hormones on nociceptive systems prior to and after L5 nerve root injury. The acute activational effects of gonadal hormones were also determined by castration of both pubertal and adult rats for a period of 2 or 6 weeks prior to L5 nerve root injury. We show, for the first time in a nerve injury model in the rat, that the activational effects of gonadal hormones mediate the female hypersensitivity observed following L5 nerve root injury.
2. Materials and methods
2.1. Animals and animal care
Male and female Sprague—Dawley rats and timed-pregnant Sprague—Dawley female rats were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Animals were housed one per cage in a controlled, supervised environment in the Animal Resource Center of Dartmouth College, New Hampshire, under a 12 h light/dark cycle. Food and water were available ad libitum. All procedures and care were approved by the Institutional Animal Care and Use Committee (IACUC) of Dartmouth College. Unless otherwise noted all surgical procedures were performed under inhalation anesthesia induced with 4% gaseous halothane in O2 and maintained at 1.5-2%.
2.2. Orchiectomy and masculinization in neonatal rats
One-day-old neonatal male pups were castrated (n=7) to remove their endogenous source of testosterone. To induce anesthesia, individual rat pups were placed onto cloth towels in contact with crushed ice (Phifer and Terry, 1986). Visual assessment of respiration and skin color was conducted every 3-5 min throughout the procedure and pedal withdrawal was monitored to ensure complete anesthesia (Park et al., 1992). A small incision in the abdomen was made, and the testes and the vas deferens were resected with a blunt forceps. The skin incision was sealed with cyanoacrylate adhesive and animals were observed during recovery as the pups were gently warmed before being returned to their litter and dam. A sham procedure, consisting of abdominal incision followed by manipulation of the gonads without resection, was performed on a second group (n=7) of neonatal male pups.
One-day-old neonatal female pups (n=7) were injected with testosterone propionate (Sigma-Aldrich, St Louis, MO) in sesame oil (SSO) vehicle (Sigma-Aldrich) subcutaneously at a concentration of 1 mg testosterone/100 μL SSO per pup in order to masculinize the female pup (Pheonix et al., 1959). The procedure was repeated 24 h later for a total of two injections. A sham procedure consisting of SSO vehicle only was injected in a control group of animals. Male and female rats were allowed to recover for 7 weeks prior to further surgical procedures.
2.3. Ovariectomy and orchiectomy in pubertal and adult rats
The activational effect of gonadal hormones was investigated in sexually immature, 6-week-old male (n=16) and female (n=16) rats and sexually mature, 12-week-old male (n=8) and female (n=8) rats by castration (performed by Harlan Sprague Dawley, Inc., Indianapolis, IN). A control group (n=16/sex) received sham surgeries consisting of manipulation of the gonads without resection. Male and female rats were allowed to recover for either 2 or 6 weeks prior to further surgical procedures. The full experimental design is shown in Fig. 1.
Fig. 1.
Schematic representation of the experimental design used to determine the organizational/activational effects of gonadal hormones in mediating sex differences in allodynia following L5 nerve root ligation. ORCH, male orchiectomy; OVX, female ovariectomy; TP, female administered testosterone propionate.
2.4. Lumbar radiculopathy
Lumbar radiculopathy (LR) was surgically induced using the procedure of Hashizume et al. (2000) in adult and pubertal rats previously having undergone manipulation of gonadal hormones either 2 or 6 weeks prior and in rats having undergone manipulation of gonadal hormones 7 weeks prior as neonates. Briefly, the L5 level was exposed by a 1-cm skin incision made at the L4-L6 level, and the muscle tissue was retracted from the superior articular processes. Following a hemi-laminectomy, the L5 dorsal and ventral nerve roots were exposed and loosely ligated with two 4-0 chromic gut ligatures. Five pieces of 5-0 chromic gut suture, 1.5-2.0 mm in length were applied beneath the loose ligatures, adjacent to the L5 nerve root, and the ligatures were secured. The wound was irrigated with sterile saline and closed using 3-0 Ethibond polyester suture followed by surgical staples.
2.5. Mechanical allodynia
The development of mechanical allodynia was assayed using 2-g (20.02 mN) and 12-g (115.3 mN) calibrated von Frey filaments (Stoelting, Wood Dale, IL) as described previously (Colburn et al., 1997; LaCroix-Fralish et al., in press) by a single investigator blinded to the hormonal and surgical status of the rats. Animals were acclimated to the testing procedure twice before the LR surgical procedure in order to record baseline values. Any animals displaying abnormal baseline paw withdrawals were excluded from the study.
2.6. Thermal hyperalgesia
The development of thermal hyperalgesia was measured by using the paw withdrawal latency test (Hargreaves et al., 1988) by a single investigator blinded to the hormonal and surgical status of the rats. A radiant heat source (24 V halogen lamp) was focused on the plantar surface of the hind paw. Withdrawal latencies were measured with a timer that was initiated at the onset of heating to the paw and terminated at the time of withdrawal from the beam of heat. A cut off time of 20 s was used to prevent tissue injury. Three trials were conducted on the hind paw ipsilateral to the L5 nerve root injury. The latencies are expressed as the median latency of the three trials. Animals were acclimated to the testing procedure twice before LR surgery in order to record baseline values.
