Abstract
Persistent replication of coxsackievirus B4 (CVB4) E2 (diabetogenic) and CVB4 JBV (nondiabetogenic) strains in thymic epithelial cell (TEC)-enriched cultures (≥95%) was proved by detection of positive- and negative-strand viral RNA by reverse transcription-PCR in extracted RNA from cell cultures, VP1 capsid protein detection by immunofluorescence (IF) staining, and release of infectious particles up to 30 days after infection without obvious cytolysis. By double-IF staining, cytokeratin-containing cells were shown to be susceptible to CVB4. The persistence of CVB4 was associated with a significantly increased rate of TEC proliferation (up to 70%) after 20 days of culture and a significantly increased chronic production of immunoreactive interleukin-6 (IL-6), leukemia inhibitory factor, and granulocyte-macrophage colony-stimulating factor in supernatant after 3 days of culture. The CVB4 replication and the release of cytokines were not restricted to the CVB4 E2 diabetogenic strain and did not depend on the genetic background of the host; however, TEC were more responsive to CVB4 E2 than CVB4 JBV as far as the production of cytokines.
Environmental factors, especially virus infections, are supposed to be involved in the pathophysiology of type 1 diabetes (16). Enteroviruses of the family Picornaviridae are small, naked icosahedral viruses ranged into four subgroups: poliovirus, coxsackievirus A, coxsackievirus B (CVB), and echovirus. Epidemiological studies have highlighted an association between incidence of type 1 diabetes and recent enterovirus infections (10). Moreover, exposure to enterovirus infection , in utero or during childhood, increases the risk of diabetes occurrence (17). Among enteroviruses, CVB is a possible etiological factor in promoting β-cell autoimmune destruction. The CVB genome and alpha interferon have been detected in peripheral blood of type 1 diabetic patients (6). The CVB4 strain E2 was isolated from the pancreas of a child who died from diabetic ketoacidosis (32), and CVB4 E2 infection induces diabetes, with hyperglycemia and β-cell autoimmunity, in some strains of mice (15, 26). The pathogenic mechanism by which CVB4 can contribute to the development of an autoimmune disease like type 1 diabetes has not been elucidated. As for other organ- or cell-specific autoimmune diseases, the development of type 1diabetes corresponds to a loss of immune self-tolerance at a central and/or peripheral level (29); however, the association between CVB4 infection and the loss of immune self-tolerance has not been investigated.
The induction of immune self-tolerance is an active multistep process already initiated within the thymus network during T-cell ontogeny (13, 14, 20). The thymus exerts a prominent role in the establishment of central T-cell self-tolerance (by clonal deletion of self-reactive T cells and generation of regulatory CD4+ CD25+ cells), as well as in the development of self major histocompatibility complex-restricted and self-tolerant T lymphocytes (1, 25). Thymic epithelial cells (TEC) play a critical role in the differentiation of T-cell precursors, providing a microenvironment with a unique capacity to generate functional and self-tolerant T cells (2). Cytokines like interleukin-6 (IL-6), leukemia inhibitory factor (LIF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) produced by TEC in the thymus (18) play an important role in this process. IL-6 has been demonstrated as a cofactor of proliferation of various thymic T-cell subpopulations (11). LIF is involved in maturation of T lymphocytes: lif−/− mice are defective in T-cell activation (30), and mice overexpressing lif in T cells display thymic and lymph node abnormalities (28). GM-CSF specifically activates the proliferation of immature thymocytes (9).
A variety of different viruses can infect human thymic epithelial tissue (22, 31). Because of the important role of the thymus in establishment of self-tolerance, viral infection of thymic tissue may play a role in the pathogenesis of autoimmune diseases. The role of a virus in the induction and/or triggering of type 1 diabetes could result from interference with the central tolerogenic role of the thymus through an interaction between the virus and TEC. Selinka et al. presented evidence of 50-, 100-, and 120-kDa CVB-binding proteins in mouse thymus (27), but nothing is known about the susceptibility of the human thymus to CVB infection. Therefore, in the present study, infection of human TEC primary cultures with CVB4 and the resulting effects on TEC function have been investigated.
CVB4 induces a persistent infection of human TEC.