2.7. Serum hormone concentration assay
The plasma concentration of gonadal hormones was assayed from blood samples acquired via cardiac puncture from all rats immediately after CO2 asphyxiation in order to ensure the efficacy of the hormonal manipulations. An extraction procedure consisting of thorough mixing of 300 μL of serum from each sample with 3 mL of diethyl ether was performed. The ether was evaporated using a gentle stream of air and the samples were then reconstituted in 300 μL of phosphate buffered saline (PBS). Following the extraction procedure, the serum concentration of testosterone and 17β-estradiol was assayed in duplicate from male and female rats, respectively, using enzyme immunoassay (EIA) kits from Cayman Chemicals (Ann Arbor, MI) according to the manufacturer's recommended protocol.
2.8. Statistical analysis
Statistical significance in the behavioral experiments was determined by repeated measures two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test and statistical significance in the steroid hormone EIA experiments was determined by two-way ANOVA analysis followed by Bonferroni post hoc test using GraphPad Prism version 4.0 a (GraphPad Software, Inc., San Diego, CA). A statistical threshold of α = 0.05 (P<0.05) was considered significant for all experiments.
3. Results
3.1. Effects of adult, pubertal, and neonatal hormonal manipulation on baseline thermal nociception
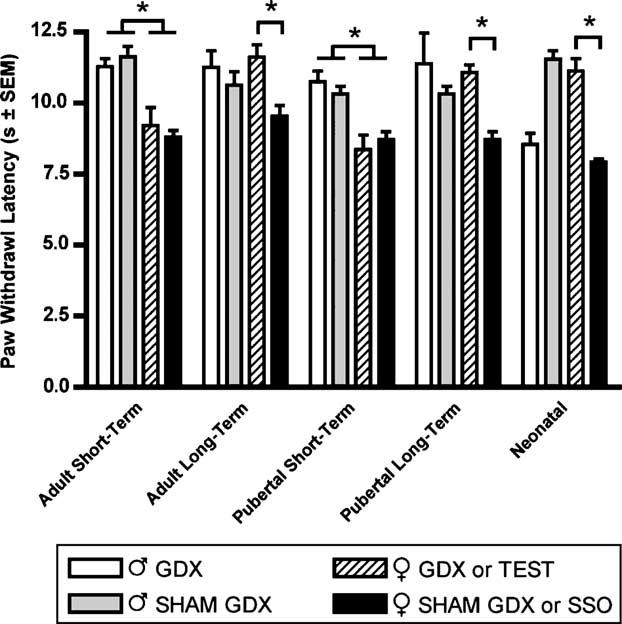
Thermal nociception was assayed in naïve (non-L5 nerve root injured), male and female rats that had previously received hormonal manipulation. The pubertal and adult groups were tested 2 weeks (short-term elimination) or 6 weeks (long-term elimination) after castration. Both the pubertal and adult ovariectomized and sham ovariectomized female rats in the 2 week post-manipulation group, and the sham ovariectomized female rats in the 6 week postmanipulation group displayed significantly lower (P<0.05) paw withdrawal latencies as compared to male rats. The group that had received neonatal manipulations was tested 7 weeks after castration/testosterone treatment. A significant decrease in paw withdrawal latency was observed in female rats receiving SSO vehicle treatments days 1 and 2 postnatally, but not in testosterone masculinized treated females. Similarly, a significant decrease in withdrawal latency was observed in day 1 postnatal orchidectomized male rats, but not in sham orchidectomized (hormonally intact) males (Fig. 2).
Fig. 2.
Baseline thermal nociception measured as latency of paw withdrawal to a constant thermal stimulus in seconds. All animals have previously received hormonal manipulations as adults 2 weeks prior to testing (Adult or Pubertal Short-Term elimination, n=8/sex) or 6 weeks prior to testing (Adult or Pubertal Long-Term elimination, n=8/sex). Animals receiving neonatal hormonal manipulations (Neonatal, n=7/sex) 7 weeks prior to testing are also shown. Responses are reported as the median of three trials per animal±group SEM. * indicates significant decrease of P<0.05.
3.2. Effects of adult (12 weeks old) hormonal manipulation on the development of tactile and thermal hypersensitivity following LR
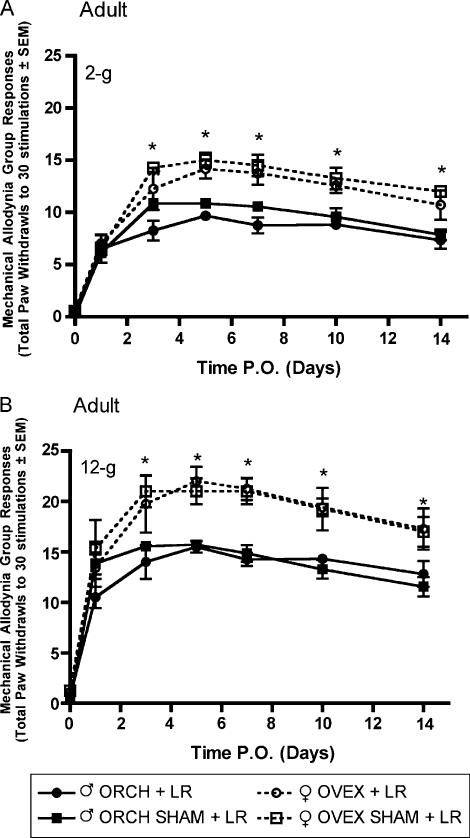
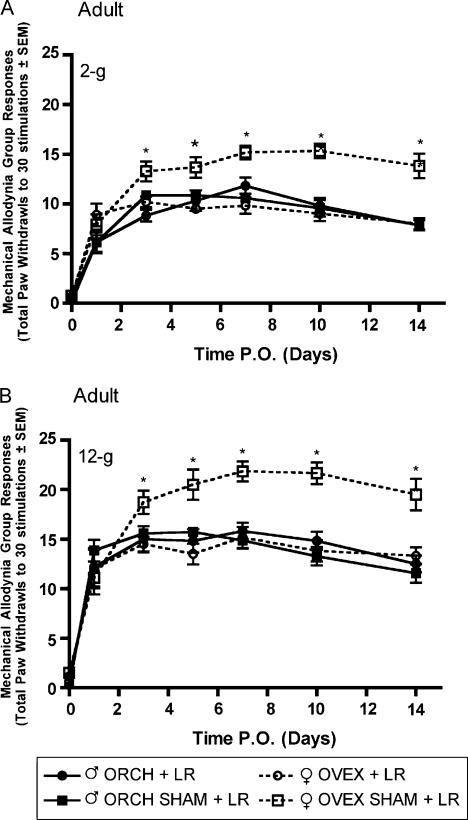
Following L5 nerve root ligation in aged matched male and female rats that had previously received gonadectomy or sham gonadectomy 2 weeks (short-term elimination) or 6 weeks (long-term elimination) prior at 12 weeks of age, robust mechanical allodynia and thermal hyperalgesia were observed. In the group receiving L5 nerve root ligation 2 weeks after hormonal manipulation, significantly (P<0.05) higher allodynic responses were observed in both ovariectomized and sham ovariectomized females as compared to males in response to 2-g (Fig. 3A) and 12-g (Fig. 3B) von Frey filaments beginning day 3 post surgery and maintained until the end of testing. In contrast, in the group receiving L5 nerve root ligation 6 weeks after hormonal manipulation, only the sham ovariectomized females displayed significantly higher allodynic responses as compared to males in response to 2-g (Fig. 4A) and 12-g (Fig. 4B) von Frey filaments.
Fig. 3.
Mechanical allodynia measured as paw withdrawal frequency to stimulation with 2-g (A) and 12-g (B) von Frey filaments in adult male and female Sprague—Dawley rats (n=8, n=8) having been castrated at 12 weeks of age, 2 weeks prior to L5 nerve root ligation. Responses are reported as the mean of 30 stimulations±group SEM. * indicates significant increase (P<0.05) compared to male.
Fig. 4.
Mechanical allodynia measured as paw withdrawal frequency to stimulation with 2-g (A) and 12-g (B) von Frey filaments in adult male and female Sprague—Dawley rats (n=7, n=7) having been castrated at 12 weeks of age, 6 weeks prior to L5 spinal root ligation. Responses are reported as the mean of 30 stimulations ± group SEM. * indicates significant increase (P<0.05) compared to male.
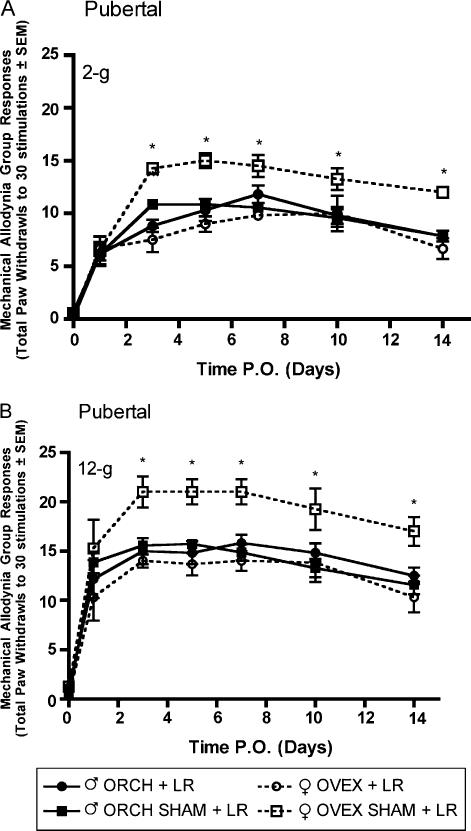
3.3. Effects of pubertal (6 weeks old) hormonal manipulation on the development of tactile and thermal hypersensitivity following LR
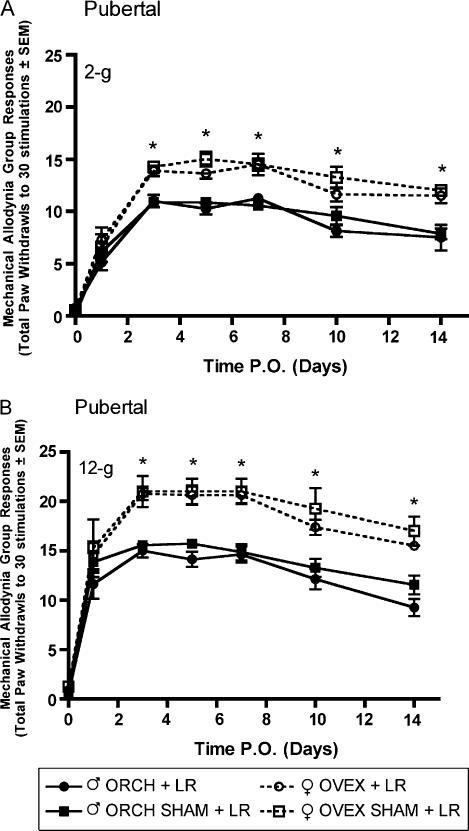
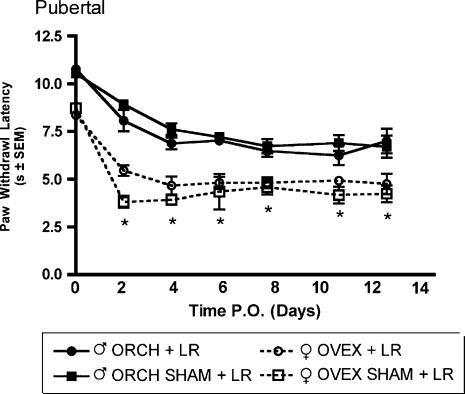
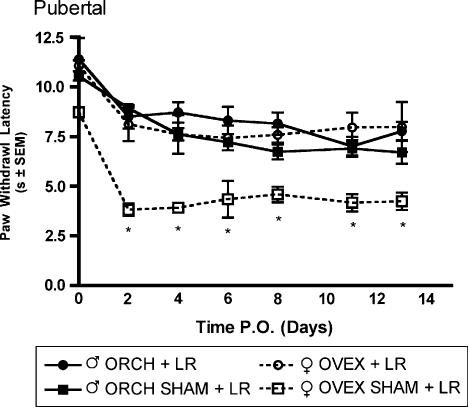