Thymus fragments were obtained from children (6 months to 3 years old) undergoing corrective cardiovascular surgery for congenital cardiopathies and processed as previously described to isolate TEC (24). Thymus fragments were cultured for 18 days, and then adherent confluent cells (TEC) were detached by treatment with a solution of trypsin-Versene (EDTA) (BioWhittaker, Verviers, Belgium) and filtered through nylon gaze to eliminate explant residues. Cultured human TEC were counted and seeded at 400,000 cells per T-25 flask (Corning, Acton, Mass.) for the dosage of infectious viral particles in TEC culture supernatants and cytokine assays for long periods postinfection. Cells were seeded at 200,000 cells per well on 6-well plates for total RNA extraction and also at 3,500 and 30,000 cells per well on 96- and 24-well culture plates for the proliferation assay and cytokine assays, respectively, in experiments with different concentrations of virus.
TEC were infected with CVB4 JBV (provided by J. Almond, Aventis Pasteur, Marcy-L'Etoile, France) and E2 strains (provided by J. W. Yoon, Julia McFarlane Diabetes Research Center, Calgary, Alberta, Canada) propagated in Hep2 cells (BioWhittaker) in Eagle's minimal essential medium (Gibco BRL, Life Technologies, Rockville, Md.) supplemented with 10% decomplemented fetal calf serum and 1% l-glutamine. TEC and Vero cell lines were inoculated with each CVB4 strain at 1 or 10 PFU/TEC and then incubated for 90 min at 37°C in a humidified atmosphere with 5% CO2. Then, TEC cultures were extensively washed with phosphate-buffered saline (BioWhittaker) and subcultured for different periods in complete culture medium and incubated at 37°C with 5% CO2.
To detect viral internalization and replication in TEC cultures, the presence of positive and negative RNA was examined by reverse transcription-PCR (RT-PCR) at different periods after infection. Total RNA was extracted with RNeasy mini kit (Qiagen, Valencia, Calif.) and resuspended in water according to the manufacturer's instructions. Then, RNA was dosed with the RiboGreen RNA quantitation kit (Molecular Probes, Eugene, Oreg.) and used in RT-PCR. Specific primers for human β-actin (upper, 5′-CTACAATGAGCTGCGTGTGG-3′; and lower, 5′-AAGGAAGGCTGGAAGAGTGC-3′) generated a 528-bp product and a 969-bp fragment if contamination by genomic DNA occurred. Primers (synthesized by Biosource, Nivelles, Belgium) used for the detection of CB4 RNAs were located within the enterovirus 5′NC region, which is highly conserved among the enterovirus serotype (upper, 5′-CAAGCACTTCTGTTTCCCCGG-3′; and lower, 5′-ATTGTCACCATAAGCAGCCA-3′). These primers generate a 435-bp product and were previously described by Leparc et al. (19). RT-PCRs were performed with Ready-To-Go RT-PCR beads (Amersham Pharmacia Biotech, Roosendaal, The Netherlands). In a final volume of 50 μl, cDNA synthesis and amplification from human β-actin and positive CB4 RNA were performed in a one-step reaction with 25 ng of total RNA, 1 μg of random hexamer primers, and 20 pmol of each specific primer, according to the manufacturer's instructions (Amersham Pharmacia Biotech). Negative CB4 RNA was evidenced in a two-step reaction. First, 25 ng of total RNA was reverse transcribed in the presence of 20 pmol of CB4 upper primer and then denatured for 10 min at 94°C. The PCR was carried out in the same tube with all of the cDNA and 20 pmol of each of the specific primers. PCR products were visualized by electrophoresis on 2% agarose gel stained with 0.5 μg of bromide of ethidium chloride per ml (Gibco BRL, Life Technologies, Rockville, Md.).