Robust mechanical allodynia and thermal hyperalgesia were observed following L5 nerve root ligation in aged matched male and female rats that had previously received gonadectomy or sham gonadectomy 2 weeks (short-term elimination) or 6 weeks (long-term elimination) prior at 6 weeks of age. In the group receiving L5 nerve root ligation 2 weeks after hormonal manipulation, significantly (P<0.05) higher allodynic responses were observed in both ovariectomized and sham ovariectomized females as compared to males in response to 2-g (Fig. 5A) and 12-g (Fig. 5B) von Frey filaments beginning day 3 post surgery and maintained until the end of testing. Significantly lower withdrawal thresholds were observed in both ovariectomized and sham ovariectomized females as compared to males in response to a noxious thermal stimulus (Fig. 6). In contrast, in the group receiving L5 nerve root ligation 6 weeks after hormonal manipulation, only the sham ovariectomized females displayed significantly higher allodynic responses as compared to males in response to 2-g (Fig. 7A) and 12-g (Fig. 7B) von Frey filaments. Significantly lower withdrawal thresholds were observed only in the sham ovariectomized females as compared to males in response to a noxious thermal stimulus (Fig. 8).
Fig. 5.
Mechanical allodynia measured as paw withdrawal frequency to stimulation with 2-g (A) and 12-g (B) von Frey filaments in pubertal male and female Sprague—Dawley rats (n=8, n=8) having been castrated at 6 weeks of age, 2 weeks prior to L5 nerve root ligation. Responses are reported as the mean of 30 stimulations ± group SEM. * indicates significant increase (P<0.05) compared to male.
Fig. 6.
Thermal hyperalgesia measured as latency of paw withdrawal to a constant thermal stimulus in pubertal male and female Sprague—Dawley rats (n=8, n=8) that had previously received hormonal manipulations 2 weeks prior to L5 nerve root ligation. Responses are reported as the median of three trials per animal ± groups SEM. * indicates significant decrease (P<0.05) compared to male.
Fig. 7.
Mechanical allodynia measured as paw withdrawal frequency to stimulation with 2-g (A) and 12-g (B) von Frey filaments in male and female Sprague—Dawley rats (n=7, n=7) having been castrated at 6 weeks of age, 6 weeks prior to L5 spinal root ligation. Responses are reported as the mean of 30 stimulations ± group SEM. * indicates significant increase (P<0.05) compared to male.
Fig. 8.
Thermal hyperalgesia measured as latency of paw withdrawal to a constant thermal stimulus in pubertal male and female Sprague—Dawley rats (n=7, n=7) that had previously received hormonal manipulations 6 weeks prior to L5 nerve root ligation. Responses are reported as the median of three trials per animal±group SEM. * indicates significant decrease (P<0.05) compared to male.