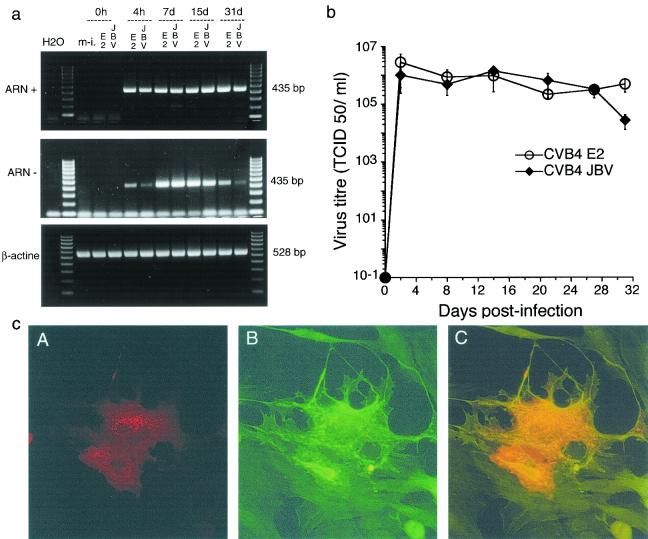
Plus and minus RNAs were detected in CVB4-infected TEC cultures as soon as 4 h after infection and during 30 days after infection (Fig. 1a). The ability of TEC to yield infectious viral particles was measured by a specific infectivity assay in Vero cells (provided by B. Rentier, University of Liege, Liege, Belgium) at several intervals postinfection (p.i.) to determine the 50% tissue culture infective dose (TCID50) (Fig. 1b). CVB4 E2 and JBV infection of TEC was productive and reached 106 TCID50s/ml at 48 h p.i. Virus production was maintained thereafter and was not stopped by serial subculture for 30 days p.i., suggesting that CVB4 E2 and JBV replicated persistently in human TEC. Enterovirus VP1 was also detected by immunocytofluorescence in human TEC cultures as previously described by using with monoclonal anti-viral protein 1 (VP1) antibody (Dako, Glostrup, Denmark) (5). To identify the nature of infected cells, we performed a double immunostaining of CVB4-infected TEC cultures. Slides were incubated with rabbit polyclonal anticytokeratin (anti-CK) antibody A575 (Dako) diluted at 1:50 overnight at 4°C. Following three washes, fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody (Jackson ImmnuoResearch Laboratories, Inc., West Grove, Pa.) was applied at a dilution of 1:50 for 1 h at room temperature. The slides were then washed and incubated with monoclonal anti-viral protein 1 (VP1) antibody diluted at 1:20 for 2 h at room temperature. Then the slides were washed and stained with tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-mouse immunoglobulin G antibody (Jackson ImmunoResearch Laboratories, Inc.) during 1 h at room temperature, washed again, and mounted with glycerol-gelatin medium, and positive cells were enumerated with a fluorescence microscope (Olympus).
FIG. 1.
TEC infected with CVB4. (a) Agarose gel electrophoresis of amplicons specific to the positive and negative strands of the enterovirus genome (435 bp). Strand-specific RT-PCR was carried out with 25 ng of total RNA taken from mock-infected TEC (m-i.) and CVB4E2-and CVB4JVB-infected TEC at 0 and 4 h and at 7, 15, and 30 days p.i. H2O, negative control. Equivalent expression of β-actin-specific RNA (528 bp) was detected in all samples. The results are representative of three independent experiments. (b) Release of infectious particles by TEC infected with CVB4 JBV and CVB4 E2. The viral titer in culture supernatants at various times p.i. was determined by the TCID50 assay with Vero cell cultures. The means ± standard deviations of three independent experiments are presented. (c) Detection of enterovirus viral protein 1 (VP1) by IF in human TEC 24 h after infection with CVB4 E2 (A, B, and C, ×200). (A) Cells indirectly stained for VP1 with TRITC-conjugated antibodies. (B) Cells indirectly stained for CK with FITC-conjugated antibodies showing the dominant epithelial phenotype of cultures. (C) Cells double stained for VP1 with TRITC-conjugated antibodies and for CK with FITC-conjugated antibodies. In double-stained cells, orange was observed. Similar results were obtained with CVB4 JBV.
Approximately 95 to 98% of cultured cells were positive for CK and thus were identified as TEC. After both infection with E2 and JBV, VP1 was located in the cytoplasm of CK-positive cells around nuclei, confirming that TEC were the VP1-producing cells (Fig. 1c). The proportions of VP1-positive TEC were quite stable: 5 to 10% at 24 h p.i. and 3 to 5% further in the culture (16 days p.i. and after).
CVB4 infection enhances the proliferation of TEC.
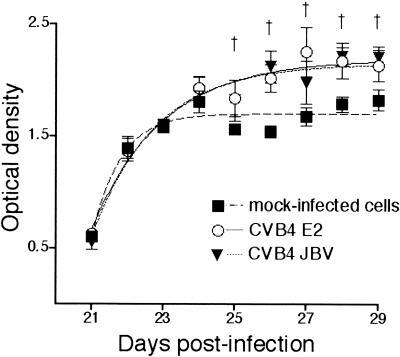
CVB4 infection did not affect the appearance of TEC by light microscopy after as many as 30 days. There was no significant change in mortality between CVB4- and mock-infected TEC cultures (<5% in both conditions). Cells were harvested from CVB4-infected or mock-infected human TEC cultures on the day of infection and 6, 13, and 20 days p.i., and then the cells were cultured in 96-well plates (3,000 cells/well) in octoplicate for 5 days. Every day, the cell proliferation reagent WST-1 (Roche, Indianapolis, Ind.), which identifies living cells, was added to the cultures, and then the cells were incubated for 4 h at 37°C with 5% CO2. The resulting formazan dye was quantitated at A450 to A690 with a spectrophotometer (Vmax kinetic microplate reader; Molecular Devices). Optical density values obtained on the day of infection and 6, 13, and 20 days p.i. were considered as baseline. The kinetics of growth was not significantly different between TEC infected with CVB4 E2 or JBV and mock-infected cells until 20 days p.i. (data not shown). After this delay, the proliferation of CVB4 JBV- and E2-infected TEC was significantly higher (20 to 70%) than that of mock-infected cells in experiments performed with cells from three different donors (P < 0.05) (Fig. 2).
FIG. 2.
Effect of CVB4 infection on TEC proliferation. Proliferation kinetics of CVB4 E2- and CVB4 JVB-infected human TEC (MOI of 10) from 20 days p.i. (20 dpi) to 30 days p.i. (end of the culture) determined with a colorimetric assay. Results of three different thymuses in eight samplings were collected (expressed as means ± standard deviations) and presented as a Boltzman sigmoidal curve. †, P < 0.05 for CVB4 E2 and JBV versus mock-infected cells (analysis of variance followed by Student-Newman-Keuls posttests).
CVB4 infection modulates TEC secretion of cytokines.
TEC cultures were infected with 1 and 10 PFU of each CVB4 strain per cell. Then, the secretion profiles of IL-6 and LIF were investigated in TEC cultures during 168 h after infection. Specific enzyme-linked immunosorbent assays were used to measure human IL-6, LIF, and GM-CSF (BioSource Europe, Nivelles, Belgium) in culture supernatant samples. The assays were performed according to the manufacturer's instructions. Absorbency was transformed to cytokine concentration by using a standard curve computed with BioSource enzyme-linked immunosorbent assay software (BioSource Europe). The levels of IL-6 and LIF in supernatants of cultures infected with CVB4 JBV at a multiplicity of infection (MOI) of 1 were not different from those of mock-infected cultures. In contrast, in supernatants of cultures infected with CVB4 JBV at an MOI of 10 or with CVB4 E2 at an MOI of 1 or 10, increased levels of IL-6 and LIF were found 96 h p.i. and thereafter compared with mock-infected cultures (Table 1). Moreover, the levels of cytokines obtained in cultures infected with CVB4 E2 at an MOI of 1 were higher than those in cultures infected with CVB4 JBV at the same MOI (P < 0.05).
TABLE 1.
Levels of IL-6 and LIF in TEC cultures infected with CVB4 E2 and JBV 168 h p.i.
| Cytokine | % Cytokine levela
|
|||
|---|---|---|---|---|
| CVB4 E2
|
CVB4 JBV
|
|||
| MOI-1 | MOI-10 | MOI-1 | MOI-10 | |
| IL-6 | 443 ± 98.5† | 391 ± 56.3* | 142 ± 10.7 | 266 ± 42.4† |
| LIF | 207 ± 7.0† | 229 ± 7.0* | 108 ± 6.9 | 172 ± 6.7† |
Results are expressed as percentages (mean ± standard error [n = 3]), with the cytokine levels in mock-infected cultures equivalent to 100. P < 0.05 (†) or P < 0.01 (*) versus mock-infected cells.