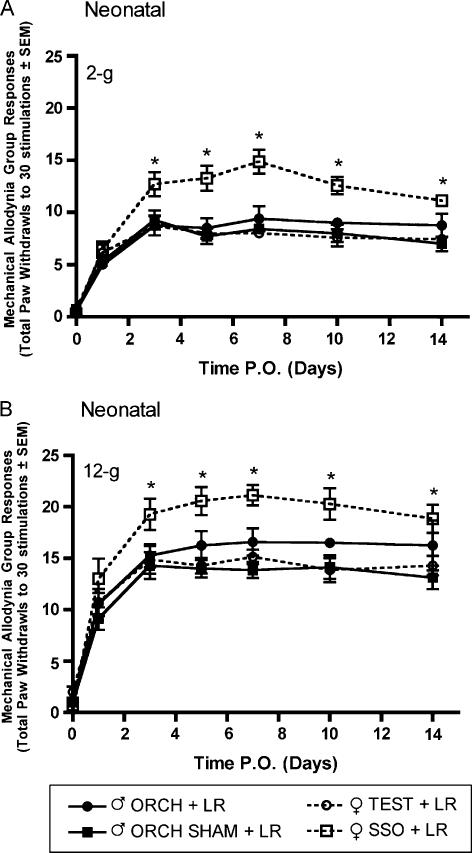
3.4. Effects of neonatal hormonal manipulation on the development of tactile and thermal hypersensitivity following LR
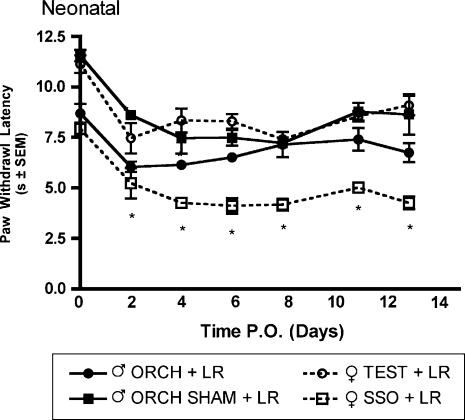
L5 nerve root ligation was performed on male and female rats that had previously undergone neonatal hormonal manipulations. Significantly higher allodynic responses to 2-g (Fig. 9A) and 12-g (Fig. 9B) von Frey filaments were observed only in the female group that had received sesame seed oil (SSO) vehicle as compared to intact and gonadectomized male rats and testosterone propionate treated females beginning day 3 post surgery. A similar result was obtained using the thermal hyperalgesia assay in which the female group that had received SSO vehicle injections on days one and two postnatal had a significantly lower mean paw withdrawal latency as compared to intact male rats and testosterone treated females (Fig. 10).
Fig. 9.
Mechanical allodynia measured as paw withdrawal frequency to stimulation with 2-g (A) and 12-g (B) von Frey filaments in male and female Sprague—Dawley rats (n=7, n=7) having previously received neonatal hormonal manipulations 7 weeks prior to L5 nerve root ligation. Responses are reported as the mean of 30 stimulations ± group SEM. * indicates significant increase (P<0.05) compared to male.
Fig. 10.
Thermal hyperalgesia measured as latency of paw withdrawal to a constant thermal stimulus in adult male and female Sprague—Dawley rats (n=5, n=7) that had previously received hormonal manipulations as neonates 7 weeks prior to L5 nerve root ligation. Responses are reported as the median of three trials per animal± group SEM. * indicates significant increase (P<0.05) compared to male.
3.5. Serum hormone assay
The concentrations of serum 17β-estradiol (Fig. 11A) and testosterone (Fig. 11B) were determined post-mortem in order to confirm the efficacy of the hormonal manipulations. Significant (P<0.05) decreases in both 17β-estradiol and testosterone was observed in all ovariectomized/orchiectomized animals while all sham ovariectomized/orchiectomized animals demonstrated no significant differences from a control sham ovariectomized/orchiectomized (Sham OVX/ORCH) group. No significant decrease in serum 17β-estradiol concentration was observed in the group of neonatal female rats that were treated with testosterone propionate.
Fig. 11.
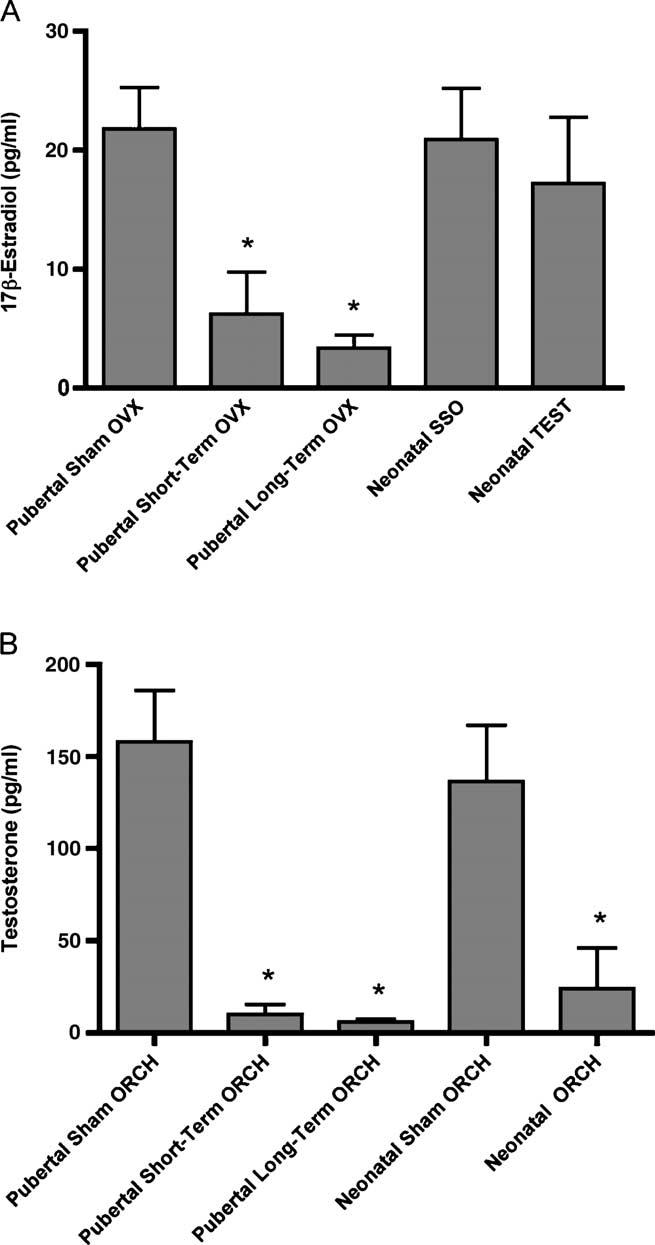
Serum concentrations of (A) 17β-estradiol and (B) testosterone from female and male rats, respectively. All animals have previously received hormonal manipulations at 6 weeks of age, 2 weeks prior to L5 nerve root ligation (Pubertal Short-Term OVX/Short-Term ORCH) or 6 weeks prior to L5 nerve root ligation (Pubertal Long-Term OVX/Long-Term ORCH). A control group consisting of sham OVX/ORCH is also shown. Animals receiving neonatal hormonal manipulations (Neonatal SSO/TEST or Neonatal Sham ORCH/ORCH) 7 weeks prior to L5 nerve root ligation are also shown. Responses are reported as the mean of duplicate samples analyzed for all animals±group SEM. * indicates statistically significant (P<0.05) decrease from control (sham OVX/ORCH).