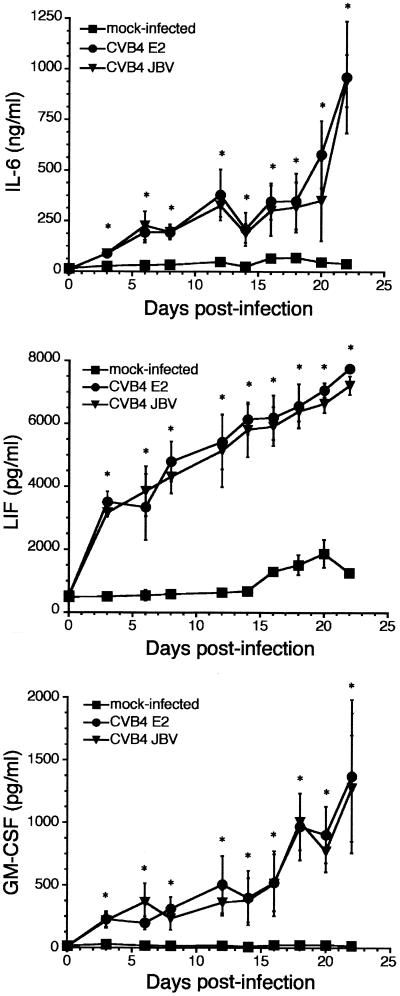
When TEC were challenged for 1 h 30 min with CVB4 JBV or CVB4 E2 (MOI of 10) and then washed and refed with complete medium every 3 days for 30 days, continuous production of IL-6, LIF, and GM-CSF was detected. Compared with mock-infected cultures, the cytokine concentrations remained significantly high and increased from day 3 p.i. to day 21 p.i (Fig. 3). Then the levels of IL-6, LIF, and GM-CSF in infected-TEC cultures decreased progressively, whereas those of mock-infected cultures were stable (data not shown).
FIG. 3.
Continuous release of IL-6, LIF, and GM-CSF in supernatants of TEC infected with CVB4 JBV and CVB4 E2 (MOI of 10) and submitted to serial subculture. Cytokine levels were detected by enzyme-linked immunosorbent assay. The results are expressed as nanograms per milliliter for IL-6 and picograms per milliliter for LIF and GM-CSF. Means ± standard deviations of three independent experiments are presented. ∗, P < 0.01 for CVB4 E2 and JBV versus mock-infected cells.
The specificity of the CVB4 effects was checked with a neutralizing polyclonal rabbit anti-CVB4 antibody (Eurobio, Les Ulis, France) at a dilution of 1:10. Both the CVB4 E2 and JBV strains were blocked 90 min at 37°C before inoculation into TEC cultures. When CVB4 E2 and JBV stock solutions used for challenging TEC were pretreated with a neutralizing antibody, the enhanced production of cytokines was inhibited (Table 2).
TABLE 2.
Effect of anti-CVB4-neutralizing antibody on IL-6 and LIF production by TEC 168 h p.i.
| Culture type | Cytokine production with anti-CVB4 antibody statusa:
|
|||
|---|---|---|---|---|
| IL-6 (ng/ml)
|
LIF (pg/ml)
|
|||
| − | + | − | + | |
| Mock infected | 16 ± 1 | 16 ± 0.01 | 430 ± 3.7 | 456 ± 5 |
| CVB4 E2 (MOI = 10) | 61 ± 0.5* | 15 ± 0.3 | 1,055 ± 1.1* | 443 ± 22.7 |
| CVB4 JBV (MOI = 10) | 51 ± 0.6* | 22 ± 0.3 | 997 ± 83.1* | 434 ± 10 |
Results are means ± standard deviations (n = 3). *, P < 0.01 versus mock-infected cells.
This study investigates the hypothesis of CVB4 infection of human TEC primary cultures. The detection of infectious particles released in the supernatants of cultures, as well as the presence of VP1 in cells and the presence of viral positive- and negative-strand RNA in cultures up to 30 days p.i., demonstrates that continuous CVB4 replication occurs in TEC cultures. CVB4 predominantly replicates in TEC in our experiments, as proved by the double-immunofluorescence (IF) of CVB-infected cells with VP1 present in CK-containing cells only and by the percentage of VP1-positive cells (10%). This excludes the role of nonepithelial cells (1 to 5%) as major host cells in TEC-enriched cultures in these experiments. The decrease in the proportion of infected cells in the course of the culture (from 10% to 5%) may be due in part to the lysis of some cells. However, according to the relatively low number of infected cells, significant lysis of infected cells did not occur. Thus, TEC cultures are capable of supporting the persistent replication of CVB4 without obvious cytopathic effect. Our results show that CVB4, usually described as a cytolytic virus, can persist in certain human cell types, which is in agreement with previous in vitro studies of CVB infection of vascular endothelial cells (7, 8) and pancreatic β-cells (5). Our data suggest that the persistence of CVB4 in TEC cultures is probably established through the mechanism of the carrier-state culture as previously reported for other cell types (8).
A relatively small proportion of TEC containing viral proteins was detected by IF in the earlier stages of infection (≤10% at 24 h p.i.) and during chronic infection (< 5%). These data suggest that there is heterogeneity in the susceptibility of TEC to CVB4 infection, which can be due to the presence of TEC subpopulations in the cultures (insofar as TEC are derived from both thymic cortex and medulla with a predominant medullary origin), or to the metabolic heterogeneity of these cells (12, 24). Further experiments are needed to clarify the mechanisms of TEC susceptibility to CVB4.
Human TEC can be infected with different viruses in vitro. Certain human immunodeficiency virus type 1 strains and isolates induce a productive and persistent infection in TEC without damaging the cells, which is consistent with the CVB4 infection of TEC observed in our studies (3). In the case of cytomegalovirus and measles virus, a persistent infection can be obtained, but morphological changes appear (22). Also, in the case of herpes simplex virus type 2 and adenovirus 7, an obvious cytopathic effect was induced (31). CVB4 replicated in TEC cultures, but it was not cytotoxic. In contrast, compared with mock-infected cells, an increased proliferation rate was observed in CVB4-infected cultures 20 days after infection. The predominant cells in these cultures were TEC, as proved by the high percentage of CK-containing cells by IF staining (>90%), which excludes the role of non-TEC-contaminating cells (<10% of the cells) as major proliferating cells in these experiments. Several factors may be responsible for the initiation of increased TEC proliferation in CVB4-infected cultures. The increased production of cytokines in CVB4-infected TEC cultures observed in the present study suggests that virus-induced factors can play a role. Whether viral components and/or virus-induced growth factors are involved in this phenomenon remains to be determined.
The CVB4 JBV strain was capable of infecting and replicating in human TEC from three different donors with the same intensity as the CVB4 diabetogenic E2 strain did. No significant difference was detected in levels of cytokines induced by these virus strains at an MOI of 10, but the level of cytokines induced by CVB4 E2 at an MOI of 1 was significantly higher than that induced by CVB4 JBV at the same MOI. Thus, the viral replication and production of cytokines in TEC cultures were not restricted to the CVB4 E2 diabetogenic strain and did not depend on the genetic background of the host. However, as far as the production of cytokines is concerned, TEC were more responsive to CVB4 E2 than CVB4 JBV. The precise mechanism remains to be elucidated; however, the capability to increase the production of cytokines in the case of CVB4 JBV and CVB4 E2 is reminiscent of the findings in other reports. Indeed, with poliovirus strains, members of the enterovirus group like CVB, it has been demonstrated that wild-type or neurovirulent strains were better interferon inducers than attenuated strains (23).
Our data confirm previous observations that TEC spontaneously produce cytokines in vitro (18, 21) and that viruses can modulate the production of cytokines by TEC, without a virus-induced cytopathic effect (31). However, our study is different from those of other investigators, because we report a significantly increased production of IL-6, LIF, and GM-CSF by TEC in response to CVB4 and because CVB4 replication was investigated at the molecular level. Indeed, the continuous increased cytokine production observed in the course of the culture of CVB4-infected TEC (up to 30 days) was correlated with the detection of VP1, viral RNA (positive and negative strands) in the cells, and viral progeny in culture supernatants, and the increased production of cytokines was inhibited by pretreatment of the virus with neutralizing antiserum against CVB4. Together, these data show that the persistent replication of CVB4 in TEC results in increased production of cytokines by these cells. Due to the increased rate of proliferation, infected TEC were confluent at 21 days p.i. in our experiments, whereas mock-infected TEC were not confluent up to 30 days p.i. That difference can explain the decreased levels of cytokines in infected cultures at 21 days p.i., whereas the values were lower, but remained unchanged in mock-infected cultures up to 30 days p.i.
Diabetogenic CVB4 can reach the thymus in the course of systemic infection of mice, which results in a significant increase in CD4− CD8− thymic T lymphocytes together with insulitis and hyperglycemia (4). The nature of infected cells within the thymus was not elucidated in the previous study, but these data suggest that CVB4 can induce abnormal T-lymphocyte maturation, which may contribute to development of diabetes. Studies are in progress in our laboratory to investigate whether, in vivo, CVB4 can infect the epithelial network of the thymus, as suggested by our in vitro studies. It will be of interest to investigate whether CVB4-infected TEC and the resulting increase in cytokine levels in the microenvironment of TEC play a role in the impairment of central T-cell self-tolerance. In our laboratory, future studies will be directed along this line to provide better knowledge about the pathogenesis of CVB4-induced diabetes type 1.
Acknowledgments
We thank H. Rubay (St. Luc Hospital, Brussels, Belgium) for providing thymuses from infants undergoing cardiac surgery.