4. Discussion
To date, there is a surprising paucity of information on the contribution of gonadal hormones to the development and maintenance of chronic pain. Using a rodent model of partial nerve injury, hormonally intact female rats were shown to be significantly more likely to develop tactile allodynia than were either male or ovariectomized female rats (Coyle et al., 1995, 1996). Similarly, the severity of experimental arthritis has been shown to be greater in gonadally intact female rats than in male rats (Green, 2001). Finally, 17β-estradiol administration to intact male rats has been shown to increase the duration of formalin-induced licking (Aloisi and Ceccarelli, 2000). Thus far, all of these studies have examined the acute, activational effects of gonadal hormones or single hormone administration. Systematic examination of the organizational—activational effects of gonadal hormones on male and female sensitivity following the induction of chronic pain has yet to be reported.
In the present study, we demonstrate in a rodent model of low back pain associated with radiculopathy that sexually dimorphic enhancement of tactile and thermal hypersensitivity in adult and pubertal female Sprague—Dawley rats can be abolished by removal of activational gonadal hormones 6 weeks, but not 2 weeks, prior to surgery. Furthermore, treatment of neonatal female rats with testosterone propionate abolishes enhancement of tactile and thermal hypersensitivity following surgery in adulthood even though circulating levels of 17β-estradiol are unchanged from sham OVX controls. This result suggests that gonadal hormones, acting during the ‘critical’ period of development, can modulate the nociceptive axis independent of circulating hormone levels in adulthood.
Classically, the organizational—activational hypothesis has stated, as a general principle, that if a behavioral effect is observed by alteration of activational gonadal hormones, then it is the activational effects of gonadal hormones that mediate the observed behavior (Arnold and Breedlove,1985). Our data, therefore suggests, that the adult activational effects of female gonadal hormones mediate the enhanced female tactile and thermal sensitivity following L5 nerve root injury. Furthermore, our results suggest a potential mechanism for gonadal hormone mediated sex differences in hypersensitivity following nerve root injury. Namely, that circulating female gonadal hormones in adult rats mediate the enhanced female hypersensitivity and are reversible after removal of the gonadal hormones. This reversal, however, is not immediate, requiring at least several weeks of time in order for modulation of tactile and thermal sensitivity.
The actions of gonadal hormones on the cells of the CNS are mediated by two distinct mechanisms. The first mechanism is via binding of the sex steroid molecules to associated receptors (estrogen receptor α/β, androgen receptor, progesterone receptor) which then bind to discrete response elements on DNA and mediate transcription or repression of target genes (Truss and Beato, 1993) including, but not limited to: neurotransmitter receptor sub-units (Cyr et al., 2000; D'Souza et al., 2003), trkA and p75NTR receptors (Lanlua et al., 2001), and peripheral myelin proteins (Melcangi et al., 2000). The second mechanism of action of gonadal hormones is via binding of the steroid molecules to membrane-associated receptors which signal through a variety of intracellular signaling cascades (Moggs and Orphanides, 2001; Simoncini and Genazzani, 2003). This mechanism of action results in rapid, short-term adaptation of cellular processes to the cellular milieu and has been demonstrated to be involved in the modulation of the γ-aminobutyric acid (GABA) type A receptor (McEwen, 1991), rapid changes in neuronal firing (Smith et al., 1988), and estrogen-mediated enhancement of long-term potentiation in the hippocampus (Bi et al., 2000). Our results suggest that the activational effects of gonadal hormones modulate female nociceptive sensitivity via genomic mechanisms based primarily on the magnitude of the temporal separation between hormone depletion and the observed changes in behavioral responses.
The present data demonstrate that activational gonadal hormones modulate nociceptive sensitivity independent of L5 nerve root injury. Specifically, long-term elimination of gonadal hormones in adult female rats increases the nociceptive threshold to thermal stimuli in naïve animals. Furthermore, when these animals are subsequently exposed to L5 nerve root injury, there is a correlative observation of decreased allodynic responses to tactile stimuli and increased withdrawal latency to thermal stimuli as compared to controls. Taken together, these data suggest that the observed female behavioral hypersensitivity is, in part, due to sex hormone-mediated alterations of the basal nociceptive set point. Namely, there is an ovarian-derived molecule or molecules that mediate the decrease in the female nociceptive set point. Alternatively, alteration of male derived sex hormones have no significant effect on the tactile and thermal sensitivity following L5 nerve root injury in any of our manipulations. This hypothesis is supported by many reports demonstrating differential pain sensitivities to a variety of stimuli across the estrus cycle in rodents, with the highest levels of nociceptive response generally noted at the time when estradiol and progesterone plasma levels peaked (reviewed by Fillingim and Ness, 2000). Future elucidation of the exact hormone molecule mediator of the observed behavioral effects is necessary, in order to begin to uncover the cellular and molecular mechanisms underlying the behavioral phenomenon of sex differences in pain.