F. Brilot was supported by the Fond pour la Recherche en Industrie et Agriculture (FRIA) and by a travel grant of the Communauté Française de Belgique. This work was supported by the Léon Frédérick Foundation; by the Association Belge du Diabète; by the European Association of Study of Diabetes (EASD); by Centre Hospitalier Régional et Universitaire (CHRU) de Lille (98/911); and by Ministère de l'Education Nationale de la Recherche et de la Technologie, UPRES EA, Université de Lille II (“Pathogenèse Virale du Diabète de Type 1”).
REFERENCES
- 1.Benoist, C., and D. Mathis. 1999. T-lymphocyte differentiation and biology, p. 367-409. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven, Philadelphia, Pa..
- 2.Bonomo, A., and P. Matzinger. 1993. Thymus epithelium induces tissue-specific tolerance. J. Exp. Med. 177:1153-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, J., H. Valentin, M. T. Nugeyre, H. Ohayon, P. Gounon, and F. Barre-Sinoussi. 1996. Productive and persistent infection of human thymic epithelial cells in vitro with HIV-1. Virology 225:413-418. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee, N. K., J. Hou, P. Dockstader, and T. Charbonneau. 1992. Coxsackievirus B4 infection alters thymic, splenic, and peripheral lymphocyte repertoire preceding onset of hyperglycemia in mice. J. Med. Virol. 38:124-131. [DOI] [PubMed] [Google Scholar]
- 5.Chehadeh, W., J. Kerr-Conte, F. Pattou, G. Alm, J. Lefebvre, P. Wattré, and D. Hober. 2000. Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in β cells. J. Virol. 74:10153-10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chehadeh, W., J. Weill, M. C. Vantyghem, G. Alm, J. Lefebvre, P. Wattre, and D. Hober. 2000. Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J. Infect. Dis. 181:1929-1939. [DOI] [PubMed] [Google Scholar]
- 7.Conaldi, P. G., L. Biancone, A. Bottelli, A. De Martino, G. Camussi, and A. Toniolo. 1997. Distinct pathogenic effects of group B coxsackieviruses on human glomerular and tubular kidney cells. J. Virol. 71:9180-9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conaldi, P. G., C. Serra, A. Mossa, V. Falcone, F. Basolo, G. Camussi, A. Dolei, and A. Toniolo. 1997. Persistent infection of human vascular endothelial cells by group B coxsackieviruses. J. Infect. Dis. 175:693-696. [DOI] [PubMed] [Google Scholar]
- 9.Denning, S. M., J. Kurtzberg, P. T. Le, D. T. Tuck, K. H. Singer, and B. F. Haynes. 1988. Human thymic epithelial cells directly induce activation of autologous immature thymocytes. Proc. Natl. Acad. Sci. USA 85:3125-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fohlman, J., and G. Friman. 1993. Is juvenile diabetes a viral disease? Ann. Med. 25:569-574. [PubMed] [Google Scholar]
- 11.Galy, A. H., C. A. Dinarello, T. S. Kupper, A. Kameda, and J. W. Hadden. 1990. Effects of cytokines on human thymic epithelial cells in culture. II. Recombinant IL1 stimulates thymic epithelial cells to produce IL6 and GM-CSF. Cell. Immunol. 129:161-175. [DOI] [PubMed] [Google Scholar]
- 12.Gay-Bellile, V., L. Boumsell, B. Caillou, and A. Bernard. 1986. Phenotypic characterization of the thymus microenvironment study of the human thymus architecture. Thymus 8:201-223. [PubMed] [Google Scholar]
- 13.Geenen, V., O. Kecha, F. Brilot, I. Hansenne, C. Renard, and H. Martens. 2001. Thymic T-cell tolerance of neuroendocrine functions: physiology and pathophysiology. Cell. Mol. Biol. 47:179-188. [PubMed] [Google Scholar]
- 14.Geenen, V., and G. Kroemer. 1993. Multiple ways to cellular immune tolerance. Immunol. Today 14:573-575. [DOI] [PubMed] [Google Scholar]
- 15.Gerling, I., N. K. Chatterjee, and C. Nejman. 1991. Coxsackievirus B4-induced development of antibodies to 64,000-Mr islet autoantigen and hyperglycemia in mice. Autoimmunity 10:49-56. [DOI] [PubMed] [Google Scholar]
- 16.Graves, P. M., J. M. Norris, M. A. Pallansch, I. C. Gerling, and M. Rewers. 1997. The role of enteroviral infections in the development of IDDM: limitations of current approaches. Diabetes 46:161-168. [DOI] [PubMed] [Google Scholar]
- 17.Hyoty, H., M. Hiltunen, M. Knip, M. Laakkonen, P. Vahasalo, J. Karjalainen, P. Koskela, M. Roivainen, P. Leinikki, T. Hovi et al. 1995. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes 44:652-657. [DOI] [PubMed] [Google Scholar]
- 18.Le, P. T., S. Lazorick, L. P. Whichard, Y. C. Yang, S. C. Clark, B. F. Haynes, and K. H. Singer. 1990. Human thymic epithelial cells produce IL-6, granulocyte-monocyte-CSF, and leukemia inhibitory factor. J. Immunol. 145:3310-3315. [PubMed] [Google Scholar]
- 19.Leparc, I., M. Aymard, and F. Fuchs. 1994. Acute, chronic and persistent enterovirus and poliovirus infections: detection of viral genome by seminested PCR amplification in culture-negative samples. Mol. Cell. Probes 8:487-495. [DOI] [PubMed] [Google Scholar]
- 20.Martens, H., B. Goxe, and V. Geenen. 1996. The thymic repertoire of neuroendocrine self-antigens: physiological implications in T-cell life and death. Immunol. Today 17:312-317. [DOI] [PubMed] [Google Scholar]
- 21.Martens, H., B. Malgrange, F. Robert, C. Charlet, D. De Groote, D. Heymann, A. Godard, J. P. Soulillou, G. Moonen, and V. Geenen. 1996. Cytokine production by human thymic epithelial cells: control by the immune recognition of the neurohypophysial self-antigen. Regul. Pept. 67:39-45. [DOI] [PubMed] [Google Scholar]
- 22.Numazaki, K., H. Goldman, X. Q. Bai, I. Wong, and M. A. Wainberg. 1989. Effects of infection by HIV-1, cytomegalovirus, and human measles virus on cultured human thymic epithelial cells. Microbiol. Immunol. 33:733-745. [DOI] [PubMed] [Google Scholar]
- 23.Pitkaranta, A., K. Linnavuori, M. Roivainen, and T. Hovi. 1988. Induction of interferon-alpha in human leukocytes by polioviruses: wild-type strains are better inducers than attenuated strains. Virology 165:476-481. [DOI] [PubMed] [Google Scholar]
- 24.Ropke, C. 1997. Thymic epithelial cell culture. Microsc. Res. Tech. 38:276-286. [DOI] [PubMed] [Google Scholar]
- 25.Sakagushi, S. 2001. Policing the regulators. Nat. Immunol. 2:283-284. [DOI] [PubMed] [Google Scholar]
- 26.See, D. M., and J. G. Tilles. 1995. Pathogenesis of virus-induced diabetes in mice. J. Infect. Dis. 171:1131-1138. [DOI] [PubMed] [Google Scholar]
- 27.Selinka, H. C., M. Huber, A. Pasch, K. Klingel, C. Aepinus, and R. Kandolf. 1998. Coxsackie B virus and its interaction with permissive host cells. Clin. Diagn. Virol. 9:115-123. [DOI] [PubMed] [Google Scholar]
- 28.Shen, M. M., R. C. Skoda, R. D. Cardiff, J. Campos-Torres, P. Leder, and D. M. Ornitz. 1994. Expression of LIF in transgenic mice results in altered thymic epithelium and apparent interconversion of thymic and lymph node morphologies. EMBO J. 13:1375-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha, A. A., M. T. Lopez, and H. O. McDevitt. 1990. Autoimmune diseases: the failure of self tolerance. Science 248:1380-1388. [DOI] [PubMed] [Google Scholar]
- 30.Towle, M. F., M. Mondragon-Escorpizo, A. Norin, and K. Fukada. 1998. Deprivation of leukemia inhibitory factor by its function-blocking antibodies augments T cell activation. J. Interferon Cytokine Res. 18:387-392. [DOI] [PubMed] [Google Scholar]
- 31.Wainberg, M. A., K. Numazaki, L. Destephano, I. Wong, and H. Goldman. 1988. Infection of human thymic epithelial cells by human cytomegalovirus and other viruses: effect on secretion of interleukin 1-like activity. Clin. Exp. Immunol. 72:415-421. [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon, J. W., M. Austin, T. Onodera, and A. L. Notkins. 1979. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 300:1173-1179. [DOI] [PubMed] [Google Scholar]