Our observation that neonatal castration in male pups lowers thermal nociceptive withdrawal latency but does not affect the subsequent thermal hyperalgesia or mechanical allodynia following L5 nerve root injury in adulthood is unexpected and is potentially significant. Other groups have previously shown that the modality (i.e. thermal or mechanical) of the nociceptive stimuli can be modulated differentially by gonadal hormones (Tall et al., 2001). Since mechanical and thermal stimuli are reported to be mediated by primarily large Aβ afferents and C-fibers, respectively (Ossipov et al., 1999), our data supports a hypothesis that gonadal hormones can differentially modulate different subtypes of neurons that have mechanistically distinct roles in mediating diverse stimuli. This observation has also been made clinically, as some types of stimuli (i.e. electrical and pressure) show greater pain responsiveness in women than others (i.e. thermal) (Lautenbacher and Rollman, 1993).
Our observation that female tactile and thermal hypersensitivity is reversible upon gonadal hormone elimination in adult rats points to the clinical application of gonadal hormone modulators as a potential therapy for chronic pain. The classical antiestrogen, tamoxifen, has shown clinical efficacy in the treatment of premenstrual and cyclical mastalgia in women (Kontostolis et al., 1997; Messinis and Lolis, 1988). Another novel target with therapeutic potential is the aromatase enzyme, which catalyzes the conversion of androgens (i.e. testosterone) into estrogens. One study, using the Japanese quail, has shown that administration of an aromatase antagonist can reverse gonadectomyinduced decreases in the nociceptive threshold (Evrard and Balthazart, 2003). Furthermore, aromatase inhibitors have shown promise in the treatment of pelvic pain associated with endometriosis (Takayama et al., 1998).
The gonadal hormones have myriad and complex actions on all levels of the nociceptive axis. There is evidence for gonadal hormone stimulation of neurogenesis (Breedlove et al., 1983; Nordeen et al., 1985) and apoptosis (Nilsen et al., 2000), synapse formation (McEwen et al., 2001) and synapse elimination (Calizo and Flanagan-Cato, 2002), neuropeptide expression (De Vries, 1990), and glial modulation (Drew and Chavis, 2000; McCarthy et al.,2002). The seemingly ubiquitous and contradictory nature of all of these actions has confounded the development of a singular mechanistic hypothesis that accounts for the effects of gonadal hormones in the modulation of nociceptive sensitivity. The present study demonstrates the importance of the temporal sequence of gonadal hormones action in the CNS in order to provide the framework necessary for further research into the role of gonadal hormone modulation of pain sensitivity.
Acknowledgements
The authors wish to thank Ann S. Clark for her technical assistance and Tracy Wynkoop and Nancy Nutile-McMenemy for their editorial assistance. This study was supported by funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases AR44757 (JAD) and the Bristol Myers-Squibb/Zimmer Orthopedic Foundation (JAD).
References
- Aloisi AM. Gonadal hormones and sex differences in pain reactivity. Clin J Pain. 2003;19:168–74. doi: 10.1097/00002508-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Ceccarelli I. Role of gonadal hormones in formalin-induced pain responses of male rats: modulation by estradiol and naloxone administration. Neuroscience. 2000;95:559–66. doi: 10.1016/s0306-4522(99)00445-5. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Fessler RG. Gonadectomy and sensitivity to electric shock in the rat. Physiol Behav. 1977;19:1–6. doi: 10.1016/0031-9384(77)90149-4. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–80. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci. 2000;97:3602–7. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn-Munro G, Blackburn-Munro R. Pain in the brain: are hormones to blame? Trends Endocrinol Metab. 2003;14:20–7. doi: 10.1016/s1043-2760(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Jordan CL, Arnold AP. Neurogenesis of motoneurons in the sexually dimorphic nerve nucleus of the bulbocavernosus in rats. Brain Res. 1983;285:39–43. doi: 10.1016/0165-3806(83)90106-2. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Cooke BM, Jordan CL. The orthodox view of brain sexual differentiation. Brain Behav Evolut. 1999;54:8–14. doi: 10.1159/000006607. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Flanagan-Cato LM. Estrogen-induced dendritic spine elimination on female rat ventromedial hypothalamic neurons that project to the periaqueductal gray. J Comp Neurol. 2002;447:234–48. doi: 10.1002/cne.10223. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O’Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmnol. 1997;79:163–75. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrin. 1998;19:323–62. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Coyle DE. Intact female rats are more susceptible to the development of tactile allodynia than ovariectomized female rats following partial sciatic nerve ligation (PSNL) Neurosci Lett. 1996;203:37–40. doi: 10.1016/0304-3940(95)12259-1. [DOI] [PubMed] [Google Scholar]
- Coyle DE, Sehlhorst CS, Mascari C. Female rats are more susceptible to the development of neuropathic pain using the partial sciatic nerve ligation (PSNL) model. Neurosci Lett. 1995;186:135–8. doi: 10.1016/0304-3940(95)11304-f. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J Neuroendocrinol. 2000;12:445–52. doi: 10.1046/j.1365-2826.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Rutkowski MD. Gender differences in rat neuropathic pain sensitivity is dependent on strain. Neurosci Lett. 2000;282:197–9. doi: 10.1016/s0304-3940(00)00880-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in neurotransmitter systems. J Neuroendocrinol. 1990;2:1–13. doi: 10.1111/j.1365-2826.1990.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111:79–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- D’Souza DN, Harlan RE, Garcia MM. Modulation of glutamate receptor expression by gonadal steroid hormones in the rat striatum. Brain Res Bull. 2003;59:289–92. doi: 10.1016/s0361-9230(02)00885-7. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Balthazart J. Aromatase (estrogen synthase) activity in the dorsal horn of the spinal cord: functional implications. Ann N Y Acad Sci. 2003;1007:263–71. doi: 10.1196/annals.1286.025. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–45. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- Giles BE, Walker JS. Sex differences in pain and analgesia. Pain Rev. 2000;7:181–93. [Google Scholar]
- Green PG. Role of adrenal medulla in development of sexual dimorphism in inflammation. Eur J Neurosci. 2001;14:1436–44. doi: 10.1046/j.0953-816x.2001.01768.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine. 2000;25:1206–17. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- Kontostolis E, Stefanidis K, Navrozoglou I, Lolis D. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393–7. doi: 10.3109/09513599709152566. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929:1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- LaCroix-Fralish ML, Rutkowski MD, Weinstein JN, Mogil JS, DeLeo JA. Spine. The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. [DOI] [PubMed] [Google Scholar]
- Lanlua P, Decorti F, Gangula PRR, Chung K, Taglialatela G, Yallampalli C. Female steroid hormones modulate receptors for nerve growth factor in rat dorsal root ganglia1. Biol Reprod. 2001;64:331–8. doi: 10.1095/biolreprod64.1.331. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Rollman GB. Sex differences in responsiveness to painful and non-painful stimuli are dependent upon the stimulation method. Pain. 1993;53:255–64. doi: 10.1016/0304-3959(93)90221-A. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Amateau SK, Mong JA. Steroid modulation of astrocytes in the neonatal brain: implications for adult reproductive function. Biol Reprod. 2002;67:691–8. doi: 10.1095/biolreprod.102.003251. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol Sci. 1991;12:141–7. doi: 10.1016/0165-6147(91)90531-v. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci. 2001;98:7093–100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi RC, Magnagh V, Galbiati M, Ghelarducci B, Sebastiani L, Martini L. The action of steroid hormones on peripheral myelin proteins: a possible new tool for the rebuilding of myelin? J Neurocytol. 2000;29:327–33. doi: 10.1023/a:1007105121765. [DOI] [PubMed] [Google Scholar]
- Messinis IE, Lolis D. Treatment of premenstrual mastalgia with tamoxifen. Acta Obstet Gynecol Scand. 1988;67:307–9. [PubMed] [Google Scholar]
- Moggs JG, Orphanides G. Estrogen receptors: orchestrators of pleiotropic cellular responses. Eur Mol Biol Org Rep. 2001;2:775–81. doi: 10.1093/embo-reports/kve185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Mor G, Naftolin F. Estrogen-regulated developmental neuronal apoptosis is determined by estrogen receptor subtype and the Fas/Fas ligand system. J Neurobiol. 2000;43:64–78. doi: 10.1002/(sici)1097-4695(200004)43:1<64::aid-neu6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–3. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Bian D, Malan TP, Jr, Lai J, Porreca F. Lack of involvement of capsaicin-sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain. 1999;79:127–33. doi: 10.1016/s0304-3959(98)00187-0. [DOI] [PubMed] [Google Scholar]
- Park CM, Clegg KE, Harvey-Clark CJ, Hollenberg MJ. Improved techniques for successful neonatal rat surgery. Lab Anim Sci. 1992;42:508–13. [PubMed] [Google Scholar]
- Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physiol Behav. 1986;38:887–90. doi: 10.1016/0031-9384(86)90058-2. [DOI] [PubMed] [Google Scholar]
- Pheonix C, Goy R, Gerall A, Young W. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinol (Baltimore) 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Genazzani AR. Non-genomic actions of sex steroid hormones. Euro J Endocrinol. 2003;148:281–92. doi: 10.1530/eje.0.1480281. [DOI] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Res. 1988;475:272–82. doi: 10.1016/0006-8993(88)90615-4. [DOI] [PubMed] [Google Scholar]
- Tall JM, Stuesse SL, Cruce WL, Crisp T. Gender and the behavioral manifestations of neuropathic pain. Pharmacol Biochem Behav. 2001;68:99–104. doi: 10.1016/s0091-3057(00)00461-5. [DOI] [PubMed] [Google Scholar]
- Takayama K, Zeitoun K, Gunby RT, Sasano H, Carr BR, Bulun SE. Treatment of severe postmenopausal endometriosis with an aromatase inhibitor. Fertil Steril. 1998;694:709–13. doi: 10.1016/s0015-0282(98)00022-3. [DOI] [PubMed] [Google Scholar]
- Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endo Rev. 1993;14:459–79. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- Yunus MB. Gender differences in fibromyalgia and other related syndromes. J Gender Spec Med. 2002;5:42–7. [PubMed] [Google Scholar]